Abstract
Objectives
The kidney is a vulnerable organ for acute lymphoblastic leukemia (ALL), by the disease, and various associated clinical pictures. This retrospective study aims to document renal ultrasound abnormalities in children with newly diagnosed ALL as well as to investigate the correlation between renal findings and clinical/laboratory/survival data.
Methods
All children (age <18 years) with ALL were included in the study. An increase in size/nephromegaly (NM) or hyperechogenicity (HE) of the kidneys at first admission was accepted as a pathological renal abnormality. The clinical/laboratory findings, survival, and long-term renal functions were compared between patients with and without NM/HE.
Results
The incidence of NM±HE was 12% in 163 patients. Enlargement of spleen, liver, or both and, hypercreatininemia was independently correlated with the presence of NM/HE. After the induction therapy, ultrasound findings were resolved in all patients, and NM/HE did not influence ALL prognosis. All survivors had normal renal functions in long term.
Conclusion
The renal ultrasound abnormalities are not uncommon in children with leukemia at admission, without a negative impact on leukemia prognosis and on long-term renal functions.
Keywords: Leukemia, nephromegaly, prognosis, renal hyperechogenecity
Acute lymphoblastic leukemia (ALL) is the most common cancer of childhood.[1,2]* Clinical picture reflects bone marrow infiltration and/or extramedullary disease. Liver, spleen, lymph nodes, kidney, testicles, central nervous system, and other organs may be involved in decreasing order of frequency.[3] There are very few imaging studies exclusively dedicated to the prevalence of renal abnormalities at the time of ALL diagnosis, and the frequency of renal radiological abnormalities was reported as 18-47%.[4-14] The most common findings are unilateral or bilateral enlargement of kidneys and/or increased renal echogenicity, of which it is impossible to reveal the etiology based on radiological findings, as renal biopsy is not routinely performed in the majority of cases, except in the autopsy series. In some reports, bilateral nephromegaly (NM) resolving after successful chemotherapy has been considered as a sign of renal leukemic involvement.[15] However, those radiological findings might also be observed due to tumor lysis syndrome (TLS), infection, and renal failure.[16] Whatever the cause, the impact of renal ultrasound abnormalities on leukemia prognosis as well as on renal functions still remains undetermined. This study aims to investigate the frequency of, and factors associated with renal ultrasonographic abnormalities before chemotherapy in children with newly diagnosed ALL, and to assess the prognostic significance of renal imaging findings at diagnosis in children with ALL. The secondary aim was to document long-term renal functions in survivors.
Methods
The study recruited all children (0-18 years) diagnosed with ALL between January 2004 and December 2017 in our center. Data were retrospectively collected from patient’s files. ALL was diagnosed by the integration of morphological, immune phenotypical, and genetic studies as described in the World Health Organization (WHO) criteria.[17] 11q23 rearrangements, i(21)amplification, t(4;11), t(9;22), and hypodiploidy were accepted as unfavorable genetics. Each patient had abdominal ultrasonography (USG) before initiation of chemotherapy as part of their initial investigation. Patients with delayed USG (i.e., after the chemotherapy started), patients with pre-existing renal disease, and patients who were referred to other centers were excluded from the study. Unilateral or bilateral NM (defined as the major axis of kidney 2SD longer than the normal age-based measurements of Turkish children[18]) and/or increase in parenchymal echogenicity by the USG report were accepted as renal abnormal findings for our study. The presence of liver and/or spleen enlargement on physical examination was also documented. Age, gender, initial leukocyte count, hemoglobin, and platelet count as well as blast immune phenotype (B or T cell) and genetic/cytogenetic abnormalities were recorded. Kidney biopsy was not performed in any patient. Initial urine analysis, blood pressure, urea, creatinine, uric acid, serum electrolytes, and lactate dehydrogenase (LDH) measurements were investigated to reveal possible clinical and laboratory correlations with renal imaging findings. Biochemical parameters other than the whole blood count were evaluated according to age-adjusted reference values set for the method of measurement. Progression-free survival and overall survival were calculated. The renal function tests (urea, creatinine, urine analysis, estimated glomerular filtration rate calculated by Schwartz formula, and urine analysis) and blood pressure measurements at the last visit were recorded in survivors of ALL. All possible contributing factors and survival rates were compared between two categories of patients described by the ultrasonographic status of kidney. All patients were treated with standard BFM backboned protocols according to assigned risk group.
The study was approved by our Sisli Hamidiye Etfal Training and Research Hospital Ethics Committee (Number 1702/2020).
Statistical Analysis
The statistical analyses were performed using the IBM Statistical Package for the Social Sciences for Windows software program version 20.0 (IBM Corp., Armonk, NY, USA). The variables were initially tested for normal distribution by Kolmogorov-Smirnov test. Descriptive statistics were presented as mean and standard deviation, median and range for continuous variables, and number and percentage for categorical variables. The study group was stratified into two categories based on presence of NM and/or increased renal echogenicity. Chi-square test was used for comparisons of categorical variables. One-way ANOVA and Mann-Whitney U tests were used for comparison of groups with symmetrical and asymmetrical distribution, respectively. Logistic regression analysis was employed to evaluate associations with NM and/or increased renal echogenicity. Survival evaluation was performed using Kaplan-Meier analysis. P<0.05 was considered statistically significant.
Results
Between January 2004 and December 2017, 185 children were diagnosed with ALL. Twenty-two patients were excluded due incomplete data. A total of 163 ALL patients (99 boys and 64 girls) with a median age of 4.89 (range 0.15-16.55) years were included in the study. Seventy percent of patients had enlarged spleen or liver or both. B-cell immunophenotype was more frequent (84%). Unfavorable genetics was present in 27 patients (16%). Only four patients had hypertension in our study, which was not enough to perform a statistical analysis. The details of study population are presented in Table 1.
Table 1.
Clinical and laboratory features of study population
| Parameters | Values/ frequencies | |
|---|---|---|
| n | % | |
| Age (median-range years) | 4.89 (0.15-16.55) | |
| Gender | ||
| Male | 99 | 60 |
| Female | 64 | 40 |
| Organomegaly | ||
| Hepatomegaly | 22 | 13.4 |
| Splenomegaly | 11 | 6.7 |
| Hepatosplenomegaly | 82 | 50.3 |
| No organomegaly | 48 | 29.4 |
| Immune phenotype | ||
| B-cell | 137 | 84 |
| T-cell | 26 | 16 |
| Genetics | ||
| Normal | 120 | 73.6 |
| Favorable | 16 | 9.8 |
| Unfavorable | 27 | 16.6 |
| White blood cell count (/mm3)* | 19,600 | |
| (900-603,000) | ||
| Hemoglobin (g/dl)* | 8.2 (3.2-14.6) | |
| Platelet (/mm3)* | 45,000 | |
| (5000-480,000) | ||
: Median-range
The incidence of abnormal findings in renal ultrasound was 17% (28/163). Eight patients had abnormal incidental findings, as presented in Table 2. Remaining 20 patients had either NM or renal hyperechogenicity (HE) or both. The incidence of any organomegaly, hyperuricemia, and elevated creatinine levels, and the median leukocyte counts were significantly higher in NM/HE group. Laboratory or clinical TLS was observed in six patients and the incidence of TLS between groups was not significant. Age, gender, immunophenotype, cytogenetics, levels of hemoglobin, platelet, serum potassium, serum calcium, urine analysis, and LDH were not correlated with the presence of NM/HE (Table 3). Only creatinine elevation and the presence of organomegaly (of spleen, liver or both) remained as significant parameters for NM/HE in regression analysis. All patients had resolution of ultrasound anomalies (other than incidental findings) in control examination at the end of induction therapy.
Table 2.
Renal ultrasound findings in our case series
| Renal ultrasound findings | n | % |
|---|---|---|
| Nephromegaly | 14 | 8.5 |
| Increase in echogenicity | 2 | 1.2 |
| Nephromegaly and increased echogenicity | 4 | 2.5 |
| Incidental findings | 8 | 4.9 |
| Urolithiasis | 1 | |
| Pelvicalyceal ectasia | 5 | |
| Hypoplastic-dysplastic kidney | 1 | |
| Extrarenal pelvis | 1 | |
| Normal | 135 | 82.8 |
Table 3.
Comparison of the study parameters between groups according to the presence of NM/HE
| Parameter | NM/HE in renal ultrasound | p | |
|---|---|---|---|
| Positive (20) | Negative (143) | ||
| Age (median-range years) | 5.0 | 4.89 | 0.83 |
| Gender (male-female) | 10/10 | 54/89 | 0.29 |
| Immune phenotype/B versus T cell | 16/4 | 121/22 | 0.59 |
| Genetics (favorable/unfavorable) | 18/2 | 118/25 | 0.39 |
| Leukocyte count (median) | 30,500/mm3 | 18,000/mm3 | 0.04 |
| Hemoglobin (median) | 7.25 g/dl | 8.2 g/dl | 0.65 |
| Platelet (median) | 45,500/mm3 | 48,000/mm3 | 0.88 |
| Any organomegaly/normal | 19/1 | 96/47 | 0.01 |
| Hypertension | 1/20 | 3/143 | 1.00 |
| Hyperuricemia* | 9/11 | 30/113 | 0.01 |
| Creatinine increase* (positive/negative) | 11/9 | 32/111 | 0.002 |
| Hyperkalemia* (positive/negative) | 0/20 | 3/140 | 1.00 |
| Hyperphosphatemia* (positive/negative) | 4/16 | 17/126 | 0.31 |
| Hypocalcemia* (positive/negative) | 0/20 | 1/142 | 1.00 |
| Laboratory TLS (positive/negative) | 1/19 | 4/139 | 0.48 |
| Urine analysis (Abnormal/normal) | 2/18 | 4/143 | 0.38 |
| Clinical TLS (positive/negative) | 0/20 | 1/142 | 1.00 |
| Lactate dehydrogenase | 1291 U/L | 867 U/L | 0.45 |
| Number of deceased patients | 7/20 | 38/143 | 0.29 |
: Age adjusted. TLS: Tumor lysis syndrome; NM: Nephromegaly; HE: Hyperechogenicity; TLS: Tumor lysis syndrome
Among 143 cases without NM/HE; median survival time was 4.34 years (range 0.1-12.7 years). In 20 cases with NM/HE; median survival time was 5.89 years (range 0.1-13.4 years) (Fig. 1). Although 10-year overall survival rate was lower in NM/HE group (61%) in comparison to that of group without anomalies (65%), the difference was not significant. After a median follow-up time of 4.47 years (range 0.1-13.4 years), none of surviving patients had abnormal clinical and laboratory findings in terms of kidney dysfunction.
Figure 1.
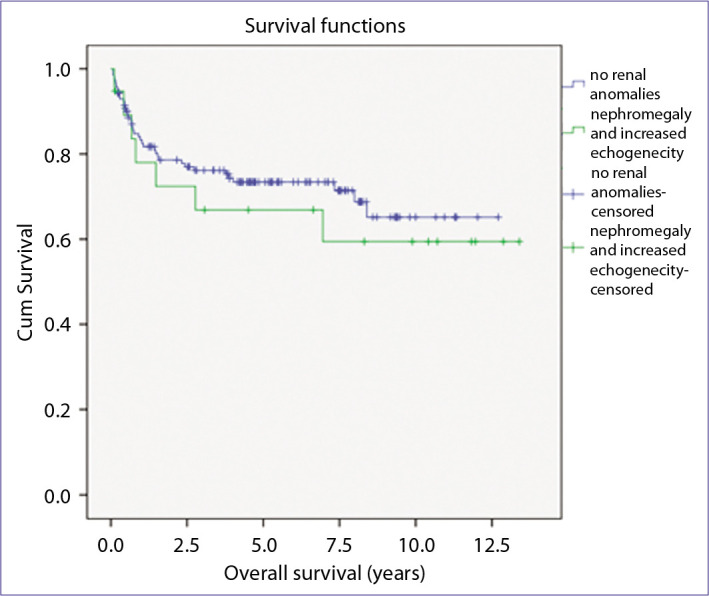
Overall survival curves according to the presence of nephromegaly/hyperechogenicity.
Discussion
In our study, we documented the incidence of NM/HE as 12% in children with ALL at diagnosis. The real prevalence of renal ultrasound anomalies in ALL patients in admission is still unknown. Our study as well as most of the studies investigating renal USG findings leukemia patients are retrospectively planned. In addition to this, different screening modalities such as intravenous pyelogram, ultrasound, and computerized tomography have been preferred in those.[4,9,14] The above circumstances might cause inadequate estimation of the frequency of renal abnormalities in ALL patients. Most commonly reported renal imaging abnormalities are bilateral, diffuse enlargement of kidneys, and/or hyperechoic pattern, and these findings have been accepted as a sign of renal leukemic involvement in the previous reports.[11,19] However, it is controversial whether kidney findings detected on imaging are indeed due to kidney involvement. Hyperechogenic kidneys presenting in childhood, particularly, accompanied by increased size of the kidneys, are more likely from acquired kidney disease, such as glomerular disorders, acute kidney injury due to various causes, infection, and infiltrative diseases.[16] The disappearance of imaging findings after therapy points out to possibility of renal leukemic disease and acute kidney injury in NM/HE patients. However, this comment remains speculative without supported by a tissue diagnosis, which is neither ethical nor necessary. As these findings resolved after induction therapy in our study group, they were interpreted as disease related.
The median age and gender distribution of our ALL patients, and the percentage of patients with hepatosplenomegaly are similar to findings reported in literature. Leukocyte counts were higher in leukemic children with NM/HE in our study. Hepatomegaly and/or splenomegaly were also more common in this group. Similar results were also documented in Hann et al.’s and Taylor et al. studies.[4,5] As the elevated leukocyte count correlates with extramedullary leukemia,[20] one might anticipate increased frequency of NM/HE in patients with higher leukocyte counts and/or organomegaly.
Parallel to current literature findings, precursor B-cell leukemia was the most frequent type of ALL in our study. The risk of extramedullary involvement is higher especially in T-cell ALL[20] but the immunophenotype of leukemia did not correlate with the presence of NM/HE in our study group.
Only 26% of our patients had cytogenetic abnormalities. At present, we know that 75% of ALL cases have evidence of molecular rearrangements.[21] The reasons for low rate of genetic abnormalities found in our study might be the inclusion of very old cases and the technical deficiencies at that time. 11q23 rearrangements and Philadelphia chromosome are known risk factors for hyperleukocytosis,[22] which might consequently increase the incidence of extramedullary involvement. However, there was no correlation between the presence of cytogenetic aberrations and positive radiological findings in our cohort.
Renal involvement in acute leukemia is generally asymptomatic, although there are a few case reports of renal failure secondary to renal leukemic involvement. Olgar et al.[11] studied 334 children with ALL. Renal leukemic infiltration, which was defined by renal ultrasound, was found to be a significant risk factor for hypertension. However, only 15.6% of such cases suffered from hypertension and the authors suggested that the renal leukemic involvement was not the only contributing factor for hypertension development. Only four patients had hypertension in our study, which was not enough to perform a statistical analysis. Hyperphosphatemia and hyperuricemia were also significantly more common in children with presumed renal leukemic infiltration in Yetgin et al.[23] study. In our study, hyperuricemia and elevated creatinine were more common in the NM/HE positive group in comparison to patients with normal ultrasound findings. We did not observe renal failure. In leukemia, deterioration of renal functions may occur due to various reasons as well as leukemic cell infiltration. Urinary tract obstruction, dehydration, immune glomerulonephritis, nephrotoxic agents, TLS, hemorrhage, and sepsis may be counted among the reasons. In our study, these situations were taken into consideration in the evaluation of the patients. Laboratory or clinical TLS was observed in only six patients and relation with the NM/HE was not significant.
The relationship between NM/HE and prognosis in ALL is still unknown. D’Angelo et al.[10] demonstrated that prognosis of patients with NM at the time of diagnosis was worse with respect to the patients without NM. Neglia et al., Fujiki et al., and Taylor et al.[5,8,14] did not consider NM as having an impact on prognosis in comparison to the presence of other well-defined prognostic criteria. Hann et al.[4] showed that the NM was associated with worse survival in cases with better risk factors defined at the era of the study. The authors concluded that the patients with low-risk features were adversely affected as they did not receive augmented therapy like high-risk patients did. In our study, we did not observe an association with between NM/HE and survival. Accordingly, with the use of contemporary risk-adapted treatment protocols, one might assume that the extramedullary leukemia is no longer a universal unfavorable prognostic factor.
Renal function tests were found within normal limits in our patients. Long-term nephrotoxicity is relatively rare in cancer survivors in comparison to other late effects such as, cardiac, endocrine, and neurological problems.[24] The most significant risk factors for future nephrotoxicity are cisplatin, and ifosfamide administration, abdominal irradiation, nephrectomy, and bone marrow transplantation.[25] Those modalities are not frequently used for the treatment of ALL, making leukemia a relatively less frequent cause of renal function loss in survivors. Long-term nephrotoxicity of high-dose methotrexate still remains controversial, as most of the patients treated with high-dose methotrexate schedules might have been exposed to many other nephrotoxic agents and situations such as antibiotics and contrast agents as well as infections.[26] The fact that the majority of our patients were treated with escalating doses of methotrexate, namely, Capizzi schemes, might be a factor limiting renal function loss.
The limitation of our study is its retrospective nature. Furthermore, treatment outcome was not categorized according to risk groups in ALL.
Conclusion
Renal ultrasound abnormalities, namely, NM and renal HE, may be observed in more than 10% of patients with ALL. Thus, the differential diagnosis of the aforementioned ultrasound abnormalities of the kidney should also include leukemia. The relationship between renal pathologies and ALL survival was well evaluated, and no negative effects were found as reported in our study. There are very few studies exclusively reporting the long-term impact of renal ultrasound abnormalities on kidney function. Our study showed that renal functions were not adversely affected in ALL survivors with renal ultrasound abnormalities in admission.
Footnotes
This study was orally presented in 9. Çocuk Dostları Kongresi which was held online between 16-20 March 2021.
Please cite this article as ”Genc DB, Akinci N, Yildirmak ZY, Vural S. Renal Ultrasonographic Abnormalities at Initial Presentation of Children Diagnosed with Acute Lymphoblastic Leukemia and Long-Term Renal Functions and Prognosis in Survivors. Med Bull Sisli Etfal Hosp 2022;56(3):421–426”.
Disclosures
Ethics Committee Approval
The study was approved by the Sisli Hamidiye Etfal Training and Research Hospital Ethics Committee (Date: 22/9/2020, no: 1702/2020).
Peer-review
Externally peer-reviewed.
Conflict of Interest
None declared.
Authorship Contributions
Concept – N.A., Z.Y.Y., D.B.G.; Design – N.A., D.B.G.; Supervision – D.B.G., Z.Y.Y.; Materials – D.B.G., Z.Y.Y., S.V.; Data collection &/or processing – D.B.G., Z.Y.Y., S.V.; Analysis and/or interpretation – D.B.G., N.A.; Literature search – N.A., D.B.G., Z.Y.Y.; Writing – N.A., D.B.G., Z.Y.Y., S.V.; Critical review – D.B.G., S.V.
References
- 1.Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. IICC-3 contributors International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol. 2017;18:719–31. doi: 10.1016/S1470-2045(17)30186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kutluk MT, Yeşilipek MA. Pediatric cancer registry in Turkey (Turkish Pediatric Oncology Group & Turkish Pediatric Hematology Association) Journal of Global Oncology. 2018;4:67. [Google Scholar]
- 3.Barcos M, Lane W, Gomez GA, Han T, Freeman A, Preisler H, et al. An autopsy study of 1206 acute and chronic leukemias (1958 to 1982) Cancer. 1987;60:827–37. doi: 10.1002/1097-0142(19870815)60:4<827::aid-cncr2820600419>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Hann IM, Lees PD, Palmer MK, Gupta S, Morris-Jones PH. Renal size as a prognostic factor in childhood acute lymphoblastic leukemia. Cancer. 1981;48:207–9. doi: 10.1002/1097-0142(19810701)48:1<207::aid-cncr2820480132>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Taylor GA, Mellits ED, Zinkham WH, Leventhal BG. Prognostic role of the intravenous urogram in children with acute leukemia. Med Pediatr Oncol. 1982;10:489–96. doi: 10.1002/mpo.2950100509. [DOI] [PubMed] [Google Scholar]
- 6.Kumari-Subaiya S, Lee WJ, Festa R, Phillips G, Pochaczevsky R. Sonographic findings in leukemic renal disease. J Clin Ultrasound. 1984;12:465–72. doi: 10.1002/jcu.1870120803. [DOI] [PubMed] [Google Scholar]
- 7.Rajantie J, Jääskelainen J, Perkkiö M, Siimes MA. Kidneys very large at diagnosis are associated with poor prognosis in children with acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1986;8:87–90. [PubMed] [Google Scholar]
- 8.Neglia JP, Day DL, Swanson TV, Ramsay NK, Robison LL, Nesbit ME., Jr Kidney size at diagnosis of childhood acute lymphocytic leukemia: lack of prognostic significance for outcome. Am J Pediatr Hematol Oncol. 1988;10:296–300. doi: 10.1097/00043426-198824000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Ojala AE, Lanning FP, Lanning BM. Abdominal sonographic findings at primary diagnosis of acute lymphoblastic leukemia in children: a comparison with different clinical risk factors. Pediatr Hematol Oncol. 1995;12:355–61. doi: 10.3109/08880019509029585. [DOI] [PubMed] [Google Scholar]
- 10.D'Angelo P, Mura R, Rizzari C, Conter V, Bellini F, Valsecchi MG, et al. Prognostic value of nephromegaly at diagnosis of childhood acute lymphoblastic leukemia. Acta Haematol. 1995;94:84–9. doi: 10.1159/000203979. [DOI] [PubMed] [Google Scholar]
- 11.Olgar S, Yetgin S, Cetin M, Aras T, Akhan O. Electrolyte abnormalities at diagnosis of acute lymphocytic leukemia may be a clue for renal damage in long-term period. J Pediatr Hematol Oncol. 2005;27:202–6. doi: 10.1097/01.mph.0000161271.68054.b9. [DOI] [PubMed] [Google Scholar]
- 12.Hilmes MA, Dillman JR, Mody RJ, Strouse PJ. Pediatric renal leukemia: spectrum of CT imaging findings. Pediatr Radiol. 2008;38:424–30. doi: 10.1007/s00247-007-0741-5. [DOI] [PubMed] [Google Scholar]
- 13.Bach AG, Behrmann C, Holzhausen HJ, Katzer M, Arnold D, Spielmann RP, et al. Prevalence and patterns of renal involvement in imaging of malignant lymphoproliferative diseases. Acta Radiol. 2012;53:343–8. doi: 10.1258/ar.2011.110523. [DOI] [PubMed] [Google Scholar]
- 14.Fujiki T, Nishimura R, Mase S, Kuroda R, Ikawa Y, Araki R, et al. Accurate detection of renal leukemic involvement in children using 3-D computed tomography modeling. Pediatr Int. 2019;61:679–87. doi: 10.1111/ped.13907. [DOI] [PubMed] [Google Scholar]
- 15.Prada Rico M, Rodríguez-Cuellar CI, Arteaga Aya LN, Nuñez Chates CL, Garces Sterling SP, Pierotty M, et al. Renal involvement at diagnosis of pediatric acute lymphoblastic leukemia. Pediatr Rep. 2020;12:8382. doi: 10.4081/pr.2020.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraus RA, Gaisie G, Young LW. Increased renal parenchymal echogenicity: causes in pediatric patients. Radiographics. 1990;10:1009–18. doi: 10.1148/radiographics.10.6.2259758. [DOI] [PubMed] [Google Scholar]
- 17.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 18.Konuş OL, Ozdemir A, Akkaya A, Erbaş G, Celik H, Işik S. Normal liver, spleen, and kidney dimensions in neonates, infants, and children: evaluation with sonography. AJR Am J Roentgenol. 1998;171:1693–8. doi: 10.2214/ajr.171.6.9843315. [DOI] [PubMed] [Google Scholar]
- 19.Pradeep R, Madhumathi DS, Lakshmidevi V, Premalata CS, Appaji L, Patil SA, et al. Bilateral nephromegaly simulating wilms tumor: a rare initial manifestation of acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30:471–3. doi: 10.1097/MPH.0b013e318168e7b3. [DOI] [PubMed] [Google Scholar]
- 20.Esparza SD, Sakamoto KM. Topics in pediatric leukemia--acute lymphoblastic leukemia. MedGenMed. 2005;7:23. [PMC free article] [PubMed] [Google Scholar]
- 21.Mullighan CG. Molecular genetics of B-precursor acute lymphoblastic leukemia. J Clin Invest. 2012;122:3407–15. doi: 10.1172/JCI61203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teachey DT, Pui CH. Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol. 2019;20:e142–54. doi: 10.1016/S1470-2045(19)30031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yetgin S, Olgar S, Aras T, Cetin M, Düzova A, Beylergil V, et al. Evaluation of kidney damage in patients with acute lymphoblastic leukemia in long-term follow-up: value of renal scan. Am J Hematol. 2004;77:132–9. doi: 10.1002/ajh.20146. [DOI] [PubMed] [Google Scholar]
- 24.Diller L, Chow EJ, Gurney JG, Hudson MM, Kadin-Lottick NS, Kawashima TI, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27:2339–55. doi: 10.1200/JCO.2008.21.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kooijmans EC, Bökenkamp A, Tjahjadi NS, Tettero JM, van Dulmen-den Broeder E, van der Pal HJ, et al. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev. 2019;3:CD008944. doi: 10.1002/14651858.CD008944.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skinner R. Late renal toxicity of treatment for childhood malignancy: risk factors, long-term outcomes, and surveillance. Pediatr Nephrol. 2018;33:215–25. doi: 10.1007/s00467-017-3662-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


