Abstract
Background/Objectives:
Excessive gestational weight gain (GWG) and pre-pregnancy obesity affect a significant portion of the US pregnant population and are linked with negative maternal and child health outcomes. The objective of this study was to explore associations of pre-pregnancy body mass index (pBMI) and GWG with longitudinally measured maternal urinary metabolites throughout pregnancy.
Subjects/Methods:
Among 652 participants in the New York University Children’s Health and Environment Study, a longitudinal pregnancy cohort, targeted metabolomics were measured in serially collected urine samples throughout pregnancy. Metabolites were measured at median 10 (T1), 21 (T2), and 29 (T3) weeks gestation using the Biocrates AbsoluteIDQ® p180 Urine Extension kit. Acylcarnitine, amino acid, biogenic amine, phosphatidylcholine, lysophosphatidylcholine, sphingolipid, and sugar levels were quantified. Pregnant people 18 years or older, without type 1 or 2 diabetes and with singleton live births and valid pBMI and metabolomics data were included. GWG and pBMI were calculated using weight and height data obtained from electronic health records. Linear mixed effects models with interactions with time were fit to determine the gestational age-specific associations of categorical pBMI and continuous interval-specific GWG with urinary metabolites. All analyses were corrected for false discovery rate.
Results:
Participants with obesity had lower long-chain acylcarnitine levels throughout pregnancy and lower phosphatidylcholine and glucogenic amino acids and higher phenylethylamine concentrations in T2 and T3 compared with participants with normal/underweight pBMI. GWG was associated with taurine in T2 and T3 and C5 acylcarnitine species, C5:1, C5-DC, and C5-M-DC, in T2.
Conclusions:
pBMI and GWG were associated with the metabolic environment of pregnant individuals, particularly in relation to mid-pregnancy. These results highlight the importance of both preconception and prenatal maternal health.
Introduction
Pre-pregnancy obesity, defined as a body mass index (BMI) ≥ 30 kg/m2, and excessive gestational weight gain (GWG), defined by the 2009 Institute of Medicine recommendations, affect approximately one third and over one half of all US pregnancies, respectively (1, 2). Both of these exposures are associated with poor pregnancy outcomes including gestational diabetes mellitus (GDM), hypertensive disorders of pregnancy, macrosomia, and large for gestational age (LGA) infants (3–5). Long-term, high pre-pregnancy BMI (pBMI) and excessive GWG have been linked with chronic obesity in the gestational parent and offspring (6–9). While both high pBMI and excessive GWG are linked with acute and long-term adverse health outcomes, their effects on the cellular environment during pregnancy are not well characterized.
Metabolomics, the measurement of low molecular weight metabolites in biospecimens, is a growing area of research to investigate disease mechanisms by providing a snapshot of metabolic perturbations that could arise from changes in the cellular environment. Various disease states, including obesity, have been linked to alterations in the ‘metabotype’ of individuals (10, 11). This research has extended to pregnant populations, where pBMI and GWG have been associated with metabolite concentrations during pregnancy (12–18).
While this research has laid the groundwork in the field, studies have suffered from small sample sizes, limited adjustment for confounders, or a narrow scope of metabolite classes (e.g., amino acids (AA), acylcarnitines, or fatty acids) (12–18). No studies have explored the association between GWG and urinary metabolites and only one has evaluated the relationship between pBMI and urinary metabolites among pregnant individuals, with most research quantifying metabolites in maternal blood (19). Additionally, studies often measure metabolites at just one time in pregnancy; a limitation, as metabolite concentrations vary over the course of gestation (15–20). This study aimed to explore the associations of pBMI and GWG with longitudinally measured metabolites throughout pregnancy.
Subjects and Methods
Study Population
This research utilized data from the New York University Children’s Health and Environment Study (NYU CHES), an ongoing, prospective longitudinal cohort study that enrolls pregnant people 18 years or older and follows them through their pregnancies. Questionnaires are administered and biospecimens are collected during prenatal care visits at three time points during pregnancy. All individuals provided written informed consent to participate in the study, which was approved by the NYU Grossman School of Medicine Institutional Review Board. The study design and cohort are described in further detail elsewhere (21).
A subset of participants with pregnancies occurring between 2016 and 2018 for which metabolomics data were available were included in this analysis. Among participants with metabolomics data (n = 680), those with stillbirths or unknown birth outcomes (n = 9), multiple births (n = 6), missing pBMI (n = 5), or pre-pregnancy type 1 or type 2 diabetes (n = 8) were excluded, resulting in an analytic sample of 652 individuals.
Pre-pregnancy BMI (pBMI)
pBMI was calculated using weight and height data abstracted from electronic health records (EHR) and questionnaires. Weights and heights collected from EHR and closest to the start of pregnancy were prioritized (Supplemental Figure 1). pBMI was categorized into three groups based on cut points defined by the U.S. Centers for Disease Control and Prevention: normal/underweight (BMI < 25.0 kg/m2), overweight (25.0 ≤ BMI < 30 kg/m2), and obese (BMI ≥ 30 kg/m2) (22). The normal and underweight pBMI categories were combined because less than two percent of the study sample had an underweight pBMI.
Gestational Weight Gain (GWG) Rate
The GWG rate between time points (e.g., GWG rate between T1 and T2) was calculated for each participant via a multi-step process. Prenatal weights obtained from EHRs were matched by gestational age (GA) at measurement to the GA at urine sample collection used for the metabolomics analysis. Urine samples with no matched weight data had weights imputed using the two weights measured closest to the GA at sample collection, using the interpolation formula below.
Approximately 10% of weights were not available on the same day as biospecimen collection, of which 8% were imputed using the interpolation formula and 2% were unable to be approximated due to insufficient weight data.
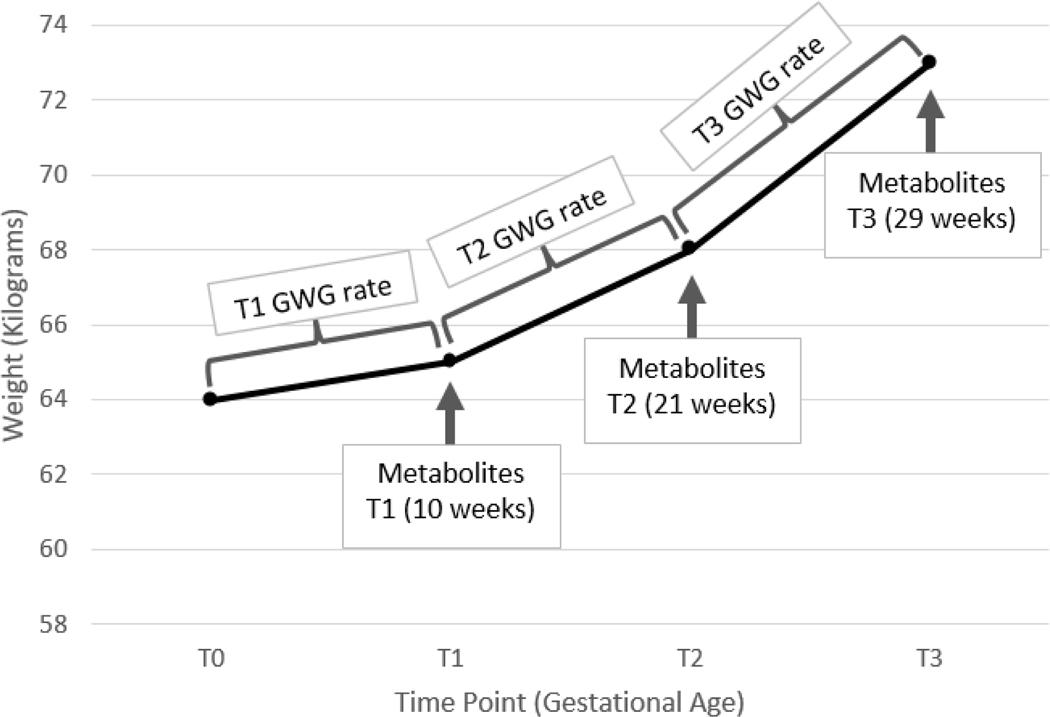
To estimate the GWG rates between time points (i.e., T0-T1, T1-T2, and T2-T3 GWG rates) for each participant, a piecewise linear mixed effects model with participant random effect was fit using the serial weights over time (i.e., pre-pregnancy, T1, T2, and T3 measured, and/or imputed), Figure 1. Because of variation in GA at sample collection, nodes were placed at the median GA at urine sample collection among all participants for T1 and T2.
Figure 1:
A simplified illustration of the GWG rate estimation approach using hypothetical data
Note: T0 corresponds to pre-pregnancy.
Metabolites
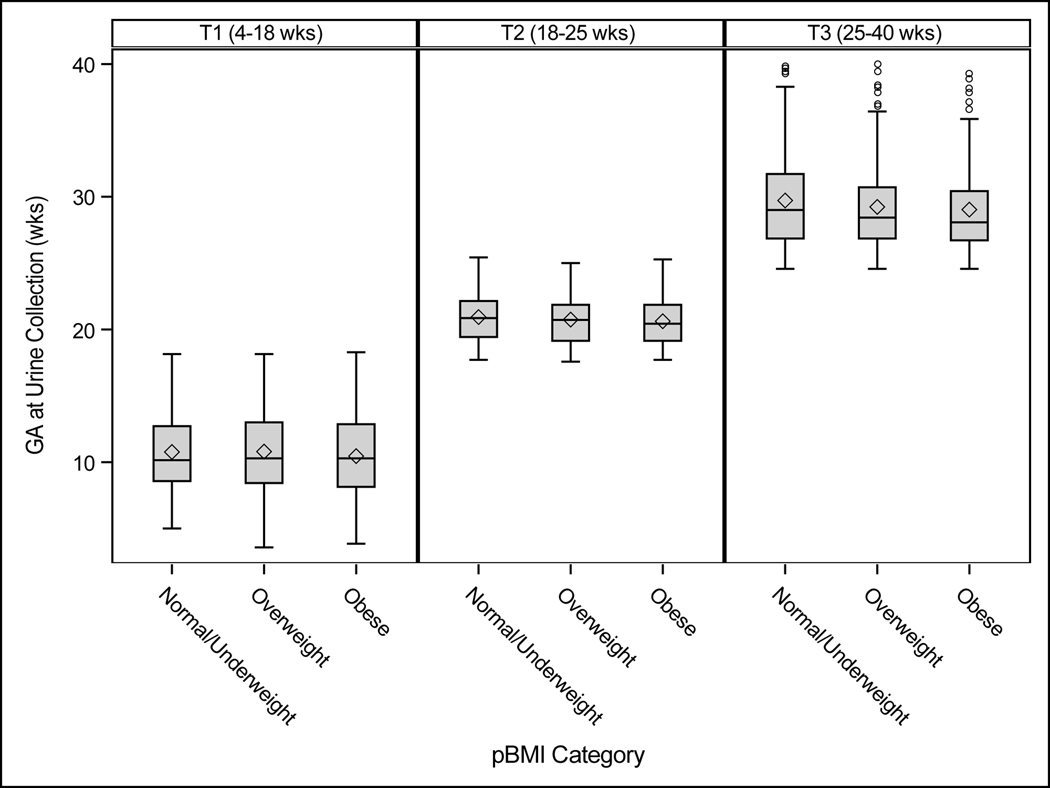
Metabolites were measured in maternal urine samples collected at three time points during pregnancy (median GA in weeks (25th, 75th percentile): T1, 10 (8, 13); T2, 21 (19, 22); T3, 29 (27, 31)), Figure 2. We used the AbsoluteIDQ® p180 Urine Extension kit (Biocrates Life Sciences AG, Innsbruck, Austria) to perform the targeted mass spectrometry (MS)-based quantitative metabolomic assay that directly measured metabolite concentrations in urine samples. This kit includes two separate parts that are analyzed by multiple reaction monitoring (MRM) tandem MS (MS\MS) analysis. The first part is a high-performance liquid chromatography (HPLC)-based method that can separate and quantify 42 metabolites (21 AA and 21 biogenic amines) and the second part is a flow injection analysis (FIA) that can simultaneously quantify up to 146 metabolites, most of which are lipids. The 146 metabolites include 40 acylcarnitines including free carnitine, 38 acyl/acyl side chain phosphatidylcholines (PC aa), 38 acyl/alkyl side chain phosphatidylcholines (PC ae), 14 lysophosphatidylcholines (lysoPC), 15 sphingolipids (SM) in the positive (+) polarity mode, and the total concentration of hexoses in the negative (–) polarity mode. The detailed preparation of the kit components, samples, and the kit plate are described in the AbsoluteIDQ® p180 user’s manual (UM_p180_Sciex_13) and the Urine Extension (Biocrates-SOP-p180-Urine) supplement. MS-based analyses were carried out on an 4000 Q-Trap® ESI-LC-MS/MS System (Sciex, Framingham, MA) equipped with an Agilent 1200 Series HPLC (Agilent Technologies, Palo Alto, CA) using an Agilent Zorbax® Eclipse XDB-C18 (3.5 μm) 3.0×100 mm column. All raw data were processed using a combination of Analyst® 1.6.2 (Sciex LP, Ontario, Canada) instrument control and data processing software, and MetIDQ Carbon 6.4.8-DB105–2809 LIMBS (Laboratory Information Management System) software (Biocrates Life Sciences AG, Innsbruck, Austria). Laboratory staff were blinded to the exposure status of samples.
Figure 2:
Boxplots of urine sample collection in weeks (wks) gestation by time point and BMI category, NYU CHES (n=652)
Assay performance monitoring was conducted by the evaluation of quality control (QC) samples. Three manufacturer-provided QC samples at three known levels of concentration for each metabolite, four replicates of the mid-level QC (QC2), and six Children’s Health Exposure Analysis Resource (CHEAR) Consortium Reference Material replicates were analyzed. QC assessments within each analytical run as well as pre- and post-analysis system suitability checks were performed. Five plates also included three QC replicates provided by NYU CHES, blinded from lab staff. The inter- and intra-coefficient of variation (CV) was calculated for each metabolite. Those with either an intra- or inter-CV > 25% (n = 28) were excluded from analysis. In addition, metabolites with > 60% of observations equal to zero (n = 5) were excluded due to poor detection levels and heavily skewed distributions, Supplemental Table 1.
Using the Ratio Explorer software package provided by Biocrates Life Sciences AG, 44 biologically relevant metabolite summations and ratios were calculated. Only summations and ratios that included valid metabolite data were explored. After exclusions, a total of 154 metabolites and 13 metabolic ratios and summations were included in the final analyses. To account for differences in urine concentration and as part of the normalization process performed by MetIDQ Carbon 6.4.8-DB105–2809 LIMBS, metabolite concentrations and ratios above the limit of detection were adjusted to have a constant creatinine concentration (i.e., 1 mmol Creatinine). Metabolite concentrations and ratios were also ln-transformed and pareto-scaled to minimize the effects of large values. Extreme outliers determined by visual inspection were removed.
Covariates
Covariates were selected based on prior literature and statistically significant associations with the exposures. Education, race, and ethnicity were reported by participants on the baseline questionnaire. Race and ethnicity were combined into five categories (i.e., non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, Hispanic, non-Hispanic other/multiracial). Maternal age, parity, insurance, and GDM diagnosis were obtained through EHRs. Other potential covariates were explored including time of urine collection, urinary cotinine levels, and maternal diet quality, total caloric intake, and choline consumption, but none was statistically significantly associated with exposures.
Statistical Analysis
We examined univariate distributions of pBMI, GWG rate, and covariates, as well as bivariate associations between covariates and pBMI category. To determine the associations of pBMI with GA-specific urinary metabolite levels throughout pregnancy, linear mixed effects models were fit allowing for interactions with time. Estimates were output comparing each pBMI category with urinary metabolites at the median GA at urine sample collection for each time point (i.e. T1, T2, T3). The model controlled for GA at sample collection, maternal age, insurance, parity, and race/ethnicity. A similar model was fit to determine the GA-specific associations of GWG rate with urinary metabolites throughout pregnancy. Differences in estimated metabolite concentrations were calculated comparing the 75th and 25th percentile of GWG rate during the interval prior (e.g., metabolite concentrations at T2 were regressed on the T1-T2 GWG rate). The GWG rate models controlled for GA at sample collection, pBMI, maternal age, insurance, parity, and race/ethnicity. All regression analyses were repeated excluding participants diagnosed with GDM (n = 561). A false discovery rate (FDR) of five percent was used to adjust for multiple testing. All statistical analyses were conducted using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Data and Code Availability
A deidentified dataset and code are available upon request from the corresponding author.
Results
Study Population Characteristics
Among the 652 participants included in this analysis, 50% had a pBMI classified as normal/underweight, 29% were overweight, and 21% were obese (Table 1). Age and GA at delivery decreased slightly across pBMI categories with obese individuals being younger and having shorter pregnancy durations than overweight and normal/underweight individuals. Individuals with a normal/underweight pBMI were more likely to be Asian or non-Hispanic White, privately insured, primipara, and have a Bachelor’s degree or higher compared with those with an overweight or obese pBMI. Overall, 14% of participants were diagnosed with GDM, with normal/underweight individuals having a lower prevalence of GDM compared with overweight and obese individuals (9.6% vs. 18.4% vs. 18.0%, respectively). Median GWG rates decreased across pBMI categories with those with obesity having the lowest GWG rate across each pregnancy interval (pre-pregnancy-T1 = −0.01 kg/weeks; T1-T2 = 0.18 kg/weeks; T2-T3 = 0.41 kg/weeks) and those with normal/underweight pBMI having the highest (pre-pregnancy-T1 = 0.03 kg/weeks; T1-T2 = 0.36 kg/weeks; T2-T3 = 0.51 kg/weeks). Across all pBMIs, GWG rates increased over pregnancy (median (25th, 75th percentile): pre-pregnancy-T1 = 0.01 (−0.06, 0.17) kg/weeks; T1-T2 = 0.33 (0.20, 0.45) kg/weeks; T2-T3 = 0.48 (0.38, 0.58) kg/weeks).
Table 1:
Study characteristics overall and by pBMI category, NYU CHES (n=652)
| Characteristics | Overall (n=652) |
pBMI | |||
|---|---|---|---|---|---|
| Normal/Underweight n=323 (49.5%) |
Overweight n=190 (29.1%) |
Obese n=139 (21.3%) |
p-value | ||
| Age (years), median (25th, 75th percentile) a | 31.9 (28.4, 35.4) | 32.1 (29.1, 35.6) | 31.8 (28.0, 35.3) | 31.4 (26.7, 34.7) | 0.08 |
| GA at Birth (wk), median (25th, 75th percentile) a | 39.4 (38.6, 40.3) | 39.4 (38.7, 40.3) | 39.4 (38.3, 40.3) | 39.3 (37.6, 40.1) | 0.08 |
| Primapara, n (%) b * | 307 (47.1) | 187 (57.9) | 77 (40.5) | 43 (30.9) | <0.01 |
| Education, n (%) b * | <0.01 | ||||
| High school or less | 243 (37.3) | 72 (22.3) | 88 (46.3) | 83 (59.7) | |
| Some college or Associate’s Degree | 97 (14.9) | 37 (11.5) | 32 (16.8) | 28 (20.1) | |
| Bachelor’s or more | 289 (44.3) | 197 (61.0) | 64 (33.7) | 28 (20.1) | |
| Race/ethnicity, n (%) b * | <0.01 | ||||
| Non-Hispanic White | 188 (28.8) | 134 (41.5) | 43 (22.6) | 11 (7.9) | |
| Non-Hispanic Black | 34 (5.2) | 13 (4.0) | 12 (6.3) | 9 (6.5) | |
| Non-Hispanic Asian | 57 (8.7) | 46 (14.2) | 9 (4.7) | 2 (1.4) | |
| Hispanic | 354 (54.3) | 122 (37.8) | 119 (62.6) | 113 (81.3) | |
| Non-Hispanic other/multi | 18 (2.8) | 8 (2.5) | 6 (3.2) | 4 (2.9) | |
| Public Insurance, n (%) b * | 371 (56.9) | 136 (42.1) | 120 (63.2) | 115 (82.7) | <0.01 |
| GDM, n (%) b * | 91 (14.0) | 31 (9.6) | 35 (18.4) | 25 (18.0) | <0.01 |
| GWG Rate (kg/wk), median (25th, 75th percentile) a | |||||
| GWG Rate T1* | 0.01 (−0.06, 0.17) | 0.03 (−0.03, 0.18) | 0.01 (−0.06, 0.16) | −0.01 (−0.12, 0.16) | 0.03 |
| GWG Rate T2* | 0.33 (0.20, 0.45) | 0.36 (0.27, 0.48) | 0.31 (0.20, 0.43) | 0.18 (0.06, 0.36) | <0.01 |
| GWG Rate T3* | 0.48 (0.38, 0.58) | 0.51 (0.41, 0.60) | 0.47 (0.38, 0.57) | 0.41 (0.31, 0.51) | <0.01 |
Kruskal-Wallis test was used
Chi-square test was used
p-value ≤ 0.05
Note: n = 23 missing education, n = 1 missing race/ethnicity, n = 13 missing all GWG rates
Metabolomics Analysis
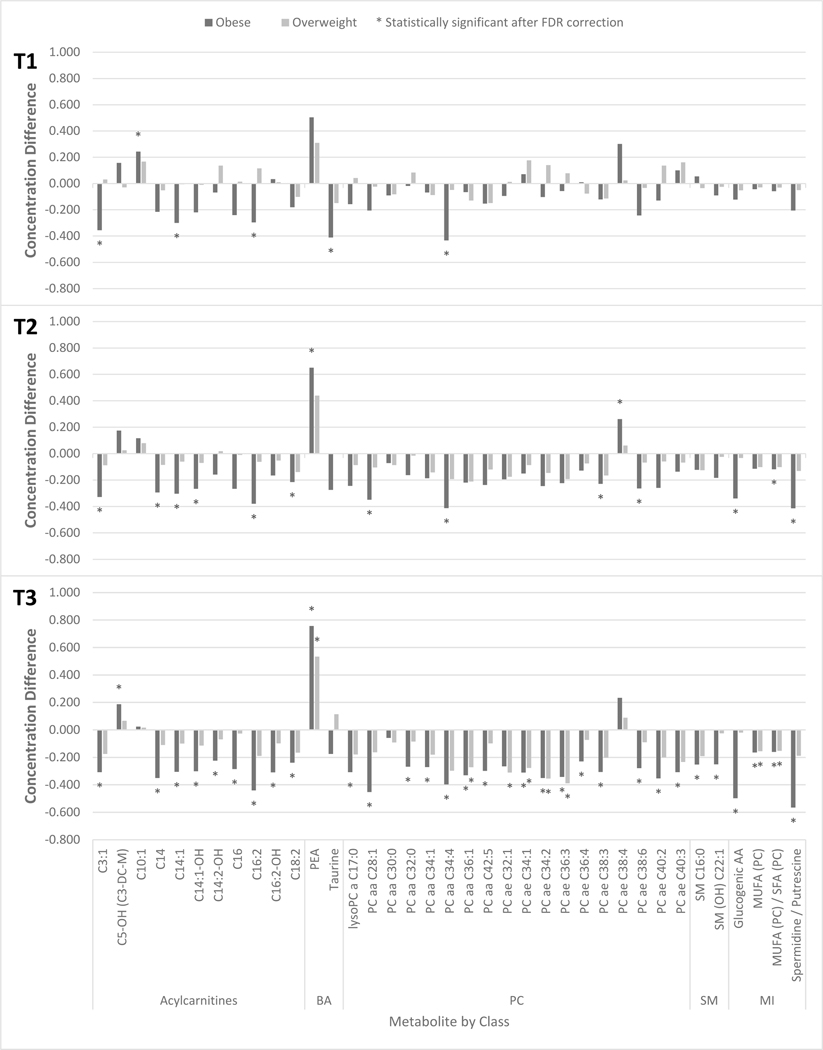
Associations of pBMI with urinary metabolites became more prominent as pregnancy progressed, with the number of significant associations increasing from T1 to T3 (Figure 3). Associations between pBMI and metabolites in the acylcarnitine class were generally negative, with the majority of results showing lower acylcarnitine concentrations in those with obesity compared with those with normal/underweight pBMI. Concentrations of C3:1, C14:1, and C16:2 were consistently lower in the obese category compared with the normal/underweight category. The majority of significant results observed during T2 and T3 in the acylcarnitine class remained statistically significant after the exclusion of GDM cases (Supplemental Table 2).
Figure 3:
Statistically significant differences in maternal urinary metabolite concentrations of participants with overweight or obese pBMI in comparison to those with normal/underweight pBMI by time point, NYU CHES (n=652)
Notes: Metabolites measured in μmol/mmol creatinine have been ln-transformed and pareto-scaled. For standard errors, see Supplemental Table 2.
Abbreviations: BA - biogenic amines, PC - phosphatidylcholines & lysophosphatidycholines, SM - sphingomyelin, MI - metabolic indicators
None of the individual AA was associated with pBMI, but glucogenic amino acid levels (a sum of glycine, serine, and alanine concentrations) were lower in obese compared with non-obese participants in T2 and T3 (Figure 3). Individually, glycine showed a non-significant negative dose response with pBMI for T1 and T2 whereas alanine’s and serine’s, as well as T3 glycine’s, associations with pBMI followed a non-monotonic pattern, with lower concentrations among normal/underweight and obese individuals compared with overweight individuals across pregnancy (Supplemental Table 2).
Compared with normal/underweight participants, those with obesity had higher phenylethylamine (PEA) concentrations in T2 and T3, persisting after exclusion of GDM cases (Figure 3, Supplemental Table 2). The majority of associations between pBMI and the phosphatidylcholine and sphingolipid classes were observed in T3, with lower metabolite levels among obese participants compared with normal/underweight participants. However, approximately half of the associations with phosphatidylcholines and with sphingolipids remained statistically significant after the removal of those with GDM diagnoses during pregnancy. Regardless of the exclusion of GDM cases, the ratio of mono-unsaturated fatty acids (MUFA (PC)) to saturated fatty acids (SFA (PC)) and the summation of MUFA (PC) were observed to be lower in both the obese and overweight categories compared with the normal/underweight category in T3.
GWG had the strongest associations with metabolite concentrations during T2, with only a few statistically significant associations in T3 and none in T1 (Table 2, Supplemental Table 3). GWG was associated with C5 and C6 chain acylcarnitine species in T2, with mixed directions in effect estimates. Associations of GWG with the three AA, tyrosine, phenylalanine, and lysine were still in the positive direction after the removal of GDM cases, but were no longer statistically significant. Taurine levels were positively associated with GWG in both T2 and T3. These results remained significant after the removal of GDM cases. One phosphatidylcholine, phosphatidylcholine diacyl C36:5, was found to be positively associated with GWG in T2, which persisted after the removal of GDM cases.
Table 2:
Statistically significant differences in maternal urinary metabolite concentrations between the 75th and 25th percentile of GWG rate by time point, NYU CHES (n=639)
| Metabolites by Class | T1, 10 weeks | T2, 21 weeks | T3, 29 weeks | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Full Sample | Without GDM | Full Sample | Without GDM | Full Sample | Without GDM | |||||||||||||
| β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value | |
| Acylcarnitines | ||||||||||||||||||
| C5-DC (C6-OH) | −0.039 | 0.012 | 0.002 | −0.041 | 0.014 | 0.003 | −0.053 | 0.013 | 0.000 | −0.054 | 0.014 | 0.000 | −0.048 | 0.016 | 0.003 | −0.047 | 0.016 | 0.004 |
| C5-M-DC | −0.015 | 0.014 | 0.285 | −0.021 | 0.016 | 0.186 | −0.058 | 0.015 | 0.000 | −0.060 | 0.016 | 0.000 | −0.069 | 0.018 | 0.000 | −0.066 | 0.018 | 0.000 |
| C5:1 | 0.033 | 0.013 | 0.010 | 0.025 | 0.014 | 0.079 | 0.050 | 0.013 | 0.000 | 0.053 | 0.014 | 0.000 | 0.048 | 0.016 | 0.003 | 0.055 | 0.016 | 0.001 |
| C6 (C4:1-DC) | 0.039 | 0.019 | 0.040 | 0.035 | 0.020 | 0.083 | 0.065 | 0.019 | 0.001 | 0.060 | 0.019 | 0.002 | 0.064 | 0.023 | 0.007 | 0.059 | 0.022 | 0.008 |
| C16:2 | 0.012 | 0.026 | 0.646 | 0.006 | 0.029 | 0.832 | 0.078 | 0.026 | 0.003 | 0.078 | 0.028 | 0.005 | 0.098 | 0.032 | 0.002 | 0.098 | 0.032 | 0.002 |
| Amino Acids | ||||||||||||||||||
| Lysine | 0.055 | 0.024 | 0.024 | 0.045 | 0.027 | 0.091 | 0.077 | 0.025 | 0.002 | 0.055 | 0.026 | 0.035 | 0.071 | 0.030 | 0.020 | 0.046 | 0.030 | 0.123 |
| Phenylalanine | 0.046 | 0.016 | 0.004 | 0.045 | 0.017 | 0.010 | 0.052 | 0.017 | 0.003 | 0.041 | 0.017 | 0.019 | 0.042 | 0.021 | 0.046 | 0.028 | 0.020 | 0.159 |
| Tyrosine | 0.029 | 0.014 | 0.036 | 0.027 | 0.015 | 0.070 | 0.051 | 0.015 | 0.001 | 0.040 | 0.015 | 0.007 | 0.051 | 0.018 | 0.004 | 0.038 | 0.017 | 0.030 |
| Biogenic Amines | ||||||||||||||||||
| Taurine | 0.078 | 0.028 | 0.006 | 0.088 | 0.031 | 0.005 | 0.160 | 0.027 | 0.000 | 0.160 | 0.028 | 0.000 | 0.169 | 0.031 | 0.000 | 0.159 | 0.032 | 0.000 |
| Phosphatidylcholines & Lysophosphatidylcholines | ||||||||||||||||||
| Phosphatidylcholine diacyl C 36:5 | 0.080 | 0.029 | 0.006 | 0.090 | 0.032 | 0.004 | 0.089 | 0.028 | 0.002 | 0.109 | 0.030 | 0.000 | 0.073 | 0.035 | 0.038 | 0.091 | 0.035 | 0.009 |
| Metabolic Indicators | ||||||||||||||||||
| C2 / C0 | 0.003 | 0.019 | 0.861 | −0.014 | 0.021 | 0.516 | −0.055 | 0.019 | 0.004 | −0.063 | 0.020 | 0.002 | −0.077 | 0.023 | 0.001 | −0.074 | 0.023 | 0.002 |
Darker grey indicates statistical significance after FDR correction
Note: Metabolites measured in εmol/mmol creatinine have been ln-transformed and pareto-scaled
Abbreviations: standard error, SE
Discussion
In this targeted metabolomics analysis, pBMI and GWG were associated with several alterations to the metabolic environment of pregnant people, highlighting the importance of both preconception and prenatal maternal health. pBMI was associated with acylcarnitine, biogenic amine, phosphatidylcholine, lysophosphatidylcholine, and sphingolipid levels, while GWG was related to acylcarnitine, amino acid, biogenic amine, and phosphatidylcholine levels. The effects of these exposures varied by GA, however, with the impacts of pBMI being most pronounced in T3 and those of GWG in T2.
Pre-pregnancy BMI and Maternal Metabolites
While pBMI did not appear to be associated with individual amino acid concentrations, we found the sum of three glucogenic AA (i.e., glycine, serine, alanine) to be lower in T2 and T3 among individuals with obesity compared with those without. Research has been mixed on the direction of the association between obesity and glucogenic AA, with glycine and serine levels typically decreased and alanine levels typically increased among those with obesity compared with those without (10). Lower concentrations of glucogenic AA in T2 and T3 among those with obesity could be related to heightened insulin resistance, which increases with pregnancy progression to account for the growing glucose demands by the fetus (23). Several studies have observed associations between glucogenic AA and various states of insulin resistance; however, findings have been inconsistent (24–28). Urine and plasma concentrations of glycine, serine, and alanine among healthy individuals have been found to be inversely associated with markers of low-grade inflammation, hypothesized to be an outcome of obesity and contributor to insulin resistance (29, 30). Low-grade inflammation is suspected to be a pathway through which fetal-programming may occur (30). Importantly in our study, the lower glucogenic amino acid levels observed among participants with obesity compared with those without remained after the exclusion of GDM cases for T3 and partially for T2.
Among the metabolite classes linked with obesity, acylcarnitines were the most consistently associated with pBMI across pregnancy. Concentrations of long-chain acylcarnitines, C14, C14:1, C14:1-OH, C14:2-OH, C16:2, C16:2-OH, and C18:2 were lower in obese participants compared with normal/underweight participants. Changes to long-chain acylcarnitine concentrations have been observed in prior studies among non-pregnant people with reported lower urinary concentrations of C14:2 in individuals with obesity and abnormal blood sugar levels compared with non-diabetic individuals with normal BMIs and higher plasma C14:1 levels among adults with obesity and those with Type 2 diabetes compared to lean adults (31, 32). Involved in fatty acid metabolism, long-chain acylcarnitines have been linked with obesity as well as prediabetes, type 2 diabetes, and metabolic syndrome in non-pregnant individuals (32–34).
We also detected primarily lower phosphatidylcholine levels in mid-pregnancy among obese participants compared with normal/underweight participants. Previous research has noted a positive association between adolescent and adult obesity and lower phosphatidylcholine concentrations (35, 36). Perturbations in phosphatidylcholine concentrations have also been associated with fetal growth restriction and birthweight, two outcomes linked with pBMI (37–39). In non-pregnant adults, certain phosphatidylcholine concentrations have been associated with diabetes and insulin resistance, including those observed by our study (40, 41). Our results mirror the findings of this prior research, as a portion of the associations between pBMI and phosphatidylcholine levels were no longer statistically significant when we excluded GDM cases. However, some phosphatidylcholine species remained altered by pre-pregnancy obesity (i.e., PC aa C34:4, PC ae 34:1, PC ae C34:2, PC ae C38:6, PC ae C38:4, and PC ae C40:3) even after the removal of patients with GDM, suggesting some overlap in pathway disruption with diabetes.
Finally, this study observed higher urinary concentrations of PEA in pregnant individuals who were obese compared with those who were normal/underweight. While few studies have looked at the implications of altered PEA concentrations during pregnancy, one study found high levels of PEA to be associated with poor neural development in mouse embryos (42). PEA is a neurotransmitter that amplifies the effects of dopamine and serotonin, with similar properties to amphetamine (43). Although this study found higher levels of PEA in participants with obesity, low levels of urinary PEA in non-pregnant individuals have been linked with attention deficit hyperactivity disorder (ADHD) and depression, two conditions linked with maternal obesity (i.e., offspring ADHD and postpartum depression) (11, 44–46). More research is needed to understand the role of PEA in pregnancy and its effects on long-term health.
Gestational Weight Gain and Maternal Metabolites
In relation to GWG, the observed positive association between GWG and taurine underscores the previously observed link between taurine levels and fetal growth (47, 48). Taurine levels are highly regulated by the placenta and play a key role in fetal development as well as placental function and development (49). Fetuses are unable to synthesize taurine and therefore rely on maternal blood to supply the nutrient (50). Inadequate taurine levels in fetal plasma have been linked to small for gestational age infants, while increased maternal urinary taurine concentrations have been positively associated with late-pregnancy fetal adiposity, a measure aimed at identifying pathogenic LGA infants (47, 48). The findings of our study further substantiate the connection between fetal growth and taurine, and point to mid-pregnancy as a critical window.
In addition, GWG was associated with concentrations of short-chain acylcarnitines, C5:1, C5-DC, and C5-M-DC. While research has not directly linked short-chain acylcarnitines with GWG, studies have found associations between short-chain acylcarnitines and birthweight, a common outcome related to GWG, as well as fetal adiposity (51, 52). One study reported correlations of neonatal plasma levels of C5:1 with LGA status as well as of C5-DC levels with ponderal index, but not in the same direction as the association we observed (51). This discrepancy could be due to differences in biofluid medium, which have been theorized to influence the directionality of effects in metabolomics research (19). The alterations in C5 acylcarnitine species may indicate a perturbation in the catabolic pathway of branched chain amino acids (BCAA) (53). Short-chain acylcarnitines are products of BCAA degradation and research has found BCAA and their metabolites to be associated with obesity and insulin resistance, both of which are linked with birthweight (8, 54–56).
Overall, the effects of GWG on metabolite concentrations were primarily observed in T2, possibly as a results of the marked increase in GWG rate between the first and second trimesters (57). Since the T2 GWG rate is derived from the weight gain between T1 and T2, it likely captures this shift in GWG rate and a corresponding transition in metabolic processes. Lindsay et al. examined changes in maternal plasma metabolite concentrations throughout pregnancy and found the majority of differences in metabolite levels between trimesters to be between the first and second trimesters and the first and third trimesters, highlighting a shift in metabolite concentrations between the first and later trimesters (5).
Strengths
This study had multiple strengths including the use of longitudinal metabolomics, model-derived GWG data, and a large and diverse study sample. The use of longitudinal data enabled us to detect differences in the associations of GWG and pBMI with metabolites across pregnancy and highlight the importance of multiple metabolomics measures throughout pregnancy. To our knowledge, only one other published study exploring pBMI and GWG and maternal metabolites has had longitudinal data (12). As for the longitudinal GWG data, by using a piecewise linear mixed effects model to estimate GWG rates as opposed to empirically calculated rates, we were able to minimize the inter-subject variations between sample collection times. We found the rates estimated using the mixed effects model were highly correlated with empirically-derived rates (T1 correlation coefficient, 0.96; T2 correlation coefficient, 0.89; T3 correlation coefficient, 0.88). Finally, the sample size of this study allowed for the control for a variety of confounders.
Limitations
Some limitations of our study include variability in urine and weight collection, poor reproducibility (i.e., high CVs) of key metabolites, adjustment for creatinine, and the potential for unmeasured confounding. Since urine was collected at varying times for each individual but the median GA at urine sample collection was used to calculate the GWG rate and effect estimates, some individuals may have had their urine collected during and not after the timeframe the GWG rate was being calculated. This variability in the timing of urine collection caused the outcome to occur during the exposure for a portion of the study population. Participant pre-pregnancy weight was also measured at varying times in relation to conception (i.e., before pregnancy, early pregnancy) leading to potential misclassification of pBMI and inaccurate T1 GWG. However, the variability in measurement timing for both pre-pregnancy weight and urine sample collection are suspected to be non-differential with respect to the outcomes. High CVs in metabolites previously observed to be associated with our exposures of interest such as isoleucine and arginine limited our ability to explore these associations. Due to limitations in the dataset, we were unable to explore other forms of creatinine adjustment that may better account for variability in urinary dilution (58). As with all observational studies, it is likely there was a degree of unmeasured confounding.
Lastly, it is important to acknowledge that while this analysis was designed to explore the effects of pBMI and GWG on maternal metabolites, the relation between the exposures and outcomes studied are complex and likely bi-directional. Additionally, due to the novelty of measuring metabolites in maternal urine, it is challenging to correlate the findings of this study with clinical outcomes; further research is needed to understand the clinical implications of this study. We also cannot speculate on downstream effects of the associations we observed on fetal development or child health, as our metabolite measures are from maternal urine, limiting their application to the fetal metabolome. For example, research has been mixed on whether maternal serum metabolite concentrations are correlated with cord blood metabolites, and urinary metabolites are not always directly related to serum metabolites (16, 19, 59, 60). Although the majority of research cited in this study utilized metabolite concentrations measured in plasma and serum and not in urine, differing metabolite concentrations between groups, regardless of directionality, indicates a potential perturbation in metabolite concentration by the exposure.
Conclusion
Both pBMI and GWG were associated with maternal metabolites to varying degrees throughout pregnancy. pBMI was associated with the metabolic environment during pregnancy, particularly in mid-pregnancy, with potential implications for the fetus, highlighting the need for interventions to improve preconception health. The GWG findings supported previous research on the relation between taurine and fetal growth and indicated possible alterations in the BCAA catabolic pathway related to short-chain acylcarnitines. However, all associations with GWG were limited to mid-pregnancy. Overall, further research is needed to confirm the findings of this study and to determine the acute and long-term health effects of the observed perturbations in metabolite concentrations.
Supplementary Material
Acknowledgements:
NYU CHES was supported by institutional funds of NYU Grossman School of Medicine as well as the NIH Office of the Director (UG3/UH3OD023305). In addition, the National Institute of Environmental Health Sciences grant number R01ES032808 and National Institute of Environmental Health Sciences Award Number K99ES030403 supported this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
COMPETING INTERESTS STATEMENT: The authors have nothing to disclose.
Competing Interests: All authors have no conflicts of interest to report.
References
- 1.Driscoll A, Gregory E. Increases in Prepregnancy Obesity: United States, 2016–2019. Centers for Disease Control and Prevention (CDC); 2020. November 2020. Report No.: 392 Contract No.: 392. [Google Scholar]
- 2.Power ML, Gaspar-Oishi M, Gibson K, Kelly EW, Lott ML, Mackeen AD, et al. A Survey of Women and Their Providers Regarding Gestational Weight Gain. Journal of women’s health (2002). 2019;28(10):1399–406. [DOI] [PubMed] [Google Scholar]
- 3.Sun Y, Shen Z, Zhan Y, Wang Y, Ma S, Zhang S, et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC pregnancy and childbirth. 2020;20(1):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu P, Xu L, Wang Y, Zhang Y, Du Y, Sun Y, et al. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2016;17(11):1091–102. [DOI] [PubMed] [Google Scholar]
- 5.Kominiarek MA, Saade G, Mele L, Bailit J, Reddy UM, Wapner RJ, et al. Association Between Gestational Weight Gain and Perinatal Outcomes. Obstetrics and gynecology. 2018;132(4):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamun AA, Kinarivala M, O’Callaghan MJ, Williams GM, Najman JM, Callaway LK. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: evidence from 21 y postpartum follow-up. Am J Clin Nutr. 2010;91(5):1336–41. [DOI] [PubMed] [Google Scholar]
- 7.Meyer DM, Stecher L, Brei C, Hauner H. Mid-pregnancy weight gain is associated with offspring adiposity outcomes in early childhood. Pediatric research. 2020. [DOI] [PubMed]
- 8.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8(4):e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endres LK, Straub H, McKinney C, Plunkett B, Minkovitz CS, Schetter CD, et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstetrics and gynecology. 2015;125(1):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rangel-Huerta OD, Pastor-Villaescusa B, Gil A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics. 2019;15(6):93-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scassellati C, Bonvicini C, Faraone SV, Gennarelli M. Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(10):1003–19.e20. [DOI] [PubMed] [Google Scholar]
- 12.Hellmuth C, Lindsay KL, Uhl O, Buss C, Wadhwa PD, Koletzko B, et al. Association of maternal prepregnancy BMI with metabolomic profile across gestation. International journal of obesity (2005). 2017;41(1):159–69. [DOI] [PubMed] [Google Scholar]
- 13.Mitro SD, Wu J, Rahman ML, Cao Y, Zhu Y, Chen Z, et al. Longitudinal Plasma Metabolomics Profile in Pregnancy-A Study in an Ethnically Diverse U.S. Pregnancy Cohort. Nutrients. 2021;13(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryckman KK, Donovan BM, Fleener DK, Bedell B, Borowski KS. Pregnancy-Related Changes of Amino Acid and Acylcarnitine Concentrations: The Impact of Obesity. AJP reports. 2016;6(3):e329–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handelman SK, Romero R, Tarca AL, Pacora P, Ingram B, Maymon E, et al. The plasma metabolome of women in early pregnancy differs from that of non-pregnant women. PLOS ONE. 2019;14(11):e0224682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shearer J, Klein MS, Vogel HJ, Mohammad S, Bainbridge S, Adamo KB. Maternal and Cord Blood Metabolite Associations with Gestational Weight Gain and Pregnancy Health Outcomes. J Proteome Res. 2021;20(3):1630–8. [DOI] [PubMed] [Google Scholar]
- 17.Cinelli G, Fabrizi M, Ravà L, Ciofi Degli Atti M, Vernocchi P, Vallone C, et al. Influence of Maternal Obesity and Gestational Weight Gain on Maternal and Foetal Lipid Profile. Nutrients. 2016;8(6):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidakovic AJ, Jaddoe VWV, Gishti O, Felix JF, Williams MA, Hofman A, et al. Body mass index, gestational weight gain and fatty acid concentrations during pregnancy: the Generation R Study. European journal of epidemiology. 2015;30(11):1175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott HD, Buchan M, Chadwick C, Field CJ, Letourneau N, Montina T, et al. Metabolic dysfunction in pregnancy: Fingerprinting the maternal metabolome using proton nuclear magnetic resonance spectroscopy. Endocrinol Diabetes Metab. 2020;4(1):e00201-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsay KL, Hellmuth C, Uhl O, Buss C, Wadhwa PD, Koletzko B, et al. Longitudinal Metabolomic Profiling of Amino Acids and Lipids across Healthy Pregnancy. PloS one. 2015;10(12):e0145794-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trasande L, Ghassabian A, Kahn LG, Jacobson MH, Afanasyeva Y, Liu M, et al. The NYU Children’s Health and Environment Study. European journal of epidemiology. 2020;35(3):305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.About Adult BMI: Centers for Disease Control and Prevention; [updated June 30, 2020. Available from: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html.
- 23.Catalano PM. Trying to understand gestational diabetes. 2014;31(3):273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walejko JM, Chelliah A, Keller-Wood M, Wasserfall C, Atkinson M, Gregg A, et al. Diabetes Leads to Alterations in Normal Metabolic Transitions of Pregnancy as Revealed by Time-Course Metabolomics. 2020;10(9):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graça G, Duarte IF, Barros AS, Goodfellow BJ, Diaz SO, Pinto J, et al. Impact of Prenatal Disorders on the Metabolic Profile of Second Trimester Amniotic Fluid: A Nuclear Magnetic Resonance Metabonomic Study. J Proteome Res. 2010;9(11):6016–24. [DOI] [PubMed] [Google Scholar]
- 26.Guasch-Ferré M, Hruby A, Toledo E, Clish CB, Martínez-González MA, Salas-Salvadó J, et al. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes care. 2016;39(5):833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messana I, Forni F, Ferrari F, Rossi C, Giardina B, Zuppi C. Proton nuclear magnetic resonance spectral profiles of urine in type II diabetic patients. Clinical chemistry. 1998;44(7):1529–34. [PubMed] [Google Scholar]
- 28.Bentley-Lewis R, Huynh J, Xiong G, Lee H, Wenger J, Clish C, et al. Metabolomic profiling in the prediction of gestational diabetes mellitus. Diabetologia. 2015;58(6):1329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietzner M, Kaul A, Henning A-K, Kastenmüller G, Artati A, Lerch MM, et al. Comprehensive metabolic profiling of chronic low-grade inflammation among generally healthy individuals. BMC Medicine. 2017;15(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantham P, Aye ILMH, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36(7):709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villarreal-Pérez JZ, Villarreal-Martínez JZ, Lavalle-González FJ, Torres-Sepúlveda MdR, Ruiz-Herrera C, Cerda-Flores RM, et al. Plasma and urine metabolic profiles are reflective of altered beta-oxidation in non-diabetic obese subjects and patients with type 2 diabetes mellitus. Diabetology & Metabolic Syndrome. 2014;6(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FGS, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring). 2010;18(9):1695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mai M, Tönjes A, Kovacs P, Stumvoll M, Fiedler GM, Leichtle AB. Serum Levels of Acylcarnitines Are Altered in Prediabetic Conditions. PLOS ONE. 2013;8(12):e82459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bene J, Márton M, Mohás M, Bagosi Z, Bujtor Z, Oroszlán T, et al. Similarities in Serum Acylcarnitine Patterns in Type 1 and Type 2 Diabetes Mellitus and in Metabolic Syndrome. Annals of Nutrition and Metabolism. 2013;62(1):80–5. [DOI] [PubMed] [Google Scholar]
- 35.Cho K, Moon JS, Kang JH, Jang HB, Lee HJ, Park SI, et al. Combined untargeted and targeted metabolomic profiling reveals urinary biomarkers for discriminating obese from normal-weight adolescents. Pediatric obesity. 2017;12(2):93–101. [DOI] [PubMed] [Google Scholar]
- 36.Wallace M, Morris C, O’Grada CM, Ryan M, Dillon ET, Coleman E, et al. Relationship between the lipidome, inflammatory markers and insulin resistance. Molecular BioSystems. 2014;10(6):1586–95. [DOI] [PubMed] [Google Scholar]
- 37.Miranda J, Simões RV, Paules C, Cañueto D, Pardo-Cea MA, García-Martín ML, et al. Metabolic profiling and targeted lipidomics reveals a disturbed lipid profile in mothers and fetuses with intrauterine growth restriction. Scientific reports. 2018;8(1):13614-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson O, Keski-Rahkonen P, Chatzi L, Kogevinas M, Nawrot T, Pizzi C, et al. Cord Blood Metabolic Signatures of Birth Weight: A Population-Based Study. J Proteome Res. 2018;17(3):1235–47. [DOI] [PubMed] [Google Scholar]
- 39.Lewandowska M Maternal Obesity and Risk of Low Birth Weight, Fetal Growth Restriction, and Macrosomia: Multiple Analyses. Nutrients. 2021;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suhre K, Meisinger C, Döring A, Altmaier E, Belcredi P, Gieger C, et al. Metabolic Footprint of Diabetes: A Multiplatform Metabolomics Study in an Epidemiological Setting. PLOS ONE. 2010;5(11):e13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semba RD, Gonzalez-Freire M, Moaddel R, Sun K, Fabbri E, Zhang P, et al. Altered Plasma Amino Acids and Lipids Associated With Abnormal Glucose Metabolism and Insulin Resistance in Older Adults. J Clin Endocrinol Metab. 2018;103(9):3331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denno KM, Sadler TW. Phenylalanine and its metabolites induce embryopathies in mouse embryos in culture. Teratology. 1990;42(5):565–70. [DOI] [PubMed] [Google Scholar]
- 43.Xie Z, Miller GM. Beta-phenylethylamine alters monoamine transporter function via trace amine-associated receptor 1: implication for modulatory roles of trace amines in brain. The Journal of pharmacology and experimental therapeutics. 2008;325(2):617–28. [DOI] [PubMed] [Google Scholar]
- 44.Molyneaux E, Poston L, Ashurst-Williams S, Howard LM. Obesity and mental disorders during pregnancy and postpartum: a systematic review and meta-analysis. Obstetrics and gynecology. 2014;123(4):857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Lagerberg T, Chang Z, Cortese S, Rosenqvist MA, Almqvist C, et al. Maternal pre-pregnancy overweight/obesity and the risk of attention-deficit/hyperactivity disorder in offspring: a systematic review, meta-analysis and quasi-experimental family-based study. International Journal of Epidemiology. 2020;49(3):857–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moises HW, Waldmeier P, Beckmann H. Urinary phenylethylamine correlates positively with hypomania, and negatively with depression, paranoia, and social introversion on the MMPI. European archives of psychiatry and neurological sciences. 1986;236(2):83–7. [DOI] [PubMed] [Google Scholar]
- 47.Walsh JM, Wallace M, Brennan L, McAuliffe FM. Early pregnancy maternal urinary metabolomic profile and later insulin resistance and fetal adiposity. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015;28(14):1697–700. [DOI] [PubMed] [Google Scholar]
- 48.Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, et al. Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. American journal of obstetrics and gynecology. 1990;162(1):253–61. [DOI] [PubMed] [Google Scholar]
- 49.Holm MB, Kristiansen O, Holme AM, Bastani NE, Horne H, Blomhoff R, et al. Placental release of taurine to both the maternal and fetal circulations in human term pregnancies. Amino Acids. 2018;50(9):1205–14. [DOI] [PubMed] [Google Scholar]
- 50.Gaull G, Sturman JA, Räihä NC. Development of mammalian sulfur metabolism: absence of cystathionase in human fetal tissues. Pediatric research. 1972;6(6):538–47. [DOI] [PubMed] [Google Scholar]
- 51.Beken S, Abali S, Yildirim Saral N, Guner B, Dinc T, Albayrak E, et al. Early Postnatal Metabolic Profile in Neonates With Different Birth Weight Status: A Pilot Study. Front Pediatr. 2021;9:646860-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sánchez-Pintos P, de Castro M-J, Roca I, Rite S, López M, Couce M-L. Similarities between acylcarnitine profiles in large for gestational age newborns and obesity. Scientific Reports. 2017;7(1):16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15(5):606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCormack SE, Shaham O, McCarthy MA, Deik AA, Wang TJ, Gerszten RE, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. 2013;8(1):52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandler V, Reisetter AC, Bain JR, Muehlbauer MJ, Nodzenski M, Stevens RD, et al. Associations of maternal BMI and insulin resistance with the maternal metabolome and newborn outcomes. Diabetologia. 2017;60(3):518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamashita H, Yasuhi I, Fukuda M, Kugishima Y, Yamauchi Y, Kuzume A, et al. The association between maternal insulin resistance in mid-pregnancy and neonatal birthweight in uncomplicated pregnancies. Endocrine journal. 2014;61(10):1019–24. [DOI] [PubMed] [Google Scholar]
- 57.Council NR. Weight gain during pregnancy: reexamining the guidelines. 2010. [PubMed]
- 58.O’Brien KM, Upson K, Cook NR, Weinberg CR. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. 2016;124(2):220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lowe WL, Bain JR, Nodzenski M, Reisetter AC, Muehlbauer MJ, Stevens RD, et al. Maternal BMI and Glycemia Impact the Fetal Metabolome. Diabetes care. 2017;40(7):902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mierzchała-Pasierb M, Lipińska-Gediga M, Fleszar MG, Lewandowski Ł, Serek P, Płaczkowska S, et al. An analysis of urine and serum amino acids in critically ill patients upon admission by means of targeted LC–MS/MS: a preliminary study. Scientific Reports. 2021;11(1):19977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A deidentified dataset and code are available upon request from the corresponding author.