Abstract
In 2017–2019, the March of Dimes convened a workgroup with biomedical, clinical, and epidemiologic expertise to review knowledge of the causes of the persistent Black-White disparity in preterm birth (PTB). Multiple databases were searched to identify hypothesized causes examined in peer-reviewed literature, 33 hypothesized causes were reviewed for whether they plausibly affect PTB and either occur more/less frequently and/or have a larger/smaller effect size among Black women vs. White women. While definitive proof is lacking for most potential causes, most are biologically plausible. No single downstream or midstream factor explains the disparity or its social patterning, however, many likely play limited roles, e.g., while genetic factors likely contribute to PTB, they explain at most a small fraction of the disparity. Research links most hypothesized midstream causes, including socioeconomic factors and stress, with the disparity through their influence on the hypothesized downstream factors. Socioeconomic factors alone cannot explain the disparity's social patterning. Chronic stress could affect PTB through neuroendocrine and immune mechanisms leading to inflammation and immune dysfunction, stress could alter a woman's microbiota, immune response to infection, chronic disease risks, and behaviors, and trigger epigenetic changes influencing PTB risk. As an upstream factor, racism in multiple forms has repeatedly been linked with the plausible midstream/downstream factors, including socioeconomic disadvantage, stress, and toxic exposures. Racism is the only factor identified that directly or indirectly could explain the racial disparities in the plausible midstream/downstream causes and the observed social patterning. Historical and contemporary systemic racism can explain the racial disparities in socioeconomic opportunities that differentially expose African Americans to lifelong financial stress and associated health-harming conditions. Segregation places Black women in stressful surroundings and exposes them to environmental hazards. Race-based discriminatory treatment is a pervasive stressor for Black women of all socioeconomic levels, considering both incidents and the constant vigilance needed to prepare oneself for potential incidents. Racism is a highly plausible, major upstream contributor to the Black-White disparity in PTB through multiple pathways and biological mechanisms. While much is unknown, existing knowledge and core values (equity, justice) support addressing racism in efforts to eliminate the racial disparity in PTB.
Keywords: preterm birth, racism, health disparities, maternal/infant health, stress, birth outcomes, social determinants of health, racial health disparities
Introduction
In 2017, the March of Dimes (MOD) (www.marchofdimes.org) launched an initiative to address the large and persistent racial disparities in preterm birth (PTB) in the United States. A multidisciplinary scientific workgroup was convened to conduct a state-of-the-science literature review of current knowledge of the causes of the Black-White disparity in PTB. This paper represents the consensus of the Workgroup's members.
Preterm birth (PTB, live birth < 37weeks) is the second leading cause of infant mortality in the United States (1) overall and the leading cause of infant mortality among African American/Black infants (2). Preterm infants are at higher risk for major adverse health outcomes in childhood (3–6) and adulthood (7–14).
While PTB has declined over the past century, African American/Black women consistently experience a rate approximately 1.5–1.6 times higher than that of Whites (15–17). Although hypotheses vary, most scientists agree that the causes of the Black-White disparity are complex and require further elucidation (18–22). This paper aims to critically review current knowledge about the Black-White disparity in PTB to inform efforts to eliminate the gap while improving birth outcomes overall. It is important to note that a cause of PTB is not necessarily a cause of the Black-White disparity in PTB. To be a cause of the disparity, a factor must not only influence PTB, it also must have a different prevalence and/or effect size among Black vs. White women.
Methods
Research assistants searched the PubMed, ERIC, Scopus, Science Direct, and Google Scholar databases using preterm, premature, prematurity, and gestational age as search terms, and risk factors suggested by the Workgroup, aiming to include all factors that have received scientific attention. Peer-reviewed, English-language articles published in the past 10 years or classic/key papers addressing whether a given factor: (a) plausibly contributes to PTB, (b) occurs more/less frequently among Blacks, and/or (c) has a larger/smaller effect among Blacks were selected. Limited web-based material from scientifically trustworthy sources [e.g., Centers for Disease Control and Prevention (CDC) reports] also were included. The Workgroup Chair led a subcommittee in drafting an initial paper and subsequent revisions for members' review. Revisions continued until consensus was reached.
As illustrated in Figure 1, based on the metaphor of a river flowing from its upstream source down to its downstream destination, we categorized potential causes as downstream, midstream or upstream, according to the proximity of their hypothesized effects to the outcome of interest (PTB). Downstream factors exert direct effects (e.g., they relatively directly trigger physiological mechanisms that result in PTB). Upstream factors have their effects further away from PTB, i.e., at or near the start of the causal chain, they may operate indirectly through midstream (intermediate) factors, which, in turn, affect downstream factors. Although interpretations have varied, the upstream/downstream theoretical framework has been widely used in public health to differentiate fundamental, underlying causes of ill health from the factors they set in motion (23).
Figure 1.
Potential causes of the Black-White disparity in PTB can be categorized by the hypothesized proximity of their effects on PTB.
Findings From the Review, Including Comments on the Plausibility of Each Hypothesized Factor
Table 1 lists all of the factors examined, organized according to whether they act primarily downstream, midstream, or upstream. Some of the factors, however, could logically be grouped in more than one category. It also is important to note that many of the factors discussed below may interact with each other (and potentially with other factors not listed) in complex ways that can alter their primary effects. Such “syndemic” (24) effects may help explain difficulties researchers have encountered in elucidating the role of these factors in PTB.
Table 1.
Summary table of factors that may influence the black-white disparity in preterm birth.
| Downstream factors hypothesized as causes of the Black-White disparity in preterm birth | Weight of the evidence that the factor contributes to the Black-White disparity in preterm birth |
|---|---|
| Prenatal care | Plausible but existing literature generally does not support a role for quantitative measures of traditional prenatal care |
| Preconception care | Plausible contributing factor but little research available |
| Elective and indicated cesarean section | Plausible contributing factors but insufficient research |
| Substance use disorders | No published studies indicate that substance use explains the Black-White disparity in PTB |
| Diet/nutrition | Plausible but more robust research needed |
| Gestational weight gain (GWG) | Unclear whether excessive or inadequate GWG contribute to the disparity |
| Obesity | Black women's higher obesity rates could contribute but cannot explain the disparity among non-obese women |
| Inter-pregnancy intervals | Potential small contributor |
| Age | Not a plausible cause of the disparity |
| Gestational diabetes (GDM) | May contribute but its greater effect size is balanced by lower prevalence among Black women |
| Hypertensive disorders of pregnancy (HDP) | Likely important contributor |
| Pre-pregnancy (pre-existing) diabetes | Plausible but low prevalence among Black women |
| Pre-pregnancy (chronic) hypertension | Highly plausible but cannot explain PTB among women without this risk factor |
| Infection | Plausible but research inconclusive |
| Microbiota | Plausible |
| Neighborhood environmental exposures | Exposures to neighborhood social and physical hazards are highly plausible and potentially major contributors |
| Cold and heat | Plausible but too few studies |
| Genetic and epigenetic factors | Genetic factors are unlikely to play a major role in the disparity (vs. in PTB), epigenetic factors may be important |
| Midstream Causes (which exert their effects through downstream factors) | |
| Stress | Likely a major contributor through neuroendocrine mechanisms. More research needed on life-course (vs. pregnancy-only) stress |
| Depression | Unlikely to explain the disparity |
| Resilience | Evidence insufficient |
| Coping | Evidence is inconsistent and insufficient |
| Social support | Plausible but research is inconsistent and has focused on support during pregnancy alone |
| Midstream paternal factors | Plausible through multiple causal pathways including those involving stress and socioeconomic factors |
| Income and education | Plausible but relationships are complex due to racism |
| Childhood and lifelong socioeconomic status | Plausible but rarely measured |
| Wealth | Plausible but we did not identify literature on wealth and the PTB disparity |
| Educational quality | Plausible but we did not identify literature on educational quality and the PTB disparity |
| Neighborhood socioeconomic disadvantage | Highly plausible, considerable literature supports |
| Upstream Causes | |
| Racism in multiple forms and through multiple pathways and biological mechanisms | Highly plausible |
As noted elsewhere in this paper, a factor may influence PTB without influencing the racial disparity, unless it is more prevalent or has a stronger effect size among African American women (or women of European descent).
DOWNSTREAM FACTORS are hypothesized to directly influence the biological mechanisms that produce PTB.
Prenatal Care
With rare exceptions, studies controlling for key maternal characteristics have not found associations between PTB and (quantitatively) inadequate prenatal care (e.g., late initiation, insufficient number of visits) (25–28). PTB rates have risen (16, 29, 30) and the racial gap in PTB has persisted (31) despite increased prenatal care usage and timely initiation overall and a narrowing of the Black-White gap in prenatal care (32). Some researchers, however, report better outcomes and smaller Black-White disparities for Black women with greater access to prenatal care (33, 34). Quality of care is another consideration. Black people receive lower quality medical services generally (35–37), and lower quality prenatal care specifically (e.g., less medical advice, fewer recommended treatments) (38–40), although not all studies have documented such differences (41, 42). The weight of evidence suggests that racial differences in traditional prenatal care are unlikely to explain the racial disparity in PTB (43, 44).
Group prenatal care, such as Centering Pregnancy (45) and Moms2Be (46), involves extended visits and more biopsychosocial support, including peer support, than traditional prenatal care. It has been associated with significantly reduced PTB and other favorable perinatal outcomes (e.g., greater pregnancy knowledge, lower stress, higher breastfeeding rates, less food insecurity, lower hospitalization costs, greater satisfaction with care, healthier behaviors) in observational (46–52), cohort (53–55) and randomized studies (56–58). Better pregnancy experiences (58) and outcomes (46, 56) in predominantly African American samples, and a smaller racial gap in PTB (55) have been linked to group prenatal care. Although literature reviews indicate that group care is a promising model, especially for higher risk groups like African Americans (59–62), more rigorous studies are needed to determine if group care can reduce PTB and the Black-White disparity in PTB, given inconsistent study results (49, 53, 54, 58, 63).
Preconception Care
African-American women of reproductive age experience elevated rates of a variety of chronic conditions that can enhance the risk of PTB. Moreover, African-American women have reduced access to efficacious health services, including contraception, control of hypertension and diabetes, preventive and therapeutic services for substance use, and mental health care, among others, that might address those risks (64–68). African Americans' lower access to and quality of medical care in general is well-documented (35–37). Screening and treatment for several conditions before pregnancy (e.g., nutritional deficiencies, STIs, hypothyroidism, hypertension, diabetes) have been associated with lower PTB rates (69–74), although some results are inconclusive (75, 76). Comprehensive preconception care programs have not, however, been associated with PTB reductions (77, 78). They typically begin only a few months before conception, however, which may be too late to mitigate risks accumulated across the life course (66). Although there is little research on whether racial disparities in preconception care contribute to disparities in PTB, it is plausible as a potential contributing factor.
Elective and Indicated Cesarean Section
Although most deliveries are vaginal, medically indicated and elective cesarean sections before 37 weeks gestation increase the PTB rate. Cesarean delivery rates are higher in Black women than Whites (35.9 and 30.7%, respectively, in 2019) (16) independent of known risk factors (79), demographic characteristics (80), and differences in labor management (81). The Black/White gap in early elective cesareans (<39 weeks) closed in Oregon after the procedure was banned statewide (82). While there is insufficient research on the extent to which disparities in cesarean rates contribute to disparities in PTB, cesarean section is plausible as a potential contributing factor. Since most women deliver vaginally, however, disparities in cesarean section would likely make a limited contribution to disparities in PTB.
Substance Use Disorders
Substance use before and during pregnancy has been linked repeatedly to adverse birth outcomes, including PTB (83–86). Racial differences in prevalence of substance use disorders, however, have been inconsistent, varying by substance, age, and sample. A nationally representative study estimated that binge-drinking-related PTB was considerably higher among White than Black women (87). At least before age 30, Black women of reproductive age are less likely than their White counterparts to smoke, engage in heavy drinking, or use marijuana (88–90) or benzodiazepines (91, 92) also found higher maternal use of alcohol and tobacco among Whites, but higher use of illicit drugs among Black mothers. Although African Americans smoke less, they are more likely to die of smoking-related causes than Whites (93). Theories explaining this paradox include: greater exposure to environmental toxins that potentiate smoking's adverse effects, melanin-related differences in nicotine metabolism, higher use of menthol cigarettes, and/or greater smoke intake per cigarette (93). To our knowledge, no published studies indicate that substance use explains the Black-White disparity in PTB.
Diet/Nutrition
Nutritional deficiencies (e.g., iron, folic acid, zinc, vitamin D, calcium, magnesium, and imbalances of ω-3 and ω-6 polyunsaturated fatty acids) are more prevalent in Black vs. White women (94, 95), despite similar prenatal vitamin use (96), and have been associated with PTB (97, 98) and bacterial vaginosis, a PTB risk factor (94, 97). Pregnant Black women have higher intake of calories, fats, carbohydrates, and sugar, higher obesity rates, and inadequate intake of nutrient-dense foods (99–101), all of which are associated with PTB (102–105). It is plausible that nutritional disparities might contribute to the PTB disparity, but more robust studies are needed.
Gestational Weight Gain (GWG)
In theory, excessive weight gain could increase PTB risk by increasing risk of hypertensive disorders (106, 107). A 2012 review by Headen and colleagues concluded that Black women are more likely than White women to have inadequate GWG, but not more likely to have excessive weight gain (108, 109). A large systematic review found that both inadequate and excessive pregnancy weight gain according to current guidelines were associated with higher risk of a number of adverse pregnancy outcomes, however, they also found that excessive weight gain was associated with lower risk of PTB (110). Analysis of data on 7,841 singleton births to U.S. Black women found that while high (>1.5 pounds/week) GWG was not associated with PTB among normal weight women, it was associated with increased risk of both spontaneous and medically indicated PTB among overweight and obese women (111). Leonard et al. (112) studied records of over 10 million non-Latino Black and non-Latino White women in the U.S. and concluded that moderate GWG was associated with reduced risk of PTB, especially the risk of early PTB among Black women. It is unclear whether excessive or inadequate weight gain contribute to the Black-White disparity in PTB.
Obesity
Excessive adipose tissue is thought to trigger inflammation and immunological changes that could lead to PTB (113). Although a 2010 meta-analysis found that overweight, obese, and normal-weight women experienced similar PTB rates (114), examinations by PTB subtype indicate that overweight/obese women experience significantly more both medically-indicated (114) and spontaneous PTB (114–116), findings are inconsistent, however (113, 117). PTB risk could increase at higher BMIs because medical risk increases (e.g., diabetes, hypertension, pre-eclampsia, lipid-induced inflammation) (107, 113, 118). Black women's significantly higher prevalence of obesity (112) (see section Nutrition) makes obesity a plausible contributor to the PTB disparity but does not explain the disparity among non-obese women.
Inter-pregnancy Intervals
Short (<12 months), very short (<6 months), and long (≥120 months) inter-pregnancy intervals (IPIs) are associated with increased odds of PTB (119–122). An IPI of 18 to 23 months has the lowest PTB odds for both Black and White women (122, 123). Black women are more likely to have suboptimal IPIs (122, 124, 125), although White women have significantly shorter median IPIs (26 months for White women vs. 30 months for Black women) (126). Hogue et al. (124) concluded that 4% of the Black-White PTB disparity could be explained by IPIs <6 months, which occur in approximately 10% of Black vs. 5% of White pregnancies. Short IPIs could contribute to the racial disparity but are unlikely to be a major cause given these findings.
Age
Age differences do not explain the PTB disparity. The racial gap in PTB is evident across all childbearing age groups (127). While Black women have higher rates of teen births than Whites, Black PTB rates are lowest among women 15–19 and steadily increase with age. Geronimus (128, 129) found, net of confounders, that LBW, VLBW, and infant mortality increased with age in Blacks but not Whites, attributing this to “weathering,” the biological result of cumulative social disadvantage, it is plausible that weathering also affects PTB. Paternal age, birthweight (130), and gestational age (131) also have been related to PTB, which could reflect biological and/or social factors.
Gestational Diabetes (GDM)
Both gestational (GDM) and chronic diabetes can lead to PTB by increasing risk of preeclampsia, which often results in preterm cesarean delivery (106, 107). GDM, although strongly associated with obesity, which is more prevalent among Black women, occurs less in Black women than in White women (112), prevalence estimates range from 4 to 4.9% and 4.5 to 7.0% among African American and White women, respectively (132–134). Odds of PTB secondary to GDM, however, are higher (aOR 1.56, 95% confidence interval 1.33–1.83) in Black women than White women (135). GDM may contribute to the PTB disparity, its lower prevalence among Black women is counterbalanced by a stronger effect size, at least in one study.
Hypertensive Disorders of Pregnancy (E.g., Gestational/Pregnancy-Associated/Pregnancy-Induced Hypertension, Preeclampsia, Eclampsia)
Hypertensive diseases of pregnancy are not uncommon complications of pregnancy (136), occurring in 6–8% of all pregnancies overall (137). Hypertensive disorders (136), have been linked repeatedly with PTB (138–141). Hypertensive disorders occur more frequently and are more severe and more likely to result in PTB in Black than White women, controlling for pre-pregnancy hypertension (137, 142–145), possibly due to unmeasured comorbidities (146, 147). A population-based longitudinal study of 2.5 million non-diabetic New York births found rates of preeclampsia of 3.2 per 100 hospitalizations among Black women and 1.8 per 100 hospitalizations among White women (137). Inflammation, thought to play an important role in PTB, may play a role in both pre-pregnancy hypertension and pregnancy-induced hypertensive disorders (148, 149). Hypertensive disorders of pregnancy likely contribute to the Black-White disparity in PTB, but the underlying causes that trigger these disorders require further elucidation.
Pre-pregnancy (Pre-existing) Diabetes
Pre-pregnancy Type I and II diabetes have been consistently associated with PTB (150–154), although the risk appears higher in women with Type II diabetes (155), who may experience other associated risks (e.g., higher weight and age, less prenatal care and glycemic control) (156). Compared with White women, Black women develop both Type I and II diabetes at higher rates and younger ages (157), and receive inferior clinical care (158). The age-standardized rate of pre-pregnancy diabetes among women giving birth in 2010 was 0.26 per 100 births among Black women and 0.16 per 100 births among non-Hispanic White women (159). Black women have worse outcomes (e.g., end-stage renal disease) than otherwise similar White women (160). Pre-pregnancy diabetes disparities may play a role in PTB disparities but the relatively low prevalence makes it unlikely to explain a major part of the disparity, rising rates over time, particularly among Black women, however, should be cause for concern (159).
Pre-pregnancy (Chronic) Hypertension
Pre-pregnancy hypertension has been linked consistently to pre-eclampsia (161–163), gestational and pre-existing diabetes (161, 164, 165), and PTB (43, 163, 166, 167). Although good blood pressure control during the first trimester can reduce a woman's risk of PTB (168), Black women not only have higher rates of pre-pregnancy and pregnancy-related hypertension than White women (169), they also appear more susceptible to their adverse effects on gestational length (170). Estimates of the prevalence of pre-pregnancy hypertension using 2011–2016 NHANES data are 18.7% among Black women and 8.2% among White women (171). Pre-pregnancy hypertension is a highly plausible contributor to the Black-White disparity in PTB.
Infection
PTB is strongly associated with periodontal and urogenital infections [e.g., bacterial vaginosis (BV)] (19, 172–180). Black women have higher prenatal infection rates (176, 177, 181–183). They have greater biological (e.g., absence of Lactobacillus ssp and high levels of Gardnerella ssp and Mobiluncus ssp), social (e.g., socioeconomic disadvantage) and psychosocial [e.g., stress (180, 181)] risk factors. A study using 2001–2004 NHANES data found a prevalence of Bacterial vaginosis of 51.4 and 23.2% among Black and White women, respectively (184). When infected, Black women may be more susceptible to PTB than White women (185).
Infection is a biologically plausible contributor to the Black-White disparity in PTB. Lack of consistent improvement in PTB with treatment, however (181, 186–188), have been attributed by some to inadequate treatment (182, 188), or our inadequate knowledge of pathogens (178). This raises the question whether infection is a cause of PTB, a marker, or an intermediate outcome of some underlying factor, such as inflammation or stress-induced immunocompromise (174, 187).
Microbiota
Microbiota are microorganisms, beneficial and harmful, living in or on the body, the microbiome is the collective genome of these microorganisms (189). The vaginal microbiota of White and Black women differ significantly (190–192). White women are more likely to have microbes that promote immunity and decrease PTB risk (e.g., BVAB3, L. crispatus, L. jensenii, L. gasseri), Black women have a higher prevalence of vaginal microbes associated with PTB (e.g., G. vaginalis, BVAB1, BVAB2, Atopobium vaginae, Megasphera, Sneathia, and Prevotella) (181, 190, 193, 194), although not all studies have reported this (195). Black women's higher douching rates could contribute to racial differences in vaginal microbiota (190), which might help to explain PTB disparities, as some studies have linked douching to PTB or PTB risk factors (e.g., urogenital infection) (180, 196–199). A person's microbiota also may be altered by oral infections (200) (see section Infection), and infections from gut-associated bacteria secondary to premature rupture of membranes (201) or inflammatory bowel disease (202), both of which have been associated with PTB (203–205).
Neighborhood Environmental Exposures
Racial residential segregation, a consequence of racism at structural and interpersonal levels, is associated not only with social and economic disadvantage, but also environmental toxicity (206–209). Black people are far more likely than Whites to live in racially segregated neighborhoods (210, 211), regardless of level of income, residential preferences, and housing affordability (212, 213). Because of environmental injustice, race/ethnicity is a stronger predictor of residence in polluted areas than low educational attainment (214), with Black people being significantly more likely to experience toxic exposures (e.g., air, traffic, water, industrial) (207, 214) linked to PTB (207, 215–218). Higher percentages of Black people in an area are associated with higher PTB rates among both Blacks and Whites, an effect that is not modified by area-level socioeconomic characteristics (219–225).
The combination of greater exposure to environmental toxins and to neighborhood social disadvantage might increase Black women's susceptibility to adverse birth outcomes (226). In a 2020 systematic review of 58 studies of air pollution and pregnancy, 84% of studies reported a significant association of air pollution with adverse birth outcomes, including PTB, with 50% also reporting significant disparities (227) (see section Neighborhood Social Disadvantage). Environmental hazards in neighborhoods are a highly plausible and potentially major contributor to the Black-White disparity in PTB.
Cold and Heat
Pregnancy exposure to excessive cold and heat has been associated with PTB (228–233). African Americans experience greater heat exposure, due to lower access to in-home central air conditioning and cooled public spaces than Whites (234–236). Bekkar et al.'s (227) systematic review of pregnancy and heat studies found that 90% of studies reported a significant association of excessive heat with PTB and most reported racial disparities. Both heat (228, 237, 238) and cold (238) may differentially impact Blacks' vs. Whites' PTB risk. Differential exposure to heat could plausibly contribute to the PTB disparity, however, there are too few studies to determine the magnitude of the contribution.
Genetic and Epigenetic Factors
Several observations indicate that genetic differences contribute to the risk of preterm birth (PTB). The likelihood of PTB is increased for women who were born preterm themselves, have a family history of PTB, or have had a prior PTB (239, 240). Family and twin studies have estimated the heritability of PTB—a measure of the relative contribution of genetics—to be in the range of 14–40% (239–241).
While these observations support inherited susceptibility to PTB, epigenetic effects may also play a role—that is, changes in gene expression as a result of environmentally mediated changes in the chromosomal matrix. Epigenetic changes at three genetic loci have been associated with risk of PTB (242–245). Social and environmental factors associated with PTB risk, such as diet and exposure to stress and environmental toxins, are potential mediators of epigenetic change. One recent study identified gene expression signatures associated with both vitamin D insufficiency (which is more common among Black women and is associated with PTB, as noted above under Diet/Nutrition) and PTB (246).
Molecular studies have identified specific inherited gene variants that are associated with a higher likelihood of PTB. However, large-scale studies have been required to reliably identify them, and the variants explain only a small proportion of the variability in PTB risk. For example, a genome-wide association study of 43,568 women of European ancestry identified and replicated variants in six genes associated with PTB risk (247, 248). The genes were involved in a range of functions, including uteroplacental circulation, development of the female reproductive system, cell energy and metabolism, adipocyte differentiation, and selenium metabolism. Although the findings were reproducible, the variants explained <1% of the variance in PTB. Other studies have implicated these and other functions, including endocrine, vascular and metabolic functions and inflammatory response, however, these studies have been limited by lack of replication or by inconsistent findings (239, 245, 249–251).
Given the documented importance of social and other environmental contributors to PTB risk, some research has focused on gene-environment interactions. A genome-wide association study of the Boston Birth Cohort, a longitudinal study of 1,733 African-American women, found an interaction between a variant in the COL24A1 gene and body mass index. Although the variant did not predict PTB risk independently, the study found that normal weight women with the variant had increased risk, while overweight and obese women with the variant had decreased risk (252). In a second cohort evaluated as part of the same study, the finding was replicated among African-American women, but not among women of European ancestry. This finding suggests that the effect of the COL24A1 variant on PTB risk is modified by exposures associated with weight status in African-American women, the negative result in European-American women could reflect differences in weight-associated exposures between African- and European-American women. Other potential interactions between genetic risk and environmental exposures have also been identified to play a role in causing spontaneous PTB, including smoking (253, 254) and bacterial vaginosis (255). The positive selection of variants in the progesterone receptor (PGR) gene observed in East Asian populations has been hypothesized to result from reduced risk of PTB associated with such variants (256). Additionally, while genetic variation in the microbiome may play a role in PTB risk, “environmental exposure factors cannot be excluded to play a role in the shaping of the cervicovaginal environment and the risk to… [spontaneous PTB]” (257).
Most research has focused on genetic susceptibility to PTB in women. The situation, however, is undoubtedly more complex, with contributions from fetal genetics (253, 258) and potential for gene-gene and gene-environmental interactions involving both fetal and maternal genomes. In addition, physiological studies suggest more than one pathway to PTB, genetic contributors to PTB and associated gene-gene and gene-environment interactions may vary for different pathways (259–261). Systems biology approaches incorporating studies of gene expression, the proteome and metabolome (262), and utilizing innovative approaches to the molecular study of pregnancy over time (263) are likely to be required to elucidate different genetic and environmental contributors to PTB. While maternal influences in predicting gestational length appear stronger, for example, indicating 1.22 additional days at birth for each additional week of the mother's gestational age, the father's gestational age has also been shown to be associated with PTB (131).
Genetic influences are a plausible contributor to the racial disparity in PTB, the magnitude of the effect, however, is likely to be small. Epigenetic effects—triggered by, e.g., environmental exposures and chronic stress associated with socioeconomic hardship and discrimination—are highly plausible as a potentially major contributor to the disparity.
MIDSTREAM FACTORS are hypothesized to affect PTB through their influence on downstream factors.
Stress
Psychosocial stress (stress) involves life demands (stressors) that strain or exceed adaptive resources, resulting in bio-psycho-social responses (the stress response) downstream that could compromise health (264, 265). Considerable research links stress to PTB, likely through stress-induced physiological mechanisms (266–269) including inflammation, immune dysregulation (270), and effects on behaviors (271, 272). Considering how stress could influence PTB by influencing behaviors, almost all of the plausible downstream factors examined here have potentially substantial behavioral influences, e.g., preconception care, nutrition, infection, microbiota, obesity (behavioral risk factors for and self-management of), pre-pregnancy hypertension, diabetes, hypertensive disorders of pregnancy, or gestational diabetes, gestational weight gain, and inter-pregnancy intervals. Heat/cold and genetics are the exceptions, however, epigenesis could have strong behavioral influences.
Although conclusions are not definitive in every case, studies have linked stress, directly or indirectly, to many of the plausible downstream factors, including: diet/nutrition (273), infection (269), microbiota (274, 275), obesity (276), pre-pregnancy hypertension (277), hypertensive disorders of pregnancy (278), pre-pregnancy diabetes (279), gestational weight gain (280), gestational diabetes (281), and epigenetic effects (282) (The many studies linking stress to the racial disparity in PTB are noted in the last sentence of the final paragraph in this section on stress).
Examining one downstream factor in more detail as an example, hypertension is a downstream risk factor plausibly influenced by stress, although further study is needed. Stress is known to induce temporary vasoconstriction and subsequent elevated blood pressure, however, the contribution of stress to the development of hypertension remains equivocal due to methodological inconsistencies (277, 283–285). Inflammation, which can result from chronic stress-related immune dysregulation (270), has repeatedly been linked to hypertension in both pregnant and non-pregnant humans (286–288). Inflammation is thought to be involved in hypertensive disorders of pregnancy (148, 149), preeclampsia itself has been considered “an excessive maternal inflammatory response to pregnancy” (289). A recent study in the Netherlands found elevated hair cortisol levels (measured from 3 months preconception to the end of the 2nd trimester) and anxiety scores (at hospital admission) in women with preeclampsia (290). A systematic review concluded that chronic stress is more likely than acute stress to result in prolonged elevated blood pressure (285).
Although Black women report more stress (291–293), this has not consistently explained PTB disparities (294–296), possibly due to inadequate measurement (297, 298), particularly a tendency to focus exclusively on stress during pregnancy. The physiologic “wear-and-tear” caused by chronic stress can compromise women's reproductive health well before they become pregnant (299). However, few studies consider life-course exposure to chronic social stressors (such as racism and/or its effects, including economic hardship), which may better explain PTB disparities than stress only during pregnancy. There is broad scientific consensus about an important role for life-course stress in the PTB disparity (66, 129, 300–302), including its biological plausibility (303, 304). Stress is a highly plausible, potentially major contributor to the racial disparity in PTB.
Depression
Depression has been associated repeatedly with PTB (305–307). Black people, however, are less likely to have a major depressive disorder diagnosis, despite higher self-reported psychological distress (308). While differences in symptomatology or reporting may explain this paradox, the literature indicates that depression does not explain PTB disparities.
Resilience
The literature on maternal resilience (as reflected by, e.g., optimism, self-efficacy, self-esteem) and PTB is inconsistent. Some studies report significant associations (309–312), but not all do (313). Others report paradoxical associations such as greater optimism among African-American women who deliver preterm (314). Wheeler et al. (315) reported a stronger positive relationship between self-efficacy and PTB in Black vs. White women. Evidence is insufficient to draw conclusions about the role in the PTB disparity of factors associated with resilience.
Coping
Research suggests that passive/avoidance coping may increase African Americans' PTB risk (314, 316), while active coping may moderate effects of stress (e.g., due to racial discrimination) on PTB (317). African Americans reportedly engage in less active (318) and more passive/avoidance coping (319) than Whites or Hispanics, particularly when facing racism (320). Given limited research on how coping could moderate stress effects in pregnancy (266, 321), coping differences might plausibly contribute to PTB disparities, the relevant literature, however, is inconsistent and insufficient.
Social Support
Extensive research indicates that social support can buffer the negative effects of stress, thereby protecting against poor health outcomes (322–330), including PTB (331, 332). Greater support has been associated with less inflammation (314) and less “weathering” (chronic stress-related biological aging) in Black people specifically (333). Lower support has been linked to shorter telomeres (an indicator of cellular aging) in perinatal women (334). Social support has been observed to be beneficial for White women with low but not high levels of stress. Among Black women, however, social support seems protective regardless of stress level (335). Many Black people draw substantial social support and health benefits from religious involvement (333, 336), and spirituality and family/friend support have been shown to predict pregnant women's anxiety, depression, and stress (337). Black people report fewer friends than Whites, but do not differ in feeling adequately supported (338). Findings on racial differences in family support vary (338, 339). It is plausible in theory that social support could contribute to the Black-White disparity in PTB. Given the inconsistencies in findings and the literature's focus only on support during pregnancy, however, it is not possible to assess the contribution of social support to Black-White disparities in PTB.
Midstream Paternal Factors
Midstream paternal factors (Note: paternal genetic factors are discussed in the section on genetic factors, and paternal age is noted among downstream factors). Midstream factors influence downstream factors and in turn are influenced by upstream factors.
Research indicates that fathers' social characteristics can influence PTB risk. Paternal hazardous occupational exposures (lead, x-rays), and age and birthweight (considered downstream factors in this paper) have been associated with PTB, although research is limited and results are inconsistent (130). Paternal education and lifetime socioeconomic factors predict PTB, independent of maternal demographics (130, 340, 341), and paternal involvement accounts for more of the racial disparity in PTB than maternal education (43). Paternal support predicts lower levels of anxiety, depression, and smoking in pregnancy (342), whereas, paternal prenatal depression, which may inhibit paternal support, predicts PTB (343). Partner-related stressors, such as separation/divorce, death, and interpersonal violence are higher at lower socioeconomic levels (344) and increase PTB risk (345–347). Although Black people are more likely to report such exposures (348), racial differences in stressful events appear to contribute little to PTB disparities (296). Theoretically, Black men's disproportionate incarceration rates could help to explain the Black-White PTB gap through various economic (349), behavioral (350), psychosocial and physical health pathways (351). Thus, it is plausible that paternal factors could contribute to the Black-White disparity in PTB through multiple causal pathways, including those involving stress and socioeconomic factors.
Socioeconomic Factors
A range of socioeconomic factors—including but not limited to income, educational quantity and quality, wealth, childhood/lifelong socioeconomic conditions, and neighborhood socioeconomic conditions—could plausibly affect PTB rates and the racial disparity, by affecting downstream factors. Research has linked one or more socioeconomic factors with most of the downstream factors, e.g., preconception care (352), cesarean section rates (353), infection (354), nutrition (355), alterations in microbiota (356, 357), obesity (358), pre-pregnancy hypertension (359, 360), pre-pregnancy diabetes (361, 362), hypertensive disorders of pregnancy (145, 363), gestational diabetes (364, 365), suboptimal gestational weight gain (366), interpregnancy intervals (367), and both of the unlikely factors, standard prenatal care (368) and substance use disorders (369, 370). Below we briefly summarize knowledge of the role of several different socioeconomic factors in PTB.
Income and Education
Black people have lower levels of income and education than Whites, and earn less for similar levels of education (371), reflecting centuries of discriminatory policies and practices (e.g., slavery, Jim Crow, redlining, segregation, mass incarceration) that have limited their socioeconomic opportunities (371–377). However, the role of socioeconomic factors in PTB disparities is not simple. Many studies have observed that differentials by race (332) and nativity (immigrant vs. US-born Black women) (378–381) persist after controlling for income and/or education. Income and education consistently predict White, but not Black, PTB rates, with the racial gap widest at the highest socioeconomic levels (382–385), and little (316, 386) to no (383, 387) racial differences in PTB rates among socioeconomically disadvantaged women. Income predicts exposure to environmental hazards and lack of exposure to health-promoting conditions in the home and neighborhood. Income, for example, is likely a major determinant of access to air conditioning, this is consistent with African Americans' markedly lower access to air conditioning (235), which could reduce the PTB risk associated with excessive heat exposure (227).
Childhood and Lifelong Socioeconomic Status (SES)
Although rarely considered, both classic (388, 389) and more contemporary studies, e.g., Dominguez et al. (300), demonstrate that childhood rather than current SES is a stronger predictor of birth outcomes. Socioeconomic disadvantage in childhood is more likely among Blacks than Whites (390), even among those of similar adult income or education (300, 391). Childhood adversity has been associated with PTB (392–394). Childhood disadvantage could affect a woman's risk of PTB in multiple ways (300, 395), including exposures to air pollution and other toxins, inadequate nutrition, and chronic stress (see Environmental Hazards and Stress sections).
By contrast, upward mobility (396), intergenerational high SES (397), and lifelong residence in high-income neighborhoods (398) are associated with better African American birth outcomes and/or smaller racial disparities, adjusting for confounders. However, upward mobility's benefits appear stronger in White mothers (397, 399, 400), this may reflect the chronic stressors that many upwardly mobile African-American women face (383, 395, 401–403).
Wealth
Wealth, the value of net assets, is a better indicator of economic security and stability than more volatile factors, such as income (404). For a given level of income and/or education, Blacks have accumulated far less wealth than similar Whites (see Racism section) (371, 376, 404, 405). Greater wealth across the life course could reduce PTB risk by reducing adverse exposures, increasing health protections, and mitigating chronic stress (66). We could not identify empiric research on wealth as a causal factor in PTB.
Educational Quality
Researchers frequently assess educational attainment (years and/or degrees), but rarely consider educational quality, which could be an important socioeconomic indicator of PTB risk. Black children are more likely than Whites to attend under-funded, lower-quality schools without advanced coursework, and to be corporally punished or suspended/expelled, all of which puts them at a disadvantage for college and the labor market (406–409). Although racial disparities in college/university enrollment have narrowed, Blacks are significantly less likely than Whites to graduate with a bachelor's degree and attend prestigious institutions (410, 411). We could not identify empiric research on educational quality as a causal factor in PTB.
Neighborhood Socioeconomic Disadvantage
Neighborhood socioeconomic disadvantage (e.g., high poverty, unemployment, racial segregation) has been repeatedly linked to PTB (412, 413). Biologically plausible pathways include stress—e.g., community violence (414)—and environmental exposures (e.g., polluted/toxic air, ground, water, housing) (see section Neighborhood Environmental Exposures). Black women are more likely to reside in disadvantaged neighborhoods, and, when doing so, are more vulnerable to PTB than their White neighbors (383, 412). Among higher-SES women, the racial disparity in LBW is lowest among those in racially congruent neighborhoods and widest among those in predominantly White neighborhoods (415).
Socioeconomic Factors Are Highly Plausible Contributors to the Racial Disparity in PTB
No study to our knowledge has measured all of the socioeconomic factors that could plausibly affect PTB. Nevertheless, the persistence of racial differences net of SES measures, and the complex patterns of SES findings indicate that socioeconomic factors alone cannot explain racial disparities in PTB.
UPSTREAM FACTORS are hypothesized to influence PTB through their influence on midstream factors, which in turn influence downstream factors.
There is considerable scientific consensus that racism, operating through multiple pathways, is a plausible upstream cause of many Black-White health disparities (416, 417) including birth outcomes (332, 418, 419). Self-reported/perceived exposure to racism in childhood (300, 420), the perinatal period (302, 421), and across the life course (316, 317, 395, 402, 422–426), as well as worry about potential exposure to racism (395) have been associated directly or indirectly (427, 428), net of confounders, with a 1.5- to 3-fold increased risk of adverse African American birth outcomes (395, 421, 429) and with the Black-White disparity in PTB (395, 424). While not all studies have observed an association between racism and PTB—e.g., Lu and Chen (296), Murrell (430)—measurement has been highly variable (298, 431, 432). Most studies, for example, have examined racial discrimination only during pregnancy or the year before delivery, fewer (317, 395, 402, 424, 429) have examined lifetime experiences, which are more likely to influence PTB based on knowledge of stress physiology (270). Mustillo et al. (424) found that the Black-White disparity in PTB became non-significant after adjusting for lifetime experiences of racial discrimination, Braveman et al. (395) had similar findings on examining worry about discrimination. Diverse experiences of racism have been studied, from overt insults and other incidents of unfair treatment to more subtle experiences and pervasive worry or vigilance in anticipation of experiencing discrimination. At the structural level, a recent study found that African American women's residence in redlined neighborhoods was associated with an increased risk of PTB (433) [Redlining is the practice of denying (or charging more for) services, such as bank loans and insurance, to residents in racially segregated areas, red lines were drawn on maps to mark areas where loans would not be given, which corresponded strongly to areas where many African Americans live].
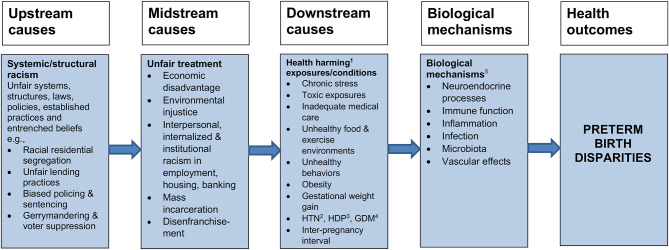
Figure 2 is a simplified schematic representation of how racism could increase risks of PTB through a range of biologically plausible pathways previously noted in this paper, operating through midstream factors such as stress and socioeconomic disadvantage, which in turn influence downstream factors that directly activate physiologic mechanisms. Racism is a pervasive system of unequal power relationships, based on ideological notions of the inherent superiority of Whites and inferiority of People of Color (POC), it unfairly advantages Whites and marginalizes POC.
Figure 2.
Racism plausibly may affect PTB as an upstream factor, acting through midstream and downstream factors that more directly trigger the physiologic mechanisms. 1. Health harming or lack of health-promoting exposures/conditions. 2. HTN: Hypertension. 3. HDP: Hypertensive disorders of pregnancy. 4. GDM: Gestational diabetes mellitus. 5. Epigenetic effects are not displayed because they may occur through exposures at each step along the causal pathways.
Racism is a chronic stressor (434–436) that could affect PTB through the body's physiologic response to stress (e.g., neuroendocrine, immune, inflammatory, and vascular mechanisms) (268, 270, 437). Racism operates not only in interpersonal interactions but also structurally via laws, policies, institutions, and practices, which historically produced and continue to perpetuate systematic disadvantage affecting POC including African Americans, examples include discriminatory lending practices and residential segregation, which produce educational and economic disadvantage, and hazardous environmental exposures. These practices perpetuate racial disparities in health and in opportunities to be healthy, even when, arguably, there is considerably less conscious intent to discriminate today than when major civil rights legislation was enacted in the 1960s.
Racial residential segregation is a salient example of structural racism, it tracks low-income and many middle-income African Americans into neighborhoods that are unhealthier in multiple ways, including exposure to environmental hazards, inferior schools, lack of employment opportunities, poor services, concentrated poverty and the accompanying sources of chronic stress (438). All of these could increase risks of PTB through a range of pathways noted in this paper. Racial segregation produces and perpetuates racial disparities in income and wealth, for example, through inferior educational and occupational opportunities, redlining, and other discriminatory banking/lending practices that make it far more difficult to own a home or to start, sustain, or expand a business (438). Discrimination in hiring, promotions, and pay also constrain income and wealth. The resultant socioeconomic disadvantage from all these sources constrains the options one has for housing and neighborhoods, and for access to health care, nutritious food, and safe green space for exercise and leisure activities, this disadvantage produces chronic stress due to constantly facing daily challenges to meet one's family's basic needs with inadequate financial resources (see Socioeconomic Factors section).
Discussion
This literature review confirms the widely held scientific view that the causes of the Black-White disparity in PTB, and the causes of PTB itself, are largely unknown. Current knowledge, however, identifies many plausible—and some implausible—potential causes, all of which warrant further research. This review also confirms that the etiology is likely to be both multifactorial and complex, which may partly reflect that PTB is not a single disease but several distinct clinical entities with varying etiologies.
While definitive proof is lacking for most hypothesized causes, most of the causes we considered are plausible—including biologically plausible—as potentially important contributors to the disparity. Most of the downstream factors and all of the midstream and upstream factors examined are plausible. Standard prenatal care and substance abuse disorders appear less likely as causes of the Black-White disparity in PTB, based on the empiric evidence to date. Despite well-documented racial and socioeconomic disparities in care, the weight of evidence has not supported lack of standard prenatal care as a cause of the PTB disparity, disparities in preconception care and in cesarean section rates, however, are plausible contributors.
While it is plausible that differences in nutrition, infections, and microbiota could potentially contribute to—and preconception care and/or group prenatal care might diminish—the Black-White disparity in PTB, evidence is limited on nutritional deficiencies and preconception care and inconsistent on infections, microbiota, and group prenatal care. Excessive gestational weight gain, gestational diabetes, hypertensive disorders of pregnancy, short inter-pregnancy intervals, and pre-pregnancy obesity, diabetes, and hypertension are plausible but cannot explain the PTB disparity among women without these conditions.
PTB has consistently been linked with exposures to toxic physical hazards, such as air pollution. Socioeconomic disadvantage is not the only cause of disproportionate exposure to hazardous neighborhood conditions. Woodruff et al. (214) found that race was a stronger predictor of residence in polluted areas than educational attainment (which is strongly correlated with income). Environmental injustice—the disproportionate location of toxic substances in Black and Brown communities—is a highly plausible contributor to the Black-White disparity in PTB. Many Black women even of relatively high SES live in racially segregated areas (439, 440) with hazardous exposures, which could contribute to the lack of expected improvement in PTB among higher-SES Black women.
Along with disparities in prenatal care and substance use, genetic differences have often been hypothesized to explain the racial disparity in PTB. When considering the potential role of genetic factors, it is important to note scientific consensus that race is primarily a social, not a biological, construct (441). Genetic variation is seen both within and across different ancestral populations. However, racially defined populations in the United States tend to demonstrate patterns of genetic variation associated with continental ancestry. Thus, the prevalence of many monogenic disorders, such as sickle cell disease and cystic fibrosis, varies among Black and White U.S. populations. If genetic research had identified common gene variants with a large independent impact on PTB risk, and if such variants differed in prevalence across racial populations, one might expect a significant genetic contribution to racial differences in PTB. However, large-scale genomic studies have failed to identify such variants. Current evidence indicates that the genetic contribution to PTB risk occurs via the aggregate effect of multiple variants, each of small effect, often involving gene-environment (and likely gene-gene) interactions. Furthermore, environmental exposures may trigger epigenetic change, environmental risks include factors as disparate as diet and smoking, the microbiome, social exposures such as racism and poverty, and, related to racism and poverty, physical exposures to toxins and air pollution (442). Even without controlling for epigenetic effects, heritability estimates suggest that the environment plays a greater role than genetics in PTB risk (443). Due to a long history of racism, exposure to most of the environmental risks associated with PTB differs markedly among U.S. Black and White populations. Furthermore, the favorable birth outcomes of Black African immigrants to the U.S. do not support a genetic basis for the PTB disparity between US-born Black and White women. If genetic differences were the basis, one would expect Black African immigrants to the United States to have PTB rates at least as unfavorable as those of US-born Black women, whereas Black African immigrants' PTB rates are similar to those of U.S. White women (444). Based on all these considerations, genetic factors contribute to PTB risk but are likely to explain at most a small fraction of the Black-White difference in PTB. Gene-environment interaction studies may help to better define PTB subtypes and their biological mechanisms, potentially informing preventive and therapeutic interventions, this should be a high priority for further research.
While many hypothesized causes are plausible, no single downstream factor explains the magnitude of the disparity, most are likely to play a small role. This suggests that PTB may reflect the combined effects of multiple causes and pathways, and that we must identify the upstream factors that initiate the harmful pathways: the causes of the causes. Identifying downstream causes can be crucial to mitigate downstream health damage and at times may help identify upstream causes. It generally does not, however, answer the question: What initiated the causal chain? What upstream forces produced these downstream conditions? Answering this is vital for prevention. Actions targeting the final steps in long, complex causal chains may be ineffective without addressing the underlying causes that initiated the chains. Furthermore, the downstream factors cannot explain the strong social patterning of the racial disparity in PTB, for example, the absence of a PTB disparity among Black African immigrants compared with U.S.-born White women.
Considering the Causes of the Causes
For example, consider racial disparities in nutrition, obesity, and environmental toxins as potential downstream causes of the racial disparity in PTB. Racial residential segregation makes Black women of both moderate and lower incomes substantially more likely to reside in areas with many fast-food outlets and convenience stores (445, 446), which are associated with obesity and poor nutrition (446, 447), and in areas less conducive to exercise, an important determinant of obesity. Black women are likely to experience chronic stress associated with racial discrimination (300, 395, 448) as well as economic disadvantage. At the same level of education, Black women have less income, at the same income level they have less wealth, coping with low financial resources is stressful. Chronic stress has been linked with less healthy behaviors in general (449) and specifically to higher consumption of and potential addiction to high-fat, high-sugar foods, leading to obesity and other metabolic diseases (450–452). Overconsumption of high-fat, high-sugar foods with little nutritious value is often accompanied by under-consumption of more nutritious foods, which increases risk of nutritional deficiencies (453, 454). Racial segregation combined with environmental injustice also means that Black women are more likely to be exposed to environmental toxins.
The literature documents several upstream/midstream causes of the downstream causes. Considerable empiric research has linked each hypothesized midstream cause (except depression, which has inconsistent results) with the racial disparity in PTB, through their influence on one or more downstream factors. Stress could affect PTB by activating neuroendocrine and immune mechanisms leading to inflammation and immune system dysfunction known to cause PTB. Stress could alter a woman's microbiota, her immune response to infection, and her susceptibility to chronic disease including pre-pregnancy conditions. Stress could trigger epigenetic changes influencing the risk of PTB.
Extensive literature also documents stress as a strong influence on behaviors, and most of the downstream factors have substantial behavioral aspects. Depression, resilience, coping, and social support are other midstream factors that could plausibly influence many downstream causes by influencing behaviors, however, research linking them with PTB is insufficient. Also based on the broader health literature rather than extensive empiric studies of PTB, it is plausible that psychosocial factors associated with resilience, coping, and social support could modify the effects of chronic stress on PTB.
Socioeconomic Disadvantage Across the Life Course
Extensive research documents pervasive socioeconomic disadvantage among Black women compared with White women, with respect to socioeconomic factors including income, education, and neighborhood conditions, and for lifetime experiences of these factors. These socioeconomic factors have been linked with PTB (383) and/or multiple downstream factors. Socioeconomic factors alone, however, cannot explain the social patterning of PTB disparities, particularly the higher than expected PTB rates among socioeconomically-better-off Black women. Forces farther upstream have produced Black women's socioeconomic disadvantage.
Looking Farther Upstream: Racism
Racism is the only factor identified by this review that directly or indirectly could explain the racial distributions of all of the downstream and midstream causes, including socioeconomic factors. Racism as a fundamental, upstream cause is a highly plausible major contributor to the Black-White disparity in PTB through multiple different causal pathways and biological mechanisms. Racism explains the racial disparity in socioeconomic factors—the legacy of slavery, 100 years of Jim Crow laws, racial residential segregation, and ongoing discrimination in employment, housing, policing, and sentencing. All of these have relentlessly deprived African Americans of socioeconomic opportunity. Socioeconomic disadvantage has differentially exposed African Americans to the chronic stress that accompanies facing daily challenges, such as childcare and feeding and sheltering one's family, with inadequate financial resources. Racial segregation has placed Black women in pervasively stressful neighborhood surroundings (often characterized by fear, deprivation, despair, and/or crime), it has systematically exposed them to environmental toxins, including air pollution. Racial discrimination is a powerful direct source of stress, considering both overt incidents and the pervasive vigilance one needs to be prepared for potential incidents affecting oneself or loved ones (455). Racism could differentially threaten or undermine resilience and coping among Black compared with White women, increasing vulnerability to the harmful effects of chronic stress.
Racism may also help to explain some of the social patterning of PTB. For example, favorable PTB rates among Black immigrants from Africa could conceivably be explained by immigrants' lack of exposure to racial discrimination earlier in their lives, during sensitive developmental periods, with its long-lasting neuroendocrine and immune sequelae. The higher than expected PTB rates among socioeconomically better-off Black women reflect the fact that even middle-class Black women often live in segregated neighborhoods, where they are exposed to the same environmental hazards and poor resources as their socioeconomically worse-off counterparts. It also could reflect cumulative physiologic wear and tear due to the chronic stress, over their lifetimes, associated with overcoming barriers to educational and occupational success. Some have hypothesized that it also may be due to higher-SES Black women experiencing more discrimination at work because of being the only or among few Black women at their professional level (383, 395, 402), this possibility is supported by studies showing that higher-income/education Black women report more discrimination than their lower-income/education Black peers (395, 456).
All of the plausible causal factors considered in this study deserve further research, especially those likely to affect many women or have large effects. More priority should be given to rigorously studying the upstream/midstream factors that are highly plausible and may present opportunities to prevent multiple destructive pathways from being set in motion. Some potential causes—such as racism, environmental injustice, and lifetime socioeconomic disadvantage—are so plausible, inter-related and amenable to policy intervention, and have such compelling reasons to address them, that they deserve particular attention. A compelling pragmatic reason to address racism, environmental injustice, and socioeconomic disadvantage is the extensive evidence that they are upstream causes for many other adverse health outcomes in addition to PTB. Another compelling reason is ethical values, which require that we not only mitigate the end-organ damage caused by unfavorable downstream factors, but also address the inequitable midstream and upstream conditions that produce downstream bodily harm through multiple pathways. While much is unknown, both existing knowledge and core ethical values can and should guide policies and research agendas.
Author Contributions
PB, TD, and WB drafted the initial manuscript, with contributions from JWC, FJ, TH, JA, PW, DS, and GS. TD and PB revised the manuscript with input from the other authors. All of the authors participated in conceptualizing the study, in reviewing drafts, and in reviewing and approving the final manuscript.
Conflict of Interest
FJ is President of Majaica, LLC. CDC funds supported March of Dime's (MOD's) Prematurity Campaign, which included convening a scientific workgroup. CDC funds were not used to directly support development of this manuscript. MOD funded a small stipend for TD to conduct a comprehensive scientific edit of the initial consensus statement to prepare it as a manuscript of publishable length. MOD funded a small stipend for TH for additional assistance with references. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study would not have been possible without the support and participation of March of Dimes and the Centers for Disease Control and Prevention's Division of Reproductive Health. The authors would like particularly to acknowledge Wanda Barfield, MD,MPH, RADM USPHS (ret) and Martha Boisseau, MPH of the Centers for Disease Control and Prevention's Division of Reproductive Health; and Rahul Gupta, MD, MPH, MBA of March of Dimes for their technical support and guidance. The authors also thank Saif Al-Amin, Abra Greenberg, Susanna Guzman, Bria Myers, Mashariki Kudumu, Molly Hayden, and Kasey Rivas, all with March of Dimes, for their assistance in reviewing references. Kweli Rashied-Henry, Gina Legaz, and Lisa Waddell were with March of Dimes at the time they participated most intensively in this effort.
References
- 1.Heron M. Deaths: Leading Causes for 2017. Washington, DC: (2019). [Google Scholar]
- 2.Ely D, Driscoll A. Infant Mortality in the United States, 2017: Data from the Period Linked Birth/Infant Death File. (2019). Retrieved from: https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_10-508.pdf [PubMed]
- 3.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. (2002) 288:728–37. 10.1001/jama.288.6.728 [DOI] [PubMed] [Google Scholar]
- 4.Dong Y, Yu JL. An overview of morbidity, mortality and long-term outcome of late preterm birth. World J Pediatr. (2011) 7:199–204. 10.1007/s12519-011-0290-8 [DOI] [PubMed] [Google Scholar]
- 5.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. (2005) 352:9–19. 10.1056/NEJMoa041367 [DOI] [PubMed] [Google Scholar]
- 6.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. (2008) 371:261–9. 10.1016/s0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- 7.Crump C, Howell EA, Stroustrup A, McLaughlin MA, Sundquist J, Sundquist K. Association of preterm birth with risk of ischemic heart disease in adulthood. JAMA Pediatr. (2019) 173:736–43. 10.1001/jamapediatrics.2019.1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of diabetes among young adults born preterm in Sweden. Diabetes Care. (2011) 34:1109–13. 10.2337/dc10-2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evensen KA, Steinshamn S, Tjonna AE, Stolen T, Hoydal MA, Wisloff U, et al. Effects of preterm birth and fetal growth retardation on cardiovascular risk factors in young adulthood. Early Hum Dev. (2009) 85:239–45. 10.1016/j.earlhumdev.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 10.Kaijser M, Edstedt Bonamy A.-K., Akre O, Cnattingius S, Granath F, et al. Perinatal risk factors for diabetes in later life. Diabetes. (2009) 58:523–6. 10.2337/db08-0558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kajantie E, Osmond C, Barker DJP, Eriksson JG. Preterm birth—a risk factor for type 2 diabetes?: the Helsinki Birth Cohort Study. Diabetes Care. (2010) 33:2623–5. 10.2337/dc10-0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerkhof GF, Breukhoven PE, Leunissen RWJ, Willemsen RH, Hokken-Koelega ACS. Does preterm birth influence cardiovascular risk in early adulthood? J Pediatr. (2012) 161:390–6.e391. 10.1016/j.jpeds.2012.03.048 [DOI] [PubMed] [Google Scholar]
- 13.Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics. (2013) 131:e1240–63. 10.1542/peds.2012-2177 [DOI] [PubMed] [Google Scholar]
- 14.Ramirez-Velez R, Correa-Bautista JE, Villa-Gonzalez E, Martinez-Torres J, Hackney AC, Garcia-Hermoso A. Effects of preterm birth and fetal growth retardation on life-course cardiovascular risk factors among schoolchildren from Colombia: the FUPRECOL study. Early Hum Dev. (2017) 106–7, 53–8. 10.1016/j.earlhumdev.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 15.Costa DL. Race and pregnancy outcomes in the twentieth century: a long-term comparison J Econ Hist. (2004) 64:1056–86. Available online at: http://www.jstor.org/stable/3874989 [Google Scholar]
- 16.Hamilton BE, Martin JA, Osterman MJK. Births: Provisional Data for 2019 (008). Washington, DC: (2020). Retrieved from: https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf [Google Scholar]
- 17.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: Final Data for 2006. Washington, DC: (2009). [PubMed] [Google Scholar]
- 18.Culhane JF, Goldenberg RL. Racial disparities in preterm birth. Semin Perinatol. (2011) 35:234–9. 10.1053/j.semperi.2011.02.020 [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. (2008) 371:75–84. 10.1016/s0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manuck TA. Racial and ethnic differences in preterm birth: a complex, multifactorial problem. Semin Perinatol. (2017) 41:511–8. 10.1053/j.semperi.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutambudzi M, Meyer JD, Reisine S, Warren N. A review of recent literature on materialist and psychosocial models for racial and ethnic disparities in birth outcomes in the US. 2000-2014. Ethn Health. (2017) 22:311–32. 10.1080/13557858.2016.1247150 [DOI] [PubMed] [Google Scholar]
- 22.Wadhwa PD, Entringer S, Buss C, Lu MC. The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol. (2011) 38:351–84. 10.1016/j.clp.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkman LF, Kawachi I, Glymour M. Social Epidemiology. 2nd ed. New York, NY: Oxford University Press; (2014). [Google Scholar]
- 24.Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet. (2017) 389:941–50. 10.1016/s0140-6736(17)30003-x [DOI] [PubMed] [Google Scholar]
- 25.Alexander GR, Korenbrot CC. The role of prenatal care in preventing low birth weight. Future Children. (1995) 5:103–20. 10.2307/1602510 [DOI] [PubMed] [Google Scholar]
- 26.Alexander GR, Kotelchuck M. Assessing the role and effectiveness of prenatal care: history, challenges, and directions for future research. Public Health Rep. (2001) 116:306–16. 10.1093/phr/116.4.306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiscella K. Does prenatal care improve birth outcomes? A critical review. Obstetr Gynecol. (1995) 85:468–79. 10.1016/0029-7844(94)00408-6 [DOI] [PubMed] [Google Scholar]
- 28.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. (2008) 371:164–75. 10.1016/S0140-6736(08)60108-7 [DOI] [PubMed] [Google Scholar]
- 29.Kogan MD, Martin JA, Alexander GR, Kotelchuck M, Ventura SJ, Frigoletto FD. The changing pattern of prenatal care utilization in the United States, 1981-1995, using different prenatal care indices. JAMA. (1998) 279:1623–8. 10.1001/jama.279.20.1623 [DOI] [PubMed] [Google Scholar]
- 30.Osterman MJK, Martin JA. SystemTiming and Adequacy of Prenatal Care in the United States, 2016. (2018). Retrieved from: https://stacks.cdc.gov/view/cdc/55174 [PubMed]
- 31.Gyamfi-Bannerman C, Ananth CV. Trends in spontaneous and indicated preterm delivery among singleton gestations in the United States, 2005-2012. Obstet Gynecol. (2014) 124:1069–74. 10.1097/aog.0000000000000546 [DOI] [PubMed] [Google Scholar]
- 32.Alexander GR, Kogan MD, Nabukera S. Racial differences in prenatal care use in the United States: are disparities decreasing? Am J Public Health. (2002) 92:1970–5. 10.2105/AJPH.92.12.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown CC, Moore JE, Felix HC, Stewart MK, Bird TM, Lowery CL, et al. Association of state medicaid expansion status with low birth weight and preterm birth. JAMA. (2019) 321:1598–609. 10.1001/jama.2019.3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray JL, Bernfield M. The differential effect of prenatal care on the incidence of low birth weight among blacks and whites in a prepaid health care plan. N Engl J Med. (1988) 319:1385–91. 10.1056/NEJM198811243192105 [DOI] [PubMed] [Google Scholar]
- 35.Institute of Medicine Committee on Understanding Eliminating Racial Ethnic Disparities in Health Care . Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; (2003). 10.17226/12875 [DOI] [PubMed] [Google Scholar]
- 36.Purnell TS, Calhoun EA, Golden SH, Halladay JR, Krok-Schoen JL, Appelhans BM, et al. Achieving health equity: closing the gaps in health care disparities, interventions, and research. Health Aff. (2016) 35:1410–5. 10.1377/hlthaff.2016.0158 [DOI] [PubMed] [Google Scholar]
- 37.Williams DR, Wyatt R. Racial bias in health care and health: challenges and opportunitiesracial bias in health care and healthracial bias in health care and health. JAMA. (2015) 314:555–6. 10.1001/jama.2015.9260 [DOI] [PubMed] [Google Scholar]
- 38.Cox RG, Zhang L, Zotti ME, Graham J. Prenatal care utilization in Mississippi: racial disparities and implications for unfavorable birth outcomes. Maternal Child Health J. (2011) 15:931–42. 10.1007/s10995-009-0542-6 [DOI] [PubMed] [Google Scholar]
- 39.Kogan MD, Kotelchuck M, Alexander GR, Johnson WE. Racial disparities in reported prenatal care advice from health care providers. Am J Public Health. (1994) 84:82–8. 10.2105/ajph.84.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paul DA, Locke R, Zook K, Leef KH, Stefano JL, Colmorgen G. Racial differences in prenatal care of mothers delivering very low birth weight infants. J Perinatol. (2006) 26:74–8. 10.1038/sj.jp.7211428 [DOI] [PubMed] [Google Scholar]
- 41.Kogan MD, Alexander GR, Kotelchuck M, Nagey DA, Jack BW. Comparing mothers' reports on the content of prenatal care received with recommended national guidelines for care. Public Health Rep. (1994) 109:637–46. [PMC free article] [PubMed] [Google Scholar]
- 42.Tran ST, Rosenberg KD, Carlson NE. Racial/ethnic disparities in the receipt of smoking cessation interventions during prenatal care. Matern Child Health J. (2010) 14:901–9. 10.1007/s10995-009-0522-x [DOI] [PubMed] [Google Scholar]
- 43.DeSisto CL, Hirai AH, Collins JW, Rankin KM. Deconstructing a disparity: explaining excess preterm birth among U.S.-born black women. Ann Epidemiol. (2018) 28:225–30. 10.1016/j.annepidem.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 44.Lhila A, Long S. What is driving the black–white difference in low birthweight in the US? Health Econ. (2012) 21:301–15. 10.1002/hec.1715 [DOI] [PubMed] [Google Scholar]
- 45.Massey Z, Rising SS, Ickovics J. CenteringPregnancy group prenatal care: promoting relationship-centered care. J Obstet Gynecol Neonatal Nurs. (2006) 35:286–94. 10.1111/j.1552-6909.2006.00040.x [DOI] [PubMed] [Google Scholar]
- 46.Gabbe PT, Reno R, Clutter C, Schottke TF, Price T, Calhoun K, et al. Improving maternal and infant child health outcomes with community-based pregnancy support groups: outcomes from Moms2B Ohio. Matern Child Health J. (2017) 21:1130–8. 10.1007/s10995-016-2211-x [DOI] [PubMed] [Google Scholar]
- 47.Crockett A, Heberlein EC, Glasscock L, Covington-Kolb S, Shea K, Khan IA. Investing in CenteringPregnancy™ group prenatal care reduces newborn hospitalization costs. Womens Health Issues. (2017) 27:60–6. 10.1016/j.whi.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 48.Heberlein EC, Frongillo EA, Picklesimer AH, Covington-Kolb S. Effects of group prenatal care on food insecurity during late pregnancy and early postpartum. Matern Child Health J. (2016) 20:1014–24. 10.1007/s10995-015-1886-8 [DOI] [PubMed] [Google Scholar]
- 49.Heberlein EC, Picklesimer AH, Billings DL, Covington-Kolb S, Farber N, Frongillo EA. The comparative effects of group prenatal care on psychosocial outcomes. Arch Womens Ment Health. (2016) 19:259–69. 10.1007/s00737-015-0564-6 [DOI] [PubMed] [Google Scholar]
- 50.Lathrop B, Pritham UA. A pilot study of prenatal care visits blended group and individual for women with low income. Nurs Womens Health. (2014) 18:462–74. 10.1111/1751-486X.12159 [DOI] [PubMed] [Google Scholar]
- 51.Novick G, Reid AE, Lewis J, Kershaw T, Rising SS, Ickovics JR. Group prenatal care: model fidelity and outcomes. J Midwifery Womens Health. (2013) 58:586. 10.1111/jmwh.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trotman G, Chhatre G, Darolia R, Tefera E, Damle L, Gomez-Lobo V. The effect of CenteringPregnancy versus traditional prenatal care models on improved adolescent health behaviors in the perinatal period. J Pediatr Adolesc Gynecol. (2015) 28:395–401. 10.1016/j.jpag.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 53.Cunningham SD, Lewis JB, Shebl FM, Boyd LM, Robinson MA, Grilo SA, et al. Group prenatal care reduces risk of preterm birth and low birth weight: a matched cohort study. J Womens Health. (2019) 28:17–22. 10.1089/jwh.2017.6817 [DOI] [PubMed] [Google Scholar]
- 54.Ickovics JR, Kershaw TS, Westdahl C, Schindler Rising S, Klima C, Reynolds H, et al. Group prenatal care and preterm birth weight: results from a matched cohort study at public clinics. Obstet Gynecol. (2003) 102(5 Pt 1):1051–7. 10.1016/S0029-7844(03)00765-8 [DOI] [PubMed] [Google Scholar]
- 55.Picklesimer AH, Billings D, Hale N, Blackhurst D, Covington-Kolb S. The effect of CenteringPregnancy group prenatal care on preterm birth in a low-income population. Am J Obstet Gynecol. (2012) 206:415.e411–7. 10.1016/j.ajog.2012.01.040 [DOI] [PubMed] [Google Scholar]
- 56.Ickovics JR, Kershaw TS, Westdahl C, Magriples U, Massey Z, Reynolds H, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. (2007) 110(2 Pt 1):330–9. 10.1097/01.AOG.0000275284.24298.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ickovics JR, Reed E, Magriples U, Westdahl C, Schindler Rising S, Kershaw TS. Effects of group prenatal care on psychosocial risk in pregnancy: results from a randomised controlled trial. Psychol Health. (2011) 26:235–50. 10.1080/08870446.2011.531577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klerman LV, Ramey SL, Goldenberg RL, Marbury S, Hou J, Cliver SP. A randomized trial of augmented prenatal care for multiple-risk, Medicaid-eligible African American women. Am J Public Health. (2001) 91:105–11. 10.2105/ajph.91.1.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Byerley BM, Haas DM. A systematic overview of the literature regarding group prenatal care for high-risk pregnant women. BMC Pregnancy Childbirth. (2017) 17:329. 10.1186/s12884-017-1522-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Catling CJ, Medley N, Foureur M, Ryan C, Leap N, Teate A, et al. Group versus conventional antenatal care for women. Cochrane Database Syst Rev. (2015) 2015:CD007622. 10.1002/14651858.CD007622.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazzoni SE, Carter EB. Group prenatal care. Am J Obstet Gynecol. (2017) 216:552–556. 10.1016/j.ajog.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 62.Ruiz-Mirazo E, Lopez-Yarto M, McDonald SD. Group prenatal care versus individual prenatal care: a systematic review and meta-analyses. J Obstet Gynaecol Can. (2012) 34:223–9. 10.1016/S1701-2163(16)35182-9 [DOI] [PubMed] [Google Scholar]
- 63.Carter EB, Barbier K, Sarabia R, Macones GA, Cahill AG, Tuuli MG. Group versus traditional prenatal care in low-risk women delivering at term: a retrospective cohort study. J Perinatol. (2017) 37:769–71. 10.1038/jp.2017.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atrash HK, Johnson K, Adams M, Cordero JF, Howse J. Preconception care for improving perinatal outcomes: the time to act. Maternal Child Health J. (2006) 10:3–11. 10.1007/s10995-006-0100-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dehlendorf C, Park SY, Emeremni CA, Comer D, Vincett K, Borrero S. Racial/ethnic disparities in contraceptive use: variation by age and women's reproductive experiences. Am J Obstet Gynecol. (2014) 210:526.e521–9. 10.1016/j.ajog.2014.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. (2003) 7:13–30. 10.1023/a:1022537516969 [DOI] [PubMed] [Google Scholar]
- 67.Lu MC, Tache V, Alexander GR, Kotelchuck M, Halfon N. Preventing low birth weight: is prenatal care the answer? J Maternal Fetal Neonatal Med. (2003) 13:362–80. 10.1080/jmf.13.6.362.380 [DOI] [PubMed] [Google Scholar]
- 68.Prather C, Fuller TR, Jeffries WL, Marshall KJ, Howell AV, et al. Racism, African American women, and their sexual and reproductive health: a review of historical and contemporary evidence and implications for health equity. Health Equity. (2018) 2:249–59. 10.1089/heq.2017.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bukowski R, Malone FD, Porter FT, Nyberg DA, Comstock CH, Hankins GDV, et al. Preconceptional folate supplementation and the risk of spontaneous preterm birth: a cohort study. PLoS Med. (2009) 6:e1000061. 10.1371/journal.pmed.1000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Catov JM, Bodnar LM, Ness RB, Markovic N, Roberts JM. Association of periconceptional multivitamin use and risk of preterm or small-for-gestational-age births. Am J Epidemiol. (2007) 166:296–303. 10.1093/aje/kwm071 [DOI] [PubMed] [Google Scholar]
- 71.Coonrod DV, Jack BW, Boggess KA, Long R, Conry JA, Cox SN, et al. The clinical content of preconception care: immunizations as part of preconception care. Am J Obstetr Gynecol. (2008) 199(6 Suppl B):S290–5. 10.1016/j.ajog.2008.08.061 [DOI] [PubMed] [Google Scholar]
- 72.Reid SM, Middleton P, Cossich MC, Crowther CA, Bain E. Interventions for clinical and subclinical hypothyroidism pre-pregnancy and during pregnancy. Cochrane Datab System Rev. (2013) CD007752. 10.1002/14651858.CD007752.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tieu J, Middleton P, Crowther CA, Shepherd E. Preconception care for diabetic women for improving maternal and infant health. Cochrane Datab System Rev. (2017) 8:CD007776. 10.1002/14651858.CD007776.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wahabi HA, Alzeidan RA, Bawazeer GA, Alansari LA, Esmaeil SA. Preconception care for diabetic women for improving maternal and fetal outcomes: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2010) 10:63. 10.1186/1471-2393-10-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Copper R, Conner M. Interconceptional antibiotics to prevent spontaneous preterm birth: a randomized clinical trial. Am J Obstetr Gynecol. (2006) 194:617–23. 10.1016/j.ajog.2005.11.049 [DOI] [PubMed] [Google Scholar]
- 76.Dean SV, Mason EM, Howson CP, Lassi ZS, Imam AM, Bhutta ZA. Born too soon: care before and between pregnancy to prevent preterm births: from evidence to action. Reproduct Health. (2013) 10:S3. 10.1186/1742-4755-10-s1-s3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elsinga J, de Jong-Potjer LC, van der Pal-de Bruin KM, le Cessie S, Assendelft WJJ, Buitendijk SE. The effect of preconception counselling on lifestyle and other behaviour before and during pregnancy. Women's Health Issues. (2008) 18(6 Suppl):S117–25. 10.1016/j.whi.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 78.Lumley J, Donohue L. Aiming to increase birth weight: a randomised trial of pre-pregnancy information, advice and counselling in inner-urban Melbourne. BMC Public Health. (2006) 6:299. 10.1186/1471-2458-6-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bryant AS, Washington S, Kuppermann M, Cheng YW, Caughey AB. Quality and equality in obstetric care: racial and ethnic differences in caesarean section delivery rates. Paediatr Perinat Epidemiol. (2009) 23:454–62. 10.1111/j.1365-3016.2009.01059.x [DOI] [PubMed] [Google Scholar]
- 80.Braveman P, Egerter S, Edmonston F, Verdon M. Racial/ethnic differences in the likelihood of cesarean delivery, California. Am J Public Health. (1995) 85:625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yee LM, Costantine MM, Rice MM, Bailit J, Reddy UM, Wapner RJ, et al. Racial and ethnic differences in utilization of labor management strategies intended to reduce cesarean delivery rates. Obstet Gynecol. (2017) 130:1285–94. 10.1097/aog.0000000000002343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kozhimannil KB, Muoto I, Darney BG, Caughey AB, Snowden JM. Early elective delivery disparities between non-Hispanic Black and White women after statewide policy implementation. Womens Health Issues. (2018) 28:224–31. 10.1016/j.whi.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chambers B, Baer RJ, Oltman SP, McLemore MR, Scott K, Karasek D, et al. 690: Racial disparities in preterm birth risk by risk factor grouping. Am J Obstetr Gynecol. (2019) 220(1 Suppl):S455–6. 10.1016/j.ajog.2018.11.713 [DOI] [Google Scholar]
- 84.Hayatbakhsh MR, Flenady VJ, Gibbons KS, Kingsbury AM, Hurrion E, Mamun AA, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. (2012) 71:215–9. 10.1038/pr.2011.25 [DOI] [PubMed] [Google Scholar]
- 85.Leemaqz SY, Dekker GA, McCowan LM, Kenny LC, Myers JE, Simpson NAB, et al. Maternal marijuana use has independent effects on risk for spontaneous preterm birth but not other common late pregnancy complications. Reproduct Toxicol. (2016) 62:77–86. 10.1016/j.reprotox.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 86.Mohlman MK, Levy DT. Disparities in maternal child and health outcomes attributable to prenatal tobacco use. Maternal Child Health J. (2016) 20:701–9. 10.1007/s10995-015-1870-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Truong KD, Reifsnider OS, Mayorga ME, Spitler H. Estimated number of preterm births and low birth weight children born in the United States due to maternal binge drinking. Matern Child Health J. (2013) 17:677–88. 10.1007/s10995-012-1048-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen P, Jacobson KC. Developmental trajectories of substance use from early adolescence to young adulthood: gender and racial/ethnic differences. J Adolescent Health. (2012) 50:154–63. 10.1016/j.jadohealth.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Evans-Polce RJ, Vasilenko SA, Lanza ST. Changes in gender and racial/ethnic disparities in rates of cigarette use, regular heavy episodic drinking, and marijuana use: ages 14 to 32. Addict Behav. (2015) 41:218–22. 10.1016/j.addbeh.2014.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keyes KM, Vo T, Wall MM, Caetano R, Suglia SF, Martins SS, et al. Racial/ethnic differences in use of alcohol, tobacco, and marijuana: Is there a cross-over from adolescence to adulthood? Soc Sci Med. (2015) 124:132–41. 10.1016/j.socscimed.2014.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cook B, Creedon T, Wang Y, Lu C, Carson N, Jules P, et al. Examining racial/ethnic differences in patterns of benzodiazepine prescription and misuse. Drug Alcohol Depend. (2018) 187:29–34. 10.1016/j.drugalcdep.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perreira KM, Cortes KE. Race/ethnicity and nativity differences in alcohol and tobacco use during pregnancy. Am J Public Health. (2006) 96:1629–36. 10.2105/AJPH.2004.056598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giovino GA, Gardiner PS. Understanding tobacco use behaviors among African Americans: progress, critical gaps, and opportunities. Nicot Tobacco Res. (2016) 18(suppl_1):S1–6. 10.1093/ntr/ntv234 [DOI] [PubMed] [Google Scholar]
- 94.Dunlop AL, Taylor RN, Tangpricha V, Fortunato S, Menon R. Maternal vitamin D. Folate, and polyunsaturated fatty acid status and bacterial vaginosis during pregnancy. Infect Dis Obstet Gynecol. (2011) 2011:216217. 10.1155/2011/216217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. (2002) 76:187–92. 10.1093/ajcn/76.1.187 [DOI] [PubMed] [Google Scholar]
- 96.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. (2007) 137:447–52. 10.1093/jn/137.2.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dunlop AL, Taylor RN, Tangpricha V, Fortunato S, Menon R. Maternal micronutrient status and preterm versus term birth for black and white US women. Reprod Sci. (2012) 19:939–48. 10.1177/1933719112438442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rogne T, Tielemans MJ, Chong MF-F, Yajnik CS, Krishnaveni GV, et al. Associations of maternal vitamin b12 concentration in pregnancy with the risks of preterm birth and low birth weight: a systematic review and meta-analysis of individual participant data. Am J Epidemiol. (2017) 185:212–23. 10.1093/aje/kww212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gennaro S, Biesecker B, Fantasia HC, Nguyen M, Garry D. Nutrition profiles of African [corrected] American women in the third trimester. MCN Am J Matern Child Nurs. (2011) 36:120–6. 10.1097/NMC.0b013e3182057a13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Myles M, Gennaro S, Dubois N, O'Connor C, Roberts K. Nutrition of black women during pregnancy. J Obstet Gynecol Neonatal Nurs. (2017) 46:e83–94. 10.1016/j.jogn.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Y, Beydoun MA. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. (2007) 29:6–28. 10.1093/epirev/mxm007 [DOI] [PubMed] [Google Scholar]
- 102.Lu M-S, He J-R, Chen Q, Lu J, Wei X, Zhou Q, et al. Maternal dietary patterns during pregnancy and preterm delivery: a large prospective cohort study in China. Nutr J. (2018) 17:71. 10.1186/s12937-018-0377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martin CL, Sotres-Alvarez D, Siega-Riz AM. Maternal dietary patterns during the second trimester are associated with preterm birth. J Nutr. (2015) 145:1857–64. 10.3945/jn.115.212019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scholl TO, Chen X. Maternal nutrition preterm delivery. In: Bendich A, Deckelbaum JR, editors. Preventive Nutrition: The Comprehensive Guide for Health Professionals. Cham: Springer International Publishing; (2015). p. 705–31. [Google Scholar]
- 105.Zerfu TA, Umeta M, Baye K. Dietary diversity during pregnancy is associated with reduced risk of maternal anemia, preterm delivery, and low birth weight in a prospective cohort study in rural Ethiopia. Am J Clin Nutr. (2016) 103:1482–8. 10.3945/ajcn.115.116798 [DOI] [PubMed] [Google Scholar]
- 106.Cunningham FG, Gant NF, Leveno KJ, Larry C. editors. Williams Obstetrics. Vol. 48. 21 ed. New York, NY: McGraw-Hill. [Google Scholar]
- 107.Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: differences among 4 racial/ethnic groups. Am J Public Health. (2005) 95:1545–51. 10.2105/ajph.2005.065680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Child Health USA (2013) . Retrieved from: https://mchb.hrsa.gov/sites/default/files/mchb/Data/Chartbooks/childhealth2013.pdf
- 109.Headen IE, Davis EM, Mujahid MS, Abrams B. Racial-ethnic differences in pregnancy-related weight. Adv Nutr. (2012) 3:83–94. 10.3945/an.111.000984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. (2017) 317:2207–25. 10.1001/jama.2017.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wise LA, Palmer JR, Heffner LJ, Rosenberg L. Prepregnancy body size, gestational weight gain, and risk of preterm birth in African-American women. Epidemiology. (2010) 21:243–52. 10.1097/EDE.0b013e3181cb61a9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leonard SA, Petito LC, Stephansson O, Hutcheon JA, Bodnar LM, Mujahid MS, et al. Weight gain during pregnancy and the black-white disparity in preterm birth. Ann Epidemiol. (2017) 27:323–8.e321. 10.1016/j.annepidem.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salihu HM, Luke S, Alio AP, Wathington D, Mbah AK, Marty PJ, et al. The superobese mother and ethnic disparities in preterm birth. J Natl Med Assoc. (2009) 101:1125–31. 10.1016/s0027-9684(15)31108-1 [DOI] [PubMed] [Google Scholar]
- 114.McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ. (2010) 341:c3428. 10.1136/bmj.c3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikstrom AK, et al. Maternal obesity and risk of preterm delivery. JAMA. (2013) 309:2362–70. 10.1001/jama.2013.6295 [DOI] [PubMed] [Google Scholar]
- 116.Tsur A, Mayo JA, Wong RJ, Shaw GM, Stevenson DK, Gould JB. ‘The obesity paradox’: a reconsideration of obesity and the risk of preterm birth. J Perinatol. (2017) 37:1088–92. 10.1038/jp.2017.104 [DOI] [PubMed] [Google Scholar]
- 117.Smith GCS, Shah I, Pell JP, Crossley JA, Dobbie R. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: a retrospective cohort study. Am J Public Health. (2007) 97:157–62. 10.2105/AJPH.2005.074294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.O'Rourke RW. Inflammation in obesity-related diseases. Surgery. (2009) 145:255–9. 10.1016/j.surg.2008.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Conde-Agudelo A, Belizan JM, Norton MH, Rosas-Bermudez A. Effect of the interpregnancy interval on perinatal outcomes in Latin America. Obstet Gynecol. (2005) 106:359–66. 10.1097/01.AOG.0000171118.79529.a3 [DOI] [PubMed] [Google Scholar]
- 120.McKinney D, House M, Chen A, Muglia L, DeFranco E. The influence of interpregnancy interval on infant mortality. Am J Obstet Gynecol. (2017) 216:316.e311–9. 10.1016/j.ajog.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.World Health Organization . Report of a WHO Technical Consultation on Birth Spacing (2005). [Google Scholar]
- 122.Zhu BP, Haines KM, Le T, McGrath-Miller K, Boulton ML. Effect of the interval between pregnancies on perinatal outcomes among white and black women. Am J Obstet Gynecol. (2001) 185:1403–10. 10.1067/mob.2001.118307 [DOI] [PubMed] [Google Scholar]
- 123.Atreya MR, Muglia LJ, Greenberg JM, DeFranco EA. Racial differences in the influence of interpregnancy interval on fetal growth. Matern Child Health J. (2017) 21:562–70. 10.1007/s10995-016-2140-8 [DOI] [PubMed] [Google Scholar]
- 124.Hogue CJ, Menon R, Dunlop AL, Kramer MR. Racial disparities in preterm birth rates and short inter-pregnancy interval: an overview. Acta Obstet Gynecol Scand. (2011) 90:1317–24. 10.1111/j.1600-0412.2011.01081.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lonhart JA, Mayo JA, Padula AM, Wise PH, Stevenson DK, Shaw GM. Short interpregnancy interval as a risk factor for preterm birth in non-Hispanic Black and White women in California. J Perinatol. (2019) 39:1175–81. 10.1038/s41372-019-0402-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Copen CE, Thoma ME, Kirmeyer S. Interpregnancy intervals in the United States: data from the birth certificate and the national survey of family growth. Natl Vital Stat Rep. (2015) 64:1–11 [PubMed] [Google Scholar]
- 127.Martin JA, Hamilton BE, Osterman MJK. Births: Final Data for 2018. (2019). Retrieved from: https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_13-508.pdf [PubMed]
- 128.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethnicity Dis. (1992) 2:207–21. [PubMed] [Google Scholar]
- 129.Geronimus AT. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc Sci Med. (1996) 42:589–97. 10.1016/0277-9536(95)00159-X [DOI] [PubMed] [Google Scholar]
- 130.Shah PS. Paternal factors and low birthweight, preterm, and small for gestational age births: a systematic review. Am J Obstet Gynecol. (2010) 202:103–23. 10.1016/j.ajog.2009.08.026 [DOI] [PubMed] [Google Scholar]
- 131.Lie RT, Wilcox AJ, Skjaerven R. Maternal and paternal influences on length of pregnancy. Obstet Gynecol. (2006) 107:880–5. 10.1097/01.Aog.0000206797.52832.36 [DOI] [PubMed] [Google Scholar]
- 132.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstetr Gynecol. (2004) 103:526–33. 10.1097/01.AOG.0000113623.18286.20 [DOI] [PubMed] [Google Scholar]
- 133.Kim S, England L, Sappenfield W, Wilson H, Bish C, Salihu H, et al. Racial/ethnic differences in the percentage of gestational diabetes mellitus cases attributable to overweight and obesity, Florida, 2004-2007. Prev Chronic Dis. (2012) 9:110249. 10.5888/pcd9.110249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pu J, Zhao B, Wang EJ, Nimbal V, Osmundson S, Kunz L, et al. Racial/ethnic differences in gestational diabetes prevalence and contribution of common risk factors. Paediatr Perinat Epidemiol. (2015) 29:436–43. 10.1111/ppe.12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nguyen BT, Cheng YW, Snowden JM, Esakoff TF, Frias AE, Caughey AB. The effect of race/ethnicity on adverse perinatal outcomes among patients with gestational diabetes mellitus. Am J Obstet Gynecol. (2012) 207:322.e321–6. 10.1016/j.ajog.2012.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. (2003) 102:181–92. 10.1016/S0029-7844(03)00475-7 [DOI] [PubMed] [Google Scholar]
- 137.Tanaka M, Jaamaa G, Kaiser M, Hills E, Soim A, Zhu M, et al. Racial disparity in hypertensive disorders of pregnancy in New York State: a 10-year longitudinal population-based study. Am J Public Health. (2007) 97:163–70. 10.2105/ajph.2005.068577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Davies EL, Bell JS, Bhattacharya S. Preeclampsia and preterm delivery: a population-based case–control study. Hypertens Pregnancy. (2016) 35:510–9. 10.1080/10641955.2016.1190846 [DOI] [PubMed] [Google Scholar]
- 139.Premkumar A, Baer RJ, Jelliffe-Pawlowski LL, Norton ME. Hypertensive disorders of pregnancy and preterm birth rates among Black women. Am J Perinatol. (2019) 36:148–54. 10.1055/s-0038-1660461 [DOI] [PubMed] [Google Scholar]
- 140.Shen M, Smith GN, Rodger M, White RR, Walker MC, Wen SW. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PLoS ONE. (2017) 12:e0175914. 10.1371/journal.pone.0175914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba'aqeel H, et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. (2006) 194:921–31. 10.1016/j.ajog.2005.10.813 [DOI] [PubMed] [Google Scholar]
- 142.Bryant AS, Seely EW, Cohen A, Lieberman E. Patterns of pregnancy-related hypertension in Black and White women. Hypertens Pregnancy. (2005) 24:281–90. 10.1080/10641950500281134 [DOI] [PubMed] [Google Scholar]
- 143.Ghosh G, Grewal J, Männistö T, Mendola P, Chen Z, Xie Y, et al. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethn Dis. (2014) 24:283–9. [PMC free article] [PubMed] [Google Scholar]
- 144.Poon LC, Kametas NA, Chelemen T, Leal A, Nicolaides KH. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. J Hum Hypertens. (2010) 24:104–10. 10.1038/jhh.2009.45 [DOI] [PubMed] [Google Scholar]
- 145.Ross KM, Dunkel Schetter C, McLemore MR, Chambers BD, Paynter RA, Baer R, et al. Socioeconomic status, preeclampsia risk and gestational length in Black and White women. J Racial Ethn Health Disparities. (2019) 6:1182–91. 10.1007/s40615-019-00619-3 [DOI] [PubMed] [Google Scholar]
- 146.Shahul S, Tung A, Minhaj M, Nizamuddin J, Wenger J, Mahmood E, et al. Racial disparities in comorbidities, complications, and maternal and fetal outcomes in women with preeclampsia/eclampsia. Hypertens Pregnancy. (2015) 34:506–15. 10.3109/10641955.2015.1090581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Speranza R, Luck M, Savitsky L, Scrivner Greiner K, Caughey AB. Racial disparities among preterm births rates in preeclampsia. Obstet Gynecol. (2018) 131:26S. 10.1097/01.Aog.0000532927.27602.46 [DOI] [Google Scholar]
- 148.Borzychowski AM, Sargent IL, Redman CWG. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. (2006) 11:309–16. 10.1016/j.siny.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 149.Ramma W, Ahmed A. Is inflammation the cause of pre-eclampsia? Biochem Soc Trans. (2011) 39:1619–27. 10.1042/bst20110672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Borsari L, Malagoli C, Werler MM, Rothman KJ, Malavolti M, Rodolfi R, et al. Joint effect of maternal tobacco smoking and pregestational diabetes on preterm births and congenital anomalies: a population-based study in Northern Italy. J Diabetes Res. (2018) 2018:2782741. 10.1155/2018/2782741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brown H, Speechley K, Macnab J, Natale R, Campbell M. Biological determinants of spontaneous late preterm and early term birth: a retrospective cohort study. BJOG. (2015) 122:491–9. 10.1111/1471-0528.13191 [DOI] [PubMed] [Google Scholar]
- 152.Köck K, Köck F, Klein K, Bancher-Todesca D, Helmer H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J Matern Fetal Med. (2010) 23:1004–8. 10.3109/14767050903551392 [DOI] [PubMed] [Google Scholar]
- 153.Ludvigsson JF, Neovius M, Söderling J, Gudbjörnsdottir S, Svensson A.-M., et al. Maternal glycemic control in type 1 diabetes and the risk for preterm birth: a population-based cohort study. Ann Intern Med. (2019) 170:691–701. 10.7326/m18-1974 [DOI] [PubMed] [Google Scholar]
- 154.Peterson C, Grosse SD, Li R, Sharma AJ, Razzaghi H, Herman WH, et al. Preventable health and cost burden of adverse birth outcomes associated with pregestational diabetes in the United States. Am J Obstetr Gynecol. (2015) 212:74.e71–9. 10.1016/j.ajog.2014.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Temple R, Murphy H. Type 2 diabetes in pregnancy – an increasing problem. Best Pract Res Clin Endocrinol Metab. (2010) 24:591–603. 10.1016/j.beem.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 156.Homko CJ, Reece EA. Development of early-onset type 2 diabetes in the young: implications for child bearing. Curr Diab Rep. (2003) 3:313–8. 10.1007/s11892-003-0023-z [DOI] [PubMed] [Google Scholar]
- 157.Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. Am J Hum Biol. (2009) 21:2–15. 10.1002/ajhb.20822 [DOI] [PubMed] [Google Scholar]
- 158.Chou AF, Brown AF, Jensen RE, Shih S, Pawlson G, Scholle SH. Gender and racial disparities in the management of diabetes mellitus among medicare patients. Womens Health Issues. (2007) 17:150–61. 10.1016/j.whi.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 159.Bardenheier BH, Imperatore G, Devlin HM, Kim SY, Cho P, Geiss LS. Trends in pre-pregnancy diabetes among deliveries in 19 states US. 2000-2010. Am J Prevent Med. (2015) 48:154–61. 10.1016/j.amepre.2014.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. (2002) 287:2519–27. 10.1001/jama.287.19.2519 [DOI] [PubMed] [Google Scholar]
- 161.Harville EW, Viikari JS, Raitakari OT. Preconception cardiovascular risk factors and pregnancy outcome. Epidemiology. (2011) 22:724–30. 10.1097/EDE.0b013e318225c960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.James PR, Nelson-Piercy C. Management of hypertension before, during, after pregnancy. Heart. (2004) 90:1499. 10.1136/hrt.2004.035444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jelliffe-Pawlowski LL, Baer RJ, Blumenfeld YJ, Ryckman KK, O'Brodovich HM, Gould JB, et al. Maternal characteristics and mid-pregnancy serum biomarkers as risk factors for subtypes of preterm birth. BJOG. (2015) 122:1484–3. 10.1111/1471-0528.13495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aubry EM, Oelhafen S, Fankhauser N, Raio L, Cignacco EL. Adverse perinatal outcomes for obese women are influenced by the presence of comorbid diabetes and hypertensive disorders. Sci Rep. (2019) 9:9793. 10.1038/s41598-019-46179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leon MG, Moussa HN, Longo M, Pedroza C, Haidar ZA, Mendez-Figueroa H, et al. Rate of gestational diabetes mellitus and pregnancy outcomes in patients with chronic hypertension. Am J Perinatol. (2016) 33:745–50. 10.1055/s-0036-1571318 [DOI] [PubMed] [Google Scholar]
- 166.Ankumah NA, Cantu J, Jauk V, Biggio J, Hauth J, Andrews W, et al. Risk of adverse pregnancy outcomes in women with mild chronic hypertension before 20 weeks of gestation. Obstet Gynecol. (2014) 123:966–72. 10.1097/aog.0000000000000205 [DOI] [PubMed] [Google Scholar]
- 167.Morgan JL, Nelson DB, Roberts SW, Wells CE, McIntire DD, Cunningham FG. Blood pressure profiles across pregnancy in women with chronic hypertension. Am J Perinatol. (2016) 33:1128–32. 10.1055/s-0036-1584581 [DOI] [PubMed] [Google Scholar]
- 168.Nzelu D, Dumitrascu-Biris D, Nicolaides KH, Kametas NA. Chronic hypertension: first-trimester blood pressure control and likelihood of severe hypertension, preeclampsia, and small for gestational age. Am J Obstet Gynecol. (2018) 218:337.e331–7. 10.1016/j.ajog.2017.12.235 [DOI] [PubMed] [Google Scholar]
- 169.Gillum RF. Epidemiology of hypertension in African American women. Am Heart J. (1996) 131:385–95. 10.1016/s0002-8703(96)90371-3 [DOI] [PubMed] [Google Scholar]
- 170.Premkumar A, Henry DE, Moghadassi M, Nakagawa S, Norton ME. The interaction between maternal race/ethnicity and chronic hypertension on preterm birth. Am J Obstet Gynecol. (2016) 215:787.e781–8. 10.1016/j.ajog.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 171.Azeez O, Kulkarni A, Kuklina EV, Kim SY, Cox S. Hypertension and diabetes in non-pregnant women of reproductive age in the United States. Prev Chronic Dis. (2019) 16:E146. 10.5888/pcd16.190105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Agger WA, Siddiqui D, Lovrich SD, Callister SM, Borgert AJ, Merkitch KW, et al. Epidemiologic factors and urogenital infections associated with preterm birth in a midwestern U.S. population. Obstetr Gynecol. (2014) 124:969–77. 10.1097/AOG.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bretelle F, Rozenberg P, Pascal A, Favre R, Bohec C, Loundou A, et al. High atopobium vaginae and gardnerella vaginalis vaginal loads are associated with preterm birth. Clin Infect Dis. (2014) 60:860–7. 10.1093/cid/ciu966 [DOI] [PubMed] [Google Scholar]
- 174.Cobb CM, Kelly PJ, Williams KB, Babbar S, Angolkar M, Derman RJ. The oral microbiome and adverse pregnancy outcomes. Int J Womens Health. (2017) 9:551–9. 10.2147/IJWH.S142730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The vaginal infections and prematurity study group. N Engl J Med. (1995) 333:1737–42. 10.1056/nejm199512283332604 [DOI] [PubMed] [Google Scholar]
- 176.Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: results of a prospective study. J Am Dent Assoc. (2001) 132:875–80. 10.14219/jada.archive.2001.0299 [DOI] [PubMed] [Google Scholar]
- 177.Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, et al. The preterm prediction study: significance of vaginal infections. Am J Obstet Gynecol. (1995) 173:4. [DOI] [PubMed] [Google Scholar]
- 178.Nadeau HCG, Subramaniam A, Andrews WW. Infection and preterm birth. Semin Fetal Neonatal Med. (2016) 21:100–5. 10.1016/j.siny.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 179.Nelson DB, Hanlon A, Nachamkin I, Haggerty C, Mastrogiannis DS, Liu C, et al. Early pregnancy changes in bacterial vaginosis-associated bacteria and preterm delivery. Paediatr Perinat Epidemiol. (2014) 28:88–96. 10.1111/ppe.12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Uscher-Pines L, Hanlon AL, Nelson DB. Racial differences in bacterial vaginosis among pregnant women: the relationship between demographic and behavioral predictors and individual BV-related microorganism levels. Matern Child Health J. (2009) 13:512–9. 10.1007/s10995-008-0372-y [DOI] [PubMed] [Google Scholar]
- 181.Foxman B, Wen A, Srinivasan U, Goldberg D, Marrs CF, Owen J, et al. Mycoplasma, bacterial vaginosis–associated bacteria BVAB3, race, and risk of preterm birth in a high-risk cohort. Am J Obstetr Gynecol. (2014) 210:226.e221–7. 10.1016/j.ajog.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Paige DM, Augustyn M, Adih WK, Witter F, Chang J. Bacterial vaginosis and preterm birth: a comprehensive review of the literature. J Nurse Midwifery. (1998) 43:83–9. 10.1016/s0091-2182(97)00161-4 [DOI] [PubMed] [Google Scholar]
- 183.Paul K, Boutain D, Manhart L, Hitti J. Racial disparity in bacterial vaginosis: the role of socioeconomic status, psychosocial stress, neighborhood characteristics and possible implications for preterm birth. Soc Sci Med. (2008) 67:824–33. 10.1016/j.socscimed.2008.05.017 [DOI] [PubMed] [Google Scholar]
- 184.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004, associations with symptoms, sexual behaviors, reproductive health. Sex Transmitted Dis. (2007) 34:864–9. 10.1097/OLQ.0b013e318074e565 [DOI] [PubMed] [Google Scholar]
- 185.Hitti J, Nugent R, Boutain D, Gardella C, Hillier SL, Eschenbach DA. Racial disparity in risk of preterm birth associated with lower genital tract infection. Paediatr Perinat Epidemiol. (2007) 21:330–7. 10.1111/j.1365-3016.2007.00807.x [DOI] [PubMed] [Google Scholar]
- 186.Lamont RF. Advances in the prevention of infection-related preterm birth. Front Immunol. (2015) 6:566. 10.3389/fimmu.2015.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Parihar AS, Katoch V, Rajguru SA, Rajpoot N, Singh P, Wakhle S. Periodontal disease: a possible risk-factor for adverse pregnancy outcome. J Int Oral Health. (2015) 7:137–42. [PMC free article] [PubMed] [Google Scholar]
- 188.Sobel R, Sobel JD. Metronidazole for the treatment of vaginal infections. Expert Opin Pharmacother. (2015) 16:1109–15. 10.1517/14656566.2015.1035255 [DOI] [PubMed] [Google Scholar]
- 189.Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. (2012) 70:S38–44. 10.1111/j.1753-4887.2012.00493.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology. (2014) 160(Pt 10):2272–82. 10.1099/mic.0.081034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. (2011) 108(Suppl 1):4680–7. 10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. (2007) 1:121. 10.1038/ismej.2007.12 [DOI] [PubMed] [Google Scholar]
- 193.Taylor-Robinson D, Lamont RF. Mycoplasmas in pregnancy. BJOG. (2011) 118:164–74. 10.1111/j.1471-0528.2010.02766.x [DOI] [PubMed] [Google Scholar]
- 194.Wen A, Srinivasan U, Goldberg D, Owen J, Marrs CF, Misra D, et al. Selected vaginal bacteria and risk of preterm birth: an ecological perspective. J Infect Dis. (2014) 209:1087–94. 10.1093/infdis/jit632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S, et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. (2017) 5:6. 10.1186/s40168-016-0223-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brotman RM, Klebanoff MA, Nansel TR, Andrews WW, Schwebke JR, Zhang J, et al. A longitudinal study of vaginal douching and bacterial vaginosis—a marginal structural modeling analysis. Am J Epidemiol. (2008) 168:188–96. 10.1093/aje/kwn103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bruce FC, Kendrick JS, Kieke BA, Jr., Jagielski S, Joshi R, et al. Is vaginal douching associated with preterm delivery? Epidemiology. (2002) 13:328–33. 10.1097/00001648-200205000-00014 [DOI] [PubMed] [Google Scholar]
- 198.Cottrell BH. An updated review of evidence to discourage douching. MCN Am J Matern Child Nurs. (2010) 35:102–7; quiz 108–9. 10.1097/NMC.0b013e3181cae9da [DOI] [PubMed] [Google Scholar]
- 199.Fiscella K, Franks P, Kendrick JS, Meldrum S, Kieke BA, Jr. Risk of preterm birth that is associated with vaginal douching. Am J Obstet Gynecol. (2002) 186:1345–50. 10.1067/mob.2002.122406 [DOI] [PubMed] [Google Scholar]
- 200.Wade WG. The oral microbiome in health and disease. Pharmacology Res. (2013) 69:137–43. 10.1016/j.phrs.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 201.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. (2010) 64:38–57. 10.1111/j.1600-0897.2010.00830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lopetuso LR, Petito V, Graziani C, Schiavoni E, Paroni Sterbini F, Poscia A, et al. Gut microbiota in health, diverticular disease, irritable bowel syndrome, and inflammatory bowel diseases: time for microbial marker of gastrointestinal disorders. Digest Dis. (2018) 36:56–65. 10.1159/000477205 [DOI] [PubMed] [Google Scholar]
- 203.Cornish J, Tan E, Teare J, Teoh TG, Rai R, Clark SK, et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut. (2007) 56:830–7. 10.1136/gut.2006.108324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mahadevan U, Sandborn WJ, Li DK, Hakimian S, Kane S, Corley DA. Pregnancy outcomes in women with inflammatory bowel disease: a large community-based study from Northern California. Gastroenterology. (2007) 133:1106–12. 10.1053/j.gastro.2007.07.019 [DOI] [PubMed] [Google Scholar]
- 205.van der Woude CJ, Ardizzone S, Bengtson MB, Fiorino G, Fraser G, Katsanos K, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. (2015) 9:107–24. 10.1093/ecco-jcc/jju006 [DOI] [PubMed] [Google Scholar]
- 206.Bravo MA, Anthopolos R, Bell ML, Miranda ML. Racial isolation and exposure to airborne particulate matter and ozone in understudied US populations: environmental justice applications of downscaled numerical model output. Environ Int. (2016) 92–93:247–55. 10.1016/j.envint.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 207.Burris HH, Hacker MR. Birth outcome racial disparities: a result of intersecting social and environmental factors. Semin Perinatol. (2017) 41:360–6. 10.1053/j.semperi.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Grove M, Ogden L, Pickett S, Boone C, Buckley G, Locke DH, et al. The legacy effect: understanding how segregation and environmental injustice unfold over time in Baltimore. Ann Am Assoc Geogr. (2018) 108:524–37. 10.1080/24694452.2017.1365585 [DOI] [Google Scholar]
- 209.Rothstein R. The Color of Law: A Forgotten History of How Our Government Segregated America. New York, NY: Liveright Publishing Corporation; (2017). [Google Scholar]
- 210.US Census Bureau . Racial and Ethnic Residential Segregation in the United States: 1980-2000. Washington, DC: (2002). Retrieved from: https://www.census.gov/prod/2002pubs/censr-3.pdf [Google Scholar]
- 211.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. (2001) 116:404–16. 10.1093/phr/116.5.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Acevedo-Garcia D, Lochner KA, Osypuk TL, Subramanian SV. Future directions in residential segregation and health research: a multilevel approach. Am J Public Health. (2003) 93:215–21. 10.2105/ajph.93.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Acevedo-Garcia D, Osypuk TL, McArdle N, Williams DR. Toward a policy-relevant analysis of geographic and racial/ethnic disparities in child health. Health Affairs. (2008) 27:321–33. 10.1377/hlthaff.27.2.321 [DOI] [PubMed] [Google Scholar]
- 214.Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC. Disparities in exposure to air pollution during pregnancy. Environ Health Perspect. (2003) 111:942–6. 10.1289/ehp.5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Casey JA, Karasek D, Ogburn EL, Goin DE, Dang K, Braveman PA, et al. Retirements of coal and oil power plants in California: association with reduced preterm birth among populations nearby. Am J Epidemiol. (2018) 187:1586–94. 10.1093/aje/kwy110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Currie J, Zivin JG, Meckel K, Neidell M, Schlenker W. Something in the water: contaminated drinking water and infant health. Can J Econ. (2013) 46:791–810. 10.1111/caje.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pergialiotis V, Kotrogianni P, Christopoulos-Timogiannakis E, Koutaki D, Daskalakis G, Papantoniou N. Bisphenol A and adverse pregnancy outcomes: a systematic review of the literature. J Matern Fetal Neonatal Med. (2018) 31:3320–7. 10.1080/14767058.2017.1368076 [DOI] [PubMed] [Google Scholar]
- 218.Wigle DT, Arbuckle TE, Turner MC, Berube A, Yang Q, Liu S, et al. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev. (2008) 11:373–517. 10.1080/10937400801921320 [DOI] [PubMed] [Google Scholar]
- 219.Anthopolos R, James SA, Gelfand AE, Miranda ML. A spatial measure of neighborhood level racial isolation applied to low birthweight, preterm birth, and birthweight in North Carolina. Spat Spatiotemporal Epidemiol. (2011) 2:235–46. 10.1016/j.sste.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 220.Kim Y, Vohra-Gupta S, Margerison CE, Cubbin C. Neighborhood racial/ethnic composition trajectories and black-white differences in preterm birth among women in Texas. J Urban Health. (2020) 97:37–51. 10.1007/s11524-019-00411-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mason SM, Messer LC, Laraia BA, Mendola P. Segregation and preterm birth: the effects of neighborhood racial composition in North Carolina. Health Place. (2009) 15:1–9. 10.1016/j.healthplace.2008.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mehra R, Boyd LM, Ickovics JR. Racial residential segregation and adverse birth outcomes: a systematic review and meta-analysis. Soc Sci Med. (2017) 191:237–50. 10.1016/j.socscimed.2017.09.018 [DOI] [PubMed] [Google Scholar]
- 223.Messer LC, Oakes JM, Mason S. Effects of socioeconomic and racial residential segregation on preterm birth: a cautionary tale of structural confounding. Am J Epidemiol. (2010) 171:664–73. 10.1093/aje/kwp435 [DOI] [PubMed] [Google Scholar]
- 224.Ncube CN, Enquobahrie DA, Burke JG, Ye F, Marx J, Albert SM. Transgenerational transmission of preterm birth risk: the role of race and generational socio-economic neighborhood context. Matern Child Health J. (2017) 21:1616–26. 10.1007/s10995-016-2251-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Osypuk TL, Acevedo-Garcia D. Are racial disparities in preterm birth larger in hypersegregated areas? Am J Epidemiol. (2008) 167:1295–304. 10.1093/aje/kwn043 [DOI] [PubMed] [Google Scholar]
- 226.Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. (2006) 114:1150–3. 10.1289/ehp.8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bekkar B, Pacheco S, Basu R, DeNicola N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Network Open. (2020) 3:e208243. 10.1001/jamanetworkopen.2020.8243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Basu R, Chen H, Li DK, Avalos LA. The impact of maternal factors on the association between temperature and preterm delivery. Environ Res. (2017) 154:109–14. 10.1016/j.envres.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kloog I. Air pollution, ambient temperature, green space and preterm birth. Curr Opin Pediatr. (2019) 31:237–43. 10.1097/mop.0000000000000736 [DOI] [PubMed] [Google Scholar]
- 230.Li S, Wang J, Xu Z, Wang X, Xu G, Zhang J, et al. Exploring associations of maternal exposure to ambient temperature with duration of gestation and birth weight: a prospective study. BMC Pregnancy Childbirth. (2018) 18:513. 10.1186/s12884-018-2100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang J, Tong S, Williams G, Pan X. Exposure to heat wave during pregnancy and adverse birth outcomes: an exploration of susceptible windows. Epidemiology. (2019) 30(Suppl. 1):S115–21. 10.1097/ede.0000000000000995 [DOI] [PubMed] [Google Scholar]
- 232.Zhang Y, Yu C, Wang L. Temperature exposure during pregnancy and birth outcomes: an updated systematic review of epidemiological evidence. Environ Pollut. (2017) 225:700–12. 10.1016/j.envpol.2017.02.066 [DOI] [PubMed] [Google Scholar]
- 233.Zhong Q, Lu C, Zhang W, Zheng X, Deng Q. Preterm birth and ambient temperature: strong association during night-time and warm seasons. J Therm Biol. (2018) 78:381–90. 10.1016/j.jtherbio.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 234.Klein Rosenthal J, Kinney PL, Metzger KB. Intra-urban vulnerability to heat-related mortality in New York City, 1997-2006. Health Place. (2014) 30:45–60. 10.1016/j.healthplace.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.O'Neill MS, Zanobetti A, Schwartz J. Disparities by race in heat-related mortality in four US cities: the role of air conditioning prevalence. J Urban Health. (2005) 82:191–7. 10.1093/jurban/jti043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Voelkel J, Hellman D, Sakuma R, Shandas V. Assessing vulnerability to urban heat: a study of disproportionate heat exposure and access to refuge by socio-demographic status in Portland, Oregon. Int J Environ Res Public Health. (2018) 15:640. 10.3390/ijerph15040640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Basu R, Malig B, Ostro B. High ambient temperature and the risk of preterm delivery. Am J Epidemiol. (2010) 172:1108–17. 10.1093/aje/kwq170 [DOI] [PubMed] [Google Scholar]
- 238.Carmichael SL, Cullen MR, Mayo JA, Gould JB, Loftus P, Stevenson DK, et al. Population-level correlates of preterm delivery among black and white women in the U.S. PLoS ONE. (2014) 9:e94153. 10.1371/journal.pone.0094153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dolan SM. Genetic and environmental contributions to racial disparities in preterm birth. Mt Sinai J Med. (2010) 77:160–5. 10.1002/msj.20169 [DOI] [PubMed] [Google Scholar]
- 240.Monangi NK, Brockway HM, House M, Zhang G, Muglia LJ. The genetics of preterm birth: progress and promise. Semin Perinatol. (2015) 39:574–83. 10.1053/j.semperi.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 241.Wu W, Witherspoon DJ, Fraser A, Clark EA, Rogers A, Stoddard GJ, et al. The heritability of gestational age in a two-million member cohort: implications for spontaneous preterm birth. Hum Genet. (2015) 134:803–8. 10.1007/s00439-015-1558-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hong X, Sherwood B, Ladd-Acosta C, Peng S, Ji H, Hao K, et al. Genome-wide DNA methylation associations with spontaneous preterm birth in US blacks: findings in maternal and cord blood samples. Epigenetics. (2018) 13:163–72. 10.1080/15592294.2017.1287654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mani S, Ghosh J, Lan Y, Senapati S, Ord T, Sapienza C, et al. Epigenetic changes in preterm birth placenta suggest a role for ADAMTS genes in spontaneous preterm birth. Hum Mol Genet. (2019) 28:84–95. 10.1093/hmg/ddy325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Parets SE, Conneely KN, Kilaru V, Menon R, Smith AK. DNA methylation provides insight into intergenerational risk for preterm birth in African Americans. Epigenetics. (2015) 10:784–92. 10.1080/15592294.2015.1062964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ribeiro de Andrade Ramos B, da Silva MG. The burden of genetic and epigenetic traits in prematurity. Reprod Sci. (2018) 25:471–9. 10.1177/1933719117718270 [DOI] [PubMed] [Google Scholar]
- 246.Yadama AP, Mirzakhani H, McElrath TF, Litonjua AA, Weiss ST. Transcriptome analysis of early pregnancy vitamin D status and spontaneous preterm birth. PLoS ONE. (2020) 15:e0227193. 10.1371/journal.pone.0227193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang G, Feenstra B, Bacelis J, Liu X, Muglia LM, Juodakis J, et al. Genetic associations with gestational duration and spontaneous preterm birth. N Engl J Med. (2017) 377:1156–67. 10.1056/NEJMoa1612665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang G, Jacobsson B, Muglia LJ. Genetic associations with spontaneous preterm birth. N Engl J Med. (2017) 377:2401–2. 10.1056/NEJMc1713902 [DOI] [PubMed] [Google Scholar]
- 249.Frey HA, Stout MJ, Pearson LN, Tuuli MG, Cahill AG, Strauss JF, et al. Genetic variation associated with preterm birth in African-American women. Am J Obstet Gynecol. (2016) 215:235.e231–8. 10.1016/j.ajog.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Rood KM, Buhimschi CS. Genetics, hormonal influences, preterm birth. Semin Perinatol. (2017) 41:401–8. 10.1053/j.semperi.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 251.Sheikh IA, Ahmad E, Jamal MS, Rehan M, Assidi M, Tayubi IA, et al. Spontaneous preterm birth and single nucleotide gene polymorphisms: a recent update. BMC Genomics. (2016) 17:759. 10.1186/s12864-016-3089-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hong X, Hao K, Ji H, Peng S, Sherwood B, Di Narzo A, et al. Genome-wide approach identifies a novel gene-maternal pre-pregnancy BMI interaction on preterm birth. Nat Commun. (2017) 8:15608. 10.1038/ncomms15608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pereyra S, Bertoni B, Sapiro R. Interactions between environmental factors and maternal-fetal genetic variations: strategies to elucidate risks of preterm birth. Eur J Obstet Gynecol Reprod Biol. (2016) 202:20–5. 10.1016/j.ejogrb.2016.04.030 [DOI] [PubMed] [Google Scholar]
- 254.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. (2002) 287:195–202. 10.1001/jama.287.2.195 [DOI] [PubMed] [Google Scholar]
- 255.Macones GA, Parry S, Elkousy M, Clothier B, Ural SH, Strauss, et al. A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol. (2004) 190:1504–8; discussion: 1503A. 10.1016/j.ajog.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 256.Li J, Hong X, Mesiano S, Muglia LJ, Wang X, Snyder M, et al. Natural selection has differentiated the progesterone receptor among human populations. Am J Hum Genet. (2018) 103:45–57. 10.1016/j.ajhg.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Elovitz MA, Gajer P, Riis V, Brown AG, Humphrys MS, Holm JB, et al. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat Commun. (2019) 10:1305. 10.1038/s41467-019-09285-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Liu X, Buil A, Feenstra B. The iPSYCH-BROAD group and early growth genetics consortium. Fetal genome-wide metaanalysis of gestational age and preterm delivery. Paper Presented at the ASHG. Orlando, FL: (2017). [Google Scholar]
- 259.Esplin MS, Manuck TA, Varner MW, Christensen B, Biggio J, Bukowski R, et al. Cluster analysis of spontaneous preterm birth phenotypes identifies potential associations among preterm birth mechanisms. Am J Obstet Gynecol. (2015) 213:429.e421–9. 10.1016/j.ajog.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. (2016) 21:68–73. 10.1016/j.siny.2015.12.011 [DOI] [PubMed] [Google Scholar]
- 261.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. (2001) 15(Suppl 2):78–89. 10.1046/j.1365-3016.2001.00010.x [DOI] [PubMed] [Google Scholar]
- 262.Argmann CA, Houten SM, Zhu J, Schadt EE. A next generation multiscale view of inborn errors of metabolism. Cell Metab. (2016) 23:13–26. 10.1016/j.cmet.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Stevenson DK, Wong RJ, Aghaeepour N, Angst MS, Darmstadt GL, DiGiulio DB, et al. Understanding health disparities. J Perinatol. (2019) 39:354–8. 10.1038/s41372-018-0298-1 [DOI] [PubMed] [Google Scholar]
- 264.Cohen S, Kessler RC, Gordon LU. Measuring Stress: A Guide for Health Social Scientists. New York, NY: Oxford University Press; (1995). [Google Scholar]
- 265.Lazarus R, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer Publishing Company; (1984). [Google Scholar]
- 266.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. (2011) 62:531–58. 10.1146/annurev.psych.031809.130727 [DOI] [PubMed] [Google Scholar]
- 267.Gennaro S, Hennessy MD. Psychological and physiological stress: impact on preterm birth. J Obstet Gynecol Neonatal Nurs. (2003) 32:668–75. 10.1177/0884217503257484 [DOI] [PubMed] [Google Scholar]
- 268.Wadhwa PD, Culhane JF, Rauh V, Barve SS. Stress and preterm birth: neuroendocrine, immune/inflammatory, vascular mechanisms. Matern Child Health J. (2001) 5:119–25. 10.1023/A:1011353216619 [DOI] [PubMed] [Google Scholar]
- 269.Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol. (2001) 15:17–29. 10.1046/j.1365-3016.2001.00005.x [DOI] [PubMed] [Google Scholar]
- 270.McEwen BS. Allostasis and the epigenetics of brain and body health over the life course: the brain on stress. JAMA Psychiatry. (2017) 74:551–2. 10.1001/jamapsychiatry.2017.0270 [DOI] [PubMed] [Google Scholar]
- 271.Goosby BJ, Cheadle JE, Mitchell C. Stress-related biosocial mechanisms of discrimination and African American health inequities. Annu Rev Sociol. (2018) 44:319–40. 10.1146/annurev-soc-060116-053403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lucini D, Di Fede G, Parati G, Pagani M. Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension. (2005) 46:1201–6. 10.1161/01.HYP.0000185147.32385.4b [DOI] [PubMed] [Google Scholar]
- 273.Lindsay KL, Buss C, Wadhwa PD, Entringer S. The interplay between maternal nutrition and stress during pregnancy: issues and considerations. Ann Nutr Metab. (2017) 70:191–200. 10.1159/000457136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. (2019) 99:1877–2013. 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- 275.Hantsoo L, Jašarević E, Criniti S, McGeehan B, Tanes C, Sammel MD, et al. Childhood adversity impact on gut microbiota and inflammatory response to stress during pregnancy. Brain Behav Immun. (2019) 75:240–50. 10.1016/j.bbi.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tomiyama AJ. Stress and obesity. Annu Rev Psychol. (2019) 70:703–18. 10.1146/annurev-psych-010418-102936 [DOI] [PubMed] [Google Scholar]
- 277.Liu MY, Li N, Li WA, Khan H. Association between psychosocial stress and hypertension: a systematic review and meta-analysis. Neurol Res. (2017) 39:573–80. 10.1080/01616412.2017.1317904 [DOI] [PubMed] [Google Scholar]
- 278.Zhang S, Ding Z, Liu H, Chen Z, Wu J, Zhang Y, et al. Association between mental stress and gestational hypertension/preeclampsia: a meta-analysis. Obstet Gynecol Surv. (2013) 68:825–34. 10.1097/ogx.0000000000000001 [DOI] [PubMed] [Google Scholar]
- 279.Kumari M, Head J, Marmot M. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med. (2004) 164:1873–80. 10.1001/archinte.164.17.1873 [DOI] [PubMed] [Google Scholar]
- 280.Kominiarek MA, Grobman W, Adam E, Buss C, Culhane J, Entringer S, et al. Stress during pregnancy and gestational weight gain. J Perinatol. (2018) 38:462–7. 10.1038/s41372-018-0051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Silveira ML, Whitcomb BW, Pekow P, Braun B, Markenson G, Dole N, et al. Perceived psychosocial stress and glucose intolerance among pregnant Hispanic women. Diabetes Metab. (2014) 40:466–75. 10.1016/j.diabet.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rogac M, Peterlin B. Epigenetic signature of chronic maternal stress load during pregnancy might be a potential biomarker for spontaneous preterm birth. Balkan J Med Genet. (2018) 21:27–33. 10.2478/bjmg-2018-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ayada C, Toru Ü, Korkut Y. The relationship of stress and blood pressure effectors. Hippokratia. (2015) 19:99–108. [PMC free article] [PubMed] [Google Scholar]
- 284.Nagele E, Jeitler K, Horvath K, Semlitsch T, Posch N, Herrmann KH, et al. Clinical effectiveness of stress-reduction techniques in patients with hypertension: systematic review and meta-analysis. J Hypertens. (2014) 32:1936–44. 10.1097/hjh.0000000000000298 [DOI] [PubMed] [Google Scholar]
- 285.Sparrenberger F, Cichelero FT, Ascoli AM, Fonseca FP, Weiss G, Berwanger O, et al. Does psychosocial stress cause hypertension? A systematic review of observational studies. J Hum Hypertens. (2009) 23:12–9. 10.1038/jhh.2008.74 [DOI] [PubMed] [Google Scholar]
- 286.Agita A, Alsagaff MT. Inflammation, immunity, and hypertension. Acta Med Indones. (2017) 49:158–65. [PubMed] [Google Scholar]
- 287.Christian LM. Psychoneuroimmunology in pregnancy: immune pathways linking stress with maternal health, adverse birth outcomes, fetal development. Neurosci Biobehav Rev. (2012) 36:350–61. 10.1016/j.neubiorev.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep. (2014) 17:507. 10.1007/s11906-014-0507-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. (1999) 180(2 Pt 1):499–506. 10.1016/s0002-9378(99)70239-5 [DOI] [PubMed] [Google Scholar]
- 290.van Esch J, Bolte A, Vandenbussche F, Schippers D, de Weerth C, Beijers R. Hair cortisol concentration and reported anxiety are elevated in preeclampsia. Pregnancy Hypertens. (2018) 13:S105. 10.1016/j.preghy.2018.08.311 [DOI] [PubMed] [Google Scholar]
- 291.Borders AEB, Wolfe K, Qadir S, Kim KY, Holl J, Grobman W. Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. J Perinatol. (2015) 35:580. 10.1038/jp.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Grobman WA, Parker C, Wadhwa PD, Willinger M, Simhan H, Silver B, et al. Racial/ethnic disparities in measures of self-reported psychosocial states and traits during pregnancy. Am J Perinatol. (2016) 33:1426–32. 10.1055/s-0036-1586510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Mukherjee S, Coxe S, Fennie K, Madhivanan P, Trepka MJ. Stressful life event experiences of pregnant women in the United States: a latent class analysis. Womens Health Issues. (2017) 27:83–92. 10.1016/j.whi.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 294.Almeida J, Bécares L, Erbetta K, Bettegowda VR, Ahluwalia IB. Racial/ethnic Inequities in low birth weight and preterm birth: The role of multiple forms of stress. Matern Child Health J. (2018) 22:1154–63. 10.1007/s10995-018-2500-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Grobman WA, Parker CB, Willinger M, Wing DA, Silver RM, Wapner RJ, et al. Racial disparities in adverse pregnancy outcomes and psychosocial stress. Obstet Gynecol. (2018) 131:328–35. 10.1097/aog.0000000000002441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lu MC, Chen B. Racial and ethnic disparities in preterm birth: the role of stressful life events. Am J Obstet Gynecol. (2004) 191:691–9. 10.1016/j.ajog.2004.04.018 [DOI] [PubMed] [Google Scholar]
- 297.Curry Owens T, Jackson FM. Examining life-course socioeconomic position, contextualized stress, and depression among well-educated african-american pregnant women. Womens Health Issues. (2015) 25:382–9. 10.1016/j.whi.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 298.Jackson FM, Hogue CR, Phillips MT. The development of a race and gender-specific stress measure for African-American women: Jackson, Hogue, Phillips contextualized stress measure. Ethnicity Dis. (2005) 15:594–600. [PubMed] [Google Scholar]
- 299.Rich-Edwards JW, Grizzard TA. Psychosocial stress and neuroendocrine mechanisms in preterm delivery. Am J Obstet Gynecol. (2005) 192(5 Suppl) S30–5. 10.1016/j.ajog.2005.01.072 [DOI] [PubMed] [Google Scholar]
- 300.Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, Sandman CA. Racial differences in birth outcomes: the role of general, pregnancy, racism stress. Health Psychol. (2008) 27:194–203. 10.1037/0278-6133.27.2.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. (2006) 96:826–33. 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Jackson FM, Phillips MT, Hogue CJ, Curry-Owens TY. Examining the burdens of gendered racism: implications for pregnancy outcomes among college-educated African American women. Matern Child Health J. (2001) 5:95–107. 10.1023/a:1011349115711 [DOI] [PubMed] [Google Scholar]
- 303.Latendresse G. The interaction between chronic stress and pregnancy: preterm birth from a biobehavioral perspective. J Midwifery Womens Health. (2009) 54:8–17. 10.1016/j.jmwh.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Olson D, Severson E, Verstraeten B, Ng J, McCreary J, Metz G. Allostatic load and preterm birth. Int J Mol Sci. (2015) 16:29856–74. 10.3390/ijms161226209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Giurgescu C, Engeland CG, Templin TN. Symptoms of depression predict negative birth outcomes in African American women: a pilot study. J Midwifery Womens Health. (2015) 60:570–577. 10.1111/jmwh.12337 [DOI] [PubMed] [Google Scholar]
- 306.Jarde A, Morais M, Kingston D, Giallo R, MacQueen GM, Giglia L, et al. Neonatal outcomes in women with untreated antenatal depression compared with women without depression: a systematic review and meta-analysis. JAMA Psychiatry. (2016) 73:826–37. 10.1001/jamapsychiatry.2016.0934 [DOI] [PubMed] [Google Scholar]
- 307.Szegda K, Markenson G, Bertone-Johnson ER, Chasan-Taber L. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J Matern Fetal Neonatal Med. (2014) 27:960–7. 10.3109/14767058.2013.845157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Barnes DM, Bates LM. Do racial patterns in psychological distress shed light on the Black-White depression paradox? A systematic review. Soc Psychiatry Psychiatr Epidemiol. (2017) 52:913–28. 10.1007/s00127-017-1394-9 [DOI] [PubMed] [Google Scholar]
- 309.Bhatia N, Chao SM, Higgins C, Patel S, Crespi CM. Association of mothers' perception of neighborhood quality and maternal resilience with risk of preterm birth. Int J Environ Res Public Health. (2015) 12:9427–43. 10.3390/ijerph120809427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Jesse DE, Seaver W, Wallace DC. Maternal psychosocial risks predict preterm birth in a group of women from Appalachia. Midwifery. (2003) 19:191–202. 10.1016/s0266-6138(03)00031-7 [DOI] [PubMed] [Google Scholar]
- 311.Pesonen AK, Lahti M, Kuusinen T, Tuovinen S, Villa P, Hamalainen E, et al. Maternal prenatal positive affect, depressive and anxiety symptoms and birth outcomes: the PREDO Study. PLoS ONE. (2016) 11:e0150058. 10.1371/journal.pone.0150058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Voellmin A, Entringer S, Moog N, Wadhwa PD, Buss C. Maternal positive affect over the course of pregnancy is associated with the length of gestation and reduced risk of preterm delivery. J Psychosom Res. (2013) 75:336–40. 10.1016/j.jpsychores.2013.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Neggers Y, Goldenberg R, Cliver S, Hauth J. The relationship between psychosocial profile, health practices, pregnancy outcomes. Acta Obstet Gynecol Scand. (2006) 85:277–85. 10.1080/00016340600566121 [DOI] [PubMed] [Google Scholar]
- 314.Giurgescu C, Sanguanklin N, Engeland CG, White-Traut RC, Park C, Mathews HL, et al. Relationships among psychosocial factors, biomarkers, preeclampsia, and preterm birth in African American women: a pilot. Appl Nurs Res. (2015) 28:e1–6. 10.1016/j.apnr.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 315.Wheeler S, Maxson P, Truong T, Swamy G. Psychosocial stress and preterm birth: The impact of parity and race. Matern Child Health J. (2018) 22:1430–5. 10.1007/s10995-018-2523-0 [DOI] [PubMed] [Google Scholar]
- 316.Dole N, Savitz DA, Siega-Riz AM, Hertz-Picciotto I, McMahon MJ, Buekens P. Psychosocial factors and preterm birth among African American and white women in central North Carolina. Am J Public Health. (2004) 94:1358–65. 10.2105/ajph.94.8.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Rankin KM, David RJ, Collins JW, Jr. African American women's exposure to interpersonal racial discrimination in public settings and preterm birth: the effect of coping behaviors. Ethn Dis. (2011) 21, 370–6. [PubMed] [Google Scholar]
- 318.Ruiz RJ, Gennaro S, O'Connor C, Marti CN, Lulloff A, Keshinover T, et al. Measuring coping in pregnant minority women. West J Nurs Res. (2015) 37:257–75. 10.1177/0193945914527176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ward EC, Wiltshire JC, Detry MA, Brown RL. African American men and women's attitude toward mental illness, perceptions of stigma, and preferred coping behaviors. Nurs Res. (2013) 62:185–94. 10.1097/NNR.0b013e31827bf533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Utsey SO, Ponterotto JG, Reynolds AL, Cancelli AA. Racial discrimination, coping, life satisfaction, and self-esteem among African Americans. J Couns Dev. (2000) 78:72–80. 10.1002/j.1556-6676.2000.tb02562.x [DOI] [Google Scholar]
- 321.Guardino CM, Schetter CD. Coping during pregnancy: a systematic review and recommendations. Health Psychol Rev. (2014) 8:70–94. 10.1080/17437199.2012.752659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Berkman LF, Glass T. Social integration, social networks, social support, health. In: Berkman LF, Kawachi I, editors. Social Epidemiology. Oxford, UK: Oxford University Press; (2000). p. 416. [Google Scholar]
- 323.Cohen S, Syme SL. Social Support and Health. San Diego, CA: Academic Press; (1985). [Google Scholar]
- 324.Gramer M, Supp N. Social support and prolonged cardiovascular reactivity: the moderating influence of relationship quality and type of support. Biol Psychol. (2014) 101:1–8. 10.1016/j.biopsycho.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 325.Hakulinen C, Pulkki-Råback L, Jokela M E, Ferrie J, Aalto M, et al. Structural and functional aspects of social support as predictors of mental and physical health trajectories: Whitehall II cohort study. J Epidemiol Commun Health. (2016) 70:710–5. 10.1136/jech-2015-206165 [DOI] [PubMed] [Google Scholar]
- 326.Howard S, Creaven AM, Hughes BM, O'Leary ÉD, James JE. Perceived social support predicts lower cardiovascular reactivity to stress in older adults. Biol Psychol. (2017) 125:70–5. 10.1016/j.biopsycho.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 327.Kaplan BH, Cassel JC, Gore S. Social support and health. Med Care. (1977) 15:47–58. [DOI] [PubMed] [Google Scholar]
- 328.Stein ER, Smith BW. Social support attenuates the harmful effects of stress in healthy adult women. Soc Sci Med. (2015) 146:129–36. 10.1016/j.socscimed.2015.10.038 [DOI] [PubMed] [Google Scholar]
- 329.Thoits PA. Mechanisms linking social ties and support to physical and mental health. J Health Soc Behav. (2011) 52:145–61. 10.1177/0022146510395592 [DOI] [PubMed] [Google Scholar]
- 330.Uchino BN, Bowen K, Carlisle M, Birmingham W. Psychological pathways linking social support to health outcomes: a visit with the “ghosts” of research past, present, and future. Soc Sci Med. (2012) 74:949–57. 10.1016/j.socscimed.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hetherington E, Doktorchik C, Premji SS, McDonald SW, Tough SC, Sauve RS. Preterm birth and social support during pregnancy: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. (2015) 29:523–35. 10.1111/ppe.12225 [DOI] [PubMed] [Google Scholar]
- 332.Institute of Medicine Committee on Understanding Premature Birth and Assuring Healthy Outcomes . Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; (2007). [Google Scholar]
- 333.Forrester S, Jacobs D, Zmora R, Schreiner P, Roger V, Kiefe CI. Racial differences in weathering and its associations with psychosocial stress: the CARDIA study. SSM Popul Health. (2019) 7:100319. 10.1016/j.ssmph.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Mitchell AM, Kowalsky JM, Epel ES, Lin J, Christian LM. Childhood adversity, social support, and telomere length among perinatal women. Psychoneuroendocrinology. (2018) 87:43–52. 10.1016/j.psyneuen.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sheffler J, Sachs-Ericsson N. Racial differences in the effect of stress on health and the moderating role of perceived social support. J Aging Health. (2016) 28:1362–81. 10.1177/0898264315618923 [DOI] [PubMed] [Google Scholar]
- 336.Krause N. Church-based social support and health in old age: exploring variations by race. J Gerontol B. (2002) 57:S332–47. 10.1093/geronb/57.6.S332 [DOI] [PubMed] [Google Scholar]
- 337.Bodaghi E, Alipour F, Bodaghi M, Nori R, Peiman N, Saeidpour S. The role of spirituality and social support in pregnant women's anxiety, depression and stress symptoms. Community Health J. (2017) 10:72–82. Available online at: http://eprints.rums.ac.ir/id/eprint/6809 [Google Scholar]
- 338.Griffin ML, Amodeo M, Clay C, Fassler I, Ellis MA. Racial differences in social support: kin versus friends. Am J Orthopsychiatry. (2006) 76:374–80. 10.1037/0002-9432.76.3.374 [DOI] [PubMed] [Google Scholar]
- 339.Flores M, Ruiz JM, Goans C, Butler EA, Uchino BN, Hirai M, et al. Racial-ethnic differences in social networks and perceived support: measurement considerations and implications for disparities research. Cultur Divers Ethnic Minor Psychol. (2019) 26:189–99. 10.1037/cdp0000283 [DOI] [PubMed] [Google Scholar]
- 340.Blumenshine PM, Egerter SA, Libet ML, Braveman PA. Father's education: an independent marker of risk for preterm birth. Matern Child Health J. (2011) 15:60–7. 10.1007/s10995-009-0559-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Collins JW, Jr, Rankin KM, David RJ. Paternal lifelong socioeconomic position and low birth weight rates: relevance to the African-American women's birth outcome disadvantage. Matern Child Health J. (2016) 20:1759–66. 10.1007/s10995-016-1981-5 [DOI] [PubMed] [Google Scholar]
- 342.Cheng ER, Rifas-Shiman SL, Perkins ME, Rich-Edwards JW, Gillman MW, Wright R, et al. The influence of antenatal partner support on pregnancy outcomes. J Womens Health. (2016) 25:672–9. 10.1089/jwh.2015.5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Liu C, Cnattingius S, Bergström M, Östberg V, Hjern A. Prenatal parental depression and preterm birth: a national cohort study. BJOG. (2016) 123:1973–82. 10.1111/1471-0528.13891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Braveman P, Marchi K, Egerter S, Kim S, Metzler M, Stancil T, et al. Poverty, near-poverty, and hardship around the time of pregnancy. Matern Child Health J. (2010) 14:20–35. 10.1007/s10995-008-0427-0 [DOI] [PubMed] [Google Scholar]
- 345.Neggers Y, Goldenberg R, Cliver S, Hauth J. Effects of domestic violence on preterm birth and low birth weight. Acta Obstetr Gynecol Scand. (2004) 83:455–60. 10.1111/j.0001-6349.2004.00458.x [DOI] [PubMed] [Google Scholar]
- 346.Shah PS, Shah J. Maternal exposure to domestic violence and pregnancy and birth outcomes: a systematic review and meta-analyses. J Womens Health. (2010) 19:2017–31. 10.1089/jwh.2010.2051 [DOI] [PubMed] [Google Scholar]
- 347.Witt WP, Cheng ER, Wisk LE, Litzelman K, Chatterjee D, Mandell K, et al. Preterm birth in the United States: the impact of stressful life events prior to conception and maternal age. Am J Public Health. (2014) 104:S73–S80. 10.2105/AJPH.2013.301688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Burns ER, Farr SL, Howards PP. Stressful life events experienced by women in the year before their infants' births—United States, 2000–2010. Morb Mortal Wkly Rep. (2015) 64:247. [PMC free article] [PubMed] [Google Scholar]
- 349.Western B. The impact of incarceration on wage mobility and inequality. Am Sociol Rev. (2010) 67:526–46. 10.2307/3088944 [DOI] [Google Scholar]
- 350.Dumont DM, Parker DR, Viner-Brown S, Clarke JG. Incarceration and perinatal smoking: a missed public health opportunity. J Epidemiol Community Health. (2015) 69:648–53. 10.1136/jech-2014-204820 [DOI] [PubMed] [Google Scholar]
- 351.deVuono-powell S, Schweidler C, Walters A, Zohrabi A. Who Pays? The True Cost of Incarceration on Families. Oakland, CA: Ella Baker Center, Forward Together, Research Action Design; (2015). Available online at: http://whopaysreport.org/wp-content/uploads/2015/09/Who-Pays-FINAL.pdf [Google Scholar]
- 352.Pazol K, Robbins CL, Black LI, Ahrens KA, Daniels K, Chandra A, et al. Receipt of selected preventive health services for women and men of reproductive age - United States, 2011-2013. MMWR Surveill Summ. (2017) 66:1–31. 10.15585/mmwr.ss6620a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Roth LM, Henley MM. Unequal motherhood: racial-ethnic and socioeconomic disparities in cesarean sections in the United States. Social Problems. (2012) 59:207–27. 10.1525/sp.2012.59.2.207 [DOI] [Google Scholar]
- 354.Cammack AL, Buss C, Entringer S, Hogue CJ, Hobel CJ, Wadhwa PD. The association between early life adversity and bacterial vaginosis during pregnancy. Am J Obstet Gynecol. (2011) 204:431.e431–8. 10.1016/j.ajog.2011.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Bodnar LM, Simhan HN, Parker CB, Meier H, Mercer BM, Grobman WA, et al. Racial or ethnic and socioeconomic inequalities in adherence to national dietary guidance in a large cohort of US pregnant women. J Acad Nutr Diet. (2017) 117:867–77.e863. 10.1016/j.jand.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Bowyer RCE, Jackson MA, Le Roy CI, Ni Lochlainn M, Spector TD, Dowd JB, et al. Socioeconomic status and the gut microbiome: a twinsUK cohort study. Microorganisms. (2019) 7:17. 10.3390/microorganisms7010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Dunlop AL, Knight AK, Satten GA, Cutler AJ, Wright ML, Mitchell RM, et al. Stability of the vaginal, oral, and gut microbiota across pregnancy among African American women: the effect of socioeconomic status and antibiotic exposure. PeerJ. (2019) 7:e8004. 10.7717/peerj.8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Singh GK, DiBari JN. Marked disparities in pre-pregnancy obesity and overweight prevalence among US women by race/ethnicity, nativity/immigrant status, sociodemographic characteristics. 2012–2014. J Obes. (2019) 2019:2419263. 10.1155/2019/2419263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Glover LM, Cain-Shields LR, Wyatt SB, Gebreab SY, Diez-Roux AV, Sims M. Life course socioeconomic status and hypertension in African American adults: the Jackson heart study. Am J Hypertens. (2020) 33:84–91. 10.1093/ajh/hpz133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Leng B, Jin Y, Li G, Chen L, Jin N. Socioeconomic status and hypertension: a meta-analysis. J Hypertens. (2015) 33:221–9. 10.1097/hjh.0000000000000428 [DOI] [PubMed] [Google Scholar]
- 361.Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. (2020). Retrieved from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 362.Krishnan S, Cozier YC, Rosenberg L, Palmer JR. Socioeconomic status and incidence of type 2 diabetes: results from the Black Women's Health Study. Am J Epidemiol. (2010) 171:564–70. 10.1093/aje/kwp443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Singh GK, Siahpush M, Liu L, Allender M. Racial/Ethnic, Nativity, and Sociodemographic Disparities in Maternal Hypertension in the United States, 2014-2015. Int J Hypertens. (2018) 2018:7897189. 10.1155/2018/7897189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. (2008) 31:2288–93. 10.2337/dc08-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis. (2014) 11:E104. 10.5888/pcd11.130415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.O'Brien EC, Alberdi G, McAuliffe FM. The influence of socioeconomic status on gestational weight gain: a systematic review. J Public Health. (2018) 40:41–55. 10.1093/pubmed/fdx038 [DOI] [PubMed] [Google Scholar]
- 367.Thoma ME, Copen CE, Kirmeyer SE. Short interpregnancy intervals in 2014: differences by maternal demographic characteristics. NCHS Data Brief . (2016) 240:1–8. [PubMed] [Google Scholar]
- 368.Green TL. Unpacking racial/ethnic disparities in prenatal care use: the role of individual-, household-, area-level characteristics. J Womens Health. (2018) 27:1124–34. 10.1089/jwh.2017.6807 [DOI] [PubMed] [Google Scholar]
- 369.Baer RJ, Chambers CD, Ryckman KK, Oltman SP, Rand L, Jelliffe-Pawlowski LL. Risk of preterm and early term birth by maternal drug use. J Perinatol. (2019) 39:286–94. 10.1038/s41372-018-0299-0 [DOI] [PubMed] [Google Scholar]
- 370.Gouin K, Murphy K, Shah PS. Effects of cocaine use during pregnancy on low birthweight and preterm birth: systematic review and metaanalyses. Am J Obstetr Gynecol. (2011) 204:340.e1–12. 10.1016/j.ajog.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 371.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, et al. Socioeconomic status in health research: one size does not fit all. JAMA. (2005) 294:2879–88. 10.1001/jama.294.22.2879 [DOI] [PubMed] [Google Scholar]
- 372.Collins JW, Jr, Herman AA, David RJ. Very-low-birthweight infants and income incongruity among African American and white parents in Chicago. Am J Public Health. (1997) 87:414–7. 10.2105/ajph.87.3.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Hanks A, Solomon D, Weller CE. Systematic Inequality: How American's Structural Racism Helped Create the Black-White Wealth Gap. Washington, DC: (2018). Retrieved from: https://www.americanprogress.org/issues/race/reports/2018/02/21/447051/systematic-inequality/ [Google Scholar]
- 374.Krieger N, Rowley DL, Herman AA, Avery B, Phillips MT. Racism, sexism, and social class: implications for studies of health, disease, and well-being. Am J Prev Med. (1993) 9:82–122. [PubMed] [Google Scholar]
- 375.Nazroo JY. The structuring of ethnic inequalities in health: economic position, racial discrimination, and racism. Am J Public Health. (2003) 93:277–84. 10.2105/ajph.93.2.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Oliver M, Shapiro TM. Black Wealth/White Wealth: A New Perspective on Racial Inequality. 2nd ed. New York, NY: Routledge; (2006). [Google Scholar]
- 377.Patten E. Racial, Gender Wage Gaps Persist in U.S. Despite Some Progress. FactTank: News in the Numbers. (2016). Retrieved from: http://www.pewresearch.org/fact-tank/2016/07/01/racial-gender-wage-gaps-persist-in-u-s-despite-some-progress/
- 378.Cabral H, Fried LE, Levenson S, Amaro H, Zuckerman B. Foreign-born and US-born black women: differences in health behaviors and birth outcomes. Am J Public Health. (1990) 80:70–2. 10.2105/ajph.80.1.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.David RJ, Collins JW, Jr. Differing birth weight among infants of U.S.-born blacks, African-born blacks, and U.S.-born whites. N Engl J Med. (1997) 337:1209–14. 10.1056/nejm199710233371706 [DOI] [PubMed] [Google Scholar]
- 380.Pallotto EK, Collins JW, Jr., David RJ. Enigma of maternal race and infant birth weight: a population-based study of US-born Black and Caribbean-born Black women. Am J Epidemiol. (2000) 151:1080–5. 10.1093/oxfordjournals.aje.a010151 [DOI] [PubMed] [Google Scholar]
- 381.Singh GK, Yu SM. Adverse pregnancy outcomes: differences between US- and foreign-born women in major US racial and ethnic groups. Am J Public Health. (1996) 86:837–43. 10.2105/ajph.86.6.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Blackmore CA, Savitz DA, Edwards LJ, Harlow SD, Bowes WA, Jr. Racial differences in the patterns of preterm delivery in central North Carolina, USA. Paediatr Perinat Epidemiol. (1995) 9:281–95. [DOI] [PubMed] [Google Scholar]
- 383.Braveman P, Heck K, Egerter S, Marchi K, Dominguez TP, Cubbin C, et al. The role of socioeconomic factors in Black–White disparities in preterm birth. Am J Public Health. (2015) 105:694–702. 10.2105/AJPH.2014.302008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Johnson JD, Green CA, Vladutiu CJ, Manuck TA. Racial disparities in prematurity persist among women of high socioeconomic status. Am J Obstetr Gynecol. (2020) 2:100104. 10.1016/j.ajogmf.2020.100104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Parker JD, Schoendorf KC, Kiely JL. Associations between measures of socioeconomic status and low birth weight, small for gestational age, and premature delivery in the United States. Ann Epidemiol. (1994) 4:271–8. 10.1016/1047-2797(94)90082-5 [DOI] [PubMed] [Google Scholar]
- 386.Lieberman E, Ryan KJ, Monson RR, Schoenbaum SC. Risk factors accounting for racial differences in the rate of premature birth. N Engl J Med. (1987) 317:743–8. [DOI] [PubMed] [Google Scholar]
- 387.Guillory VJ, Samuels ME, Probst JC, Sharp G. Prenatal care and infant birth outcomes among medicaid recipients. J Health Care Poor Underserved. (2003) 14:272–89. 10.1353/hpu.2010.0734 [DOI] [PubMed] [Google Scholar]
- 388.Drillien CM. The social and economic factors affecting the incidence of premature birth. BJOG. (1957) 64:161–84. 10.1111/j.1471-0528.1957.tb02618.x [DOI] [PubMed] [Google Scholar]
- 389.Illsley R. Social class selection and class differences in relation to stillbirths and infant deaths. Brit Med J. (1955) 2:1520–4. 10.1136/bmj.2.4955.1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Braveman P, Heck K, Egerter S, Rinki C, Marchi K, Curtis M. Economic hardship in childhood: a neglected issue in ACE studies? Matern Child Health J. (2018) 22:308–17. 10.1007/s10995-017-2368-y [DOI] [PubMed] [Google Scholar]
- 391.Williams DR. Racial/ethnic variations in women's health: the social embeddedness of health. Am J Public Health. (2002) 92:588–97. 10.2105/ajph.92.4.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Christiaens I, Hegadoren K, Olson DM. Adverse childhood experiences are associated with spontaneous preterm birth: a case–control study. BMC Med. (2015) 13:124. 10.1186/s12916-015-0353-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Gillespie SL, Christian LM, Alston AD, Salsberry PJ. Childhood stress and birth timing among African American women: Cortisol as biological mediator. Psychoneuroendocrinology. (2017) 84:32–41. 10.1016/j.psyneuen.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Margerison-Zilko CE, Strutz KL, Li Y, Holzman C. Stressors across the life-course and preterm delivery: evidence from a pregnancy cohort. Matern Child Health J. (2017) 21:648–58. 10.1007/s10995-016-2151-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Braveman P, Heck K, Egerter S, Dominguez TP, Rinki C, Marchi KS, et al. Worry about racial discrimination: a missing piece of the puzzle of Black-White disparities in preterm birth? PLoS ONE. (2017) 12:e0186151. 10.1371/journal.pone.0186151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Collins JW, Jr., Rankin KM, David RJ. African American women's lifetime upward economic mobility and preterm birth: the effect of fetal programming. Am J Public Health. (2011) 101:714–9. 10.2105/AJPH.2010.195024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Foster HW, Wu L, Bracken MB, Semenya K, Thomas J, Thomas J. Intergenerational effects of high socioeconomic status on low birthweight and preterm birth in African Americans. J Natl Med Assoc. (2000) 92:213–21. [PMC free article] [PubMed] [Google Scholar]
- 398.Collins JW, Jr., David RJ, Simon DM, Prachand NG. Preterm birth among African American and white women with a lifelong residence in high-income Chicago neighborhoods: an exploratory study. Ethnicity Dis. (2007) 17:113–7. [PubMed] [Google Scholar]
- 399.Pearl M, Ahern J, Hubbard A, Laraia B, Shrimali BP, Poon V, et al. Life-course neighbourhood opportunity and racial-ethnic disparities in risk of preterm birth. Paediatr Perinat Epidemiol. (2018) 32:412–9. 10.1111/ppe.12482 [DOI] [PubMed] [Google Scholar]
- 400.Tian Y, Holzman C, Slaughter-Acey J, Margerison-Zilko C, Luo Z, Todem D. Maternal socioeconomic mobility and preterm delivery: a latent class analysis. Matern Child Health J. (2018) 22:1647–58. 10.1007/s10995-018-2562-6 [DOI] [PubMed] [Google Scholar]
- 401.Gamst G, Arellano-Morales L, Meyers LS, Serpas DG, Balla J, Diaz A, et al. Shifting can be stressful for African American women: a structural mediation model. J Black Psychol. (2020) 46:364–87. 10.1177/0095798420939721 [DOI] [Google Scholar]
- 402.Jackson FM, Rowley DL, Curry Owens T. Contextualized stress, global stress, and depression in well-educated, pregnant, African-American women. Womens Health Issues. (2012) 22:e329–6. 10.1016/j.whi.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 403.Myers HF, Lewis T, Parker-Dominguez T. Stress, coping & minority health. In: Bernal G, Trimble J, Burlew K, Leong F, editors. The Handbook of Racial and Ethnic Minority Psychology. Thousand Oaks, CA: Sage Publications; (2003). p. 377–400. [Google Scholar]
- 404.Shapiro T, Meschede T, Osoro S. The Roots of the Widening Racial Wealth Gap: Explaining the Black-White Economic Divide. Research and Policy Brief . (2013). Retrieved from https://heller.brandeis.edu/iere/pdfs/racial-wealth-equity/racial-wealth-gap/roots-widening-racial-wealth-gap.pdf
- 405.Orzechowski S, Sepielli P. Net Worth and Asset Ownership of Households: 1998 and 2000. Washington, DC: (2003). [Google Scholar]
- 406.Darling-Hammond L. Unequal Opportunity: Race and Education. (1998). Retrieved from: https://www.brookings.edu/articles/unequal-opportunity-race-and-education/
- 407.Kozol J. Savage Inequalities: Children in America's Schools. New York, NY: Crown; (1991). [Google Scholar]
- 408.Kozol J. The Shame of the Nation: The Restoration of Apartheid Schooling in America. New York, NY: Crown; (2005). [Google Scholar]
- 409.Startz D. Schools, Black Children, Corporal Punishment. (2016). Retrieved from: https://www.brookings.edu/blog/brown-center-chalkboard/2016/01/14/schools-black-children-and-corporal-punishment/
- 410.Baker R, Klasik D, Reardon SF. Race and stratification in college enrollment over time. AERA Open. (2018) 4:2332858417751896. 10.1177/2332858417751896 [DOI] [Google Scholar]
- 411.Sablich L. 7 Findings That Illustrate Racial Disparities in Eduction. (2016). Retrieved from: https://thepolicy.us/7-findings-that-illustrate-racial-disparities-in-education-aba8cb1a9591
- 412.Lorch SA, Enlow E. The role of social determinants in explaining racial/ethnic disparities in perinatal outcomes. Pediatr Res. (2015) 79:141–7. 10.1038/pr.2015.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.O'Campo P, Burke JG, Culhane J, Elo IT, Eyster J, Holzman C. Neighborhood deprivation and preterm birth among non-hispanic black and white women in eight geographic areas in the United States. Am J Epidemiol. (2007) 167:155–63. 10.1093/aje/kwm277 [DOI] [PubMed] [Google Scholar]
- 414.Masho SW, Cha S, Chapman DA, Chelmow D. Understanding the role of violence as a social determinant of preterm birth. Am J Obstetr Gynecol. (2017) 216:183.e1–7. 10.1016/j.ajog.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 415.Kothari CL, Paul R, Dormitorio B, Ospina F, James A, Lenz D, et al. The interplay of race, socioeconomic status and neighborhood residence upon birth outcomes in a high black infant mortality community. SSM Popul Health. (2016) 2:859–67. 10.1016/j.ssmph.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Giurgescu C, McFarlin BL, Lomax J, Craddock C, Albrecht A. Racial discrimination and the black-white gap in adverse birth outcomes: a review. J Midwifery Womens Health. (2011) 56:362–70. 10.1111/j.1542-2011.2011.00034.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.James SA. Confronting the moral economy of US racial/ethnic health disparities. Am J Public Health. (2003) 93:189. 10.2105/ajph.93.2.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Hogue CJR, Bremner JD. Stress model for research into preterm delivery among black women. Am J Obstet Gynecol. (2005) 192:S47–55. 10.1016/j.ajog.2005.01.073 [DOI] [PubMed] [Google Scholar]
- 419.Hogue CR, Kramer MR. What causes racial disparities in very preterm birth? A biosocial perspective. Epidemiol Rev. (2009) 31:84–98. 10.1093/ajerev/mxp003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Hilmert CJ, Dominguez TP, Schetter CD, Srinivas SK, Glynn LM, Hobel CJ, et al. Lifetime racism and blood pressure changes during pregnancy: implications for fetal growth. Health Psychol. (2014) 33:43–51. 10.1037/a0031160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Collins JW, Jr., David RJ, Symons R, Handler A, Wall SN, et al. Low-income African-American mothers' perception of exposure to racial discrimination and infant birth weight. Epidemiology. (2000) 11:337–9. 10.1097/00001648-200005000-00019 [DOI] [PubMed] [Google Scholar]
- 422.Bower KM, Geller RJ, Perrin NA, Alhusen J. Experiences of racism and preterm birth: findings from a pregnancy risk assessment monitoring system, 2004 through 2012. Womens Health Issues. (2018) 28:495–501. 10.1016/j.whi.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 423.Jackson FM, James SA, Owens TC, Bryan AF. Anticipated negative police-youth encounters and depressive symptoms among pregnant African American Women: a brief report. J Urban Health Bull N Y Acad Med. (2017) 94:259–65. 10.1007/s11524-017-0136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, Kiefe CI. Self-reported experiences of racial discrimination and Black-White differences in preterm and low-birthweight deliveries: the CARDIA Study. Am J Public Health. (2004) 94:2125–31. 10.2105/ajph.94.12.2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Rosenberg L, Palmer JR, Wise LA, Horton NJ, Corwin MJ. Perceptions of racial discrimination and the risk of preterm birth. Epidemiology. (2002) 13:646–52. 10.1097/01.Ede.0000030929.51122.20 [DOI] [PubMed] [Google Scholar]
- 426.Slaughter-Acey JC, Sealy-Jefferson S, Helmkamp L, Caldwell CH, Osypuk TL, Platt RW, et al. Racism in the form of micro aggressions and the risk of preterm birth among Black women. Ann Epidemiol. (2016) 26:7–13.e11. 10.1016/j.annepidem.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Giurgescu C, Zenk SN, Dancy BL, Park CG, Dieber W, Block R. Relationships among neighborhood environment, racial discrimination, psychological distress, and preterm birth in African American women. J Obstet Gynecol Neonatal Nurs. (2012) 41:E51–61. 10.1111/j.1552-6909.2012.01409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Misra D, Strobino D, Trabert B. Effects of social and psychosocial factors on risk of preterm birth in Black women. Paediatr Perinat Epidemiol. (2010) 24:546–54. 10.1111/j.1365-3016.2010.01148.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Collins JW, Jr, David RJ, Handler A, Wall S, Andes S. Very low birthweight in African American infants: the role of maternal exposure to interpersonal racial discrimination. Am J Public Health. (2004) 94:2132–8. 10.2105/ajph.94.12.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Murrell NL. Stress, self-esteem, and racism: relationships with low birth weight and preterm delivery in African American women. J Natl Black Nurses Assoc. (1996) 8:45–53. [PubMed] [Google Scholar]
- 431.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. (2005) 61:1576–96. 10.1016/j.socscimed.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 432.Williams DR, Yan Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. (1997) 2:335–51. 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- 433.Matoba N, Suprenant S, Rankin K, Yu H, Collins JW. Mortgage discrimination and preterm birth among African American women: an exploratory study. Health Place. (2019) 59:102193. 10.1016/j.healthplace.2019.102193 [DOI] [PubMed] [Google Scholar]
- 434.Berger M, Sarnyai Z. “More than skin deep”: stress neurobiology and mental health consequences of racial discrimination. Stress. (2015) 18:1–10. 10.3109/10253890.2014.989204 [DOI] [PubMed] [Google Scholar]
- 435.Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans. A biopsychosocial model. Am Psychol. (1999) 54:805–6. [DOI] [PubMed] [Google Scholar]
- 436.Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0138511. 10.1371/journal.pone.0138511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. (2007) 87:873–904. 10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- 438.Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. (2019) 40:105–25. 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Anthopolos R, Kaufman JS, Messer LC, Miranda ML. Racial residential segregation and preterm birth: built environment as a mediator. Epidemiology. (2014) 25:397–405. 10.1097/EDE.0000000000000079 [DOI] [PubMed] [Google Scholar]
- 440.Grady SC, McLafferty S. Segregation, nativity, and health: reproductive health inequalities for immigrant and native-born black women in New York City. Urban Geogr. (2007) 28:377–97. 10.2747/0272-3638.28.4.377 [DOI] [Google Scholar]
- 441.Yudell M, Roberts D, DeSalle R, Tishkoff S. Taking race out of human genetics. Science. (2016) 351:564. 10.1126/science.aac4951 [DOI] [PubMed] [Google Scholar]
- 442.Burris HH, Lorch SA, Kirpalani H, Pursley DM, Elovitz MA, Clougherty JE. Racial disparities in preterm birth in USA: a biosensor of physical and social environmental exposures. Arch Dis Child. (2019) 104:931–5. 10.1136/archdischild-2018-316486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Parets SE, Knight AK, Smith AK. Insights into genetic susceptibility in the etiology of spontaneous preterm birth. Appl Clin Genet. (2015) 8:283–90. 10.2147/tacg.S58612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.David R, Collins J, Jr. Disparities in infant mortality: what's genetics got to do with it? Am J Public Health. (2007) 97:1191–7. 10.2105/AJPH.2005.068387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Kwate NOA. Fried chicken and fresh apples: Racial segregation as a fundamental cause of fast food density in black neighborhoods. Health Place. (2008) 14:32–44. 10.1016/j.healthplace.2007.04.001 [DOI] [PubMed] [Google Scholar]
- 446.Morland K, Filomena S. Disparities in the availability of fruits and vegetables between racially segregated urban neighbourhoods. Public Health Nutr. (2007) 10:1481–89. 10.1017/S1368980007000079 [DOI] [PubMed] [Google Scholar]
- 447.Corral I, Landrine H, Hao Y, Zhao L, Mellerson JL, Cooper DL. Residential segregation, health behavior and overweight/obesity among a national sample of African American adults. J Health Psychol. (2012) 17:371–8. 10.1177/1359105311417191 [DOI] [PubMed] [Google Scholar]
- 448.Lu MC, Kotelchuck M, Hogan V, Jones L, Wright K, Halfon N. Closing the Black-White gap in birth outcomes: a life-course approach. Ethn Dis. (2010) 20(1 Suppl 2):S2-62-76. [PMC free article] [PubMed] [Google Scholar]
- 449.Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. (2010) 100:933–9. 10.2105/ajph.2008.143446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Kuo LE, Czarnecka M, Kitlinska JB, Tilan JU, Kvetnanský R, Zukowska Z. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann N Y Acad Sci. (2008) 1148:232–7. 10.1196/annals.1410.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry. (2013) 73:827–35. 10.1016/j.biopsych.2013.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Yau YH, Potenza MN. Stress and eating behaviors. Minerva Endocrinol. (2013) 38:255–67. [PMC free article] [PubMed] [Google Scholar]
- 453.Astrup A, Bügel S. Overfed but undernourished: recognizing nutritional inadequacies/deficiencies in patients with overweight or obesity. Int J Obes. (2019) 43:219–32. 10.1038/s41366-018-0143-9 [DOI] [PubMed] [Google Scholar]
- 454.DiNicolantonio JJ, Berger A. Added sugars drive nutrient and energy deficit in obesity: a new paradigm. Open heart. (2016) 3:e000469. 10.1136/openhrt-2016-000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Nuru-Jeter A, Dominguez TP, Hammond WP, Leu J, Skaff M, Egerter S, et al. “It's the skin you're in”: African-American women talk about their experiences of racism. an exploratory study to develop measures of racism for birth outcome studies. Matern Child Health J. (2009) 13:29–39. 10.1007/s10995-008-0357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Lewis TT, Cogburn CD, Williams DR. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, emerging issues. Annu Rev Clin Psychol. (2015) 11:407–40. 10.1146/annurev-clinpsy-032814-112728 [DOI] [PMC free article] [PubMed] [Google Scholar]