Abstract
Seven species-specific monoclonal antibodies (MAbs) to Bartonella quintana were produced and characterized. The MAbs were of the immunoglobulin G class and reacted only with 13 B. quintana strains in indirect microimmunofluorescence and Western immunoblotting assays. They did not react with eight other Bartonella spp., including Bartonella henselae, the most closely related species, and a selected MAb did also not react with nine other strains of gram-negative bacteria. The MAbs reacted mainly with a 34-kDa protein epitope of B. quintana which was shown to be species specific by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Four of five body lice experimentally infected with B. quintana were found to be positive for the organism in microimmunofluorescence assays with one MAb. These MAbs may provide a specific, simple, rapid, and low-cost tool for the identification of B. quintana and the diagnosis of infections due to the microorganism.
Bartonella spp. are gram-negative, short-rod bacteria. Presently there are 14 recognized species within the genus Bartonella (2, 3, 9, 11, 12), and of these, four species are currently recognized as human pathogens: B. bacilliformis, B. quintana, B. henselae, and B. elizabethae (6). B. bacilliformis was the earliest species of this genus to be described (23) and is the agent of Carrion's disease. Infections with B. bacilliformis have yet to be reported from outside a very restricted geographic region in the Andes of western South America. B. quintana was first recognized during World War I as the etiological agent of trench fever. Although Vinson and Fuller (28) isolated the organism in 1961, there was little scientific interest in the organism or trench fever for the next 20 years, as they were apparently only very rarely encountered. Recent investigations, however, have led to the reemergence of B. quintana as an organism of medical importance. Bacillary angiomatosis was initially characterized by the appearance of multiple cutaneous lesions, which were assumed to be infectious because these lesions contained bacilli that stained with Warthin-Starry stain (1, 5, 16) and resolved with antibiotic treatment (5). Subsequently the observed bacillus was characterized by PCR and 16S rRNA gene sequencing, which showed it to be a new organism closely related to B. quintana (22), and in 1992 B. quintana was isolated from skin lesions of bacillary angiomatosis patients (14). The organism has also been found to be associated with other, less specific clinical syndromes, such as bacteremia (26), endocarditis (7, 19, 27), chronic lymphadenopathy (20), neurological disorders (29), and chronic bacteremia in homeless patients (4). There is a need, then, for rapid and specific methods to identify B. quintana and differentiate it from other Bartonella species. In this report we describe the characteristics and specificities of seven species-specific monoclonal antibodies (MAbs) that we produced against B. quintana.
MATERIAL AND METHODS
Bartonella strains.
The sources of the Bartonella strains used in the study are listed in Table 1. Bartonella isolates were grown on Columbia blood agar containing 5% whole sheep blood (BioMerieux, Marcy l'Etoile, France) at 37°C with a 5% carbon dioxide atmosphere, except for B. bacilliformis, which was grown at 32°C. After 5 to 7 days of culture, the organisms were harvested and suspended in sterile deionized water for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or with phosphate-buffered saline (pH 6.8) (PBS) for microimmunofluorescence (MIF).
TABLE 1.
Strains of Bartonella used for screening and determination of specificity of MAbs
| Species | Strain | Sourcea |
|---|---|---|
| B. bacilliformis | Monzon 812 | |
| Monzon 269 | ||
| B. henselae | Houston-1T | ATCC 49882 |
| URBHLLY8 | CSD; Marseille, France | |
| URBHLIE9 | IE; Marseille, France | |
| B. quintana | FullerT | ATCC VR-358 |
| URBQMLY4 | LY; Marseille, France | |
| Oklahoma | Chronic adenopathy; Oklahoma City, Okla. | |
| URBQPIEH2 | IE; Marseille, France | |
| B. elizabethae | F9251T | ATCC 49927 |
| B. grahamii | V2T | NCTC 12860 |
| B. taylorii | M6T | NCTC 12861 |
| B. doshiae | R18T | NCTC 12862 |
| B. vinsonii | BakerT | ATCC VR-152 |
| B. henselae | Cat 6 | South Africa |
| Fizz | South Africa | |
| King | United States | |
| SA2 | United States | |
| CAL-1 | United States | |
| B. quintana | SH-perm | Russia |
| URBQTBAAH1 | BA; Marseille, France | |
| URBQMLYI5 | LY; Marseille, France | |
| URBQPBAA7 | BA; Marseille, France | |
| URBQLIEH6 | IE; Marseille, France | |
| URBQMTF14 | TF; Marseille, France | |
| URBQMTF15 | TF; Marseille, France | |
| URBQMTF17 | TF; Marseille, France | |
| URBQMTF12 | TF; Marseille, France | |
| B. clarridgeiae | URBCMNHC26 | Cat's blood, France |
BA, bacillary angiomatosis; CSD, cat scratch disease; IE, infective endocarditis; LY, lymphadenopathy; TF, trench fever.
Production of MAbs.
For production of MAbs (10), 6-week-old female BALB/c mice were inoculated three times intraperitoneally with 2 × 104 B. quintana Fuller organisms, suspended in 0.5 ml of PBS, at 7-day intervals. One week after the final intraperitoneal inoculation the mice were injected intravenously with 4 × 103 organisms suspension in 0.1 ml of PBS. Three days later, spleen cells from the mice were fused with SP2/0-Ag14 myeloma cells (10:1) by using 50% polyethylene glycol (molecular weight, 1,300 to 1,600; Sigma Chemical Co., St. Louis, Mo.). Fusion cells were grown in hybridoma medium (Seromed, Berlin, Germany) with 17% fetal bovine serum (Gibco BRL) and hypoxanthine-aminopterin-thymidine selective medium (Sigma Chemical Co.) at 37°C in a humidified atmosphere supplemented with 5% CO2. The supernatants were screened for antibodies to B. quintana by MIF, and positive hybridomas were subcloned twice by limiting dilution. Isotypes of MAbs were determined with an ImmunoType Mouse Monoclonal Antibody Isotyping Kit with antisera to mouse immunoglobulin M (IgM), IgA, IgG1, IgG2a, IgG2b, and IgG3 (Sigma Chemical Co.). Specificities of MAbs were tested by Western immunoblotting.
MIF assay.
The MIF assay (18) was used to screen hybridoma clones and to determine the specificities of MAbs. Antigens were placed on 24-well microscope slides with a pen nib. The antigens were fixed in methanol for 10 min at room temperature and incubated in a humidified chamber at 37°C for 30 min. After two washes in PBS (5 min each) and rinsing in sterile distilled water, the slides were air dried at 37°C. Following incubation at 37°C for 30 min with dechlorotriazinyl amino fluorescein-conjugated goat anti-mouse IgG plus IgM (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) diluted 1:200 in PBS with 0.2% Evans blue (BioMerieux), the slides were washed as described above and mounted with Fluoprep (BioMerieux) before being read under an epifluorescence microscope (Axioskop20; Carl Zeiss, Gottingen, Germany) at a magnification of ×400. Sera from immunized mice were used as positive controls, and sera from healthy unexposed mice were used as negative controls.
SDS-PAGE and Western immunoblotting.
Antigens were suspended in an equal volume of sample buffer (0.0625 M Tris hydrochloride [pH 8.0], 2% SDS, 5% 2-mercaptoethanol, 10% glycerol, 0.02% bromophenol blue) (15) and separated electrophoretically in 12% resolving gels with 5% stacking gels at a constant current of 8 to 10 mA per gel for 3 to 4 h in running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS) in a Mini Protein II apparatus (Bio-Rad, Richmond, Calif.). Prestained SDS-PAGE standards (low range; Bio-Rad) were used as a reference. The separated antigens were transferred to 0.45-μm-pore-size nitrocellulose membranes (Hybond-C; Amersham, Little Chalfont, United Kingdom) at 50 V for 1 h at 4°C in an electrophoretic transfer cell (Mini Trans-Blot; Bio-Rad). After transfer, the nitrocellulose membranes were incubated overnight in PBS with 5% nonfat dry milk to block nonspecific binding sites. After three 10-min washes in PBS, the membranes were air dried and cut into strips, which were incubated with MAbs diluted 1:10 in PBS containing 3% nonfat dry milk at room temperature for 1 h and washed as described above. After incubation at room temperature for 1 h with peroxidase-conjugated F(ab′)2 fragment goat anti-mouse IgG (heavy and light chains) (AffiniPure; Jackson ImmunoResearch) diluted 1:200 in PBS with 3% nonfat dry milk and three washes in PBS, color was developed with coloring buffer containing 0.015% 4-chloro-1-naphthol and 0.015% hydrogen peroxide in 16.7% methanol in Tris-buffered saline.
Blind testing of bacteria with MAbs.
Blind testing of 26 bacteria by MIF with MAb Bq73H4 was carried out with 13 B. quintana strains isolated from patients in Marseille, France, by our laboratory; 5 Bartonella strains of other species; and Escherichia coli, Desulfovibrio fairfieldensis, Enterococcus faecalis, Afipia clevelandensis, Pseudomonas aeruginosa, Stenotrophomonas maltophila, Afipia felis, and Klebsiella pneumoniae.
Detection of Bartonella in lice preparations.
Twenty milliliters of a suspension of 1010 B. quintana Oklahoma organisms per ml was injected intravenously into a New Zealand White rabbit over 30 min. Following the injection, five human body lice were applied to the previously shaved belly of the rabbit and allowed to feed for 15 min. After being crushed and smeared onto microscope slides, these body lice were tested for B. quintana by MIF with undiluted hybridoma Bq73H4 supernatant as described above.
RESULTS
MIF reactivities and isotypes of MAbs.
Seven MAbs of subclasses IgG3, IgG1, and IgG2a (Table 2) produced from subcloned hybridomas were examined for their reactivities with Bartonella strains. These MAbs were reactive at identical titers with all B. quintana strains tested, except strain URBQPIEH2 (Table 2), and did not react at all with the other Bartonella species tested.
TABLE 2.
Subclasses of MAbs and titers to different B. quintana isolates
| MAb | IgG subclass | Titer to B. quintana straina:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
| Bq73H4 | 1 | 1,280 | 1,280 | 1,280 | 640 | 1,280 | 1,280 | 1,280 | 1,280 | 1,280 | 1,280 | 1,280 | 1,280 | 1,280 |
| Bq85G7 | 3 | 1,280 | 1,280 | 1,280 | 320 | 1,280 | 1,280 | 1,280 | 1,280 | 1,280 | 1,280 | 1,280 | 1,280 | 1,280 |
| Bq86D2 | 3 | 320 | 320 | 320 | 40 | 320 | 320 | 320 | 320 | 320 | 320 | 320 | 320 | 320 |
| Bq90H6 | 2a | 320 | 320 | 320 | 160 | 320 | 320 | 320 | 320 | 320 | 320 | 320 | 320 | 320 |
| Bq99C9 | 3 | 2,560 | 2,560 | 320 | 160 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 |
| Bq100E9 | 3 | 2,560 | 2,560 | 640 | 160 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 |
| Bq100G5 | 3 | 2,560 | 2,560 | 320 | 1,280 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 | 2,560 |
B. quintana strains: 1, Fuller; 2, URBQMLY4; 3, Oklahoma; 4, URBQPIEH2; 5, SH-perm; 6, URBQTBAAH1; 7, URBQMTF17; 8, URBQMTF12; 9, URBQMLYI5; 10, URBQPBAA7; 11, URBQLIEH6; 12, URBQMTF14; 13, URBQMTF15.
SDS-PAGE.
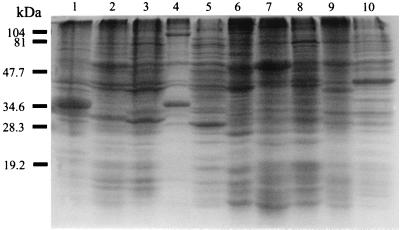
Although the SDS-PAGE profiles of the different species of Bartonella differed (Fig. 1), a 34-kDa protein appeared to be specific for B. quintana, and this was also the most prominent band in the SDS-PAGE profiles.
FIG. 1.
Coomassie brilliant blue staining profiles of Bartonella strains on SDS-PAGE. Lanes: 1, B. bacilliformis; 2, B. henselae subspecies houstoniae; 3, B. henselae subspecies massiliae; 4, B. clarridgeiae; 5, B. quintana; 6, B. elizabethae; 7, B. grahamii; 8, B. taylorii; 9, B. doshiae; 10, B. vinsonii. Molecular mass markers were loaded on the left.
Western immunoblotting.
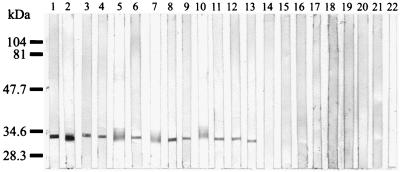
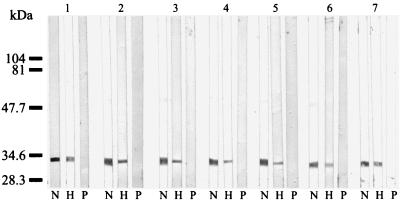
The seven MAbs reacted with a 34-kDa epitope of all B. quintana strains but did not react with any antigens of the other Bartonella species (Fig. 2). In Western blots of B. quintana antigens that had been digested with proteinase K, the MAbs failed to react with the 34-kDa epitope. Heating of the antigens (100°C for 10 min) before immunoblotting did not, however, affect the reactivities of the MAbs (Fig. 3).
FIG. 2.
Western immunoblotting of MAb Bq100G5 with Bartonella antigens. Lanes: 1, B. quintana Fuller; 2, B. quintana URBQMLY4; 3, B. quintana Oklahoma; 4, B. quintana URBQPIEH2; 5, B. quintana SH-perm; 6, B. quintana URBQTBAAH1; 7, B. quintana URBQMTF17; 8, B. quintana URBQMTF12; 9, B. quintana URBQMLYI5; 10, B. quintana URBQPBAA7; 11, B. quintana URBQLIEH6; 12, B. quintana URBQMTF14; 13, B. quintana URBQMTF15; 14, B. bacilliformis Monzon 812; 15, B. henselae Hounton-1; 16, B. henselae URBHLLY 8; 17, B. elizabethae; 18, B. grahamii V2; 19, B. taylorii M6; 20, B. doshiae R18; 21, B. vinsonii Baker; 22, B. clarridgeiae. Molecular mass markers were loaded on the left.
FIG. 3.
Immunoblotting of B. quintana Fuller antigens treated with MAbs in different ways. Lanes N, native antigens without treatment; lanes P, antigens treated with proteinase K (Boehringer, Mannheim, Germany) at 1.5 mg/ml at 37°C for 1.5 h; lanes H, antigens heated at 100°C for 5 min. MAbs: group 1, Bq73H4; group 2, Bq85G7; group 3, Bq86D12; group 4, Bq90H6; group 5, Bq99C9; group 6, Bq100E9; group 7, Bq100G5. Molecular mass markers were loaded on the left.
Blind testing of bacteria with MAbs.
The supernatant from hybridoma Bq73H4 reacted with all of the strains of B. quintana tested but did not react with the other Bartonella species or with the other bacteria used in the study.
Detection of B. quintana in lice.
Bartonella could be demonstrated in four of the five infected lice by MIF with MAbs Bq73H4 (Fig. 4), Bq85G7, and Bq100E9.
FIG. 4.
Fluorescent antibody-stained organisms of B. quintana on a crushed infected-lice smear with supernatant of undiluted hybridoma Bq73H4.
DISCUSSION
Several methods for confirming the identification of presumptive Bartonella spp. have been described, ranging from the comparison of the biochemical reactivities of isolates to more complex genotypic and phenotypic analyses. While Bartonella spp. were once considered to be relative inert, our laboratory (8) found that the introduction of 100 μg of hemin per ml to the test media resulted in the bacteria giving positive reactions in a number of biochemical tests. This then enabled the effective differentiation of B. quintana, B. henselae, and B. vinsonii from one another. The differentiation of Bartonella spp. has also been shown to be possible by using an extensive range of genotypic analyses, and DNA-DNA hybridization has clearly demonstrated the taxonomic position of Bartonella spp. (3, 6, 21). The 16S rRNA gene sequences of Bartonella spp. have been shown to be similar but unique for each species (3, 6, 17, 21), and Bartonella spp. can be differentiated by restriction fragment length polymorphism analyses of amplified 16S rRNA gene products with a combination of DdeI and MnlI (2). Similarly, PCR-restriction fragment length polymorphism analysis of the citrate synthase gene enables the identification of Bartonella spp. (13, 17), which can also be differentiated by pulsed-field gel electrophoresis with various restriction enzymes (17).
Only a few phenotypic characteristics have been reported for the identification of Bartonella at the species level (17, 25). In our experiments, we were able to produce and characterize species-specific MAbs to B. quintana. All seven of our MAbs reacted with various antigens of B. quintana in MIF and Western immunoblotting assays. The MAb (Bq73H4) selected for diagnostic purpose did not react with antigens of other Bartonella species as determined with eight strains of other bacteria, which clearly indicates that it is species specific for B. quintana. We note that the reactivity of our MAbs with B. quintana isolate URBQPIEH2 was relatively weak. This indicates that there might be antigenic differences between strains of B. quintana, and further research to resolve this issue is under way in our laboratory.
The MAbs that we describe in this report appear, then, to be suitable for use in rapid, simple, accurate, and low-cost tests such as direct and indirect MIF assays to differentiate Bartonella spp. The use of the MAbs would appear to be particularly useful in differentiating B. quintana and B. henselae, organisms which cause similar clinical signs in patients and have to date been differentiated only by using more expensive and complicated genotypic analyses. The MAbs could also be of use in epidemiological studies, as we were able to use MAbs Bq73H4, Bq85G7, and Bq100E9 to detect B. quintana by MIF in four of the five lice that we experimentally infected with the organism, and could be complementary with PCR detection (24).
ACKNOWLEDGMENTS
We thank Armand Tasmadjian for assistance with the experiments, P. E. Fournier for assistance with the photography, and P. Kelly for reviewing the manuscript.
REFERENCES
- 1.Angritt P, Tuur S M, Macher A M, Smith K J, Park C S, Hobin F P, Myrie-Williams C. Epithelioid angiomatosis in HIV infection: neoplasm or cat-scratch disease. Lancet. 1988;i:996. doi: 10.1016/s0140-6736(88)91813-2. [DOI] [PubMed] [Google Scholar]
- 2.Birtles R J, Harrison T G, Saunders N A, Molyneux D H. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taytorii sp. nov., and Bartonella doshiae sp. nov. Int J Syst Bacteriol. 1995;45:1–8. doi: 10.1099/00207713-45-1-1. [DOI] [PubMed] [Google Scholar]
- 3.Brenner D J, O'Connor S P, Winkler H H, Steigerwalt A G. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Bacteriol. 1993;43:777–786. doi: 10.1099/00207713-43-4-777. [DOI] [PubMed] [Google Scholar]
- 4.Brouqui P, Lascola B, Roux V, Raoult D. Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med. 1999;340:184–189. doi: 10.1056/NEJM199901213400303. [DOI] [PubMed] [Google Scholar]
- 5.Cockerell C J, Webster G F, Whitlow M A, Friedman-Kien A E. Epithelioid angiomatosis: a distinct vascular disorder in patients with the acquired immunodeficiency syndrome or AIDS-related complex. Lancet. 1987;ii:654–656. doi: 10.1016/s0140-6736(87)92442-1. [DOI] [PubMed] [Google Scholar]
- 6.Daly J S, Worthington M G, Brenner D J, Moss C M, Hollis D G, Weyant R S, Steigerwalt A G, Weaver R E, Daneshvar M I, O'Connor S P. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drancourt M, Mainardi J L, Brouqui P, Vandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult D. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 8.Drancourt M, Raoult D. Proposed tests for the routine identification of Rochalimaea species. Eur J Clin Microbiol Infect Dis. 1993;12:710–713. doi: 10.1007/BF02009387. [DOI] [PubMed] [Google Scholar]
- 9.Droz S, Chi B, Horn E, Steigerwalt A G, Whitney A M, Brenner D J. Bartonella koehlerae sp. nov., isolated from cats. J Clin Microbiol. 1999;37:1117–1122. doi: 10.1128/jcm.37.4.1117-1122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harlow E, Lane D. Monoclonal antibodies and growing hybridomas. In: Harlow E, Lane D, editors. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. pp. 139–282. [Google Scholar]
- 11.Heller R, Riegel P, Hansmann Y, Delacour G, Bermond D, Dehio C, Lamarque F, Monteil H, Chomel B, Piemont Y. Bartonella tribocorum sp. nov., a new Bartonella species isolated from the blood of wild rats. Int J Syst Bacteriol. 1998;48:1333–1339. doi: 10.1099/00207713-48-4-1333. [DOI] [PubMed] [Google Scholar]
- 12.Heller R, Kubina M, Mariet P, Riegel P, Delacour G, Dehio C, Lamarque F, Kasten R, Boulouis H J, Monteil H, Chomel B, Piemont Y. Bartonella alsatica sp. nov., a new Bartonella species isolated from the blood of wild rabbits. Int J Syst Bacteriol. 1999;49:283–288. doi: 10.1099/00207713-49-1-283. [DOI] [PubMed] [Google Scholar]
- 13.Joblet C, Roux V, Drancourt M, Gouvernet J, Raoult D. Identification of Bartonella (Rochalimaea) species among fastidious gram-negative bacteria on the basis of the partial sequence of the citrate-synthase gene. J Clin Microbiol. 1995;33:1879–1883. doi: 10.1128/jcm.33.7.1879-1883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koehler J E, Quinn F D, Berger T G, LeBoit P E, Tappero J W. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.LeBiot P E, Berger T G, Egbert B M, Yen Y S B, Stoler M H, Bonfiglio T A. Epithelioid hemangioma-like vascular proliferation in AIDS: manifestations of cat scratch disease bacillus infection. Lancet. 1988;i:960–963. doi: 10.1016/s0140-6736(88)91779-5. [DOI] [PubMed] [Google Scholar]
- 17.Maurin M, Roux V, Stein A, Ferrier F, Viraben R, Raoult D. Isolation and characterization by immunofluorescence, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blot, restriction fragment length polymorphism-PCR, 16S rRNA gene sequencing, and pulsed-field gel electrophoresis of Rochalimaea quintana from a patient with bacillary angiomatosis. J Clin Microbiol. 1994;32:1166–1171. doi: 10.1128/jcm.32.5.1166-1171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philip R N, Casper E A, Burgdorfer W, Gerloff R K, Hughes L B, Bell E J. Serologic typing of Rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978;121:1961–1968. [PubMed] [Google Scholar]
- 19.Raoult D, Fournier P E, Drancourt M, Marrie T J, Etienne J, Cosserat J, Cacoub P, Poinsignon Y, Leclercq P, Sefton A M. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125:646–652. doi: 10.7326/0003-4819-125-8-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Raoult D, Drancourt M, Carta A, Gastaut J A. Bartonella (Rochalimaea) quintana isolation in patient with chronic adenopathy, lymphopenia, and a cat. Lancet. 1994;343:977. doi: 10.1016/s0140-6736(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 21.Regnery R L, Anderson B E, Clarridge J E, Rodriguez-Barradas M C, Jones D C, Carr J H. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J Clin Microbiol. 1992;30:265–274. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Relman D A, Loutit J S, Schmith T M, Falkow S, Tompkins L S. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med. 1990;323:1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 23.Ristic R, Kreier J P. Family II. Bartonellaceae. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 717–719. [Google Scholar]
- 24.Roux V, Raoult D. Body lice as tools for diagnosis and surveillance of reemerging diseases. J Clin Microbiol. 1999;37:596–599. doi: 10.1128/jcm.37.3.596-599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slater L N, Coody D W, Woolridge L K, Welch D F. Maurine antibody responses distinguish Rochalimaea henselae from Rochalimaea quintana. J Clin Microbiol. 1992;30:1722–1727. doi: 10.1128/jcm.30.7.1722-1727.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spach D H, Kanter A S, Dougherty M J, Larson A M, Coyle M B, Brenner D J, Swaminathan B, Matar G M, Welch D F, Root R K, Stamm W E. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N Engl J Med. 1995;332:424–428. doi: 10.1056/NEJM199502163320703. [DOI] [PubMed] [Google Scholar]
- 27.Spach D H, Callis K P, Paauw D S, Houze Y B, Schoenknecht F D, Welch D F, Rosen H, Brenner D J. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J Clin Microbiol. 1993;31:692–694. doi: 10.1128/jcm.31.3.692-694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinson J W, Fuller H S. Studies on trench fever. 1. Propagation of Rickettsia-like organisms from a patient's blood. Pathol Microbiol. 1961;24:S152–S166. [PubMed] [Google Scholar]
- 29.Wong M T, Dolan M J, Lattuada C P, Jr, Regnery R L, Garcia M L, Mokulis E C, LaBarre R C, Ascher D P, Delmar J A, Kelly J W, Leigh D R, McRae A C, Reed J B, Smith R E, Melcher G P. Neuroretinitis, aseptic meningitis, and lymphadenitis associated with Bartonella (Rochalimaea) henselae infection in immunocompetent patients and patients infected with human immunodeficiency virus type 1. Clin Infect Dis. 1995;21:352–360. doi: 10.1093/clinids/21.2.352. [DOI] [PubMed] [Google Scholar]