Abstract
Background
Fear of pain during insertion of intrauterine contraception (IUC) is a barrier to use of this method. IUC includes copper‐containing intrauterine devices and levonorgestrel‐releasing intrauterine systems. Interventions for pain control during IUC insertion include non‐steroidal anti‐inflammatory drugs (NSAIDs), local cervical anesthetics, and cervical ripening agents such as misoprostol.
Objectives
To review randomized controlled trials (RCTs) of interventions for reducing IUC insertion‐related pain
Search methods
We searched for trials in CENTRAL, MEDLINE, EMBASE, POPLINE, ClinicalTrials.gov, and ICTRP. The most recent search was 22 June 2015. We examined reference lists of pertinent articles. For the initial review, we wrote to investigators to find other published or unpublished trials.
Selection criteria
We included RCTs that evaluated an intervention for preventing IUC insertion‐related pain. The comparison could have been a placebo, no intervention, or another active intervention. The primary outcomes were self‐reported pain at tenaculum placement, during IUC insertion, and after IUC insertion (up to six hours).
Data collection and analysis
Two authors extracted data from eligible trials. For dichotomous variables, we calculated the Mantel‐Haenszel odds ratio (OR) with 95% confidence interval (CI). For continuous variables, we computed the mean difference (MD) with 95% CI. In meta‐analysis of trials with different measurement scales, we used the standardized mean difference (SMD).
Main results
We included 33 trials with 5710 participants total; 29 were published from 2010 to 2015. Studies examined lidocaine, misoprostol, NSAIDs, and other interventions. Here we synthesize results from trials with sufficient outcome data and moderate‐ or high‐quality evidence.
For lidocaine, meta‐analysis showed topical 2% gel had no effect on pain at tenaculum placement (two trials) or on pain during IUC insertion (three trials). Other formulations were effective compared with placebo in individual trials. Mean score for IUC‐insertion pain was lower with lidocaine and prilocaine cream (MD ‐1.96, 95% CI ‐3.00 to ‐0.92). Among nulliparous women, topical 4% formulation showed lower scores for IUC‐insertion pain assessed within 10 minutes (MD ‐15.90, 95% CI ‐22.77 to ‐9.03) and at 30 minutes later (MD ‐11.10, 95% CI ‐19.05 to ‐3.15). Among parous women, IUC‐insertion pain was lower with 10% spray (median 1.00 versus 3.00). Compared with no intervention, pain at tenaculum placement was lower with 1% paracervical block (median 12 versus 28).
For misoprostol, meta‐analysis showed a higher mean score for IUC insertion compared with placebo (SMD 0.27, 95% CI 0.07 to 0.46; four studies). In meta‐analysis, cramping was more likely with misoprostol (OR 2.64, 95% CI 1.46 to 4.76; four studies). A trial with nulliparous women found a higher score for IUC‐insertion pain with misoprostol (median 46 versus 34). Pain before leaving the clinic was higher for misoprostol in two trials with nulliparous women (MD 7.60, 95% CI 6.48 to 8.72; medians 35.5 versus 20.5). In one trial with nulliparous women, moderate or severe pain at IUC insertion was less likely with misoprostol (OR 0.30, 95% CI 0.16 to 0.55). In the same trial, the misoprostol group was more likely to rate the experience favorably. Within two trials of misoprostol plus diclofenac, shivering, headache, or abdominal pain were more likely with misoprostol. Participants had no vaginal delivery. One trial showed the misoprostol group less likely to choose or recommend the treatment.
Among multiparous women, mean score for IUC‐insertion pain was lower for tramadol 50 mg versus naproxen 550 mg (MD ‐0.63, 95% CI ‐0.94 to ‐0.32) and for naproxen versus placebo (MD ‐1.94, 95% CI ‐2.35 to ‐1.53). The naproxen group was less likely than the placebo group to report the insertion experience as unpleasant and not want the medication in the future. An older trial showed repeated doses of naproxen 300 mg led to lower pain scores at one hour (MD ‐1.04, 95% CI ‐1.67 to ‐0.41) and two hours (MD ‐0.98, 95% CI ‐1.64 to ‐0.32) after insertion. Most women were nulliparous and also had lidocaine paracervical block.
Authors' conclusions
Nearly all trials used modern IUC. Most effectiveness evidence was of moderate quality, having come from single trials. Lidocaine 2% gel, misoprostol, and most NSAIDs did not help reduce pain. Some lidocaine formulations, tramadol, and naproxen had some effect on reducing IUC insertion‐related pain in specific groups. The ineffective interventions do not need further research.
Plain language summary
Methods to reduce pain with insertion of intrauterine contraception
Fear of pain with insertion of intrauterine contraception (IUC) may cause women to avoid using this very effective method of birth control. IUC includes devices with copper and with the hormone levonorgestrel. Researchers have studied many ways of reducing pain with IUC insertion. These include drugs that lessen uterine cramps, soften and open the cervix (uterus opening), or numb the cervix.
We reviewed randomized trials of reducing pain during IUC insertion through 22 June 2015. We found 33 studies with a total of 5710 women. Most were recent trials. Methods tested were nonsteroidal anti‐inflammatory drugs (NSAIDs), lidocaine, misoprostol, and other treatments. Lidocaine 2% gel had no effect on pain during IUC insertion (three trials) or pain with tenaculum (type of forceps) placement (two trials). Other types of lidocaine showed some effect. Pain score for IUC insertion was lower with a lidocaine and prilocaine cream and with 10% lidocaine spray. With 4% lidocaine gel, pain scores were lower shortly after IUC insertion. With 1% lidocaine injection, pain score at tenaculum placement was lower compared with no intervention.
With four misoprostol trials, the pain score with IUC insertion was higher for misoprostol versus placebo ('dummy' treatment). Two other trials showed higher pain scores with misoprostol versus placebo either at IUC insertion or after. However, another study showed the misoprostol group had less serious IUC‐insertion pain. Also, the misoprostol group rated the insertion more favorably. In analysis of four trials, cramping was more likely with misoprostol versus placebo. Within two other trials, the misoprostol group was more likely to have shivering, headache, or abdominal pain. In one study, the misoprostol group was less likely to choose the treatment again or recommend it.
Pain score during IUC insertion was lower for the opioid tramadol versus naproxen. In the same trial, pain was lower for naproxen versus placebo. The naproxen group was less likely than the placebo group to rate the experience as unpleasant and not want the treatment in the future. In another trial, women with several naproxen doses had lower pain scores after IUC insertion than the placebo group.
Overall, the effectiveness results were of moderate quality, having come from single studies. Trials of lidocaine, tramadol and naproxen showed some effect on reducing pain from IUC insertion.
Summary of findings
Background
Description of the condition
Intrauterine contraception (IUC) provides long‐term, reversible contraception equal in efficacy to tubal sterilization (Grimes 2008). IUC includes copper‐containing intrauterine devices (IUDs) and levonorgestrel‐releasing intrauterine systems (LNG‐IUS). The term 'IUD' is often used to include both types. Depending on the country, the use of IUDs worldwide ranges from 2% to 75%. On average, 15% of reproductive‐aged women in "developing regions" and 9% in "developed regions" use IUDs (UN 2013). Increasing the number of women using IUC is an important public health goal. IUDs are considered appropriate for most women, including nulliparous women and adolescents (Comm Adolescent Health 2012; Ott 2014). One barrier to IUC is the fear of pain during insertion (Asker 2006). Components of the insertion procedure that may cause pain include the application of the tenaculum to the cervix to stabilize the uterus and provide traction for straightening the cervical canal, passing the uterine sound, advancing the inserter tube through the cervix, and irritation of the endometrial cavity when the device is deployed. Cervical pain is mediated by S2 to S4 parasympathetic nerves, and the T10 to L1 sympathetic fibers innervate the uterine fundus. While some IUDs are inserted postpartum or postabortal, most are inserted more than four weeks after pregnancy as a clinic‐based procedure. The levels of pain that women experience during IUD insertion vary in published reports. Most women experience mild to moderate discomfort during IUD insertion. Rarely, the pain is severe and associated with nausea and weakness. Pain may persist for a few days after insertion. Predictors of pain during IUC insertion include nulliparity, age greater than 30 years, a longer interval since last pregnancy or menses, history of dysmenorrhea, and not currently breastfeeding (Hubacher 2006; Kaislasuo 2014). Psychosocial factors, such as expected pain, also influence the pain perceived by women undergoing the procedure (Goldstuck 1985; Murty 2003).
Description of the intervention
Pharmacological methods of pain control used for IUC insertion commonly include non‐steroidal anti‐inflammatory drugs (NSAIDs), anxiolytics, opioids, and local anesthetics in the form of intracervical gel, cervical and paracervical block, and intrauterine instillation. A survey of UK physicians found a wide variation in the use of analgesia or anesthesia for IUC insertions from no routine use to always using prophylactic NSAIDs or 2% lidocaine gel intracervically during the procedure (Tolcher 2003). Other interventions to ease IUC insertion include the use of prostaglandins or nitric oxide donors such as nitroprusside and nitroglycerin.
How the intervention might work
Local anesthetics that are administered topically or through injection may decrease cervical pain by blocking nerve fibers. Anxiolytics reduce pre‐insertion anxiety and may lead to decreased pain perception by the woman (Murty 2003). NSAIDs have been shown to reduce pain associated with IUD use (Grimes 2006). In the context of IUD insertion, NSAIDs and opioids may reduce cervical or uterine pain. Misoprostol, a prostaglandin E1 analogue, may decrease pain by dilating and softening the cervix prior to insertion (Goldberg 2003). Nitric oxide donors are smooth muscle relaxants that may also soften the cervix but without the uterine cramping found with misoprostol (Thomson 1997; Bednarek 2013).
Why it is important to do this review
Pain at insertion of an IUD can be distressing and may deter women from using the method. Since the publication of our initial review in 2009 that called for more research, numerous trials have examined various interventions for pain with IUD insertion. This review evaluates both prophylactic and procedural interventions to reduce pain. Determining the optimal method for reducing pain during IUD insertion will benefit women and may increase the uptake of IUDs as a contraceptive method. Alternatively, ineffective interventions may only increase costs and delay initiation of the insertion procedure.
Objectives
To review randomized controlled trials (RCTs) of interventions for reducing IUC insertion‐related pain
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials in any language that evaluated an intervention for preventing IUC insertion‐related pain. The intervention could be compared with a placebo or another active intervention.
Types of participants
Women having any type of IUC inserted
Types of interventions
We included any pharmacological or other intervention administered prior to, or during, IUC insertion in order to reduce pain at the time of insertion and up to six hours afterward.
Types of outcome measures
Primary outcomes
For this review, the primary outcomes were self‐reported pain scores related to IUC insertion: at tenaculum placement, during IUC insertion, and after IUC insertion (up to six hours). The trials may have had a different primary outcome, such as ease of insertion for the provider.
Secondary outcomes
Side effects, adverse events, and participant satisfaction
Search methods for identification of studies
Electronic searches
We searched for trials until 22 June 2015. Databases included the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), EMBASE, POPLINE, ClinicalTrials.gov and WHO ICTRP. Strategies for this version are shown in Appendix 1. The search strategies for the initial review are shown in Appendix 2.
Searching other resources
We searched the reference lists of retrieved titles and relevant review articles for additional studies. For the initial review, we wrote to investigators of published trials to solicit information regarding other published or unpublished trials that may have been missed in our initial search.
Data collection and analysis
Selection of studies
We assessed all titles and abstracts identified during the literature searches for potential eligibility.
Data extraction and management
Two authors independently assessed and extracted data from the studies. We resolved any discrepancies or disagreements through discussion or a third author if needed. One author entered data into Review Manager 5 (RevMan 2014) and a second confirmed correct data entry. We list the specifics tasks by author in Contributions of authors.
Assessment of risk of bias in included studies
We examined the trial methodology according to recommended guidelines (Higgins 2011). Factors that we considered for potential bias were study design, randomization process, allocation concealment, blinding, early discontinuation and loss to follow‐up rates.
Measures of treatment effect
For continuous variables, we computed the mean difference (MD) with 95% confidence interval (CI). If trials in meta‐analysis used different measurement scales, we used the standardized mean difference (SMD). RevMan uses the inverse variance approach. For dichotomous outcomes, we calculated the Mantel‐Haenszel odds ratio (OR) with 95% CI. In meta‐analysis, we used a random‐effects model. When a comparison includes only one study, fixed and random effects give the same result; no heterogeneity exists. Where the trial report provided only the medians and ranges, we present the data as provided by the investigators.
Dealing with missing data
We contacted trial investigators as needed to supplement published information.
Data synthesis
We applied principles from GRADE to assess the evidence quality and address confidence in the effect estimates (Balshem 2011; Higgins 2011). When a meta‐analysis is not viable due to varied interventions, a 'Summary of findings' table is not feasible. Therefore, not every trial or outcome is part of a formal GRADE assessment with an evidence profile and 'Summary of findings' table (Guyatt 2011).
Our assessment of evidence quality, which could be high, moderate, low, or very low, was based on the evidence from the individual studies. We considered the evidence from RCTs to be high quality initially, then downgraded as follows: 1) moderate quality if risk of bias (RoB) is high for one factor assessed or unclear for two; 2) low quality if RoB is high for two items or if high and unclear risk totals three; 3) very low if RoB is high for three factors or if high and unclear risk totals at least four. Follow‐up was less an issue for this review, since the primary outcomes of interest were measured on the procedure day.
For the 'Summary of findings' tables, we downgraded one level if the evidence came from only one trial. We could not examine consistency across trials with only one study. Further research may change the estimate due to having a different population, intervention, or outcome measure. Examples include nulliparous women versus both parous and nulliparous women, different timing or application of the intervention, and different timing or assessment for the pain outcome.
Sensitivity analysis
We synthesized results from trials with sufficient outcome data and evidence of moderate or high quality.
Results
Description of studies
Results of the search
For the initial review in 2009, the search strategy yielded 349 articles. Four randomized controlled trials met the inclusion criteria for the review.
For the 2015 version, the database searches produced 102 unduplicated citations (Figure 1). We had removed 51 duplicates, either electronically or by hand. With six items identified from other sources, the total of unduplicated references was 108. After discarding 65 references, we reviewed the text of 43 articles or abstracts (primary and secondary). We added 29 new trials (Included studies): 27 full‐text primary reports plus two primary conference abstracts, both of which also had listings in a clinical trial register. For secondary reports, we included four full‐text articles and seven conference abstracts, some of which led us to the full‐text reports. We excluded two primary reports.
1.
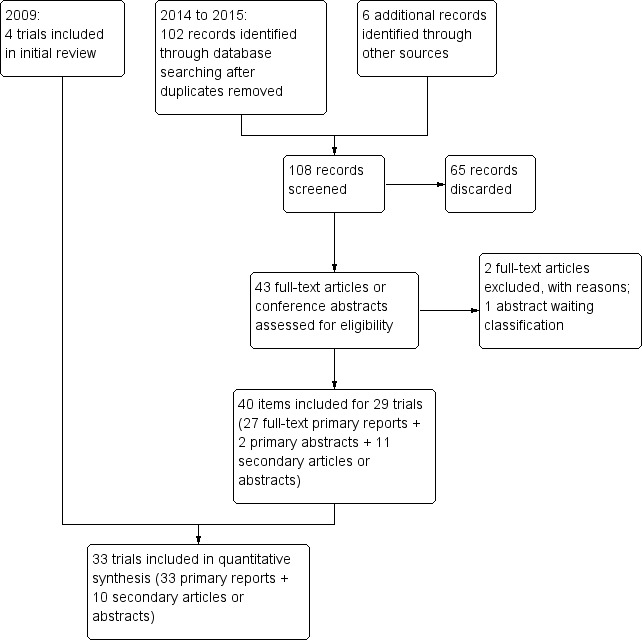
Study flow diagram
We categorized four trials as Studies awaiting classification: two with manuscripts reportedly in progress; one with no current information; and one with a conference abstract identified while this manuscript was under peer review. Also while under peer review, we identified conference abstracts from trials that examined alternatives to the standard tenaculum. We did not include them, because we had not searched for such studies and may only have seen a select sample. For the next update, we will consider whether to expand the search strategy with the terminology for that type of intervention.
From recent clinical trial registers, we obtained 95 unduplicated trials. Two led us to primary reports included above. Three trials are Ongoing studies. We excluded two listings for trials that never started. The remaining listings were not relevant or represented completed trials that were already included.
Included studies
We identified 33 trials that met our eligibility criteria, after adding 29 new studies to the original four. The trials had a total of 5710 participants, with a mean of 178 and the median at 95. The number of participants in each trial ranged from 24 to 2019: 18 trials had fewer than 100 participants, 14 had from 100 to 300 women, and one had 2019 participants. The reports were published from 1974 to 2015, with 29 published from 2010 to 2015. The trials were conducted in Eastern and Western Europe, the Middle East, South America, and the USA. Of the 33 trials, 7 evaluated non‐steroidal anti‐inflammatory drugs (NSAIDs), 12 examined lidocaine (one used an NSAID for comparison), 10 studied misoprostol, and 5 evaluated other interventions (one used NSAID as a comparison as well as placebo).
Table 6 summarizes the experimental and comparison interventions for the 33 trials, along with the delivery method and timing. Additional trial details are given in Characteristics of included studies.
1. Intervention summary.
| Study | Experimental intervention | Comparison intervention | Delivery | Timing before procedure | Parity |
| NSAID | |||||
| Massey 1974 | Naproxen 300 mg x 4 doses (+ paracervical block, 1% lidocaine, 8 mL) | Placebo (+ paracervical block, 1% lidocaine, 8 mL) | Tablet, oral | Previous night and 1.5 h prior; 2 and 6 h after | (96% nulliparous) |
| Karabayirli 2012 | Naproxen 550 mg | Placebo | Tablet | 1 h | Multiparous |
| Hubacher 2006 | Ibuprofen 400 mg | Placebo | Tablet, oral | ≥ 45 min | _ |
| Jensen 1998 | Ibuprofen 600 mg | Placebo | Tablet, oral | 1 to 4 h (also 4 to 6 h after) | _ |
| Bednarek 2015 | Ibuprofen 800 mg | Placebo | Tablet, oral | 30 to 45 min | (Requesting abortion) |
| Chor 2012 | Ibuprofen 800 mg | Placebo | Tablet, oral | 45 min | _ |
| Ngo 2014a | Ketorolac 30 mg | Saline | intramuscular injection | 30 min | _ |
| Lidocaine | |||||
| Mohammad‐Alizadeh‐C 2010 | 2% gel (amount unspecified) | Lubricant gel or no intervention | Swab | > 1 min | _ |
| Maguire 2012 | 2% gel (1 mL) | Placebo gel | Swab | prior to uterine sounding | _ |
| Allen 2013 | 2% gel (6 mL) | Placebo gel | 2 sites, 3 mL each, via syringe | 3 min | _ |
| McNicholas 2012 | 2% gel (2.5 to 4 mL) | Placebo gel | 0.5 to 1 mL topical; 2 to 3 mL inserted | 3 min | Randomization stratified by parity |
| Rapkin 2014a | 2% gel (5 mL) | Placebo gel | Self‐inserted vaginally | 5 min | Nulliparous |
| Nelson 2013 | 2% (1.2 mL) | Saline placebo | Infused, 3 sites | 3 min | _ |
| Tornblom‐Paulander 2015 | 4% gel (8.5 mL) | Placebo | Topical, 3 sites | 5 min | Nulliparous |
| Aksoy 2015 | 10% spray (40 mg) | Saline placebo | Topical, 2 sites; 4 puffs total | 3 min | Parous |
| Ahmadi Doulabi 2013 | EMLA cream 5% (lidocaine + prilocaine) (5 g) | Placebo cream | Swab on cervix and cervical opening | 7 min | _ |
| Mody 2012 | 1% paracervical block (10 mL) | No analgesia (no intervention) | 2 sites, 5 mL each | 3 min | _ |
| Cırık 2013 | 1% paracervical block (10 mL) | Saline or no intervention | 2 sites, 5 mL each | 5 min | _ |
| Castro 2014 | 2% intracervical block (1.8 mL) | Ibuprofen 400 mg | 4 sites | 5 min (lidocaine); 1 h (ibuprofen) | Nulliparous or no vaginal delivery |
| Misoprostol | |||||
| Sääv 2007 | 400 μg + diclofenac | Diclofenac | Sublingual | 1 h | Nulliparous |
| Ibrahim 2013 | 400 µg + diclofenac | Diclofenac | Sublingual | 1 h | Prior cesarean delivery only |
| Heikinheimo 2010 | 400 µg | Placebo | Sublingual | 3 h | _ |
| Edelman 2011 | 400 µg | Placebo | Buccal | 90 min | Nulliparous |
| Lathrop 2013 | 400 µg | Placebo | Buccal | 2 to 4 h | No pregnancy > 19 6/7 weeks |
| Espey 2014 | 400 µg | Placebo | Buccal | 2 to 8 h | Nulliparous |
| Lotke 2013 | 400 μg | Placebo | Vaginal or buccal | 2 h | Nulliparous |
| Swenson 2012 | 400 μg | Placebo | Vaginal or buccal | 3 to 4 h | No pregnancy > 13 6/7 weeks |
| Dijkhuizen 2011 | 400 μg | Placebo | Vaginal | 3 h | _ |
| Scavuzzi 2013 | 400 μg | Placebo | Vaginal | 4 h | Nulligravida |
| Other | |||||
| Bednarek 2013 | Nitroprusside gel 1% | Placebo gel | 1 mL | immediately prior | Nulliparous |
| Micks 2014 | Nitroglycerin ointment 0.5 mg | Placebo ointment | 1 mL | 35 to 40 min | Nulliparous |
| Karabayirli 2012 | Tramadol 50 mg | Naproxen 550 mg | Tablet | 1 h | Multiparous |
| Shahnazi 2012 | Lavender 10 drops in diluted milk | Diluted milk | 3 drops on cotton, inhaled | 30 min (also during) | _ |
| Cameron 2013 | Bladder emptying delayed (after IUC insertion) | Bladder emptying immediate (before IUC insertion) | Water 1L, orally, 1 h before appointment | _ | _ |
aNo full report; sources included conference abstract and clinical trial listing
Seven trials examined NSAIDs, i.e., naproxen, ibuprofen, and ketorolac. For naproxen, the oral doses were 300 mg (four times) (Massey 1974) and 550 mg (Karabayirli 2012). Oral doses for ibuprofen were 400 mg (Hubacher 2006), 600 mg (Jensen 1998) and 800 mg (Bednarek 2015; Chor 2012). The ketorolac dose was 30 mg by intramuscular injection (Ngo 2014). In addition, a lidocaine trial used ibuprofen as the comparator (Castro 2014); results are presented with the other lidocaine trials.
Twelve trials examined the effects of various lidocaine formulations. These include 2% gel (Allen 2013; Maguire 2012; McNicholas 2012; Mohammad‐Alizadeh‐C 2010; Rapkin 2014), 4% gel (Tornblom‐Paulander 2015), a cream containing lidocaine and prilocaine (Ahmadi Doulabi 2013), 2% solution for infusion (Nelson 2013), 10% spray (Aksoy 2015), 1% paracervical block (Cırık 2013; Mody 2012), and 2% intracervical block (Castro 2014).
Ten trials evaluated misoprostol 400 µg. Administration was sublingual (Heikinheimo 2010; Ibrahim 2013; Sääv 2007), buccal (Edelman 2011; Espey 2014; Lathrop 2013), vaginal (Dijkhuizen 2011; Scavuzzi 2013), or either vaginal or buccal (Lotke 2013; Swenson 2012). Five of these trials were part of a prospective meta‐analysis as described in Turok 2011 (Edelman 2011; Espey 2014; Lathrop 2013; Lotke 2013; Swenson 2012).
Five trials studied other interventions. Two examined nitric oxide donors, i.e., nitroprusside gel 1% (Bednarek 2013) and nitroglycerin ointment 0.5 mg (Micks 2014). Others studied tramadol 50 mg and naproxen (Karabayirli 2012), delayed bladder emptying (Cameron 2013), and lavender essence (Shahnazi 2012).
Excluded studies
We excluded 10 studies. Some lacked randomization (Hepburn 1980; Thiery 1985); one used alternate assignment (Newton 1977) and another assigned by date of birth (Oloto 1997). One report was a review article rather than intervention study (Hollingworth 1995). Two RCTs lacked our primary outcomes (Goldstuck 1983; Jafari 2014). For another study, the analysis did not appear to account for clustering effects (Mirmohamad Aliei 2013). Lastly, two trials never started (Stephenson 2010; Teal 2012).
Risk of bias in included studies
Details for each study are shown in Characteristics of included studies. Figure 2 shows the overall risk of bias for evidence in this review. Risk of bias by trial can be seen in Figure 3.
2.

Risk of bias graph: review authors' judgements about risk of bias as percentages across all 33 included studies
3.
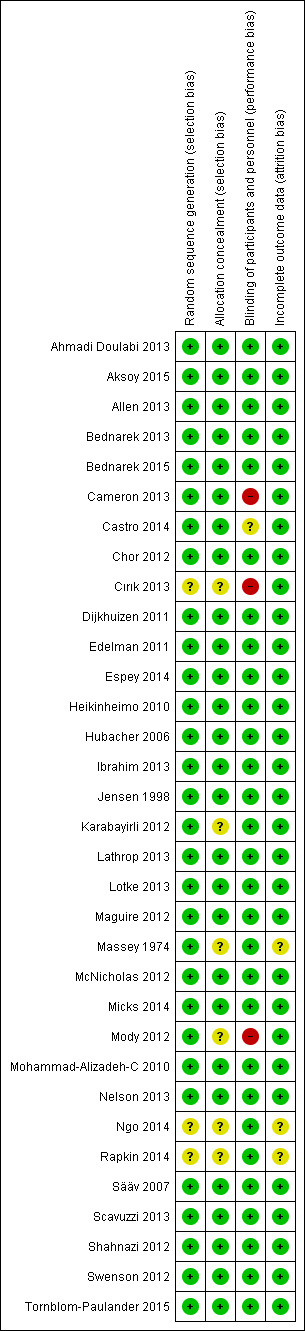
Risk of bias summary: review authors' judgements about risk of bias for each included study
Allocation
Most included trials mentioned some type of computer‐generated randomization scheme and many also noted block size. Those lacking information were two conference abstracts (Ngo 2014; Rapkin 2014) and one full report (Cırık 2013). Similarly, most trials reported adequate allocation concealment, such as pharmacy‐blinding packages or sequentially numbered, sealed, opaque envelopes. The exceptions were the two abstracts and one report noted above, an older report (Massey 1974) and two recent trials (Karabayirli 2012; Mody 2012).
Blinding
Three studies did not appear to use any blinding (Cameron 2013; Cırık 2013; Mody 2012). The others reported some blinding, e.g., participants and providers, and some mentioned research staff or the analyst.
Incomplete outcome data
Loss to follow‐up was a minor issue for the primary outcomes in this review. The main outcome of pain with IUD insertion was assessed during or immediately after the procedure. However, the assessment of pain up to six hours after the procedure could involve losses, as could side effect or satisfaction data. Loss data were unavailable from the conference abstracts (Ngo 2014; Rapkin 2014).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Summary of findings: lidocaine 2% gel.
| Lidocaine 2% gel compared with placebo for pain with IUC insertion | ||||
|
Patient or population: women with IUC being inserted Settings: clinic Intervention: lidocaine 2% gel, topical Comparison: placebo | ||||
| Outcomes | Relative effect (95% CI) | No. of participants (studies) | Quality of evidence (GRADE) | Notes on parity |
| Pain score at tenaculum placement (10 cm or 100 mm VAS) | SMD ‐0.03 (‐0.25 to 0.18) | 345 (2 studies) | ⊕⊕⊕⊕ high | No limitation |
| Pain score during IUC insertion (10 cm or 100 mm VAS) | SMD ‐0.02 (‐0.21 to 0.18) | 409 (3 studies) | ⊕⊕⊕⊕ high | No limitation |
| IUC: intrauterine contraception: VAS: visual analog scale; SMD: Standardized mean difference; CI: Confidence interval | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Summary of findings 2. Summary of findings: other lidocaine.
| Lidocaine formulations for pain with IUC insertion | ||||
|
Patient or population: women with IUC being inserted Settings: clinic Intervention: lidocaine formulations (see Notes) Comparison: see Notes | ||||
| Outcomes | Relative effect | No. of participants (studies) | Quality of evidencea (GRADE) | Notes on intervention (parity specification) |
| Pain score at tenaculum placement (100 mm VAS) |
Median (1st, 3rd quartiles): 12 (4, 27) vs 28 (14.5, 40.5) |
50 (Mody 2012) | ⊕⊕⊝⊝ low | Lidocaine 1% paracervical block vs no intervention |
| Pain score for IUC insertion (10 cm VAS) | MD ‐1.96 (95% CI ‐3.00 to ‐0.92) | 92 (Ahmadi Doulabi 2013) | ⊕⊕⊕⊝ moderate | EMLA cream 5% (lidocaine + prilocaine) vs placebo cream |
| Pain score for IUC insertion (10 cm VAS) |
Median (range): 1.00 (0 to 6) vs 3.00 (0 to 7) |
200 (Aksoy 2015) | ⊕⊕⊕⊝ moderate | Lidocaine 10% spray vs saline placebo (parous) |
| Pain score for IUC insertion (within 10 min) (100 mm VAS) | MD ‐15.90 (95% CI ‐22.77 to ‐9.03) | 209 (Tornblom‐Paulander 2015) |
⊕⊕⊕⊝ moderate | Lidocaine 4% short‐acting gel vs placebo gel (nulliparous) |
| Pain score at 30 min post‐insertion (100 mm VAS) | MD ‐11.10 (95% CI ‐19.05 to ‐3.15) | 114 (Tornblom‐Paulander 2015) |
⊕⊕⊕⊝ moderate | Lidocaine 4% short‐acting gel vs placebo gel (nulliparous) |
| IUC: intrauterine contraception: VAS: visual analog scale; MD: Mean difference; CI: Confidence interval | ||||
| GRADE Working Group grades of evidence Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | ||||
aWhen the evidence came from only one trial, we downgraded one level; further research may change the estimate.
Summary of findings 3. Summary of findings: naproxen or tramadol.
| Naproxen or tramadol for pain with IUC insertion | ||||
|
Patient or population: women with IUC being inserted Settings: clinic Intervention: naproxen or tramadol Comparison: see Notes | ||||
| Outcomes | Relative effect (95% CI) | No. of participants (studies) | Quality of evidencea (GRADE) | Notes on intervention (parity) |
| Pain score during IUC insertion (10‐point VAS) | MD ‐0.63 (‐0.94 to ‐0.32) | 69 (Karabayirli 2012) |
⊕⊕⊕⊝ moderate | Tramadol 50 mg vs naproxen 550 mg (multiparous) |
| Pain score during IUC insertion (10‐point VAS) | MD ‐1.94 (‐2.35 to ‐1.53) | 68 (Karabayirli 2012) |
⊕⊕⊕⊝ moderate | Naproxen 550 mg vs placebo (multiparous) |
| Satisfaction: insertion experience was unpleasant | OR 0.02 (0.01 to 0.09) | 68 (Karabayirli 2012) |
⊕⊕⊕⊝ moderate | Naproxen 550 mg vs placebo (multiparous) |
| Satisfaction: would not want treatment in future | OR 0.02 (0.00 to 0.08) | 68 (Karabayirli 2012) |
⊕⊕⊕⊝ moderate | Naproxen 550 mg vs placebo (multiparous) |
| Pain score 1 hour after IUC insertion (5‐point scale) | MD ‐1.04 (‐1.67 to ‐0.41) | 50 (Massey 1974) |
⊕⊕⊝⊝ low | Naproxen 300 mg (4 doses) vs placebo; both had paracervical block (48 nulliparous/50) |
| Pain score 2 hours after IUC insertion (5‐point scale) | MD ‐0.98 (‐1.64 to ‐0.32) | 41 (Massey 1974) |
⊕⊕⊝⊝ low | Naproxen 300 mg (4 doses) vs placebo; both had paracervical block (48 nulliparous/50) |
| IUC: intrauterine contraception: VAS: visual analog scale; MD: Mean difference; CI: Confidence interval: OR: Odds ratio | ||||
| GRADE Working Group grades of evidence Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | ||||
aWhen the evidence came from only one trial, we downgraded one level; further research may change the estimate.
Summary of findings 4. Summary of findings: misoprostol 400 µg.
| Misoprostol 400 µg compared with placebo for pain with IUC insertion | ||||
|
Patient or population: women with IUC being inserted Settings: clinic Intervention: misoprostol 400 µg Comparison: placebo | ||||
| Outcomes | Relative effect | No. of participants (studies) | Quality of evidencea (GRADE) | Notes on administration (parity) |
| Pain score during IUC insertion (100 mm VAS) | SMD 0.27 (95% CI 0.07 to 0.46) | 400 (4 studies) | ⊕⊕⊕⊕ high | Vaginal or buccal (parity varied by trial) |
| Side effect: cramping (before IUC insertion) | OR 2.64 (95% CI 1.46 to 4.76) | 466 (4 studies) | ⊕⊕⊕⊕ high | Vaginal or buccal (parity varied by trial) |
| Moderate to severe pain at IUC insertion (dichotomous) | OR 0.30 (95% CI 0.16 to 0.55) | 179 (Scavuzzi 2013) | ⊕⊕⊝⊝ low | Vaginal (nulligravida) |
|
Satisfaction: experience was slightly or not disagreeable |
OR 4.34 (95% CI 2.32 to 8.12) | 179 (Scavuzzi 2013) | ⊕⊕⊕⊝ moderate | Vaginal (nulligravida) |
| Pain score at IUC insertion (100 mm VAS) |
Median (range): 46 (11 to 92) vs 34 (0 to 90) |
73 (Lathrop 2013) |
⊕⊕⊕⊝ moderate | Buccal (no pregnancy >= 20 weeks) |
| Pain score before leaving clinic (100 mm VAS) |
Median (range): 35.5 (1 to 100) vs 20.5 (0 to 86) |
73 (Lathrop 2013) |
⊕⊕⊕⊝ moderate | Buccal (no pregnancy >= 20 weeks) |
| Pain score for highest level before leaving clinic (100 mm VAS) | MD 7.60 (95% CI 6.48 to 8.72) | 105 (Swenson 2012) | ⊕⊕⊕⊝ moderate | Vaginal or buccal (no pregnancy >= 14 weeks) |
| IUC: intrauterine contraception: VAS: visual analog scale; SMD: Standardized mean difference; MD: Mean difference; CI: Confidence interval; OR: Odds Ratio | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | ||||
aWhen the evidence came from only one trial, we downgraded one level; further research may change the estimate.
Summary of findings 5. Summary of findings: misoprostol 400 µg + diclofenac.
| Misoprostol 400 µg + diclofenac compared with diclofenac for pain with IUC insertion | ||||
|
Patient or population: women with IUC being inserted Settings: clinic Intervention: misoprostol 400 µg (sublingual) + diclofenac 100 mg Comparison: diclofenac 100 mg | ||||
| Outcomes | Relative effect (95% CI) | No. of participants (studies) | Quality of evidencea (GRADE) | Notes on parity |
| Side effect: headache | OR 5.68 (1.23 to 26.19) | 255 (Ibrahim 2013) |
⊕⊕⊕⊝ moderate | Prior cesarean only |
| Side effect: abdominal pain | OR 3.93 (1.41 to 10.97) | 230 (Ibrahim 2013) |
⊕⊕⊕⊝ moderate | Prior cesarean only |
| Side effect: shivering | OR 5.48 (1.41 to 21.33) | 79 (Sääv 2007) |
⊕⊕⊕⊝ moderate | Nulliparous |
| Satisfaction: would choose treatment again | OR 0.30 (0.14 to 0.65) | 255 (Ibrahim 2013) |
⊕⊕⊕⊝ moderate | Prior cesarean only |
| Satisfaction: would recommend treatment to friend | OR 0.36 (0.16 to 0.81) | 255 (Ibrahim 2013) |
⊕⊕⊕⊝ moderate | Prior cesarean only |
| IUC: intrauterine contraception: CI: Confidence interval; OR: Odds Ratio | ||||
| GRADE Working Group grades of evidence Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | ||||
aWhen the evidence came from only one trial, we downgraded one level; further research may change the estimate.
Pain data for each study are summarized in Table 7, which also includes sample sizes. Most trials assessed pain level using a visual analog scale (VAS), e.g., 10 cm or 10 points or 100 mm. Specifics are given below. Data on satisfaction and side effects are summarized in Table 8.
2. Outcome summary: pain.
| Study | Total N | Experimental intervention | Delivery | Pain at tenaculum placement | Pain during IUC insertion | Pain after IUC insertion |
| NSAID | ||||||
| Massey 1974 | 50 | Naproxen 300 mg (x 4 doses) + paracervical block (1% lidocaine 8 mL) | Tablet, oral | _ | NSa | Naproxen < placebo (1 h, 2 h) |
| Karabayirli 2012 | 103 | Naproxen 550 mg | Tablet, oral | _ | Naproxen < placebo | _ |
| Hubacher 2006 | 2019 | Ibuprofen 400 mg | Tablet, oral | _ | NS | _ |
| Jensen 1998 | 55 | Ibuprofen 600 mg | Tablet, oral | _ | NS | NS (4 to 6 h) |
| Bednarek 2015 | 202 | Ibuprofen 800 mg | Tablet, oral | _ | NS | _ |
| Chor 2012 | 87 | Ibuprofen 800 mg | Tablet, oral | NS | NS | _ |
| Ngo 2014b | 67 | Ketorolac 30 mg | Intramuscular injection | NS | NS | Ketorolac < placebo (5 min, 15 min) |
| Lidocaine | ||||||
| Maguire 2012 | 200 | 2% gel (1 mL) | Swab | NS | NS | _ |
| Mohammad‐Alizadeh‐C 2010 | 96 | 2% gel | Swab | _ | NS | _ |
| Allen 2013 | 150 | 2% gel (6 mL) | 2 sites, 3 mL each | NS | NS | _ |
| Nelson 2013 | 40 | 2% (1.2 mL) | Infused, 3 sites | NS | NS | NS (global, visit end) |
| McNicholas 2012 | 200 | 2% gel (2.5 to 4 mL) | 0.5 to 1 mL topical; 2 to 3 mL inserted | NS | NS | _ |
| Rapkin 2014b | 64 | 2% gel (5 mL) | Self‐inserted vaginally | Lidocaine < placebo | NS | _ |
| Tornblom‐Paulander 2015 | 218 | 4% gel (8.5 mL) | Topical, 3 sites | _ | Lidocaine < placebo (within 10 min) | Lidocaine < placebo (30 min); NS (1 h) |
| Aksoy 2015 | 200 | 10% spray (40 mg) | Topical, 2 sites; 4 puffs total | _ | Lidocaine < placebo (immediately after) | _ |
| Ahmadi Doulabi 2013 | 92 | EMLA cream 5% (lidocaine + prilocaine) (5 g) | Swab | EMLA < placebo | EMLA < placebo (immediately after) | _ |
| Mody 2012 | 50 | 1% paracervical block (10 mL) | 2 sites, 5 mL each | Lidocaine < control | NS | NS (5 min) |
| Cırık 2013b | 95 | 1% paracervical block (10 mL) | 2 sites, 5 mL each | Lidocaine < placebo or control | Lidocaine < placebo or control | Lidocaine < placebo or control (5 min) |
| Castro 2014 | 100 | 2% intracervical block (1.8 mL) | 4 sites | _ | NS | NS (2 h, 6 h) |
| Misoprostol | ||||||
| Sääv 2007 | 80 | 400 μg + diclofenac | Sublingual | _ | NS | _ |
| Ibrahim 2013 | 274 | 400 µg + diclofenac | Sublingual | _ | NS | _ |
| Heikinheimo 2010 | 89 | 400 µg | Sublingual | _ | NS | _ |
| Edelman 2011 | 40 | 400 µg | Buccal | NS | NS | NS (5 min) |
| Lathrop 2013 | 73 | 400 µg | Buccal | _ | Misoprostol > placebo | Misoprostol > placebo (before clinic departure) |
| Espey 2014 | 83 | 400 µg | Buccal | _ | NS (immediately after) | NS (before clinic discharge) |
| Lotke 2013 | 61 | 400 μg | Vaginal or buccal | _ | NS | _ |
| Swenson 2012 | 108 | 400 μg | Vaginal or buccal | _ | NS | Misoprostol > placebo (before leaving clinic) |
| Dijkhuizen 2011 | 270 | 400 μg | Vaginal | _ | NS | _ |
| Scavuzzi 2013 | 190 | 400 μg | Vaginal | _ | Misoprostol < placebo | _ |
| Other | ||||||
| Bednarek 2013 | 24 | Nitroprusside gel 1% | 1 mL | NS | NS | NS (30 min) |
| Micks 2014 | 24 | Nitroglycerin ointment 0.5 mg | 1 mL | NS | NS | NS (30 min) |
| Karabayirli 2012 | 103 | Tramadol 50 mg | Tablet, oral | _ | Tramadol < naproxen | _ |
| Shahnazi 2012 | 106 | Lavender 10 drops in diluted milk | 3 drops on cotton, inhaled | _ | NS | _ |
| Cameron 2013 | 200 | Delayed bladder emptying (after IUC insertion) | Oral (water 1 L) | _ | NS | _ |
aNS = no significant difference between study arms bExcluded from sensitivity analysis due to insufficient outcome data or low quality evidence
3. Outcome summary: side effects and satisfaction.
| Study | N | Experimental intervention | Delivery | Side effects or adverse events | Satisfaction or acceptability |
| NSAID | |||||
| Karabayirli 2012 | 103 | Naproxen 550 mg | Tablet | 0 | Unsatisfied: naproxen < placebo |
| Hubacher 2006 | 2019 | Ibuprofen 400 mg | Tablet, oral | _ | _ |
| Jensen 1998 | 55 | Ibuprofen 600 mg | Tablet, oral | _ | _ |
| Bednarek 2015 | 202 | Ibuprofen 800 mg | Tablet, oral | _ | _ |
| Chor 2012 | 87 | Ibuprofen 800 mg | Tablet, oral | 0 | _ |
| Massey 1974 | 58 | Naproxen 300 mg (x 4 doses) + paracervical block (1% lidocaine 8 mL) | Tablet, oral | NAa | _ |
| Ngo 2014 | 67 | Ketorolac 30 mg | Intramuscular injection | NA | NA |
| Lidocaine | |||||
| Maguire 2012 | 200 | 2% gel (1 mL) | Swab | NA | NA |
| Mohammad‐Alizadeh‐C 2010 | 96 | 2% gel | Swab | _ | _ |
| Allen 2013 | 150 | 2% gel (6 mL) | 2 sites, 3 mL each | NSc | NS |
| Nelson 2013 | 40 | 2% (1.2 mL) | Infused, 3 sites | _ | _ |
| McNicholas 2012 | 200 | 2% gel (2.5 to 4 mL) | 0.5 to 1 mL Topical; 2 to 3 mL inserted | NA | _ |
| Rapkin 2014 | 64 | 2% gel (5 mL) | Self‐inserted vaginally | _ | NA |
| Tornblom‐Paulander 2015 | 218 | 4% gel (8.5 mL) | Topical, 3 sites | NS | _ |
| Aksoy 2015 | 200 | 10% spray (40 mg) | Topical, 2 sites; 4 puffs total | NA | _ |
| Ahmadi Doulabi 2013 | 92 | EMLA cream 5% (lidocaine + prilocaine) (5 g) | Swab | _ | _ |
| Mody 2012 | 50 | 1% paracervical block (10 mL) | 2 sites, 5 mL each | NS | _ |
| Cırık 2013b | 95 | 1% paracervical block (10 mL) | 2 sites, 5 mL each | NS | _ |
| Castro 2014 | 100 | 2% intracervical block (1.8 mL) | 4 sites | _ | NS |
| Misoprostol | |||||
| Sääv 2007 | 80 | 400 μg + diclofenac | Sublingual | Shivering: misoprostol > placebo; Other: NS | NS |
| Ibrahim 2013 | 274 | 400 µg + diclofenac | sublingual | Headache or abdominal pain: misoprostol > placebo; Other: NS | Choose again or recommend to friend: misoprostol < control |
| Heikinheimo 2010 | 89 | 400 µg | Sublingual | NS | _ |
| Edelman 2011 | 40 | 400 µg | Buccal | NS | _ |
| Lathrop 2013 | 73 | 400 µg | Buccal | _ | NS |
| Espey 2014 | 83 | 400 µg | Buccal | NS | NS |
| Lotke 2013 | 61 | 400 μg | Vaginal or buccal | NS | NS |
| Swenson 2012 | 108 | 400 μg | Vaginal or buccal | NA | NS |
| Dijkhuizen 2011 | 270 | 400 μg | Vaginal | NS | _ |
| Scavuzzi 2013 | 190 | 400 μg | Vaginal | NS | Misoprostol > placebo |
| Other | |||||
| Bednarek 2013 | 24 | Nitroprusside gel 1% | 1 mL | NS | Satisfaction with pain control: NS; Satisfaction with procedure: nitroprusside < placebo |
| Micks 2014 | 24 | Nitroglycerin ointment 0.5 mg | 1 mL | NS | Satisfaction with pain control or procedure: NS |
| Karabayirli 2012 | 103 | Tramadol 50 mg | Tablet | None | NS |
| Shahnazi 2012 | 106 | Lavender 10 drops in diluted milk | 3 drops on cotton, inhaled | None | _ |
| Cameron 2013 | 200 | Delayed bladder emptying (after IUC insertion) | Oral (water 1 L) | _ | _ |
aNA = not available (assessed but not reported, or provided insufficient data) bExcluded from sensitivity analysis due to low quality evidence cNS = no significant difference between study arms
Non‐steroidal anti‐inflammatory drugs (NSAIDs)
Pain: at tenaculum placement, during IUC insertion, and after (up to 6 hours)
Seven trials examined non‐steroidal anti‐inflammatory drugs (NSAIDs): two of oral naproxen, four of oral ibuprofen, and one of ketorolac injected. All assessed pain during IUC insertion. Two recent trials also assessed pain at tenaculum placement. Three studies of varying age examined pain after IUC insertion.
Naproxen
Two trials compared different doses of naproxen versus placebo. Massey 1974 examined naproxen 300 mg, while Karabayirli 2012 used naproxen 550 mg. For Massey 1974, the medication was taken the night before and 90 minutes prior to IUC insertion. Both groups received a lidocaine paracervical block prior to IUD insertion. Two more doses of naproxen were scheduled for two and six hours after IUC insertion. Of 50 participants, 48 were nulliparous. The study groups did not differ significantly in mean pain score during or immediately after IUD insertion (Analysis 1.1). However, the naproxen group had lower mean pain scores than the placebo group at one and two hours after IUC insertion: mean difference (MD) ‐1.04 (95% CI ‐1.67 to ‐0.41; participants = 50) and MD ‐0.98 (95% CI ‐1.64 to ‐0.32; participants = 41), respectively (Analysis 1.2). Group means did not differ significantly at later time points (Analysis 1.2). Women could request additional pain medication, but were then considered to be dropouts. The investigators analyzed length of time in study without needing additional analgesia. Within the 24‐hour study period, 7 of 24 participants assigned to naproxen asked for additional medication compared with 17 of 26 in the placebo group (reported P = 0.01). Karabayirli 2012 administered 550 mg of naproxen one hour before IUD insertion. Participants were multiparous. The naproxen group had a lower mean for pain during IUD insertion compared with the placebo group (MD ‐1.94, 95% CI ‐2.35 to ‐1.53; participants = 68) (Analysis 2.1). This trial also studied tramadol; those results are shown in 'Other interventions' below.
1.1. Analysis.
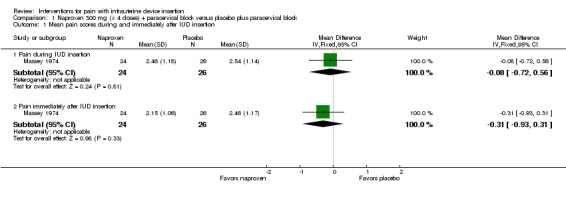
Comparison 1 Naproxen 300 mg (x 4 doses) + paracervical block versus placebo plus paracervical block, Outcome 1 Mean pain scores during and immediately after IUD insertion.
1.2. Analysis.
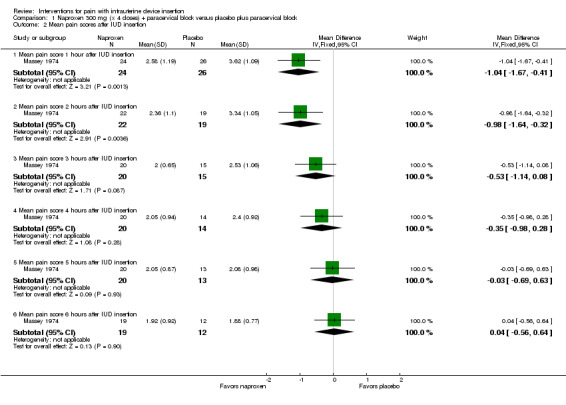
Comparison 1 Naproxen 300 mg (x 4 doses) + paracervical block versus placebo plus paracervical block, Outcome 2 Mean pain scores after IUD insertion.
2.1. Analysis.
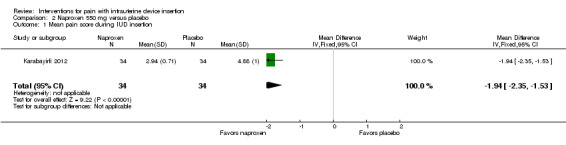
Comparison 2 Naproxen 550 mg versus placebo, Outcome 1 Mean pain score during IUD insertion.
Ibuprofen
Four trials examined ibuprofen (400 mg to 800 mg) versus placebo. Administration of 400 mg ibuprofen at least 45 minutes prior to IUD insertion had no effect on pain during IUD insertion (Hubacher 2006). Pain scores were not normally distributed. Median pain scores during IUD insertion were 1.0 for both the ibuprofen and control arms, using a 10 cm VAS (Analysis 3.1; participants = 2018). Increasing age, lower parity, longer time since last pregnancy, and no lactation were associated with increased pain, but ibuprofen was no more effective in any of those groups. Similarly, 600 mg of ibuprofen administered one to four hours prior to insertion did not show an effect on pain (Jensen 1998). The median pain scores for the ibuprofen and placebo groups at IUD insertion were 3.3 and 2.5 (Analysis 4.1; participants = 55). Of 27 women in the ibuprofen arm, 18 reported moderate to severe pain (3 or greater) compared with 14 women of 28 in the placebo arm (Analysis 4.2). Median pain scores after four to six hours did not differ significantly (Analysis 4.1). Women were allowed to take additional pain medication after IUD insertion if needed. Three women in the ibuprofen group and four in the placebo group did so, and the investigators excluded them from further analysis of pain data.
3.1. Analysis.
Comparison 3 Ibuprofen 400 mg versus placebo, Outcome 1 Median pain score during IUD insertion.
| Median pain score during IUD insertion | ||
|---|---|---|
| Study | Ibuprofen (N = 1010) | Control (N = 1008) |
| Hubacher 2006 | 1 | 1 |
4.1. Analysis.
Comparison 4 Ibuprofen 600 mg versus placebo, Outcome 1 Median pain scores.
| Median pain scores | |||
|---|---|---|---|
| Study | Time frame | Ibuprofen | Placebo |
| Jensen 1998 | At insertion | 3.3 | 2.5 |
| Jensen 1998 | After insertion, 4 to 6 hours | 1.7 | 1.8 |
4.2. Analysis.
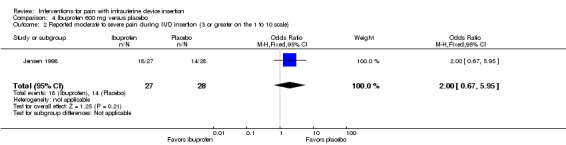
Comparison 4 Ibuprofen 600 mg versus placebo, Outcome 2 Reported moderate to severe pain during IUD insertion (3 or greater on the 1 to 10 scale).
The other two ibuprofen trials compared ibuprofen 800 mg versus placebo, administered 30 to 45 minutes prior to the procedure (Bednarek 2015; Chor 2012). The study arms did not differ significantly in mean scores for pain with tenaculum placement or during IUD insertion (Analysis 5.1; Analysis 5.2; participants = 81) (Chor 2012). Median scores for pain during IUD insertion reportedly did not differ significantly between the groups overall nor when the analysis was stratified by parity (Analysis 5.3; participants = 202) (Bednarek 2015).
5.1. Analysis.
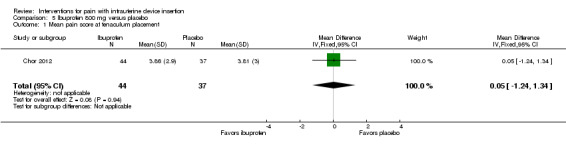
Comparison 5 Ibuprofen 800 mg versus placebo, Outcome 1 Mean pain score at tenaculum placement.
5.2. Analysis.
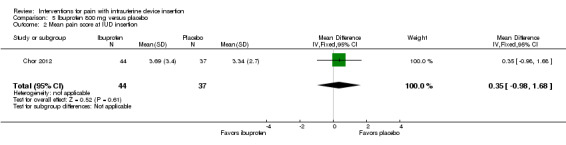
Comparison 5 Ibuprofen 800 mg versus placebo, Outcome 2 Mean pain score at IUD insertion.
5.3. Analysis.
Comparison 5 Ibuprofen 800 mg versus placebo, Outcome 3 Median pain score at IUD insertion.
| Median pain score at IUD insertion | ||||
|---|---|---|---|---|
| Study | Groups | Ibuprofen Median (range) | Placebo Median (range) | Reported P |
| Bednarek 2015 | All women | 38 (0 to 100) | 41.5 (0 to 100) | 0.5 |
| Bednarek 2015 | ‐ | N = 101 | N = 101 | ‐ |
| Bednarek 2015 | Nulliparous | 59 (0 to 97) | 60 (0 to 100) | 0.60 |
| Bednarek 2015 | ‐ | N = 37 | N = 36 | ‐ |
| Bednarek 2015 | Parous | 29 (1 to 100) | 34 (1 to 96) | 0.34 |
| Bednarek 2015 | ‐ | N = 64 | N = 65 | ‐ |
Ketorolac
For Ngo 2014, results came from a conference abstract. Ketorolac 30 mg was injected intramuscularly, 30 minutes before IUD insertion. The placebo was saline injection. The ketorolac group had a lower median pain score than the placebo group at five minutes after IUD insertion (1.1 versus 2.5; reported P = 0.003; participants = 67) (Analysis 6.1) and at 15 minutes after IUD insertion (0.6 versus 2.5; reported P < 0.001) (Analysis 6.1). The study arms did not differ significantly in mean scores during tenaculum placement or during IUD insertion (data not provided). Within the nulliparous subgroup, women treated with ketorolac had a lower median score for pain at IUD insertion (5.8 versus 8.2; reported P = 0.016) (Analysis 6.1).
6.1. Analysis.
Comparison 6 Ketorolac 30 mg versus placebo, Outcome 1 Median pain scores.
| Median pain scores | |||||
|---|---|---|---|---|---|
| Study | Group | Time point | Ketorolac (N = 33) | Placebo (N = 34) | Reported P |
| Ngo 2014 | All women | 5 min after insertion | 1.1 | 2.5 | 0.003 |
| Ngo 2014 | All women | 15 min after insertion | 0.6 | 2.5 | < 0.001 |
| Ngo 2014 | Nulliparous | At insertion | 5.8 | 8.2 | 0.016 |
Side effects or adverse events
Three NSAID trials had information on side effects. Two stated no adverse events occurred (Chor 2012; Karabayirli 2012) with ibuprofen and naproxen respectively. In Massey 1974, participants recorded a list of symptoms several times during the study of naproxen with lidocaine paracervical block. The report abstract noted no "untoward effects" occurred.
Satisfaction
One NSAID trial with multiparous women had satisfaction data. For Karabayirli 2012, women in the naproxen group were less likely to report the insertion as 'unpleasant' compared with those in the placebo group (OR 0.02, 95% CI 0.01 to 0.09; participants = 68) (Analysis 2.2). The women who received naproxen were also less likely to "not prefer" the medication for future IUD insertion (OR 0.02, 95% CI 0.00 to 0.08; participants = 68) (Analysis 2.2).
2.2. Analysis.
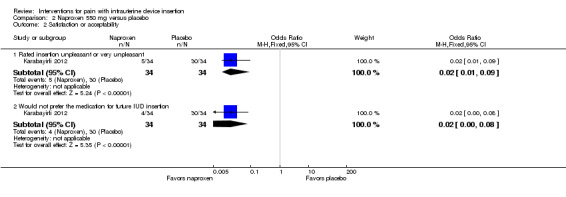
Comparison 2 Naproxen 550 mg versus placebo, Outcome 2 Satisfaction or acceptability.
Lidocaine
Pain: at tenaculum placement, during IUC insertion, and after (up to 6 hours)
Of 12 trials, 8 assessed pain at tenaculum placement and 11 measured pain during IUC insertion. Five reported on pain after IUC insertion, from five minutes to six hours post‐procedure.
2% gel versus placebo (topical)
Five trials compared a 2% gel versus placebo; one also included a group with no intervention. We combined three studies in meta‐analysis (Allen 2013; Maguire 2012; Mohammad‐Alizadeh‐C 2010). Mohammad‐Alizadeh‐C 2010 and Maguire 2012 applied the gel with a swab, one minute before tenaculum placement or sounding the uterus respectively. Three minutes before tenaculum placement, Allen 2013 used a syringe to apply 3 mL of gel and insert another 3 mL. In meta‐analysis with two of the trials, mean pain at tenaculum placement did not differ significantly between the groups (Analysis 7.1; participants = 345). Pain during IUD insertion did not differ significantly in meta‐analysis of the three trials (Analysis 7.2; participants = 409). Other analyses within individual trials showed that the study arms did not differ significantly, including mean pain scores at 20 minutes after IUD insertion (Analysis 8.1; participants = 145) (Allen 2013). When the analysis was stratified by parity in Maguire 2012, mean scores for pain during IUD insertion did not differ significantly by study arm (reported P = 0.87 for nulliparous; reported P = 0.39 for parous; participants = 200). One trial also compared the 2% gel with no intervention; the study arms did not differ significantly for pain scores during IUD insertion (Analysis 9.1; participants = 63) (Mohammad‐Alizadeh‐C 2010).
7.1. Analysis.
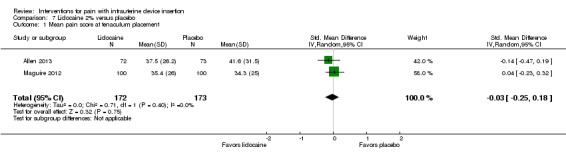
Comparison 7 Lidocaine 2% versus placebo, Outcome 1 Mean pain score at tenaculum placement.
7.2. Analysis.
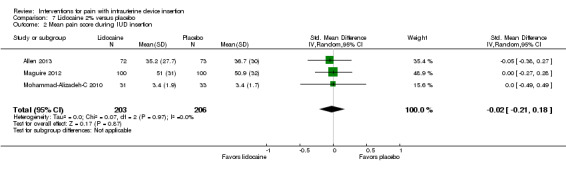
Comparison 7 Lidocaine 2% versus placebo, Outcome 2 Mean pain score during IUD insertion.
8.1. Analysis.
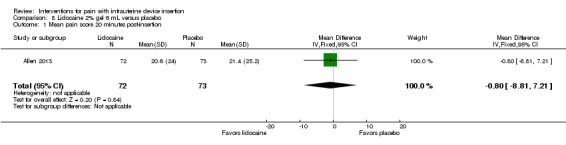
Comparison 8 Lidocaine 2% gel 6 mL versus placebo, Outcome 1 Mean pain score 20 minutes post‐insertion.
9.1. Analysis.
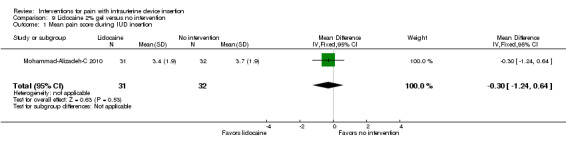
Comparison 9 Lidocaine 2% gel versus no intervention, Outcome 1 Mean pain score during IUD insertion.
Two trials reported median pain scores (McNicholas 2012; Rapkin 2014). McNicholas 2012 applied 0.5 to 1 mL of gel topically and inserted 2 to 3 mL with an angiocatheter, three minutes before starting the IUD insertion. Pain scores were not normally distributed. The study groups did not differ significantly in median pain scores at tenaculum placement or during IUD insertion (Analysis 10.1; participants = 200). When stratified by parity, the study arms did not differ significantly either (Analysis 10.2). In Rapkin 2014, participants self administered 5 mL of gel vaginally at least five minutes prior to IUD insertion. Results were in a conference abstract. Participants were nulliparous. The median difference in pain between baseline and tenaculum placement was lower for the lidocaine group compared with the placebo group (32 versus 56; reported P = 0.030; participants = 64) (Analysis 10.3). For median difference in pain between baseline and IUD insertion, the groups were not significantly different (Analysis 10.3).
10.1. Analysis.
Comparison 10 Lidocaine 2% gel, 2.5 to 5 mL, versus placebo, Outcome 1 Median pain scores.
| Median pain scores | ||||
|---|---|---|---|---|
| Study | Time period | Lidocaine (N = 100) Median (range) | Placebo (N = 99) Median (range) | Reported P |
| McNicholas 2012 | At tenaculum placement | 4 (0 to 10) | 4 (0 to 10) | 0.15 |
| McNicholas 2012 | During IUD insertion | 5 (0 to 10) | 6 (0 to 10) | 0.16 |
10.2. Analysis.
Comparison 10 Lidocaine 2% gel, 2.5 to 5 mL, versus placebo, Outcome 2 Median pain scores by parity.
| Median pain scores by parity | |||||
|---|---|---|---|---|---|
| Study | Parity | Time period | Lidocaine Median (range) | Placebo Median (range) | Reported P |
| McNicholas 2012 | Nulliparous | Tenaculum placement | 4 (0 to 10) | 4 (0 to 10) | 0.54 |
| McNicholas 2012 | Nulliparous | IUD insertion | 6 (2 to 10) | 6 (2 to 10) | 0.18 |
| McNicholas 2012 | Parous | Tenaculum placement | 3 (0 to 9) | 4 (0 to 10) | 0.23 |
| McNicholas 2012 | Parous | IUD insertion | 4 (1 to 10) | 5 (0 to 9) | 0.72 |
10.3. Analysis.
Comparison 10 Lidocaine 2% gel, 2.5 to 5 mL, versus placebo, Outcome 3 Median difference in pain scores from baseline.
| Median difference in pain scores from baseline | ||||
|---|---|---|---|---|
| Study | Time period | Lidocaine | Placebo | Reported P |
| Rapkin 2014 | Tenaculum placement | 32 mm | 56 mm | 0.030 |
| Rapkin 2014 | IUD insertion | 61 mm | 68 mm | 0.133 |
Other lidocaine formulations versus placebo (topical or infused)
Four trials used other lidocaine formulations or application methods. Two studies examined a novel cream or gel versus placebo (Ahmadi Doulabi 2013; Tornblom‐Paulander 2015). Tornblom‐Paulander 2015 used a short‐acting 4% lidocaine gel. Participants were nulliparous. Using an applicator at five minutes before IUC insertion, the investigators applied 8 mL total (on surface of portio, in cervical canal, and into uterine cavity). Compared with the placebo group, the lidocaine group had lower mean scores for pain with IUC insertion as assessed within 10 minutes (MD ‐15.90, 95% CI ‐22.77 to ‐9.03; participants = 209) (Analysis 11.1) and for pain at 30 minutes post‐insertion (MD ‐11.10, 95% CI ‐19.05 to ‐3.15; participants = 114) (Analysis 11.2). Mean pain scores did not differ significantly at one hour post‐procedure (Analysis 11.3; participants = 208). Women in the lidocaine group were less likely than those in the placebo group to receive additional analgesic while in the clinic (OR 0.42, 95% CI 0.21 to 0.80; participants = 218) (Analysis 11.4). Ahmadi Doulabi 2013 examined EMLA cream, consisting of 2.5% lidocaine and 2.5% prilocaine. Investigators applied 5 grams on the cervix and cervical opening, seven minutes before IUD insertion. The EMLA group had a lower mean pain score for tenaculum use compared with the placebo group (MD ‐2.78, 95% CI ‐3.66 to ‐1.90; participants = 92) (Analysis 12.1). Mean pain score related to IUC insertion was assessed after removing the insertion tube. The EMLA group had a lower pain score than the placebo group (MD ‐1.96, 95% CI ‐3.00 to ‐0.92; participants = 92) (Analysis 12.2).
11.1. Analysis.
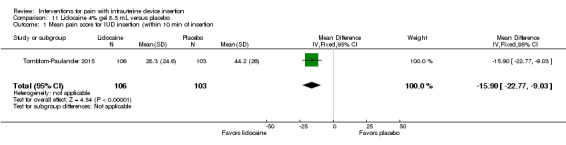
Comparison 11 Lidocaine 4% gel 8.5 mL versus placebo, Outcome 1 Mean pain score for IUD insertion (within 10 min of insertion.
11.2. Analysis.
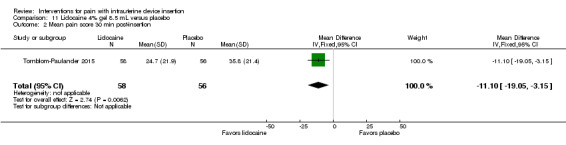
Comparison 11 Lidocaine 4% gel 8.5 mL versus placebo, Outcome 2 Mean pain score 30 min post‐insertion.
11.3. Analysis.
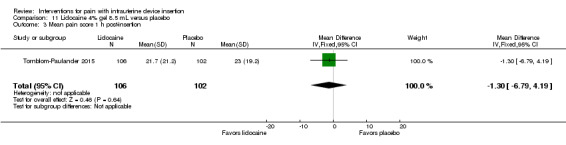
Comparison 11 Lidocaine 4% gel 8.5 mL versus placebo, Outcome 3 Mean pain score 1 h post‐insertion.
11.4. Analysis.
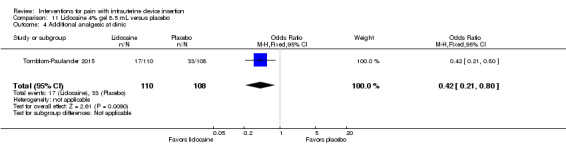
Comparison 11 Lidocaine 4% gel 8.5 mL versus placebo, Outcome 4 Additional analgesic at clinic.
12.1. Analysis.
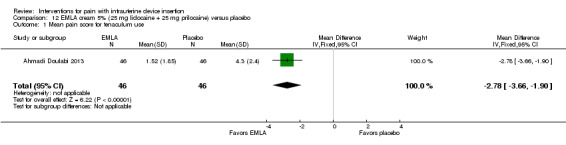
Comparison 12 EMLA cream 5% (25 mg lidocaine + 25 mg prilocaine) versus placebo, Outcome 1 Mean pain score for tenaculum use.
12.2. Analysis.
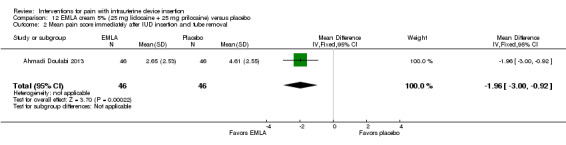
Comparison 12 EMLA cream 5% (25 mg lidocaine + 25 mg prilocaine) versus placebo, Outcome 2 Mean pain score immediately after IUD insertion and tube removal.
Two trials compared a different application method, i.e., infusion or spray, versus saline as placebo (Aksoy 2015; Nelson 2013). Nelson 2013 examined intrauterine infusion of 2% lidocaine. Three minutes before IUD insertion, the investigator infused 1.2 mL into the endometrial cavity (lower third, middle, and top of cavity). The study arms did not differ significantly in mean pain scores at tenaculum placement (Analysis 13.1), with IUD insertion (Analysis 13.2), or for "global score" at end of visit (Analysis 13.3) (participants = 40 for each analysis). In Nelson 2013, 11 women took NSAIDs prior to enrollment, but pain scores did not differ by NSAID use (Analysis 13.4). Aksoy 2015 used a 10% lidocaine spray; the participants were parous. Three minutes before tenaculum placement, investigators administered three puffs to the cervical surface and one puff towards the cervical os for a total of 40 mg. Median score for pain during IUD insertion was lower for the lidocaine group than for the placebo group (1.00 versus 3.00; reported P < 0.001; participants = 200) (Analysis 14.1).
13.1. Analysis.
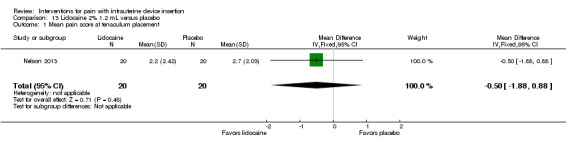
Comparison 13 Lidocaine 2% 1.2 mL versus placebo, Outcome 1 Mean pain score at tenaculum placement.
13.2. Analysis.
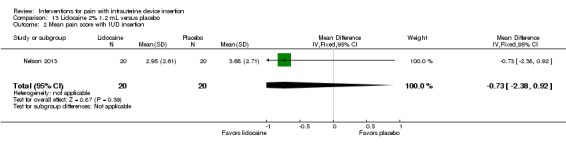
Comparison 13 Lidocaine 2% 1.2 mL versus placebo, Outcome 2 Mean pain score with IUD insertion.
13.3. Analysis.
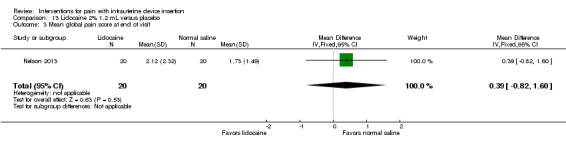
Comparison 13 Lidocaine 2% 1.2 mL versus placebo, Outcome 3 Mean global pain score at end of visit.
13.4. Analysis.
Comparison 13 Lidocaine 2% 1.2 mL versus placebo, Outcome 4 Mean pain scores by NSAID intake.
| Mean pain scores by NSAID intake | ||||
|---|---|---|---|---|
| Study | Study group | NSAID | No NSAID | Reported P |
| Nelson 2013 | Overall | 3.89 | 3.25 | < 0.76 |
| Nelson 2013 | Lidocaine | 3.8a | ‐ | ‐ |
| Nelson 2013 | Saline | ‐ | 3.7b | 0.86 (a versus b) |
14.1. Analysis.
Comparison 14 Lidocaine 10% spray, 40 mg, versus placebo, Outcome 1 Median pain scores.
| Median pain scores | ||||
|---|---|---|---|---|
| Study | Time period | Lidocaine (N = 100) Median (range) | Placebo (N = 100) Median (range) | Reported P |
| Aksoy 2015 | During IUD insertion | 1.00 (0 to 6) | 3.00 (0 to 7) | < 0.001 |
1% paracervical or 2% intracervical block
Three trials injected lidocaine; the comparisons were no intervention, placebo, or oral ibuprofen. Two trials compared a paracervical block of 1% lidocaine (10 mL) to no intervention (control) (Cırık 2013; Mody 2012) or to saline as placebo (Cırık 2013). The intervention occurred three minutes before starting the IUC insertion (Mody 2012) or five minutes before IUC insertion (Cırık 2013). In Mody 2012, the lidocaine group had a lower median pain score at tenaculum placement compared with the no‐intervention group (12 versus 28; reported P = 0.008; participants = 50) (Analysis 15.1). The study arms did not differ significantly for pain with IUD insertion or at five minutes after the procedure (Analysis 15.1). In Cırık 2013, median pain scores were lower with lidocaine compared with saline placebo or no intervention at tenaculum placement (4 versus 7), at IUD insertion (2 versus 6), and at five minutes post‐procedure (1 versus 4) (reported P < 0.01; participants = 95) (Analysis 15.1).
15.1. Analysis.
Comparison 15 Lidocaine 1% 10 mL paracervical block versus no paracervical block, Outcome 1 Median pain scores.
| Median pain scores | |||||
|---|---|---|---|---|---|
| Study | Time period and scale | Lidocaine 1% paracervical block | Placebo (saline injection) | No intervention | Reported P |
| Cırık 2013 | VAS 0 to 10 | N = 34 | N = 30 | N = 31 | ‐ |
| Cırık 2013 | Immediately after tenaculum placement | 4 (range 0 to 6) | 7 (range 4 to 9) | 7 (range 5 to 8) | < 0.01 |
| Cırık 2013 | Immediately after IUD insertion | 2 (range 0 to 5) | 6 (range 2 to 7) | 6 (range 3 to 7) | < 0.01 |
| Cırık 2013 | 5 min after procedure | 1 (range 0 to 4) | 4 (range 1 to 6) | 4 (range 1 to 6) | < 0.01 |
| Mody 2012 | Scale 0 to 100 | N = 26 | ‐ | N = 24 | ‐ |
| Mody 2012 | At tenaculum placement | 12 (1st, 3rd quartiles: 4, 27) | _ | 28 (1st, 3rd quartiles: 14.5, 40.5) | 0.008 |
| Mody 2012 | At IUD insertion | 24 (1st, 3rd quartiles: 3, 73) | _ | 62 (1st, 3rd quartiles: 8, 77) | 0.09 |
| Mody 2012 | 5 min after procedure | 12 (1st, 3rd quartiles: 2, 25) | _ | 17 (1st, 3rd quartiles: 3, 35) | 0.72 |
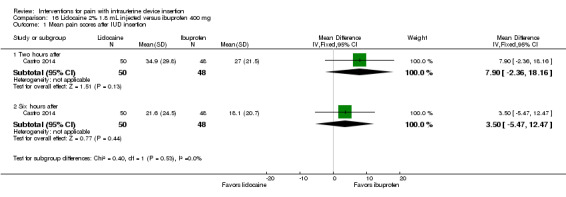
Castro 2014 compared an intracervical block of 2% lidocaine (1.8 mL), five minutes before LNG‐IUS insertion, versus oral ibuprofen 400 mg at one hour prior to LNG‐IUS insertion. Participants were nulliparous or without a previous vaginal delivery. For pain immediately after IUS insertion, the investigators analyzed the VAS scores as mild (0 to 30 mm), moderate (40 to 60 mm), or severe (70 to 100 mm). Presumably these categories were only for pain immediately after IUS insertion, as means were not shown for that time point but were available for all others. The study arms did not differ significantly in mean pain assessed at two and six hours after IUS insertion (participants = 98; Analysis 16.1). The investigators also grouped the VAS scores as mild, moderate, or severe pain. The proportions reporting moderate or severe pain did not differ significantly (Analysis 16.2).
16.1. Analysis.
Comparison 16 Lidocaine 2% 1.8 mL injected versus ibuprofen 400 mg, Outcome 1 Mean pain scores after IUD insertion.
16.2. Analysis.
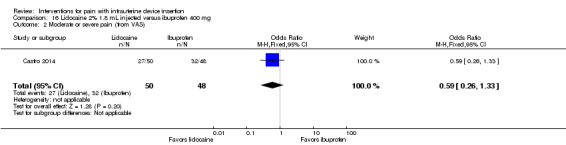
Comparison 16 Lidocaine 2% 1.8 mL injected versus ibuprofen 400 mg, Outcome 2 Moderate or severe pain (from VAS).
Side effects or adverse events
Six trials, four of which studied lidocaine gel formulations, provided varying information on side effects or adverse events. In a trial of 2% lidocaine gel, Allen 2013 assessed nausea and dizziness with four‐point scales. The study arms did not differ significantly in the proportions with moderate or severe nausea or who were moderately or severely dizzy (participants = 145; Analysis 8.2). Complications were one vasovagal reaction and one IUD inadvertently pulled out with scissors and replaced (groups not specified). Two other studies of 2% lidocaine gel provided limited information. In McNicholas 2012, adverse events were reported over six months and included five expulsions (one for lidocaine and four for placebo), as well as one perforation and one case of pelvic inflammatory disease (groups not specified). Maguire 2012 stated the groups were "highly similar" for side effects, e.g., nausea, vomiting, and dizziness. In Tornblom‐Paulander 2015, the proportions of women with at least one adverse event did not differ significantly between the 4% lidocaine group and the placebo group (participants = 218; Analysis 11.5).
8.2. Analysis.
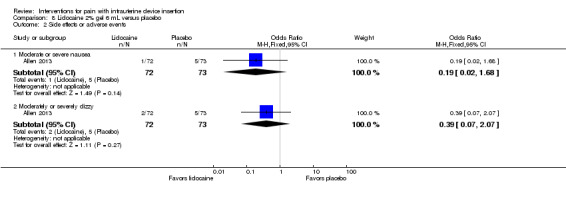
Comparison 8 Lidocaine 2% gel 6 mL versus placebo, Outcome 2 Side effects or adverse events.
11.5. Analysis.
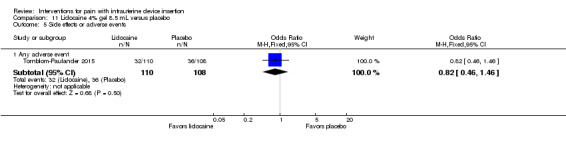
Comparison 11 Lidocaine 4% gel 8.5 mL versus placebo, Outcome 5 Side effects or adverse events.
Two studies of lidocaine paracervical block reported on side effects. In Mody 2012, vasovagal symptoms and bleeding did not differ significantly between the group with paracervical block and the group with no intervention (participants = 50; Analysis 15.2). No vasovagal syncope occurred, nor any uterine perforation. The study groups in Cırık 2013 did not differ significantly for complications (paracervical block of lidocaine or of saline or no intervention). Vasovagal syncope occurred in five participants (reported P = 0.36; participants = 95) (Analysis 15.3). The groups were similar for vasovagal symptoms, e.g., nausea and vomiting (reported P = 0.06). No bleeding or uterine perforation occurred.
15.2. Analysis.
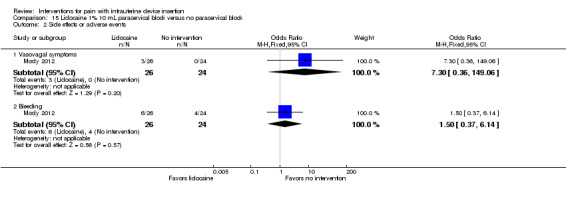
Comparison 15 Lidocaine 1% 10 mL paracervical block versus no paracervical block, Outcome 2 Side effects or adverse events.
15.3. Analysis.
Comparison 15 Lidocaine 1% 10 mL paracervical block versus no paracervical block, Outcome 3 Vasovagal syncope.
| Vasovagal syncope | ||||
|---|---|---|---|---|
| Study | Lidocaine 1% paracervical block | Placebo (saline 0.9% injection) | Control (no intervention) | Reported P |
| Cırık 2013 | N = 34 | N = 30 | N = 31 | ‐ |
| Cırık 2013 | 1 | 1 | 2 | 0.36 |
Satisfaction or acceptability
Of four trials of lidocaine 2% that assessed satisfaction or acceptability, two had comparative data. Allen 2013 assessed acceptability with a five‐point scale. The lidocaine gel group did not differ significantly from the placebo group in the proportion finding the pain during IUD insertion to be mostly or completely acceptable (participants = 143; Analysis 8.3). In Castro 2014, the group with lidocaine injected did not differ significantly from the ibuprofen group in the proportion rating the experience as uncomfortable or very uncomfortable (participants = 98; Analysis 16.3). Two trials provided percentages for satisfaction overall rather than by study arm (Maguire 2012; Rapkin 2014).
8.3. Analysis.
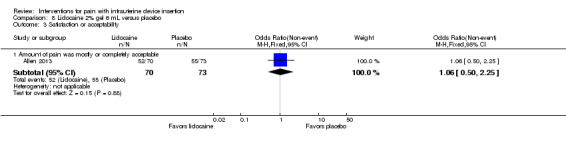
Comparison 8 Lidocaine 2% gel 6 mL versus placebo, Outcome 3 Satisfaction or acceptability.
16.3. Analysis.
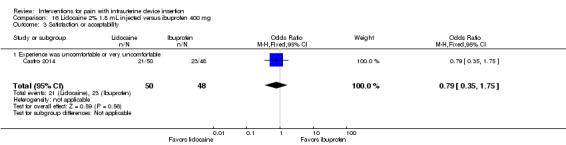
Comparison 16 Lidocaine 2% 1.8 mL injected versus ibuprofen 400 mg, Outcome 3 Satisfaction or acceptability.
Misoprostol
Pain: at tenaculum placement, during IUC insertion, and after (up to 6 hours)
All 10 trials assessed pain during IUC insertion. One measured pain at tenaculum placement. Four assessed pain after IUC insertion, ranging from five minutes to before clinic departure.
Misoprostol plus diclofenac versus diclofenac
Two trials compared misoprostol 400 µg sublingually plus diclofenac versus diclofenac alone at one hour before IUC insertion (Ibrahim 2013; Sääv 2007). Median pain scores for the misoprostol and control groups at the time of insertion did not differ significantly in either study (255 and 59 participants respectively; Analysis 17.1). Further, the misoprostol groups in these trials had the same median and range as did the placebo groups. Participants in Sääv 2007 were nulliparous.
17.1. Analysis.
Comparison 17 Misoprostol 400 µg + diclofenac 100 mg versus diclofenac 100 mg alone, Outcome 1 Median pain score at IUD insertion.
| Median pain score at IUD insertion | |||
|---|---|---|---|
| Study | Misoprostol + diclofenac | Diclofenac | Reported P |
| Ibrahim 2013 | 7 (range 2.5 to 10) | 6.5 (range 0 to 10) | 0.8 |
| Ibrahim 2013 | N = 130 | N = 125 | ‐ |
| Sääv 2007 | 7 (range 2.5 to 10) | 6.5 (range 0 to 10) | 0.20 |
| Sääv 2007 | N = 29 | N = 30 | ‐ |
Misoprostol versus placebo
Eight trials compared misoprostol 400 µg versus placebo. Participants were instructed to administer the medication via a specific route and at a specific time prior to IUC insertion: vaginally at three hours (Dijkhuizen 2011) or four hours (Scavuzzi 2013); sublingually at three hours (Heikinheimo 2010); buccally at 90 minutes (Edelman 2011), two to four hours (Lathrop 2013), two to eight hours (Espey 2014); or either buccally or vaginally at three to four hours (Swenson 2012) or at two hours (Lotke 2013).
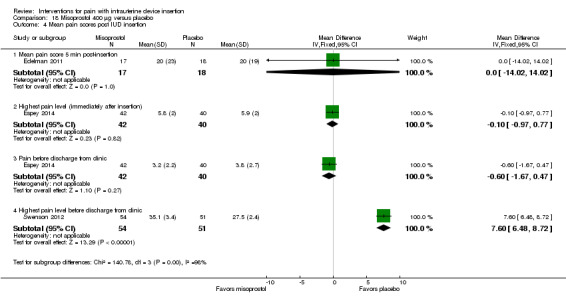
We combined four trials in a meta‐analysis (Dijkhuizen 2011; Edelman 2011; Lotke 2013; Swenson 2012). The misoprostol group had a higher mean score for pain during IUD insertion (standardized mean difference (SMD) 0.27, 95% CI 0.07 to 0.46; participants = 400) (Analysis 18.2). When the analysis was stratified by parity in Dijkhuizen 2011, mean scores for pain during IUD insertion did not differ significantly by study arm (participants = 199; Analysis 18.3). Participants in Edelman 2011 and Lotke 2013 were nulliparous. In Edelman 2011, the study arms did not differ significantly for pain with tenaculum placement (participants = 35; Analysis 18.1) or pain five minutes after IUD insertion (Analysis 18.4). In Swenson 2012, mean score for highest pain before discharge was higher for the misoprostol group (MD 7.60, 95% CI 6.48 to 8.72; participants = 105) (Analysis 18.4). The women had no pregnancy of 14 weeks or longer.
18.2. Analysis.
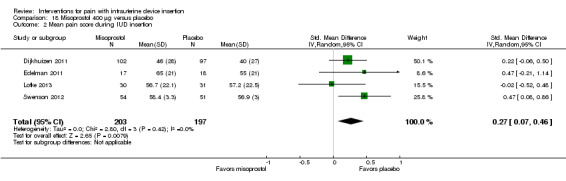
Comparison 18 Misoprostol 400 μg versus placebo, Outcome 2 Mean pain score during IUD insertion.
18.3. Analysis.
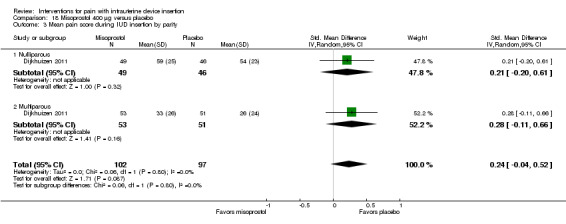
Comparison 18 Misoprostol 400 μg versus placebo, Outcome 3 Mean pain score during IUD insertion by parity.
18.1. Analysis.
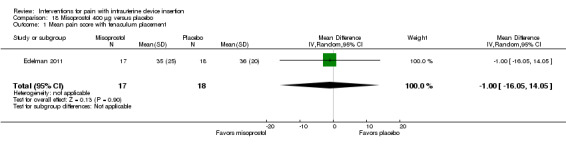
Comparison 18 Misoprostol 400 μg versus placebo, Outcome 1 Mean pain score with tenaculum placement.
18.4. Analysis.
Comparison 18 Misoprostol 400 μg versus placebo, Outcome 4 Mean pain scores post IUD insertion.
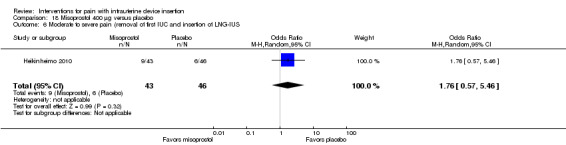
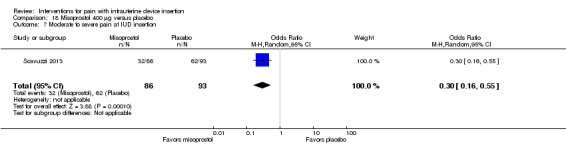
The other four trials of misoprostol 400 µg versus placebo had varied outcome measures and analyses (Espey 2014; Heikinheimo 2010; Lathrop 2013; Scavuzzi 2013). Participants were nulliparous in Espey 2014 and Scavuzzi 2013. In Espey 2014, the groups did not differ significantly for mean score for "highest pain level," assessed immediately after IUD insertion (after instrument removal) or mean pain score before discharge from clinic (participants = 82; Analysis 18.4). After IUC insertion, most women in Espey 2014 took additional pain medication: 68% of misoprostol group; 65% of placebo group). Of the misoprostol group, 8% took NSAIDs prior to IUC insertion, as did 5% of the placebo group. In Lathrop 2013, the misoprostol group had a higher median score for pain immediately after IUD insertion (46 versus 34; reported P = 0.044) and prior to discharge from clinic (35.5 versus 20.5; reported P = 0.024) (participants = 73; Analysis 18.5). The women had no pregnancy of 20 weeks or longer. Two trials reported pain scores as categorical variables. Heikinheimo 2010 apparently used four categories. Participants had the IUC removed and then the LNG‐IUS inserted. The groups did not differ significantly in pain assessments (participants = 89; Analysis 18.6). Scavuzzi 2013 used a scale of 0 to 10, but analyzed pain dichotomously as absent or mild (0 to 5) versus moderate or severe (6 to 10). Women in the misoprostol group were less likely to have moderate or severe pain compared with those in the placebo group (OR 0.30, 95% CI 0.16 to 0.55; participants = 179) (Analysis 18.7).
18.5. Analysis.
Comparison 18 Misoprostol 400 μg versus placebo, Outcome 5 Median pain scores.
| Median pain scores | ||||
|---|---|---|---|---|
| Study | Time frame | Misoprostol (N = 37) | Placebo (N = 36) | Reported P |
| Lathrop 2013 | Immediately after insertion | 46 (range 11 to 92) | 34 (range 0 to 90) | 0.044 |
| Lathrop 2013 | Before discharge from clinic | 35.5 (range 1 to 100) | 20.5 (range 0 to 86) | 0.024 |
18.6. Analysis.
Comparison 18 Misoprostol 400 μg versus placebo, Outcome 6 Moderate to severe pain (removal of first IUC and insertion of LNG‐IUS.
18.7. Analysis.
Comparison 18 Misoprostol 400 μg versus placebo, Outcome 7 Moderate to severe pain at IUD insertion.
Side effects or adverse events
Misoprostol plus diclofenac versus diclofenac
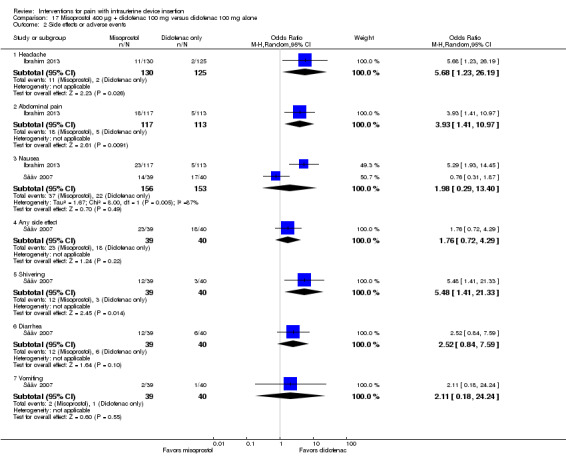
The two studies of misoprostol plus diclofenac reported side effects. In Sääv 2007, the study groups did not differ significantly for "any side effect," which included shivering, diarrhea, nausea, and vomiting (Analysis 17.2). The only side effect that differed significantly between the two groups was shivering (OR 5.48, 95% CI 1.41 to 21.33; participants = 79) (Analysis 17.2). Side effects were measured after IUD insertion. For Ibrahim 2013, women in the misoprostol group were more likely than those in the control group to report having had a headache (OR 5.68, 95% CI 1.23 to 26.19; participants = 255) or abdominal pain (OR 3.93, 95% CI 1.41 to 10.97; participants = 230) (Analysis 17.2). While the misoprostol group was also more likely to have nausea in Ibrahim 2013, the meta‐analysis with Sääv 2007 did not show a significant difference (participants = 308; Analysis 17.2).
17.2. Analysis.
Comparison 17 Misoprostol 400 µg + diclofenac 100 mg versus diclofenac 100 mg alone, Outcome 2 Side effects or adverse events.
Misoprostol versus placebo
Of eight trials with this comparison, six provided data on side effects. A meta‐analysis of four trials showed women in the misoprostol group were more likely to report abdominal cramping than those in the placebo group (OR 2.64, 95% CI 1.46 to 4.76; participants = 466) (Analysis 18.8). None of the other side effects differed significantly between the groups, including results of various meta‐analyses (Analysis 18.8). Two trials did not have data on side effects or complications. Swenson 2012 assessed side effects one week after IUD insertion but did not provide results. Lotke 2013 did not gather data on specific side effects.
18.8. Analysis.
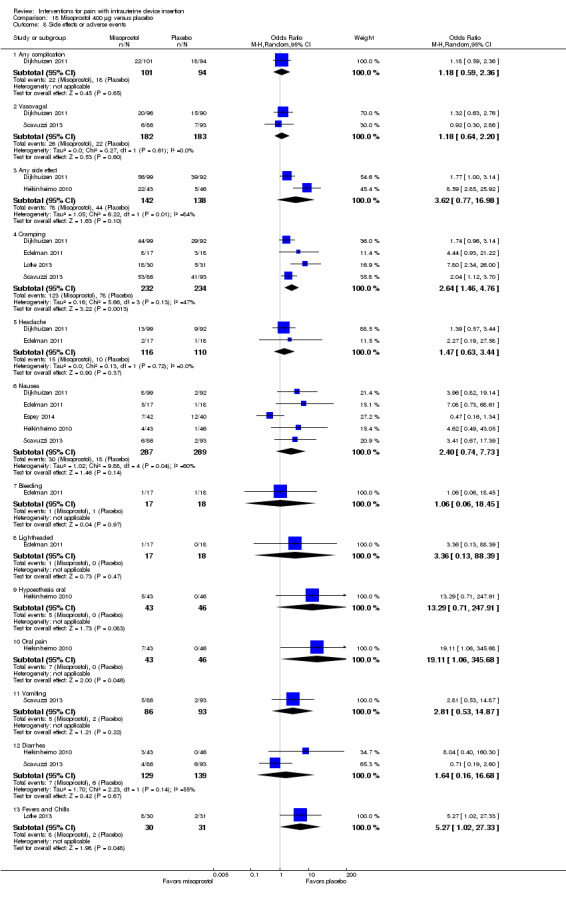
Comparison 18 Misoprostol 400 μg versus placebo, Outcome 8 Side effects or adverse events.
Satisfaction
Misoprostol plus diclofenac versus diclofenac
For these two studies, the study arms did not differ significantly for being "satisfied" with the insertion experience (participants = 255; Analysis 17.3) (Ibrahim 2013) or for rating the insertion experience as "very little unpleasant" (participants = 79; Analysis 17.3) (Sääv 2007). However, in Ibrahim 2013, the group with misoprostol plus diclofenac was less likely than the diclofenac‐only group to choose the treatment again (OR 0.30, 95% CI 0.14 to 0.65; participants = 255) or to recommend it to a friend (OR 0.36, 95% CI 0.16 to 0.81; participants = 255) (Analysis 17.3).
17.3. Analysis.
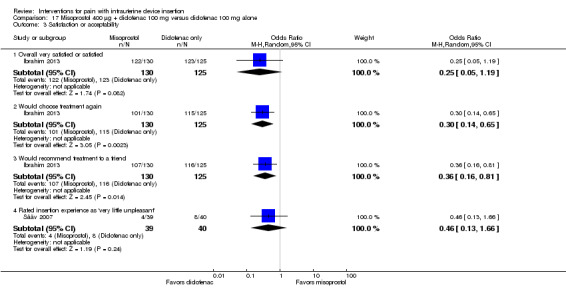
Comparison 17 Misoprostol 400 µg + diclofenac 100 mg versus diclofenac 100 mg alone, Outcome 3 Satisfaction or acceptability.
Misoprostol versus placebo
Five of these eight trials had data on satisfaction. Women in the misoprostol group were more likely to rate the IUD insertion experience as slightly disagreeable or not disagreeable in Scavuzzi 2013 (OR 4.34, 95% CI 2.32 to 8.12; participants = 179) (Analysis 18.9). At one week after IUC insertion, the study groups did not differ significantly for satisfaction in Lathrop 2013 (participants = 73; Analysis 18.9) nor for likelihood of having another IUD inserted in Swenson 2012 (participants = 102; Analysis 18.9). The study arms also did not differ in the likelihood of recommending IUD insertion to a friend in meta‐analysis (participants = 167; Analysis 18.9).
18.9. Analysis.
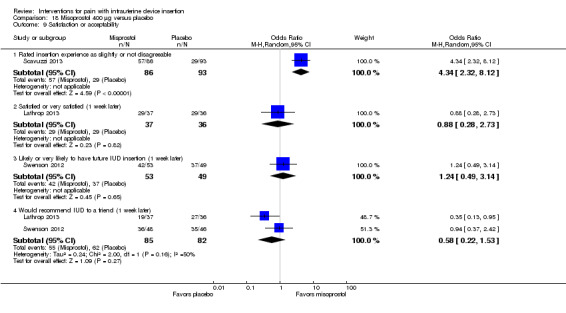
Comparison 18 Misoprostol 400 μg versus placebo, Outcome 9 Satisfaction or acceptability.
The remaining two trials assessed satisfaction one to two weeks after IUC insertion (Espey 2014; Lotke 2013). The reports had percentages for each study arm and P values but no actual counts (Analysis 18.10). Reportedly, the study arms did not differ significantly for satisfaction in either study, for pain not influencing future IUD use in Espey 2014 (participants = 83), and for definitely recommending IUD use to a friend in Lotke 2013 (participants = 61).
18.10. Analysis.
Comparison 18 Misoprostol 400 μg versus placebo, Outcome 10 Satisfaction or acceptability (1 week later).
| Satisfaction or acceptability (1 week later) | ||||
|---|---|---|---|---|
| Study | Outcome | Misoprostol | Placebo | Reported P |
| Espey 2014 | Pain would not influence future IUD use (at 1 to 2 weeks) | 65% | 65% | > 0.99 |
| Espey 2014 | Satisfied or very satisfied with using IUD (at 1 to 2 weeks) | 98% | 88% | 0.12 |
| Lotke 2013 | Satisfied or very satisfied with their IUD (at 1 week) | 77% | 84% | 0.94 |
| Lotke 2013 | Would definitely recommend IUD to a friend (at 1 month) | 87% | 87% | 0.61 |
Other interventions
Pain: at tenaculum placement, during IUC insertion, and after (up to six hours)
These five trials assessed pain during IUC insertion. However, only the studies of nitric oxide donors reported on pain at tenaculum placement and pain after IUC insertion (30 minutes post‐procedure).
Two pilot studies examined nitric oxide donors versus placebo among nulliparous women. The experimental interventions were nitroprusside 10 mg as 1% aqueous gel administered immediately prior to IUC insertion (Bednarek 2013) and 1 mL of 0.5 mg nitroglycerin ointment at 30 to 45 minutes before the procedure (Micks 2014). Bednarek 2013 showed no significant difference between the groups in pain scores at tenaculum placement (participants = 23; Analysis 19.1). Meta‐analysis of these two small trials showed no effect of the intervention on pain during IUD insertion (participants = 47; Analysis 19.2). In other analyses, the groups did not differ significantly in Bednarek 2013 for pain 30 minutes after IUD insertion (Analysis 19.3). Micks 2014 also reported no significant difference in pain at those time points; results were shown in a figure without actual values.
19.1. Analysis.
Comparison 19 Nitric oxide donors versus placebo, Outcome 1 Mean pain score at tenaculum placement.
19.2. Analysis.
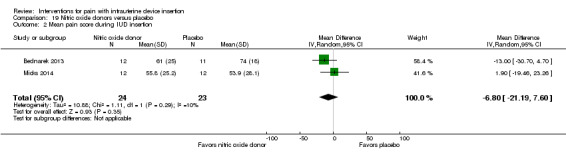
Comparison 19 Nitric oxide donors versus placebo, Outcome 2 Mean pain score during IUD insertion.
19.3. Analysis.
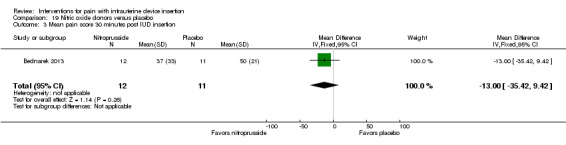
Comparison 19 Nitric oxide donors versus placebo, Outcome 3 Mean pain score 30 minutes post IUD insertion.
Among multiparous women, Karabayirli 2012 compared tramadol 50 mg versus naproxen 500 mg, administered one hour before IUD insertion. Results for naproxen versus placebo are shown in the section on NSAIDs. The tramadol group had a lower mean score than the naproxen group for pain during IUD insertion (MD ‐0.63, 95% CI ‐0.94 to ‐0.32; participants = 69) (Analysis 20.1).
20.1. Analysis.
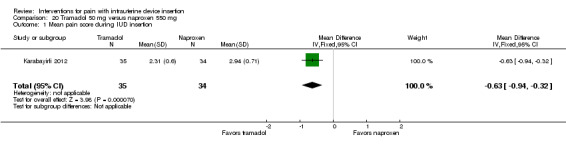
Comparison 20 Tramadol 50 mg versus naproxen 550 mg, Outcome 1 Mean pain score during IUD insertion.
Two trials had non‐pharmacological interventions. Shahnazi 2012 used lavender oil, three drops on cotton, inhaled 30 minutes before and during the procedure. Women in the lavender group were no more likely than those in the placebo group to have medium or severe pain scores after the intervention (participants = 106; Analysis 21.1). Median pain scores and interquartile ranges were the same for both study groups (Analysis 21.2). In Cameron 2013, the participants consumed one liter of water an hour before the appointment. The investigators examined the effect of bladder emptying before IUD insertion (immediate) versus after IUD insertion (delayed). The study arms did not show a difference in pain during IUD insertion (participants = 196; Analysis 22.1).
21.1. Analysis.
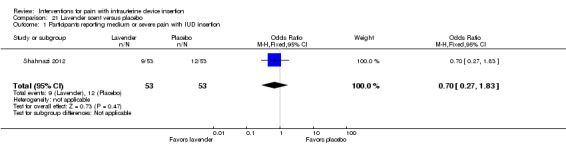
Comparison 21 Lavender scent versus placebo, Outcome 1 Participants reporting medium or severe pain with IUD insertion.
21.2. Analysis.
Comparison 21 Lavender scent versus placebo, Outcome 2 Median pain score during IUD insertion.
| Median pain score during IUD insertion | ||
|---|---|---|
| Study | Lavender (N = 53) | Placebo (N = 53) |
| Shahnazi 2012 | 1 (1st, 3rd quartiles: 0, 3) | 1 (1st, 3rd quartiles: 0, 3) |
22.1. Analysis.
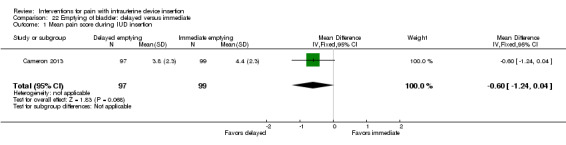
Comparison 22 Emptying of bladder: delayed versus immediate, Outcome 1 Mean pain score during IUD insertion.
Side effects or adverse events
Four trials reported on side effects. The two studies of nitric oxide donors reported no significant difference between the study groups in side effects at any time point (Bednarek 2013; Micks 2014). Both provided a list of potential side effects assessed. In Bednarek 2013, the nitroprusside group had two participants with vasovagal reactions while the placebo group had none. Micks 2014 had no vasovagal reactions or other complications in the trial of nitroglycerin ointment.
The other two trials reported not having any side effects. These were the studies of lavender essence (Shahnazi 2012) and of tramadol versus naproxen versus placebo (Karabayirli 2012).
Satisfaction or acceptability
Three trials had data in this area. In meta‐analysis, the two pilot trials of nitric oxide donors showed no significant difference in mean scores for satisfaction with pain control or satisfaction with the procedure (participants = 48; Analysis 19.4). However, in Bednarek 2013, the nitroprusside group appeared to have a lower mean score for satisfaction with the procedure. In Karabayirli 2012, the tramadol and naproxen groups did not differ significantly in rating the experience as unpleasant or in not preferring the medication for future IUD insertion (participants = 69; Analysis 20.2).
19.4. Analysis.
Comparison 19 Nitric oxide donors versus placebo, Outcome 4 Satisfaction.
20.2. Analysis.
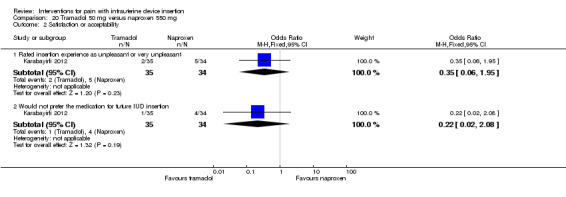
Comparison 20 Tramadol 50 mg versus naproxen 550 mg, Outcome 2 Satisfaction or acceptability.
Discussion
Summary of main results
This section emphasizes our sensitivity analysis or examination of trials with sufficient outcome data and evidence of moderate or high quality. We focus on meta‐analyses as well as significant differences within individual trials. Pain results for each study are summarized in Table 7. Results for side effects and for satisfaction are summarized in Table 8. The tables identify results excluded from this synthesis.
Lidocaine formulations
Meta‐analysis of three trials showed that 2% gel had no effect on pain during IUC insertion (Table 1). Pain at tenaculum placement did not differ between the study arms in meta‐analysis of two trials.
-
Four other studies indicated some effect of the lidocaine on pain (Table 2).
One trial applied a lidocaine and prilocaine cream on the cervix and cervical opening. The experimental group had a lower mean score for pain with IUC insertion compared with the placebo group.
A study with nulliparous women applied a short‐acting 4% lidocaine formulation on the surface of the portio, in the cervical canal, and into the uterine cavity. The lidocaine group had a lower mean pain score than the placebo group at 10 and 30 minutes after IUC insertion.
A trial with parous women sprayed 10% lidocaine on the cervical surface and toward the cervical os. The lidocaine group had a lower median pain score for IUC insertion compared with the placebo group.
The remaining trial used a 1% paracervical block, and reported a lower median pain score at tenaculum placement for the lidocaine group versus a group with no intervention.
NSAID or tramadol
Of the NSAID trials, two had some significant differences in pain scores between the groups; one also examined tramadol (Table 3). For pain during IUC insertion among multiparous women, a group with tramadol 50 mg had a lower mean score compared with a group that received naproxen 550 mg. Further, the naproxen group had a lower mean score than a placebo group. In another study, multiple doses of naproxen 300 mg led to lower pain scores at one and two hours after IUC insertion. Nearly all the women were nulliparous in that small study.
For satisfaction, the naproxen 550 mg group was more likely than the placebo group to be satisfied with the experience (Table 3). Specifically, the naproxen group was less likely to report the insertion experience as "unpleasant," and less likely to "not prefer" the treatment for future IUC insertion. The women were multiparous,
Misoprostol 400 mg
Four trials were included in meta‐analysis. The misoprostol group had a higher mean pain score with IUC insertion compared with the placebo group (Table 4).
-
Three individual studies showed some differences in pain between the misoprostol and placebo groups (Table 4).
In one trial, the misoprostol group had a higher median score for pain at IUC insertion and for pain score before leaving the clinic. Participants had no pregnancy of 20 weeks or more.
In another study, the misoprostol group had a higher mean score for pain before leaving the clinic. Participants had no pregnancy of 14 weeks or more.
A trial with nulliparous women showed fewer in the misoprostol group reported moderate or severe pain at IUC insertion than those in the placebo group.
-
The misoprostol trials assessed side effects and some examined satisfaction (Table 4).
In meta‐analysis of four trials, cramping was more likely in the misoprostol group than the placebo group.
Regarding satisfaction, the trial with nulliparous women mentioned above showed the misoprostol group was more likely than the placebo group to rate the experience more favorably, i.e., as not disagreeable or slightly disagreeable.
-
Two trials of misoprostol also used diclofenac with both groups (Table 5).
Both studies had some side effects. The misoprostol group was more likely to report shivering in the trial with nulliparous women. In the other trial, the misoprostol group was more likely to report headache or abdominal pain; the women had prior cesarean delivery only.
For satisfaction in the trial with women who had cesarean delivery only, the misoprostol group was less likely to choose the treatment again or to recommend it to a friend.
Other results
We excluded from the sensitivity analysis two conference abstracts and one full article (Table 7).
Preliminary results indicated a group that received the NSAID ketorolac had a lower pain score than the placebo group shortly after IUC insertion.
A trial of 1% lidocaine paracervical block had limited reporting. However, the lidocaine group had a lower pain score at tenaculum placement, during IUC insertion, and shortly afterward.
Overall completeness and applicability of evidence
Of the 33 included trials, 29 were published in the last five years. Nearly all used modern IUC, either levonorgestrel‐releasing or copper‐containing. Many trials allowed the participants to choose the type of IUC. No study compared insertion‐related pain with devices of smaller versus larger diameter. An early trial showing a benefit of naproxen may not be relevant (Massey 1974). The IUD used (the Dalkon Shield) had unique insertion mechanics and is no longer available.
The interventions covered four large categories: NSAIDs, lidocaine, misoprostol, and 'other.' Of the NSAIDs studied, naproxen and ibuprofen are commonly available but ketorolac less so. In some cases, the dose may have been lower than that sometimes used in clinical practice for acute gynecological pain. Various lidocaine formulations were examined, using varied amounts and administration methods: 2% gel; 1% and 2% formulations injected or infused; 4% gel; 10% spray; and a cream containing lidocaine and prilocaine. The misoprostol dose was standard across trials; administration was sublingual, buccal, or vaginal. The 'other' interventions included two nitric oxide donors (nitroprusside and nitroglycerine), an opioid (tramadol), a physical intervention (bladder emptying time) and an essential oil (lavender).
Pain measurements were taken at various time points, making comparisons difficult. Assessment of pain during or immediately after a procedure may differ from assessment of the pain at some later time. For pain after insertion, measurements ranged from five minutes to several hours later. A few trials assessed 'highest' level of pain after IUC insertion and before leaving the clinic, which was not comparable to measurement at a specific time. Most reports presented mean scores, while some noted the pain scores were not normally distributed. In those cases, the investigators appropriately used a nonparametric method for analysis. We did not combine results from those trials in meta‐analysis, even if means were also reported. In addition, a few trials provided categorical analysis of pain, e.g., moderate or severe pain, and two also reported means. We did not focus on levels of pain; the choice and meaning of cut‐offs may vary across trials.
We did not search for trials with comparative data by parity. Inclusion criteria regarding parity varied across studies (Table 6): 16 did not specify parity (one with 48 nulliparous/50 women), 10 were limited to nulliparous women, two included only parous women, and one stratified the sample by parity. Four trials had one of the following limitations: women with a prior cesarean delivery, those requesting abortion, or women having no pregnancy longer than 13 6/7 weeks or no longer than 20 6/7 weeks. A few studies analyzed the outcomes by parity; others examined overall pain scores by parity. Two ibuprofen trials found the median pain score for nulliparous women was approximately twice that for parous women (Bednarek 2015; Hubacher 2006). A misoprostol trial showed mean pain scores for nulliparous women that were twice those for multiparous women (Dijkhuizen 2011). Median pain scores did not differ much by parity in a lidocaine trial (McNicholas 2012). A cohort study of pain with IUD insertion compared women with and without previous vaginal delivery (Allen 2014). Pain scores were lower for women with versus without vaginal delivery. Pain was also higher among women with cesarean delivery who had experienced labor and some cervical dilation. Multivariate analysis indicated that expected pain and baseline anxiety were among the significant predictors of pain scores.
Quality of the evidence
Overall, the quality of evidence was high from the individual trials. More than 80% of trials had low risk of bias for the identified criteria (Figure 2). Loss to follow‐up was a minor issue for this review, given our primary outcomes were assessed while the participants were still in the clinical setting. A few studies did not use any blinding. Three trials had limited design information; two had conference abstracts and listings in a clinical trial register, and one was a full report (Figure 3).
However, we considered most of the effectiveness evidence to be of moderate quality. For the 'Summary of findings' tables, we downgraded one level if the evidence came from only one trial, as explained earlier (Data synthesis). Further research may change the estimate if trials vary by types of participants (e.g., parous versus nulliparous women), timing or application of the intervention, or timing or assessment of the outcome. In some cases, the results were imprecise due to a wide confidence interval, or a large range when medians were used.
Many trials were small studies without sufficient power to detect significant differences in pain scores. Most had a priori sample size calculations, but some were based on differences in pain scores larger than those found. However, smaller differences in VAS pain scores may not be clinically significant. For other trials, the primary outcomes did not include pain but rather provider's ease of insertion or successful insertion.
Agreements and disagreements with other studies or reviews
Several reviews have examined the use of misoprostol with IUD insertion for improving providers' ease of insertion or reducing pain for the women. Because misoprostol did not improve insertion ease and also led to more side effects, Waddington 2012 recommended ceasing use of misoprostol for IUD insertion. From a systematic review of pain management for IUC insertion, Gemzell‐Danielsson 2013 concluded that no evidence supported the use of misoprostol 400 µg for reducing pain. They also noted the likelihood of harm due to misoprostol. Pergialiotis 2014 reviewed RCTs and controlled trials of analgesia for pain reduction with IUC insertion. They concluded that misoprostol led to higher pain scores and more side effects. Several trials in our review are part of a prospective meta‐analysis using individual patient data (Turok 2011). The trials examine use of misoprostol for providers' ease of insertion and for pain as reported by the women. That work may provide further evidence of whether misoprostol is helpful or harmful at specific time points or within certain subgroups of women.
Some reviews addressed analgesics for reducing pain with IUC insertion. A systematic review examined RCTs that used intrauterine local anesthesia for pain with a range of gynecologic procedures, including IUC insertion (Mercier 2012). That review included one trial of IUD insertion, which was not eligible for our review (Oloto 1997). Women were randomized by birth date to a lignocaine 2% gel (lidocaine), a gel without the lignocaine, or usual insertion procedures. Women with the active gel had lower pain scores compared with women in the other two groups. In Gemzell‐Danielsson 2013, the RCTs of pre‐insertion interventions for pain control were all included in our review. The researchers did not find any evidence to support use of pre‐insertion ibuprofen. As we noted, they stated that naproxen and the opioid tramadol may be beneficial but also that larger studies were needed. No local anesthesia appeared to be helpful. However, because of variable needs and reactions, they believed injectable local anesthesia should be available and used as the situation warranted. Pergialiotis 2014 included RCTs and controlled clinical trials that had single treatments and used currently available IUC. The 13 trials in their meta‐analysis were also in our review. The researchers identified paracervical lidocaine as effective for reducing pain at tenaculum placement. However, they analyzed means for those two trials that reported medians for their primary outcomes (Cırık 2013; Mody 2012), one of which stated that the data were not normally distributed.
Authors' conclusions
Implications for practice.
A few treatments made a difference in pain control. Of the NSAIDs, naproxen may decrease pain during IUC insertion among parous women (550 mg) and in the first hours afterward in nulliparous women (300 mg; two separate doses). Most trials show no benefit of ibuprofen. The opioid tramadol (50 mg) may reduce pain during insertion among parous women but only slightly more than naproxen does. Misoprostol did not help with pain; it may even increase pain and cause more side effects. Lidocaine 2% gel showed no effect on pain with tenaculum placement or during IUC insertion. Some other lidocaine formulations may lessen pain during IUC insertion and shortly thereafter. These include 4% topical gel studied in nulliparous women, 10% spray examined in parous women, lidocaine and prilocaine cream, and 1% paracervical block. The wait time between application and intervention for these medications to act ranged from three to seven minutes. Practitioners still need better interventions than those generally used.
Two recent papers discuss the evidence regarding pain management for practical purposes. Kass‐Wolff 2014 examined interventions for pain related to endometrial biopsies and IUC insertion. The intent was to inform advance practice nurses of the available evidence. Bahamondes 2014 focused on pain related to IUC insertion with the intent of providing recommendations for practice. The researchers reviewed pharmacological interventions, and addressed insertion methods and pre‐insertion counseling.
Implications for research.
From 2010 to 2015, 29 RCTs were completed that evaluated interventions for pain with IUC insertion. While most were high‐quality trials, we downgraded the evidence quality if the effectiveness data came from a single trial. Future research may change the estimate. The studies tested several NSAIDs, various lidocaine formulations and administration methods, misoprostol, and other interventions. Most trials used the modern levonorgestrel‐releasing intrauterine system (LNG‐IUS) or the copper T 380A IUD. The studies measured pain at tenaculum placement as well as pain during and after IUD insertion. We considered most evidence of effectiveness to be of moderate quality, having come from single studies. Several interventions had no effect on pain; these included lidocaine 2% gel, misoprostol, and most NSAIDs. Therefore, many interventions do not require further research.
A few interventions helped reduce pain among specific groups of women, e.g., parous or nulliparous women. Those interventions were naproxen 550 mg, tramadol 50 mg, and some lidocaine formulations (i.e., lidocaine and prilocaine cream, short‐acting 4% gel, 10% spray, and 1% paracervical block). Trials with participants of differing parity would help determine if results are consistent. Use of the same scales and measurement times across trials would facilitate interpretation of results. To make the procedure acceptable to women, we need a greater understanding of how much pain reduction women expect and want from these interventions.
What's new
| Date | Event | Description |
|---|---|---|
| 22 June 2015 | New search has been performed | Search updated |
| 2 June 2015 | New search has been performed | Search updated; added English‐language report of included trial (Ahmadi Doulabi 2013) |
| 16 March 2015 | Amended | New trial included that had been awaiting classification; total of 29 new trials |
| 27 January 2015 | New citation required and conclusions have changed | Added 28 new Included studies, 4 Ongoing studies, and 4 Studies awaiting classification |
| 16 December 2014 | Amended | Added 'pain at tenaculum placement' to primary outcomes. For initial review, no such data were available. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 12 December 2007 | New citation required and major changes | Substantive amendment |
Acknowledgements
In 2014, Thomas Grey of FHI 360 helped check the Study Characteristics data and the outcome data for pain with IUC insertion. For the 2009 review, Carol Manion of FHI 360 and Carol Mita of Harvard Medical School assisted with the literature searches.
Appendices
Appendix 1. Search 2015
PubMed (1 June 2015)
(iud* OR iucd* OR ius* OR intrauterine devices OR intrauterine device*) AND insert* AND (pain OR cramping OR discomfort) AND (control* OR therapy OR treat* OR alleviate* OR ameliorate* OR reduc* OR minimiz* OR analgesics OR analgesic* OR anodyne* OR anesthesia and analgesia)
AND ( ( "2007/01/01"[PDat] : "3000/12/31"[PDat] ) )
Article types: Clinical trial
CENTRAL (The Cochrane Library 2015, issue 5 (22 June 2015)
Title, Abstract, Keywords: intrauterine AND (device* OR contracept* OR system*) AND Title, Abstract, Keywords: pain AND insert*
Publication date: 2007 to 2015
POPLINE (3 February 2015)
Keyword: IUD AND Keyword: Pain AND Keyword: Insertion AND Keyword: Clinical trials
Years: 2007 to 2014
EMBASE (5 August 2014)
'intrauterine devices'/exp OR 'intrauterine devices' AND 'insertion' AND ('pain'/exp OR 'pain') AND ('analgesic agent'/exp OR 'analgesic agent' OR 'anesthetic agent'/exp OR 'anesthetic agent')
Publication year: 2007 to 2014
ClinicalTrials.gov (5 January 2015)
Search terms: intrauterine device OR IUD OR IUS Study type: Interventional Conditions: contraception OR pregnancy OR pain OR IUD Outcome measures: pain Received on or after 01/01/2007
ICTRP (27 January 2014)
Title: (intrauterine OR IUD OR IUS) AND pain Recruitment status: all Date of registration: between 01 January 2007 and 27 January 2014
Appendix 2. Search 2008
See: Cochrane Fertility Regulation Group methods used in reviews
MEDLINE via PubMed
(iud* OR iucd* OR ius* OR intrauterine devices OR intrauterine device*) AND insert* AND (pain OR cramping OR discomfort) AND (control* OR therapy OR treat* OR alleviate* OR ameliorate* OR reduc* OR minimiz* OR analgesics OR analgesic* OR anodynes OR anesthesia and analgesia)
CENTRAL
pain and (intrauterine device* or intrauterine contraception* or intrauterine system*) and (analgesia* or therapy) and insert*
POPLINE
(intrauterine device*/iud*/iucd*/ius*/intrauterine contracept*) & (analgesi*/nsaids/local anesthesia/cervical block/therapy/treat*/alleviat*/ameliorat*/reduc*/minimiz*/control*) & (pain/discomfort/cramping/analgesi*/nsaids/local anesthesia/cervical block) & insert* & clinical trial*
EMBASE
(intrauterine(w)contraceptive(w)device? or intrauterine(w)device? or iud? or iucd? or ius? or intrauterine(w)contracept?) AND insert? AND ((analgesic? or analgesic agent! or local anesthesia agent or local anesthesia! or cervical(w)block) OR (pain or pain/ or pain/ or pain! or cramping or discomfort)) AND ((analgesic? or analgesic agent! or local anesthesia agent or local anesthesia! or cervical(w)block) OR (control? or treat? or alleviat? or ameliorat? or reduc? or limit? or minimiz?))
ClinicalTrials.gov
"intrauterine device"
Data and analyses
Comparison 1. Naproxen 300 mg (x 4 doses) + paracervical block versus placebo plus paracervical block.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain scores during and immediately after IUD insertion | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Pain during IUD insertion | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.72, 0.56] |
| 1.2 Pain immediately after IUD insertion | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.93, 0.31] |
| 2 Mean pain scores after IUD insertion | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Mean pain score 1 hour after IUD insertion | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.67, ‐0.41] |
| 2.2 Mean pain score 2 hours after IUD insertion | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.98 [‐1.64, ‐0.32] |
| 2.3 Mean pain score 3 hours after IUD insertion | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.14, 0.08] |
| 2.4 Mean pain score 4 hours after IUD insertion | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.98, 0.28] |
| 2.5 Mean pain score 5 hours after IUD insertion | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.69, 0.63] |
| 2.6 Mean pain score 6 hours after IUD insertion | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.56, 0.64] |
Comparison 2. Naproxen 550 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score during IUD insertion | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐1.94 [‐2.35, ‐1.53] |
| 2 Satisfaction or acceptability | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Rated insertion unpleasant or very unpleasant | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.01, 0.09] |
| 2.2 Would not prefer the medication for future IUD insertion | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.08] |
Comparison 3. Ibuprofen 400 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Median pain score during IUD insertion | Other data | No numeric data |
Comparison 4. Ibuprofen 600 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Median pain scores | Other data | No numeric data | ||
| 2 Reported moderate to severe pain during IUD insertion (3 or greater on the 1 to 10 scale) | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.67, 5.95] |
Comparison 5. Ibuprofen 800 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score at tenaculum placement | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.24, 1.34] |
| 2 Mean pain score at IUD insertion | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.98, 1.68] |
| 3 Median pain score at IUD insertion | Other data | No numeric data |
Comparison 6. Ketorolac 30 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Median pain scores | Other data | No numeric data |
Comparison 7. Lidocaine 2% versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score at tenaculum placement | 2 | 345 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.25, 0.18] |
| 2 Mean pain score during IUD insertion | 3 | 409 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.21, 0.18] |
Comparison 8. Lidocaine 2% gel 6 mL versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score 20 minutes post‐insertion | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐8.81, 7.21] |
| 2 Side effects or adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Moderate or severe nausea | 1 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.68] |
| 2.2 Moderately or severely dizzy | 1 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.07, 2.07] |
| 3 Satisfaction or acceptability | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Amount of pain was mostly or completely acceptable | 1 | 143 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.50, 2.25] |
Comparison 9. Lidocaine 2% gel versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score during IUD insertion | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.24, 0.64] |
Comparison 10. Lidocaine 2% gel, 2.5 to 5 mL, versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Median pain scores | Other data | No numeric data | ||
| 2 Median pain scores by parity | Other data | No numeric data | ||
| 3 Median difference in pain scores from baseline | Other data | No numeric data |
Comparison 11. Lidocaine 4% gel 8.5 mL versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score for IUD insertion (within 10 min of insertion | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | ‐15.90 [‐22.77, ‐9.03] |
| 2 Mean pain score 30 min post‐insertion | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐11.10 [‐19.05, ‐3.15] |
| 3 Mean pain score 1 h post‐insertion | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐6.79, 4.19] |
| 4 Additional analgesic at clinic | 1 | 218 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.21, 0.80] |
| 5 Side effects or adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Any adverse event | 1 | 218 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.46] |
Comparison 12. EMLA cream 5% (25 mg lidocaine + 25 mg prilocaine) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score for tenaculum use | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐2.78 [‐3.66, ‐1.90] |
| 2 Mean pain score immediately after IUD insertion and tube removal | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐1.96 [‐1.00, ‐0.92] |
Comparison 13. Lidocaine 2% 1.2 mL versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score at tenaculum placement | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.88, 0.88] |
| 2 Mean pain score with IUD insertion | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐2.38, 0.92] |
| 3 Mean global pain score at end of visit | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.82, 1.60] |
| 4 Mean pain scores by NSAID intake | Other data | No numeric data |
Comparison 14. Lidocaine 10% spray, 40 mg, versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Median pain scores | Other data | No numeric data |
Comparison 15. Lidocaine 1% 10 mL paracervical block versus no paracervical block.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Median pain scores | Other data | No numeric data | ||
| 2 Side effects or adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Vasovagal symptoms | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.30 [0.36, 149.06] |
| 2.2 Bleeding | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.37, 6.14] |
| 3 Vasovagal syncope | Other data | No numeric data |
Comparison 16. Lidocaine 2% 1.8 mL injected versus ibuprofen 400 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain scores after IUD insertion | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Two hours after | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 7.90 [‐2.36, 18.16] |
| 1.2 Six hours after | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | 3.5 [‐5.47, 12.47] |
| 2 Moderate or severe pain (from VAS) | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.26, 1.33] |
| 3 Satisfaction or acceptability | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.35, 1.75] |
| 3.1 Experience was uncomfortable or very uncomfortable | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.35, 1.75] |
Comparison 17. Misoprostol 400 µg + diclofenac 100 mg versus diclofenac 100 mg alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Median pain score at IUD insertion | Other data | No numeric data | ||
| 2 Side effects or adverse events | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Headache | 1 | 255 | Odds Ratio (M‐H, Random, 95% CI) | 5.68 [1.23, 26.19] |
| 2.2 Abdominal pain | 1 | 230 | Odds Ratio (M‐H, Random, 95% CI) | 3.93 [1.41, 10.97] |
| 2.3 Nausea | 2 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 1.98 [0.29, 13.40] |
| 2.4 Any side effect | 1 | 79 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.72, 4.29] |
| 2.5 Shivering | 1 | 79 | Odds Ratio (M‐H, Random, 95% CI) | 5.48 [1.41, 21.33] |
| 2.6 Diarrhea | 1 | 79 | Odds Ratio (M‐H, Random, 95% CI) | 2.52 [0.84, 7.59] |
| 2.7 Vomiting | 1 | 79 | Odds Ratio (M‐H, Random, 95% CI) | 2.11 [0.18, 24.24] |
| 3 Satisfaction or acceptability | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Overall very satisfied or satisfied | 1 | 255 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.05, 1.19] |
| 3.2 Would choose treatment again | 1 | 255 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.14, 0.65] |
| 3.3 Would recommend treatment to a friend | 1 | 255 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.16, 0.81] |
| 3.4 Rated insertion experience as 'very little unpleasant' | 1 | 79 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.13, 1.66] |
Comparison 18. Misoprostol 400 μg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score with tenaculum placement | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐16.05, 14.05] |
| 2 Mean pain score during IUD insertion | 4 | 400 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.07, 0.46] |
| 3 Mean pain score during IUD insertion by parity | 1 | 199 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.04, 0.52] |
| 3.1 Nulliparous | 1 | 95 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.20, 0.61] |
| 3.2 Multiparous | 1 | 104 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.11, 0.66] |
| 4 Mean pain scores post IUD insertion | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Mean pain score 5 min post‐insertion | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐14.02, 14.02] |
| 4.2 Highest pain level (immediately after insertion) | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.97, 0.77] |
| 4.3 Pain before discharge from clinic | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.67, 0.47] |
| 4.4 Highest pain level before discharge from clinic | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 7.60 [6.48, 8.72] |
| 5 Median pain scores | Other data | No numeric data | ||
| 6 Moderate to severe pain (removal of first IUC and insertion of LNG‐IUS | 1 | 89 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.57, 5.46] |
| 7 Moderate to severe pain at IUD insertion | 1 | 179 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.16, 0.55] |
| 8 Side effects or adverse events | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Any complication | 1 | 195 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.59, 2.36] |
| 8.2 Vasovagal | 2 | 365 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.64, 2.20] |
| 8.3 Any side effect | 2 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 3.62 [0.77, 16.98] |
| 8.4 Cramping | 4 | 466 | Odds Ratio (M‐H, Random, 95% CI) | 2.64 [1.46, 4.76] |
| 8.5 Headache | 2 | 226 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.63, 3.44] |
| 8.6 Nausea | 5 | 576 | Odds Ratio (M‐H, Random, 95% CI) | 2.40 [0.74, 7.73] |
| 8.7 Bleeding | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.06, 18.45] |
| 8.8 Lightheaded | 1 | 35 | Odds Ratio (M‐H, Random, 95% CI) | 3.36 [0.13, 88.39] |
| 8.9 Hypoesthesia oral | 1 | 89 | Odds Ratio (M‐H, Random, 95% CI) | 13.29 [0.71, 247.91] |
| 8.10 Oral pain | 1 | 89 | Odds Ratio (M‐H, Random, 95% CI) | 19.11 [1.06, 345.68] |
| 8.11 Vomiting | 1 | 179 | Odds Ratio (M‐H, Random, 95% CI) | 2.81 [0.53, 14.87] |
| 8.12 Diarrhea | 2 | 268 | Odds Ratio (M‐H, Random, 95% CI) | 1.64 [0.16, 16.68] |
| 8.13 Fevers and Chills | 1 | 61 | Odds Ratio (M‐H, Random, 95% CI) | 5.27 [1.02, 27.33] |
| 9 Satisfaction or acceptability | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Rated insertion experience as slightly or not disagreeable | 1 | 179 | Odds Ratio (M‐H, Random, 95% CI) | 4.34 [2.32, 8.12] |
| 9.2 Satisfied or very satisfied (1 week later) | 1 | 73 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.28, 2.73] |
| 9.3 Likely or very likely to have future IUD insertion (1 week later) | 1 | 102 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.49, 3.14] |
| 9.4 Would recommend IUD to a friend (1 week later) | 2 | 167 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.22, 1.53] |
| 10 Satisfaction or acceptability (1 week later) | Other data | No numeric data |
Comparison 19. Nitric oxide donors versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score at tenaculum placement | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐32.09, 12.09] |
| 2 Mean pain score during IUD insertion | 2 | 47 | Mean Difference (IV, Random, 95% CI) | ‐6.80 [‐21.19, 7.60] |
| 3 Mean pain score 30 minutes post IUD insertion | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐35.42, 9.42] |
| 4 Satisfaction | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Mean score for satisfaction with pain control | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐8.39 [‐24.55, 7.77] |
| 4.2 Mean score for overall satisfaction with procedure | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐6.53 [‐19.80, 6.75] |
Comparison 20. Tramadol 50 mg versus naproxen 550 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score during IUD insertion | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐0.94, ‐0.32] |
| 2 Satisfaction or acceptability | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Rated insertion experience as unpleasant or very unpleasant | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.06, 1.95] |
| 2.2 Would not prefer the medication for future IUD insertion | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.02, 2.08] |
Comparison 21. Lavender scent versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants reporting medium or severe pain with IUD insertion | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.27, 1.83] |
| 2 Median pain score during IUD insertion | Other data | No numeric data |
Comparison 22. Emptying of bladder: delayed versus immediate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pain score during IUD insertion | 1 | 196 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.24, 0.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmadi Doulabi 2013.
| Methods | Location: Hamadan, Iran Recruitment time: September 2012 to October 2012 Sample size calculation and outcome of focus: assume SD 2.55 and mean 3.5; 46 per group for 92 total |
|
| Participants | General with N: 92 women, IUD candidates Source: health center in Hamadan, Iran Inclusion criteria: 20 to 35 years, no contraindication to IUD insertion, between 2 and 5 days of menstruation, no analgesic drug (acetaminophen, ibuprofen, mefenamic acid) for 6 h prior, no sedative drug use for 24 h prior, no severe mental stress for 2 months prior Exclusion criteria: sensitivity to EMLA cream, cervix size < 6 cm or > 9 cm, cervical stenosis |
|
| Interventions | On cervix and cervical opening with cotton swab: 1) EMLA cream (lidocaine 2.5% + prilocaine 2.5%), 5 g 2) Placebo cream Timing: 7 min before IUD insertion |
|
| Outcomes | Primary: pain intensity during 3 stages of IUD insertion (10 cm VAS): after tenaculum use, after hysterometer insertion, after IUD insertion and removal of tube Secondary: no mention |
|
| Notes | IUC used: CuT 380A Initial article in Persian; information from translation and clinical trial listing; English‐language article available 2015 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized stratified block allocation;" block size 4; each block "matched" for age and for number and type of deliveries |
| Allocation concealment (selection bias) | Low risk | Placebo prepared by pharmacy laboratory at investigators' university |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Triple blind"; placebo cream comparable to EMLA in appearance, consistency, color, and smell; similar containers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: all participants had procedure; analysis included all women randomized |
Aksoy 2015.
| Methods | Location: Kayseri, Turkey Recruitment time: December 2013 to January 2014 Sample size calculation and outcome of focus: pain with IUD insertion; reference values from published study. Assuming pain reduction by 0.5 SD was acceptable, at least 95 required in each arm to detect clinically significant difference between groups on 10 cm VAS scale when assuming 80% power to detect primary hypothesis and type I error of 0.05. Assuming 5% dropout, planned to recruit 200 (100 per study arm) |
|
| Participants | General with N: 200 parous women, 18 to 49 years old Source: family planning clinic at tertiary care center Inclusion criteria: age ≥ 18 years; accepting IUD as method of contraception; no known previous allergic reaction or sensitivity to lidocaine or placebo spray; no accompanying extraordinary medical or surgical conditions needing special attention; no specific request for anesthesia, or suspected pathology necessitating anesthesia; no history of chronic pelvic pain or dysmenorrhea Exclusion criteria: currently pregnant or within 2 weeks of pregnancy conclusion; presence of known uterine anomaly or fibroid distorting uterine cavity; contraindication to copper IUD based on CDC medical eligibility criteria; untreated acute cervicitis or PID; known cervical stenosis or extraordinary surgical conditions necessitating cervical dilators; any systemic diseases or medications that would affect perception of pain; current or past history of illegal drug or narcotic use; inability to understand how to score 10 cm VAS for pain; and VAS pain score other than 0 (no pain) just before IUD insertion |
|
| Interventions | 1) Lidocaine spray 10% (10 mg/mL); 4 pumps, with 3 to cervical surface and 1 toward cervical os (net 40 mg) 2) Saline spray placebo Timing: 3 min before tenaculum placement |
|
| Outcomes | Primary: pain during IUD insertion (10 cm VAS), assessed immediately after Secondary: no mention |
|
| Notes | IUC used: CuT 380A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number chart (via SPSS) |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Spray bottles wrapped in black paper. Sprays were identical in appearance, color, and consistency. Participants, anesthesia technician, and gynecologist performing procedure were blinded to bottle contents. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Allen 2013.
| Methods | Location: Providence RI, USA Recruitment time: March 2011 to July 2012 Sample size calculation and outcome of focus: Based on previous clinic study, assuming alpha 0.05, 80% power and SD 32 mm, adding 5% to account for dropouts, 150 participants needed to detect 15 mm mean difference between groups on 0 to 100 mm VAS |
|
| Participants | General with N: 150 women Source: university obstetrics and gynecology practice Inclusion criteria: English‐ or Spanish‐speaking women, 18 to 49 years old, requesting IUD insertion for contraception or abnormal uterine bleeding, no prior IUD use, > 6 weeks postpartum or 2 weeks postabortion if recently pregnant, no analgesics or anxiolytics in previous 12 h and no misoprostol use prior to insertion Exclusion criteria: any contraindication to IUD placement, allergy to lidocaine or sensitivities to components of lidocaine or placebo gel and chronic narcotic, benzodiazepine or barbiturate use within past year |
|
| Interventions | 1) 2% lidocaine gel (3 mL at anterior lip of cervix; 3 mL in cervical canal) 2) Placebo gel Timing: after speculum insertion and 3 min before tenaculum was placed and IUD inserted |
|
| Outcomes | Primary: pain score with tenaculum placement, during IUD insertion, and 20 min post insertion (100 mm VAS) Secondary: side effects and acceptability |
|
| Notes | IUC used: CuT 380A and LNG‐IUS Insertions at 6 to 12 weeks postpartum, 2 to 4 weeks postabortion or interval |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally computer‐generated with 1:1 allocation ratio in alternating blocks of 4 and 6 |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐prepared identical syringes were labeled only with study name and sequential number according to randomization list. Participants assigned study number in order of recruitment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and providers were blind to treatment assignment. The study gels were indistinguishable in appearance. No identifiers of treatment group were placed on participant data sheets or medications. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Bednarek 2013.
| Methods | Location: Portland Oregon, USA Recruitment time: December 2010 to June 2011 Sample size calculation and the outcome of focus: Assuming one‐sided alpha of 0.05, pooled SD 28 mm and power 82%, a sample size of 12 in each group was calculated to detect 30 mm pain score difference between groups. |
|
| Participants | General with N: 24 nulliparous women Source: academic medical clinic and local family planning clinic Inclusion criteria: nulliparous women, age 18 to 45 years, requesting LNG‐IUS for contraception Exclusion criteria: previous pregnancy > 20 weeks; previous IUD placement or attempted placement; contraindication to nitroprusside or LNG‐IUS; history of migraine, cluster or vascular headaches; blood pressure < 90/55 or > 150/100 at beginning of study visit |
|
| Interventions | 1) 10 mg nitroprusside, 1% aqueous gel 2) Placebo gel Timing: immediately prior to IUD placement |
|
| Outcomes | Primary: pain score at tenaculum placement, with IUD insertion, and 30 min post‐insertion (100 mm VAS with 0 = no pain and 100 = worst imaginable pain) Secondary: side effects, satisfaction (100 mm VAS) |
|
| Notes | IUC used: LNG‐IUS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list, source not specified |
| Allocation concealment (selection bias) | Low risk | Study drug prepared and packaged at a separate site |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and all study staff involved in the care of the participant were blinded to study arm allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Bednarek 2015.
| Methods | Location: Portland OR, Pittsburgh PA, Atlanta GA, Albuquerque NM; USA Recruitment time: June 2007 to February 2009 Sample size calculation and the outcome of focus: estimated 266 women would return in main study for delayed IUD insertion; 80% power at alpha .05 for 7 mm difference on 100 mm VAS with SD 20 mm (change of 9 to 14 mm on 100 mm VAS considered clinically important) |
|
| Participants | General with N: 202 women requesting uterine aspiration for spontaneous or induced abortion Source: 4 academic medical centers Inclusion criteria: ≥ 18 years of age, presented to participating sites requesting uterine aspiration for induced or spontaneous abortion between 5 and 12 weeks of gestation, desired intrauterine contraception Exclusion criteria: cervicitis or PID, uterine anomaly or fibroid distorting the cavity, known or suspected molar or ectopic pregnancy, PID or sexually transmitted infection in previous 3 months |
|
| Interventions | 1) Ibuprofen 800 mg 2) Placebo Timing: 30 to 45 min before insertion |
|
| Outcomes | Primary: pain during IUD insertion (100 mm VAS) Secondary: not specified |
|
| Notes | IUC used: LNG‐IUS or CuT 380A (choice) Planned substudy within primary study of immediate versus delayed IUC insertion |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocks of varying size, stratified by center, equal allocation to arms |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque envelopes; pharmacy‐prepared and distributed packages, identical in appearance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind (not specified) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Cameron 2013.
| Methods | Location: Scotland, UK Recruitment time: from March 2009 to March 2011 Sample size calculation and the outcome of focus: Assuming two‐sided alpha of 0.05 and power of 80%, a sample size of 100 participants in each arm was calculated to detect difference between 90% easy insertions with empty bladder and 99% easy insertions with filled bladder. |
|
| Participants | General with N: 200 women Source: family planning clinic in Edinburgh, Scotland, UK Inclusion criteria: wished to have an intrauterine method of contraception, routinely attended clinic for counseling about method by clinician, before being given an appointment for subsequent insertion of device, agreed to attend for IUD or IUS insertion with full bladder Exclusion criteria: no other criteria |
|
| Interventions | Women drank 1 liter (L) (or 6 glasses) of fluid in hour before appointment.
Timing of bladder emptying: 1) Immediate: went to toilet immediately prior to IUC insertion 2) Delayed: went to toilet after IUC insertion |
|
| Outcomes | Primary: pain during IUD insertion (10‐point scale where 0 = no pain and 10 = agony) Secondary: ease of insertion (primary for trial) |
|
| Notes | IUC used: Mirena, Slimline TT380, Nova T 380, Multiload 375, UT 380 and Mini TT 380 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Castro 2014.
| Methods | Location: São Paulo, Brazil Recruitment time: February 2012 to July 2013 Sample size calculation: Pilot study of 6 women in each group gave mean 68 ± 11 mm for ibuprofen and 28 ± 10 mm with anesthetic. Considering 10% difference to be clinically relevant, alpha = 5%, and power 80%, 40 women per group would be required. Tried to include 100 women to account for sample loss and protocol violations. |
|
| Participants | General with N: 100 women Source: clinic of Medical School of Ribeirao Preto, University of São Paulo Inclusion criteria: age 18 to 45 years, no previous IUC use, wanted to use LNG‐IUS, no previous vaginal delivery or nulliparous, FSH ≤ 10 ng/mL Exclusion criteria: category 3 or 4 for LNG‐IUS use according to medical eligibility criteria (WHO 2009), illicit drug or alcohol users, allergy to NSAID or lidocaine, presence of chronic pelvic pain, presence of abnormality in cervix, previous abortion or miscarriage with or without uterine curettage, women with continued use of medications that interfere with pain threshold, psychiatric disorders |
|
| Interventions | 1) 1.8 ml 2% lidocaine without epinephrine; intracervical block with carpule syringe in uterine cervix 5 min prior to LNG‐IUS insertion (position 3, 6, 9, and 12 o'clock) 2) Ibuprofen 400 mg, 1 h before LNG‐IUS insertion |
|
| Outcomes | Primary: pain of LNG‐IUS insertion (100 mm VAS); 'facial pain scale' used but method not described Assessed immediately after insertion and 2 and 6 h after insertion Secondary: overall discomfort with LNG‐IUS insertion; provider's ease of inserting LNG‐IUS |
|
| Notes | IUC used: LNG‐IUS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by computer program (www.randomizer.org) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes; opened by investigator who performed intervention at time of study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Statistical analysis was blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none (2 dropped out after randomization to NSAID group but before LNG‐IUS insertion) |
Chor 2012.
| Methods | Location: Chicago, IL, USA Recruitment time: from April 2007 to January 2010 Sample size calculation and the outcome of focus: Based on published literature, and assuming a SD of 23 mm, an alpha of 0.05 and power of 80%, a sample size of 37 participants in each group was calculated to detect a difference of 1.5 cm in the VAS assessment of pain at the time of IUD insertion. |
|
| Participants | General with N: 87 women enrolled; 81 received IUDs Source: University of Illinois Medical Center Inclusion criteria: 18 years or older, desiring LNG‐IUS for contraception, without contraindications to using LNG‐IUS, without medical contraindications to NSAIDs, able to provide phone number for follow‐up questions, and had not taken pain medications on day of enrollment Exclusion criteria: not reported |
|
| Interventions | 1) Ibuprofen 800 mg 2) Placebo containing lactose Timing: 45 min prior to IUD insertion |
|
| Outcomes | Primary: Pain at time of tenaculum placement, pain at time of IUD insertion (10 cm VAS; 0 = no pain and 10 = unbearable) Secondary: provider's ease of insertion |
|
| Notes | IUC used: LNG‐IUS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, source not specified |
| Allocation concealment (selection bias) | Low risk | Both placebo and ibuprofen were identical in appearance, and were packaged in serially‐numbered envelopes and used in order. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, providers and study recruiters were blinded to medication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Cırık 2013.
| Methods | Location: Samsun, Turkey Recruitment time: December 2012 to March 2013 Sample size calculation and outcome of focus: not reported |
|
| Participants | General with N: 95 women Source: Samsun Maternity Hospital Inclusion criteria: 18 to 45 years old, presenting at hospital family planning unit for IUD insertion Exclusion criteria: lidocaine or copper allergy, uterine Mullerian anomalies, cervicitis, uterus bigger than 3 months of gestation, fibroids or polyps; had analgesic medication within 6 h of procedure or drug for cervical dilatation such as misoprostol; PID history in last 3 months; pregnancy within 6 weeks |
|
| Interventions | 1) 10 ml 1% lidocaine paracervical block 2) 10 mm 0.9% saline solution, paracervical injection 3) No analgesia Timing: 5 min before IUD insertion Injections: 5 ml at 3 o'clock and 5 ml at 9 o'clock positions of cervix |
|
| Outcomes | Primary: pain score immediately after tenaculum placement, immediately after IUD insertion, and 5 min after procedure; 10‐point VAS (0 = no pain to 10 = worst pain ever felt) Secondary: side effects |
|
| Notes | IUC used: CuT 380 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Family Planning Unit providers were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Dijkhuizen 2011.
| Methods | Location: Leiden, the Netherlands Recruitment time: May 2007 to December 2008 Sample size calculation and outcome of focus: Based on published literature, assuming type I error .05 and power .80: 266 needed to detect difference of expected failed insertions of 1.3% versus 8.8%. |
|
| Participants | General with N: 270 women randomized; analysis based on 199 who received IUDs Source: outpatient gynecology department of university medical center and 4 affiliated hospitals Inclusion criteria: nulliparous or multiparous, ≥ 18 years, IUD to be inserted (regardless of indication and type of IUD), or IUD to be replaced Exclusion criteria: contraindications for misoprostol use (pregnancy, prostaglandin allergy) or contraindications for IUD use (6 weeks postpartum, gynecologic malignancy, PID, unexplained vaginal bleeding and pregnancy) |
|
| Interventions | 1) Misoprostol 400 μg (in 2 tablets), vaginally 2) Placebo Timing: 3 h before insertion |
|
| Outcomes | Primary: pain during insertion (10 cm line for VAS, read in mm according to investigator communication) Secondary: side effects (before insertion), insertion‐related complications |
|
| Notes | IUC used: LNG‐IUS and Copper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list; source not specified |
| Allocation concealment (selection bias) | Low risk | Sealed opaque medication packets, numbered and used consecutively |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded: neither clinician nor participant knew whether placebo or misoprostol was administered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none for pain during insertion; 17.6% (35/199) by 6‐week follow‐up (17 misoprostol and 18 placebo) |
Edelman 2011.
| Methods | Location: Portland, OR, USA Recruitment time: February 2007 to March 2010 Sample size calculation and outcome of focus: Assuming 80% power, one‐sided alpha .05, taking dropout or disqualification into account, 40 needed to detect 20 mm decrease in pain with IUD insertion in misoprostol group |
|
| Participants | General with N: 40 nulliparous women Source: Oregon Health and Science University in Portland, OR Inclusion criteria: nulliparous women, aged 18 to 45 years, requesting IUD for contraception Exclusion criteria: prior pregnancy > 20 weeks; pregnant within 6 weeks of study entry; prior attempted or successful IUD insertion; history of cervical procedure such as cone biopsy, Loop electrosurgical excision procedure, cryotherapy; WHO Medical Eligibility Criteria category 3 or 4 for IUD use |
|
| Interventions | 1) Misoprostol 400 µg, buccally 2) Placebo Timing: 90 min before insertion |
|
| Outcomes | Primary: pain at tenaculum placement, during IUD insertion, and 5 min post‐insertion (100 mm VAS) Secondary: side effects (prior to insertion); provider's ease of insertion |
|
| Notes | IUC used: LNG‐IUS and CuT 380A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization scheme was computer‐generated and obtained by phone. |
| Allocation concealment (selection bias) | Low risk | Placebo was similar in shape, size, taste and color and given to participants in an opaque envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and providers blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Espey 2014.
| Methods | Location: Albuquerque, NM, USA Recruitment time: January 2010 to January 2013 Sample size calculation and outcome of focus: Assuming two‐sided alpha of .05 and a power of 80%, sample size of 80 women needed to detect 0.8 cm ± 1.25 difference in maximum pain with IUD insertion. |
|
| Participants | General with N: 83 nulliparous women Source: university reproductive health clinic Inclusion criteria: nulliparous women, desiring LNG‐IUS or CuT 380A IUD for contraception, English‐speaking, age ≥ 18 or age 14 to 17 with parental consent Exclusion criteria: history of pregnancy lasting beyond 19 6/7 weeks, any pregnancy in last 4 weeks, active genital infection or cervicitis, undiagnosed abnormal uterine bleeding, fibroids distorting uterine cavity, history of cervical or uterine cancer, uterine anomaly, PID within last 3 months, and ibuprofen or copper allergy |
|
| Interventions | 1) Misoprostol 400 μg, buccally 2) Placebo Timing: 2 to 8 h before the IUD insertion |
|
| Outcomes | Primary: pain related to IUD insertion, assessed immediately after insertion (instruments removed) and pain before clinic discharge (10 cm VAS with 0 = none and 10 = worst imaginable pain) Secondary: satisfaction and side effects (1 to 2 weeks later) |
|
| Notes | IUC used: LNG‐IUS or CuT 380A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, 8‐block randomization sequence |
| Allocation concealment (selection bias) | Low risk | Pharmacy prepared medication at another university. Drug and placebo tablets packaged according to randomization list, labeled with consecutive numbers, and pulled in sequential order |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded to assignment to treatment groups. Drug and placebo tablets were identical in appearance, taste and smell, and packaging. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none for pain related to IUD insertion; also had follow‐up at 1 to 2 weeks |
Heikinheimo 2010.
| Methods | Location: Finland, France, Ireland and Sweden Recruitment time: 2006 to 2007 Sample size calculation and outcome of focus: Assuming proportion of easy insertions = 0.99 misoprostol and 0.79 placebo, two‐sided significance level .05, 2 dropouts per treatment group, power 80%: 86 needed to test difference in ease of LNG‐IUS insertion. |
|
| Participants | General with N: 89 women Source: 17 clinics in 4 countries Inclusion criteria: used first LNG‐IUS for 4 years plus 3 to 9 months and opted for immediate replacement with second LNG‐IUS, good general health, 23 to 45 years of age, normal cervical smear result, clinically normal breast examination, normal size uterus, uterine cavity sound measure 6 to 10 cm, and willing and able to participate Exclusion criteria: any signs of genital infection, menopausal symptoms, body mass index (BMI) > 35 kg/m², ovarian cysts > 30 mm transverse diameter, contraindications to misoprostol or LNG‐IUS or positive pregnancy test |
|
| Interventions | 1) Misoprostol 400 μg (in 2 tablets), sublingually
2) Placebo Timing: 3 h before the removal and insertion procedure |
|
| Outcomes | Primary: pain at removal of first IUC and insertion of LNG‐IUS (4 categories: none, mild, moderate and severe) Secondary: adverse events (on occurrence and 2 h after medication) |
|
| Notes | IUC used: LNG‐IUS Study was subset of multi‐site trial that evaluated bleeding profile and safety of repeat use of LNG‐IUS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Created by SAS randomization program at ratio of 1:1, stratified by site |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes, numbered and used consecutively |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, doctors and staff performing the insertion were blinded. Tablets looked identical. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none (not applicable) |
Hubacher 2006.
| Methods | Location: Santiago, Chile Recruitment time: June 2002 to August 2003 Sample size calculation: none (sub‐analysis), trial size determined by main outcome |
|
| Participants | General with N: 2019 women (204 nulliparous) Source: 42 Ministry of Health facilities and 1 private clinic Inclusion criteria: aged 18 to 49 years, literate, menstruated in last 6 weeks, never used an IUD, > 6 weeks postpartum if recently pregnant Exclusion criteria: medical contraindications to IUDs or ibuprofen, had used IUD before |
|
| Interventions | 1) Ibuprofen 400 mg 2) Placebo Timing: at least 45 min before insertion |
|
| Outcomes | Pain score during IUD insertion (10 cm VAS) | |
| Notes | IUC used: CuT 380A 7 subjects also received paracervical block 94% of insertions were done within 5 days of menstrual cycle start |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random permuted blocks with block sizes of 20, 10, 4, and 2 |
| Allocation concealment (selection bias) | Low risk | Central pharmacy dispensed sealed pill bottles; labeled group A and group B |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were unaware of assignment; identical‐appearing placebo and ibuprofen tablets |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 1 in ibuprofen arm did not have pain score data and was not analyzed. |
Ibrahim 2013.
| Methods | Location: Ismailia, Egypt Recruitment time: July 2010 to December 2011 Sample size calculation and outcome of focus: not reported; outcome of focus was success or failure of IUD insertion |
|
| Participants | General with N: 274 women (1:1 ratio) Source: Gynaecology Clinic of Suez Canal University Hospital Inclusion criteria: delivered previously by cesarean section, requesting IUD insertion Exclusion criteria: previous vaginal delivery or contraindication for IUD insertion (e.g., uterine bleeding of undetermined origin, fibroids or other uterine abnormalities, active vaginitis or cervicitis, history of PID or puerperal sepsis); < 4 weeks postpartum; pregnant; on anticoagulant therapy |
|
| Interventions | 1) Misoprostol 400 μg (in 2 tablets) sublingually + diclofenac potassium 100 mg orally 2) Control: 100 mg diclofenac oral route Timing: 1 h before IUD insertion |
|
| Outcomes | Primary: pain during IUD insertion (0 to 10 VAS); follow‐up 1 week and 1 month later Secondary: satisfaction and side effects For trial, primary was success or failure of insertion. Secondary were provider's ease of insertion, woman's satisfaction with procedure, and complications or side effects until follow‐up at 1 month. |
|
| Notes | IUC used: CuT 380A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number table, source not specified |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes, numbered and used consecutively |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blinded Drugs administered were unknown (blinded) to investigating doctors who inserted IUDs but not to participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 30 intervention and 25 control had incomplete data for follow‐up, but this does not affect data for pain during IUD insertion |
Jensen 1998.
| Methods | Location: Herlev, Denmark Recruitment time: May 1994 to May 1995 Sample size calculation: formal a priori sample size calculation performed |
|
| Participants | General with N: 55 women (3 nulliparous) Source: 1 family planning clinic Inclusion criteria: Danish‐speaking women in good health desiring IUD insertion Exclusion criteria: age < 18 years, serious illness, allergy to NSAID or aspirin, dyspepsia or peptic ulcer disease, medication of any kind except for oral contraceptives |
|
| Interventions | 1) Ibuprofen 600 mg 2) Placebo Timing: 1 to 4 h before, 4 to 6 h after, and morning after insertion |
|
| Outcomes | Primary: pain score during IUD insertion (scale 1 to 10, from least to most intense pain) Secondary: pain score in first 4 to 6 h after insertion and over following 3 days. Correspondence with investigator indicated that participants measured pain before ingesting study medication scheduled at 4 to 6 h after insertion. |
|
| Notes | IUC used: Nova‐T or TCu 380S Authors of initial review corresponded with investigator to obtain additional information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Correspondence with investigator indicated use of a computer‐generated scheme; stratified by IUD type |
| Allocation concealment (selection bias) | Low risk | Central pharmacy packaging of drug in opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were unaware of the assignment, tablets were of same size and shape, but taste may have differed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Karabayirli 2012.
| Methods | Location: probably Turkey (location of investigators) Recruitment time: not reported Sample size calculation and outcome of focus: Based on initial pilot measurements, assuming SD within each group approximately 1.8, maximum difference of mean pain score (10‐point VAS) was 1.5, sample size of 29 in each group was calculated with power 80% and alpha .05 |
|
| Participants | General with N: 103 women Source: not reported; possibly university clinic Inclusion criteria: healthy (physical status ASA I) multiparous women of childbearing age (18 to 49 years), scheduled for IUD insertion Exclusion criteria: known allergy or hypersensitivity to NSAIDs or tramadol hydrochloride; current use of narcotic or NSAID; history of epilepsy, peptic ulcer disease, bleeding disorder, asthma, or hepatic or renal failure; nulliparity; delivery within 12 months, and breastfeeding at time of insertion |
|
| Interventions | 1) Naproxen sodium 550 mg (Apranax Fort tablets) 2) Tramadol HCl 50 mg (Contramal capsules) 3) Placebo (empty capsules) Timing: 1 h before insertion of IUD |
|
| Outcomes | Primary: pain score immediately after IUD insertion (10‐point VAS, with 10 meaning 'worst imaginable pain') Secondary: side effects, satisfaction and preference |
|
| Notes | IUC used: Multiload Cu 375 Standard | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and involved physicians of obstetrics and gynecology were blinded to the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Lathrop 2013.
| Methods | Location: Atlanta, GA Recruitment time: from May 2010 to December 2010 Sample size calculation and outcome of focus: Assuming 23 mm SD, alpha .05 and power > 80%, sample size of total 70 was calculated to detect mean difference of at least 16 mm in primary outcome of pain using 100 mm VAS. |
|
| Participants | General with N: 73 women Source: Emory Clinic, faculty practice affiliated with Emory University Inclusion criteria: desired IUD, ≥ 18 years old, negative pregnancy test with no prior pregnancies beyond 19 6/7 weeks Exclusion criteria: PID diagnosed in last 3 months, cervicitis, currently pregnant or had pregnancy in past 14 days, sepsis associated with most recent pregnancy, known uterine anomaly or fibroid that distorts uterine cavity, contraindication to desired IUD (copper or levonorgestrel) based upon CDC medical eligibility criteria, inflammatory bowel disease or known allergy to misoprostol |
|
| Interventions | 1) Misoprostol 400 μg, buccally 2) Placebo Timing: 2 to 4 h prior to IUD insertion |
|
| Outcomes | Primary: pain scores immediately after IUD insertion and before discharge from clinic (100 mm VAS with 0 = none, 100 mm = worst imaginable) Secondary: side effects and satisfaction (1 week and 1 month later); ease of insertion (not powered to detect) |
|
| Notes | IUC used: CuT 380A and LNG‐IUS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, block randomization in blocks of 8 |
| Allocation concealment (selection bias) | Low risk | Medication and control had identical appearance and absorption into a single troche and were prepackaged in identical plastic containers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, research staff and providers were blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Lotke 2013.
| Methods | Location: Tucson, AZ, USA Recruitment time: January 2009 to January 2011 Sample size calculation and outcome of focus: Assuming 23 mm SD, alpha 0.05 and power > 80%: 60 participants needed to detect mean difference ≥ 15 mm in primary outcome of participant‐reported pain using 100 mm VAS. |
|
| Participants | General with N: 61 women Source: 3 University of Arizona gynecology clinic locations Inclusion criteria: nulliparous, 18 to 45 years old, interested in IUD for contraception Exclusion criteria: previous pregnancy > 14 weeks gestation, active pelvic infection or cervicitis, uterine anomaly, fibroid uterus, copper allergy or Wilson's disease (for Paragard only), undiagnosed abnormal uterine bleeding, cervical or uterine cancer |
|
| Interventions | 1) Misoprostol 400 μg, vaginally or buccally 2) Placebo Timing: 2 h prior to IUD insertion |
|
| Outcomes | Primary: pain scores (100 mm VAS with 0 = none, 100 mm = worst imaginable), immediately following IUD insertion Secondary: side effects (prior to IUD insertion), satisfaction measured on 5‐point scale (1 = very unsatisfied, 5 = very satisfied); provider‐rated ease of insertion follow‐up at 1 week and 1 month |
|
| Notes | IUC used: Paragard (CuT 380A) or Mirena (LNG‐IUS) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomization in blocks of 8 by clinical pharmacy at another university |
| Allocation concealment (selection bias) | Low risk | Pills were sequentially numbered in identical pill vials |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: data reported for 30 women in each group (of 30 or 31 initially) |
Maguire 2012.
| Methods | Location: New York, NY, USA Recruitment time: October 2010 to March 2011 Sample size calculation and outcome of focus: Assuming 10% dropout, two‐sided alpha .05 and 90% power, sample size of 110 women was calculated to detect 20 mm pain (100 mm VAS) reduction. To evaluate subgroups, sample size was increased to 200. |
|
| Participants | General with N: 200 women (60 nulliparous) Source: 2 sites (New York hospital family planning clinic and university family planning faculty practice) Inclusion criteria: speak English or Spanish, age 18 to 45 years, chose IUD for birth control, could undergo IUD insertion during that clinic visit as determined by health care provider Exclusion criteria: current participation in other clinical research, pregnancy ending in past 4 weeks or lidocaine allergy |
|
| Interventions | 1) Lidocaine 2% gel; cotton swab soaked in 1 mL 2) Placebo gel; cotton swab soaked in 1 mL Timing: 60 seconds before clinician sounded uterus and then inserted the IUD; swab remained in cervix for 60 seconds |
|
| Outcomes | Primary: pain during sounding, tenaculum placement, and IUD insertion as measured by 100 mm VAS Secondary: side effects and satisfaction |
|
| Notes | IUC used: CuT 380A IUD (Paragard) and LNG‐IUS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Fixed block sizes of 10, 1:1 allocation ratio, created by SAS 9.2 |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant, provider and researcher analyzing data remained blinded to treatment allocation. Lidocaine gel and placebo gel were both colorless and odorless and of similar consistency. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Massey 1974.
| Methods | Location: Palo Alto, CA, USA Recruitment time: not reported Sample size calculation: not reported |
|
| Participants | General with N: 50 women (48 nulliparous) Source: student health center Inclusion criteria: IUD use deemed appropriate, normal physical examination and laboratory tests Exclusion criteria: severe painful menses, or premenstrual tension |
|
| Interventions | 1) Naproxen 300 mg (x 4 doses) + paracervical block (8 mL 1% lidocaine) 2) Placebo + paracervical block (8 mL 1% lidocaine) Timing: oral medications at 10:00 PM the night before and 1.5 h before IUD insertion; oral medications scheduled for 2 and 6 h after IUD insertion |
|
| Outcomes | Primary: pain score during and immediately after IUD insertion, at hourly intervals afterward for 10 h, and at 24 h (scale 1 to 5: 1 = no discomfort, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe discomfort) Secondary: requirement for additional analgesia, abdominal cramping, backache, headache, cold sweats, nausea, and vomiting; recorded before and after taking first 2 oral medications, before and immediately after IUD insertion, at hourly intervals thereafter for 10 h, and at 24 h Study recorded side‐effect data but report did not have those results. |
|
| Notes | IUC used: Dalkon Shield; dilated to 4 mm Authors of initial review corresponded with investigator to obtain additional information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permuted blocks of 10; source not specified |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators unaware of assignment; identical appearing placebo and naproxen tablets |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: none for pain during or immediately after IUD insertion; thereafter, women requiring additional medications were considered dropouts (investigators analyzed time in study without needing additional analgesia) |
McNicholas 2012.
| Methods | Location: St. Louis, MO, USA Recruitment time: 1 August 2011 to 1 December 2011 Sample size calculation and outcome of focus: Based on previous study, assuming alpha .05, 90% power and SD 2.5 (10‐point scale), adding 15% for dropout, stratifying for parity, sample size of 100 nulliparous and 100 parous women calculated to detect 50% reduction in mean pain score. |
|
| Participants | General with N: 200 women Source: Contraceptive CHOICE Project at Washington University Inclusion criteria: age 18 to 45 years presenting to CHOICE Project; ability to give written informed consent in English; willing to be randomized and complete study questionnaires; no contraindication to or history of allergic reaction to lidocaine Exclusion criteria: not reported |
|
| Interventions | 1) 2% lidocaine gel (2.5 to 4 mL over 2 sites) 2) Placebo gel (2.5 to 4 mL over 2 sites) Timing: 3 min before the initiation of IUD insertion |
|
| Outcomes | Primary: pain with tenaculum placement and pain with IUD insertion (10‐point scale) Secondary: side effects, adverse events |
|
| Notes | IUC used: LNG and Copper All participants received ibuprofen approximately 10 min prior to procedure to minimize post‐procedure cramping. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted varying block size randomization scheme with nQuery software; stratified by parity |
| Allocation concealment (selection bias) | Low risk | Labeled and sealed opaque envelopes Gels were indistinguishable in appearance by color and consistency. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant and clinician placing the IUD were blinded to identity of gel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Micks 2014.
| Methods | Location: Portland, OR, USA Recruitment time: from March 2012 to November 2012 Sample size calculation: Assuming pooled SD 28 mm, one‐sided alpha 0.05, power 82%, and superiority of treatment, sample size of 12 in each group was calculated to detect 30 mm pain score difference between groups. This pilot study was not powered to detect clinically significant decrease in pain. |
|
| Participants | General with N: 24 nulliparous women Source: Oregon Health and Science University (OHSU) and Planned Parenthood Columbia Willamette (PPCW) Inclusion criteria: aged 18 to 45 years, generally healthy and were requesting LNG‐IUS, (Bayer Healthcare Pharmaceuticals) for contraception Exclusion criteria: 1) previous pregnancy beyond 20 weeks; 2) previous IUD placement or attempted IUD placement; 3) previous cervical cold knife cone or loop electrosurgical excision procedure; 4) contraindication to LNG‐IUS or nitroglycerin; 5) history of hypertensive or hypotensive disorder; 6) history of migraine, cluster headaches or vascular headaches; and 7) blood pressure < 90/55 or > 150/100 in office prior to speculum exam |
|
| Interventions | 1) 1 mL of 0.5 mg nitroglycerin ointment 2) Placebo Timing: 30 to 45 min before procedure Participants were given option of premedication with ibuprofen 800 mg prior to receiving study medication. |
|
| Outcomes | Primary: pain at tenaculum placement, during IUD insertion, and 30 min post‐procedure (100 mm VAS with 0 mm = no pain, 100 mm = most pain imaginable) Secondary: satisfaction (100 mm VAS), side effects and ease of insertion |
|
| Notes | IUC used: LNG‐IUS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated; source not specified |
| Allocation concealment (selection bias) | Low risk | Central randomization through research pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and all study staff involved in care of participants were blinded to study arm allocation. Placebo was identical. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Mody 2012.
| Methods | Location: Chicago, IL, USA Recruitment time: July 2010 to February 2011 Sample size calculation and outcome of focus: from published literature, assuming alpha .05, 80% power and SD 2.2 cm, and accounting for dropout, sample size of 50 participants calculated to detect 20 mm mean pain score difference between groups (0 to 100 mm VAS) |
|
| Participants | General with N: 50 women Source: Obstetrics and Gynecology practice, Northwestern Medical Faculty Foundation Inclusion criteria: seeking IUD and expressed interest in participating Exclusion criteria: copper allergy, current cervicitis, levonorgestrel allergy, lidocaine allergy, misoprostol use within 24 h prior to IUD insertion, pain medication use within 6 h prior to IUD insertion, PID within 3 months, pregnancy within 6 weeks, prior successful IUD insertion or prior IUD insertion attempt, uterine anomaly or distortion of uterine cavity |
|
| Interventions | 1) 10 mL 1% lidocaine paracervical block 2) No analgesia (standard of care) Timing: 3 min before initiation of IUC insertion Injections: cervical‐vaginal junction; 2 mL at 12 o'clock, 5 mL at 4 o'clock, 5 mL at 8 o'clock |
|
| Outcomes | Primary: pain with tenaculum placement, during IUD insertion, and 5 min post‐procedure (100 mm VAS) Secondary: side effects |
|
| Notes | IUC used: levonorgestrel‐releasing, Copper T 380A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permuted blocks of 4, stratified by parity, source not specified |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Providers not blinded Participants apparently not blinded; controls received no analgesia. Pain data collected by clinician not involved in procedure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Mohammad‐Alizadeh‐C 2010.
| Methods | Location: Tabriz, Iran Recruitment time: June 2010 to October 2010 Sample size calculation and outcome of focus: Based on published literature, assuming mean of IUD insertion pain 3.6 with SD = 1.1 for group with no intervention and using software Stata 9.2, sample size of 32 calculated for each group to detect at least 20% reduction in pain levels with two‐sided 5% significant level and power 90%. |
|
| Participants | General with N: 96 women (33 lubricant, 31 lidocaine, 32 control) Source: public health center in Tabriz with highest IUD insertion in city Inclusion criteria: referred to health center for IUD insertion during study period, no contraindications for IUD insertion in accordance with national guidelines, signed informed consent form Exclusion criteria: difficulty in inserting IUD and uterine depth < 6 cm or > 9 cm |
|
| Interventions | 1) 2% lidocaine gel on swab; quantity not specified 2) Lubricant gel 3) No intervention Timing: 1 min before tenaculum placement and then IUD insertion; swab left 1 min in cervical canal |
|
| Outcomes | Pain score during IUD insertion (0 to 10 VAS); measured pain intensity in "entire IUD insertion process"' | |
| Notes | IUC used: CuT 380A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation sequence was generated using 6 and 9 block sizes and computer‐generated random digits |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, assessor and data analyst were blinded to 3 groups. IUD inserter was blind to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Nelson 2013.
| Methods | Location: Los Angeles, CA, USA Recruitment time: from June 2008 to December 2008 Sample size calculation: Sample size arbitrarily selected for convenience to meet time and financial constraints in this investigator‐funded study. |
|
| Participants | General with N: 40 women Source: Women's Health Care Clinic (WHCC) at the Los Angeles Biomedical Research Institute at Harbor‐UCLA Medical Center Inclusion criteria: women identified as candidates for IUD use following clinic protocols, had given consent for IUD insertion and expressed interest in participating Exclusion criteria: not reported |
|
| Interventions | 1) 2% lidocaine (1.2 mL) infused into endometrial cavity (lower third, middle, and top of cavity) 2) Saline (1.2 mL) Timing: 3 min before IUD was inserted |
|
| Outcomes | Primary: pain score after tenaculum placement, after liquid infusion and IUD insertion, pain score of overall procedure (0 to 9 scale) Secondary: pain score comparisons by IUD types, prior IUD use, NSAID use, and by timing of placement during menses or not |
|
| Notes | IUC used: CuT 380A or LNG‐IUS 11 women took NSAIDs 15 min to 2 h prior to IUD insertion |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done in a 1:1 ratio using a random number generator. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered and sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blinded" 2 clinicians who inserted IUDs were blinded to study group; participants apparently blinded. Research nurse placed study medication into tubing out of sight of provider and participant. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Ngo 2014.
| Methods | Location: San Diego, CA, USA Recruitment time: July 2012 to May 2013 Sample size calculation and outcome of focus: not reported |
|
| Participants | General with N: 67 women Source: university medical center Inclusion criteria: nulliparous and multiparous women ages 18 to 50, English‐ or Spanish‐speaking, presenting for IUD insertion for contraception or menorrhagia (in the case of Mirena IUD insertion) Exclusion criteria: pregnancy, any diagnosed pain issues, if the patient has taken any pain medications within 6 h of enrollment (including aspirin or other NSAIDs), misoprostol within 24 h of enrollment, history of prior IUD insertion, known allergy to NSAIDs (including diagnosis of aspirin or NSAID‐induced asthma or urticaria), known contraindications to NSAIDs (including bile acid sequestrants, cyclosporine, drotrecogin, floctafenine, lithium, methotrexate, pentoxifylline, probenecid, rivaroxaban, SSRIs, warfarin), renal insufficiency, peptic ulcer disease or history of significant gastrointestinal bleeding, known thrombocytopenia, known coagulopathy, or known bleeding disorder, and known contraindications to IUD |
|
| Interventions | 1) Ketorolac (Toradol) injection (30 mg, 1 cc volume) 2) Placebo 0.9% normal saline injection (1 cc volume) Timing: 30 min prior to IUD insertion |
|
| Outcomes | Primary: pain score with IUD insertion 5 min after insertion and 15 min after insertion (10 cm VAS) Secondary: side effects and satisfaction (assessed, according to ClinicalTrials.gov listing, but not in abstract) |
|
| Notes | IUC used: LNG‐IUS; unclear whether copper‐containing IUD also used Information from conference abstract and ClinicalTrial.gov listing |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
Rapkin 2014.
| Methods | Location: Pittsburgh, PA, USA Recruitment time: June 2012 to May 2013 Sample size calculation: not reported |
|
| Participants | General with N: 64 nulliparous women, 14 to 55 years of age Source: Women's hospital Inclusion criteria: nulliparous (no history of pregnancy ≥ 24 weeks gestational age), desires insertion of either LNG‐IUD or copper T380A IUD (Cu‐IUD), no history of pregnancy in last 6 weeks, able to provide written informed consent in English and comply with study procedures Exclusion criteria: known allergy or hypersensitivity to lidocaine or other amino amide local anesthetics, prior failed IUD insertion, prior IUD use, use of narcotic or benzodiazepine medication in last 24 h, US CDC Medical Eligibility Criteria (MEC) category 3 or 4 classification for use of IUD, positive pregnancy test or reasonable risk of pregnancy due to unprotected heterosexual intercourse since last menstrual period |
|
| Interventions | Self‐administered: 1) 2% lidocaine gel, 5 mL (investigator confirmed 2% used, not 1% as abstract stated) 2) Placebo gel Timing: ≥ 5 min before IUD insertion |
|
| Outcomes | Primary: change in pain from baseline to speculum and tenaculum placement and IUD insertion (100 mm VAS) Secondary: acceptability of self‐inserting gel prior to IUD (assessed, according to ClinicalTrials.gov listing, but not in abstract); ease of IUD insertion as reported by physicians |
|
| Notes | IUC used: LNG‐IUD or CuT 380A Information from abstract and ClinicalTrial.gov listing 14 October 2014: Investigator communicated that report is in progress and will be submitted for publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind (participant, caregiver, investigator) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
Scavuzzi 2013.
| Methods | Location: Recife, Pernambuco, Brazil Recruitment time: January 2009 to November 2011 Sample size calculation and outcome of focus: Considering frequency of subjective difficulty in inserting IUD of 45% in placebo and 50% reduction in rate with use of misoprostol, adding 20% for dropout: 190 calculated using OpenEpi software program. |
|
| Participants | General with N: 190 nulligravid women Source: family planning clinic of Institute of Medicine in Recife Inclusion criteria: nulligravidas, reproductive age, never had surgery of uterine cervix and requested IUD as contraceptive method Exclusion criteria: contraindication to IUD use as defined in categories 3 and 4 of medical eligibility criteria for contraceptive use (WHO 2004) |
|
| Interventions | 1) Misoprostol 400 μg, vaginally 2) Placebo Timing: 4 h prior to IUD insertion |
|
| Outcomes | Primary: pain at insertion (0 to 10 scale, 0 = absence of pain and 10 = worst pain imaginable); categorized as 'absent or mild' (0 to 5) and 'moderate or severe' (6 to 10) Secondary: side effects (prior to (used in this review), during, and 24 h after IUD insertion) and satisfaction |
|
| Notes | IUC used: CuT 380A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization carried out (1:1) using block randomization method by Random Allocation Software program, version 1.0 (Isfahan, Iran), and labeled using letters A and B. List sent to pharmaceutical company, where coding (misoprostol or placebo) of each letter A and B, was randomly selected. |
| Allocation concealment (selection bias) | Low risk | Each woman was identified by sequential ordinal number corresponding to sealed box containing medication or placebo. Each box was identified with woman’s name and registration number, and only opened when tablets had to be inserted into vagina. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither investigator nor woman was aware if misoprostol or placebo was to be administered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 1 woman in misoprostol group discontinued study after having medication |
Shahnazi 2012.
| Methods | Location: Ardebil, Iran Recruitment time: 2011 Sample size calculation and outcome of focus: Stata software used; sample based on 'findings of survey', i.e., 53 each group |
|
| Participants | General with N: 106 married women, 15 to 49 years old Source: health care center Inclusion criteria: no contraindications for IUD insertion; no history of cervical surgery; Spielberger score > 30 of 20 questions; understanding the consent; no severe pain while completing questionnaire; no use of benzodiazepines, tranquilizers, narcotics or analgesics; no eczema or its past history; no asthma, allergy, migraines or chronic headaches according to participant report; no active mental diseases; no impaired sense of smell based on participant report Exclusion criteria: not specified |
|
| Interventions | 1) Lavender 10 drops with diluted milk; inhale 3 drops on cotton and add drops if needed 2) Placebo (diluted milk) Timing: 30 min before and during IUD insertion |
|
| Outcomes | Primary: anxiety; pain immediately after IUD insertion (VAS 0 to 10) Secondary: no mention |
|
| Notes | IUC used: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized table of random numbers; blocks of 4 and 6 |
| Allocation concealment (selection bias) | Low risk | Sealed bottles handed to participants with numbers from 1 to 106. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not aware of allocation: person in charge of IUD insertion and person who measured anxiety and pain Participants informed of random selection and researcher did not know who would be assigned to which group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none |
Swenson 2012.
| Methods | Location: Salt Lake City, UT, USA Recruitment time: January 2009 to November 2010 Sample size calculation and outcome of focus: From published literature, assuming standard deviation 23, alpha .05, beta 0.10, and power 90%, sample size of total 100 women needed to detect 15 mm difference in primary outcome of participant‐perceived pain using the 100 mm VAS. |
|
| Participants | General with N: 108 women Source: University outpatient obstetrics and gynecology clinic Inclusion criteria: seeking intrauterine contraception, ≥ 18 years of age, negative pregnancy test, no prior pregnancies beyond 13 6/7 weeks of gestation, willing to follow up in 1 month for IUD string check Exclusion criteria: active cervical infection, PID in last 3 months, current pregnancy, prior pregnancy beyond 14 weeks of gestation, known uterine anomaly, uterine leiomyoma that distorts uterine cavity, copper allergy or Wilson’s disease (for copper T380A), abnormal bleeding, history of genital tract cancer, used narcotics or benzodiazepines on day of procedure |
|
| Interventions | 1) Misoprostol 400 μg, vaginally or buccally 2) Placebo Timing: 3 to 4 h before the IUD insertion |
|
| Outcomes | Primary: pain during IUD insertion and highest pain level after insertion (before leaving clinic) (100 mm VAS with 0 = extremely easy and 100 = worst imaginable) Secondary: side effects and satisfaction (1 week later); ease of insertion |
|
| Notes | IUC used: CuT 380A and LNG‐IUS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, blocks of 4 |
| Allocation concealment (selection bias) | Low risk | University pharmacy generated random allocation sequence and dispensed medication. Medication was identical appearance, taste, and smell as well as absorption; formulated by study pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and healthcare providers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 2 placebo participants received pre‐medication for pain and were excluded, and 1 placebo participant did not return for IUD insertion. |
Sääv 2007.
| Methods | Location: Stockholm, Sweden Recruitment time: September 2004 to July 2006 Sample size calculation: based on other published research |
|
| Participants | General with N: 80 women Source: Karolinska University Hospital Inclusion criteria: nulliparous, general good health, age ≥ 18 years Exclusion criteria: signs of genital infection, contraindication to misoprostol, positive pregnancy test |
|
| Interventions | 1) Misoprostol 400 µg sublingually + diclofenac 100 mg 2) Diclofenac 100 mg Timing: 1 h before insertion |
|
| Outcomes | Primary: difficulty of insertion rated by provider (easy, moderate, difficult) Secondary: baseline cervical dilation, pain score with IUD insertion (10 cm VAS), general insertion experience rated by woman (very unpleasant, unpleasant, or 'very little unpleasant'); side effects (shivering, diarrhea, nausea, vomiting), pain, and bleeding (after insertion and up to 1 month) |
|
| Notes | IUC used: Nova‐T Protocol violations: 2 in misoprostol group and 3 in diclofenac‐only group received 2 tablets of acetaminophen/codeine (strength not reported) instead of diclofenac due to history of asthma. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number table by study nurse not directly involved in study |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes numbered and used consecutively |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators, but not participants, were unaware of assignment; nurse administered study medication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up: 2 in diclofenac‐only arm had failed insertions but were included in analysis; 1 in misoprostol arm withdrew consent prior to insertion and was not analyzed; 1 in misoprostol arm lost after insertion and 1‐month data were not analyzed. |
Tornblom‐Paulander 2015.
| Methods | Location: Stockholm, Norrkӧping, Linkӧping; Sweden Recruitment time: June 2012 to May 2013 Sample size calculation: From pilot study, SD for pain in 10 min was 40 mm. Assuming VAS difference 20 mm between arms, 86 needed per group at 5% level, 90% power; considering withdrawals, overall recruitment target was 200. |
|
| Participants | General with N: 218 women Source: 3 hospitals Inclusion criteria: nulliparous women who want IUC; at least 18 years of age a) If regular menstruation, in menstrual cycle day 1 to 6 at insertion or pregnancy reliably excluded b) If no or small irregular bleedings due to hormonal contraception or other reasons, insertion may take place any day if pregnancy reliably excluded. • understand Swedish language for study procedures • give written informed consent after verbal and written information Exclusion criteria: • clinical evidence of, or ongoing treatment for, active cervical infection • positive pregnancy test • PID in past month • history of uterine colonization • known uterine anomaly that contraindicates IUD insertion • copper allergy or Wilson’s disease • cervical or uterine cancer • known allergy to lidocaine • known intolerance to paracetamol (rescue medication) • recidivating porphyria • significant morbidity interfering with study drug or procedures • took any analgesics 24 h prior to IUD insertion • previous pregnancy > 13 gestational weeks |
|
| Interventions | 1) SHACT: 4% lidocaine formulation, short‐acting; viscosity increases with increasing temperature; 8.5 mL applied (1 mL on surface of portio, 2 mL in cervical canal, 5.5 mL in uterine cavity) 2) Placebo gel Timing: 5 min before IUC insertion |
|
| Outcomes | Primary: pain during insertion (reported within 10 min), 30 min after and 1 h after IUC insertion (assessed with 100 mm VAS); later pain recorded in diary on day 1 (2 h after IUC insertion) and on days 2 to 4 Secondary: safety and tolerability, assessed 10 min after and 1 h after IUC insertion; asked participants to report adverse events up to 10 days, excluding menstrual pain, abdominal pain, and abdominal discomfort related to IUC insertion; participants recorded use of acetaminophen (provided) for 4 days after IUC insertion |
|
| Notes | IUC used: LNG‐IUS or Nova T 380 Investigator provided SDs and Ns needed for analysis that were not in the report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by the study statistician using nQuery Advisor; 1:1 randomization |
| Allocation concealment (selection bias) | Low risk | From code numbers on randomization list, contract manufacturing organization prepared labeled vials for hospital pharmacies. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded (no detail on who) "Randomization list concealed from all study personnel until study completion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: none mentioned (no flow chart) Exclusions: 9 of 218 (4%) women from efficacy analysis because no IUC placed (uterus too small for insertion): 4 from lidocaine group and 5 from placebo group, according to Ns provided by investigator. |
CDC: Centers for Disease Control h: hour(s) LNG‐IUS: levonorgestrel‐releasing intrauterine system min: minute(s) NSAID: nonsteroidal anti‐inflammatory drug PID: pelvic inflammatory disease SD: standard deviation VAS: visual analogue scale WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Goldstuck 1983 | Examined pain in the first 7 days after IUD insertion, not during or after IUD insertion up to 6 h |
| Hepburn 1980 | Full text indicated not a randomized controlled trial |
| Hollingworth 1995 | No abstract; full text indicated this was review article |
| Jafari 2014 | Intervention did not meet eligibility criterion; began at the first clinic visit after IUD insertion. |
| Mirmohamad Aliei 2013 | Random assignment by day; analysis did not appear to account for clustering effects. Article written in Persian; information extracted from English translation. |
| Newton 1977 | Full text indicated alternate assignment to study arms |
| Oloto 1997 | Randomized by birth date |
| Stephenson 2010 | Investigator communicated that trial never started; funding source withdrawn. |
| Teal 2012 | Investigator communicated that study never started. Had been planned as part of Turok 2011. Providers had little difficulty inserting IUDs; had no interest in a misoprostol arm. |
| Thiery 1985 | Full text indicated this was not a randomized controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
Brody 2011.
| Methods | Location: Chattanooga, TN, USA Recruitment time: August 2010 to September 2012 Sample size calculation and outcome of focus: no mention |
| Participants | General with N: 210 women Source: University department of obstetrics and gynecology Inclusion criteria: receiving Mirena for standard indications, did not receive NSAID Exclusion criteria: do not desire to be in study, have taken narcotics, Mirena not able to be placed, cervical dilation required, allergy to lidocaine |
| Interventions | 1) 5 cc 2% lidocaine gel on cervix and intracervically 2) Lubricant: KY gel Timing: no mention |
| Outcomes | Primary: VAS (0 to 10) pain scores in 3 categories of 0 to 2, 3 to 4, and ≥ 5; assessed at time of insertion and at 5 and 10 min after insertion Secondary: no mention |
| Notes | IUC used: LNG‐IUS (Mirena) Estimated completion September 2012 Could not find any publication; unable to obtain information from investigator (15 October 2014) |
Elsafty 2011.
| Methods | Location: Cairo, Egypt Recruitment time: July 2011 to December 2011 Sample size calculation and outcome of focus: no mention |
| Participants | General with N: 200 Source: University Hospital Inclusion criteria: age 18 to 45, speak Arabic or English Exclusion criteria: history of cervical surgery, known hypersensitivity to topical analgesics, first trimester abortion or miscarriage in previous 6 weeks, second trimester abortion or miscarriage in previous 12 weeks |
| Interventions | 1) Lidocaine spray 10% 2) Saline spray Timing: unclear |
| Outcomes | Primary: VAS score of pain 5 min from spraying and after application of tenaculum Secondary: no mention |
| Notes | IUC used: no mention 19 October 2014: Investigator communicated that trial was completed; report written but not yet published. Contact: Mohamed Ibrahim Emeira +201224456471 emeira2@gmail.com |
Khodokarami 2011.
| Methods | Location: Karaj, Alborz, Iran Recruitment time: October 2010 to October 2011 Sample size calculation and outcome of focus: no mention |
| Participants | General with N: 150 women Source: Shohadaye Fardis health services, Shohadaye Aghtape health services Inclusion criteria: Age 18 to 48 years, Iranian, speak Farsi, read and write, no cold or smelling disorder, no use of 'alleviating' during last 24 h, no smoking or opiate, no asthma Exclusion criteria: allergy to chamomile, misuse of chamomile |
| Interventions | 1) Chamomile essential oil, 3 drops on cotton; 7 to 10 cm from nose 2) Placebo: propylene glycol aroma 3) Control group: nothing Timing: 'inhale for 5 min' |
| Outcomes | Primary: pain immediately after IUD insertion Secondary: no mention |
| Notes | IUC used: no mention 15 October 2014: Investigator communicated that report will soon be published in Farsi. Plan to translate into English for submission to international journal. Contact: Nahid Khodakarami, Shahid Beheshti University of Medical Sciences, Taleghani hospital; Tehran, Iran; khodakarami@sbmu.ac.ir |
Singh 2015.
| Methods | Location: Albuquerque, NM, USA Recruitment time: October 2013 to August 2014 Sample size calculation and outcome of focus: no mention |
| Participants | General with N: 80 women, 13 to 45 years old Source: reproductive health center Inclusion criteria: age > 18 OR age 12 to 17 with parent or legal guardian who can consent; English‐speaking; desires Mirena® or ParaGard® IUD; nulliparous; can use laughing gas; no narcotic pain medication prior to procedure Exclusion criteria: currently pregnant; ever pregnant > 19 weeks, 6 days; < 4 weeks since spontaneous abortion or medical abortion; desires Skyla® IUD; PID in last 3 months; current mucopurulent discharge; uterine anomaly that distorts uterine cavity; known uterine fibroid with disruption of uterine cavity; copper allergy or Wilson's disease (for ParaGard®); current cervical or uterine cancer; inability to breathe through nose; significant active upper airway infection |
| Interventions | 1) 50% nitrous oxide and 50% oxygen via nasal mask 2) 100% oxygen via nasal mask Timing: 2 min before and throughout procedure; 100% oxygen after procedure for 3 to 5 minutes |
| Outcomes | Primary: maximum pain score during IUD insertion (100 mm VAS); clinical trial listing states "change from baseline" at 2 min after procedure and prior to clinic discharge Secondary: satisfaction with overall pain control (VAS and 5‐point Likert), provider ease of insertion |
| Notes | IUC used: LNG‐IUS (Mirena) or Cu T380A (Paragard) Information obtained from conference abstract (May 2015), and clinical trial listing (retrospective, March 2015). Abstract noted mean maximal pain scores at IUD insertion were "similar between groups". Outcome measures inconsistent between abstract and clinical trial listing. Trial can be assessed for next review update; full report may be available then. |
LNG‐IUS: levonorgestrel‐releasing intrauterine system PID: pelvic inflammatory disease
Characteristics of ongoing studies [ordered by study ID]
Fouda 2015.
| Trial name or title | Diclofenac plus lidocaine gel for pain relief during intrauterine device insertion (IUD) |
| Methods | Location: Cairo, Egypt Recruitment time: January 2015 to July 2015 Sample size calculation and outcome of focus: no mention |
| Participants | General with N: 90 women, 18 to 50 years old Source: Obstetrics and Gynecology Department, Cairo University Inclusion criteria: reproductive age, requesting IUD for contraception Exclusion criteria: contraindication to IUD insertion (< 6 weeks postpartum, < 2 weeks after abortion, uterine anomalies, fibroid distorting uterine cavity, pregnancy, PID, cervicitis, uterine depth < 6 cm or > 9 cm) , previous IUD insertion, allergy to diclofenac or lidocaine, peptic ulcer, asthma, bleeding disorders, cardiac, liver or kidney diseases) |
| Interventions | 1) Diclofenac (100 mg) 1 hour before IUD insertion; lidocaine gel placed on cervix 3 min before IUD insertion 2) Placebo tablet 1 hour before IUD insertion; placebo gel placed on cervix 3 min before IUD insertion |
| Outcomes | Primary: intensity of pain during procedure; assessed by VAS Secondary: adverse effects of diclofenac and lidocaine |
| Starting date | January 2015 |
| Contact information | Usama M Fouda, MD, PhD; 01095401375 ext 2; umfrfouda@yahoo.com |
| Notes |
Jamshidi 2014.
| Trial name or title | Study of local anesthesia as a method to decrease IUD insertion related pain |
| Methods | Location: Baltimore, MD, USA Recruitment time: December 2013 to December 2014 Sample size calculation and outcome of focus: no mention |
| Participants | General with N: 50 women Source: University medical center Inclusion criteria: ages 18 to 45 years, nulliparous and "functionally nulliparous" (women who never had vaginal delivery nor previous significant cervical dilation, i.e., women who had miscarriage or abortion < 24 weeks gestation or cesarean section while not in active labor, defined as < 4 cm dilation) Exclusion criteria: presence of CDC Medical Eligibility Criteria for Contraceptive Use category 3 or 4 precaution to levonorgestrel IUD; chronic narcotic use; current or past history of illegal drug use, excluding marijuana; allergy to lidocaine |
| Interventions | 1) Lidocaine: paracervical block using 15 ml 1% lidocaine 2) Placebo: paracervical block using 15 ml bacteriostatic saline Timing: no mention |
| Outcomes | Primary: pain at time of IUD insertion Secondary: pain at other time points of pelvic exam (up to 15 min after IUD inserted); ease of insertion as rated by provider |
| Starting date | December 2013; estimated completion December 2014 |
| Contact information | Roxanne Jamshidi, MD; 410‐550‐0336; rjamshi1@jhmi.edu |
| Notes | IUC used: levonorgestrel IUD |
Mody 2014.
| Trial name or title | Pain control for intrauterine device placement using paracervical block |
| Methods | Location: San Diego, CA, USA Recruitment time: August 2014 to August 2015 Sample size calculation and outcome of focus: no mention |
| Participants | General with N: 144 women Source: University and family planning clinics Inclusion criteria: 18 to 45 years old, nulliparous, English‐ or Spanish‐speaking, present for IUD device placement for contraception or menorrhagia (with LNG‐IUS) Exclusion criteria: pregnancy, diagnosed chronic pain issue, pain medication within 6 h of enrollment, misoprostol administration within 24 h of enrollment, prior IUD insertion, known contraindications to IUD |
| Interventions | Both groups: 2 mL 1% buffered lidocaine anesthetic at anterior lip of cervix, where tenaculum will be placed 1) Paracervical block (PCB) of 18 mL 1% buffered lidocaine, distributed evenly between 4 o'clock and 8 o'clock positions of cervix 2) No PCB (sham PCB: capped spinal needle at places that PCB would have been injected) Timing: no mention |
| Outcomes | Primary: pain with IUD placement (100 mm VAS scale); recorded at time of IUD placement Secondary: baseline and reported pain at different time points (100 mm VAS), i.e., anticipated pain recorded prior to the procedure, at time of speculum insertion, placement of the PCB or sham, tenaculum placement, sounding, and 5 min after IUD placement; intrapersonal change in pain during IUD placement, i.e., ANOVA of pain scores for participants over several time points; overall pain with IUD insertion procedure, recorded 5 min after IUD placement; post‐IUD insertion questionnaire on satisfaction with pain control method |
| Starting date | August 2014; estimated completion August 2015 |
| Contact information | Sheila Mody, MD MPH; 619‐543‐6777; smody@ucsd.edu John Paul Farala; 619‐739‐0262; johnpaul.farala@ucsf.edu |
| Notes | IUC used: Mirena (LNG‐IUS) and Paragard (CuT 380A) mentioned, but unclear whether one or both were used. |
LNG‐IUS: levonorgestrel‐releasing intrauterine system PCB: paracervical block PID: pelvic inflammatory disease
Contributions of authors
2015
LM Lopez ran the searches and reviewed the results, and wrote to investigators for additional information. She did the secondary data extraction for side effects, satisfaction, pain with tenaculum placement, and 'Risk of bias'. She and A Bernholc developed the summary tables. LM Lopez drafted the Results and Discussion.
A Bernholc did the primary extraction and entry of data on side effects, satisfaction, and pain with tenaculum placement. She added Characteristics and pain data for several new trials. A Bernholc checked the Results text, and refined the interpretation of outcome data. She and LM Lopez developed the summary tables.
Y Zeng did the primary data extraction and entry for Characteristics and for pain with IUC insertion. This work included most of the new trials.
RH Allen and D Hubacher updated the Background. RH Allen and PA O'Brien provided feedback on outcomes for inclusion and provided comments on the manuscript.
D Bartz reviewed and commented on the manuscript.
2009
RH Allen and D Bartz developed the protocol, extracted the data, and drafted the review.
DA Grimes (formerly of FHI 360), D Hubacher, and PA O'Brien provided editorial assistance.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Child Health and Human Development, USA.
2008 to 2014: Support for conducting the review and updates at FHI 360
-
US Agency for International Development, USA.
2014: This report is made possible by the generous support of the American people through the United States Agency for International Development (USAID) under the terms of The Evidence Project, co‐operative agreement no. AID‐OAA‐A‐13‐00087. The findings and conclusions are the sole responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government. 2008 to 2009: Support for conducting the review and updates at FHI 360
Declarations of interest
LM Lopez, A Bernholc, Y Zeng are employed at FHI 360, where Hubacher 2006 was conducted. They were not involved in that trial.
RH Allen has served as a clinical trainer for Merck and as a consultant for Actavis and Bayer. She was an investigator of Allen 2013, an included trial.
D Hubacher has served on Advisory Boards for Bayer HealthCare Pharmaceuticals, Inc., Teva Pharmaceuticals, and OCON Medical. He has received product donation from Bayer HealthCare Pharmaceuticals, Inc., Teva Pharmaceuticals, and Merck Sharp & Dohme Corp. He has received research funding from Bayer HealthCare Pharmaceuticals, Inc. and Duramed. He was an investigator of Hubacher 2006, an included trial.
D Bartz and PA O'Brien have no known conflicts of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ahmadi Doulabi 2013 {published data only}
- Ahmadi Doulabi M, Tavakolian S, Mortazavi A, Akbarzade Baghban A. Effect of EMLA cream on pain of Copper IUD insertion [article in Persian]. Scientific Journal of Hamadan Nursing & Midwifery Faculty 2013;21(3):24‐30. [Google Scholar]
- Ahmadi M. A comparison of EMLA cream and placebo on pain during IUD insertion in women referred to health centers of Imam Hussein. www.irct.ir/searchresult.php?id=5698&number=4 (accessed 23 September 2014). [IRCT201209015698N4]
- Tavakolian S, Ghorbani M, Doulabi MA, Baghban AA, Mortazavi A. Lidocaine‐prilocaine cream as analgesia for IUD insertion: a prospective, randomized, controlled, triple blinded study. Global Journal of Health Science 2015;7(4):399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aksoy 2015 {published data only}
- Acmaz G. Lidocaine 10% spray reduces pain during intrauterine device insertion. clinicaltrials.gov/ct2/show/NCT02020551 (accessed 11 September 2014).
- Aksoy H, Aksoy Ü, Ozyurt S, Açmaz G, Babayigit M. Lidocaine 10% spray to the cervix reduces pain during intrauterine device insertion: a double‐blind randomised controlled trial. Journal of Family Planning and Reproductive Health Care 2015 Mar 10 [Epub ahead of print]:1‐5. [DOI] [PubMed]
Allen 2013 {published data only}
Bednarek 2013 {published data only}
- Bednarek PH, Micks EA, Edelman AB, Li H, Jensen JT. The effect of nitroprusside on IUD insertion experience in nulliparous women: A pilot study. Contraception 2013;87(4):421‐5. [DOI] [PubMed] [Google Scholar]
Bednarek 2015 {published data only}
- Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT. Immediate versus delayed IUD insertion after uterine aspiration. New England Journal of Medicine 2011;364(23):2208‐17. [DOI] [PubMed] [Google Scholar]
- Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT, Post‐Aspiration IUD Randomization (PAIR) Study Trial Group. Prophylactic ibuprofen does not improve pain with IUD insertion: a randomized trial. Contraception 2015;91(3):193‐7. [DOI] [PubMed] [Google Scholar]
- Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Mayo J, et al. Pain with IUD insertion following prophylactic ibuprofen: A randomized trial (abstract). Contraception 2010;82(2):194‐5. [DOI] [PubMed] [Google Scholar]
Cameron 2013 {published data only}
- Cameron ST, Glasier A, Cooper A, Johnstone A. Does a full bladder assist insertion of intrauterine contraception?: a randomised trial. Journal of Family Planning and Reproductive Health Care 2013;39(3):207‐10. [DOI] [PubMed] [Google Scholar]
Castro 2014 {published data only}
- Castro TV, Franceschini SA, Poli‐Neto O, Ferriani RA, Silva de Sa MF, Vieira CS. Effect of intracervical anesthesia on pain associated with the insertion of the levonorgestrel‐releasing intrauterine system in women without previous vaginal delivery: a RCT. Human Reproduction 2014;29(11):2439‐45. [DOI] [PubMed] [Google Scholar]
- Vitti T, Ferriani R, Vieira C. Local anaesthetic in uterine cervix is not associated with lower pain score in levonorgestrel‐releasing intrauterine system insertion in women without previous vaginal delivery (abstract). European Journal of Contraception and Reproductive Health Care 2012;17(S1):S104. [Google Scholar]
Chor 2012 {published data only}
- Chor J, Bregand‐White J, Golobof A, Harwood B, Cowett A. Ibuprofen prophylaxis for levonorgestrel‐releasing intrauterine system insertion: A randomized controlled trial. Contraception 2012;85(6):558‐62. [DOI] [PubMed] [Google Scholar]
- Chor J, Harwood B, Cowett A. Ibuprofen prophylaxis for levonorgestrel‐releasing intrauterine system insertion: A randomized controlled trial (abstract). Contraception 2010;82(2):201‐2. [DOI] [PubMed] [Google Scholar]
Cırık 2013 {published data only}
- Cırık DA, Taşkın EF, Tuğlu A, Ortaç AS, Dai O. Paracervical block with 1% lidocaine for pain control during intrauterine device insertion: a prospective, single‐blinded, controlled study. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2013;2(3):263‐7. [Google Scholar]
Dijkhuizen 2011 {published and unpublished data}
- Dijkhuizen K, Dekkers OM, Holleboom CA, Groot CJ, Hellebrekers BW, Roosmalen GJ, et al. Vaginal misoprostol prior to insertion of an intrauterine device: an RCT. Human Reproduction 2011;26(2):323‐9. [DOI] [PubMed] [Google Scholar]
Edelman 2011 {published data only}
- Edelman AB, Schaefer E, Olson A, Houten L, Bednarek P, Leclair C, et al. Effects of prophylactic misoprostol administration prior to intrauterine device insertion in nulliparous women. Contraception 2011;84(3):234‐9. [DOI] [PubMed] [Google Scholar]
Espey 2014 {published data only}
- Espey E, Leeman L, Greene H, Singh R, Ogburn T, Fowler K. Misoprostol for IUD insertion in nulliparous women: A randomized controlled trial (abstract). Contraception 2013;88(3):456‐7. [DOI] [PubMed] [Google Scholar]
- Espey E, Singh RH, Leeman L, Ogburn T, Fowler K, Greene H. Misoprostol for intrauterine device insertion in nulliparous women: A randomized controlled trial. American Journal of Obstetrics and Gynecology 2014;210(3):208.e1‐5. [DOI] [PubMed] [Google Scholar]
Heikinheimo 2010 {published data only}
- Gemzell‐Danielsson K, Inki P, Boubli L, O'Flynn M, Kunz M, Heikinheimo O. Bleeding pattern and safety of consecutive use of the levonorgestrel‐releasing intrauterine system (LNG‐IUS)‐‐a multicentre prospective study. Human Reproduction 2010;25(2):354‐9. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O, Inki P, Kunz M, Parmhed S, Anttila AM, Olsson SE, et al. Double‐blind, randomized, placebo‐controlled study on the effect of misoprostol on ease of consecutive insertion of the levonorgestrel‐releasing intrauterine system. Contraception 2010;81(6):481‐6. [DOI] [PubMed] [Google Scholar]
Hubacher 2006 {published data only}
- Hubacher D, Reyes V, Lillo S, Zepeda A, Chen P, Croxatto H. Pain from copper intrauterine device insertion: randomized trial of prophylactic ibuprofen. American Journal of Obstetrics and Gynecology 2006;195(5):1272‐7. [DOI] [PubMed] [Google Scholar]
Ibrahim 2013 {published data only}
- Ibrahim ZM, Sayed Ahmed WA. Sublingual misoprostol prior to insertion of a T380A intrauterine device in women with no previous vaginal delivery. European Journal of Contraception and Reproductive Health Care 2013;18(4):300‐8. [DOI] [PubMed] [Google Scholar]
Jensen 1998 {published and unpublished data}
- Jensen HH, Blaabjerg J, Lyndrup J. Prophylactic use of prostaglandin synthesis inhibitors in connection with IUD insertion. Ugeskrift for Laeger 1998;160(48):6958‐61. [PubMed] [Google Scholar]
Karabayirli 2012 {published data only}
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. Journal of Minimally Invasive Gynecology 2012;19(5):581‐4. [DOI] [PubMed] [Google Scholar]
Lathrop 2013 {published data only}
- Lathrop E, Haddad L, McWhorter CP, Goedken P. Self‐administration of misoprostol prior to intrauterine device insertion among nulliparous women: a randomized controlled trial. Contraception 2013;88(6):725‐9. [DOI] [PubMed] [Google Scholar]
Lotke 2013 {published data only}
- Lotke P. Use of misoprostol for intrauterine device (IUD) insertion in nulliparous women. clinicaltrials.gov/show/NCT01001897 (accessed 28 October 2014).
- Lotke PS, Tiwari A, Nuño VL. Inserting intrauterine devices in nulliparous women: is misoprostol beneficial? A registered clinical trial. American Journal of Clinical and Experimental Obstetrics Gynecology 2013;1(1):62‐8. [Google Scholar]
Maguire 2012 {published data only}
- Maguire K, Davis A, Rosario Tejeda L, Westhoff C. Intracervical lidocaine gel for intrauterine device insertion: a randomized controlled trial. Contraception 2012;86(3):214‐9. [DOI] [PubMed] [Google Scholar]
- Maguire K, Davis A, Rosario Tejeda L, Westhoff CL. Intracervical 2% lidocaine gel as an analgesic during intrauterine device insertion: A randomized controlled trial (abstract). Contraception 2012;85(3):324. [DOI] [PubMed] [Google Scholar]
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers' assessment of pain during intrauterine device insertion. Contraception 2014;89(1):22‐4. [DOI] [PubMed] [Google Scholar]
Massey 1974 {published data only}
- Massey SE, Varady JC, Henzl MR. Pain relief with naproxen following insertion of an intrauterine device. Journal of Reproductive Medicine 1974;13(6):226‐31. [PubMed] [Google Scholar]
McNicholas 2012 {published data only}
- McNicholas C, Madden T, Zhao Q, Secura G, Allsworth J, Peipert J. Cervical lidocaine for IUD insertional pain: A randomized control trial (abstract). Contraception 2012;86(3):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas CP, Madden T, Zhao Q, Secura G, Allsworth JE, Peipert JF. Cervical lidocaine for IUD insertional pain: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2012;207(5):384.e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Micks 2014 {published data only}
- Micks EA, Jensen JT, Bednarek PH. The effect of nitroglycerin on the IUD insertion experience in nulliparous women: a pilot study. Contraception 2014;90(1):60‐5. [DOI] [PubMed] [Google Scholar]
Mody 2012 {published data only}
- Krishnan S, Kiley J, Rademaker A, Gawron L, Stika C, Hammond C. Pain control for intrauterine device insertion: A randomized trial of 1% lidocaine paracervical block (abstract). Contraception 2011;84(3):319. [DOI] [PubMed] [Google Scholar]
- Mody SK, Kiley J, Rademaker A, Gawron L, Stika C, Hammond C. Pain control for intrauterine device insertion: A randomized trial of 1% lidocaine paracervical block. Contraception 2012;86(6):704‐9. [DOI] [PubMed] [Google Scholar]
Mohammad‐Alizadeh‐C 2010 {published data only}
- Mohammad‐Alizadeh‐Charandabi S, Seidi S, Kazemi F. Effect of lidocaine gel on pain from copper IUD insertion: a randomized double‐blind controlled trial. Indian Journal of Medical Sciences 2010;64(8):349‐55. [PubMed] [Google Scholar]
Nelson 2013 {published data only}
- Nelson AL, Fong JK. Intrauterine infusion of lidocaine does not reduce pain scores during IUD insertion. Contraception 2013;88(1):37‐40. [DOI] [PubMed] [Google Scholar]
Ngo 2014 {published data only}
- Mody S. Pain control for intrauterine device placement: a Ttial of ketorolac prior to intrauterine device placement. clinicaltrials.gov/show/NCT01664559 (accessed 11 September 2014). [NCT01664559]
- Ngo L, Ward K, Mody S. Pain control for intrauterine device insertion: a randomized double‐blind controlled trial of ketorolac (abstract). Contraception 2014;90(3):297. [Google Scholar]
Rapkin 2014 {published and unpublished data}
- Rapkin RB. Self‐administered intravaginal 2% lidocaine gel prior to intrauterine device insertion in nulliparous women (LIVIIN). clinicaltrials.gov/show/NCT01534520 (accessed 4 September 2014).
- Rapkin RB, Achilles SL, Boraas C, Cremer M, Schwarz EB, Chen BA. Self‐administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial (abstract). Obstetrics and Gynecology 2014;123(5 (Suppl)):110S. [NCT01534520] [DOI] [PubMed] [Google Scholar]
Sääv 2007 {published data only}
- Sääv I, Aronsson A, Marions L, Stephansson O, Gemzell‐Danielsson K. Cervical priming with sublingual misoprostol prior to insertion of an intrauterine device in nulliparous women: a randomized controlled trial. Human Reproduction 2007;22(10):2647‐52. [DOI] [PubMed] [Google Scholar]
Scavuzzi 2013 {published data only}
- Scavuzzi A, Souza AS, Costa AA, Amorim MM. Misoprostol prior to inserting an intrauterine device in nulligravidas: a randomized clinical trial. Human Reproduction 2013;28(8):2118‐25. [DOI] [PubMed] [Google Scholar]
Shahnazi 2012 {published data only}
- Shahnazi M, Nikjoo R, Yavarikia P, Mohammad‐Alizadeh‐Charandabi S. Inhaled lavender effect on anxiety and pain caused from intrauterine device insertion. Journal of Caring Sciences 2012;1(4):255‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swenson 2012 {published data only}
- Swenson C, Turok DK, Ward K, Jacobson JC, Dermish A. Self‐administered misoprostol or placebo before intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstetrics and Gynecology 2012;120(2 Part 1):341‐7. [DOI] [PubMed] [Google Scholar]
Tornblom‐Paulander 2015 {published and unpublished data}
- Pharmanest AB 2013. A randomized, double blind, placebo controlled, parallel group clinical study investigating the analgesic effect and safety of a topical formulation of lidocaine (SHACT) during and after insertion of an Intra Uterine Device. A phase II study. www.clinicaltrialsregister.eu/ctr‐search/trial/2011‐006220‐20/SE (accessed 22 January 2015).
- Tingaker B, Brodin A, Irestedt L, Ekman‐Ordeberg G. A novel mucosal pain relief drug candidate ‐ SHACT ‐‐ gives highly significant analgesia at insertion of intra uterine device (IUD) (abstract). Reproductive Sciences 2014;21(3):318A. [Google Scholar]
- Tornblom‐Paulander S, Tingåker BK, Werner A, Liliecreutz C, Conner P, Wessel H, et al. Novel topical formulation of lidocaine provides significant pain relief for intrauterine device insertion: pharmacokinetic evaluation and randomized placebo‐controlled trial. Fertility and Sterility 2015;103(2):422‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Goldstuck 1983 {published data only}
- Goldstuck ND. Treatment of pain following IUD insertion with meptazinol‐a new centrally acting analgesic. Contraceptive Delivery Systems: An International Journal 1983;4(1):33‐7. [PubMed] [Google Scholar]
Hepburn 1980 {published data only}
- Hepburn S. Method of local anesthesia for IUD insertion. Contraceptive Delivery Systems 1980;1(4):371‐82. [PubMed] [Google Scholar]
Hollingworth 1995 {published data only}
- Hollingworth B. Pain control during insertion of intrauterine device. British Journal of Family Planning 1995;21(3):102‐3. [Google Scholar]
Jafari 2014 {published data only}
- Jafari A, Sayyahi M, Torkashvand R, Piryaei H. Effect of vitamin B1 on pain after insertion of intrauterine device [article in Persian]. Journal of Babol University of Medical Sciences 2014;16(3):13‐20. [Google Scholar]
Mirmohamad Aliei 2013 {published data only}
- Mirmohamad Aliei M, Khazaie F, Rahnama P, Rahimikian F, Modarres M, Bekhradi R, et al. Effect of lavender on pain during insertion of intrauterine device: A clinical trial [article in Persian]. Journal of Babol University of Medical Sciences 2013;15(4):93‐9. [Google Scholar]
- Mirmohammadali AM. Effect of lavender essential oil on anxiety and pain during insertion of intrauterine device. apps.who.int/trialsearch/trial.aspx?trialid=IRCT201209266206N4 (accessed 29 July 2014). [IRCT201209266206N4]
Newton 1977 {published data only}
- Newton JR, Reading AE. The effects of psychological preparation on pain at intrauterine device insertion. Contraception 1977;16(5):523‐32. [DOI] [PubMed] [Google Scholar]
Oloto 1997 {published data only}
- Oloto EJ, Bromham DR, Murty JA. Pain and discomfort perception at IUD insertion‐‐effect of short‐duration, low‐volume, intracervical application of two percent lignocaine gel (Instillagel™)‐‐a preliminary study. British Journal of Family Planning 1997;22(4):177‐80. [Google Scholar]
Stephenson 2010 {published data only}
- Stephenson J. Does topical anaesthetic gel reduce pain/discomfort during intrauterine device (IUD)/intrauterine system (IUD) insertion? A randomised, placebo‐controlled, double‐blind trial. www.controlled‐trials.com/ISRCTN83819888 (accessed 23 September 2014).
Teal 2012 {published data only}
- Teal S. IUD insertion in nulliparous women. clinicaltrials.gov/show/NCT01422226 (accessed 19 August 2014).
Thiery 1985 {published data only}
- Thiery M. Pain relief at insertion and removal of an IUD: a simplified technique for paracervical block. Advances in Contraception 1985;1(2):167‐70. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Brody 2011 {published data only}
- Brody K. Topical Lidocaine on the Cervix and Intra‐Cervical Prior to Insertion of Intrauterine Devices (10‐053). http://clinicaltrials.gov/show/NCT01192490 (accessed 17 September 2014).
Elsafty 2011 {published data only}
- Elsafty ASE. Efficacy study of topical application of lidocaine spray prior to IUD insertion. clinicaltrials.gov/show/NCT01496105 (accessed 18 September 2014).
Khodokarami 2011 {published data only}
- Khodokarami N. The effect of chamomile essential oil on pain intensity of IUD insertion. www.irct.ir/searchresult.php?id=8950&number=1 (accessed 23 September 2014).
Singh 2015 {published data only}
- Singh R. Nitrous oxide for pain management of IUD insertion (NIUD). clinicaltrials.gov/ct2/show/NCT02391714 (accessed 4 May 2015). [DOI: ]
- Singh RH, Thaxton LD, Carr S, Leeman LM, Schneider E, Espey E. Nitrous oxide for intrauterine device insertion in nulliparous women: a randomized controlled trial [22]. Obstetrics and Gynecology 2015;125(Suppl 1):7S. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Fouda 2015 {published data only}
- Fouda UM. Diclofenac plus lidocaine gel for pain relief during intrauterine device insertion (IUD). clinicaltrials.gov/show/NCT02332057 (accessed 27 January 2015).
Jamshidi 2014 {published data only}
- Jamshidi R. Study of local anesthesia as a method to decrease IUD insertion related pain. clinicaltrials.gov/show/NCT01967017 (accessed 11 September 2013).
Mody 2014 {published data only}
- Mody S, Culwell K. Pain control for intrauterine device placement using paracervical block. clinicaltrials.gov/show/NCT02219308 (accessed 11 September 2014).
Additional references
Allen 2014
- Allen RH, Carey MS, Raker C, Goyal V, Matteson K. A prospective cohort study of pain with intrauterine device insertion among women with and without vaginal deliveries. Journal of Obstetrics and Gynaecology 2014;34(3):263‐7. [DOI] [PubMed] [Google Scholar]
Asker 2006
- Asker C, Stokes‐Lampard H, Beavan J, Wilson S. What is it about intrauterine devices that women find unacceptable? Factors that make women non‐users: a qualitative study. Journal of Family Planning and Reproductive Health Care 2006;32(2):89‐94. [DOI] [PubMed] [Google Scholar]
Bahamondes 2014
- Bahamondes L, Mansour D, Fiala C, Kaunitz AM, Gemzell‐Danielsson K. Practical advice for avoidance of pain associated with insertion of intrauterine contraceptives. Journal of Family Planning and Reproductive Health Care 2014;40(1):54‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Comm Adolescent Health 2012
- Committee on Adolescent Health Care, Long‐Acting Reversible Contraception Working Group. American College of Obstetricians Gynecologists Committee Opinion no. 539: Adolescents and long‐acting reversible contraception: implants and intrauterine devices. Obstetrics and Gynecology 2012;120(4):983‐8. [DOI] [PubMed] [Google Scholar]
Gemzell‐Danielsson 2013
- Gemzell‐Danielsson K, Mansour D, Fiala C, Kaunitz AM, Bahamondes L. Management of pain associated with the insertion of intrauterine contraceptives. Human Reproduction Update 2013;19(4):419‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 2003
- Goldberg AB, Carusi DA, Meckstroth KR. Misoprostol in gynecology. Current Women's Health Reports 2003;3(6):475‐83. [PubMed] [Google Scholar]
Goldstuck 1985
- Goldstuck ND, Mathews ML. A comparison of the actual and expected pain response following insertion of an intrauterine contraceptive device. Clinical Reproduction and Fertility 1985;3(1):65‐71. [PubMed] [Google Scholar]
Grimes 2006
- Grimes DA, Hubacher D, Lopez LM, Schulz KF. Non‐steroidal anti‐inflammatory drugs for heavy bleeding or pain associated with IUD use. Cochrane Database of Systematic Reviews 2006;4:CD006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimes 2008
- Grimes DA, Mishell DR. Intrauterine contraception as an alternative to interval tubal sterilization. Contraception 2008;77(1):6‐9. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org (accessed 25 March 2014).
Kaislasuo 2014
- Kaislasuo J, Heikinheimo O, Lähteenmäki P, Suhonen S. Predicting painful or difficult intrauterine device insertion in nulligravid women. Obstetrics and Gynecology 2014;124(2 Pt 1):345‐53. [DOI] [PubMed] [Google Scholar]
Kass‐Wolff 2014
- Kass‐Wolff JH, Fisher JE. Evidence‐based pain management for endometrial biopsies and IUD insertions. Nurse Practitioner 2014;39(3):43‐50. [DOI] [PubMed] [Google Scholar]
Mercier 2012
- Mercier RJ, Zerden ML. Intrauterine anesthesia for gynecologic procedures: a systematic review. Obstetrics and Gynecology 2012;120(3):669‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murty 2003
- Murty J. Use and effectiveness of oral analgesia when fitting an intrauterine device. Journal of Family Planning and Reproductive Health Care 2003;29(3):150‐1. [DOI] [PubMed] [Google Scholar]
Ott 2014
- Ott MA, Sucato GS, Committee on Adolescence. Contraception for adolescents. Pediatrics 2014;134(4):e1257‐81. [DOI] [PubMed] [Google Scholar]
Pergialiotis 2014
- Pergialiotis V, Vlachos DG, Protopappas A, Vlachos GD. Analgesic options for placement of an intrauterine contraceptive: a meta‐analysis. European Journal of Contraception and Reproductive Health Care 2014;19(3):149‐60. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Thomson 1997
- Thomson AJ, Lunan CB, Cameron AD, Cameron IT, Greer IA, Norman JE. Nitric oxide donors induce ripening of the human uterine cervix: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 1997;104(9):1054‐1057. [DOI] [PubMed] [Google Scholar]
Tolcher 2003
- Tolcher R. Intrauterine techniques: contentious or consensus opinion?. Journal of Family Planning and Reproductive Health Care 2003;29(1):21‐4. [DOI] [PubMed] [Google Scholar]
Turok 2011
- Turok DK, Espey E, Edelman AB, Lotke PS, Lathrop EH, Teal SB, et al. The methodology for developing a prospective meta‐analysis in the family planning community. Trials 2011;12:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
UN 2013
- United Nations Population Division. World Contraceptive Patterns 2013. www.un.org/en/development/desa/population/publications/family/contraceptive‐wallchart‐2013.shtml (accessed 11 February 2015).
Waddington 2012
- Waddington A, Reid R. More harm than good: the lack of evidence for administering misoprostol prior to IUD insertion. Journal of Obstetrics and Gynaecology Canada 2012;34(12):1177‐9. [DOI] [PubMed] [Google Scholar]


