Abstract
The aim of this clinical trial was to assess the efficacy and safety of low‐dose triple combinations of amlodipine, telmisartan, and chlorthalidone in patients with essential hypertension. After a 2‐week placebo run‐in period, 176 patients were randomized to seven treatment groups (placebo, quarter‐dose combination, third‐dose combination, half‐dose combination, amlodipine 5 mg, amlodipine 10 mg, and telmisartan 80 mg) and administered the assigned study drug orally for 8 weeks. The primary efficacy endpoint was the change in the mean sitting systolic blood pressure (BP) (MSSBP) at Week 8. The MSSBP and mean sitting diastolic BP in the quarter‐dose and half‐dose groups were significantly lower compared to the placebo and amlodipine 5 mg groups, with similar BP‐lowering effects observed compared to the amlodipine 10 mg and telmisartan 80 mg groups. However, the third‐dose group showed significant BP improvement only compared to the placebo group. A similar pattern was observed for the control rate of hypertension and response rates. Additional analysis was conducted after correcting for gender and age effects, and, as a result, the third‐dose group showed similar results with regard to the BP‐lowering effect as the quarter‐dose and half‐dose groups. In terms of safety, no special adverse events and clinically significant results were noted, and all dose groups of the triple combination are considered safe for use in essential hypertension patients. The current findings indicated that low‐dose triple combination of amlodipine, telmisartan, and chlorthalidone over 8 weeks effectively improved the BP‐lowering effect in patients with essential hypertension without any safety concerns.
Keywords: amlodipine, chlorthalidone, hypertension, low‐dose combination, telmisartan
1. INTRODUCTION
Hypertension is among the most prevalent conditions closely related to cardiovascular and cerebrovascular disease, which account for the largest number of deaths among adults in Korea. According to a 6‐year follow‐up study (Korean Medical Insurance Corporation study, KMIC) of a specific male adult population, hypertensive patients with a blood pressure (BP) of 140/90 mmHg or higher were reported to have a 2.6 times higher risk of cardiovascular disease than those with BP lower than 130/85 mmHg. 1 , 2 Therefore, the prevention and management of high BP are recognized as major priorities for the promotion of public health. 3
Hypertension treatment generally consists of monotherapy in the first stage, and dual combination therapy at the second stage or higher. However, more than 60% of hypertensive patients fail to control their target BP via monotherapy, with this being more frequently found in patients with higher clinical risk and a lower target BP. In this case, co‐administration of two or more antihypertensive agents with different mechanisms of action is expected to enhance the BP‐lowering effect and avoid adverse effects as opposed to higher doses from a single agent. 4 , 5
The European Society of Cardiology recommends low‐dose combination therapy rather than monotherapy for the early treatment of hypertension, and The British Hypertension Society as well as The National Clinical Guideline Center recommend that patients with uncontrolled hypertension under dual combination therapy use triple combination therapy with diuretics or calcium channel blocker (CCB) added to the angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB). 6
In the TRIUMPH study, patients receiving telmisartan/amlodipine/hydrochlorothiazide triple low‐dose combination therapy had significantly lower systolic BP (SBP) and diastolic BP (DBP) (by 9.8 mmHg and 5.0 mmHg, respectively) compared to those receiving standard care alone (P < .001), and there was no significant difference in the frequency of adverse events (AEs) between the two groups (6.6% for the triple combination vs. 6.8% for standard care). 7
Law et al. 8 reported that the average BP‐lowering effect of single‐component antihypertensive agents in a meta‐analysis of 354 randomized controlled trial (RCT) studies was only 9.1/5.50 (SBP/DBP) mmHg, whereas low‐dose triple combination therapy exhibited superior BP reduction and reduced the risk of stroke and ischemic heart disease (IHD) events by a half and one‐third, respectively. Further, in a previous meta‐analysis study on combination therapy reduced by 1/4 compared to the standard dose monotherapy, SBP and DBP were significantly reduced, and the incidence of AEs was lower than that under any antihypertensive alone. 9
Atkins and Chow 10 reported that low‐dose combination therapy is a promising option for the initial treatment of hypertension that appears to be safe and effective, emphasizing the need for larger trials of triple and quadruple low‐dose combination studies that would provide stronger evidence for efficacy as well as safety.
Thus far, studies have explored various combinations, but there are no reports evaluating the efficacy and safety of the low‐dose combination of amlodipine, telmisartan, and chlorthalidone. The aim of this clinical trial was to assess the efficacy and safety of three types (quarter‐, third‐, and half‐dose of the standard dose) of low‐dose triple combinations of amlodipine, telmisartan, and chlorthalidone after 8 weeks of administration in patients with essential hypertension.
2. METHODS
2.1. Study design
This 8‐week, double‐blind, parallel design, phase 2 study was conducted in the Republic of Korea between February 2020 and February 2021 (ClinicalTrials.gov registration number: NCT04218552). The study was conducted in compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines. At each site, the study was carried out under approval of the site‐specific institutional review board (IRB). Prior to study enrollment and any of the study procedures, written informed consent was obtained from all subjects. In compliance with Declaration of Helsinki, Good Clinical Practice, and all applicable regulations, the study was conducted in an ethical and scientific manner.
Patients with essential hypertension who met the inclusion criteria took placebo during the run‐in period for 2 weeks. At the end of the run‐in period, subjects deemed eligible based on the final inclusion and exclusion criteria were randomized in a 1:1:1:1:1:1:1 ratio to the seven treatment groups (placebo, quarter‐dose combination [telmisartan/amlodipine/chlorthalidone 10/1.25/3.125 mg], third‐dose combination [telmisartan/amlodipine/chlorthalidone 13.333/1.667/4.167 mg], half‐dose combination [telmisartan/amlodipine/chlorthalidone 20/2.5/6.25 mg], amlodipine 5 mg, amlodipine 10 mg, and telmisartan 80 mg), and were administered the assigned study drug orally for 8 weeks in a double‐blind manner. During the treatment period, subjects visited the clinical trial institution at Week 4 and Week 8 for the evaluation of efficacy and safety (Figure 1).
FIGURE 1.
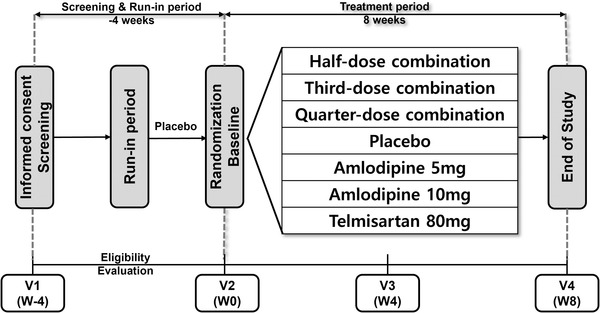
Flowchart of the study design
During the clinical trial, investigational product (IP) compliance of subjects was managed to maintain at least 75% of the placebo run‐in period and at least 80% of the treatment period, and the final IP compliance was at least 80% of the treatment period. IP compliance was assessed for each of the four IPs in treatment period.
2.2. Study population
This study enrolled Korean patients with essential hypertension at the age of ≥19 to <75 who met the criteria of 140 mmHg ≤ mean sitting SBP (MSSBP) < 180 mmHg if not receiving antihypertensive drugs, 130 mmHg ≤ MSSBP < 180 mmHg if receiving antihypertensive drugs at screening, and 140 mmHg ≤ MSSBP < 180 mmHg at the randomization visit. In the case of diabetic or chronic renal disease patients, the criterium of 130 mmHg ≤ MSSBP < 180 mmHg had to be satisfied.
Meanwhile, patients with mean sitting DBP (MSDBP) ≥ 110 mmHg measured at screening and the randomization visit were excluded from participation. Other important exclusion criteria were as follows: history of secondary hypertension or suspected secondary hypertension (aortic stenosis, hyperaldosteronemia, renal stenosis, Cushing disease, chromium‐friendly cell carcinoma, polycystic nephrotic disease, etc.), severe cardiac disease (NYHA Class 3 and 4), ischemic cardiac disease and peripheral arterial disease within the past 6 months, orthostatic hypotension with symptoms, and need for the concomitant administration of other antihypertensive drugs (diuretics, β‐blockers, ACEIs, ARBs, CCBs, renin inhibitors, etc.) during the clinical trial participation period.
2.3. Outcome measures
The primary efficacy endpoint was the change in MSSBP from baseline to Week 8. The secondary efficacy endpoints were: (1) the change in MSSBP from baseline to Week 4, (2) the changes in MSDBP from baseline to Week 4 and Week 8, (3) the percentage of patients who reached the target BP (MSSBP < 140 mmHg and MSDBP < 90 mmHg, in case of diabetic or chronic renal disease patients, MSSBP < 130 mmHg and MSDBP < 80 mmHg) at Week 4 and Week 8, and (4) the proportion of patients whose MSSBP decreased by 20 mmHg or more and/or MSDBP decreased by 10 mmHg or more at Week 4 and Week 8 compared to the baseline.
During screening, BP was measured three times at 2‐min intervals in both arms in the sitting position, and the average of the three measurements was calculated. At each visit, The BP was measured when the patient was relaxed for at least 5 min using the same arm. BP was measured with a calibrated sphygmomanometer (Watch BP Home, Microlife, Shenzhen, China) supplied by sponsor.
Additional analyses were performed on the primary endpoints by correcting for gender, age, and both in order to confirm the effects of these factors. The safety of subjects was assessed based on treatment‐emergent adverse event (TEAE). TEAE includes vital signs, electrocardiograms (ECG), and laboratory test abnormalities, etc.
2.4. Statistical analysis
As this was an exploratory clinical trial planned for the purpose of exploring and evaluating the safety and efficacy of three low‐dose triple combinations, it was conducted with the minimum sample size generally required based on experience.
All statistical analyses were performed using SAS 9.4 64bit (SAS Institute Inc., Cary, NC, USA).
Continuous variables were analyzed via descriptive statistics (number of subjects, mean ± standard deviation), and categorical variables were presented as frequency and percentage. The efficacy set included all patients who received at least one dose of study drug after being randomly assigned and subjected to efficacy evaluation at least once after baseline. All patients who received at least one dose of study drug for safety analysis were included after being randomly assigned to treatment. All missing data for efficacy variables were replaced via the last observation carried forward (LOCF) method.
For efficacy endpoints, descriptive statistics (number of subjects, mean ± standard deviation) were presented for baseline and MSSBP measurements at evaluation visits compared to baseline, and the difference between each test group and the control group was analyzed via analysis of covariance (ANCOVA).
3. RESULTS
3.1. Patient disposition and baseline characteristics
A total of 318 subjects were screened, of which 176 subjects were randomized. Among them, 26 subjects were assigned to the amlodipine 5 mg group, and 25 subjects were randomly assigned to each of the other six treatment groups (Figure 2).
FIGURE 2.
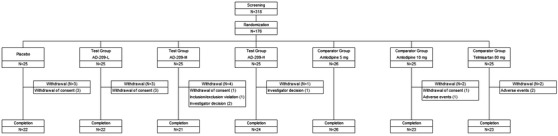
Subject disposition
The baseline characteristics of patients are summarized in Table 1. Demographics and baseline information were generally comparable between all groups, but there were some differences in the distribution of gender and age among the triple combination groups. Of the subjects who participated in the clinical trial, none had a history of chronic kidney disease or clinically significant albuminuria within 6 months.
TABLE 1.
Baseline demographic and clinical characteristics of subjects
| Placebo (n = 25) | Quarter‐dose (n = 25) | Third‐dose (n = 25) | Half‐dose (n = 25) | Amlodipine 5 mg (n = 26) | Amlodipine 10 mg (n = 25) | Telmisartan 80 mg (n = 25) | Total (n = 176) | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 59.96 ± 13.19 | 63.88 ± 8.45 | 58.84 ± 10.15 | 64.16 ± 6.68 | 61.65 ± 9.67 | 59.88 ± 10.82 | 58.12 ± 9.12 | 60.93 ± 9.98 |
| Age group (years) | ||||||||
| <65 | 13 (52.00) | 10 (40.00) | 17 (68.00) | 9 (36.00) | 14 (53.85) | 15 (60.00) | 18 (72.00) | 96 (54.55) |
| ≥65 | 12 (48.00) | 15 (60.00) | 8 (32.00) | 16 (64.00) | 12 (46.15) | 10 (40.00) | 7 (28.00) | 80 (45.45) |
| Gender | ||||||||
| Male | 18 (72.00) | 10 (40.00) | 20 (80.00) | 15 (60.00) | 18 (69.23) | 17 (68.00) | 17 (68.00) | 115 (65.34) |
| Female | 7 (28.00) | 15 (60.00) | 5 (20.00) | 10 (40.00) | 8 (30.77) | 8 (32.00) | 8 (32.00) | 61 (34.66) |
| Height (cm) | 165.53 ± 9.47 | 159.38 ± 8.83 | 165.37 ± 6.93 | 163.03 ± 8.49 | 166.76 ± 9.22 | 165.32 ± 8.88 | 165.08 ± 7.96 | 164.37 ± 8.74 |
| Weight (kg) | 70.70 ± 12.31 | 63.18 ± 10.21 | 72.92 ± 11.08 | 68.64 ± 12.38 | 71.42 ± 13.40 | 74.34 ± 14.24 | 71.32 ± 15.10 | 70.36 ± 13.00 |
| Body mass index (kg/m2) | 25.71 ± 3.29 | 24.82 ± 3.07 | 26.65 ± 3.50 | 25.80 ± 4.18 | 25.55 ± 3.28 | 27.02 ± 3.62 | 25.97 ± 3.95 | 25.93 ± 3.58 |
| Smoking | ||||||||
| Non‐smoker | 16 (64.00) | 18 (72.00) | 13 (52.00) | 17 (68.00) | 12 (46.15) | 14 (56.00) | 13 (52.00) | 103 (58.52) |
| Ex‐smoker | 5 (20.00) | 4 (16.00) | 8 (32.00) | 2 (8.00) | 7 (26.92) | 10 (40.00) | 4 (16.00) | 40 (22.73) |
| Smoker | 4 (16.00) | 3 (12.00) | 4 (16.00) | 6 (24.00) | 7 (26.92) | 1 (4.00) | 8 (32.00) | 33 (18.75) |
| Duration of disease (years)a | 10.35 ± 6.05 | 10.24 ± 7.04 | 9.78 ± 4.64 | 10.15 ± 8.24 | 11.90 ± 9.04 | 8.29 ± 7.89 | 9.71 ± 7.68 | 10.07 ± 7.30 |
| Antihypertensives | ||||||||
| Yes | 24 (96.00) | 22 (88.00) | 24 (96.00) | 23 (92.00) | 22 (84.62) | 20 (80.00) | 23 (92.00) | 158 (89.77) |
| No | 1 (4.00) | 3 (12.00) | 1 (4.00) | 2 (8.00) | 4 (15.38) | 5 (20.00) | 2 (8.00) | 18 (10.23) |
| History of diabetes | ||||||||
| Yes | 4 (16.00) | 5 (20.00) | 9 (36.00) | 7 (28.00) | 7 (26.92) | 5 (20.00) | 3 (12.00) | 40 (22.73) |
| No | 21 (84.00) | 20 (80.00) | 16 (64.00) | 18 (72.00) | 19 (73.08) | 20 (80.00) | 22 (88.00) | 136 (77.27) |
| Baseline MSSBP | ||||||||
| Mean ± SD | 148.15 ± 8.45 | 151.77 ± 12.65 | 149.65 ± 12.45 | 153.17 ± 10.39 | 150.98 ± 10.30 | 149.87 ± 12.89 | 151.38 ± 10.55 | |
| Baseline MSDBP | ||||||||
| Mean ± SD | 89.77 ± 5.88 | 90.76 ± 7.97 | 91.30 ± 9.98 | 91.96 ± 8.84 | 93.68 ± 8.96 | 90.80 ± 10.12 | 94.38 ± 10.15 | |
| Baseline pulse rate | ||||||||
| Mean ± SD | 70.04 ± 11.27 | 73.20 ± 9.46 | 75.80 ± 11.08 | 76.20 ± 12.98 | 78.15 ± 12.42 | 74.00 ± 16.29 | 76.44 ± 7.70 | |
Data are presented as mean ± standard deviation or number (%). The percentage was calculated using the number of subjects for each parameter in each group as a denominator. MSDBP, mean sitting diastolic BP; MSSBP, mean sitting systolic BP; SD, standard deviation.
aDuration of disease: (date of screening − date of diagnosis of hypertension)/365.25.
3.2. Efficacy outcomes
The change (mean ± SD) in MSSBP from baseline to Week 8 was −5.85 ± 10.74 mmHg in the placebo group, −18.87 ± 16.87 mmHg in the quarter‐dose group, −14.55 ± 14.84 mmHg in the third‐dose group, −19.55 ± 14.75 mmHg in the half‐dose group, −8.27 ± 13.79 mmHg in the amlodipine 5 mg group, −17.07 ± 13.92 mmHg in the amlodipine 10 mg group, and −10.23 ± 18.54 mmHg in the telmisartan 80 mg group (Figure 3).
FIGURE 3.
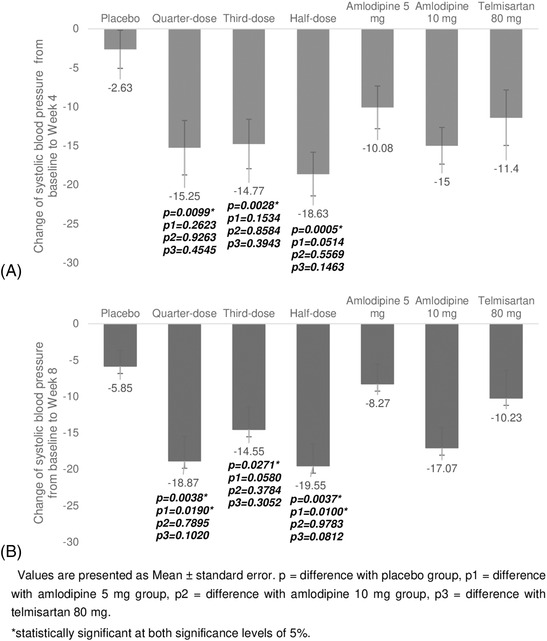
Changes in systolic blood pressure (BP) from baseline to Week 4 (A) and Week 8 (B). Values are presented as the mean ± standard error. p = difference with the placebo group, p1 = difference with the amlodipine 5 mg group, p2 = difference with the amlodipine 10 mg group, p3 = difference with the telmisartan 80 mg. *Significant at the 5% level
The differences between changes in each triple combination group compared to the placebo group were −12.62 ± 4.14 mmHg (P = .0038) in the quarter‐dose group, −7.38 ± 3.23 mmHg (P = .0271) in the third‐dose group, and −10.85 ± 3.54 mmHg (P = .0037) in the half‐dose group, with all three being significant.
The differences between each triple combination group (quarter‐dose, third‐dose, half‐dose) and active reference groups were as follows: −10.42 ± 4.29 mmHg (P = .0190), −6.73 ± 3.46 mmHg (P = .0580), −9.80 ± 3.65 mmHg (P = .0100) for amlodipine 5 mg, −1.14 ± 4.25 mmHg (P = .7895), 2.85 ± 3.20 mmHg (P = .3784), −.09 ± 3.45 mmHg (P = .9783) for amlodipine 10 mg, and −8.62 ± 5.16 mmHg (P = .1020), −4.87 ± 4.69 mmHg (P = .3052), −8.52 ± 4.78 mmHg (P = .0812) for telmisartan 80 mg. A statistically significant decrease in BP compared to the amlodipine 5 mg group was not confirmed only in the third‐dose group. There was no statistically significant decrease in BP in all triple combination groups compared to the amlodipine 10 mg and telmisartan 80 mg groups.
The change (mean ± SD) in MSSBP from baseline to Week 4 was −2.63 ± 11.97 mmHg in the placebo group, −15.25 ± 17.47 mmHg in the quarter‐dose group, −14.77 ± 15.22 mmHg in the third‐dose group, −18.63 ± 13.73 mmHg in the half‐dose group, −10.08 ± 13.96 mmHg in the amlodipine 5 mg group, −15.00 ± 11.77 mmHg in the amlodipine 10 mg group, and −11.40 ± 17.05 mmHg in the telmisartan 80 mg group.
All triple combination groups showed a significant BP reduction compared to the placebo group at 4 weeks, but none showed a significant reduction compared to the amlodipine 5 mg, 10 mg, and telmisartan 80 mg groups.
The change in MSDBP from baseline to Week 4 and Week 8 for the triple combination group was not statistically significant compared to the placebo group only for the third‐dose group at both evaluation visits.
The percentage of patients who reached the target BP at Week 4 and Week 8 was 20.83% and 25.00% in the placebo group, 60.00% and 68.00% in the quarter‐dose group, 22.73% and 17.39% in the third‐dose group, 58.33% and 58.33% in the half‐dose group, 19.23% and 23.08% in the amlodipine 5 mg group, 45.83% and 52.00% in the amlodipine 10 mg group, as well as 43.48% and 43.48% in the telmisartan 80 mg group, respectively (Figure 4).
FIGURE 4.
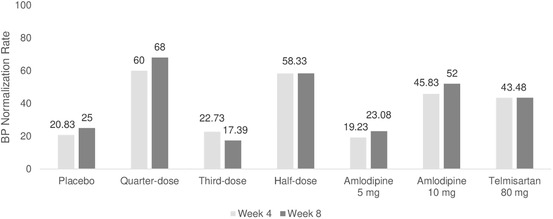
Control rate of blood pressure at Week 4 and Week 8
No triple combination group showed a significantly higher control rate of hypertension compared to the amlodipine 10 mg and telmisartan 80 mg dose groups. Further, only the third‐dose group failed to show a significantly higher control rate of hypertension compared to the placebo and amlodipine 5 mg groups.
The BP response rate, defined as a reduction of MSSBP by more than 20 mmHg and a reduction of MSDBP by more than 10 mmHg at Week 4 and Week 8, respectively, compared to the baseline, was 8.33% and 12.50% in the placebo group, 24.00% and 32.00% in the quarter‐dose group, 9.09% and 8.70% in the third‐dose group, 41.67% and 45.83% in the half‐dose group, 11.54% and 19.23% in the amlodipine 5 mg group, 12.50% and 24.00% in the amlodipine 10 mg group, as well as 26.09% and 30.43% in the telmisartan 80 mg group. No triple combination group showed a statistically significantly higher response rate compared to the amlodipine 10 mg and telmisartan 80 mg dose groups. Further, the quarter‐dose and third‐dose groups did not show a significantly higher response rate compared to the placebo and amlodipine 5 mg groups (Figure 5).
FIGURE 5.
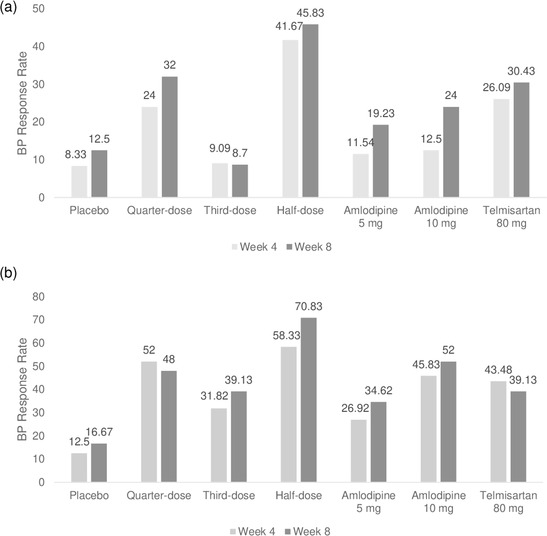
BP response rate defined as a reduction of MSSBP by more than 20 mmHg and (A)/or (B) a reduction of MSDBP by more than 10 mmHg. BP, blood pressure; MSDBP, mean sitting diastolic BP; MSSBP, mean sitting systolic BP
Even when the response rate was defined as a case where only one of the two criteria is satisfied, the third‐dose group did not show a significantly higher response rate compared to the placebo and amlodipine 5 mg groups. The quarter‐dose group showed a significantly higher BP response rate than the placebo group but did not show a significant difference compared to the amlodipine 5 mg group (Figure 5).
The efficacy evaluation indicated that the triple combination group showed a significant improvement in BP compared to placebo overall, but the third‐dose group showed a relatively low improvement in BP compared to quarter‐dose and half‐dose group. Considering that the high ratio of men and low age distribution in the third‐dose group may have affected results, analysis of variance (ANOVA) was conducted after correcting for age, gender, and age + gender.
Similar BP reduction effects were confirmed, without statistically significant differences between the third‐dose, quarter‐dose, and half‐dose groups (Figure 6 and Table 2).
FIGURE 6.
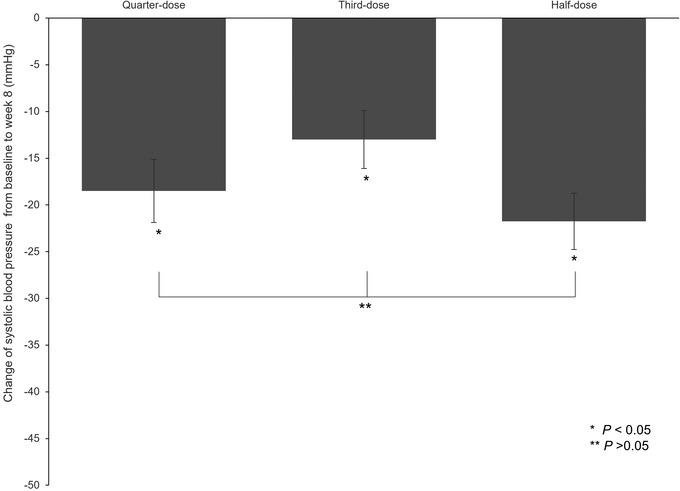
Change of systolic blood pressure (BP) from baseline at Week 8 (additional analysis)
TABLE 2.
Change of systolic BP from baseline at Week 8 (additional analysis)
| Quarter‐dose (n = 25) | Third‐dose (n = 23) | Half‐dose (n = 24) | |
|---|---|---|---|
| Baseline | |||
| Number of subjects | 25 | 23 | 24 |
| Mean ± SD | 151.77 ± 12.65 | 150.32 ± 12.61 | 153.34 ± 10.58 |
| Median | 151.50 | 147.50 | 153.10 |
| Minimum–maximum | 131.50–175.70 | 132.00–175.00 | 132.00–173.00 |
| Week 8 | |||
| Number of subjects | 25 | 23 | 24 |
| Mean ± SD | 132.90 ± 20.79 | 135.77 ± 10.84 | 133.79 ± 12.70 |
| Median | 130.00 | 135.50 | 135.50 |
| Minimum–maximum | 101.50–183.50 | 121.30–159.00 | 116.50–161.50 |
| Change from baseline to Week 8 | |||
| Number of subjects | 25 | 23 | 24 |
| Mean ± SD | −18.87 ± 16.87 | −14.55 ± 14.84 | −19.55 ± 14.75 |
| Median | −18.50 | −13.00 | −21.75 |
| Minimum–maximum | −44.00–18.30 | −52.50–12.00 | −45.00–13.20 |
| Model‐adjusted change from baseline to Week 8 | |||
| Number of subjects | 25 | 23 | 24 |
| LS mean ± SE | −19.07 ± 2.97 | −15.31 ± 3.34 | −19.30 ± 3.03 |
| 95% Confidence interval | [−25.01, −13.13] | [−21.97, −8.64] | [−25.36, −13.24] |
| P‐value | <.0001 | <.0001 | <.0001 |
| Treatment differencea | |||
| Difference of LS means ± SE | 3.76 ± 4.62 | −.23 ± 4.26 | |
| 95% Confidence interval | [−5.47, 13.00] | [−8.73, 8.27] | |
| P‐valueb | .4187 | .9575 | |
| Treatment differencec | |||
| Difference of LS means ± SE | −3.99 ± 4.51 | ||
| 95% Confidence interval | [−13.00, 5.02] | ||
| P‐valueb | .3796 |
Abbreviations: BP, blood pressure; LS mean, least squares mean; MSSBP, mean sitting systolic BP; SD, standard deviation; SE, standard error.
aTreatment difference: third‐dose – quarter‐dose, half‐dose – quarter‐dose.
bAnalysis of covariance: change = baseline MSSBP + treatment + age + gender + age*gender.
cTreatment difference: half‐dose – third‐dose.
3.3. Safety outcomes
During the 8‐week study period, a total of 18 AEs were reported in 27 patients, with an incidence rate of 10.53%, whereas 10 adverse drug reactions (ADRs) were reported in 12 (5.85%) of the 171 patients in the safety set (Table 3).
TABLE 3.
Summary of overall safety
| Placebo (n = 24) | Quarter‐dose (n = 25) | Third‐dose (n = 25) | Half‐dose (n = 25) | Amlodipine 5 mg (n = 26) | Amlodipine 10 mg (n = 25) | Telmisartan 80 mg (n = 24) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | No. of events | n (%) | No. of events | n (%) | No. of events | n (%) | No. of events | n (%) | No. of events | n (%) | No. of events | n (%) | No. of events | |
| Adverse events | 2 (8.33) | 2 | 1 (4.00) | 1 | 3 (13.04) | 5 | 3 (12.50) | 3 | 2 (7.69) | 4 | 3 (12.00) | 4 | 4 (16.67) | 8 |
| Adverse drug reactions | 0 | 0 | 1 (4.00) | 1 | 2 (8.70) | 3 | 2 (8.33) | 2 | 2 (7.69) | 2 | 1 (4.00) | 2 | 2 (8.33) | 2 |
| Severity | ||||||||||||||
| Mild | 0 | 0 | 0 | 0 | 2 (8.70) | 3 | 2 (8.33) | 2 | 2 (7.69) | 2 | 1 (4.00) | 2 | 2 (8.33) | 2 |
| Moderate | 0 | 0 | 1 (4.00) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Specific term | ||||||||||||||
| Chest pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (7.69) | 2 | 0 | 0 | 0 | 0 |
| Fatigue | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.00) | 1 | 0 | 0 |
| Peripheral swelling | 0 | 0 | 0 | 0 | 1 (4.35) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chronic gastritis | 0 | 0 | 0 | 0 | 1 (4.35) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspepsia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.17) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eye swelling | 0 | 0 | 0 | 0 | 1 (4.35) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blood potassium increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.17) | 1 |
| Headache | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.17) | 1 |
| Insomnia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.00) | 1 | 0 | 0 |
| Pruritus | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.17) | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Flushing | 0 | 0 | 1 (4.00) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Serious adverse events | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.85) | 1 | 0 | 0 | 2 (8.33) | 2 |
| Serious adverse drug reactions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The percentage was calculated using the number of subjects for each parameter in each group as a denominator. Multiple adverse events may be collected from one subject. MedDRA version 24.0.
There was no significant difference in the incidence of AEs and ADRs between groups. The incidence of severe adverse events (SAEs) was 1.75% (3/171 patients, three cases), all of which had no causal relationship with the investigated drugs.
There was no significant difference in the ADR incidence rate between groups, and no serious adverse drug reactions (SADRs) were recorded. ADRs in the low‐dose triple combination groups included one case in one subject (4.00%), three cases in two subjects (8.70%), and two cases in two subjects (8.33%) in the quarter‐dose, third‐dose, and half‐dose groups, respectively, of which only one case (flushing) in the quarter‐dose group was evaluated as “moderate,” while all other cases were “mild.” Laboratory testing indicated that potassium (K) was significantly changed from normal (NCS) to abnormal (CS) in one subject of the telmisartan 80 mg group at Week 8. This abnormality in the potassium (K) result was reported as “blood potassium increased” and was evaluated as “mild” in the severity assessment and as an ADR, which had a causal relationship with the study drug.
4. DISCUSSION
This clinical trial demonstrated that the low‐dose triple combination of amlodipine, telmisartan, and chlorthalidone effectively lowered BP in essential hypertension patients. We could study unique low dose triple combination drug, including quarter does, with individually the most powerful and well‐researched, amlodipine, telmisartan, and chlorthalidone for the first time in Asian subjects.
After an 8‐week administration of the triple combination, the change in MSSBP from baseline was −18.87 ± 16.87 mmHg, −14.55 ± 14.84 mmHg, and −19.55 ± 14.75 mmHg in the quarter‐dose, third‐dose, and half‐dose group, respectively.
Following administration of the triple combination, the MSSBP and MSDBP at 8 weeks in the quarter‐dose and half‐dose groups decreased significantly compared to the placebo and amlodipine 5 mg groups, and exhibited stronger BP‐lowering effects compared to the amlodipine 10 mg and telmisartan 80 mg groups. The third‐dose group showed a statistically significant BP improvement when compared to the placebo.
The average change of BP and the control rate of hypertension response, all triple combination groups showed superior results compared to the placebo group but only the third‐dose group showed relatively poor result.
In the third‐dose group, the proportion of women and the percentage of patients aged ≥65 years were relatively low compared to the quarter‐dose and half‐dose groups. But after correcting for age, gender, and age + gender BP‐lowering effect observed in the third‐dose group was not significantly different from those observed in the quarter‐dose and half‐dose groups (third‐dose group vs. quarter‐dose group, P = .3471, third‐dose group vs. half‐dose group, P = .4766). Accordingly, gender and age influenced the BP‐lowering effect, which was in agreement with the notion that gender and age are the main factors affecting BP, as previously reported. 11 , 12
Previous clinical trial showed MSSBP lowering effect with low‐dose triple combination of amlodipine, losartan, and chlorthalidone 13 which was correspond with the present clinical trial.
The incidence rate of AEs after study drug administration was 8.33%, 4.00%, 13.04%, 12.50%, 7.69%, 12.00%, and 16.67% in the placebo, quarter‐dose, third‐dose, half‐dose, amlodipine 5 mg, amlodipine 10 mg, and telmisartan 80 mg groups, respectively. There was no significant difference in the incidence rate of AEs between these groups.
The “flushing” reported in the quarter‐dose group was evaluated as “moderate” and is known as the most common ADR after amlodipine administration. 14 All other reported ADRs were evaluated as “mild,” with most being tolerable and safe, when referring to the AEs described in the amlodipine and telmisartan drug information.
In terms of safety, no special AEs and clinically significant results were noted, indicating that all triple combination regimens are safe for use in essential hypertension patients.
To our knowledge, this is the first multicenter, randomized, parallel clinical trial to evaluate the efficacy and safety of half‐, third‐, and quarter‐dose antihypertensive combinations of amlodipine, telmisartan, and chlorthalidone.
The “stepped care,” which is most commonly used for the treatment of hypertension according to medical guidelines, requires more time and money from both physicians and patients. Frequent hospital visits are required for drug administration and titration, with repeated up‐titration potentially leading to inappropriate BP control.
Therefore, the need for early combination therapy has long been discussed. However, there have not been many large‐scale studies of combination therapy from treatment initiation, such as the ADVANCE (ACEI + diuretics vs. background therapy), 15 FEVER (Felodipine + diuretics vs. diuretics), 16 ACCOMPLISH (ACE inhibitor + CCB vs. ACE inhibitor vs. diuretics), 17 and TRIUMPH studies, 7 of which only ACCOMPLISH confirmed the efficacy for cardiovascular disease prevention. 18 In addition, the recently published QUARTET study established the efficacy, tolerability, and simplicity of the quadruple combination at quarter‐dose (irbesartan at 37.5 mg, amlodipine at 1.25 mg, indapamide at .625 mg, and bisoprolol at 2.5 mg) compared to the monotherapy control (irbesartan 150 mg). 19
In the present study, the BP‐lowering effect of low‐dose triple combinations indicated that these regimens could be useful in the treatment of hypertension, thus representing an alternative treatment option.
The limitations of this study include a relatively short study period and a small sample size. The 8‐week study period is considered rather short for confirming the BP‐lowering effect of study drugs, considering that hypertension medication is administered for relatively long periods. While previous clinical trials were also conducted with about 25 patients per group to explore study drug efficacy, larger‐scale future studies are warranted. In the current study, low‐dose triple combinations were compared to single control drugs but no comparison with usual care, such as additions from dual to triple or sequential addition from a single drug to triple combination, was performed. Thus dose‐dependent effects were not confirmed since there was no reference group for the dual combination. Further studies on the direct cardiovascular disease prevention effect are necessary. Recently most of the antihypertensive trials use ambulatory BP or home BP, so using only clinic BP might be one important limitation of this trial.
Despite these limitations, our study showed that the low‐dose triple combination of amlodipine, telmisartan, and chlorthalidone exhibited a greater BP‐lowering effect over 8 weeks relative to monotherapy, without any safety concerns in patients with essential hypertension.
AUTHOR CONTRIBUTIONS
Ki‐Chul Sung contributed to the design and conduct of the study, interpretation of data, and drafted the manuscript. Jung Hoon Sung, Eun Joo Cho, Jeong Cheon Ahn, Seung Hwan Han, Weon Kim, Kye Hun Kim, Il Suk Sohn, Jinho Shin, Seok Yeon Kim, Kwang‐il Kim, Seok Min Kang, Sung‐Ji Park, Yong‐Jin Kim, Joon‐Han Shin, and Seong‐Mi Park conduct of the study and drafted the manuscript. Chang‐Gyu Park contributed to the design, conduct of the study, the acquisition, analysis, and interpretation of data, and drafted the manuscript. All authors reviewed and approved the final manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
PATIENT CONSENT STATEMENT
Prior to study enrollment and any of the study procedures, written informed consent was obtained from all subjects.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
We have no material that require a permission.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov identifier NCT04218552.
ACKNOWLEDGMENTS
Sponsorship for this study and article processing charges were covered by Addpharma. Inc., Gyeonggi‐do, Republic of Korea. All authors had full access to all of the data in this study and take complete responsibility for the integrity of data and the accuracy of data analysis.
Sung K‐C, Sung JH, Cho EJ, et al. Efficacy and safety of low‐dose antihypertensive combination of amlodipine, telmisartan, and chlorthalidone: A randomized, double‐blind, parallel, phase II trial. J Clin Hypertens. 2022;24:1298–1309. 10.1111/jch.14570
REFERENCES
- 1. Jee SH, Appel LJ, Suh I, Whelton PK, Kim IS. Prevalence of cardiovascular risk factors in South Korean adults: results from the Korea Medical Insurance Corporation (KMIC) Study. Ann Epidemiol. 1998;8:14‐21. [DOI] [PubMed] [Google Scholar]
- 2. Park JK, Kim CB, Kim KS, Kang MG, Jee SH. Meta‐analysis of hypertension as a risk factor of cerebrovascular disorders in Koreans. J Korean Med Sci. 2001;16:2‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and control of hypertension: JACC health promotion series. J Am Coll Cardiol. 2018;72(11):1278‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee JE, Choi JY, Yum YJ, et al. Clinical effectiveness and safety of amlodipine/losartan‐based single‐pill combination therapy in patients with hypertension: findings from real‐world, multicenter observational databases. J Clin Hypertens (Greenwich). 2021;23(11):1975‐1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sung KC, Oh YS, Cha DH, et al. Efficacy and tolerability of telmisartan/amlodipine + hydrochlorothiazide versus telmisartan/amlodipine combination therapy for essential hypertension uncontrolled with telmisartan/amlodipine: the phase III, multicenter, randomized, double‐blind TAHYTI study. Clin Ther. 2018;40(1):50‐63. [DOI] [PubMed] [Google Scholar]
- 6. Unger T, Borghi C, Charchar F, et al. 2020 International society of hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982‐1004. [DOI] [PubMed] [Google Scholar]
- 7. Webster R, Salam A, de Silva HA, et al. Fixed low‐dose triple combination antihypertensive medication vs usual care for blood pressure control in patients with mild to moderate hypertension in Sri Lanka: a randomized clinical trial. JAMA. 2018;320:566‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bennett A, Chow CK, Chou M, et al. Efficacy and safety of quarter‐dose blood pressure‐lowering agents: a systematic review and meta‐analysis of randomized controlled trials. Hypertension. 2017;70:85‐93. [DOI] [PubMed] [Google Scholar]
- 10. Atkins ER, Chow CK. Low‐dose combination therapy for initial treatment of hypertension. Curr Hypertens Rep. 2020;22:65. [DOI] [PubMed] [Google Scholar]
- 11. Martins D, Nelson K, Pan D, Tareen N, Norris K. The effect of gender on age‐related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J Gend Specif Med. 2001;4:20. [PubMed] [Google Scholar]
- 12. Jaquet F, Goldstein IB, Shapiro D. Effects of age and gender on ambulatory blood pressure and heart rate. J Hum Hypertens. 1998;12:253‐257. [DOI] [PubMed] [Google Scholar]
- 13. Hong SJ, Sung KC, Lim SW, et al. Low‐dose triple antihypertensive combination therapy in patients with hypertension: a randomized, double‐blind, phase II study. Drug Des Devel Ther. 2020;14:5735‐5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. US Food & Drug Administration . Highlights of prescribing information: Norvasc (amlodipine besylate) tablets for oral administration initial US Approval: 1987. 2010.
- 15. Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829‐840. [DOI] [PubMed] [Google Scholar]
- 16. Liu L, Zhang Y, Liu G, Li W, Zhang X, Zanchetti A. The felodipine event reduction (FEVER) study: a randomized long‐term placebo‐controlled trial in Chinese hypertensive patients. J Hypertens. 2005;23:2157‐2172. [DOI] [PubMed] [Google Scholar]
- 17. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359:2417‐2428. [DOI] [PubMed] [Google Scholar]
- 18. Korean Academy of Medical Sciences , Korean Centers for Disease Control and Prevention . Evidence‐Based Guideline for Hypertension in Primary Care . Korean Academy of Medical Sciences; 2020.
- 19. Chow CK, Atkins ER, Hillis GS, et al. Initial treatment with a single pill containing quadruple combination of quarter doses of blood pressure medicines versus standard dose monotherapy in patients with hypertension (QUARTET): a phase 3, randomised, double‐blind, active‐controlled trial. Lancet. 2021;398:1043‐1052. [DOI] [PubMed] [Google Scholar]


