Abstract
Lifestyle recommendations are first‐line elements in the management of arterial hypertension. This cross‐sectional study aimed to analyze the level to which lifestyle recommendations are used in hypertension management in France, using data from the Esteban study, which was implemented by Santé Publique France, France's public health agency, from 2014 to 2016 on a representative sample of the French population. The study sample comprised 440 adult Esteban participants who were aware they had hypertension and were aged 18–74 years old. The main outcomes were the proportion of participants who received lifestyle recommendations in their hypertension management plan, and the proportion of recommendations according to the three following dimensions: physical activity, weight loss, and changes in diet. Over half (57.0%) of the 440 participants declared they did not receive lifestyle recommendations as part of their hypertension management plan in the year preceding the study. Of these, 39.0% did not receive pharmacological treatment either. Physical activity was recommended to 31.8% of sedentary participants and weight loss to 26.8% of participants with overweight or obesity. One‐fifth of the study sample (20.1%) received dietary recommendations. Of these, 69% and 10.7% were advised to limit their salt and alcohol intake, respectively. Lifestyle interventions are too rarely recommended in hypertension management plans in France. Adherence to lifestyle recommendations needs in‐depth discussion not only at the time of diagnosis but also throughout follow‐up.
Keywords: healthy diet, hypertension management, lifestyle recommendations, physical activity, weight loss
1. INTRODUCTION
Hypertension (HTN) is defined by an office blood pressure (BP) measure ≥ 140/90 mm Hg for the systolic and diastolic BP, respectively, after repeated consultation. 1 , 2 , 3 , 4 It is the main cause of cardiovascular (CV) diseases and premature deaths worldwide with a HTN‐related complications account over 10 million deaths every year. 5 However, the number of people with HTN doubled in the last three decades and exceeded 1 billion in 2019. 6 , 7
International guidelines agree on two lines of HTN treatment: recommendations for lifestyle changes and drug treatments. 1 , 2 , 3 Lifestyle recommendations prevent HTN and constitute the first‐line treatment for grade I hypertension. When drug treatments are necessary, lifestyle recommendations remain part of HTN management and prevent other CV risk factors, such as diabetes, dyslipidemia, and overweight/obesity.
International and European guidelines have long agreed on the major lifestyle recommendations needed in HTN management: physical activity, the Dietary Approaches to Stop Hypertension diet (ie, the DASH diet, which limits salt and alcohol consumption, and promotes greater consumption of fruits and vegetables), and body weight control. Lifestyle recommendations are becoming ever more detailed in HTN management guidelines. Recent additions include stress reduction, practicing mindfulness, and reducing exposure to air pollution and cold temperatures. 1 , 2 , 3 , 4 Regarding to healthy diet recommendation in French HTN management, French guidelines rely on both DASH diet, which is recommended in European and International guidelines and remains the main dietary recommendation in the management of hypertension in France, and a French Nutrition and Health Program (Programme National Nutrition Santé or PNNS) implemented in several countries with the aims of preventing chronic diseases. It considers salt, alcohol, and physical activity but is not specifically intended for hypertensive people. 8
The effects of lifestyle interventions on BP level are well described. 9 , 10 , 11 , 12 , 13 , 14 A meta‐analysis of 25 studies showed that as body weight decreased by 1 kg, SBP and DBP decreased by − 1.05 mm Hg and − .92 mm Hg, respectively. 15 , 16 A review published in 2020 highlighted that regular exercise can have an impact of overall 5 mm Hg decrease in BP. With a decrease of 5 mm Hg in systolic BP, mortality due to coronary heart disease decreases by 9%, mortality due to stroke decreases by 14% and all‐cause mortality decreases by 7%. 17
A report from the American College of Cardiology (ACC)/American Heart Association (AHA), published in 2019, showed that a healthy diet is the most efficient lifestyle recommendation for hypertensive adults (with an impact of 11 mm Hg on BP level), followed by physical activity, weight loss, limiting salt, and alcohol moderation (each with an impact between 4 and 8 mm Hg on BP level). 11 A review published in 2018 found that the DASH diet was as efficient on reducing BP level as a monotherapy, while physical activity and weight loss were the second and third most efficient lifestyle changes for lowering BP level. The review confirmed that lifestyle recommendations can help to control stage 1 hypertension and avoid CV complications. 12 Smoking cessation has no direct impact on BP level. Nevertheless, its presence in lifestyle recommendations is justified by the fact that it is a major CV risk factor and the cause of 10% of CV deaths worldwide. 18
In France, the Esteban study (2014–2016) found that 48.6% of hypertensive adults were pharmacologically treated, but only 24.3% had controlled hypertension. 19 In general, data about lifestyle recommendations in overall HTN management in France are lacking.
We aimed to describe the proportion of lifestyle recommendations in global HTN management in France, and the characteristics of patients who receive these recommendations.
2. METHODS
2.1. Study design
Esteban was a cross‐sectional study conducted between 2014 and 2016 on a representative sample of the French population. It aimed to optimize knowledge about various dimensions of the French population's lifestyle (eg, eating habits, physical activity) and dimensions of specific chronic diseases (eg, screening rate, prevalence, treatment). 20
The nationwide cross‐sectional Esteban study was carried out with a three‐stage sampling design. In the first stage, a stratified sample of primary units (municipality or groups of municipalities) was randomly drawn. In the second stage, households were randomly selected in each primary unit using random generation of phone numbers. Finally, in the third stage, one individual was randomly selected from among eligible household members using the Kish method to participate in the study.
The stratification was performed according to two variables: the region (eight geographical areas) and the degree of urbanization (5 degrees: rural, < 20 000 inhabitants, 20 000–100 000 inhabitants, greater than 100 000 inhabitants, Paris and its suburbs).
This complex survey design was taken into account in the estimation of the initial weighting applied to each person who participated in the first visit. This weighting corresponded to the number of eligible persons in the household, multiplied by the inverse of the probability of drawing from the household and by the inverse of the probability of drawing from the primary unit.
All participants gave their informed consent prior to their inclusion in the study. For each of them, data were collected in several stages: (1) a home visit with a face‐to‐face questionnaire; (2) a remote (ie, phone/internet) diet survey by a dietician; (3) a self‐administered questionnaire; and (4) a health examination. 20 Participants who gave their consent for data‐matching (87.5%) were matched to the Système National des données de santé database (SNDS, or National Health Insurance Information System), which gives access to all reimbursements for health care expenditure, including prescribed drugs and outpatient care provided by general practitioners or specialists. 21 To account for adults who withdrew the study between the first visit and the health examination or did not give their consent for matching to the SNDS database a non‐response correction was performed using the score method. Finally, a recalibration was made using the margin calibration method. The margins used in the calibration were taken from the 2012 Census of Population and were based on the following data: age, sex, diploma of the household reference person and whether the reference person lived in a couple or not and period of data collection. Esteban's aims and study protocol are described in greater detail elsewhere. 20
2.2. Ethical compliance
Esteban received approval from the committee for the protection of persons (no. 2012‐A00456‐34), from the Advisory Committee on the processing of research information in the health sector, from the National Commission for Information and Freedoms, and finally from a specific Decree of the Council of State which gave approval for the study's data to be matched with data from the SNDS.
2.3. Study population
Of the 3021 adults participating in Esteban, 19 2105 had a health examination as part of the study with at least 2 BP level measurements, and gave consent for data matching with the SNDS (Figure 1).
FIGURE 1.
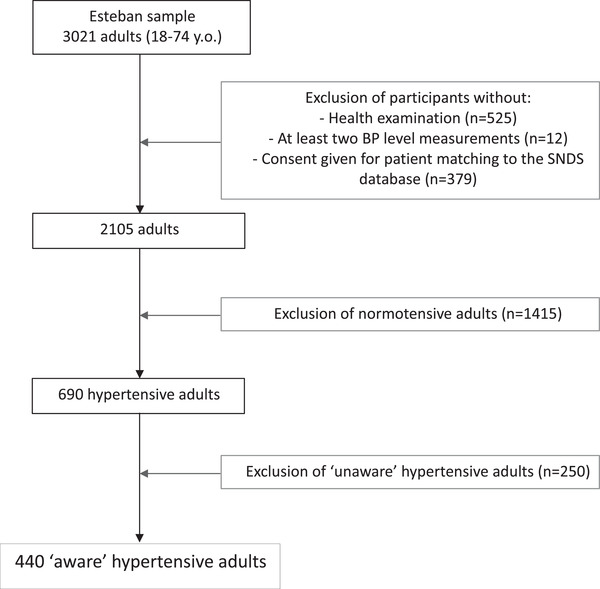
Study flowchart
Participants who met the following criteria were considered to have HTN: declaring that they had been previously diagnosed with HTN by a physician (either home visit or self‐administered questionnaire), and/or currently taking antihypertensive treatment (SNDS database), and/or having a BP ≥ 140/90 mm Hg (Esteban health examination). ESTEBAN study's overall hypertensive population is described in greater details in another review paper. 19
Of the 2105 participants, 690 were considered to have HTN according to the above‐mentioned criteria. Of these, 440 participants were considered to be aware of their HTN. That is to say, they answered “yes” to at least one of the following two questions: “Have you been previously diagnosed with HTN by a physician?” (home visit), and “Has a doctor ever told you that your BP is too high?” (self‐administered questionnaire). These 440 “aware” participants were included in the present analysis (ie, study sample).
2.4. Data collection and treatment
Recommending physical activity as part of their HTN management was evaluated in Esteban with the question “In the last 12 months, has your doctor recommended you increase your physical activity to lower your BP?” (self‐administered questionnaire).
Physical activity (work, leisure, sports, and finally domestic activities) in the 4 weeks preceding the survey was also assessed in the self‐administered questionnaire. Data were divided into three categories: a low, intermediate, or high overall level of physical activity, as per the World Health Organization (WHO) recommendations for physical activity in adults. 22
Recommending weight loss as part of their HTN management was evaluated with the question “In the last 12 months, has your doctor recommended you lose weight to lower your BP?” (self‐administered questionnaire). We only considered overweight/obese participants for this specific recommendation in the data analysis.
Body size was assessed using the body mass index (BMI). The BMI was calculated using anthropometric measurements collected during the Esteban study's health examination by a health professional according to a standardized protocol. Body size was also divided into three categories according to corpulence: normal corpulence (BMI < 25), overweight (25 ≤ BMI < 30), and obesity (BMI ≥ 30).
Implementation of this lifestyle recommendation was evaluated with the question “In the last year, have you tried to (i) lose weight? (ii) not gain weight? (iii) gain weight? (iv) you have not worried about your weight” (self‐administered questionnaire).
Recommending a healthy diet as part of their HTN management was evaluated with two questions. The first was “In the last 12 months, has your doctor recommended you follow a specific diet to lower your BP?”. Participants who answered “yes” then answered the second question which detailed the dietary recommendations made, as follows: “Did he/she recommend you (i) moderate your delicatessen, cheese, dairy product consumption?, (ii) moderate your meat consumption?, (iii) moderate your salt consumption?, (iv) moderate your alcohol consumption?, (v) increase your fruit and vegetable consumption?, (vi) increase your fish consumption?, and (vii) other ?” (self‐administered questionnaire).
For the present analysis, only four of these categories were considered as follows: salt moderation, alcohol moderation, increased fruit and vegetable consumption, and other.
Salt consumption was evaluated with the urinary sample taken during the health examination.
BP was measured in a sitting position during the health examination by a medical staff, with an Omron® 705_IT BP monitor on the right arm with a cuff adapted to the circumference of the arm in accordance with to the following standard protocol: three measurements 1 min apart after 5 min of rest and after 30 min from the blood sample was taken, in the same position. We considered the systolic and diastolic average BP levels in the last two measurements only.
Hypertensive adults were considered treated if the SNDS database indicated that they had received one or more antihypertensive drugs in the 6 months prior to the study. Treated hypertensive adults were considered adherent to therapy if the antihypertensive drugs dispensed covered at least 80% of the year preceding the health examination.
The CV risk factors considered were diabetes, high low‐density lipoprotein (LDL) cholesterol, current smoking or quitting in the previous 6 months, overweight/obesity, and a family history of early CV onset. Participants were considered diabetic if they met at least one of the following criteria: diagnosed with diabetes by a physician prior to the study (self‐administered questionnaire), currently taking anti‐diabetic treatment (SNDS), and having a fasting blood glucose (FBG) ≥ 7.0 mmol/l in blood test results. Finally, participants were considered to have hypercholesterolemia if they met at least one of the following criteria: currently taking anti‐hypercholesterolemia treatment (SNDS), and an LDL cholesterol > 1.6 g/l in blood test results (health examination).
Health perceived were evaluated with the question “What is your general state of health?” (self‐administered questionnaire). Participants could answer “very god”, “good”, “good enough”, “bad”, “very bad”, “refusal”, “do not know”. Data were put into two categories for this study: very good/good, good enough/bad/very bad.
2.5. Statistical analysis
To take into account non‐responses and withdrawals during the Esteban study, the characteristics of the 2105 participants were weighted to ensure the study was representative of the French population. These weights were calculated using data from the National Institute of Statistics and Economic Studies on the French population for 2012.
Quantitative variables were expressed as weighted means and standard deviation (SD), while qualitative variables were expressed as weighted percentages. Student's t‐test was used to compare means and either the Chi‐squared test or Fisher's exact test (depending on conditions) was used to compare percentages. Univariate logistic regressions were used to determine the characteristics associated with the recommendation of at least one lifestyle change in patients’ HTN management plan. Variables with a p‐value < .15 were included in the multivariate logistic regressions. Only variables with a p‐value < .05 in the latter were considered significant. Similar regressions were performed to determine the factors associated with the recommendation of each type of lifestyle change (ie, physical activity, weight loss, healthy diet).
We performed a subgroup analysis with participants who had overweight/obesity because it is the only sub‐population for whom all three recommendations (physical activity practice, weight loss, healthy diet with a focus on salt consumption moderation) were needed.
All statistical analyses were performed using SAS Enterprise® Guide Version 7.1 software.
3. RESULTS
Among the 440 adults considered to be aware of their hypertension, mean age was 58.4 ± 13.9 years. Two‐thirds (66%) were treated pharmacologically and less than half (43.6%) had controlled hypertension. Eighty‐seven percent had at least one other CV risk factor associated with their HTN: 18.4% were diabetic, and 38.4% had hypercholesterolemia. Three quarters had overweight/obesity (75.1%), and 39.1% had a low level of physical activity. Just under two‐thirds (62.9%) of the participants felt they were in good or very good health (Table 1).
TABLE 1.
Sociodemographic and health characteristics of “aware” hypertensive adults in our study sample
| Characteristic | Weighted % |
|---|---|
| Sex | |
| Men | 50.8 |
| Women | 49.2 |
| Age (y.o.) ‐ mean (SD) | 58.4 (13.9) |
| Education level | |
| Primary or lower than secondary school certificate | 19.9 |
| Secondary school certificate | 53.5 |
| > secondary school certificate | 26.5 |
| Marital status | |
| Single, divorced or separated, widowed | 26.6 |
| Couple (married or not) | 73.4 |
| Perceived financial status | |
| Very comfortable | 18.6 |
| Comfortable | 35.5 |
| Difficult / Very difficult | 46.0 |
| Systolic BP level (mm Hg) ‐ mean (SD) | 141.6 (24.9) |
| Diastolic BP level (mm Hg) ‐ mean (SD) | 83.1 (14.3) |
| Pharmacologically‐treated HTN | 66.4 |
| Controlled HTN | 43.6 |
| Diabetes | 18,4 |
| Hypercholesterolemia | 38.4 |
| Current smoker or quit smoking in previous 6 months | 12.6 |
| BMI (kg/m2) ‐ mean (SD) | 29.7 (8.2) |
| Corpulence | |
| Normal | 24.87 |
| Overweight | 36.9 |
| Obesity | 38.2 |
| Level of physical activity a | |
| Low | 39.1 |
| Moderate | 55.2 |
| High | 5.7 |
| Alcohol consumption (g per day) | 9.6 |
| Family history of early cardiovascular disease b | 15.3 |
| Secondary CV prevention | 8.5 |
| Good therapeutic adherence (≥ 80%) | 12.4 |
| At least one chronic disease | 32.7 |
| Perceived health status | |
| Very good / Good | 62.9 |
| Quite good / Poor/ Very poor | 37.1 |
| Functional limitations | |
| None | 71.6 |
| Yes, some | 17.4 |
| Yes, very functionally limited | 11 |
Level of physical activity based on WHO recommendations. An ideal level of physical activity corresponds to the global recommendations for physical activity in adults as defined by the WHO.
Family history of myocardial infarction, sudden death and/or stroke in a close relative (parents, siblings) before the age of 65 and 55 in men and women, respectively.
3.1. Hypertension management
Among aware hypertensive adults, 57% declared they did not receive any lifestyle recommendation as part of their HTN management plan in the year preceding the study (Figure 2). Of these, 61.0% only received pharmacological therapy and the other 39.0% received nor lifestyle recommendations nor pharmacological therapy. These 39.0% of aware hypertensive adults with nor lifestyle recommendation nor pharmacological therapy represent one in four of our study sample (23.7%).
FIGURE 2.
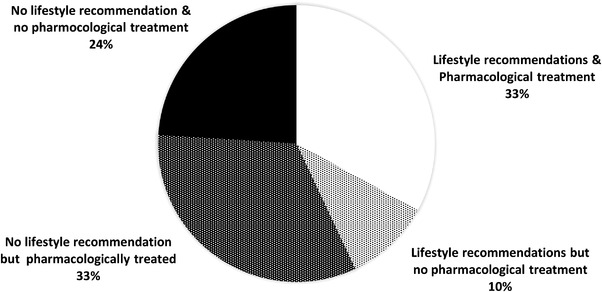
Distribution of “aware” hypertensive adults according to their overall HTN management program
Of the 43% of our study sample who had received at least one lifestyle recommendation, 22.7% were treated only with lifestyle recommendations.
Among the participants with both lifestyle recommendations and pharmacological drugs, mean BP level was 143.6 +/‐ 52.4 mm Hg for systolic and 87.2 +/‐ 25.2 mm Hg for diastolic, respectively (Supporting information 1). One‐fifth (21.9%) of them were considered adherent versus 15.6% of those only provided pharmacological treatment.
3.2. Lifestyle recommendations in hypertension management
Physical activity was the lifestyle intervention most recommended in our study sample (26.0%). Weight loss and healthy diet were recommended to one fifth of the sample (20.5% and 20.1%, respectively).
Logistic regressions showed that the likelihood of receiving at least one lifestyle recommendation increased with BMI (OR = 1.55 in persons with overweight, OR = 3.37 in persons with obesity) (p = .0183) and age (OR = 1.04) (p = .0216) (Table 2).
TABLE 2.
Characteristics associated with receiving at least one lifestyle recommendation as part of HTN management program
| Univariate logistic regression | Multivariate logistic regression | |||
|---|---|---|---|---|
| Crude OR [95% CI] | p | Adjusted OR [95% CI] | p | |
| Age | 1.04 [1.01‐1.07] | <.0001 | 1.04 [1.01‐1.07] | .0216 |
| Pharmacologically treated HTN | ||||
| No | 1,00 [ref] | 0,0008 | 1,00 [ref] | 0,5055 |
| Yes | 2,75 [1,52‐4,98] | 1,33 [0,57‐3,08] | ||
| Education level | ||||
| Primary or lower than secondary school certificate | 1,00 [ref] | 0,0847 | 1,00 [ref] | .1031 |
| Secondary school certificate | .44 [.21‐.94] | .52 [.2‐1.32] | ||
| > secondary school certificate | .48 [.23‐1] | .97 [.37‐2.55] | ||
| Diabetes | ||||
| No | 1.00 [ref] | .0019 | 1.00 [ref] | .0577 |
| Yes | 3.19 [1.53‐6.63] | 2.69 [.97‐7.49] | ||
| Hypercholesterolemia | ||||
| No | 1.00 [ref] | .0748 | 1.00 [ref] | .6322 |
| Yes | 1.62 [.95‐2.74] | 1.17 [.62‐2.21] | ||
| Corpulence | ||||
| Normal | 1.00 [ref] | .0015 | 1.00 [ref] | .0183 |
| Overweight | 1.56 [.8‐3.07] | 1.55 [.66‐3.65] | ||
| Obesity | 3.27 [1.69‐6.35] | 3.37 [1.39‐8.19] | ||
| Good therapeutic adherence (≥80%) | ||||
| No | 1.00 [ref] | .0748 | 1.00 [ref] | .3616 |
| Yes | 2.08 [.93‐4.65] | 1.65 [.56‐4.87] | ||
| At least one chronic disease | ||||
| No | 1.00 [ref] | .0014 | 1.00 [ref] | .135 |
| Yes | 2.49 [1.42‐4.36] | 1.71 [.84‐3.48] | ||
| Perceived health status | ||||
| Very good / Good | 1.00 [ref] | .0571 | 1.00 [ref] | .4948 |
| Quite good / Poor / Very poor | 1.68 [.98‐2.86] | 1,26 [0,64‐2,48] | ||
3.2.1. Physical activity recommendation
In terms of physical activity, 5.7%, 55.2%, and 39.1% of our sample had a high, moderate, and low level, respectively. Among the latter, 31.8% declared they had received this recommendation as part of their HTN management plan.
Logistic regressions showed that the likelihood of being recommended to do physical activity increased with BMI (OR = 2.50 in those with overweight, and OR = 5.14 in those with obesity) (p = .0056) in diabetics (OR = 3.13) (p = .0069), and in adults with a personal history of CV disease (OR = 2.88) (p = .0056). By contrast, active smoking was significantly less associated with the recommendation to do physical activity (OR = .32) (p = .0404) (Supporting information 2).
3.2.2. Healthy diet recommendations
Among all the participants in the study sample who declared they had received one or more recommendations to modify their diet, 69.0% mentioned they had been recommended to moderate their salt consumption, 18.7% to increase their fruit and vegetable consumption, and 10.7% to moderate their alcohol consumption. Moreover, 77.8% of those who did not receive this specific recommendation consumed more than 6 g of salt per day (15.4% consuming 10 g or more) versus 71.4% of those who received it (3% consuming 10 g or more).
Corpulence was the only characteristic significantly associated with the likelihood of receiving at least one recommendation (OR = 1.79 in those with overweight, OR = 3.54 in those with obesity) (p = .0184) (Supporting information 3).
3.2.3. Weight loss recommendation
Among persons with overweight or obesity, respectively, 15.7% and 37.6% declared they had been recommended to lose weight to reduce their BP. Fifty‐five percent of the latter had tried to lose weight in the previous 12 months (57.4% and 54.4% in those with overweight and obesity, respectively).
Perceived financial status and corpulence were significantly correlated with the likelihood of being advised to lose weight as part of the healthy diet. The more difficult the financial situation, the greater the likelihood of being advised to commence the healthy diet to lose weight (OR = 3.65 in difficult/very difficult) (p‐value = .031). Likewise, the greater the person's weight, the greater the probability of being asked to lose weight (OR = 12.91 in those with overweight, OR = 42.12 in those with obesity) (p‐value < .0001) (Supporting information 4).
3.2.4. Persons with overweight and with obesity
Our sample comprised 75.1% of persons with overweight or obesity (our 36.9% and 38.2%, respectively). In this subgroup, the mean BP level was 141.4/84.5 mm Hg, with 10.4% having stage II HTN (160 < Systolic BP < 179 and/or 100 < Diastolic BP < 109), and 5.7% with stage III HTN (Systolic BP≥180 and/or Diastolic BP≥110). Just under half (46.5%) had received one or more lifestyle recommendations as part of their HTN management plan (specifically, 52.7% of those pharmacologically treated and 30.7% of those not treated pharmacologically). Physical activity (30.9%) was the intervention most recommended, followed by weight loss (26.8%), and salt moderation (26.4%). Among persons who declared they had received one or more lifestyle recommendations, 26.1% received all three (Supporting information 5).
4. DISCUSSION
Over half (57.0%) of our study sample of hypertensive participants declared they did not receive any lifestyle recommendation as part of their hypertension management plan in the year preceding the Esteban study. Of these, 39% did not receive pharmacological treatment either. Physical activity was recommended to 31.8% of sedentary aware hypertensive adults, and weight loss to 26.8% of those with overweight. One fifth (20.1%) of the study sample were advised to adopt a healthy diet. Of these, 69.0% were advised to limit their salt intake, and 10.7% their alcohol consumption.
4.1. Lifestyle recommendations in hypertension management
Physical activity was only recommended to a quarter of our study sample, despite the fact that it reduces CV risk, helps regulate and lower BP, and constitutes an integral part of HTN treatment. 23 , 24 The benefits of physical activity concern all hypertensive persons, irrespective of age and body weight. Even people with functional limitations can be physically active, but in this case, it is preferable to seek professional help for an adapted and personalized program. 25 , 26 A meta‐analysis published in 2021 had evidence of moderate certainty that walking reduces at least systolic BP for all ages and both sexes, and that it may also reduce diastolic BP. 27
The healthy diet was recommended to only a fifth of our sample. This lifestyle change is also beneficial for all types of hypertensive patients. A 2020 meta‐analysis showed that DASH might be the most effective intervention in lowering BP levels in adults with prehypertension and established hypertension. 28 Another study highlighted that the combination of moderating salt consumption and the DASH diet lowered BP levels in persons with pre‐HTN and stage I HTN ranges, and that the greater the systolic BP level, the greater this effect. 10
Despite the evidence of their benefits in HTN management, all these lifestyle recommendations remain under‐addressed in our study, constituting a real loss of care opportunity for patients. A French study published in 2020 based on a sample of 10 710 volunteer hypertensive participants showed that unhealthy behaviors (heavy alcohol consumption, non‐adherence to dietary recommendations, and overweight) were associated with uncontrolled hypertension, both separately and combined. 29
Our results also showed that participants who were advised to limit their salt intake had lower salt consumption than those not advised to do so, although the levels for both were still higher the recommended 5–6 g of salt per day. 2 This highlights the need to educate adults about the hidden salts in processed products (which can constitute 80% of our salt intake) and inform them of alternatives to salt (spices, aromatic herbs, etc.) which can ensure taste pleasure. This can encourage good therapeutic adherence. 30
Our results showed that alcohol consumption restriction was the least recommended dietary intervention, even though its effects on BP were proven in a meta‐analysis published in 2017. More specifically, moderating alcohol intake was found to lower BP level in a dose‐dependent manner. 31
Our study also showed that weight loss and healthy diet were both recommended in the same low proportions in HTN patients with overweight/obesity. A 2016 systematic review and meta‐analysis of randomized controlled trials showed that besides lowering BP, the effect of a low‐calorie DASH diet was greater than other low‐calorie diets and typical ‐ more specifically, Western or populations’ usual ‐ diets in participants with overweight or obesity. 32
Our results showed that approximately half of our overweight/obese participants who received the recommendation to lose weight had tried to do so in the previous 12 months. This supports the hypothesis that participants can have difficulty to change their habits to lose weight and need greater support.
Physical activity was too rarely discussed with hypertensive adults with overweight/obesity despite the fact that, in combination with a healthy diet, it remains the major line of weight loss. 33
We also found that most of the characteristics associated with a greater likelihood of receiving lifestyle recommendations were generally linked to fragility: overweight/obesity, old age, secondary cardiovascular prevention, and diabetes. As populations characterized by these factors are more prone to developing CV and other diseases, they are more likely to consult more often and are therefore more likely to receive lifestyle recommendations. Besides HTN, lifestyle recommendations can also prevent/control other chronic pathologies and dependence, making them particularly indicated for these fragile populations. 34 , 35
Our French study corroborates results from studies in other European nations which found that physical activity was the most indicated recommendation for HTN, followed by weight loss and nutritional. 36 , 37 In contrast, the proportion of patients receiving lifestyle recommendations were higher in other European studies. 36 , 37 These differences may be accounted for by possible memory bias in our study sample, or more probably by the simple fact that French doctors prescribe lifestyle recommendations less than their counterparts in other European countries. The French society of HTN recommended at least 30 min for a medical visit of a newly diagnosed patient with HTN in its 2013 guidelines. It has also become a recommendation of the National Health Authority (Haute Autorité de la Santé, HAS) in its 2016 guidelines, but in the absence of financial incentives for doctors to encourage change in medical practice, we can assume that there has been no change in the management of HTN since these recommendations. 4 , 38 Indeed, a survey conducted by Santé Publique France among French general practitioners in 2019 showed that 45% prioritized lifestyle recommendations and 3% only offered first‐line antihypertensive pharmacological treatment. 39 Cross‐sectional studies in 2013 and 2020, highlighted that general practitioners mentioned not having enough time to propose and discuss lifestyle recommendations with their patients, which may explain the lower level of lifestyle recommendations declared by aware hypertensive adults in our study. 39 , 40 This lack of time could be resolved using a more multidisciplinary approach to HTN management, which involves advanced practice nurses, dieticians, psychologists, physiotherapists, and other healthcare professionals. Specifically, such an approach could ensure improved long‐term follow‐up, better adherence to HTN management, and greater control of BP levels. A sedentary lifestyle, an unhealthy diet, and overweight/obesity are public health problems which are not exclusive to HTN patients. In 2020, the WHO declared that these problems were leading global risks to health. 22 , 30 , 33 , 41 Sedentary lifestyle causes up to five million deaths every year worldwide. With respect to obesity, worldwide prevalence nearly tripled between 1975 and 2016 and continues to increase today. 33 Moderating salt consumption could avoid 2.5 million deaths every year worldwide. 30 For all these reasons, lifestyle recommendations must become a priority in the management of chronic diseases, especially HTN. This affirmation simply reflects calls which public health organizations and international guidelines have been making for years regarding HTN management.
4.2. Hypertension management
A quarter of our sample was not treated either with lifestyle recommendations or with pharmacological treatment. The management of these hypertensive patients raises many questions about post‐diagnosis follow‐up in terms of medical deserts, lack of awareness of the disease, lack of patients’ involvement in the management of chronic disease, and treatment refusal, among other things. The high percentage of participants who declared they were in good/very good health in our study may support the hypothesis that some hypertensive patients neglect their HTN management because they are asymptomatic and do not feel ill. This highlights the importance of therapeutic education from diagnosis through to follow‐up, to optimize hypertensive patients’ involvement in their disease management. An American cross‐sectional study published in 2021 confirmed the benefits of therapeutic education on hypertensive and diabetic patients in terms of health behaviors. 42 A randomized controlled trial published in 2016 proved that therapeutic education leads to a better level of knowledge and empowerment of hypertensive patients, as well as better adherence to lifestyle recommendations. 43
Given that less than half of aware hypertensive adults in our study had the disease under control in 2015, patient involvement and therapeutic adherence must become a priority in French HTN management. 19 The 2018 European Society of Hypertension (ESH)/European Society of Cardiology (ESC) guidelines highlight the importance of improving patients’ therapeutic adherence. 2 , 44 A Swiss review paper published in 2019 suggested that general practitioners should promote a bio‐psycho‐social approach, focused on patient empowerment and multidisciplinary HTN management with other care providers to encourage adherence. 44
4.3. Strengths and limitations
This study provides new results for the proportion of lifestyle recommendations in the management of HTN in France. Very few studies to date have focused on this aspect of HTN management. The data were collected from 2014 to 2016 and reflected the circumstances of 6–8 years ago. We cannot exclude that the situation has improved since the beginning of this study. However, since no additional efforts have been made to make these recommendations widely available and no financial incentives have been offered to physicians and/or dietitians to assist in their implementation as is the case in other countries, there is little chance that the situation has changed significantly since. The main limitation of our study is its lack of power due to the relatively small size of the sample. It limits the scope of our results.
Patient‐based reports may involve memory and social desirability biases. However, the latter was limited in our study using a self‐administered questionnaire. This last allow us to describe the lifestyle recommendations in HTN management only but we cannot exclude that some participants could have received one or more lifestyle recommendation in another disease management. Furthermore, Esteban's cross‐sectional design and the study protocol did not allow us to analyze adherence to each lifestyle recommendation or to interpret the factors associated with the likelihood of receiving lifestyle recommendations, because we cannot exclude reverse causality. It would be interesting to design a study to evaluate adherence to lifestyle recommendations and potential changes in health habits over a determined period to identify obstacles and levers to lifestyle changes. It would also be interesting to compare blood pressure control and blood pressure‐related organ damage with or without each lifestyle intervention.
5. CONCLUSIONS
Lifestyle recommendations were underused in the management of HTN in our study sample in France, despite being omnipresent in disease treatment guidelines for several decades. Dedicated time during consultations should be regularly given to hypertensive patients to promote and discuss these recommendations, either with general practitioners or paramedic staff. Multidisciplinary HTN management could be a solution to offer more medical time and resources to patients. It could optimize therapeutic education, provide better follow‐up, stronger therapeutic adherence, and ensure greater control of BP levels.
CONFLICTS OF INTEREST
Juliette VAY‐DEMOUY reports, outside the submitted work, financial support from Boston Scientific SAS and AstraZeneca. Jacques BLACHER reports, outside the submitted work, personal fees from Abbott, Bayer, Bottu, Ferring, Steripharma, Kantar, Teriak, personal fees and non‐financial support from Pfizer, Quantum Genomics, Sanofi and Servier. Pauline NEUDORFF, Valérie OLIE, Clémence GRAVE and Amélie GABET declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Juliette Vay‐Demouy participated to the conception of the presented idea, developed the theory, analyzed the data and wrote the manuscript. Hélène Lelong verified the analytical methods, discussed the results, and commented on the manuscript. Pauline Neudorff discussed the results and commented on the manuscript. Amélie Gabet and Clémence Grave verified the analytical methods and commented the manuscript. Jacques Blacher and Valérie Olié conceived the presented idea and designed the study. They both helped to develop the theory, analyze the data, and verified its. They also contributed to the writing of the manuscript. All authors discussed the results and commented on the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors thank the Centers for Health Examinations, the Cetaf and the laboratories involved in the collection, as well as the entire Esteban team and study participants. Our thanks also to Jude Sweeney (Milan, Italy) for copyediting and revising the manuscript.
Vay‐Demouy J, Lelong H, Neudorff P, et al. Underuse of lifestyle recommendations in hypertension management in France: The Esteban study. J Clin Hypertens. 2022;24:1266–1275. 10.1111/jch.14576
REFERENCES
- 1. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982‐1004. [DOI] [PubMed] [Google Scholar]
- 2. Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC task force for the management of arterial hypertension. J Hypertens. 2018;36(12):2284‐2309. [DOI] [PubMed] [Google Scholar]
- 3. Mancia G, Fagard R. Practice guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013(31):1925‐1938. [DOI] [PubMed] [Google Scholar]
- 4. Blacher J, Halimi JM, Hanon O, et al. Management of arterial hypertension in adults: 2013 guidelines of the French society of arterial hypertension. Presse Med. 2013;42(5):819‐825. [DOI] [PubMed] [Google Scholar]
- 5. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population‐representative studies with 104 million participants. Lancet. 2021;398(10304):957‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317(2):165‐182. [DOI] [PubMed] [Google Scholar]
- 8. Lelong H, Blacher J, Menai M, et al. Association between blood pressure and adherence to French dietary guidelines. Am J Hypertens. 2016;29(8):948‐958. [DOI] [PubMed] [Google Scholar]
- 9. Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ. Dietary patterns and blood pressure in adults: a systematic review and meta‐analysis of randomized controlled trials. Adv Nutr Bethesda Md. 2016;7(1):76‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juraschek SP, Miller ER, Weaver CM, Appel LJ. Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J Am Coll Cardiol. 2017;70(23):2841‐2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnett DK, Blumenthal RS, Albert MA, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140(11):596‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahmood S, Shah KU, Khan TM, et al. Non‐pharmacological management of hypertension: in the light of current research. Ir J Med Sci. 2019;188(2):437‐452. [DOI] [PubMed] [Google Scholar]
- 13. Ozemek C, Tiwari S, Sabbahi A, Carbone S, Lavie CJ. Impact of therapeutic lifestyle changes in resistant hypertension. Prog Cardiovasc Dis. 2020;63(1):4‑9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dickinson HO, Mason JM, Nicolson DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24(2):215‐233. [DOI] [PubMed] [Google Scholar]
- 15. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta‐analysis of randomized controlled trials. Hypertension. 2003;42(5):878‐884. [DOI] [PubMed] [Google Scholar]
- 16. Kokubo Y, Padmanabhan S, Iwashima Y, Yamagishi K, Goto A. Gene and environmental interactions according to the components of lifestyle modifications in hypertension guidelines. Environ Health Prev Med. 2019;24(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alpsoy Ş. Exercise and hypertension. Adv Exp Med Biol. 2020;1228: 153‐167. [DOI] [PubMed] [Google Scholar]
- 18. Linneberg A, Jacobsen RK, Skaaby T, et al. Effect of smoking on blood pressure and resting heart rate: a mendelian randomization meta‐analysis in the CARTA consortium. Circ Cardiovasc Genet. 2015;8(6):832‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vallée A, Gabet A, Grave C, Sorbets E, Blacher J, Olié V. Patterns of hypertension management in France in 2015: the ESTEBAN survey. J Clin Hypertens (Greenwich). 2020;22(4):663‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balicco A, Oleko A, Szego E, et al. Esteban design: a cross‐sectional health survey about environment, biomonitoring, physical activity and nutrition. 2017; [Internet] https://www‐em‐premium‐com.ezproxy.univ‐paris13.fr/article/1183047/resultatrecherche/1
- 21. Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the système national d'information interrégimes de l'Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique. 2017; 65 Suppl 4: S149‑S167. French. [DOI] [PubMed] [Google Scholar]
- 22. WHO, World Health Organization. Physical activity. 2020; [Internet] https://www.who.int/news‐room/fact‐sheets/detail/physical‐activity
- 23. AFSSAPS . The 2005 guidelines for dyslipidemia management. 2005; [Internet] https://apimedpl.org/contenu/uploads/2019/12/RCV_Afssaps_2005_tt_dyslipidemie_reco.pdf. French. [Google Scholar]
- 24. Sharman JE, La Gerche A, Coombes JS. Exercise and cardiovascular risk in patients with hypertension. Am J Hypertens. 2015;28(2):147‐158. [DOI] [PubMed] [Google Scholar]
- 25. Sosner P, Gremeaux V, Bosquet L, Herpin D. High blood pressure and physical exercise. Ann Cardiol Angeiol (Paris). 2014;63(3):197‐203. [DOI] [PubMed] [Google Scholar]
- 26. Gremeaux V, Gayda M, Lepers R, Sosner P, Juneau M, Nigam A. Exercise and longevity. Maturitas. 2012;73(4):312‐317. [DOI] [PubMed] [Google Scholar]
- 27. Lee LL, Mulvaney CA, Wong YKY, Chan ES, Watson MC, Lin HH. Walking for hypertension. Cochrane Database Syst Rev. 2021;2: CD008823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fu J, Liu Y, Zhang L, et al. Nonpharmacologic interventions for reducing blood pressure in adults with prehypertension to established hypertension. J Am Heart Assoc. 2020;9(19):e016804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cherfan M, Vallée A, Kab S, et al. Unhealthy behaviors and risk of uncontrolled hypertension among treated individuals‐the CONSTANCES population‐based study. Sci Rep. 2020;10(1):1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. WHO, World Health Organization . Salt reduction. 2020; [Internet] https://www.who.int/news‐room/fact‐sheets/detail/salt‐reduction
- 31. Roerecke M, Kaczorowski J, Tobe SW, Gmel G, Hasan OSM, Rehm J. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta‐analysis. Lancet Public Health. 2017;2(2):e108‑120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soltani S, Shirani F, Chitsazi MJ, Salehi‐Abargouei A. The effect of dietary approaches to stop hypertension (DASH) diet on weight and body composition in adults: a systematic review and meta‐analysis of randomized controlled clinical trials. Obes Rev Off J Int Assoc Study Obes. 2016;17(5):442‐454. [DOI] [PubMed] [Google Scholar]
- 33. WHO, World Health Organization . Obesity and overweight. 2021; [Internet]. https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight
- 34. Cesena FHY. Lifestyle in the very elderly matters. Arq Bras Cardiol. 2020;115(5):882‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doughty KN, Del Pilar NX, Audette A, Katz DL. Lifestyle medicine and the management of cardiovascular disease. Curr Cardiol Rep. 2017;19(11):116. [DOI] [PubMed] [Google Scholar]
- 36. Bolbrinker J, Zaidi Touis L, Gohlke H, Weisser B, Kreutz R. European guidelines on lifestyle changes for management of hypertension : awareness and implementation of recommendations among German and European physicians. Herz. 2018;43(4):352‐358. [DOI] [PubMed] [Google Scholar]
- 37. Zaidi Touis L, Bolbrinker J, Riemer TG, Kreutz R. Moderation of alcohol consumption as a recommendation in European hypertension management guidelines: a survey on awareness, screening and implementation among European physicians. BMJ Open. 2018;8(10):e022026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. HAS, Haute Autorité de la Santé . Prise en charge de l'hypertension artérielle de l'adulte. 2016; [Internet] https://www.has‐sante.fr/jcms/c_2059286/fr/prise‐en‐charge‐de‐l‐hypertension‐arterielle‐de‐l‐adulte French. [Google Scholar]
- 39. Grave G, Gautier A, Gane J, Gabet A, Lacoin F, Olié V. Prévention, dépistage et prise en charge de l'HTA en France, le point de vue des médecins généralistes, France, 2019. Bull Epidémiol Hebd;2020(5):115‐123. French. [Google Scholar]
- 40. Rigal L, Falcoff H, Rahy Z, Flores P, Saurel‐Cubizolles MJ, Ringa V. Lack of dietary and lifestyle advice given to hypertension patients, their characteristics and those of their general practitioner. Glob Health Promot. 2013;20: 33‐42. 2 Suppl. [DOI] [PubMed] [Google Scholar]
- 41. WHO, World Health Organization . Healthy diet. 2020; [Internet] https://www.who.int/news‐room/fact‐sheets/detail/healthy‐diet
- 42. Williams AR, Wilson‐Genderson M, Thomson MD. A cross‐sectional analysis of associations between lifestyle advice and behavior changes in patients with hypertension or diabetes: nHANES 2015–2018. Prev Med. 2021;145: 106426. [DOI] [PubMed] [Google Scholar]
- 43. Perl S, Niederl E, Kos C, et al. Randomized evaluation of the effectiveness of a structured educational program for patients with essential hypertension. Am J Hypertens. 2016;29(7):866‐872. [DOI] [PubMed] [Google Scholar]
- 44. Celi L, John V, Pechère‐Bertschi A, Zisimopoulou S. [Therapeutic adherence in the treatment of hypertension in primary healthcare]. Rev Med Suisse. 2019;15(668):1946‐1949. French. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


