Abstract
Introduction
This trial evaluates whether daily low-dose aspirin initiated before 16 weeks of gestation can reduce preeclampsia and fetal growth restriction in nulliparous women identified by first-trimester uterine artery Dopplers as at high risk of preeclampsia.
Methods
This randomized, blinded, placebo-controlled, parallel-group trial took place in 17 French obstetric departments providing antenatal care. Pregnant nulliparous women aged ≥ 18 years with a singleton pregnancy at a gestational age < 16 weeks of gestation with a lowest pulsatility index ≥ 1.7 or a bilateral protodiastolic notching for both uterine arteries on an ultrasound performed between 11+0 and 13+6 weeks by a certified sonographer were randomized at a 1:1 ratio to 160 mg of low-dose aspirin or to placebo to be taken daily from inclusion to their 34th week of gestation. The main outcome was preeclampsia or a birthweight ≤ 5th percentile. Other outcomes included preeclampsia, severe preeclampsia, preterm preeclampsia, preterm delivery before 34 weeks, mode of delivery, type of anesthesia, birthweight ≤ 5th percentile and perinatal death.
Results
The trial was interrupted due to recruiting difficulties. Between June 2012 and June 2016, 1104 women were randomized, two withdrew consent, and two had terminations of pregnancies. Preeclampsia or a birthweight ≤ 5th percentile occurred in 88 (16.0%) women in the low-dose aspirin group and in 79 (14.4%) in the placebo group (proportion difference 1.6 [-2.6; 5.9] p = 0.45). The two groups did not differ significantly for the secondary outcomes.
Conclusion
Low-dose aspirin was not associated with a lower rate of either preeclampsia or birthweight ≤ 5th percentile in women identified by their first-trimester uterine artery Doppler as at high risk of preeclampsia.
Trial registration
(NCT0172946).
Introduction
Preeclampsia complicates around 2 to 8% of pregnancies world wild [1, 2]. It can lead to maternal mortality and morbidity, which in turn lead to perinatal morbidity and mortality due to fetal growth restriction (FGR) and medically indicated prematurity [3, 4]. Antiplatelet agents are known to prevent preeclampsia and its consequences when they are administered before 16 weeks of gestation [5, 6]. The difficulty, however, is early identification of pregnancies at high risk of preeclampsia that could benefit from this preventive treatment [7, 8]. Parous women with a history of preeclampsia are candidates, but what is challenging is identifying nulliparous women who should receive this preventive treatment as they have roughly twice the risk to develop preeclampsia when compared to parous women [9, 10].
Preeclampsia and growth restriction are the consequence of abnormal placental implantation and inadequate utero-placental blood flow. Normal placentation comprises trophoblast cell invasion of the decidual and myometrial segments of spiral arteries, which induces reversible changes in the normal arterial wall architecture. Trophoblastic invasion starts from eight weeks’ gestation and studies have shown that abnormal uterine artery Doppler as early as the first trimester can identify women at high risk of preeclampsia and fetal growth restriction [11]. The pulsatility index, alone or combined with notching, are the most predictive uterine artery Doppler indices in the first trimester [12].
As women who develop preeclampsia have different uterine artery Doppler patterns on their first-trimester ultrasound [13, 14], we decided to conduct a trial to test the efficacy of low-dose aspirin to reduce the incidence of preeclampsia or growth restriction in nulliparous pregnant women identified at "high-risk" by their first-trimester ultrasound Doppler findings.
Material and methods
Study design
We conducted a randomized, blinded, placebo-controlled, parallel-group trial.
Population
Eligible women were those aged ≥ 18 years, with a singleton pregnancy, nulliparous at a gestational age < 16 weeks of gestation with an ultrasound performed between 11+0 and 13+6 weeks showing a lowest pulsatility index ≥ 1.7 for both uterine arteries or bilateral protodiastolic notching [15]. Women carrying a fetus with severe congenital anomalies (with or without termination of pregnancy) diagnosed on this first-trimester ultrasound, women receiving anticoagulant treatments or with any contraindications to antiplatelet treatment, and those with coagulopathies, lupus, or antiphospholipid syndrome were not included.
Setting
Recruitment took place in 16 French maternity units including tertiary university hospitals, general hospitals, and private obstetrics departments, and one private imaging center.
Screening for participants
As the inclusion criteria involved sonographic parameters, sonographers (physicians or midwives) screened the women. All sonographers had validated an online certificate (Collège Français d’Échographie Fœtale) for first-trimester uterine artery Doppler examinations ([13] https://www.epp-echofoetale.fr). Sonographers were invited to an information meeting held in each participating unit before the trial began to review the aims and the protocol of the study. Eligible women were screened and informed of the study during their first trimester ultrasound. All eligible women were seen in a specific consultation with a physician or midwife in the participating unit. This provider explained the aims and scope of the study, checked inclusion and exclusion criteria, and obtained written consent from the women who met all inclusion and no exclusion criteria and were willing to participate.
Randomization and blinding
Women were randomly allocated in a 1:1 ratio to 160 mg of low-dose aspirin daily or to a placebo. A secure computer-generated, online, centralized web-based system managed the randomization (with a fixed block size of four units, in a sequence generated by a statistician from INSERM CIC 1415 not involved in patient recruitment) and concealment processes. Women, clinicians, and outcome assessors were blinded to allocation. The dataset was unblinded for analysis once the data collection was finalized. In case of a serious adverse event which would require unblinding, this was possible at any time of the study; however this has never been necessary.
Intervention group
After randomization, hospital pharmacists dispensed to women in the experimental group a sufficient quantity of low-dose aspirin to be taken daily during their evening meal from the day of inclusion until they reached their 34th week of gestation. The purchased product was in the form of 288-g sachets of DLlysine acetylsalicylate powder, corresponding to 160 mg of low-dose aspirin (Kardegic®), to be dissolved in water for administration. They were asked to store it in a dry place at a temperature less than 25° Celsius.
Control group
Women randomized to the control group received placebo powder (purchased from Bertin Pharma), in similar-looking sachets as the intervention group, to be stored and taken according to the same protocol.
Follow-up
The women received a notebook, which they had to return to the investigating center after childbirth, to indicate any possible side effects, together with a card stating that they were participating in the study, with the contact details required in case of a medical emergency for which an unblinding procedure was absolutely necessary. Follow-up was identical in the two groups and women’s pregnancies were monitored and managed by their physician or midwife according to French guidelines. After childbirth, women were asked to bring back all the remaining treatments and packaging to the pharmacists of their center to assess adherence to the treatment.
Outcomes
The main outcome was a composite of preeclampsia or newborn with a birthweight ≤ 5th percentile. Preeclampsia was defined by hypertension in pregnancy (systolic blood pressure ≥ 140 mmHg or diastolic pressure ≥ 90 mmHg, measured twice at least 4 hours apart), and proteinuria greater than 300 mg/24 hour after 20 weeks or in the post-partum period [16, 17]. To determine birthweights ≤ 5th percentile, we used the EPOPé growth curves, adjusted for the newborn’s gestational age and sex and the mother’s parity, height, and weight [18].
The secondary outcomes were preeclampsia, severe preeclampsia, defined as women with preeclampsia and any of the following: systolic blood pressure ≥ 160 mmHg or diastolic blood pressure ≥ 110 mmHg, proteinuria ≥ 5 g/day, oliguria, HELLP syndrome, eclampsia, acute pulmonary edema, placental abruption, or stillbirth. The rate of preterm preeclampsia (<37 weeks’ gestation) was added as a secondary outcome after the trial’s registration at clinicaltrials.gov to be able to compare results with those of the ASPRE trial [19]. Secondary outcomes also included preterm delivery before 34 weeks, mode of delivery (vaginal or caesarean) and the type of anaesthesia for delivery. Secondary outcomes for infants included a birthweight ≤ 5th percentile or perinatal death, defined as a stillbirth from 22 weeks through a neonatal death in the first 7 days. Any kind of bleeding was also studied as a secondary outcome. Serious adverse events, defined as any event, reaction or unexpected adverse reaction that results in death, is life threatening or requires hospitalization were also reported and coded according to MedDra dictionary (version 19.1) (https://admin.meddra.org/sites/default/files/guidance/file/94911910_termselptc_r4_12_sep2016.pdf.)
Sample size
According to an unpublished study conducted in our unit, the presence of first trimester bilateral notching and/or a high pulsatility index among nulliparas is predictive of the occurrence of pre-eclampsia with a sensitivity of 75% and a specificity of 67% and predictive of growth restriction with a sensitivity of 55% and a specificity of 67%. Based on this performance we hypothesized that low-dose aspirin could reduce the primary outcome, expected to be 21.2% in the control group (protocol in S3 File), by 15% [20–22]. To show a relative reduction from 21.2% to 18.0% in the occurrence of preeclampsia or birthweight ≤ 5th percentile with a power of 80% and a two-tailed type I error of 5%, we required 2415 women in each group. As we expected that some pregnancies would end in termination, and we planned four intermediate analyses (every 1000 inclusions) to counter the unknown precise expected rates of outcome in the control group, we planned to include 2486 women in each group. The study protocol (S3 File) describes the sample size calculation in fuller detail.
Analysis
A statistical analysis plan was finalized before the dataset was frozen. Because the trial was stopped prematurely, no interim analysis was performed. Women were analysed according to their randomization group (intention to treat). Baseline characteristics were reported per group with numbers and percentages for categorical variables and with medians and interquartile ranges for continuous variables. For the primary outcome, missing data were managed with simple imputation by assuming that women with missing data for the primary outcome had preeclampsia or that their infant had a birthweight < 5th percentile. A complete-case analysis was also conducted. Rates were then compared with the Chi-square test. The between-group difference in proportions was estimated as well as its 95% confidence interval (CI) (Wald method). Results were also presented as crude odd ratios (ORs) with their 95% CIs. No imputation was performed for secondary outcomes, which were also compared with Chi-square tests. The between-group difference and odds ratios were also presented for secondary outcomes. Statistical analyses were performed with SAS version 9.4 and R version 3.3.1 software.
Ethics
The study protocol (S3 File) and patient information documents were approved by the competent French authorities: Agence Nationale de Sécurité du Médicament et des produits de santé (number of approval A120316-72 on the 07/05/2012) and Comité de Protection des Personnes (number 2012-R8; 27/03/2012). The study protocol was registered at ClinicalTrials.gov (NCT01729468) and in the European EudraCT database (2011-003536-30).
Results
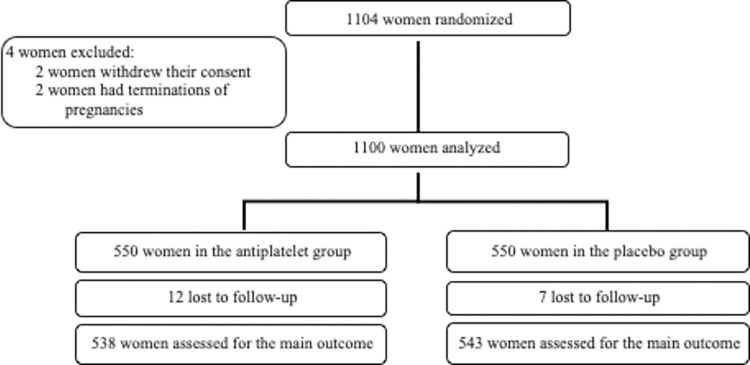
Between June 2012 and June 2016, 1104 women were randomized. The trial was stopped after 4 years due to recruiting difficulties resulting in a failure to conduct any of the four initially planned intermediate analyses. Because two women withdrew their consent and two terminations of pregnancy occurred after randomization, 1100 women were finally analysed: 550 in the intervention (low-dose aspirin) and 550 in the control (placebo) group (Fig 1).
Fig 1.
The mean age of the women were 28.3 ± 4.9 years and 28.7 ± 4.7 years in the aspirin and the placebo group, respectively. Eight percent of women were of white ethnicity, S1 Table. The median gestational age at delivery were 39.7 [38.5–40.6] and 39.7 [38.5–40.8] in the aspirin and the placebo group, respectively. The number (rate) of cases of preeclampsia or infants with a birthweight ≤ 5th percentile was 88 (16.0%) in the low-dose aspirin group versus 79 (14.4%) in the placebo group (proportion difference 1.6 [-2.6; 5.9], p = 0.45 OR 1.14 95%CI (0.82–1.58)), S2 Table. These findings were supported by our complete-case analysis, which showed 76 (14.1%) and 72 (13.3%) with preeclampsia or infants with birthweight ≤ 5th percentile in the low-dose aspirin and the placebo groups, respectively (P = 0.68). The two groups did not differ for secondary outcomes. Also in each group, during treatment, around 25% of the women reported bleeding (epistaxis or gingival bleeding (80%) and metrorrhagia (20%)) as an adverse effect. Overall, 112 (20%) and 120 (22%) women, respectively, experienced at least one serious adverse event (S3 Table). In all, around 25% of the women in each group stopped their treatment before their 34th week.
Discussion
In this trial conducted among women identified at high risk of preeclampsia by their first-trimester uterine artery Doppler examination, the rate of preeclampsia or birthweight<5th percentile in women who received low-dose aspirin before 16 weeks gestation was not statistically different from that of women receiving placebo.
This trial was planned before the results from the ASPRE trial which also evaluated an intervention among women at high risk of preeclampsia [19]. They identified women by using an algorithm including demographic, clinical, biological, and Doppler findings, which produced a population very different from ours, with 30% of the women parous and 10% with a history of preeclampsia. In the ASPRE trial, approximately forty percent of the primary outcomes occurred in parous women. Being limited to nulliparas is the main strength of our trial, as parous women differ substantially in terms of risk assessment for preeclampsia and are not the women for whom the decision about preventive treatment is challenging. Other strengths include that all sonographers were certified for uterine artery Dopplers and also that all women were administered their treatment before 16 weeks of gestation which is the only way the preventive treatment can be effective.
Nevertheless, our trial has limitations, including that we were not able to reach the number of inclusions initially planned, although we extended our inclusion period by one year. This may be explained partly by an inaccurate initial assessment of feasibility and the lower than expected number of eligible women. Recruitment may also have been difficult as healthy nulliparous women may not have understood how their participation could improve outcomes for them or the baby as, by definition, nulliparas have never experienced adverse pregnancy outcomes. Another limitation was the lower than expected rate for the main outcome in the control group (14% of events versus 21% expected). Our hypothesis and sample size were built on numbers of outcomes which were not specific from the French population. A French study conducted when the trial was designed found that preeclampsia affected only 1.7% of nulliparous women whereas we used an expected rate of 6% [10]. It is likely that the lower than expected rate for the main outcome may be explained, in part, by this erroneous assessment of the rate of preeclampsia in nulliparous women in France [9]. Thus, we can speculate that if an intermediate analysis would have been conducted, it is likely that the trial would have been stopped for futility. One in four woman interrupted their treatment before their 34th week of gestation which is concordant with compliance findings from metanalysis evaluating the effect of aspirin to prevent preeclampsia in the pregnant population [5]. The reported rates of women who were fully compliant in the studies included in the metanalysis varied between 60 and 90%. Detailed compliance was not assessed precisely or regularly through the follow up of the pregnancy so we are unable to explain these adherence rates. Also, the screening took place in 17 participating units and we were not able to assess how many first-trimester ultrasound examinations of nulliparous women were conducted by sonographers during the inclusion period. We are therefore unable to estimate how many women were screened. Finally, we suspect some of the adverse events may have been under-reported. Indeed, some of the adverse events were also secondary outcomes (metrorrhagia and preterm delivery) and there are some discrepancies between the numbers of outcomes and adverse events which is why we are cautious in the interpretation our adverse event data.
The observed rate of preterm preeclampsia in our control group was half that in the ASPRE trial control group, which means that their algorithm was more successful in identifying women at risk of preeclampsia. We found another study also focusing on the evaluation of first trimester uterine Doppler among a population of nulliparas who were not particularly at high risk [23]. They found a rate of preeclampsia in their population (4.9%) which was close to what we observed in our study thereby confirming that we were unable to identify a high-risk population. Bujold et al.’s meta-analysis which evaluated acetylsalicylic acid for the prevention of preeclampsia and intra-uterine growth restriction in women with abnormal uterine artery doppler found that this treatment, when initiated early, could reduce the incidence of preeclampsia and in particular severe preeclampsia [24]. It turns out that most of the observed cases of term preeclampsia and was mild which may explain partly the lack effect of the treatment in our trial.
Algorithms that aim to identify women at high risk of preeclampsia by clinical, biological, and ultrasound parameters have been published and have sparked enthusiasm, but the cost effectiveness of their implementation at a population level is still debated [25–28]. The trial was designed in 2008 and at the time, none of those algorithms were recommended which is why we selected our population differently. Until now, those algorithms are still not recommended by national guidelines even if recommended by the International Federation of Gynecology and Obstetrics, the International Society of ultrasound in obstetrics and Gynecology and the International Society for the Study of Hypertension in Pregnancy [29, 30]. Many studies were published during the elapsed time between de design of the trial (2008) and the publication (2022). This delay was mainly due to motivation issues and organisation difficulties. Inclusion criteria to select women at high risk of preeclampsia which seemed appropriate at the time are now obsolete. Because women in most high-income countries are advised to undergo a first-trimester ultrasound, screening for preeclampsia through clinical and Doppler findings would not require a major increase in cost [31]. Accordingly, a combination of clinical and Doppler findings should be used in further studies to evaluate interventions to reduce preeclampsia.
Conclusion
In our study, low-dose aspirin was not associated with a lower rate than placebo of either preeclampsia or FGR in women identified as at high risk of preeclampsia during a first-trimester uterine artery Doppler examination. We interpret our results cautiously because of the lack of power related to insufficient number of patients recruited.
Supporting information
Results are numbers and percentages unless stated otherwise.
(DOCX)
(DOCX)
(DOCX)
(DOC)
(XLSX)
(DOC)
Acknowledgments
We thank all the women who agreed to participate in the study. We would like to thank Anne Rebion for her analysis, Hélène Bourgoin for her help with the pharmacy. We also thank the research assistants who helped to conduct this trial Yoann Desvignes and Catherine Fermont and the participating members of the “Groupe de Recherche en Obstétrique et Gynécologie (GROG)”, Thomas Schmitz, Elie Azria, Catherine Deneux-tharaux, Anne Ego, Francois Goffinet, Cyril Huissoud, Gilles Kayem, Bruno Langer, Camille Le Ray, Olivier Morel, Marie-Victoire Senat and Damien Subtil.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The trial was funded by a government grant “Programme Hospitalier de Recherche Clinique National” PHRCN-2008. The government did not have a role in designing the study, interpreting the results or writing the manuscript.
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009. Jun;33(3):130–7. doi: 10.1053/j.semperi.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 2.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013. Sep;170(1):1–7. doi: 10.1016/j.ejogrb.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 3.Steegers EAP, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010. Aug 21;376(9741):631–44. doi: 10.1016/S0140-6736(10)60279-6 [DOI] [PubMed] [Google Scholar]
- 4.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014. Jun;2(6):e323–333. doi: 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 5.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018. Mar;218(3):287–293.e1. doi: 10.1016/j.ajog.2017.11.561 [DOI] [PubMed] [Google Scholar]
- 6.Roberge S, Sibai B, McCaw-Binns A, Bujold E. Low-Dose Aspirin in Early Gestation for Prevention of Preeclampsia and Small-for-Gestational-Age Neonates: Meta-analysis of Large Randomized Trials. Am J Perinatol. 2016. Jul;33(8):781–5. doi: 10.1055/s-0036-1572495 [DOI] [PubMed] [Google Scholar]
- 7.Man R, Hodgetts Morton V, Devani P, Morris RK. Aspirin for preventing adverse outcomes in low risk nulliparous women with singleton pregnancies: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021. Jul;262:105–12. doi: 10.1016/j.ejogrb.2021.05.017 [DOI] [PubMed] [Google Scholar]
- 8.Brunelli E, Seidenari A, Germano C, Prefumo F, Cavoretto P, Di Martino D, et al. External validation of a simple risk score based on the ASPRE trial algorithm for preterm pre-eclampsia considering maternal characteristics in nulliparous pregnant women: a multicentre retrospective cohort study. BJOG. 2020. Sep;127(10):1210–5. doi: 10.1111/1471-0528.16246 [DOI] [PubMed] [Google Scholar]
- 9.Subtil D, Goeusse P, Puech F, Lequien P, Biausque S, Breart G, et al. Aspirin (100 mg) used for prevention of pre-eclampsia in nulliparous women: the Essai Régional Aspirine Mère-Enfant study (Part 1). BJOG. 2003. May;110(5):475–84. doi: 10.1046/j.1471-0528.2003.02096.x [DOI] [PubMed] [Google Scholar]
- 10.Goffinet F, Aboulker D, Paris-Llado J, Bucourt M, Uzan M, Papiernik E, et al. Screening with a uterine Doppler in low risk pregnant women followed by low dose aspirin in women with abnormal results: a multicenter randomised controlled trial. BJOG. 2001. May;108(5):510–8. doi: 10.1111/j.1471-0528.2001.00116.x [DOI] [PubMed] [Google Scholar]
- 11.Vainio M, Kujansuu E, Koivisto AM, Mäenpää J. Bilateral notching of uterine arteries at 12–14 weeks of gestation for prediction of hypertensive disorders of pregnancy. Acta Obstet Gynecol Scand. 2005. Nov;84(11):1062–7. doi: 10.1111/j.0001-6349.2005.00889.x [DOI] [PubMed] [Google Scholar]
- 12.Cnossen JS, Morris RK, ter Riet G, Mol BWJ, van der Post JAM, Coomarasamy A, et al. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ. 2008. Mar 11;178(6):701–11. doi: 10.1503/cmaj.070430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velauthar L, Plana MN, Kalidindi M, Zamora J, Thilaganathan B, Illanes SE, et al. First-trimester uterine artery Doppler and adverse pregnancy outcome: a meta-analysis involving 55,974 women. Ultrasound Obstet Gynecol. 2014. May;43(5):500–7. doi: 10.1002/uog.13275 [DOI] [PubMed] [Google Scholar]
- 14.Yu CKH, Khouri O, Onwudiwe N, Spiliopoulos Y, Nicolaides KH. Prediction of pre-eclampsia by uterine artery Doppler imaging: relationship to gestational age at delivery and small-for-gestational age. Ultrasound Obstet Gynecol. 2008. Mar;31(3):310–3. doi: 10.1002/uog.5252 [DOI] [PubMed] [Google Scholar]
- 15.Prefumo F, Sebire NJ, Thilaganathan B. Decreased endovascular trophoblast invasion in first trimester pregnancies with high-resistance uterine artery Doppler indices. Hum Reprod. 2004. Jan;19(1):206–9. doi: 10.1093/humrep/deh037 [DOI] [PubMed] [Google Scholar]
- 16.Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018. Jul;13:291–310. [DOI] [PubMed] [Google Scholar]
- 17.ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. 2019. Jan;133(1):1. [DOI] [PubMed] [Google Scholar]
- 18.Ego A, Prunet C, Lebreton E, Blondel B, Kaminski M, Goffinet F, et al. [Customized and non-customized French intrauterine growth curves. I - Methodology]. J Gynecol Obstet Biol Reprod (Paris). 2016. Feb;45(2):155–64. [DOI] [PubMed] [Google Scholar]
- 19.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017. Aug 17;377(7):613–22. doi: 10.1056/NEJMoa1704559 [DOI] [PubMed] [Google Scholar]
- 20.Poon LCY, Staboulidou I, Maiz N, Plasencia W, Nicolaides KH. Hypertensive disorders in pregnancy: screening by uterine artery Doppler at 11–13 weeks. Ultrasound Obstet Gynecol. 2009. Aug;34(2):142–8. doi: 10.1002/uog.6452 [DOI] [PubMed] [Google Scholar]
- 21.Poon LCY, Akolekar R, Lachmann R, Beta J, Nicolaides KH. Hypertensive disorders in pregnancy: screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound Obstet Gynecol. 2010. Jun;35(6):662–70. doi: 10.1002/uog.7628 [DOI] [PubMed] [Google Scholar]
- 22.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007. May 26;369(9575):1791–8. doi: 10.1016/S0140-6736(07)60712-0 [DOI] [PubMed] [Google Scholar]
- 23.Demers S, Boutin A, Gasse C, Drouin O, Girard M, Bujold E. First-Trimester Uterine Artery Doppler for the Prediction of Preeclampsia in Nulliparous Women: The Great Obstetrical Syndrome Study. Am J Perinatol. 2019. Jul;36(9):930–5. doi: 10.1055/s-0038-1675209 [DOI] [PubMed] [Google Scholar]
- 24.Bujold E, Morency AM, Roberge S, Lacasse Y, Forest JC, Giguère Y. Acetylsalicylic acid for the prevention of preeclampsia and intra-uterine growth restriction in women with abnormal uterine artery Doppler: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2009. Sep;31(9):818–26. doi: 10.1016/S1701-2163(16)34300-6 [DOI] [PubMed] [Google Scholar]
- 25.Chaemsaithong P, Sahota DS, Poon LC. First trimester preeclampsia screening and prediction. Am J Obstet Gynecol. 2020. Jul 16; doi: 10.1016/j.ajog.2020.07.020 [DOI] [PubMed] [Google Scholar]
- 26.Sentilhes L, Azria E, Schmitz T. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017. Dec 14;377(24):2399–400. doi: 10.1056/NEJMc1713798 [DOI] [PubMed] [Google Scholar]
- 27.Mallampati D, Grobman W, Rouse DJ, Werner EF. Strategies for Prescribing Aspirin to Prevent Preeclampsia: A Cost-Effectiveness Analysis. Obstet Gynecol. 2019. Sep;134(3):537–44. doi: 10.1097/AOG.0000000000003413 [DOI] [PubMed] [Google Scholar]
- 28.Dubon Garcia A, Devlieger R, Redekop K, Vandeweyer K, Verlohren S, Poon LC. Cost-utility of a first-trimester screening strategy versus the standard of care for nulliparous women to prevent pre-term pre-eclampsia in Belgium. Pregnancy Hypertens. 2021. Aug;25:219–24. doi: 10.1016/j.preghy.2021.06.012 [DOI] [PubMed] [Google Scholar]
- 29.Henderson JT, Thompson JH, Burda BU, Cantor A. Preeclampsia Screening: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017. Apr 25;317(16):1668–83. doi: 10.1001/jama.2016.18315 [DOI] [PubMed] [Google Scholar]
- 30.Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019. May;145 Suppl 1(Suppl 1):1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016. Apr 19;353:i1753. doi: 10.1136/bmj.i1753 [DOI] [PMC free article] [PubMed] [Google Scholar]