Abstract
Introduction:
Percutaneous auricular nerve stimulation has been used for the treatment of symptoms associated with opioid withdrawal, including abdominal pain, nausea, and general discomfort. However, its potential utility for pain management and opioid minimization after surgery has not been investigated. The purpose of this study was to test the feasibility and acceptability of a trial protocol designed to assess the effectiveness of the NSS2-Bridge device as a non-pharmacologic alternative to opioids after cesarean delivery.
Methods:
In a randomized control design, healthy women receiving cesarean delivery were randomized to receive the active device, placebo device, or no device. Devices were placed on the ear following cesarean delivery and left in place for 5 days. Feasibility and acceptability of the device was assessed by patient reports of device tolerability (rated on a 100mm visual analog scale where 0 is not tolerable at all and 100 is the most tolerable) as well as qualitative reporting. Additional outcomes assessed included proportion of patients not using opioids in hospital, as well as pain at rest, pain with movement, and total opioid consumption in the hospital and for the first 5 days after surgery.
Results:
There were 60 patients included in the final analysis. Device tolerability was rated highly, with an average daily score of >75 mm on the visual analog scale. The trial retention rate was 89.7% with most exclusions (42.9%) occurring due to unanticipated development of care complexity (e.g., hemorrhage and additional surgical procedures), with only 1 exclusion (14.3%) due to device discomfort. The active device group achieved the highest proportion of opioid-free hospitalizations (40%) compared to placebo (20%) and no device groups (30%). Pain at rest and with movement was similar between treatment groups.
Conclusions:
This trial protocol designed to test the efficacy of NSS2-Bridge device for post-cesarean pain management is feasible and acceptable. Larger proportions of patients not using opioids in the active device group justifies additional investigation on device effectiveness in pregnant and postpartum people at highest risk for pain.
Keywords: NSS-Bridge Device, Post-Cesarean Delivery, Analgesia
Introduction
Pregnancy-associated mortality involving opioids have more than doubled over the past decade. Persistent post-cesarean pain occurs in up to 11% of women and people with opioid use disorder (OUD) may experience worse pain [1], [2] including during and after childbirth [3]. These pain exacerbations can lead to increased opioid requirements for breakthrough pain, potentially increasing risk for developing new OUD [4]. Many people with OUD desire strict opioid avoidance even in the context of pain [5], making effective non-opioid alternative pain therapies an important area for investigation.
Complementary and alternative medicine approaches have been successful in pain management in some treatment settings. However, there is currently a knowledge gap on the role of these therapies for pain management after cesarean delivery, which is a more painful mode of delivery than vaginal delivery [6], [7]. The World Health Organization opioid ladder recommends emphasizing non-opioid therapies for pain management, to reduce inappropriate opioid prescribing and its risk for subsequent new OUD [8]. For women with OUD, alternative therapies – both pharmacological and non-pharmacological – are critically needed, to avert the risks and consequences of opioid exposure and abuse.
To address this need, identifying the therapeutic effectiveness of novel and alternative devices on post-cesarean pain management is important. The NSS2-Bridge device is a disposable, percutaneous nerve field stimulator device cleared by the FDA for the treatment of symptoms associated with opioid withdrawal [9], [10]. Evidence supports the concept that these stimulations are transmitted to the cranial nerve nucleus located in the brainstem and the spine, modulating the pain pathways via the limbic system [11]–[15]. Our early research has suggested that the NSS2-Bridge successfully reduces postoperative pain and opioid requirements by up to 67% after major abdominal surgery (such as laparotomies, colectomies, and Whipple procedures) [16], [17] as well as gastric bypass surgeries18 and kidney donor surgery [18].
The purpose of this study was to test the feasibility and acceptability of a randomized placebo-controlled and natural-history (no intervention arm) trial protocol that tests the effectiveness of the NSS2-Bridge device in postpartum women after cesarean delivery. The hypothesis for the current study is that such a trial protocol is feasible and acceptable to participants, thereby supporting fully powered trials to identify the role that the NSS2-Bridge device may have in reducing opioid requirements and pain after cesarean delivery. The full trial hypothesis informing the current trial design is that women treated with the NSS2-Bridge will have less pain and reduced opioid requirements after cesarean delivery.
Methods
The study was approved by the University of Pittsburgh Institutional Review Board (STUDY19110257) and all participants gave written informed consent to participate in the study. All methods were carried out in accordance with relevant guidelines and regulations including CONSORT 2010 guidelines. The trial was registered on Clinical Trials. Gov (NCT04365465 registered 28/04/2020).
Healthy women at term gestation with singleton pregnancies who presented for planned, scheduled cesarean delivery to UPMC Magee-Women’s Hospital under planned spinal anesthesia were eligible to participate. Subjects were included if they were pregnant, in the third trimester, having a planned and scheduled cesarean delivery under spinal anesthesia with neuraxial morphine, had clean and healthy skin amenable to device application, and were 18 years old or older. Subjects were excluded if they were not fluent in English (surveys used were validated in the English language), unable to participate in informed consent, unable to give informed consent for any reason, unable to participate fully in all study procedures for any reason, unable to participate fully in all study procedures for any reason, having a cesarean delivery under general anesthesia, had a history of hemophilia, pacemakers or implantable electronic devices, had a history of psoriasis or other skin conditions precluding device application, needed to receive rescue abdominal wall block for any reason, and any cesarean delivery that had unanticipated additional procedures including but not limited to hysterectomy, cystoscopy, and other procedures for management of postpartum hemorrhage.
Study Design
This study design was a 3-arm parallel randomized control trial by RedCAP to the following groups: 1) active device; 2) placebo device; 3) no intervention (active control). Enrolled subjects were randomized to one of the three study groups using a computed-generated randomization scheme. Randomization was assigned by an investigator blinded to the patient and clinical activities. Devices were placed immediately on arrival to Phase I recovery, while subjects were still at a minimum T6 level of a spinal anesthetic block and were not yet experiencing any pain. Device placement was by an investigator who was blinded to group assignment. Both patients and care providers were blinded to group assignment for the active and placebo devices.
Active and Placebo Devices
The NSS2-Bridge device (Figure 1) is an FDA approved device for treatment of clinical symptoms associated with opioid withdrawal, including pain, nausea, and vomiting9. The active device consists of a small battery-powered and disposable stimulator operating for 5 days and activated by its connection to 4 electrodes (3 active, 1 ground). Each electrode is implanted to a different part of the ear. The active device is a nerve stimulator that stimulates the nerves present in the ear, including branches of the vagal, trigeminal, facial, and glossopharyngeal nerves. The proposed mechanism of action is that the device stimulates the terminal branches of these nerves innervating the ear; that nerve impulse is then transmitted to the corresponding nuclei of these cranial nerves in the brain stem, thereby modulating pain pathways through the limbic system [11]–[14].
Figure 1:
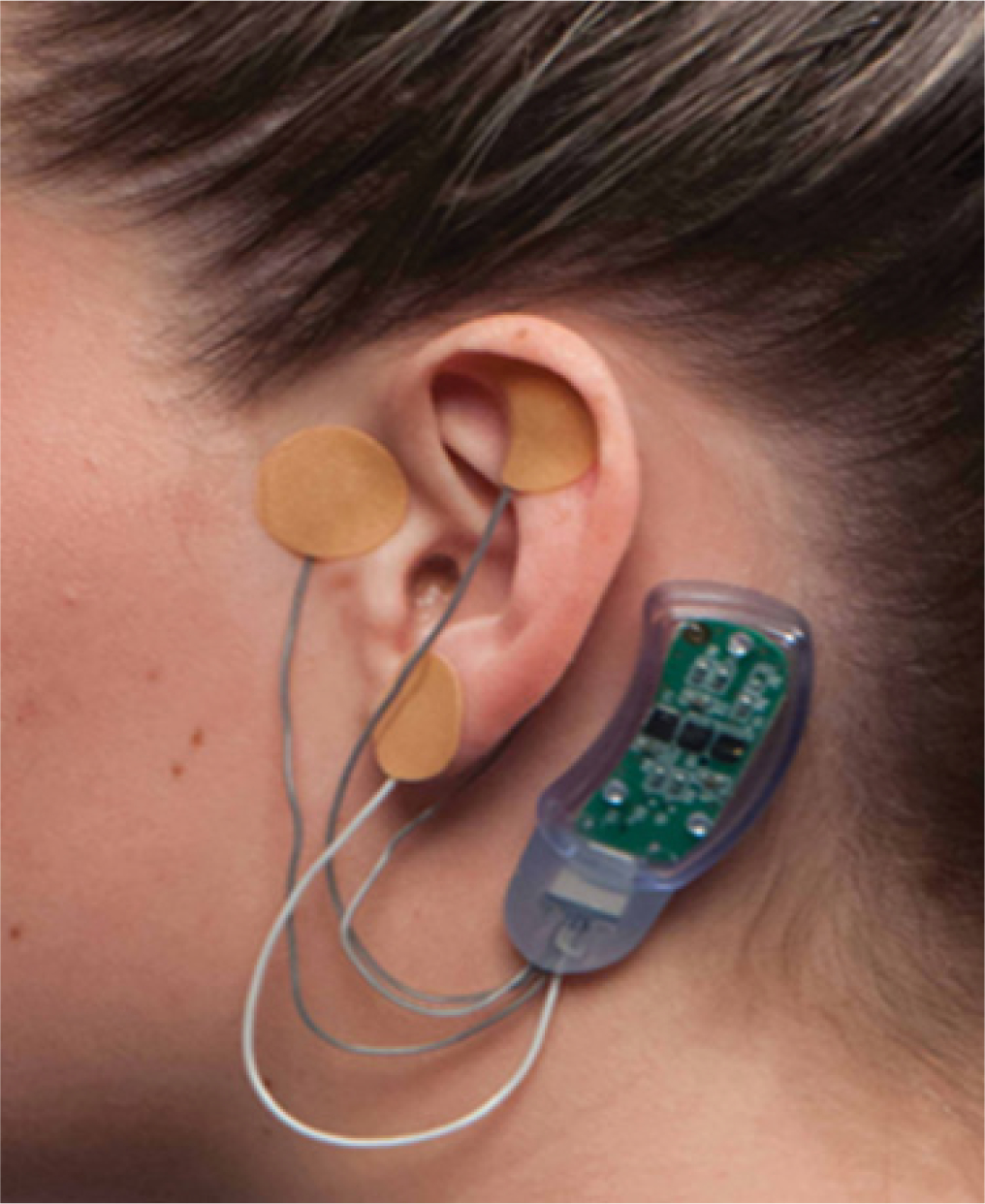
NSS2-Bridge Device.
The NSS2-Bridge placebo device is similar to the active device, except that the stimulator does not deliver any current, and the electrodes are smooth. After placement, each electrode of both active and placebo devices are covered by a small brown circular tape, which maintains blinding.
Spinal anesthesia and postpartum analgesia
Spinal anesthesia consisted of hyperbaric bupivacaine 12mg, fentanyl 15mcg, and morphine 150mcg. Postpartum analgesia followed our typical standardized clinical care protocols for cesarean delivery and consisted of intravenous ketorolac 30mg q8h for 24 hours, followed by conversion to ibuprofen 600mg PO q8h; acetaminophen 1000mg PO q8h. Oral oxycodone 5–10mg is typically given for pain rated 7 or higher on a 0–10 numeric rating scale, or for any pain that the patient reports as intolerable.
Data collection
Subject data were collected including age, race, ethnicity, education level, income level, body mass index, gravidity, parity, anxiety/depression history, mental illness history, prior cesarean deliveries, opioid use disorder history. Device tolerability was assessed by asking the patients who withdrew from the study, to indicate their reasons for withdrawal from the study. Acceptability of the device was assessed each day for 5 days using a visual analog scale for the question, “To what extent do you find the BRIDGE device to be tolerable?” where 0 was completely intolerable, 100 was completely tolerable, and 50 was neutral. Feasibility was assessed by rates of participant withdrawal and reasons for withdrawal, and completion of enrollment meeting pre-specified target of 60 participants with at least 80% retention rates. Acceptability was also assessed by patient comments on an open-ended question, “Please share any other thoughts that you have about the device.”
Other data recorded included rates of patients not requiring any opioid during hospitalization, total milligram morphine equivalents (MME) consumed in the hospital, patient-reported outcomes included daily PROMIS pain intensity (pain at rest) and pain interference (pain with movement) inventories on postpartum days 0 through 5.
Statistical Analysis
Based on our internal preliminary data, approximately 25 participants were eligible for recruitment per month. Based on an anticipated 76% recruitment rate established from our previous studies, we anticipated that we would successfully enroll our target sample size of 60 women (20 per group) over the feasibility period of 6 months. As a feasibility investigation, we expected this number would be sufficient to assess feasibility and acceptability of the study protocol.
Feasibility and acceptability measures were described or compared as follows. Acceptability scales on the 0–100mm visual analog scale were summarized for the active device group over postpartum days 0 to 3 and a score of >70 was considered highly acceptable. Qualitative comments by subjects were assessed. Descriptive statistics were applied to assess rates and reasons for participant withdrawal.
Descriptive statistics were used to compare demographic characteristics between treatment groups by intent to treat principles. We explored relationships between treatment groups and changes over time with pain with movement and pain at rest, using violin plots for pain over postpartum days 0 to 5. Proportions of opioid-free hospitalization and trends for total MME consumption were calculated by treatment group.
Sample size calculations for a fully powered study were determined based on pain score means and variances/standard deviations with a priori specified effect size as detailed below (2-tailed α = 0.05, 80% power). The primary outcome for a full trial was considered evoked pain (pain with movement), measured by 0–10 numeric rating scale at 72 hours after delivery. Additional outcomes assessed included pain with movement over postpartum days 1 through 5, MME consumption, and proportions of opioid-free hospitalizations. All analyses were conducted using Stata SE 17 (StataCorp LP, 1985, College Station, TX) and XLSTAT (Microsoft Inc., USA).
Results
There were 68 women enrolled and randomized (Figure 2). A total of 60 subjects (20 active, 20 placebo, 20 in no device) were included in the analysis. Baseline characteristics of subjects are shown in Table 1 and were not significantly different between treatment groups.
Figure 2:
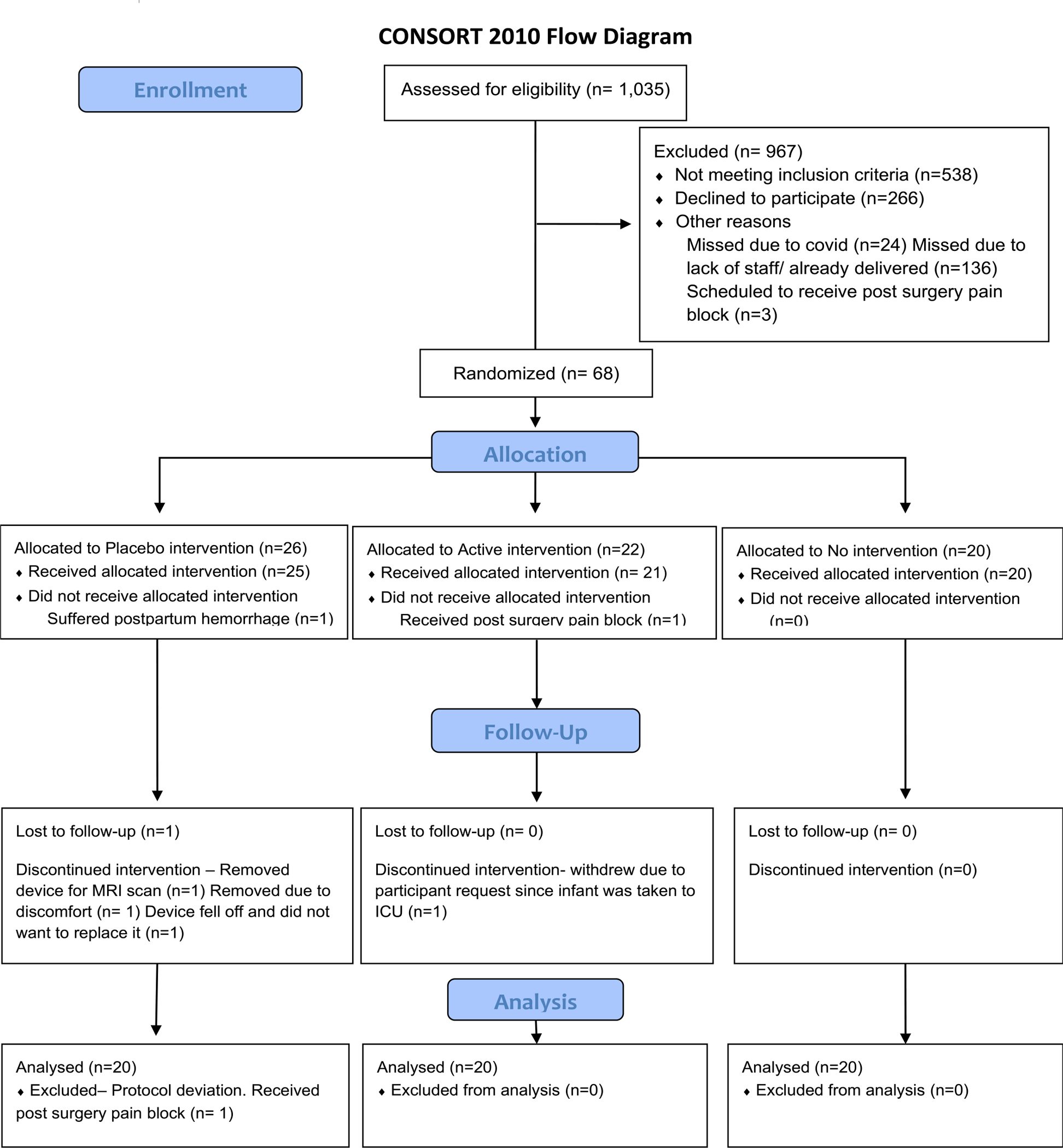
Consort Flow Diagram
Table 1:
Demographic characteristics of the study cohort. IQR, inter-quartile range
| Characteristics | Active Device (N = 20) | No Device (N = 20) | Placebo Device (N = 20) |
|---|---|---|---|
| Demographics: | |||
| Mean Age Mean (Std Dev) | 32.7 (5.5) | 31.4(5.8) | 32.1 (4.7) |
| Race – no./total no. (%) | |||
| White | 18/20 (90) | 17/20 (85) | 17/20 (85) |
| Black/African American | 1/20 (5) | 2/20 (10) | 2/20 (10) |
| Asian | 1/20 (5) | 1/20 (5) | 0/20 (0) |
| Other | 0/20 (0) | 0/20 (0) | 1/20 (5) |
| Ethnicity – no./total no. (%) | |||
| Hispanic | 0/20 (0) | 1/20 (5) | 1/19 (5) |
| Non-Hispanic | 20/20 (100) | 17/20 (85) | 18/19 (94) |
| Not Reported | 0/20 (0) | 2/20 (10) | 0/19 (0) |
| Education level – no./total no. (%) | |||
| High school graduate, diploma, or equivalent | 4/ 20 (20) | 1/ 19 (5) | 1/ 19 (5) |
| Trade/ Technical/ Vocational Training | 1/20 (5) | 3/19 (15) | 3/19 (15) |
| Some college credit, no degree | 3/20 (15) | 3/19 (15) | 3/19 (15) |
| Associate degree | 3/20 (15) | 1/19 (5) | 1/19 (5) |
| Bachelor’s degree | 3/ 20 (15) | 2/ 19 (10) | 2/ 19 (10) |
| Master’s degree | 1/ 20 (5) | 4/ 19 (21) | 5/ 19 (26) |
| Doctorate degree | |||
| Income level – no./total no. (%) | |||
| Less than $10,000 | 1/ 20 (5) | 2/ 19 (10) | 2/ 20 (10) |
| $10,000 to $19,999 | 2/20 (10) | 1/19 (5) | 0/20 (0) |
| $20,000 to $29,999 | 1/20 (5) | 1/19 (5) | 2/20 (10) |
| $30,000 to $39,999 | 1/20 (5) | 0/19 (0) | 0/20 (0) |
| $40,000 to $49,999 | 1/20 (5) | 1/ 19 (5) | 1/20 (10) |
| $50,000 to $59,999 | 1/20 (5) | 0/ 19 (0) | 0/ 20 (0) |
| $60,000 to $69,999 | 2/ 20 (10) | 0/19 (0) | 2/20 (10) |
| $70,000 to $79,999 | 0/20 (0) | 4/19 (21) | 2/20 (10) |
| $80,000 to $89,999 | 2/20 (10) | 1/19 (5) | 3/20 (15) |
| $90,000 to $99,999 | 1/20 (5) | 1/19 (5) | 4/20 (20) |
| $100,000 to $149,999 | 6/20 (30) | 3/19 (15) | 2/20 (10) |
| $150,000 or more | 2/20 (5) | 5/19 (26) | 2/20 (10) |
| Median Body Mass Index (IQR) | 31.9 [21.8– 44.4] | 34.8 [24.2 – 51.9] | 29.7 [25.8 – 34.5] |
| Median Gravidity (IQR) | 2 [1– 5] | 2 [1– 8] | 2.5 [1– 8] |
| Parity (IQR) | 1[0–2] | 1 [0–5] | 1 [0– 3] |
| History of Anxiety/ Depression no./total no. (%) | 14/20 (70) | 10/20 (50) | 10/20 (50) |
| History of mental illness no./total no. (%) | 4/20 (20) | 1/19 (5) | 3/20 (15) |
| Median Prior Cesareans (IQR) | 1 [0– 2] | 1 [0–5] | 1 [0–3] |
| Opioid use disorder no./total no. (%) | 0/20 (0) | 1/20 (5) | 0/20 (0) |
| Medication for opioid use disorder no./total no. (%) | 0/20 (0) | 1/20 (5) | 0/20 (0) |
| Substance use disorder no./total no. (%) | 4/20 (20) | 3/20 (15) | 1/20 (5) |
Acceptability and Feasibility
The mean ± standard deviation device tolerability in the active groups were 86.2 ± 27.0 on day 0, 76.8 ± 20.5 on day 1, 76.5 ± 20.0 on day 2, and 76.8 ± 22.1 on day 3. Qualitative comments on acceptability ranged positive (e.g., “It’s great” and “I don’t even notice it”) to formative (e.g., “Needs to be waterproof” “Stuck to my hair”). There were 7 subjects withdrawn (7/68, 10.3% withdrawal rate, 89.7% retention rate) due to development of clinical care complexity conferring inability to participate in follow-up procedures (n=3), device discomfort (n=1), receipt of additional pain care outside of the trial protocol (n=1), and survey non-adherence (n=2) (Table 2).
Table 2:
Reasons for participant withdrawal
| Frequency (n = 7) | Percent % | |
|---|---|---|
| Hemorrhage or complex care, unable to participate in follow-up procedures | 3 | 42.90% |
| Survey non-adherence | 2 | 28.60% |
| Complex pain receiving additional care influencing pain experience | 1 | 14.30% |
| Device discomfort | 1 | 14.30% |
Opioid-Free Hospitalization Outcome, Pain Outcomes, and MME consumption
The active device group achieved the highest proportion (40%) of opioid-free hospitalization compared to placebo and standard therapy (Table 3). Pain with movement over the first 72 hours trended similarly between groups (active group mean 4.6 ± 2.3, placebo group 4.6 ± 2.5, control group 4.2 ± 2.3) (Figure 3). Pain at rest and with movement were lowest on day 0 for all treatment groups and trended similarly between treatment groups across days 1 through 5 (Figure 3). Opioid consumption averaged 51.1 ± 56.6 MME in the active device group, 71.6 ± 90.3 MME in the placebo device group, and 42.8 ± 44.0 MME in the no device group.
Table 3:
Proportion of opioid-free hospitalizations across treatment groups. The active group achieved highest proportion of opioid-free hospital stay compared to placebo or standard group
| Active (n=20) | Placebo (n=20) | Standard (n=20) | |
|---|---|---|---|
| Opioid-free | 8 (40%) | 4 (20%) | 6 (30%) |
| Used opioids | 12 (60%) | 16 (80%) | 14 (70%) |
Figure 3:
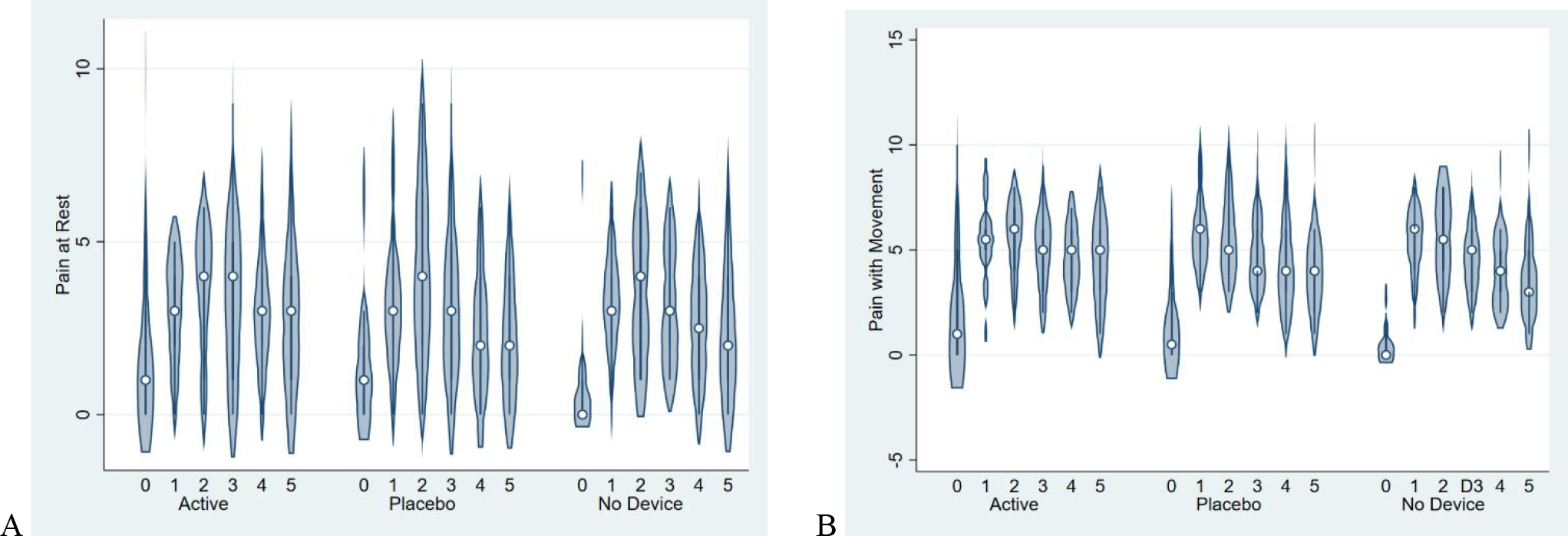
Pain with movement (A) and at rest (B) between treatment groups over the first 5 days after delivery
Sample Size Calculation for Future Trial
For a primary outcome of evoked pain (pain with movement) measured by 0–10 numeric rating scale in the first 72 hours, based on the data in this study, a sample size of 179 in each treatment group is estimated to detect a 3.5-point difference between groups for the outcome of pain with movement in the first 72 hours, with 80% power and significance level alpha = 0.05, a difference that we also considered clinically meaningful. Based on the data in this study, a sample size of 276 in each group is estimated to detect a 7.5-milligram difference between groups for the outcome of milligram morphine equivalents, with 80% power and significance level alpha = 0.05. Adjustments to this sample size should be made for planned multivariable regression analysis using conventional 10 events per adjusted variable [19].
Discussion
The primary findings of this study are that cesarean delivery analgesia with the NSS2-Bridge device is feasible and acceptable to participants. There was an 89.7% participant retention rate, and average device tolerability ratings were highly acceptable throughout the postpartum period. Trials on alternative methods for pain management should be pursued for women in the postpartum period, particularly for those who prioritize alternative treatments.
Auricular nerve stimulation has been described for neuromodulation of several different medical conditions, including pain, substance use disorders, depression, cardiovascular arrhythmias, and peripheral arterial disease [13], [18], [20]–[23]. There are anatomic and physiologic underpinnings for auricular nerve stimulation[13], [24]. Simulation of cranial nerves evokes responses in the brainstem and cortical structures, triggering reflex responses through somatosensory cortex, prefrontal cortex, and limbic structures which regulate pain experience and modulation. The neuromodulation that ensues is suggested to be a potential way of limiting medication requirements, including opioids for pain management. Our study advances scientific knowledge about the role that percutaneous auricular nerve stimulation may have in reducing postpartum pain and opioid needs among postpartum women at high and low risk for severe pain and opioid misuse. Treatment services, technologies, and preventative interventions that drive acute pain management services have the potential to be changed as a result of these kinds of investigations.
Complementary and alternative medicine (CAM) approaches are well-known to anesthesiology and perioperative practices, gaining in popularity among the general public[25], [26] and include modalities such as acupuncture, aromatherapy, hypnosis, and music therapy[27], [28]. However, limited data on CAM efficacy and treatment responsiveness limits its adoption in most hospital-based care environments. In one systematic review [29], the use of CAM in otolaryngologic surgeries was associated with reduced preoperative anxiety, postoperative pain, and nausea and vomiting, with limited adverse effects. In obstetrics [30], CAM interventions include acupressure and ginger for nausea, moxibustion for version of breech presentation, and sterile water injections for back pain during labor, although the evidence to support these modalities is limited. Our study provides additional information about the feasibility and acceptability of CAM trials in the cesarean delivery population. Our data supports the conduct of future trials that are focused on the effectiveness and predictors of treatment response and non-response among pregnant and postpartum patients.
The strengths of this study include the inclusion of both placebo and natural history arms, which enables assessment of active device effects vs. placebo analgesia effects. Limitations include the limited sample size which limits definitive conclusions regarding the effectiveness of the intervention on pain and opioid consumption. We also included the general cesarean population, while a future design may consider targeted enrollment of a more homogeneous group of patients at high risk for severe pain and opioid use, such as those with preoperative anxiety or smoking.
In summary, we demonstrate feasibility and acceptability of a study protocol using the NSS2-Bridge device for post-cesarean pain management. This project supports the pursuit of larger studies that investigates the effectiveness of CAM for postpartum people with and without OUD, thereby shifting current postpartum pain approaches toward innovative multimodal complementary pain treatment strategies. Future rigorous trials in these special populations will provide better evidence to patients, their support persons, and providers regarding the utility of CAM for pain and symptom management.
Acknowledgements
We are grateful to Masimo for their support of this study through the provision of the devices.
Funding
This project was supported by the CRISP Grant by National Institutes of Health (UL1 TR001857). Dr. Lim is supported in part by the Department of Anesthesiology & Perioperative Medicine, and by NIH (K12HD043441)
Footnotes
Competing interests
None
Ethics approval and consent to participate
This study was approved by the University of Pittsburgh Institutional Review Board (STUDY19110257). Written informed consent was obtained from each participant. All methods were carried out in accordance with relevant guidelines and regulations
Consent for publication
No details, images, or videos related to an individual person apply to this manuscript
Availability of data and materials
Availability of raw data and materials are available to scientists at the discretion of the corresponding author who wishes to use them for non-commercial purposes without breaching participant confidentiality. Data supporting the findings of this study are available from the corresponding author, but restrictions apply to the availability of these data which were used under license for the current study so are not publicly available. The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
- 1.Weibel S, Neubert K, Jelting Y, et al. Incidence and severity of chronic pain after caesarean section: A systematic review with meta-analysis. European journal of anaesthesiology 33 (2016): 853–865. [DOI] [PubMed] [Google Scholar]
- 2.Manhapra A Complex Persistent Opioid Dependence-an Opioid-induced Chronic Pain Syndrome. Current treatment options in oncology (2022). [DOI] [PubMed] [Google Scholar]
- 3.Komatsu R, Ando K, Flood PD. Factors associated with persistent pain after childbirth: a narrative review. Br J Anaesth. 124 (2020): 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prabhu M, Dolisca S, Wood R, et al. Postoperative Opioid Consumption After Scheduled Compared With Unscheduled Cesarean Delivery. Obstetrics and gynecology 133 (2019): 354–363. [DOI] [PubMed] [Google Scholar]
- 5.Weiss RD, Potter JS, Griffin ML, et al. Reasons for opioid use among patients with dependence on prescription opioids: the role of chronic pain. Journal of substance abuse treatment 47 (2014): 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald EA, Gartland D, Small R, Brown SJ. Dyspareunia and childbirth: a prospective cohort study. BJOG : an international journal of obstetrics and gynaecology 122 (2015): 672–679. [DOI] [PubMed] [Google Scholar]
- 7.Keag OE, Norman JE, Stock S J Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis. PLoS medicine 15 (2018): 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anekar AA, Cascella M WHO Analgesic Ladder. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; (2022). [PubMed] [Google Scholar]
- 9.FDA. FDA grants marketing authorization of the first device for use in helping people to reduce the symptoms of opioid withdrawal [News Release]. Available from: www.fda.gov/news-events-press-announcements/fda-grants-marketing-authorization-first-device-use-helping-reduce-symptoms-opioid-withdrawal. Accessed (2022).
- 10.Miranda A, Taca A. Neuromodulation with percutaneous electrical nerve field stimulation is associated with reduction in signs and symptoms of opioid withdrawal: a multisite, retrospective assessment. Am J Drug Alcohol Abuse 44 (2018): 56–63. [DOI] [PubMed] [Google Scholar]
- 11.Alimi D L’auriculothérapie médicale: bases scientifiques, principes, indications et stratégies thérapeutiques. Parice, France: Elsevier Masson SAS; (2017). [Google Scholar]
- 12.Anthwal N, Thompson H. The development of the mammalian outer and middle ear. J Anat 228 (2016): 217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercante B, Ginatempo F, Manca A, Melis F, Enrico P, Deriu F. Anatomo-Physiologic Basis for Auricular Stimulation. Medical acupuncture 30 (2018): 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peuker ET, Filler T J The nerve supply of the human auricle. Clinical anatomy (New York, NY) 15 (2002): 35–37. [DOI] [PubMed] [Google Scholar]
- 15.Babygirija R, Sood M, Kannampalli P, et al. Percutaneous electrical nerve field stimulation modulates central pain pathways and attenuates post-inflammatory visceral and somatic hyperalgesia in rats. Neuroscience 356 (2017): 11–21. [DOI] [PubMed] [Google Scholar]
- 16.Chelly J, Holtzman M, Bartlett D, et al. Reduction in postoperative opioid requirement associated with use of the NSS-2® Bridge device, a disposable auriculo-nerve field stimulator, and factors affecting the response in cancer patients undergoing abdominal surgical procedures (2022).
- 17.Blank JJ, Liu Y, Yin Z, et al. Impact of Auricular Neurostimulation in Patients Undergoing Colorectal Surgery with an Enhanced Recovery Protocol: A Pilot Randomized, Controlled Trial. Dis Colon Rectum 64 (2021): 225–233. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed BH, Courcoulas AP, Monroe AL, et al. Auricular nerve stimulation using the NSS-2 BRIDGE device to reduce opioid requirement following laparoscopic Roux-en-Y gastric bypass. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 17 (2021): 2040–2046. [DOI] [PubMed] [Google Scholar]
- 19.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. American journal of epidemiology 165 (2007): 710–718. [DOI] [PubMed] [Google Scholar]
- 20.Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. Journal of neural transmission (Vienna, Austria: 1996) 120 (2013): 821–827. [DOI] [PubMed] [Google Scholar]
- 21.Hackl G, Prenner A, Jud P, et al. Auricular vagal nerve stimulation in peripheral arterial disease patients. VASA Zeitschrift fur Gefasskrankheiten 46 (2017): 462–470. [DOI] [PubMed] [Google Scholar]
- 22.Mahadi KM, Lall VK, Deuchars SA, et al. Cardiovascular autonomic effects of transcutaneous auricular nerve stimulation via the tragus in the rat involve spinal cervical sensory afferent pathways. Brain stimulation 12 (2019): 1151–1158. [DOI] [PubMed] [Google Scholar]
- 23.Mahoney JJ 3rd, Hanlon CA, Marshalek PJ, et al. Transcranial magnetic stimulation, deep brain stimulation, and other forms of neuromodulation for substance use disorders: Review of modalities and implications for treatment. Journal of the neurological sciences (2020) 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butt MF, Albusoda A, Farmer AD, Aziz Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat 235 (2020): 588–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astin J A Why patients use alternative medicine: results of a national study. Jama 279 (1998): 1548–1553. [DOI] [PubMed] [Google Scholar]
- 26.Ventola C L Current Issues Regarding Complementary and Alternative Medicine (CAM) in the United States: Part 1: The Widespread Use of CAM and the Need for Better-Informed Health Care Professionals to Provide Patient Counseling. P & T : a peer-reviewed journal for formulary management 35 (2010): 461–468. [PMC free article] [PubMed] [Google Scholar]
- 27.Fleckenstein J, Baeumler PI, Gurschler C, et al. Acupuncture for post anaesthetic recovery and postoperative pain: study protocol for a randomised controlled trial. Trials (2014):215–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shakeel M, Bruce J, Jehan S, et al. Use of complementary and alternative medicine by patients admitted to a surgical unit in Scotland. Annals of the Royal College of Surgeons of England 90 (2008): 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kallush A, Riley CA, Kacker A. Role of Complementary and Alternative Medicine in Otolaryngologic Perioperative Care. The Ochsner journal 18 (2018): 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson FW, Johnson CT. Complementary and alternative medicine in obstetrics. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 91 (2005):116–124. [DOI] [PubMed] [Google Scholar]


