Abstract
A flow cytometric phagocytosis assay was established to investigate the role of anti-merozoite antibody, complement, and cytokines on the phagocytosis of Plasmodium falciparum merozoites by human neutrophils. This assay involved allowing fluorescein isothiocyanate-labeled merozoites to interact with phagocytes and analysis of the cells on a FACScan with Lysis II software. To differentiate the proportion of neutrophil surface-bound merozoites from the merozoites ingested by neutrophils, the fluorescence of bound merozoites was quenched by adding trypan blue. The data showed that sera from malaria-immune individuals in the Solomon Islands and Papua New Guinea promoted merozoite engulfment by neutrophils. The cytokines tumor necrosis factor alpha, gamma interferon, granulocyte-macrophage colony-stimulating factor, and interleukin-1β significantly increased the amount and the rate of merozoite phagocytosis by neutrophils. Optimum merozoite phagocytosis occurred when both cytokines and anti-malarial antibody were present.
Immune responses to the merozoite stage are important in resistance to malaria, since this transitory stage, which emerges from schizonts, is responsible for the initiation and perpetuation of both the asexual and sexual parasite life cycles in the host's blood. Vaccine strategies aim to direct the immune response against merozoites (3, 4, 10, 11), since antibodies to merozoite surface proteins have been shown to block adhesion and invasion of host erythrocytes (RBC) (1, 5, 8, 22, 27). However, vaccine strategies need to take into consideration not only the merozoite invasion inhibition activity of the antibody but also its ability to promote phagocytosis of merozoites. Phagocytosis of Plasmodium falciparum merozoites has been observed in infected individuals (29, 31). Rapid phagocytosis not only prevents merozoite invasion of RBC but also reduces toxic effects known to be caused by the membrane anchor molecules of merozoite surface proteins (9, 25).
Studies of merozoite phagocytosis have been hampered for several reasons. The studies carried out on human monocytes (2, 13, 14) and neutrophils (19) depended on light-microscopic observations. Morphology-based microscopic examination of merozoites (size, 1.0 to 1.2 μm) is tedious, subjective, and time-consuming. Studies which used purified merozoites are rare, and in fact, mature schizonts have been commonly used as a source of merozoites (1, 4, 8, 27).
We have now developed a nonsubjective and versatile method for measuring phagocytosis of P. falciparum merozoites by human neutrophils by flow cytometric analysis. Purified merozoites were used to ensure that merozoite-specific immune mechanisms could be studied. Using this system, we have reexamined and extended the known immune factors which influence phagocytosis of P. falciparum merozoites by human neutrophils.
MATERIALS AND METHODS
Reagents.
Human recombinant interleukin-1β (IL-1β) (specific activity, 3.4 × 104 U by lymphocyte-activating assay; >99% purity), recombinant tumor necrosis factor alpha (rTNF-α) (specific activity, 6 × 107 U/mg; >99% purity), and recombinant gamma interferon (rIFN-γ) (specific activity, 2 × 107 U/mg; >99% purity) were produced by Genentech Inc. (South San Francisco, Calif.) and kindly provided by G. R. Adolf (Ernst Boehringer Ingelheim Institut, Vienna, Austria). Human recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF) was kindly provided by H. Aschauer (Sandoz Forschungsinstitut, Vienna, Austria). The preparation contained a specific activity of 6.2 × 107 U/mg when assayed for colony formation by a chronic myeloid leukemia cell line. Murine monoclonal antibody (MAb) immunoglobulin G (IgG) to human heat-labile enterotoxin, TNF-α, and IFN-γ were provided by G. R. Adolf, and MAb (IgG) to IL-1β was obtained from Haem Pty. Ltd., Camberwell, Victoria, Australia. These products contained <0.01 ng of endotoxin/ml, as determined by Limulus amoebocyte lysate assay.
RPMI 1640 medium, medium 199, and Hanks balanced salt solution (Cytosystems, Castle Hill, Australia), Percoll (Pharmacia LKB Biotechnology, Uppsala, Sweden), and [3H]hypoxanthine (Amersham Corp., Arlington Heights, Ill.) were purchased. Trypan blue, d-glucose, N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES buffer), and fluorescein isothiocyanate (FITC) were purchased from Sigma Chemical Co., St. Louis, Mo. All reagents were prepared in pyrogen-free distilled water according to the manufacturers' instructions. Percoll (75%; d = 1.126) was prepared in 10× phosphate-buffered saline (PBS). The media, sera, buffers, and cytokines were tested regularly for endotoxin contamination by Limulus amoebocyte lysate assay.
Sera.
Immune plasma or sera were obtained from 30 adults who were long-term residents in malaria-endemic areas in the Solomon Islands and Papua New Guinea. These samples, referred to as immune sera (IS), contained high titers of anti-MSA-2 (merozoite surface antigen-2) antibody as detected by enzyme-linked immunosorbent assay compared to sera obtained from healthy individuals who had not been to a malaria-endemic area (normal sera [NS]). Sera from five adults that contained high MSA-2 antibody levels were pooled and used in this study. Both IS and NS were heated at 56°C for 30 min to inactivate complement.
Merozoites.
Synchronized P. falciparum (3D7 and FC27 strains) in vitro cultures were maintained in fresh human RBC (blood group O+; RBC with less than 1 week of storage at 4°C) in RPMI 1640 medium supplemented with 10% heat-inactivated human group AB serum, 0.25% d-glucose, and 0.85% TES buffer as described previously (17, 18). The cultures were maintained in 60-ml volumes in culture flasks (175-cm2; Nunc Co., Roskilde, Denmark) with daily culture changes and gassing (1% O2 and 5% CO2 in N2). Giemsa-stained smears were prepared to detect mature schizont counts. Spontaneously released merozoites were collected from the cultures containing 6 to 15% parasitized RBC with over 80% mature schizonts. Merozoites were separated by layering 7 ml of cultures on 3 ml of Percoll (75%) and centrifuging the tubes at 750 × g for 5 min (19). The merozoite band (a dark band in the liquid phase above Percoll) was suspended in PBS (9 ml) and subjected to slow centrifugation (235 × g for 4 min) to remove any contaminatory RBC. The supernatant containing merozoites was aspirated into a syringe and filtered through two interconnected filters (Swinnex filters; 3- and 1.2-μm pore size in filter holders; Millipore Corp., Bedford, Mass.) to remove any contaminatory RBC or infected RBC (21). The filtrate was centrifuged at 2,110 × g for 10 min, and the pellet was reconstituted in a small volume of PBS. Comparisons were carried out of the merozoites fixed in 1% formalin and washed several times, those killed instantly by heating (at either 50 or 60°C for 1 min in a water bath while being shaken gently), and those prepared from culture supernatants without the use of Percoll gradient.
Preparation of FITC-conjugated merozoites.
Purified merozoites (108/200 μl of PBS free of Ca2+ and Mg2+) were labeled with FITC (0.1 mg/ml) for 30 min at 37°C in the dark. Unbound FITC was removed by washing the merozoites twice with PBS by centrifugation (2,113 × g for 10 min). The merozoites were dispensed into polystyrene tubes (9 by 76 mm) at 5 × 106 in 100 μl of PBS/tube.
Preparation of neutrophils.
Neutrophils were prepared by the rapid single-step technique (7). Freshly drawn peripheral blood from healthy volunteers was layered onto Ficoll-Hypaque (density, 1.114) and centrifuged at 400 × g for 30 min. The neutrophils, which resolved in the second leukocyte band, were harvested and washed three times. These were >97% pure and >99% viable as determined by the trypan blue exclusion method. The neutrophils were suspended in PBS at 106/100 μl and mixed with 100 μl of cytokines (0.10 to 1,000 U) or PBS (control) and incubated at 37°C for 30 min.
Opsonization and interaction of merozoites with neutrophils.
Merozoites (5 × 105 in 100 μl) were mixed with 50 μl of sera (IS/NS/heat-inactivated NS [HNS]/HBSS diluted 1:3 with RPMI 1640 medium), and the tubes were incubated at 37°C for 20 min. They were then mixed with neutrophils (106 in 100 μl) and incubated for various times (1, 5, 10, and 30 min) at 37°C in the dark, with gentle mixing of the tubes after every 5 min. The tubes were placed on ice following incubation, and flow cytometry analysis was conducted immediately.
Flow cytometry analysis of phagocytosis.
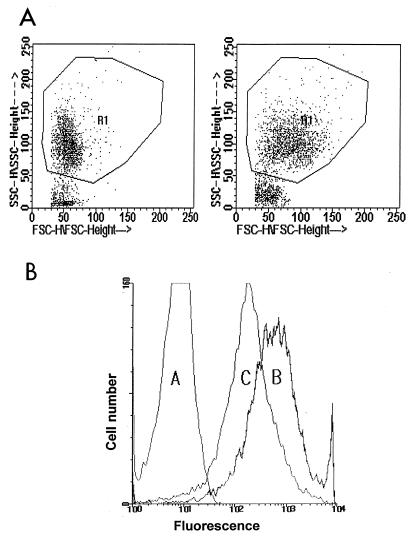
Neutrophils that had interacted with merozoites were analyzed on a fluorescence activated cell scan (FACScan) with Lysis II software (Becton Dickinson). Typically, 10,000 neutrophils were counted per tube. Debris and nonadhered (free) merozoites were excluded by using forward scatter and side scatter. The region for the neutrophil population was then selected (Fig. 1A). In addition, to ascertain that cells in the selected region were neutrophils, we also stained the neutrophils by combined staining with CD16 FITC (Becton Dickinson Immunocytometry Systems, San Jose, Calif.)–CD14 phycoerythrin (Dako Co., Glostrup, Denmark)–CD45 peridinin chlorophyll protein. Neutrophils were identified as CD16 bright, CD45 bright, high side scatter events. Monocytes were excluded as CD14 bright events.
FIG. 1.
(A) Dot plot of neutrophils that have interacted with P. falciparum merozoites (left) and neutrophils pretreated with the cytokine TNF-α that have interacted with merozoites opsonized with IS (right). The neutrophil gate (R1) was defined as described in Materials and Methods. SSC, side scatter; FSC, forward scatter. (B) Histograms showing the fluorescence intensity of FITC-conjugated merozoite association with neutrophils. Histogram A, neutrophils incubated with merozoites in the absence of opsonin or cytokines. Histogram B, cytokine (TNF-α)-pretreated neutrophils when allowed to interact with IS-opsonized merozoites, representing adhering and ingested parasites. Histogram C, after trypan blue quenching of the neutrophils from histogram B, representing only the ingested merozoites. In every reading, the autofluorescence of neutrophils was deducted from the median fluorescence intensity of histograms A, B, and C.
Median fluorescence intensity (MFI) was recorded (Fig. 1B), and then 50 μl of trypan blue (0.4%) was added to the tubes to quench the fluorescence of adhered (23, 30) merozoites. In preliminary experiments, 0.1 to 4.0% trypan blue was used, and 0.4% was selected as the optimum concentration which quenches the fluorescence from adhered parasites without affecting that of ingested merozoites. Histograms (Fig. 1B) were drawn based on MFI, and to remove the autofluorescence of neutrophils from the neutrophils that had interacted with FITC-conjugated merozoites, the value for neutrophils alone was deducted.
Statistics.
The data are presented as means with standard errors of the means (SEM) of triplicates and analyzed by the t test for paired data.
RESULTS
Flow cytometry analysis of phagocytosis.
The flow cytometric method enabled us to study at least 10,000 neutrophils per treatment for the event of phagocytosis of P. falciparum merozoites. As shown in Fig. 1A, there was an increase in the forward scatter due to cytokine treatment of neutrophils and attachment of opsonized merozoites to neutrophils. These differences were also reflected in the histogram drawn on the basis of the median fluorescence intensity (Fig. 1B). The method allowed the differentiation of merozoites ingested by neutrophils from those adhering to neutrophils (Fig. 1B). Trypan blue (0.4%) addition effectively quenched the fluorescence of merozoites adhering to neutrophils without affecting the fluorescence of merozoites ingested by the neutrophils (Fig. 1B). This was also confirmed by examining the samples treated or not treated with trypan blue by microscopy under fluorescence (data not presented). While heat-killed merozoites and the merozoites which were not subjected to any treatment produced the same results, formalin-fixed merozoites were not suitable for phagocytic studies (data not presented).
Comparison of the effects of NS, IS, HNS, and HIS.
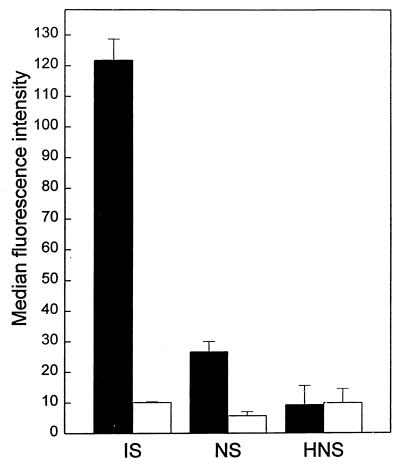
Neutrophils were allowed to react with merozoites which had been pretreated with either IS, heat-inactivated IS (HIS), NS, or HNS. The neutrophils were examined for ingestion and adherence of merozoites. The results showed that IS markedly increased phagocytosis of merozoites compared to NS (6-fold more) and HNS (12-fold more) (Fig. 2). When the data for individual samples were assessed, 93% of the IS samples showed a greater degree of phagocytosis-promoting activity than NS and 100% more than HNS. Heat inactivation of IS did not significantly affect the IS-enhanced phagocytosis of merozoites (data not presented).
FIG. 2.
Effect of pooled IS, NS, and HNS (five samples from different individuals) on phagocytosis of P. falciparum merozoites by human neutrophils. Merozoites (5 × 105) were incubated with 20% serum before being added to the neutrophils. The means + SEM of four experiments are given. The statistics for merozoite ingestion are as follows: IS versus NS or HNS, P < 0.001; NS versus HNS, P < 0.01. Open bars, bound merozoites; solid bars, ingested merozoites.
Effects of cytokines.
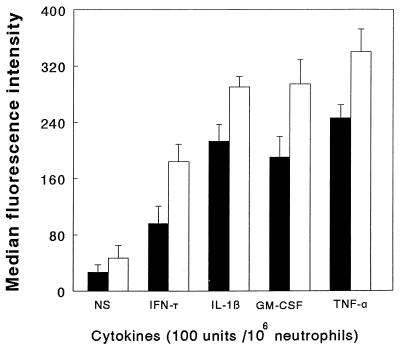
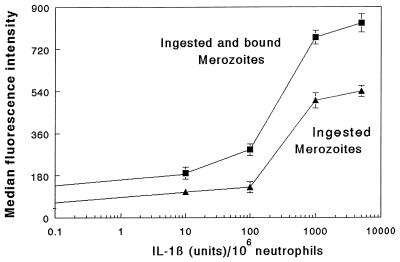
The results shown in Fig. 3 indicate that the cytokines TNF-α, GM-CSF, IL-1β, and IFN-γ significantly enhanced phagocytosis of merozoites by neutrophils in the presence of NS. The effect of these cytokines was concentration dependent and is illustrated for IL-1β (Fig. 4). Treatment of the cytokine preparation with cytokine-specific MAbs or heating at 80°C for 30 min abolished the cytokine-induced increase in merozoite phagocytosis (data not shown).
FIG. 3.
Effects of cytokine (100 U) pretreatment of human neutrophils on the phagocytosis of P. falciparum merozoites (5 × 105) in the presence of NS. The results are presented as the means + SEM of three experiments, each conducted with neutrophils from a different individual. The statistics of merozoite ingestion are as follows: diluent versus TNF-α, IFN-γ, GM-CSF, and IL-1β, P < 0.025. Open bars, bound merozoites; solid bars, ingested merozoites.
FIG. 4.
Effects of various doses of IL-1β on neutrophil-mediated phagocytosis of P. falciparum merozoites in the presence of NS. The results are expressed as the means ± standard deviations of triplicates, which are representative of three experiments. The diluent-treated neutrophils showed results similar to those with IL-1β at 0.1 U/106 neutrophils. Neutrophils pretreated with IL-1β at 100 and 1,000 U showed significantly higher merozoite phagocytosis than diluent-treated cells (P < 0.01 and P < 0.001, respectively).
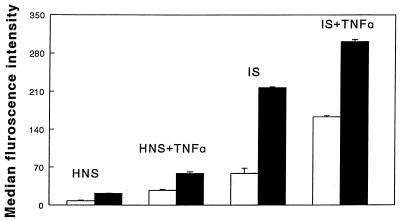
The results of the combined effects of the cytokine (TNF-α) pretreatment of neutrophils and IS opsonization of merozoites are shown in Fig. 5. Optimum merozoite ingestion was seen in the presence of both TNF-α and IS. This effect was significantly higher than the TNF-α-induced effects (P < 0.025) or IS effects (P < 0.01).
FIG. 5.
Combined effects of cytokine (100 U) pretreatment of human neutrophils and opsonization of merozoites with pooled HIS and HNS on the phagocytosis of P. falciparum merozoites by human neutrophils. The results are presented as the means + SEM of three experiments, each conducted with neutrophils from a different individual. The statistics of merozoite ingestion are as follows: TNF-α + IS versus TNF-α + HNS, P < 0.025. TNF-α + IS versus HIS, P < 0.01. Open bars, bound merozoites; solid bars, ingested merozoites.
Kinetics of merozoite phagocytosis.
The kinetics of merozoite phagocytosis of neutrophils over a 30-min incubation was assessed. As shown in Fig. 6, adhesion of parasites occurred within minutes of contact with neutrophils. In the presence of HNS, insignificant merozoite ingestion occurred at all time points. Merozoites opsonized with NS were ingested at a higher rate than those treated with HNS. In comparison, neutrophils pretreated with TNF-α (in the presence of HNS) or neutrophils that had interacted with merozoites opsonized with IS showed significantly higher ingestion of merozoites than the neutrophils that had interacted with merozoites exposed to NS or HNS.
FIG. 6.
Time-related effect of P. falciparum merozoite ingestion by human neutrophils. Comparisons were made with parasites opsonized with IS-NS-HNS without cytokine pretreatment of the neutrophils or with parasites opsonized with HNS and allowed to interact with TNF-α (100 U/106 neutrophils). The results are expressed as the means of triplicates which are representative of two experiments.
DISCUSSION
Insight into the immune effector mechanisms against the merozoite stage of P. falciparum is essential to develop strategies for vaccine development. In the present study we have argued that in addition to the merozoite agglutination properties of antibody, other immune responses against merozoites should also be elucidated. Our results show that phagocytosis of P. falciparum merozoites can be influenced by many immune factors and that phagocytosis can be efficiently measured by flow cytometry.
Although the immune factors which affect the P. falciparum intraerythrocytic-stage elimination by the host have been described (16), the factors which affect merozoite phagocytosis remain to be appropriately studied. The studies carried out with monocyte phagocytosis of merozoites have described the effects of antibody, the soluble factor(s) which is released during this interaction, and how these factors cause degeneration of intraerythrocytic forms of P. falciparum (2, 26). The importance of complement, antimalarial antibody, and cytokines in the phagocytosis of merozoites by human neutrophils was clarified in the present study. The data showed that the cytokines IFN-γ, TNF-α, IL-1β, and GM-CSF enhance merozoite phagocytosis by human neutrophils. These cytokines are produced during malaria episodes, and their roles in relation to immunity and the pathology of malaria have been discussed (6, 16, 28). This is consistent with previous findings which showed that TNF-α and IFN-γ enhance the neutrophil respiratory burst in response to P. falciparum merozoites, as analyzed by chemiluminescence (19). Results of studies of the kinetics of phagocytosis showed that antibody and cytokines markedly increased the rate of neutrophil phagocytosis of merozoites. The results also showed that the maximum merozoite phagocytosis of neutrophils occurred in the presence of both cytokines and opsonic antibody.
The flow cytometry assay described in this paper was done with purified merozoites devoid of contamination with other parasitic stages, while previous studies based on chemiluminescence (12, 19, 20, 24) or morphology (13, 14, 19) have not addressed this aspect. The flow cytometry method offers a simpler, rapid, more reproducible, and less subjective method to study phagocytosis compared to previous methods. An added advantage is the ability to distinguish adhering merozoites from ingested merozoites by trypan blue quenching. In contrast, a chemiluminescence response can be a result of nonadhering, adhering, or ingested parasites. Compared to morphology, flow cytometry allows the study of a larger number of neutrophils (10,000 in this study) at a time. Previous morphology-based phagocytic studies have described the fate of phagocytic cells or intraerythrocytic parasitic stages rather than the fate of the merozoite (2, 13, 14, 19). It is difficult to quantitate how many merozoites have been ingested; therefore, the studies have focused on how many phagocytes are involved in phagocytosis of merozoites (19), while some others addressed the fate of intraerythrocytic stages of P. falciparum during phagocytosis of merozoites (2, 13, 14, 26). In contrast, the present study provides a method to follow the fate of the merozoites, and the extent of merozoite adherence or ingestion.
This work not only illustrates the importance of immune serum (containing high titers of MSA-2) in promoting merozoite phagocytosis but also shows a role for agonist cytokines. The action of both of these factors is more likely to ensure removal of the merozoites than either of them alone. The data parallel previous findings with the intraerythrocytic asexual stage of these parasites, where phagocytosis and killing of the organism were optimal when both antibody and cytokines were present (16, 17). While aspects of the relationship between opsonic antibody types and cytokine-induced heavy-chain gene switching remain speculative, it is evident that certain cytokine patterns give rise to opsonic antibodies and others give rise to nonopsonic antibodies (15). Thus, in the context of the present findings with merozoites and previous reports on intraerythrocytic asexual blood stages, a delicate balance seems to be required between the type of cytokine pattern and the type of IgG subclass response induced by parasite antigens. Based on the results of this study and those of previous studies of P. falciparum intraerythrocytic stages (17, 18), this may best fit the type 1 cytokine pattern (Th1).
ACKNOWLEDGMENTS
We thank Greg Hodge, Department of Haematology, Women's and Children's Hospital, Adelaide, Australia, for his help with the FACScan. Our thanks also extend to Christine Rzepczyk (Queensland Institute of Medical Research, Herston, Brisbane, Australia) for providing malaria immune sera. We are grateful to the South Australian Branch of the Red Cross Blood Transfusion Centre for providing blood and plasma packs for parasite cultures.
This work was supported by the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases and a University of Adelaide Research Grant.
REFERENCES
- 1.Bhatia A, Vernes A. Antigen diversity amongst ten geographic isolates of Plasmodium falciparum defined by inhibition assay. Indian J Malariol. 1992;29:23–28. [PubMed] [Google Scholar]
- 2.Bouharoun-tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung A, Leban J, Shaw A R, Merkli B, Stocker J, Chizzolini C, Sander C, Perrin L H. Immunization with synthetic peptides of Plasmodium falciparum surface antigen induces antimerozoite antibodies. Proc Natl Acad Sci USA. 1986;83:8328–8332. doi: 10.1073/pnas.83.21.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen S. Immunity to malaria. Proc R Soc Lond Biol Sci. 1979;15:203–245. doi: 10.1098/rspb.1979.0001. [DOI] [PubMed] [Google Scholar]
- 5.Colman J P, Jenson J B. Affinity-purified antibodies to ring-infected erythrocyte surface antigen do not correlate with merozoite invasion inhibition in Plasmodium falciparum. Infect Immun. 1988;56:457–461. doi: 10.1128/iai.56.2.457-461.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrante A, Kumaratilake L M, Rathjen D A. The role of cytokine-activated phagocytic cells in immunity to malaria. In: Good M F, Saul F J, editors. Molecular immunological considerations in malaria vaccine development. Boca Raton, Fla: CRC Press; 1994. pp. 47–95. [Google Scholar]
- 7.Ferrante A, Thong Y H. Separation of mononuclear leucocytes from human blood by the one-step Hypaque-Ficoll method is dependent on blood column height. J Immunol Methods. 1982;48:81–85. doi: 10.1016/0022-1759(82)90212-5. [DOI] [PubMed] [Google Scholar]
- 8.Franzen L, Wahlin B, Wahlgren M, Aslund L, Perlmann P, Wigzell H, Petterson U. Enhancement of inhibition of Plasmodium falciparum erythrocyte reinvasion in vitro by antibodies to an asparagine rich protein. Mol Biochem Parasitol. 1989;32:201–211. doi: 10.1016/0166-6851(89)90071-6. [DOI] [PubMed] [Google Scholar]
- 9.Gerold P, Schofield L, Blackman M J, Holder A A, Schwarz R T. Structural analysis of the glycosyl-phosphatidylinositol membrane anchor of the merozoite surface proteins-1 and -2 of Plasmodium falciparum. Mol Biochem Parasitol. 1996;75:131–143. doi: 10.1016/0166-6851(95)02518-9. [DOI] [PubMed] [Google Scholar]
- 10.Howard R J, Pasloscke B L. Target antigens for asexual malaria vaccine development. Parasitol Today. 1993;10:369–372. doi: 10.1016/0169-4758(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 11.Jones T R, Hoffman S L. Malaria vaccine development. Clin Microbiol Rev. 1994;7:303–310. doi: 10.1128/cmr.7.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kharazmi A, Jepsen S, Andersen B J. Generation of reactive oxygen radicals by human phagocytic cells activated by Plasmodium falciparum. Scand J Immunol. 1987;25:335–341. doi: 10.1111/j.1365-3083.1987.tb02198.x. [DOI] [PubMed] [Google Scholar]
- 13.Khusmith S, Druilhe P, Gentilini M. Enhanced Plasmodium falciparum merozoite phagocytosis by monocytes from immune individuals. Infect Immun. 1982;35:874–879. doi: 10.1128/iai.35.3.874-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khusmith S, Druilhe P. Antibody-dependent ingestion of P. falciparum merozoites by human blood monocytes. Parasite Immunol. 1983;5:357–368. doi: 10.1111/j.1365-3024.1983.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumaratilake L M, Ferrante A. Unravelling the cytokine network in malaria. Parasitol Today. 1993;9:56–57. doi: 10.1016/0169-4758(93)90034-d. [DOI] [PubMed] [Google Scholar]
- 16.Kumaratilake L M, Ferrante A. T cell cytokines in malaria: their role in the regulation of neutrophil- and macrophage-mediated killing of Plasmodium falciparum asexual blood forms. Res Immunol. 1994;145:423–429. doi: 10.1016/s0923-2494(94)80172-x. [DOI] [PubMed] [Google Scholar]
- 17.Kumaratilake L M, Ferrante A, Rzepczyk C M. Tumor necrosis factor enhances neutrophil-mediated killing of Plasmodium falciparum. Infect Immun. 1990;58:788–793. doi: 10.1128/iai.58.3.788-793.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumaratilake L M, Ferrante A, Rzepczyk C M. The role of T lymphocytes in immunity to Plasmodium falciparum. Enhancement of neutrophil-mediated parasite killing by lymphotoxin and interferon-γ: comparisons with tumor necrosis factor effects. J Immunol. 1991;146:762–767. [PubMed] [Google Scholar]
- 19.Kumaratilake L M, Ferrante A, Jaeger T, Rzepczyk C M. Effects of cytokines, complement, and antibody on the neutrophil respiratory burst and phagocytic response to Plasmodium falciparum merozoites. Infect Immun. 1992;60:3731–3738. doi: 10.1128/iai.60.9.3731-3738.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lunel F, Descamps L B, Druilhe P. Activation of phagocyte oxidative metabolism by opsonized Plasmodium falciparum merozoites. Acta Trop. 1990;47:61–68. doi: 10.1016/0001-706x(90)90068-b. [DOI] [PubMed] [Google Scholar]
- 21.Mrema J E K, Langreth S G, Jost R C, Riekmann K H, Heidrich H G. Plasmodium falciparum: isolation and purification of spontaneously released merozoites by nylon membrane sieves. Exp Parasitol. 1982;54:285–295. doi: 10.1016/0014-4894(82)90037-6. [DOI] [PubMed] [Google Scholar]
- 22.Reeder J, Brown G V. Antigenic variation and immune evasion in Plasmodium falciparum malaria. Immunol Cell Biol. 1996;74:546–554. doi: 10.1038/icb.1996.88. [DOI] [PubMed] [Google Scholar]
- 23.Sahlin S, Hed J, Rundquist I. Differentiation between attached and ingested immune complexes by a fluorescence quenching cytofluorometric assay. J Immunol Methods. 1983;60:115–124. doi: 10.1016/0022-1759(83)90340-x. [DOI] [PubMed] [Google Scholar]
- 24.Salmon D, Vilde J L, Andrieu B, Simonovic R, Lebras J. Role of immune serum and complement in stimulation of the metabolic burst of human neutrophils by Plasmodium falciparum. Infect Immun. 1986;51:801–806. doi: 10.1128/iai.51.3.801-806.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schofield L, Novakovic S, Gerold P, Schwarz R T, McConville M J, Tachado S D. Glycosylphosphatidylinositol toxin of Plasmodium up-regulates intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via tyrosine kinase-dependent signal transduction. J Immunol. 1996;156:1886–1896. [PubMed] [Google Scholar]
- 26.Shi Y P, Udhayakumar V, Oloo A J, Nahlen B L, Lal A A. Differential effect and interaction of monocytes, hyperimmune sera, and immunoglobulin G on the growth of asexual stage Plasmodium falciparum parasites. Am J Trop Med Hyg. 1999;60:135–141. doi: 10.4269/ajtmh.1999.60.135. [DOI] [PubMed] [Google Scholar]
- 27.Sjoberg K, Hosein Z, Wahlin B, Carlsson J, Wahlgren M, Troy-Blomerg M, Berzins K, Perlman P. Plasmodium falciparum: an invasion inhibitory human monoclonal antibody is directed against a malarial glycolipid antigen. Exp Parasitol. 1991;73:317–325. doi: 10.1016/0014-4894(91)90103-4. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson M M, Nowotarski M, Yap G. Cytokines and malaria. Clin Investig Med. 1990;13:353–359. [PubMed] [Google Scholar]
- 29.Sun T, Chakrabarti C. Schizonts, merozoites and phagocytosis in falciparum malaria. Ann Clin Lab Sci. 1985;15:465–469. [PubMed] [Google Scholar]
- 30.Tan A M, Ferrante A, Goh D H, Roberton D M, Cripps A W. Activation of the neutrophil bactericial activity for nontypable Haemophilus influenzae by tumor necrosis factor and lymphotoxin. Paed Res. 1995;37:155–159. doi: 10.1203/00006450-199502000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Wickramasinghe S N, Phillips R E, Looareesuwan S, Warrell D A, Hughes M. The bone marrow in human cerebral malaria: parasite sequestration within sinusoids. Br J Haem. 1987;66:295–306. doi: 10.1111/j.1365-2141.1987.tb06913.x. [DOI] [PubMed] [Google Scholar]