Abstract
In recent years research on natural speech processing has benefited from recognizing that low-frequency cortical activity tracks the amplitude envelope of natural speech. However, it remains unclear to what extent this tracking reflects speech-specific processing beyond the analysis of the stimulus acoustics. In the present study, we aimed to disentangle contributions to cortical envelope tracking that reflect general acoustic processing from those that are functionally related to processing speech. To do so, we recorded EEG from subjects as they listened to auditory chimeras, stimuli composed of the temporal fine structure of one speech stimulus modulated by the amplitude envelope (ENV) of another speech stimulus. By varying the number of frequency bands used in making the chimeras, we obtained some control over which speech stimulus was recognized by the listener. No matter which stimulus was recognized, envelope tracking was always strongest for the ENV stimulus, indicating a dominant contribution from acoustic processing. However, there was also a positive relationship between intelligibility and the tracking of the perceived speech, indicating a contribution from speech-specific processing. These findings were supported by a follow-up analysis that assessed envelope tracking as a function of the (estimated) output of the cochlea rather than the original stimuli used in creating the chimeras. Finally, we sought to isolate the speech-specific contribution to envelope tracking using forward encoding models and found that indices of phonetic feature processing tracked reliably with intelligibility. Together these results show that cortical speech tracking is dominated by acoustic processing but also reflects speech-specific processing.
SIGNIFICANCE STATEMENT Activity in auditory cortex is known to dynamically track the energy fluctuations, or amplitude envelope, of speech. Measures of this tracking are now widely used in research on hearing and language and have had a substantial influence on theories of how auditory cortex parses and processes speech. But how much of this speech tracking is actually driven by speech-specific processing rather than general acoustic processing is unclear, limiting its interpretability and its usefulness. Here, by merging two speech stimuli together to form so-called auditory chimeras, we show that EEG tracking of the speech envelope is dominated by acoustic processing but also reflects linguistic analysis. This has important implications for theories of cortical speech tracking and for using measures of that tracking in applied research.
Keywords: auditory processing, envelope tracking, language, phonetic features, speech decoding, speech intelligibility
Introduction
Over the past few years research on natural speech processing has benefited from recognizing that low-frequency cortical activity tracks the amplitude envelope of natural speech (Ahissar et al., 2001; Luo and Poeppel, 2007; Lalor and Foxe, 2010). This has been useful for investigating the mechanisms underlying speech processing (Peelle and Davis, 2012), how such processing is affected by attention (Ding and Simon, 2012; Power et al., 2012; Zion-Golumbic et al., 2013), and how audio and visual speech interact (Luo et al., 2010; Zion-Golumbic et al., 2013; Crosse et al., 2015, 2016a). However, it remains unclear to what extent these cortical measures reflect higher-level speech-specific processing versus lower-level processing of the spectrotemporal/acoustic stimulus dynamics.
There has been some evidence that speech intelligibility affects these envelope tracking measures (Peelle et al., 2013), suggesting that they may indeed index speech-specific processing. But precisely what aspects of speech processing are reflected in envelope tracking measures, or even how specifically the measures reflect speech processing at all, is unclear. It has been suggested that different neural populations, having different functional roles in receptive speech processing, may simultaneously contribute to envelope tracking measures (Ding and Simon, 2014). Furthermore, specific mechanistic theories have been proposed, suggesting that envelope tracking (or envelope entrainment, more specifically) represents a more active process for parsing speech into discrete chunks for further processing (Giraud and Poeppel, 2012). However, drawing definitive inferences about the meaning of cortical speech tracking must contend with the likelihood that much of the speech-tracking signal will derive from general auditory processing of the stimulus acoustics by cortical regions that are agnostic to the special nature of speech. Indeed, a wealth of evidence has been amassed suggesting that speech is processed by a hierarchically organized network of cortical regions with responses in earlier stages (including primary auditory cortex) being well accounted for based on the spectrotemporal acoustics of the stimulus and later stages being invariant to those acoustics and involved in more abstract linguistic processing (Davis and Johnsrude, 2003; DeWitt and Rauschecker, 2012; Huth et al., 2016; de Heer et al., 2017; Kell et al., 2018; Norman-Haignere and McDermott, 2018). This is consistent with the idea that speech sounds are perceived using mechanisms that evolved to process environmental sounds more generally (Diehl et al., 2004), with additional linguistic processing occurring in specialized downstream pathways (Hickok and Poeppel, 2007; Rauschecker and Scott, 2009).
Indeed, this notion that cortical tracking of speech might reflect (perhaps a lot of) general acoustic processing as well as (perhaps a more limited contribution from) linguistic processing helps to explain several other findings in the literature. For example, although cortical envelope tracking sometimes shows sensitivity to speech intelligibility as mentioned above (Peelle et al., 2013), this is definitely not always the case (Howard and Poeppel, 2010). Indeed, robust cortical tracking has been observed for completely unintelligible speech, including vocoded and backward speech (Howard and Poeppel, 2010; Di Liberto et al., 2015, 2018), as well as very general auditory stimuli such as amplitude modulated broadband noise (Lalor et al., 2009). So acoustic processing definitely makes a substantial contribution. In the present study, we aim to explore this idea of dissociable contributions to envelope tracking using so-called auditory chimeras (Smith et al., 2002). In particular, we record EEG from subjects as they listen to speech–speech chimeras: stimuli composed of the temporal fine structure (TFS) of one speech stimulus modulated by the amplitude envelope (ENV) of another speech stimulus. By varying how these chimeras are constructed, we obtain some control over which stimulus is recognized by the listener, allowing us to decouple the processing of the acoustic envelope from the speech content. We hypothesize that envelope tracking will be dominated by the dynamic changes in the acoustic energy of the signal, with a smaller component reflecting speech-specific processing.
Materials and Methods
Subjects
Seventeen native English speakers (mean age 24.9 years; SD, 3.7; range 20–30; 8 males) participated in the experiment. Participants reported no neurologic diseases and self-reported normal hearing. Informed consent was obtained from all participants before the experiment, and subjects received monetary compensation for their time. The study was conducted in accordance with protocols approved by the Research Subjects Review Board at the University of Rochester.
Stimuli and experimental procedure
In our experiment, we wanted to decouple the acoustic amplitude fluctuations of the stimuli from their speech content. To do this, we used so-called auditory chimeras (Smith et al., 2002). These are stimuli in which the envelope of one sound is used to modulate the temporal fine structure of a second sound. Importantly, this can be done after first filtering the sounds into complementary frequency bands using a filter bank. Then, each filter output is Hilbert transformed to derive its analytic signal, and the envelope (calculated as the magnitude of the analytic signal) of the first sound is used to modulate the fine structure (calculated as the cosine of the phase of the analytic signal) of the other sound within each band, giving a series of partial chimeras. Finally, the partial chimeras are summed over all frequency bands to produce the final chimera (Fig. 1). Critically for our experiment, the number of frequency bands used has a marked effect on which original sound source is actually perceived (Smith et al., 2002). If the original sounds are both speech, and just one band is used, then listeners will partially recognize the speech content corresponding to the temporal fine structure. They will (obviously) fail to understand any of the speech content corresponding to the source of the broadband envelope. But as the number of frequency bands grows, listeners will increasingly understand the speech content relating to the source of the envelope and will no longer perceive the speech content of the temporal fine structure source.
Figure 1.
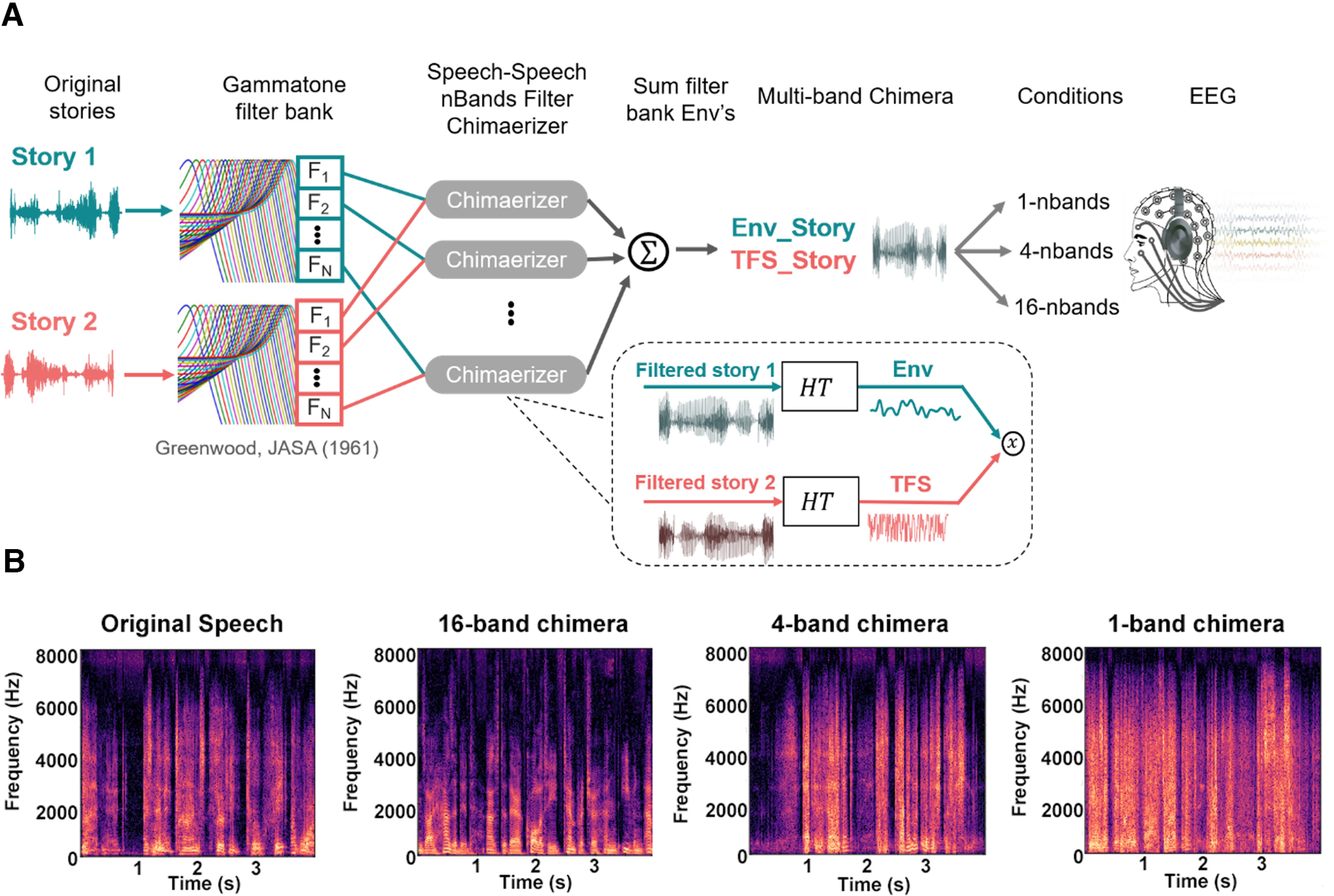
Speech-speech chimera generation. A, Two audiobooks were passed through Gammatone filter banks with different numbers of frequency bands (1, 4, 16) for our different experimental conditions. The outputs of these filterbanks were Hilbert transformed (HT) allowing us to calculate the envelope of story 1 and a temporal fine structure representation of story 2. The envelope of story 1 was used to modulate the temporal fine structure of story 2 within each frequency band, and the resulting partial chimeras were summed to produce a final multiband chimera that was played to the subject. B, Spectrogram of an example segment of story 1 (left) and of the three types of chimera corresponding to that segment.
For our experiment, we generated auditory chimeras from two speech sources. These were audiobooks of two classic works of fiction [Env_Story and TFS_Story], which were read in English by two different male speakers and sampled at 48 kHz. Our study consisted of three experimental conditions each involving the presentation of a speech–speech chimera generated using a filter bank with a different number of frequency bands, specifically 1, 4, and 16. These filter banks were composed of finite impulse response bandpass filters that were spectrally divided into logarithmically spaced filters along the cochlear frequency map from 80 to 8020 Hz (Greenwood, 1990). Each filter had a nearly rectangular response, and adjacent filters overlapped by 25% of the bandwidth of the narrower filter. All stimulus processing was performed using MATLAB (release 2016b) software (MathWorks). And stimuli were presented to subjects using the Psychophysics toolbox (Brainard and Vision, 1997) within the MATLAB environment along with custom code.
Forty-five separate 1 min speech segments were randomly selected from each audiobook and were used to generate the three types of chimera (1, 4, and 16 bands), resulting in 15 trials per chimeric condition. All the chimera stimuli were made by using the envelope of the Env_Story segment to modulate the TFS of the TFS_Story segments. We chose to always use one audiobook as the Env_Story and the other audiobook as the TFS_Story. This was mostly driven by a desire to not divide our EEG data into an extra set of subconditions. We also wanted to be consistent in the behavioral task in having subjects always answering questions on one story for the envelope and on the other story for TFS. Importantly, we used these same two audiobooks in previous studies (Power et al., 2012; O'Sullivan et al., 2015) and saw no systematic difference in the strength of neural tracking to each story. As such, we think it is unlikely that we have introduced any bias into our results by choosing not to counterbalance the audiobooks across the Env and TFS conditions. All stimuli were spatialized by convolution with a head-related transfer function, simulating a scenario in which each stimulus appears to be spatially located directly in front of the subject (Algazi et al., 2001). Incidentally, we selected an additional 10 segments from Env_Story and five segments from TFS_Story, and we presented these, unmodified, to the subjects as a control condition. Ultimately, however, we did not include any analysis of these control data in our results below.
Subjects were instructed to attend to the audio stimulus and maintain visual fixation for the duration of each trial on a crosshair centered on the screen and to minimize eye blinking and all other motor activities. To quantify speech intelligibility, after each trial, subjects were required to answer four multiple-choice questions (MCQs) on both stories (i.e., four from the Env_Story and four from the TFS_Story). Each question had four possible answers. MCQs, answer choices and chimera condition order were all pseudorandomized across subjects. Stimulus presentation and data recording took place in an audiometric grade sound attenuated and electromagnetically shielded room (120a Series, IAC Acoustics). The visual stimuli (crosshair, MCQs, and answer choices) were presented on a 24 inch LCD monitor (ASUS Predator), operating at a refresh rate of 60 Hz, and participants were seated at a distance of 70 cm from the display. All audio stimuli were normalized to have the same root mean square intensity and were presented binaurally through Sennheiser HD650 headphones at a self-adjusted comfortable level.
EEG acquisition and preprocessing
EEG was recorded from 130 channels at 512 Hz using a BioSemi ActiveTwo system. One hundred twenty-eight cephalic electrodes were positioned according to the BioSemi Equiradial system, with another two electrodes located over the left and right mastoids. Triggers indicating the start of each trial were presented using Psychophysics toolbox in MATLAB for synchronous recording along with the EEG.
The EEG data were first resampled to 128 Hz using the decimate function in MATLAB. The decimate function incorporates an eighth-order low-pass Chebyshev type I infinite impulse response anti-aliasing filter. Consistent with previous research suggesting that speech tracking is strongest in the delta band (1–4 Hz) and theta band (4–8 Hz), we focused our analysis on frequencies below 8 Hz. Specifically, we used a zero phase-shift Chebyshev type 2 bandpass filter with pass bands between 1 and 8 Hz. Subsequent preprocessing was performed using the FieldTrip toolbox (Oostenveld et al., 2011) and custom code in MATLAB. After filtering, bad channels were defined as those whose variance was either less than half or greater than twice that of the surrounding three to seven channels (depending on location in the montage). These channels were then replaced through spherical spline interpolation (FieldTrip). Next, after removing bad channels, we applied denoising using independent component analysis, usually only removing one or two components reflecting eye-movement-related artifacts, which were determined following definitions provided in (Debener et al., 2010).
Indexing cortical speech tracking using the temporal response function
The goal of this study was to examine how cortical activity tracks the envelope of speech and how that tracking might derive from acoustic versus speech-specific processing. To index cortical speech tracking, we used the temporal response function (TRF) framework (Crosse et al., 2016b). The general idea of this framework is to use linear regression to map between ongoing speech features (e.g., the envelope) and ongoing neural responses. This can be done either in the forward direction by examining how well different speech features can explain variance in EEG responses on individual channels (a forward encoding model approach). Or it can be done in the backward direction by attempting to reconstruct an estimate of a speech feature using all the EEG channels (a decoding approach). It provides a number of dependent measures, that is, the accuracy of EEG predictions based on a forward encoding model, the accuracy of stimulus reconstructions based on a backward decoding model, or the weights that are applied to the stimulus features (forward) or EEG data (backward) on different channels and at different time lags between stimulus and response (Crosse et al., 2016b). In the forward direction, the TRF can be described via the following equation:
where is the neural response at time point t on channel n, s(t) is the stimulus feature at time t, which can be a univariate (e.g., the envelope) or multivariate (e.g., the spectrogram) representation of the speech, τ indexes the relative time lag between the speech stimulus feature and the neural response in samples, and is an error term. In our analysis, t runs from 1 to the length of the trial (i.e., 60 s), 128 channels, and τ = –100–500 ms, indicating that we are exploring the impact of the stimulus on the EEG data at lags from –100 ms to +500 ms. We then estimated the unknown TRF, using regularized (ridge) linear regression (Crosse et al., 2016b). As mentioned above, this enabled us to use the weights of the TRF itself () as dependent measures and to test how well different speech features were represented in the EEG by seeing how well the TRF can predict EEG responses to held-out trials (Crosse et al., 2016b). In cases where the speech representation is multivariate, the resulting TRF is often known as a multivariate TRF (mTRF). We also conducted a backward decoding analysis described by the following equation:
where the main difference is that the TRF, , is a multivariate function (often known as a decoder) that was fit on all EEG channels at the same time. In general, this approach is more sensitive as it makes more effective use of the available data, but it is also limited in terms of what speech features, can be reconstructed. In our case, we restricted ourselves to decoding based on the univariate amplitude envelope of the speech stimuli (see below, Speech stimulus representations). Again, the weights of the decoder and the ability of the decoder to reconstruct the stimulus were then available to us as dependent measures.
Speech stimulus representations
To use the TRF framework to examine how cortical activity tracks the amplitude envelope of our speech chimeras, we first needed to calculate the amplitude envelope of our stimuli. Moreover, we were specifically interested in how this cortical tracking might reflect contributions from acoustic and speech-specific processing. To do this, we also wished to use the TRF to explore how cortical activity reflects the processing of other acoustic and speech-specific features of our stimuli. Importantly, for other acoustic and speech-specific features to show up in measures of envelope tracking, their temporal dynamics would need to correlate with those of the envelope. This is true for the spectrogram and phonemes of speech (and less true for higher-level representations of speech based on semantic content; Broderick et al., 2018). As such, to assess how contributions from spectrotemporal and phoneme processing might contribute to envelope tracking, we derived the following acoustic and phonemic feature representations of our speech stimuli:
The Envelope
We calculated the amplitude envelope (Env) for each of the 45 one-min speech segments from both Env_Story and TFS_Story. We did this by first bandpass filtering the segments into 128 logarithmically spaced frequency bands spanning 80 and 8000 Hz using a cochlear filter bank (Greenwood, 1990). The envelope for each band was computed using the Hilbert transform, then the broadband envelope was obtained by averaging over the 128 narrowband envelopes. The output of this process was then logarithmically transformed in an effort to account for the nonlinear relationship between electrophysiological responses and stimulus amplitude (Aiken and Picton, 2008). Specifically, dB envelope representations were generated by taking 20log10 of the broadband envelope (Aiken and Picton, 2008).
Recovered envelope
One important issue with modeling our EEG data as a function of the envelopes of the original stories used in creating the chimeras is that the subjects were not actually presented with these original stories. So, when considering how our EEG might track the envelope of the stories in a chimera, we wanted to understand how the cortex might be able to recover the envelope of the TFS story (TFS_Story) from the output of the cochlea. To estimate this recovered envelope (RE-Env), we did the following. We first determined the TFS of the chimera stimuli for all three chimera conditions (1, 4, and 16 bands) by calculating the Hilbert transform of those chimera stimuli and then determining the cosine of the phase of the resulting analytic signal. We then filtered the TFS signal into 128 bands using a cochlear frequency map spanning a range of 80 to 8020 Hz (Greenwood, 1990), and we created analytical signals for each frequency band. Finally, the envelope of each of these narrow bands (calculated as the absolute values of its analytical signal) was summed to generate the broadband RE-Env (Smith et al., 2002; Zeng et al., 2004).
Spectrogram
The envelope is a very impoverished measure of a speech signal. To more fully explore the relationship between envelope tracking and the acoustic processing of speech, we sought to more richly represent the speech acoustics. We did this by computing the Log-Mel spectrogram of our speech stimuli (Chi et al., 2005; Schädler et al., 2012; Verhulst et al., 2018). This involved passing the speech signals through a bank of 64 filters that spanned from 124.1 to 7284.1 Hz and were organized according to the Mel scale (i.e., the scale of pitches judged by listeners to be equal in distance one from another). The output of these filters was then scaled by a logarithmic compressive nonlinearity to convert it to the final Log-Mel spectrogram (Sgram). The choice of the Log-Mel spectrogram was made because it incorporates several properties of the auditory system, nonlinear frequency scaling, and compression of amplitude values.
Phonemes
We were also interested to explore how envelope tracking might relate to speech-specific processing in the form of a sensitivity to phonemes (Ph) within the speech. To derive a representation of phonemes, we used the Montreal Forced Aligner (McAuliffe et al., 2017), a Python-based open source tool based on the Kaldi ASR toolkit that relies on triphone-based hidden Markov models to create statistical models associating phonetic symbols to speech signals (http://kaldi-asr.org/). The aligner, given an audio speech file and corresponding textual orthographical transcription, partitions each word into phonemes from the American English International Phonetic Alphabet (IPA) and performs forced-alignment (Yuan and Liberman, 2008), returning the starting and ending time points for each phoneme. This information was then converted into a multivariate time series composed of indicator variables, which are binary arrays (one for each phoneme). These are active for the time points in which phonemes occurred. The phonemes are mutually exclusive, so only one can be active at each sample point. We selected a subset of the IPA comprising the 35 most frequent phonemes in the presented speech stimuli (3 of 38 IPA phonemes were excluded as being outliers in terms of how rare they were). Ph is a language dependent representation of speech.
Using the mTRF to assess the sensitivity of EEG to different speech features
We use the TRF framework to assess how well different speech features are represented in the EEG data (see below, Results). As discussed above, two of our dependent measures are (1) how well we can reconstruct a speech envelope from the EEG responses (i.e., backward modeling) and (2) how well we can predict data on different EEG channels using the stimulus spectrogram and phonemes (i.e., forward modeling). Two key related considerations for using these dependent measures are (1) how to train and test the mTRF models to trust in the generalizability of the findings, and (2) how to regularize the mTRF models to make them robust to EEG fluctuations that are unrelated to the speech, as well as to noise. In terms of the latter issue, we used ridge regression. In brief, this approach penalizes large values in the mTRF, meaning that we are reducing the variance of our mTRF weights by adding a bias term. Ultimately, this makes for a more generalizable model (Crosse et al., 2016b). However, care must be taken not to over-regularize. In what follows, we describe how we determined the ridge regression parameters (known as λ values) that allowed us to best map between speech features and EEG.
Our general strategy for training, regularizing, and testing was to use the following cross-validation procedure. For each of our four stimulus representations, a separate TRF was fit to each of M trials for a range of λ values. One trial was left out to be used as a test set, with the remaining M-1 trials to be used for the inner cross-validation. Next, one of these inner M-1 trials was chosen to be left out and used as a validation set. The remaining M-2 trials were used as a training set. An average model was obtained by averaging over the single-trial models in the training set. This was done for each λ value. Next, this average model was used to either reconstruct the speech envelope by convolving it with the EEG data (decoding) or predict the EEG responses by convolving it with the chosen speech representations (forward modeling) associated with the validation set. The accuracy of this reconstruction or prediction (of selected EEG channels; see below) was assessed by comparing it with the real speech envelope or EEG using Pearson's correlation coefficient. This procedure was repeated so that each of the M-1 trials was left out of the training set once. The λ value that produced the highest average reconstruction or prediction accuracy across all the validation sets was then chosen as the optimal λ. Please note, this could mean different optimal values of λ for reconstruction, prediction, each subject, and each set of speech features.
Next, using the optimal λ value chosen above, another average model was obtained by averaging over the single-trial models in both the validation and training sets and using that model on the data from the test set. Model performance was assessed by quantifying how accurately the reconstructed envelope or predicted EEG correlated with the actual stimulus envelope or the actual recorded EEG response from the test set, again using Pearson's r. This entire procedure was repeated M times so that each trial was left out of the inner-cross validation procedure once. As before, the overall model performance was then finally assessed by averaging the overall individual model performances for each trial. Again, λ parameter optimization was done separately for each stimulus representation and subject (i.e., each model was based on its respective optimal performance). Our modeling procedures were subjected to permutation testing to quantify a null distribution; 95% quartiles, demarcated by gray boxes, are reported in the figures.
To evaluate whether either phonetic or spectral acoustic features contributed independently to predicting the neural responses across conditions, we computed the partial correlation coefficients (Pearson's r) between the EEG predicted by either phonological (or spectral acoustics) feature model with the actual recorded EEG after controlling for the effects of spectral acoustics (or phonological features). Specifically, we fitted separate cross-validated forward model TRFs on each of the two speech representations (Sgram, Ph) and predicted EEG based on those models. Then we used the built-in MATLAB function partialcorr (X, Y, Z), where X = actual recorded EEG, Y = predicted EEG in response to the feature of interest (the feature whose unique contribution is to be identified), and Z = concatenated predicted EEGs in response to the other feature (which is to be partialled out). This function computes the partial correlation coefficients between X and Y, while controlling for the variables in Z (Fisher, 1924).
Statistics
Significance at the group level of either decoder or encoder accuracies was evaluated using nonparametric permutation statistics. The neural responses were permuted across trials so that they were matched to features from a different trial, and the same leave-one-out cross-validation procedure as described above was performed to compute TRFs and either reconstruction or prediction accuracies. This was done 2000 times for each subject to establish a distribution of chance-level prediction accuracies. By randomizing the data across trials and recalculating the test statistic, we obtained a reference distribution to evaluate the statistic of the actual data. We also ran randomization using cluster-based statistics (Monte Carlo procedure) across channels and time for topographical demarcation of significant time-sensor clusters to be used as regions of interest for further analyses (Maris and Oostenveld, 2007). Furthermore, we adopted the same procedures for the partial correlation analyses, based on the prediction accuracies (phonetic/spectral acoustic encoders). We also computed a distribution of partial correlations for the phonetic (or spectral acoustics) features measures (i.e., we partialled out the contributions of all other features). Unless otherwise stated, further analyses were done on significant channels following permutation tests or computed on 12 temporal parietal channels (six symmetric pairs over left and right hemispheres).
Linear mixed-effects models (LME) were implemented to explore behavioral and EEG results and their interrelationship, via the fitlme function in MATLAB using the restricted maximum likelihood method. Advantages over standard ANOVA approaches have been previously reported (Krueger and Tian, 2004; Wainwright et al., 2007; Luke, 2017). After visual inspection of the residual plots, it was clear there were no obvious deviations from homoscedasticity or normality. All p values were estimated using the Satterthwaite approximations. Post hoc analyses were performed using linear hypothesis testing on linear regression model coefficients (coeftest). Mixed-effects models account for multiple comparisons. Subjects were treated as random factors according to the following linear-model expression: () and (), where RA stands for reconstruction accuracy and Beh corresponds to behavioral task performance.
As well as using frequentist probability-based statistics, we also used the Bayesian analog of an ANOVA (anovanBF) to allow us to explicitly determine the amount of evidence in favor of the null hypothesis ( no interaction). We estimated the Bayes factors ) using MATLAB code adapted from R Studio (https://www.rstudio.com) and the function anovanBF in the toolbox Bayes factor (https://cran.r-project.org/web/packages/BayesFactor/index.html). We adopted the commonly used Jeffrey–Zellner–Siow prior with a scaling factor of 0.707 (Rouder et al., 2009, 2012; Schönbrodt et al., 2017). Monte Carlo resampling with iterations was used for the estimation. Subjects represented the random factor. Importantly, this estimation allows us to quantify evidence that our experimental factors and interactions explain variance in the data above the random between-subject variations. Standard convention stipulates that any exceeding three is evidence in favor of the alternative hypothesis (), whereas below 0.33 is in support of the null hypothesis (), and ranging between one and three is taken as weak anecdotal evidence in favor of the alternative hypothesis, as is widely reported in the literature (Wagenmakers et al., 2011).
Results
One-hundred-twenty-eight-channel EEG was recorded from 17 subjects as they listened to 60 s auditory chimeras composed of segments of narrative speech from two audiobooks. In particular, the envelope of Env_Story was used to modulate the temporal fine structure of TFS_Story. By varying the number of frequency bands used in making the chimeras (1, 4, or 16 bands), we aimed to manipulate which story was understood by the subjects. Following Smith et al. (2002), we expected subjects to (partially) recognize TFS_Story for the 1-band chimeras, to (partially) recognize Env_Story for the 4-band chimeras, and to clearly recognize Env_Story for the 16-band chimeras.
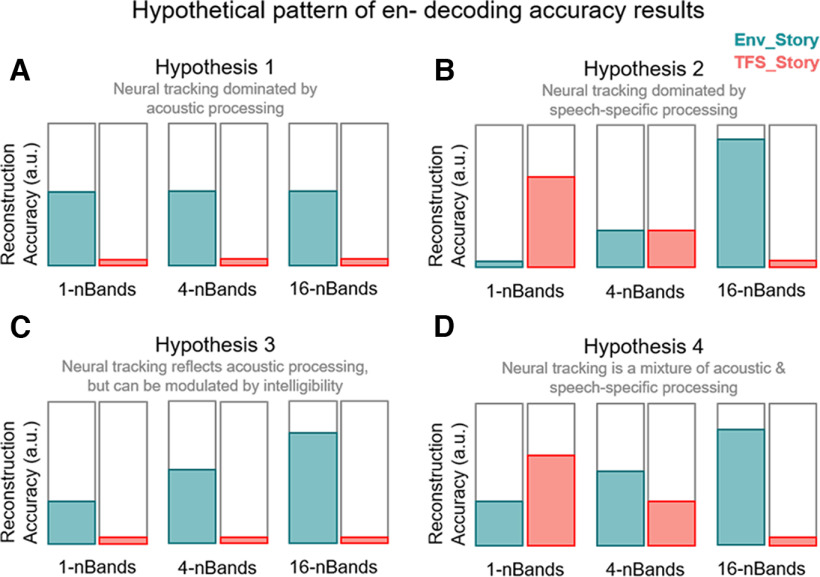
With such a pattern of behavioral results, what might we expect in terms of variation in how cortical signals track the envelopes of the Env_Story and the TFS_Story? Based on previous studies, several plausible alternative hypotheses can be proposed as to how such signals might vary across our chimera conditions. We briefly suggest four alternative hypotheses that could be plausibly advanced based on previous research.
Hypothesis 1 (Fig. 2A) is that neural tracking of speech is overwhelmingly dominated by acoustic processing. Were this true, one might expect the acoustic energy fluctuations (i.e., the envelope) to play such a dominant role that neural tracking would reflect the processing of only the Env_Story across chimera conditions. An example of a previous study that might motivate a hypothesis like this is Howard and Poeppel (2010), which reported that discrimination of speech stimuli based on neuronal response phase patterns depends on acoustics but not comprehension. Hypothesis 2 (Fig. 2B) is that neural tracking of speech is dominated by speech-specific processing. Were this hypothesis true, one might expect neural tracking to very closely mirror speech intelligibility, with no tracking for the unintelligible stimulus despite its acoustic energy fluctuations (e.g., no tracking of the Env_Story in the 1-band condition). This would be a reasonable hypothesis based on a series of studies by Zoefel and VanRullen (2015, 2016), who explored speech entrainment using novel speech/noise speech stimuli that were constructed to have no systematic changes in sound amplitude and spectral content. It might also be a reasonable hypothesis coming from studies that have focused on how speech-specific acoustic edges might be driving neural entrainment to that speech (Doelling et al., 2014). Hypothesis 3 (Fig. 2C) is that neural tracking of speech is dominated by general acoustic processing that can be enhanced by intelligibility through either predictive processing related to the speech content (Broderick et al., 2018), by attention (O'Sullivan et al., 2015), or higher order statistical structure in the acoustics (Zuk et al., 2020). Were this hypothesis true, one might expect that neural tracking would again be dominated by the acoustic energy of the Env_Story, but the tracking of Env_Story would increase across chimera conditions. One would expect no discernible tracking of the TFS_Story in this case. Finally, hypothesis 4 (Fig. 2D) is that neural tracking of speech contains contributions from low-level neural populations that are responsive to general acoustic input and from higher-level neural populations that are specifically tuned to speech sounds. Were this hypothesis true, one would expect the Env_Story to contribute to all chimera conditions, but more so in chimera conditions where the Env_Story is intelligible. And one would expect the TFS_Story to contribute only in those conditions where the TFS_Story is intelligible. This is the hypothesis we favor based on the previous literature (and a synthesis thereof). If the data support this hypothesis, it will also be of interest to see the relative size of the contributions to the EEG that appear to derive from general acoustic processing versus speech-specific processing.
Figure 2.
Hypothetical pattern of results. A, Decoding accuracy is mainly driven by the speech envelope and is not modulated as a function of speech intelligibility. TFS is poorly decoded as there is no envelope feature. B, Speech is the dominant factor here, neural tracking is speech specific and is related to the parsing of the structure of higher-order speech features. In this instance, it would be predicted that there is stronger tracking for the most intelligible conditions, that is, 1 band for the TFS_Story and 16 bands for the Env_Story. The lack of tracking of an unintelligible stimulus component that varies in its acoustic energy could be explained by comodulation masking release, wherein coherent fluctuations in a masker can improve signal detection by suppressing that masker (Dau et al., 2009). C, The hypothesis that obligatory sensory tracking of the acoustics of speech stimulus is enhanced by speech intelligibility. Tracking is dominated by the speech envelope as shown in the Env_Story, whereas there is little to no cortical tracking of the TFS_Story. D, Neural tracking is a mixture of both general acoustic processing and speech-specific processing. In this case, we would expect to see tracking of the Env_Story across all conditions, with stronger tracking as that story becomes more intelligible. We would also expect to see some tracking of the TFS_Story in conditions where that story is intelligible. How strongly the tracking of the Env_Story will be relative to the TFS_Story is an open question.
Speech comprehension varies across speech–speech chimera conditions
Speech comprehension was assessed by asking multiple-choice questions at the end of each trial. There were four possible answers to each question, setting the theoretical chance level at 25%. However, we determined that, at the group level, a score of 27.5% was significantly greater than chance () based on a binomial distribution using all 840 trials (14 subjects × 60 questions) per condition. We then tested our distribution of subject scores against 27.5% using a one-sided Wilcoxon signed rank test. The pattern of behavior was largely consistent with what we expected based on Smith et al. (2002; Fig. 3A). In particular, performance on the questions for Env_Story in the 1-band condition was 29.43% ± 2.11 (percentage mean ± SEM) and was not significantly greater than chance (p = 0.28). However, as the number of chimera bands increased, so did performance with subjects achieving 33.43% ± 1.57 and 68.43% ± 2.09 for the 4-band and 16-band conditions, respectively, both of which were significantly greater than chance and Wilcoxon signed rank test). Meanwhile, the performance on TFS_Story showed largely the opposite effect. Performance on the questions were 37.86% ± 1.41, 33.71% ± 1.16, and 22.79% ± 1.73 for the 1-band, 4-band, and 16-band conditions, respectively. Importantly, these scores were significantly above chance for the 1-band () and 4-band conditions (). However, as expected, they were not significantly above chance for the 16-band condition ). We wanted to more explicitly test for the interaction effect between chimera condition and story that we expected from Smith et al. (2002). To do this we used a two-way repeated-measures ANOVA (rmANOVA; with factors of chimera condition, 1-, 4-, 16-band; and story, ENV Env_Story, TFS TFS_Story). This analysis showed main effects of chimera condition ( and story (. Importantly, it also revealed a significant interaction between chimera condition and story (. Post hoc analyses were conducted using Bonferroni-corrected pairwise t tests, which showed that Env_Story intelligibility scores in the 16-band condition were significantly higher than those in the 1-band condition (, and TFS_Story scores in the 1 band were significantly higher than 16 band (. This pattern of results generally replicates the findings from Smith et al. (2002), although we assessed speech intelligibility by means of comprehension questions, whereas Smith et al. (2002) probed word recall.
Figure 3.
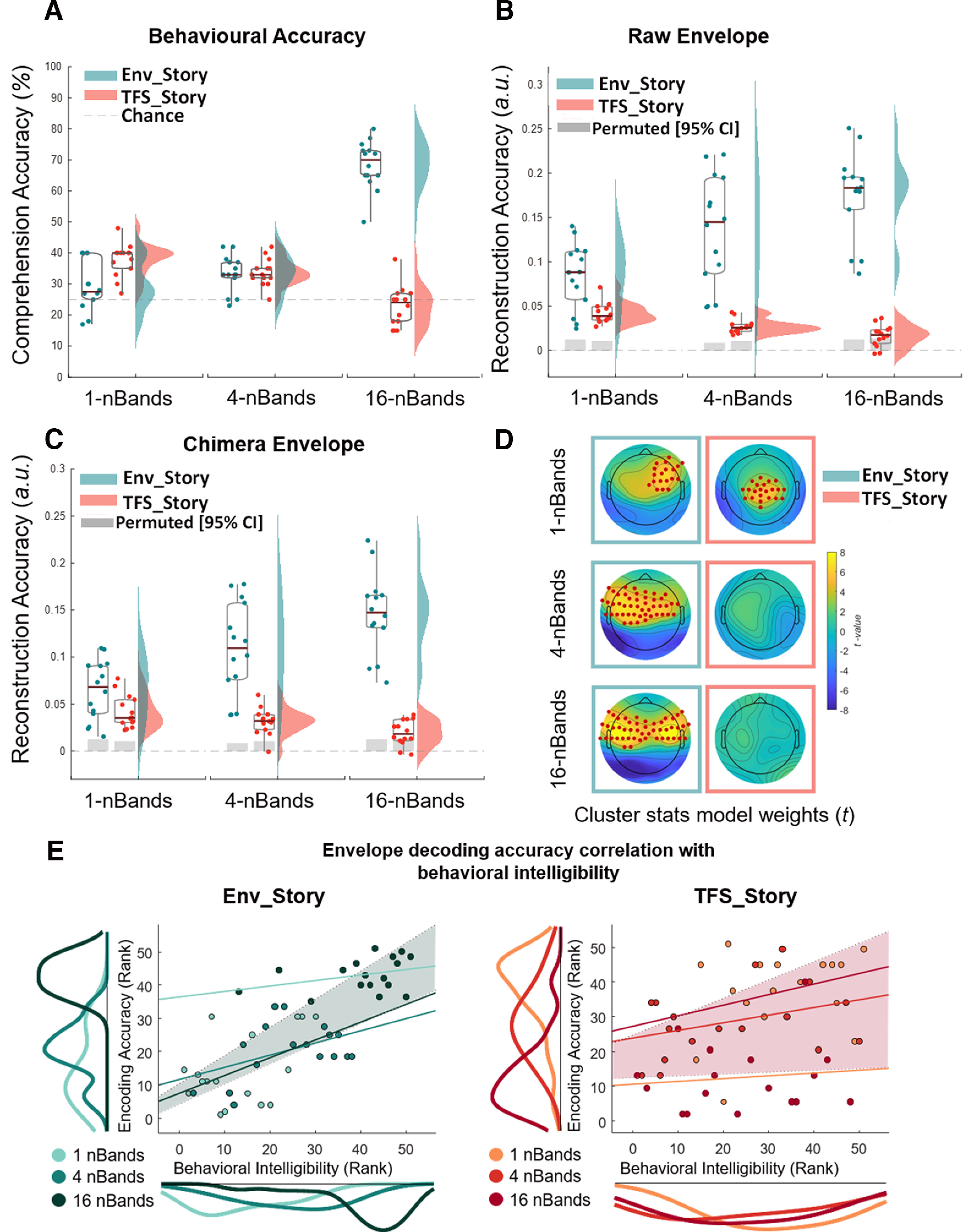
Cortical tracking of speech envelopes reflects both acoustics and speech intelligibility across chimera conditions. A–C, Box plots (mean ± SEM) and rain kernel density estimates of speech intelligibility and reconstruction decoding accuracy (Rho) values for Env_Story (turquoise) and TFS_Story (peach). Group and individual statistics; black line in box plot indicates mean across subjects, and gray box demarcates single-subject-level statistical significance above chance (permutation test based on shuffling trial labels 1000 times before reconstructing the envelopes, *p< 0.05). Single dots represent single-subject data. Behavioral intelligibility (A), original envelope reconstruction accuracies (B), and chimera envelope reconstruction accuracies across bands, conditions, and stories (C). D, Topographical plots show forward transformed decoder weights across all channels for Env_Story and TFS_Story over a time-lag window of 80–120 ms. Red dots indicate significant effects at the group level (one-tailed cluster-based permutation test, N = 2000, p< 0.05). E, Left, Env_Story; right. TFS_Story brain-behavior correlations. Individual dots represent subjects and are color coded according to condition. Correlations were assessed using robust Pearson's correlation (bootstrap permutation test p< 0.05). This was done for each individual condition (colored lines), as well as collapsed across all conditions (shaded area representing 95% confidence interval). Subplots show the distribution of the data in terms of both envelope decoding (left) and behavior (below) using the same color code as the subject dots.
Cortical tracking of the original story envelopes varies with understanding of speech–speech chimeras
To adjudicate among the four hypotheses introduced above (Fig. 2), and to test hypothesis 4 in particular, we wished to explore how cortical tracking of speech envelopes might reflect acoustic and speech-specific processing. Importantly, all three of our stimulus conditions (1-, 4-, and 16-band chimeras) have envelopes that are derived from Env_Story. As such, their acoustic energy will be dominated by that of Env_Story. However, as shown in the previous section, for the 1-band condition, subjects are more likely to understand the speech content from TFS_Story. How might envelope tracking reflect these acoustic and behavioral inconsistencies? We investigated this by attempting to reconstruct estimates of the speech envelopes corresponding to Env_Story and TFS_Story from the EEG recorded during each of our chimera conditions. As discussed above, we did this for all 15 trials for each condition using a cross-validation training and testing procedure. Importantly, for this first analysis, we simply sought to reconstruct the envelopes of the original speech segments, not of the chimeras themselves. Then we compared these reconstructions to the two original envelopes using Pearson's correlation. We also determined a baseline level of reconstruction performance by generating a null distribution of Pearson's r values by shuffling the labels between trials 1000 times and attempting to reconstruct the envelopes using the same procedure.
The general pattern of stimulus reconstruction measures mirrored that of the behavioral results and supported hypothesis 4 (Fig. 2D). Specifically, as the number of frequency bands in the chimeras increased, envelope reconstructions for Env_Story tended to increase, and reconstructions for TFS_Story decreased (Fig. 3B). We assessed this pattern of results using a two-way rmANOVA (with factors of chimera condition and story). As with the behavioral results, we found significant main effects for chimera condition ( and story (. And, mirroring our key behavioral result, we found a significant interaction between chimera condition and story (. Again, we conducted post hoc analyses using Bonferroni-corrected pairwise t tests. These also tracked with the behavioral results in that reconstructions for Env_Story were significantly higher for the 16-band condition than the 1-band condition (, and TFS_Story reconstructions were significantly higher for the 1-band condition than the 16-band condition (.
One important discrepancy between the behavioral and cortical tracking results is that there is robust cortical tracking of Env_Story for the 1-band condition (Fig. 3B, left, blue cloud), despite it being entirely unintelligible (Fig. 3A, left, blue cloud). Indeed, the cortical tracking of Env_Story in this condition is significantly stronger than that for TFS_Story (, despite TFS_Story being the one that is better understood. Of course, this is easily explained by reminding ourselves that the envelope of the chimera in this condition is determined by Env_Story. This highlights that fluctuations in the acoustic energy of an auditory stimulus will robustly drive cortical tracking, completely independently of any speech-specific activity. That said, the similar pattern of interaction effects for behavior and envelope tracking also supports the notion that cortical envelope tracking does, at least to some extent, index speech-specific processing. Env_Story tracking improved across conditions as Env_Story understanding increased, and TFS_Story tracking decreased as TFS_Story understanding fell. On this issue, it is particularly interesting to consider the case of the cortical tracking of TFS_Story in the 1-band condition. Again, the envelope of the chimera stimulus in this condition is dominated by Env_Story, and, yet, we have significant cortical tracking of the envelope of TFS_Story. How might this come about? We examine this further in the next section, where we discuss the results of Figure 3C.
Before that, to visualize which EEG channels were contributing to the pattern of envelope reconstructions reported above, we transformed the backward decoding model weights (Haufe et al., 2014) into a pattern of forward model weights (Fig. 3D). Visualizing these weights across the scalp is more analogous to exploring the topographical distribution of event-related potentials (Lalor et al., 2009). Using a cluster-based nonparametric permutation (N = 2000) analysis (Maris and Oostenveld, 2007) we identified model weights that were significantly different from zero across subjects and trials over a window of 80–120 ms time lags. We chose these time lags based on a prominent peak in the forward TRF model over these time lags (see below). For Env_Story there was an increase in the number of channels with significant model weights as intelligibility increased across conditions. These channels were largely over central and temporal scalp. For TFS_Story, significant model weights were found only for the 1-band condition, that is, the condition for which TFS_Story was most intelligible. They were centered over central scalp regions.
We also sought to explore the correspondence in the pattern of results between behavior and stimulus reconstruction more directly (Fig. 3E,F). Specifically, we used robust correlation analysis with bootstrap resampling (Pernet et al., 2013) to check for correlations between these measures across subjects. We did so in one analysis that included all conditions and found a strong positive correlation between decoding accuracy and behavioral intelligibility scores as a function of chimera condition for Env_Story and TFS_Story . We tested this relationship further using a linear mixed effect model with subjects as a random factor () with comprehension accuracy scores and envelope reconstruction values as dependent and predictor variables, respectively. The LME models variability because of stimulus conditions and subjects simultaneously. We found a significant positive relationship between reconstruction accuracy and comprehension scores for Env_Story ( and for TFS_Story (
An important aspect of the previous result is that as mentioned it was conducted across all stimulus conditions collapsed together. We also wished to explore the possibility that there might be a correlation between behavior and reconstruction accuracy within each condition across subjects. That said, we were not especially confident about finding such a result. This is because EEG envelope tracking measures can vary greatly between subjects based on factors that are completely unrelated to their ability to understand speech. For example, basic biophysical differences in cortical folding and brain/skull conductivity properties likely drive a very large percentage of the variance in EEG responses across subjects. With n = 17 in the present study, we expected such causes of variance might obscure any variance related to speech intelligibility across subjects. Indeed, using permutation testing while controlling for multiple comparisons across conditions, we found that there were no significant correlations within any one condition alone, for either the Env_Story , for 1 band, 4 band, and 16 band, respectively) or the TFS_Story , for 1 band, 4 band, and 16 band, respectively).
Cortical tracking of envelopes recovered from the chimeras also varies with understanding of speech–speech chimeras
In the previous section, we wondered about how we might see significant cortical tracking of the TFS_Story envelope to a 1-band chimera whose envelope is determined by Env_Story (Fig. 3B). The answer likely lies in the fact that each subject's auditory cortex does not actually operate on the chimera stimuli that we present. Rather, each stimulus first undergoes extensive peripheral and subcortical processing. Indeed, the first stage of this processing is the fine-grained filtering by the cochlea. This produces a representation in the early auditory system that will be exquisitely sensitive to the temporal fine structure in the stimulus. It may be that some of the computations conducted by the cortex serve to synthesize a coherent TFS_Story speech object from these earlier representations, a process sometimes referred to as analysis by synthesis (Halle and Stevens, 1959, 1962; Bever and Poeppel, 2010; Poeppel and Monahan, 2011; Ding and Simon, 2013). This synthesis of an auditory object for TFS_Story from the temporal fine structure of the chimera is likely what our cortical envelope tracking measure is indexing in this case.
To explore this more directly, we conducted an additional analysis aimed at examining how cortical activity might track with the envelopes of Env_Story and TFS_Story that we recovered from the actual chimera stimuli that were presented to the subjects. We determined the envelope of the chimera by passing it through a filter bank, determining the Hilbert transform of each narrowband frequency, and averaging the envelopes across frequency bands (see above, Materials and Methods). Next, we computed the ReEnv of the TFS component from the chimeras using an approach based on the Hilbert transform and cochlear scaled filtering (Patterson, 1987; Irino and Patterson, 1997; Patterson et al., 2002; Apoux et al., 2011). The overall pattern of envelope reconstruction results for the chimera envelopes (which should be very similar to those for Env_Story) and Re-Env (which we expected to resemble the envelopes for TFS_Story) was very similar to that for the original envelopes (Fig. 3C). Specifically, a two-way rmANOVA (with factors chimera condition and story) showed main effects for chimera condition ( and story (. And there was a significant two-way interaction between chimera condition and story (. Post hoc Bonferroni-corrected t tests showed that Env_Story reconstruction accuracies for the 16-band condition were significantly higher than those for the 1-band condition (, and TFS_Story reconstruction accuracies for the 1-band condition were significantly higher than that for the 16-band condition (
For Env_Story, the fact that the pattern of stimulus reconstruction results was so similar for the original and recovered envelopes (Fig. 3B,C, compare turquoise rain cloud plots) was entirely unsurprising. This is because the envelopes from the original Env_Story were used to create the chimeras in the first place. As such, the raw stimulus envelope for the Env_Story and the envelope of the chimeras were highly correlated for all conditions (Table 1, second column). For the same reason, the correlation between the raw stimulus envelope for the TFS_Story and the envelope of the chimeras was very low (in fact, it was slightly negatively correlated). Meanwhile, the similar pattern of stimulus reconstruction results for the original and recovered envelopes for TFS_Story (Fig. 3B,C, compare turquoise rain cloud plots) was more interesting. Indeed, it was not obvious in advance that this would be the result, given that the temporal fine structure of TFS_Story was so heavily modulated by the envelope of Env_Story for the chimeras. Moreover, this pattern cannot be because the Re-Env was correlated with the raw TFS_Story envelope because, as mentioned above, if anything, they are weakly negatively correlated with each other (Table 1, third column). As such, it appears that this result supports that notion that cortex has been able to synthesize a coherent representation of the TFS_Story from its (partial) temporal fine structure. However, the fact that the pattern of TFS_Story results appears qualitatively similar for the raw envelope analysis (Fig. 3B) and the Re-Env analysis (Fig. 3C) does not allow us to conclude that the signals being tapped into by the two analyses are actually the same. In other words, it could be that the analysis based on the Re-Env is driven by EEG responses to speech features that are different from those that are driving the raw envelope analysis. To explore this, we directly compared the stimulus reconstructions that were obtained using the raw envelope of the TFS_Story with those obtained using the envelope recovered from the TFS structure of the chimera. There was a significant positive correlation between these reconstructions only for the 1-band condition [, 95% CI (0.16–0.20)]; Table 1, right column). And although this correlation was relatively low, it was substantially higher than the correlations between the reconstructions and the original or reconstructed envelopes shown in Figure 3, B and C. This suggests that the information being recovered by cortex, as indexed by the Re-Env-based reconstruction analysis, is not identical to that being indexed by the raw envelope analysis, but that there is some overlap between the two. Again, it is not surprising that they are not identical given that the temporal fine structure of TFS_Story was so heavily modulated by the envelope of Env_Story for the chimeras. And, again, this is the reason why Env_Story reconstructions based on the raw and chimera envelopes are so highly correlated with each other (Table 1, fourth column). A final observation is the correspondence between the behavior and speech reconstructions for TFS_Story in the 16-band condition. At the group level, behavioral performance was not above chance, indicating a general lack of understanding of TFS_Story in this condition, consistent with previous research (Smith et al., 2002). This was mirrored in the failure to reconstruct the recovered envelope of TFS_Story in this condition (Fig. 3C).
Table 1.
Stimulus and reconstructed envelope correlations
| Stimulus |
TRF reconstruction |
|||
|---|---|---|---|---|
| Env_Story | TFS_Story | Env_Story | TFS_Story | |
| Chimera condition | Raw, chimera | Raw, chimera | Raw, chimera | Raw, chimera |
| 1-Band | 0.91 (0.001) | −0.20 (0.001) | 0.91 (0.001) | 0.18 (0.002) |
| 4-Bands | 0.90 (0.001) | −0.11 (0.001) | 0.97 (0.001) | 0.08 (0.114) |
| 16-Bands | 0.73 (0.001) | −0.10 (0.001) | 0.95 (0.001) | −0.10 (0.019) |
Computed between raw and chimera-derived envelopes for both the original stimulus and TRF reconstructed versions. Statistics reported as Pearson's ρ (p value). Correlations computed using robust correlation analyses with boostrap resampling across subjects (Pernet et al., 2013).
Intelligibility of speech–speech chimeras is reflected more strongly by cortical speech tracking in the delta band than the theta band
A substantial amount of previous research has sought to distinguish the roles of delta and theta band cortical activity in speech processing (Ding and Simon, 2013, 2014; Peelle et al., 2013; Doelling et al., 2014; Etard and Reichenbach, 2019). With that in mind, we sought to explore the sensitivity of delta and theta band speech-tracking measures across our different chimera conditions. We did this by attempting to reconstruct the envelopes of Env_Story and TFS_Story from EEG that had first been filtered into either the delta (0.05–4 Hz) or theta (4-8 Hz) bands (using zero phase-shift Chebyshev type 2 bandpass filters). We found that the pattern of reconstruction accuracies based on delta band EEG (Fig. 4, left) corresponded to that seen for speech intelligibility (Fig. 3A). However, this was much less clear for reconstructions based on theta band activity (Fig. 4, right).
Figure 4.
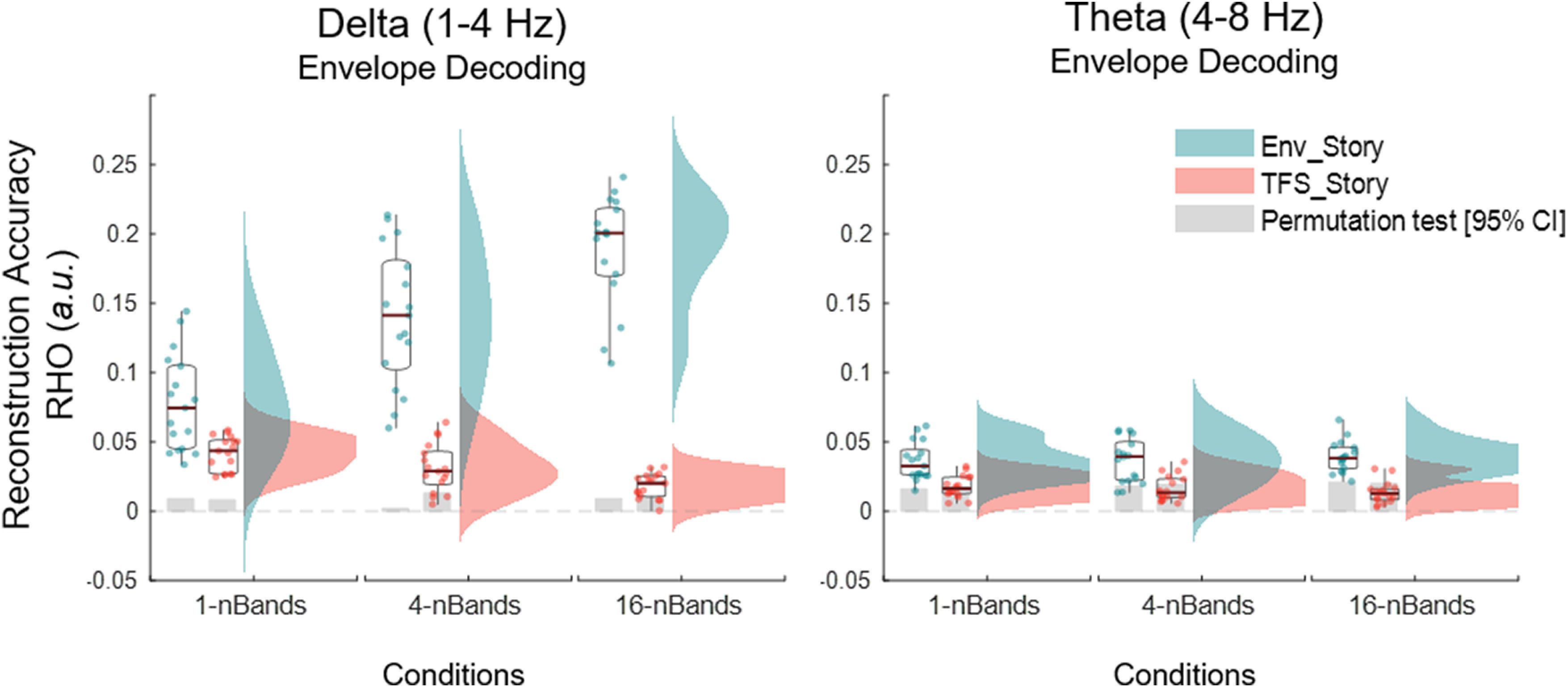
Envelope reconstructions based on delta and theta band EEG. Box plots (mean ± SEM) and rain kernel density estimates of reconstruction decoding accuracy (Rho) values for Env_Story (turquoise) and TFS_Story (peach). Group and individual statistics; black line in box plot indicates mean across subjects, and gray box demarcates single-subject-level statistical significance above chance (permutation test based on shuffling trial labels 1000 times before reconstructing the envelopes, *p< 0.05). Single dots represent single-subject data.
Indeed, for delta band reconstructions, as with both the broadband EEG decoding of the envelopes of the original speech (Fig. 3B) and the chimera speech (Fig. 3C), we found significant main effects for chimera condition ( and story (. And, we again saw a significant two-way interaction between chimera condition and story (. Again, post hoc Bonferroni-corrected pairwise t tests revealed that reconstructions for Env_Story were significantly higher for the 16-band condition than 1-band condition (, and TFS_Story reconstructions were significantly higher for the 1-band condition than the 16-band condition (, as we had found with broadband EEG. In contrast, theta-based reconstructions did not track significantly with speech intelligibility in the chimera stimuli, although there was a main effect for story (, there was no main effect for chimera condition (, and no interaction effect (. Post hoc Bonferroni-corrected t tests showed that Env_Story reconstruction accuracies for the 16-band condition were not significantly different (or higher) than the 1-band condition (. Similarly, there was no significant difference between 1-band and 16-bands conditions in TFS_Story reconstruction accuracies .
Exploring the relationship between the intelligibility of speech–speech chimeras and cortical speech tracking at different latencies
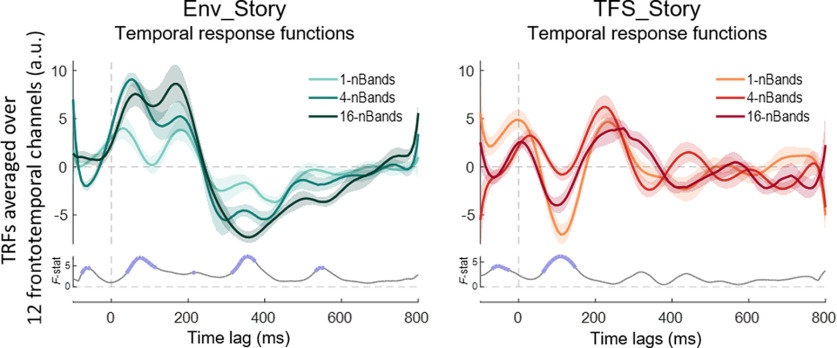
Previous research has suggested that different hierarchical stages of acoustic and linguistic speech processing can be approximately indexed by exploring brain responses to speech at different latencies (Salmelin, 2007). Given that we are interested in disambiguating general acoustic processing from speech-specific processing, we sought to explore the possibility that speech tracking at different time lags might be differentially sensitive to the intelligibility of our various speech chimeras. To do this, we plotted the (forward) TRF by learning a linear mapping from the envelope of our different chimera stimuli to the corresponding EEG responses (Crosse et al., 2016b). If acoustic processing is indexed at shorter latencies and speech-specific processing at longer latencies, we might expect to see strong Env_Story TRF responses at short latencies for all conditions, with variations in longer latency TRF components that mirror our behavioral measures of speech intelligibility. And we might expect to see relatively weak early TRF responses to the TFS_Story with longer latency TRF components again reflecting our speech intelligibility measures.
Figure 5A displays TRFs averaged over 12 frontotemporal channels (six from the left scalp and their symmetrical counterparts on the right), chosen based on previous literature (Di Liberto et al., 2015; Crosse et al., 2016b; Broderick et al., 2018; Teoh et al., 2022). Robust TRFs were visible for most conditions. In general, the TRFs for the Env_Story appeared to increase in amplitude from the 1-band condition to the 16-band condition. However, there was no clear difference in the pattern across conditions for early TRF components compared with later components. Indeed, Monte Carlo cluster-based permutation statistics show main effects of chimera condition at a number of latencies both early and late (Fig. 5. subplot). For the TFS_Story, the 1-band (most intelligible) condition displayed the largest TRF at a latency of ∼100–170 ms. However, this result needs to be treated with caution given that the (somewhat intelligible) 4-band response appeared smaller than the (completely unintelligible) 16-band response.
Figure 5.
TRFs indexing the relationship between the envelope of the different chimera stimuli and the corresponding EEG response. TRFs are averaged over a set of 12 electrodes with high prediction accuracies over frontotemporal scalp (6 on the left side of the scalp, and their symmetrical counterparts on the right), without biasing any of the TRF models. Shaded lines demarcate SEM. Subplots show Monte Carlo cluster-based permutation statistics, main effect of chimera condition (F value), with the thick blue lines indicating significance (p< 0.05).
Modeling speech responses in terms of both acoustics and phonetic features can disambiguate acoustic and speech–specific processing
The envelope reconstruction results above (Fig. 3) highlight one of the key limitations of using envelope tracking as a measure of speech processing. The general correspondence in the patterns of results for behavior and tracking indicate a sensitivity to speech-related processing. However, the robust tracking of speech that is either completely or almost completely unintelligible (i.e., Env_Story in the 1-band and 4-band conditions; Fig. 3B) highlights that much of the cortical tracking is simply driven by acoustic energy, which is consistent with previous work (Lalor et al., 2009; Howard and Poeppel, 2010). In the previous section, we attempted to approximately index EEG activity that is driven by the stimulus acoustics and reflects speech-specific processing by exploring TRF components at different latencies. However, that analysis is still based on a very coarse representation of the stimulus (i.e., its envelope) whose dynamics necessarily correlate with those of many acoustic and linguistic features. And, as such, the results are difficult to interpret.
An alternative approach is to use forward encoding models to explicitly model EEG responses in terms of specific acoustic and linguistic features of the speech stimulus (Di Liberto et al., 2015). Here, we explore this issue with a focus on acoustic and phonemic features whose dynamics likely correlate with those of the envelope. In particular, we aimed to model EEG responses in terms of both the spectrogram and phonemes of speech and to try to identify unique variance in the EEG responses that can be explained by each. In doing so, we aim to disentangle the encoding of acoustic features from speech-specific processing that likely jointly contribute to the envelope reconstruction measures investigated above. Specifically, the two feature spaces are highly correlated, but not perfectly so. And here we wished to isolate the unique contributions of each feature.
To explicitly quantify the unique contributions of acoustic and phonemic processing to the EEG, we computed the partial correlation coefficients (Pearson's r) between the EEG predicted by either the Phoneme (Ph) or Spectrogram (Sgram) model with the actual recorded EEG, after controlling for the effects of the other feature. The unique predictive power of each model is shown in Figure 6 for phonemes and spectrogram across each band condition (shown here for an average across 12 channels, six symmetric pairs on the left and right scalp as previously used in Di Liberto et al., 2015, 2018; although including all 128 channels revealed the same qualitative pattern of results). In particular, we conducted two separate ANOVAs, one focused on Ph predictions (while controlling for Sgram) and one focused on Sgram (while controlling for Ph). Both ANOVAs (rmANOVA) had factors of chimera condition, 1-, 4-, 16-bands, and story, Env_Story, TFS_Story). Both models showed a generally similar pattern to our envelope-based results above, with improvements in prediction with increasing chimera bands for Env_Story and decreases in prediction with increasing chimera bands for TFS_Story (Fig. 3). Indeed, for both models, we found a significant main effect of chimera condition (Ph, ; Sgram, and Story (Ph, ; Sgram, , and a significant interaction of chimera condition and story (Ph, ; Sgram, . Additionally, post hoc Bonferroni-corrected pairwise t tests revealed that Env_Story partial correlation coefficient scores in the 16-band condition were significantly higher than those scores in the 1-band condition for both models (Ph, ; Sgram, , and TFS_Story scores in the 1-band were significantly higher than the 16-bands for both models (Ph, Sgram,
Figure 6.
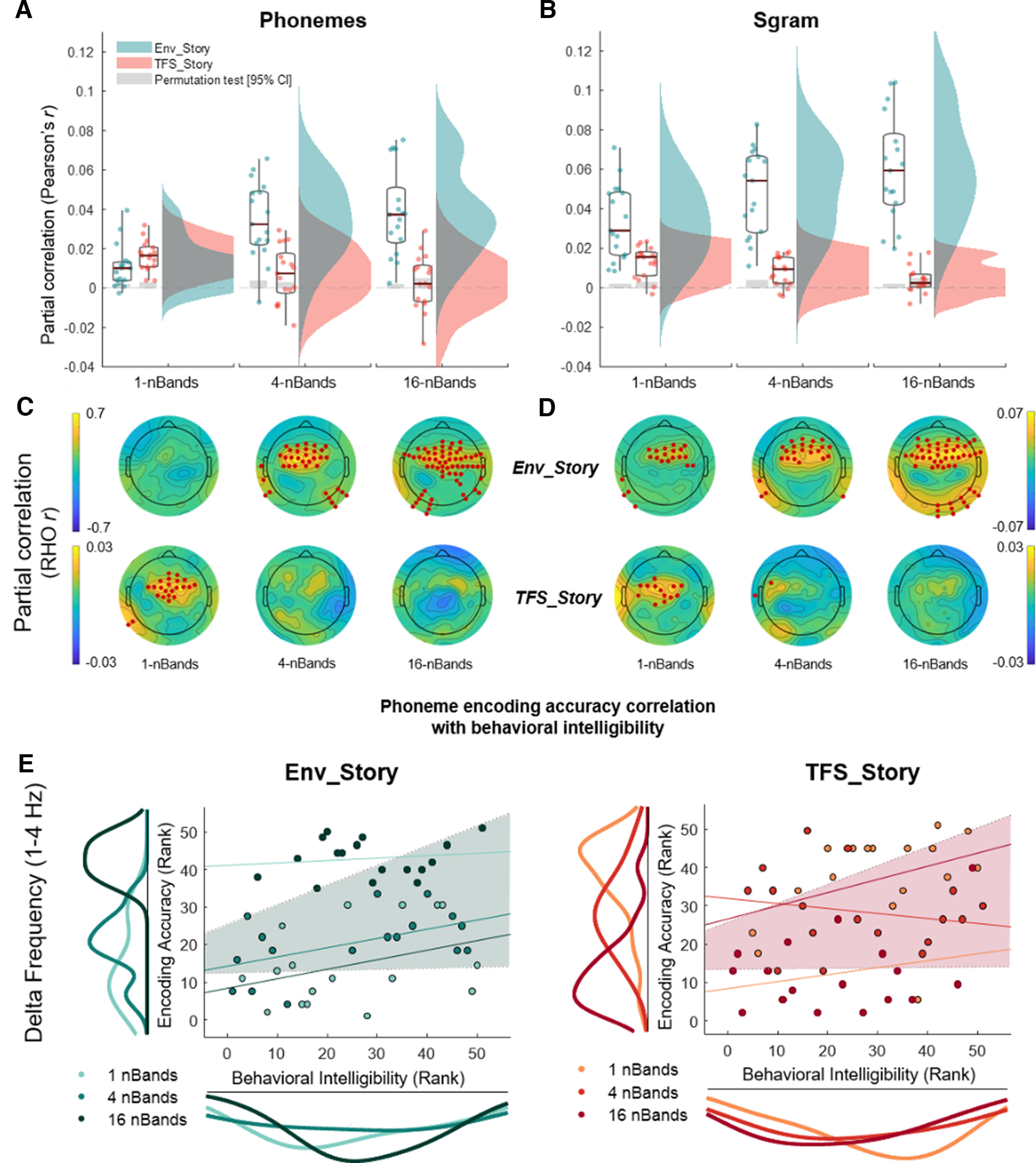
Partial correlations of real EEG and EEG predicted using forward encoding models based on different stimulus feature representations. A, Partial correlation of real EEG and EEG predicted using the phonetic feature representation (Phonemes) while controlling for the acoustic representation (Sgram). Correlations are averaged over the same set of 12 electrodes as in Figure 5, that is, a set with high prediction accuracies over frontotemporal scalp (6 on the left side of the scalp, and their symmetrical counterparts on the right). B, Partial correlation of real EEG and EEG predicted using the Sgram representation while controlling for the Phon representation. Box plots (mean ± SEM) and rain kernel density estimates of partial correlation Rho (Pearson's r, two tailed) values for Env_Story (turquoise) and TFS_Story (peach). Group and individual statistics; black line in box plot indicates mean across subjects, and gray box demarcates single-subject-level statistical significance above chance (permutation test based on shuffling trial labels 1000 times before predicting EEG, *p< 0.05). Single dots represent single-subject data. C, D, Topographical plots show partial correlation Rho across channels for Env_Story (C) and TFS_Story (D). Red dot indicates significant effects at group level statistics (one-tailed cluster-based permutation test, *p< 0.05). E, Phoneme encoding according to accuracy relationship with behavioral intelligibility scores for delta (1–4 Hz) frequency band. Left, Env_Story; right, TFS_Story. Individual dots represent subjects and are color coded according to condition. Using robust Spearman's correlations (bootstrap permutation test p< 0.05, confidence interval 95%). This was done for each individual condition (colored lines), as well as collapsed across all conditions (shaded area representing confidence interval 95%). Subplots show the distribution of the data in terms of both envelope decoding (left) and behavior (below) using the same color code as the subject dots.
Although these data show that both acoustic and phonemic features track with our original envelope findings, there are a couple of notable differences in the performances of the two models. First, in the 1-band condition, the Env_Story is completely unintelligible, so any prediction based on Env_Story must be driven by acoustic processing. This is reflected in the fact that the acoustic Sgram model (Fig. 6B,D) predictions are significantly above zero (). Meanwhile, the unique contribution of the Ph model (Fig. 6A,C), is not significantly above zero for this condition (), which again makes sense given that there is no phonemic information in this chimera for Env_Story as speech was degraded and unintelligible in this band. Conversely, for TFS_Story in the 1-band condition, the Ph model adds unique predictive power (Fig. 6A,C), again, reflecting the fact that this chimera contains phonemic information for TFS_Story. The unique contribution of the Ph model disappears for the 4-band and 16-band conditions, again supporting the idea that it represents a measure of speech-specific processing, separable from more general acoustic processing. The differences between the Ph and Sgram models can also be seen when visualizing which EEG channels are significantly predicted by each model (Fig. 6C,D). Although the performance of the Sgram model is qualitatively similar to that of the envelope, the unique contributions of the Ph model seem to track more specifically with speech intelligibility.
Next, under the assumption that the phonetic feature model performance might more closely relate to speech intelligibility, we assessed the relationship between EEG phonetic feature encoding (while controlling for Sgram) and behavioral intelligibility score (Fig. 6E). In particular, we ran a Spearman's robust correlation analysis with bootstrap resampling (Pernet et al., 2013) and specifically looked at delta band EEG (given our results in Fig. 4). As before, we conducted the analysis in one correlation collapsing across conditions and found a significant positive correlation between phonetic encoding and behavioral intelligibility score as a function of condition, for Env_Story [] and TFS_Story ). As we collapsed across conditions, we wanted to provide further support for this relationship using a LME model with behavioral intelligibility score and phonetic feature encoding as dependent and predictor variables, respectively. The LME models variability because of stimulus conditions and subjects simultaneously. We found a significant positive relationship between reconstruction accuracy and intelligibility score for Env_Story ( and for TFS_Story (. Nonsignificant within-condition correlation lines are plotted for completeness.
Discussion
In this study, we have aimed to disentangle the contributions to cortical speech envelope tracking that derive from acoustic and speech-specific processing. Using speech–speech chimeras, we aimed to decouple the acoustic envelope fluctuations of a stimulus from its speech content. We found evidence of robust envelope tracking based on acoustic energy fluctuations. We also found that changes in the strength of envelope tracking correlate with speech intelligibility across conditions. Altogether, we conclude that the cortical tracking of speech envelopes contains a large contribution from general auditory processing, with additional contributions from neural populations that are specifically tuned to speech, consistent with our original hypothesis (Fig. 2, hypothesis 4).
The notion of a substantial general acoustic contribution to cortical speech responses is in line with the idea that speech sounds are perceived using mechanisms that evolved to process environmental sounds more generally (Diehl et al., 2004), with additional linguistic processing occurring in specialized downstream pathways (Hickok and Poeppel, 2007; Rauschecker and Scott, 2009). Indeed, there is a wealth of evidence suggesting that speech is processed by a hierarchically organized network of cortical regions with responses in earlier stages (including primary auditory cortex) being well accounted for based on the spectrotemporal acoustics of the stimulus and later stages being invariant to those acoustics and involved in more abstract linguistic processing (Davis and Johnsrude, 2003; DeWitt and Rauschecker, 2012; Huth et al., 2016; de Heer et al., 2017; Kell et al., 2018; Norman-Haignere and McDermott, 2018). Of course, to contribute to envelope tracking, any such linguistic processing must involve speech features whose dynamics correlate with the envelope. One candidate set of features would be phonemes, whose onsets and offsets will often coincide with fluctuations in the envelope. The superior temporal gyrus, an auditory association area whose activity is not especially well captured based on a spectrotemporal representation of speech (Davis and Johnsrude, 2003; Norman-Haignere and McDermott, 2018), has shown a high degree of tuning for phonetic features (Chang et al., 2010; Mesgarani et al., 2014).
The idea that envelope tracking consists of general acoustic processing contributions from primary auditory areas and temporally correlated speech-specific contributions from areas like superior temporal gyrus (STG), explains many of the features of our results. For example, we see robust envelope tracking of Env_Story in the 1-band condition, when it is completely unintelligible. This must necessarily be because of simple auditory responses to changes in acoustic energy and fits with previous work showing robust envelope tracking to nonspeech stimuli (Lalor et al., 2009). The tracking of the Env_Story envelope then increases in strength across the 4-band and 16-band conditions as subjects increasingly understand the speech content of story 1, which likely reflects the increased contribution from speech-specific neuronal populations across these conditions. Conversely, we see significantly lower envelope tracking for TFS_Story, which makes sense as the energy fluctuations of the stimulus are dominated by Env_Story. Thus, the general acoustic processing contribution will be relatively insensitive to the dynamics of TFS_Story. However, in the 1-band (and 4 band) condition, subjects can partially understand the speech content of TFS_Story. And this leads to significant envelope tracking for TFS_Story in that condition, likely driven by contributions from STG and other speech-specific areas. That said, we also found that the EEG tracked the envelope that was recovered from the chimera stimuli (RE-Env), meaning that there could be tracking of lower-level acoustic features that have been resynthesized from the chimera after it passes through the cochlea.
We attempted to parse the contributions of acoustic and speech-specific processing using forward encoding models. We did this in two ways. First, we explored whether we might see differential sensitivity to variations in intelligibility as a function of latency between stimulus and response (Fig. 4). Although no particularly clear pattern emerged, it is important to bear in mind that the envelope of speech is a very compressed measure of a speech signal. Indeed, any speech features (acoustic or linguistic) that covary with the speech envelope are likely to contribute to the output measure. As such, the TRF in this case is very difficult to interpret. Second, we explored how the EEG across our different conditions reflected acoustic and phonetic feature processing by explicitly modeling those features. In general, both sets of features contributed uniquely to predicting EEG responses, with both increasing across conditions for Env_Story and decreasing across conditions for TFS_Story. Notably, the unique contribution for the phoneme features (Ph) was only significant for individual EEG channels for Env_Story in the 4-band and 16-band conditions, that is, where Env_Story could be partially understood, and TFS_Story in the 1-band condition, i.e., were TFS_Story could be partially understood. The phoneme model added no value in conditions where Env_Story (1 band) and TFS_Story (16 band) could not be understood. This suggests that modeling the responses to speech in this way has enabled us to tap into contributions from speech-specific areas, like STG.
The idea of general and speech-specific contributions to envelope tracking helps to explain why it can be quite difficult to link cortical envelope tracking measures with measures of speech understanding (Howard and Poeppel, 2010). For example, some studies have reported that cortical envelope tracking shows sensitivity to speech intelligibility (Peelle et al., 2013; Vanthornhout et al., 2018), whereas others have failed to find it (Howard and Poeppel, 2010). It may be that the large general auditory processing contribution to envelope tracking that is common to both intelligible and unintelligible speech somewhat masks a smaller contribution from task-based speech-specific processing. Indeed, in studies that have tried to control or account for the acoustic contributions to speech perception, correlations with behavior have been reported for phoneme-level processing (Di Liberto et al., 2018), including in STG (Leonard et al., 2016).
It has been well established that cortical envelope tracking is strongly affected by selective attention (Kerlin et al., 2010; Ding and Simon, 2012; Power et al., 2012; O'Sullivan et al., 2015). However, correlations between envelope tracking and behavioral measures of cocktail party attention have been difficult to identify (O'Sullivan et al., 2015; Tune et al., 2020). Again, if we consider envelope tracking as the combination of general acoustic and speech-specific processing, this makes sense. Using invasive recordings, it has recently been shown that cocktail party attention effects vary substantially in their strength across the cortical hierarchy, with weak effects in early auditory areas like Heschl's gyrus and much stronger effects in areas like STG (O'Sullivan et al., 2019). And EEG studies have suggested something similar on the basis of examining envelope tracking at different latencies (Power et al., 2012) or based on trying to isolate markers of acoustic and phonetic feature processing (Teoh et al., 2022). Indeed, focusing specifically on cortical responses to higher-level linguistic speech features, including those at the lexical and semantic levels, researchers have often found strong correlations with attention (Brodbeck et al., 2018; Broderick et al., 2018) and speech understanding, more generally (Broderick et al., 2018). Incidentally, it is worth reflecting on the possible role of attention in the pattern of results we see in the present study. For example, one might wonder whether changes in the amount of attention being paid to Env_Story or TFS_Story across conditions is driving the changes in envelope reconstruction accuracy we see (Fig. 2). We think this is unlikely. This is because, in our experiment, there was always just a single chimera stimulus being presented at any one time. Thus, any increase in attention to the speech content is likely to enhance the response to the acoustic energy changes. Of course, this is not guaranteed. Feature-based attention has been shown to affect the processing of some features more than others within the same object (Maunsell and Treue, 2006). However, the limited literature exploring how different feature-based attention tasks within a cocktail party environment have shown no clear effects on envelope tracking (Lauteslager et al., 2014).
The approach and results in this study have implications for theories of so-called speech entrainment (Giraud and Poeppel, 2012; Obleser and Kayser, 2019). One such prominent theory posits that intrinsic, ongoing oscillatory brain rhythms entrain to the rhythms of the speech signal by aligning their phase with the stimulus in an anticipatory, behaviorally effective manner. The core idea of this model is that salient points (edges) in the speech signal cause phase resetting of ongoing oscillatory activity. The realignment of the phase of these oscillations, which has been linked to fluctuations in cortical excitability (Lakatos et al., 2005), then enables the parsing and chunking of the continuous speech signal into discrete linguistic units for further processing by faster oscillations (Ghitza, 2011; Giraud and Poeppel, 2012; Rimmele et al., 2018). This theory links to fundamental electrophysiological observations in nonhuman studies (Lakatos et al., 2008). However, when it comes to the specific case of speech processing in humans, it has largely been built from observations that cortical activity (often measured noninvasively) shows consistent phasic fluctuations in theta band activity across repeated presentations of a speech stimulus (Luo and Poeppel, 2007). The fact that theta band activity appears special in this regard has led to the suggestion that this entrainment facilitates the parsing of continuous speech into syllables, given that the time scale of syllables is in the range of 4–8 Hz. However, basing this theory on the idea of consistent fluctuations in noninvasively recorded brain responses to speech is problematic. As we have shown in the present study, much of the variance in these fluctuations is driven by amplitude modulations of the stimulus, again, in line with previous research (Lalor et al., 2009). If acoustic edges (perhaps corresponding to syllable boundaries) were driving this cortical tracking, we might expect to see stronger tracking for TFS_Story than Env_Story in our 1-band condition. Of course, it may be that different low-frequency oscillators, each with a different specific role, are concurrently active in different early cortical areas. Indeed, recent research (also using stimuli where the ENV and TFS have been decoupled) has reported cortical entrainment in the theta range to temporal fine structure, distinct from that to the envelope (Teng et al., 2019). In particular, that study showed robust MEG tracking of unintelligible stimuli composed entirely of the TFS of speech (i.e., with no envelope fluctuations). Furthermore, behavioral results showed that TFS could help improve the recognition of speech whose envelope had been temporally distorted. The authors interpreted these findings as evidence that cortical entrainment to speech reflects the tracking of both the temporal and spectral structure of speech. That finding agrees well with our data showing EEG tracking of both Env_Story and TFS_Story. However, differences in the stimuli and tasks between the two studies led to different interpretations of the results. Teng et al. (2019) interpret their findings as evidence for two complementary mechanisms through which neural entrainment can facilitate the segmentation of speech (for further processing). On the other hand, our somewhat more direct linking of envelope and TFS tracking to speech comprehension in one experiment has led us to the interpretation that these neural measures index not just the segmentation of speech but dissociable contributions from general acoustic and speech-specific processing. Specifically, our interpretation is that acoustic energy fluctuations drive evoked responses in early auditory areas, with speech-specific tuning leading to additional contributions from auditory association areas like STG. We think this interpretation can also explain the cortical tracking of TFS reported by Teng et al. (2019). Although decisively adjudicating on the relative contributions of entrained oscillations versus evoked responses is not straightforward (Obleser and Kayser, 2019).
The variation in speech intelligibility across chimera conditions was reflected more strongly in delta band EEG frequencies than in theta band. This agrees with previous studies highlighting a specific correspondence between speech intelligibility/comprehension in challenging listening environments and delta band tracking (Ding et al., 2014; Etard and Reichenbach, 2019; Mai and Wang, 2019). For example, Etard and Reichenbach (2019) explored EEG responses to native and foreign languages in different levels of background noise and found that cortical tracking in the theta band was mainly correlated with clarity, whereas the delta band was most closely related to speech comprehension. Ding and Simon (2014), who dissociated the envelope and temporal fine structure of speech using noise vocoding, found that cortical tracking in the delta band predicted speech recognition scores for individual listeners. Indeed, more generally, a slew of previous research has linked cortical activity in the delta band with the tracking of linguistic features, even if these features have no acoustic correlates (Buiatti et al., 2009; Ding et al., 2016; Makov et al., 2017; Brodbeck et al., 2018; Broderick et al., 2018; Jin et al., 2018; Sheng et al., 2019).
Although the correspondence of delta band activity to behavior was not unexpected, we were somewhat surprised that the delta band tracking of the chimera stimuli was generally so much stronger than for the theta band. We sought to understand this through the lens of our previously mentioned interpretation that cortical speech tracking is largely driven by evoked responses. To that end, we calculated the mean amplitude of the modulation spectrum of our chimera stimuli in the delta and theta frequency ranges and found that delta band stimulus modulations were in excess of three times as large as modulations in the theta range (1 band, 3.15; 4 band, 3.39; 16 band, 3.34). This compares to a ratio of only 1.88 in (clean speech) stimuli we have used in previous research and that produced generally stronger tracking in the theta band than the delta band (Di Liberto et al., 2015). As such, the stronger tracking of speech in the delta band in the present study may simply reflect differences in evoked response amplitude based on more energetic modulations in that frequency range. The 1/f noise characteristics of the EEG itself (our current EEG dataset had an average delta/theta ratio across subjects of 6.18) could also be a contributory, interacting factor. That all said, we also found some evidence for preferential, speech-specific tracking in the delta range in our data. Specifically, the (demeaned) reconstructed envelopes for the broadband chimeras had delta/theta ratios of 7.00 for the 1-band stimuli, 12.65 for the 4-band stimuli, and 12.71 for the 16-band stimuli. These ratios are much higher than those of the stimuli. And they increase with the number of bands in the stimuli, as does the tracking and the intelligibility of the Env_Story. This suggests that there is a relative increase in delta tracking that may relate to behavior and that cannot be explained solely by the statistics of the stimuli and the EEG. Such a dissociation between delta and theta when using degraded/noisy speech stimuli would be consistent with several previous studies. For example, when the spectral resolution of speech decreases, theta band cortical tracking has been shown to be reduced (Peelle et al., 2013; Ding et al., 2014), but delta band tracking can be enhanced (Ding et al., 2014). Furthermore, when speech is presented in competing speech streams, delta band frequency (1–4 Hz) cortical tracking has been reported to be robust, whereas theta frequency band (4–8 Hz) cortical tracking often decreases as the level of the competing stream is increased (Ding and Simon, 2013). Further work is needed to more fully explore the roles of evoked responses and entrained oscillations in the delta and theta bands, bearing in mind that both general auditory and speech-specific activity are likely to be occurring within those frequency ranges.
Footnotes
This work was supported by a Career Development Award from Science Foundation Ireland (CDA/15/3316) and a grant from the National Institutes of Health–National Institute on Deafness and Other Communication Disorders (DC016297). We thank Dr. Aaron Nidiffer, Dr. Aisling O'Sullivan, Thomas Stoll, and Lauren Szymula for assistance with data collection and Dr. Nathaniel Zuk, Dr. Aaron Nidiffer, and Dr. Aisling O'Sullivan for comments on this manuscript.
References
- Ahissar E, Nagarajan S, Ahissar M, Protopapas A, Mahncke H, Merzenich MM (2001) Speech comprehension is correlated with temporal response patterns recorded from auditory cortex. Proc Natl Acad Sci USA 98:13367–13372. 10.1073/pnas.201400998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken SJ, Picton TW (2008) Envelope and spectral frequency-following responses to vowel sounds. Hear Res 245:35–47. [DOI] [PubMed] [Google Scholar]
- Algazi VR, Duda RO, Thompson DM, Avendano C (2001) The CIPIC HRTF database. Proceedings of the 2001 IEEE Workshop on the Applications of Signal Processing to Audio and Acoustics 2001:99–102. 10.1109/ASPAA.2001.969552 [DOI] [Google Scholar]
- Apoux F, Millman RE, Viemeister NF, Brown CA, Bacon SP (2011) On the mechanisms involved in the recovery of envelope information from temporal fine structure. J Acoust Soc Am 130:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever TG, Poeppel D (2010) Analysis by synthesis: a (re-)emerging program of research for language and vision. Bioling 4:174–200. 10.5964/bioling.8783 [DOI] [Google Scholar]
- Brainard DH, Vision S (1997) The psychophysics toolbox. Spat Vis 10:433–436. [PubMed] [Google Scholar]
- Brodbeck C, Hong LE, Simon JZ (2018) Rapid transformation from auditory to linguistic representations of continuous speech. Curr Biol 28:3976–3983.e5. 10.1016/j.cub.2018.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick MP, Anderson AJ, Di Liberto GM, Crosse MJ, Lalor EC (2018) Electrophysiological correlates of semantic dissimilarity reflect the comprehension of natural, narrative speech. Curr Biol 28:803–809.e3. [DOI] [PubMed] [Google Scholar]
- Buiatti M, Pena M, Dehaenelambertz G (2009) Investigating the neural correlates of continuous speech computation with frequency-tagged neuroelectric responses. NeuroImage 44:509–519. [DOI] [PubMed] [Google Scholar]
- Chang EF, Rieger JW, Johnson K, Berger MS, Barbaro NM, Knight RT (2010) Categorical speech representation in human superior temporal gyrus. Nat Neurosci 13:1428–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi T, Ru P, Shamma SA (2005). Multiresolution spectrotemporal analysis of complex sounds. J Acoust Soc Am 118:887–906. [DOI] [PubMed] [Google Scholar]
- Crosse MJ, Butler JS, Lalor EC (2015) Congruent visual speech enhances cortical entrainment to continuous auditory speech in noise-free conditions. J Neurosci 35:14195–14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosse MJ, Di Liberto GM, Lalor EC (2016a) Eye can hear clearly now: inverse effectiveness in natural audiovisual speech processing relies on long-term crossmodal temporal integration. J Neurosci 36:9888–9895. 10.1523/JNEUROSCI.1396-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosse MJ, Di Liberto GM, Bednar A, Lalor EC (2016b) The multivariate temporal response function (mTRF) toolbox: a MATLAB toolbox for relating neural signals to continuous stimuli. Front Hum Neurosci 10:604. 10.3389/fnhum.2016.00604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dau T, Ewert S, Oxenham AJ (2009) Auditory stream formation affects comodulation masking release retroactively. J Acoust Soc Am 125:2182–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS (2003) Hierarchical processing in spoken language comprehension. J Neurosci 23:3423–3431. 10.1523/JNEUROSCI.23-08-03423.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heer WA, Huth AG, Griffiths TL, Gallant JL, Theunissen FE (2017) The hierarchical cortical organization of human speech processing. J Neurosci 6539–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener TJ, Schneider TR, Viola FC (2010) Using ICA for the analysis of multi-channel EEG data. In: Simultaneous EEG and fMRI (Ullsperger M, Debener S, eds), pp 121–134. Oxford: Oxford UP. [Google Scholar]
- DeWitt I, Rauschecker JP (2012) Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci USA 109:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Liberto GM, O'Sullivan JA, Lalor EC (2015) Low-frequency cortical entrainment to speech reflects phoneme-level processing. Curr Biol 25:2457–2465. [DOI] [PubMed] [Google Scholar]
- Di Liberto GM, Crosse MJ, Lalor EC (2018) Cortical measures of phoneme-level speech encoding correlate with the perceived clarity of natural speech. eNeuro 5:ENEURO.0084-18.2018. 10.1523/ENEURO.0084-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl RL, Lotto AJ, Holt LL (2004) Speech perception. Annu Rev Psychol 55:149–179. [DOI] [PubMed] [Google Scholar]
- Ding N, Simon JZ (2012) Emergence of neural encoding of auditory objects while listening to competing speakers. Proc Natl Acad Sci USA 109:11854–11859. 10.1073/pnas.1205381109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Simon JZ (2013) Adaptive temporal encoding leads to a background-insensitive cortical representation of speech. J Neurosci 33:5728–5735. 10.1523/JNEUROSCI.5297-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Simon JZ (2014) Cortical entrainment to continuous speech: functional roles and interpretations. Front Hum Neurosci 8:311. 10.3389/fnhum.2014.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Chatterjee M, Simon JZ (2014) Robust cortical entrainment to the speech envelope relies on the spectro-temporal fine structure. Neuroimage 88:41–46. 10.1016/j.neuroimage.2013.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Melloni L, Zhang H, Tian X, Poeppel D (2016) Cortical tracking of hierarchical linguistic structures in connected speech. Nat Neurosci 19:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doelling KB, Arnal LH, Ghitza O, Poeppel D (2014) Acoustic landmarks drive delta–theta oscillations to enable speech comprehension by facilitating perceptual parsing. Neuroimage 85:761–768. 10.1016/j.neuroimage.2013.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etard O, Reichenbach T (2019) Neural speech tracking in the theta and in the delta frequency band differentially encode clarity and comprehension of speech in noise. J Neurosci 39:5750–5759. 10.1523/JNEUROSCI.1828-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA (1924) The distribution of the partial correlation coefficient. Metron 3:329–332. [Google Scholar]
- Ghitza O (2011) Linking speech perception and neurophysiology: speech decoding guided by cascaded oscillators locked to the input rhythm. Front Psychology 2:130. 10.3389/fpsyg.2011.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A-L, Poeppel D (2012) Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci 15:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DD (1990) A cochlear frequency-position function for several species—29 years later. J Acoust Soc Am 87:2592–2605. 10.1121/1.399052 [DOI] [PubMed] [Google Scholar]
- Halle M, Stevens K (1959). Analysis by synthesis. In: Proceeding of the seminar on speech compression and processing (Wathen-Dunn W, Woods LE, eds), Vol II, paper D7. [Google Scholar]
- Halle M, Stevens K (1962) Speech recognition: a model and a program for research. IEEE Trans Inform Theory 8:155–159. 10.1109/TIT.1962.1057686 [DOI] [Google Scholar]
- Haufe S, Meinecke F, Görgen K, Dähne S, Haynes JD, Blankertz B, Bießmann F (2014) On the interpretation of weight vectors of linear models in multivariate neuroimaging. Neuroimage 87:96–110. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D (2007) The cortical organization of speech processing. Nat Rev Neurosci 8:393–402. [DOI] [PubMed] [Google Scholar]
- Howard MF, Poeppel D (2010) Discrimination of speech stimuli based on neuronal response phase patterns depends on acoustics but not comprehension. J Neurophysiol 104:2500–2511. 10.1152/jn.00251.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth AG, de Heer WA, Griffiths TL, Theunissen FE, Gallant JL (2016) Natural speech reveals the semantic maps that tile human cerebral cortex. Nature 532:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irino T, Patterson RD (1997) A time-domain, level-dependent auditory filter: the gammachirp. J Acoust Soc Am 101:412–419. 10.1121/1.417975 [DOI] [Google Scholar]
- Jin P, Zou J, Zhou T, Ding N (2018) Eye activity tracks task-relevant structures during speech and auditory sequence perception. Nat Commun 9:5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell AJ, Yamins DL, Shook EN, Norman-Haignere SV, McDermott JH (2018) A task-optimized neural network replicates human auditory behavior, predicts brain responses, and reveals a cortical processing hierarchy. Neuron 98:630–644.e16. [DOI] [PubMed] [Google Scholar]
- Kerlin JR, Shahin AJ, Miller LM (2010) Attentional gain control of ongoing cortical speech representations in a “cocktail party”. J Neurosci 30:620–628. 10.1523/JNEUROSCI.3631-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger C, Tian L (2004) A comparison of the general linear mixed model and repeated measures ANOVA using a dataset with multiple missing data points. Biol Res Nurs 6:151–157. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE (2005) An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol 94:1904–1911. 10.1152/jn.00263.2005 [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE (2008) Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320:110–113. [DOI] [PubMed] [Google Scholar]
- Lalor EC, Foxe JJ (2010) Neural responses to uninterrupted natural speech can be extracted with precise temporal resolution. Eur J Neurosci 31:189–193. [DOI] [PubMed] [Google Scholar]
- Lalor EC, Power AJ, Reilly RB, Foxe JJ (2009) Resolving precise temporal processing properties of the auditory system using continuous stimuli. J Neurophysiol 102:349–359. [DOI] [PubMed] [Google Scholar]
- Lauteslager T, O'Sullivan JA, Reilly RB, Lalor EC (2014). Decoding of attentional selection in a cocktail party environment from single-trial EEG is robust to task. Annu Int Conf IEEE Eng Med Biol Soc 2014:1318–1321. [DOI] [PubMed] [Google Scholar]
- Leonard MK, Baud MO, Sjerps MJ, Chang EF (2016) Perceptual restoration of masked speech in human cortex. Nat Commun 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke SG (2017) Evaluating significance in linear mixed-effects models in R. Behav Res Methods 49:1494–1502. 10.3758/s13428-016-0809-y [DOI] [PubMed] [Google Scholar]
- Luo H, Poeppel D (2007) Phase patterns of neuronal responses reliably discriminate speech in human auditory cortex. Neuron 54:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Liu ZX, Poeppel D (2010) Auditory cortex tracks both auditory and visual stimulus dynamics using low-frequency neuronal phase modulation. Plos Biol 8:e1000445. 10.1371/journal.pbio.1000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai G, Wang WS (2019) Delta and theta neural entrainment during phonological and semantic processing in speech perception. bioRxiv. doi: 10.1101/556837. 10.1101/556837 [DOI] [Google Scholar]
- Makov S, Sharon O, Ding N, Benshachar M, Nir Y, Golumbic EZ (2017) Sleep disrupts high-level speech parsing despite significant basic auditory processing. J Neurosci 37:7772–7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R (2007). Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164:177–190. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Treue S (2006) Feature-based attention in visual cortex. Trends Neurosci 29:317–322. [DOI] [PubMed] [Google Scholar]
- McAuliffe M, Socolof M, Mihuc S, Wagner M, Sonderegger M (2017). Montreal Forced Aligner: trainable text-speech alignment using Kaldi. Proc. Interspeech 2017:498–502. [Google Scholar]
- Mesgarani N, Cheung C, Johnson K, Chang EF (2014) Phonetic feature encoding in human superior temporal gyrus. Science 343:1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman-Haignere SV, McDermott JH (2018) Neural responses to natural and model-matched stimuli reveal distinct computations in primary and nonprimary auditory cortex. PLoS Biol 16:e2005127. 10.1371/journal.pbio.2005127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan JA, Power AJ, Mesgarani N, Rajaram S, Foxe JJ, Shinn-Cunningham BG, Slaney M, Shamma SA, Lalor EC (2015) Attentional selection in a cocktail party environment can be decoded from single-trial EEG. Cereb Cortex 25:1697–1706. 10.1093/cercor/bht355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan J, Herrero J, Smith E, Schevon C, McKhann GM, Sheth SA, Mehta AD, Mesgarani N (2019) Hierarchical encoding of attended auditory objects in multi-talker speech perception. Neuron 104:1195–1209.e3. 10.1016/j.neuron.2019.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obleser J, Kayser C (2019) Neural entrainment and attentional selection in the listening brain. Trends Cogn Sci 23:913–926. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M (2011) FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011:156869. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RD (1987) A pulse ribbon model of monaural phase perception. J Acoust Soc Am 82:1560–1586. 10.1121/1.395146 [DOI] [PubMed] [Google Scholar]
- Patterson RD, Uppenkamp S, Johnsrude IS, Griffiths TD (2002) The processing of temporal pitch and melody information in auditory cortex. Neuron 36:767–776. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Davis MH (2012) Neural oscillations carry speech rhythm through to comprehension. Front Psychology 3:320. 10.3389/fpsyg.2012.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Gross J, Davis MH (2013) Phase-locked responses to speech in human auditory cortex are enhanced during comprehension. Cereb Cortex 23:1378–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet CR, Wilcox RR, Rousselet GA (2013) Robust correlation analyses: false positive and power validation using a new open source matlab toolbox. Front Psychol 3:606. 10.3389/fpsyg.2012.00606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D, Monahan PJ (2011) Feedforward and feedback in speech perception: revisiting analysis by synthesis. Lang Cogn Process 26:935–951. 10.1080/01690965.2010.493301 [DOI] [Google Scholar]
- Power AJ, Foxe JJ, Forde EJ, Reilly RB, Lalor EC (2012) At what time is the cocktail party? A late locus of selective attention to natural speech. Eur J Neurosci 35:1497–1503. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK (2009) Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci 12:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele JM, Morillon B, Poeppel D, Arnal LH (2018) Proactive sensing of periodic and aperiodic auditory patterns. Trends Cogn Sci 22:870–882. [DOI] [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G (2009) Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev 16:225–237. 10.3758/PBR.16.2.225 [DOI] [PubMed] [Google Scholar]
- Rouder JN, Morey RD, Speckman PL, Province JM (2012) Default Bayes factors for ANOVA designs. J Math Psychol 56:356–374. 10.1016/j.jmp.2012.08.001 [DOI] [Google Scholar]
- Salmelin R (2007) Clinical neurophysiology of language: the MEG approach. Clin Neurophysiol 118:237–254. 10.1016/j.clinph.2006.07.316 [DOI] [PubMed] [Google Scholar]
- Schädler MR, Meyer BT, Kollmeier B (2012). Spectro-temporal modulation subspace-spanning filter bank features for robust automatic speech recognition. J Acoust Soc Am 131:4134–4151. [DOI] [PubMed] [Google Scholar]
- Schönbrodt FD, Wagenmakers E-J, Zehetleitner M, Perugini M (2017) Sequential hypothesis testing with Bayes factors: efficiently testing mean differences. Psychol Methods 22:322–339. [DOI] [PubMed] [Google Scholar]
- Sheng J, Zheng L, Lyu B, Cen Z, Qin L, Tan LH, Huang M, Ding N, Gao J (2019) The cortical maps of hierarchical linguistic structures during speech perception. Cereb Cortex 29:3232–3240. [DOI] [PubMed] [Google Scholar]
- Smith ZM, Delgutte B, Oxenham AJ (2002) Chimaeric sounds reveal dichotomies in auditory perception. Nature 416:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng X, Cogan GB, Poeppel D (2019) Speech fine structure contains critical temporal cues to support speech segmentation. Neuroimage 202:116152. [DOI] [PubMed] [Google Scholar]
- Teoh ES, Ahmed F, Lalor EC (2022) Attention differentially affects acoustic and phonetic feature encoding in a multispeaker environment. J Neurosci 42:682–691. 10.1523/JNEUROSCI.1455-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tune S, Alavash M, Fiedler L, Obleser J (2020) Neural attention filters do not predict behavioral success in a large cohort of aging listeners. bioRxiv. doi: 10.1101/2020.05.20.105874. 10.1101/2020.05.20.105874 [DOI] [Google Scholar]
- Vanthornhout J, Decruy L, Wouters J, Simon JZ, Francart T (2018) Speech intelligibility predicted from neural entrainment of the speech envelope. J Assoc Res Otolaryngol 19:181–191. 10.1007/s10162-018-0654-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S, Altoè A, Vasilkov V (2018) Computational modeling of the human auditory periphery: Auditory-nerve responses, evoked potentials and hearing loss. Hear Res 360:55–75. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E-J, Wetzels R, Borsboom D, Van Der Maas HL (2011) Why psychologists must change the way they analyze their data: the case of psi: comment on Bem (2011). J Pers Soc Psychol 100:426–432. [DOI] [PubMed] [Google Scholar]
- Wainwright PE, Leatherdale ST, Dubin JA (2007) Advantages of mixed effects models over traditional ANOVA models in developmental studies: a worked example in a mouse model of fetal alcohol syndrome. Dev Psychobiol 49:664–674. 10.1002/dev.20245 [DOI] [PubMed] [Google Scholar]
- Yuan J, Liberman M (2008) Speaker identification on the SCOTUS corpus. J Acoust Soc Am 123:3878. [Google Scholar]
- Zeng F-G, Nie K, Liu S, Stickney G, Rio ED, Kong Y-Y (2004). On the dichotomy in auditory perception between temporal envelope and fine structure cues (L). J Acoust Soc AM 116:1351–1354. [DOI] [PubMed] [Google Scholar]
- Zion-Golumbic EM, Ding N, Bickel S, Lakatos P, Schevon CA, McKhann GM, Goodman RR, Emerson R, Mehta AD, Simon JZ, Poeppel D, Schroeder CE (2013) Mechanisms underlying selective neuronal tracking of attended speech at a “cocktail party”. Neuron 77:980–991. 10.1016/j.neuron.2012.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoefel B, VanRullen R (2015) Selective perceptual phase entrainment to speech rhythm in the absence of spectral energy fluctuations. J Neurosci 35:1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoefel B, VanRullen R (2016) EEG oscillations entrain their phase to high-level features of speech sound. Neuroimage 124:16–23. [DOI] [PubMed] [Google Scholar]
- Zuk NJ, Teoh ES, Lalor EC (2020) EEG-based classification of natural sounds reveals specialized responses to speech and music. NeuroImage 210:116558. [DOI] [PubMed] [Google Scholar]