Abstract
Food-borne disease due to intestinal parasites (IPs) and enteric bacterial infections (EBIs) remain a major public health problem. Food handlers, individuals involved in preparing and serving food, working with poor personal hygiene could pose a potential threat of spreading IPs and EBIs to the public. The aim of this study was to examine the overall prevalence and risk factors of IPs and EBIs among food handlers in four selected regions of Ethiopia. Scientific articles written in English were recovered from PubMed, ScienceDirect, Web of Science, Google Scholar, Cochrane Library, and other sources from Google Engine and University Library Databases. “Prevalence,” “Intestinal Parasites,” “Enteric Bacterial Infections,” “Associated Factors,” “Food Handlers,” and “Ethiopia” were the search terms used for this study. For critical appraisal, PRISMA 2009 was applied. Stata software version 16 was used to perform the meta-analysis. Heterogeneity and publication bias were evaluated using Cochran's Q, inverse variance (I2), and funnel plot asymmetry tests. A random-effects model was used to calculate the pooled burden of IPs and EBIs and its associated factors among food handlers, along with the parallel odds ratio (OR) and 95% confidence interval (CI). For this meta-analysis, a total of 5844 food handlers were included in the 20 eligible studies. The overall pooled prevalence of IPs and EBIs among food handlers in four selected regions of Ethiopia was 29.16% (95% CI: 22.61, 35.71), with covering (25.77%) and (3.39%) by IPs and EBIs, respectively. Ascaris lumbricoides, Entamoeba histolytica/dispar, Giardia lamblia, and hookworm were the most prevalent IPs among food handlers with a pooled prevalence of 7.58%, 6.78%, 3.67%, and 2.70%, respectively. Salmonella and Shigella spp. were the most prevalent EBIs among food handlers with a pooled prevalence of 2.78% and 0.61%, respectively. A high prevalence of IPs and EBIs among food handlers was observed in Oromia (38.56%; 95% CI: 29.98, 47.14), while a low prevalence was observed in the Tigray region (19.45%; 95% CI: 6.08, 32.82). Food handlers who had not taken food hygiene training (OR: 0.68, 95% CI: −0.34, 1.69), untrimmed finger nail (OR: 2.23, 95% CI: 1.47, 2.99), lack of periodic medical checkup (OR: 1.52, 95% CI: 0.41, 2.64), lack of handwashing habits (OR: 1.97, 95% CI: 0.53, 3.41), and eating raw vegetables and meat (OR: 2.63, 95% CI: 0.92, 4.34) were factors significantly associated with the prevalence of IPs and EBIs. The prevalence of IPs and EBIs was high in the selected Ethiopian region (Amhara, Oromia, SNNPR, and Tigray) food handlers along an increasing prevalence trend from 2014 to 2022. Therefore, this study recommends the provision of proper health education and training regarding personal hygiene, hand washing, food handling, medical checks, as well as raw vegetable and meat safety.
1. Introduction
Food-borne diseases are illnesses caused by ingesting pathogenic microorganisms (bacteria, fungi, viruses, and parasites) or their toxins (for bacteria and fungi) [1, 2]. Food-borne outbreaks can result in both health and economic losses. According to the World Health Organization (WHO) [3], 600 million people worldwide become severely ill each year, with 420,000 dying as a result of food contamination. Food-borne infections affect an estimated 48 million people in the United States each year, resulting in 128,000 hospitalizations and 3,000 fatalities [4, 5]. Food-borne and waterborne infections were also projected to cause approximately 700,000 deaths each year in Africa [6] and cause both short-term (nausea, vomiting, and diarrhea) and long-term (tissue damage, cancer, kidney or liver failure, brain disorders, and neural disorders) disorders.
Gastrointestinal parasitic infections are prevalent throughout the world, with the highest prevalence in poorer nations due to poor personal hygiene, environmental sanitation, socioeconomic, demographic, and health-related behaviors [7]. The most common way for intestinal parasitic infections to spread is through contaminated food and water, but they can further transmit from person-to-person through fecal-oral contact [8]. In the world, intestinal parasites infect approximately one-third of the total population, with the tropics and subtropics bearing the greatest load [9]. Ascaris lumbricoides, Trichuris trichiura, hookworm, Entamoeba histolytica, and Giardia lamblia infect an estimated 1.2 billion, 795 million, 740 million, 500 million, and 2.8 million people worldwide [10, 11]. In Ethiopia, the burden of intestinal parasites (IPs) is extremely high. A third (26 million), a quarter (21 million), and one in every eight (11 million) Ethiopians are infected with Ascaris lumbricoides, Trichuris trichiura, and hookworm [12], respectively. As a result, Ethiopia has the second, third, and fourth largest burdens of ascariasis, hookworm, and trichuriasis, in sub-Saharan Africa, respectively.
Enteric bacterial infection (EBI)-causing microbes, namely, the genus of Salmonella (Salmonellosis) and Shigella (Shigellosis) are also the most important sources of food-borne diseases. As a result, they continue to be significant public health issues. Furthermore, clinical prevention and control of typhoid fever are difficult, particularly in Africa, due to the development of antibiotic resistance, and vaccines are not immunogenic to young children. Globally, there are 93.8 million cases of gastroenteritis caused by Salmonella species each year, with 155,000 deaths. An estimated 80.3 million of these cases were food-borne [13]. Shigella species are more common in temperate and tropical areas. Shigella species causes an estimated 80–165 million cases of disease and 600,000 deaths worldwide each year [14, 15].
Food handlers are individuals engaged in preparing and serving foods, infected with gastrointestinal parasitic and bacterial infections, and practicing poor personal hygiene could be serious sources of transmission of IPs and EBIs to the public. Since food handlers infected with IPs and EBIs exhibit subclinical symptoms and are asymptomatic carriers, they are unaware of their possible role in the spread of infection, hinders the pathogens, and challenges in the integrated control and elimination of infections [8]. Furthermore, food handlers have a large impact on the spread of IPs and EBIs as they can directly or indirectly transmit infections to a large number of foods and drink consumers in food service establishments namely restaurants, hotels, factories, canteens, schools, hospitals, prisons, or other places where food is prepared and served to a range of users [16, 17].
Aside from socioeconomic issues, other factors including the availability of clean water, the survival of pathogenic parasites and bacteria in different environmental conditions, and personal and public hygiene habits all play an important role in the transmission of IPs and EBIs [18, 19]. Ethiopia has one of the lowest rates of clean water supply and toilet coverage [20]. Ethiopian studies on personal hygiene factors, for example, hand washing after toilet use, medical cheek examinations including stool exams, eating raw vegetables and meat, hand washing before food handling and meal, finger nail status, food hygiene training, and knowledge of enteric parasites and bacteria were found to contribute to the prevalence of IPs and EBIs among food handlers [12, 21, 22].
Numerous studies have been conducted in Africa, to investigate the prevalence of intestinal parasitic and enteric bacterial infections among handlers of food. In Ethiopia, the risk of Salmonella and Shigella infection among food handlers ranged from 1 to 7.5% [21, 23, 24]. However, the prevalence of intestinal parasitic infections among food handlers varied and was inconsistent across studies: 10.9 to 45.3% in Ethiopian university cafeterias [25–30], 43.9% in street dwellers [31], 48.1% in prisons [32], 35% in orphanage centers [33, 34], 32.3% in public hospitals [34], and 14.5 to 44% in restaurants and cafeterias [12, 22]. IPs and EBIs are one of the common public health issues in Ethiopia, with varying levels of prevalence throughout the country. In Ethiopia, only one systematic review and meta-analysis study were conducted on the prevalence of IPs among food handlers by Yimam et al. [12]. However, the prevalence and risk factors of both IPs and EBIs among selected region food handlers is not collected, well-organized, or recorded as a systematic review and meta-analysis. As a result, the objective of this study was to provide tangible evidence on the overall prevalence and risk factors for IPs and EBIs among food handlers using previously conducted research articles in four (Amhara, Oromia, SNNPR, and Tigray) regions of Ethiopia. Furthermore, the results obtained in the current investigation could significantly contribute to healthcare providers, users, and policy makers.
2. Methods
2.1. Profile of the Country
Ethiopia measures 1,104,300 square kilometers and is located in the Horn of Africa (total land area is 1,000,000 square kilometers) (386,102 square miles). Ethiopia is bordered in the north by Eritrea, in the east by Djibouti and Somalia, in the west by Sudan and South Sudan, and in the south by Kenya. According to Worldometer's elaboration of the most recent United Nations data, Ethiopia's current population was 113,881,451 in 2020, which is comparable to 1.47%. Furthermore, according to the aforementioned report, approximately, 21.3% of the population (24, 463, 423) will live in urban areas by 2020 [35, 36].
2.2. Search Strategy
This systematic review and meta-analysis were performed according to the Guidelines and checklists for Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2009 [37]. An extensive search was conducted in international databases (PubMed, ScienceDirect, Web of Science, Google Scholar, and Cochrane Library) and other sources (Google Engine and University Library Databases). Articles were searched using MeSH key terms and phrases in combination or separate using Boolean operators (“OR”/“AND”) such as “Prevalence,” “Intestinal Parasites,” “Enteric Bacterial Infections,” “Associated Factors,” “Food Handlers,” and “Ethiopia.” The study was carried out from November 2021 to June 2022. The search process was presented following PRISMA flow chart 2009 guidelines along the studies included and excluded with reasons of exclusion (Figure 1).
Figure 1.
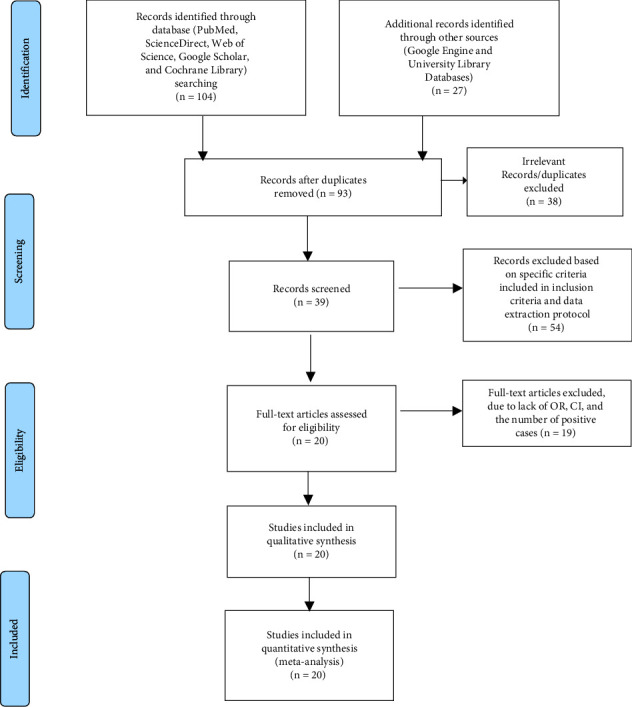
PRISMA 2009 flow diagram of eligible studies.
2.3. Criteria for Inclusion and Exclusion of Studies
In this systematic and meta-analysis review, institutional and hospital-based studies were included. Articles collected through the searches were evaluated for inclusion in the meta-analysis based on the following criteria: (i) Selected Ethiopian region (Amhara, Oromia, SNNPR, and Tigray) studies on the prevalence of IPs and EBIs as well as their risk factors among food handlers with a sample size of at least 100 observations; (ii) only human studies category and reported in English; (iii) cross-sectional and case-control studies; (iv) recent journals studied from 2014 to 2022; (v) only bacterial and parasitic etiological agents; and (vi) articles published and available online were included in this study. However, reports about knowledge and practice of food handlers towards IPs and EBIs, investigation on patterns of antimicrobial resistance of EBIs only, studies conducted outside selected regions and duplicate publications or extensions of analysis from original studies, as well as studies that were incompletely presented, were excluded from the review process. Four Ethiopian regions were included in the study due to their large population size, increasing urbanization and cover a wide range of geographical locations, along with many infrastructures, namely, hotels, resorts, cafeterias, and organizations (governmental and nongovernmental). Among many of the previously published articles, only 20 met the meta-analysis selection criteria (Figure 1).
2.4. Data Extraction
The data extraction protocol consists of the name of the country, author and year of publication, study setting, study area, sample size, number of positive cases, prevalence and their associated risk factors. If the study was conducted over a range of years, then the latest year of the stated range was used. The period from January 1 to March 30, 2022, was used for study selection, quality evaluation, and data extraction.
2.5. Quality Assessment of Individual Studies
The general quality of the evidence was assessed using the GRADE approach (Grading of Recommendations Assessment, Development, and Evaluation) [38]. Using the three (methodological quality, comparability, and the outcome and statistical analysis of the study) main assessment tools, the quality of each study was determined. High-quality publications received five up to six points, moderate-quality publications received four points, and low-quality articles received zero up to three points. The choice and evaluation of the articles' quality were performed independently by the two reviewers (AG and AA). The articles were added after agreement was reached and the discrepancies between the reviewers were resolved through discussion.
2.6. Risk of Publication Bias
Using funnel plot symmetry, Cochran's Q test, and the I2 test, the risks of publication bias in articles were analyzed.
2.7. Statistical Analysis
The pooled prevalence of IPs and EBIs among food handlers was calculated by dividing the total positive cases by the total study subjects included in this meta-analysis and multiplying by a factor of a hundred. We used a random-effects model to estimate the pooled effect size. To sort out the causes of heterogeneity, subgroup analysis was conducted based on sample size, region of the study, year of publication, and study area. The Cochran Q statistic with inverse variance and funnel plot symmetry was used to assess the existence of statistical heterogeneity. A log odds ratio was used to decide the association between IPs and EBIs with the associated risk factors among food handler respondents included in the studies. Meta-analysis was performed using Stata software version 16, where P ≤ 0.05 was considered statistically significant.
3. Results
A total of 131 articles on the prevalence and associated risk factors of IPs and EBIs among food handlers in Ethiopia were recovered. Thirty-eight of these articles were excluded due to duplicates. Of the remaining 93 articles, 54 were excluded based on specific criteria included in the inclusion criteria and the data extraction protocol. Of the remaining 39 articles, 19 articles were further excluded due to the fact that they did not have OR, 95% CI, and the number of positive cases (meaning the report was based only on the estimated prevalence percentage). Therefore, only 20 of the studies met the eligibility criteria and were included in the final systematic review and meta-analysis study (Figure 1).
The prevalence of intestinal parasites among Ethiopian food handlers were assessed from 20 eligible studies conducted from 2014 to 2022 (9 year study). Helminthes, protozoan, and enteric bacteria were the most prevalent intestinal parasites and enteric bacterial infections among Ethiopian food handlers with a pooled prevalence of 13.89%, 11.88%, and 3.39%, respectively. 7.58%, 6.78%, 3.67%, 2.78%, and 2.70% was, respectively, the pooled prevalence of Ascaris lumbricoides, Entamoeba histolytica/dispar, Giardia lamblia, Salmonella spp., and hookworm (Table 1).
Table 1.
Prevalence of intestinal parasites and enteric bacteria among food handlers.
| Category | Pooled number (%) | |
|---|---|---|
| Helminthes | 724 (13.89) | |
| Ascaris lumbricoides | 395 (7.58) | |
| Hookworm | 141 (2.70) | |
| Taenia species | 58 (1.11) | |
| Trichuris trichiura | 53 (1.02) | |
| Enterobius vermicularis | 29 (0.56) | |
| Schistosoma mansoni | 26 (0.50) | |
| Hymenolepis nana | 22 (0.42) | |
|
| ||
| Protozoan | 619 (11.88) | |
| Entamoeba histolytica/dispar | 353 (6.78) | |
| Giardia lamblia | 191 (3.67) | |
| Entamoeba coli | 50 (0.96) | |
| Entamoeba hartmanni | 18 (0.34) | |
| Giardia intestinalis | 7 (0.13) | |
|
| ||
| Enteric bacteria | 177 (3.39) | |
| Salmonella spp. | 145 (2.78) | |
| Shigella spp. | 32 (0.61) | |
| Total | 1,520 (29.16) | |
3.1. Characteristics of the Eligible Studies
Table 2 presents the characteristics of the studies required for analysis. Twenty studies were eligible and thus were included in the meta-analysis. Studies were conducted between 2014 and 2022, and all of them were cross-sectional studies. Seven and 13 studies were carried out between 2014 and 2018, as well as between 2019 and 2022, respectively. Based on the criteria, four regions, namely, Tigray (3 articles), Oromia (5 articles), Amhara (6 articles), and SNNPR (6 articles) were involved. The prevalence of intestinal parasites (IPs) and enteric bacterial infections (EBIs) among eligible studies ranged from 9.9% to 61.9% (Table 2).
Table 2.
List and characteristics of 20 eligible studies conducted from 2014 to 2022 among food handlers.
| Authors | Year | Region | Study Area | Sample Size | Case | Prevalence (95% CI) | Quality Score |
|---|---|---|---|---|---|---|---|
| Tefera and mebrie | 2014 [39] | Oromia | Yebu town | 118 | 52 | 44.1 (36.6–45.8) | 4 |
| Mama and alemu | 2015 [40] | SNNPR | Arba minch university | 345 | 123 | 36.0 (32.7–39.8) | 4 |
| Mama and alemu | 2016 [41] | SNNPR | Arba minch university | 345 | 34 | 9.9 (6.3–12.7) | 4 |
| Abera et al. | 2016 [42] | Amhara | Bahir dar university | 410 | 53 | 12.9 (9.8–15.7) | 4 |
| Gezehegn et al. | 2017 [43] | Tigray | Aksum town | 400 | 58 | 14.5 (11.3–18.0) | 6 |
| Girma et al. | 2017 [44] | Oromia | Jimma university | 148 | 31 | 33.0 (28.2–37.5) | 5 |
| Solomon et al. | 2018 [45] | SNNPR | Wolaita sodo town | 387 | 159 | 41.0 (35.4–45.2) | 5 |
| Asires et al. | 2019 [46] | Amhara | East and west gojjam | 416 | 213 | 61.9 (51.2–68.7) | 4 |
| Alemnew et al. | 2019 [47] | Amhara | Woldia university | 256 | 43 | 16.8 (13.1–21.2) | 5 |
| Kebede et al. | 2019 [48] | Amhara | Wollo university | 200 | 30 | 15.0 (12.1–19.3) | 5 |
| Kumma et al. | 2019 [49] | SNNPR | Wolaita sodo university | 233 | 55 | 23.6 (18.2–29.1) | 6 |
| Alemu et al. | 2019 [50] | Amhara | Chagni town | 422 | 62 | 14.8 (11.5–18.0) | 6 |
| Kuti et al. | 2020 [51] | Oromia | Madda walabu university | 198 | 50 | 25.3 (21.9–29.6) | 5 |
| Diriba et al. | 2020 [52] | SNNPR | Dilla university | 220 | 113 | 51.3 (40.9–62.7) | 4 |
| Legese et al. | 2020 [53] | Tigray | Adigrat university | 301 | 33 | 11.0 (6.1–14.6) | 5 |
| Yesigat et al. | 2020 [54] | Amhara | Motta town | 243 | 67 | 26.6 (21.5–32.9) | 6 |
| Regassa et al. | 2021 [55] | Tigray | Medebay zana district | 401 | 129 | 33.2 (27.3–38.2) | 5 |
| Yeshanew et al. | 2021 [56] | Oromia | Mettu town | 139 | 62 | 44.6 (34.6–52.3) | 5 |
| Kumalo et al. | 2021 [57] | SNNPR | Dawuro zone | 402 | 108 | 26.9 (22.7–30.6) | 5 |
| Gemechu et al. | 2022 [58] | Oromia | Jimma town | 260 | 125 | 48.5 (38.7–57.5) | 6 |
3.2. Pooled Prevalence of IPs and EBIs
A random-effects model was employed to estimate the pooled prevalence of IPs and EBIs among food handlers in Ethiopia. The total national prevalence of IPs and EBIs among food handlers was 29.16 (95% CI: 22.61–35.71) (Figure 2).
Figure 2.
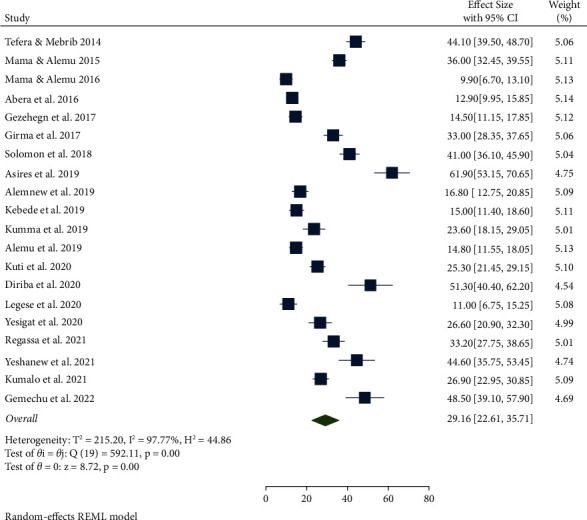
Forest plot of the pooled prevalence of IPs and EBIs among food handlers in Ethiopia from 2014 to 2022.
3.2.1. Subgroup Analysis
The high pooled prevalence of IPs and EBIs was reported from sample size <200; 36.39% (95% CI: 27.18, 45.60), followed by > 200 sample size; 27.34% (95% CI: 19.64, 35.04) (Table 3 and S1). Regarding the regional distribution, a high prevalence of IPs and EBIs among food handlers was observed in Oromia (38.56%; 95% CI: 29.98, 47.14), followed by SNNPR (31.03%; 95% CI: 19.66, 42.40), and Amhara (24.36%; 95% CI: 9.67, 29.05), while a low prevalence of IPs and EBIs was observed in the Tigray region (19.45%; 95% CI: 6.08, 32.82) (Table 3 and Figure 3). High heterogeneity in the prevalence of IPs and EBIs was observed across studies within and between three regions (Amhara, SNNPR, and Tigray) (I2 > 96% and P < 0.001) (Figure 3)
Table 3.
Prevalence of IPs and EBIs among food handlers in Ethiopia by subgroup analysis.
| Variables | Characteristics | Number of studies | Sample size | Prevalence(95% CI) | I 2, P value |
|---|---|---|---|---|---|
| Sample size | <200 | 4 | 603 | 36.39 (95% CI: 27.18, 45.60) | 92.56%, P < 0.001 |
| >200 | 16 | 5241 | 27.34 (95% CI: 19.64, 35.04) | 98.07%, P < 0.001 | |
|
| |||||
| Pooled prevalence of IPs and EBIs by region | Amhara | 6 | 1947 | 24.36 (95% CI: 9.67, 39.65) | 98.73%, P < 0.001 |
| Oromia | 5 | 863 | 38.56 (95% CI: 29.98, 47.14) | 91.68%, P < 0.001 | |
| SNNPR | 6 | 1932 | 31.03 (95% CI: 19.66, 42.40) | 97.42%, P < 0.001 | |
| Tigray | 3 | 1102 | 19.45 (95% CI: 6.08, 32.83) | 96.60%, P < 0.001 | |
|
| |||||
| Pooled prevalence of IPs and EBIs by year | 2014–2018 | 7 | 2153 | 27.26 (95% CI: 16.55, 37.97) | 98.27%, P < 0.001 |
| 2019–2022 | 14 | 3691 | 30.16 (95% CI: 21.68, 38.83) | 97.46%, P < 0.001 | |
|
| |||||
| Pooled prevalence of IPs and EBIs by study area | University | 10 | 2656 | 23.04 (95% CI: 15.24, 30.84) | 97.34%, P < 0.001 |
| Town/district/zone | 10 | 3188 | 35.25 (95% CI: 25.87, 44.63) | 97.23%, P < 0.001 | |
|
| |||||
| Overall | 20 | 5844 | 29.16 (95% CI: 22.61, 35.71) | 97.77%, P < 0.001 | |
Figure 3.
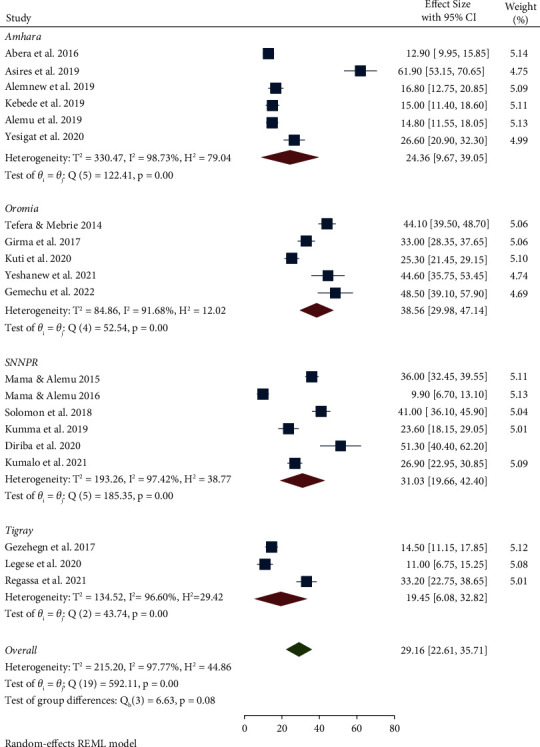
Pooled prevalence of IPs and EBIs among food handlers by region.
3.3. Factors Associated with IPs and EBIs among Food Handlers in Ethiopia
In this meta-analysis, food hygiene training, finger nail status, medical checkup, hand washing before food handling, hand washing before meals, and eating raw vegetables and meat were significantly associated with IPs and EBIs among food handlers (S4, S5, S6, S7, S8, and S9).
The results from five studies revealed that food hygiene training was strongly associated with IPs and EBIs. Food handlers who did not receive food hygiene training were 0.68 times more likely to have IPs and EBIs than those who had received food hygiene training (OR: 0.68, 95% CI: −0.34, 1.69, p=0.03) (S4).
The association between fingernail trimming and hygiene habits, as well as IPs and EBIs in Ethiopian food handlers was calculated from 15 studies. The pooled results showed that food handlers with untrimmed fingernail and poor cleanness habits were 2.23 times more likely to be infected with IPs and EBIs than their counterparts (OR: 2.23, 95% CI: 1.47, 2.99, and p < 0.001) (S5).
The pooled results of eight studies showed that medical checkup were strongly associated with IPs and EBIs among food handlers. The probability of IPs and EBIs was 1.52 times higher among food handlers without attending medical checkup than their counterparts (OR: 1.52, 95% CI: 0.41, 2.64, p < 0.001) (S6).
The odds of having IPs and EBIs were 1.97 times higher among food handlers who did not wash their hands before food handling than people who wash their hands (OR: 1.97, 95% CI: 0.53, 3.41, p=0.01) (S7).
The association between raw meat and vegetable eating habits with IPs and EBIs was evaluated in four studies. The odds of having IPs and EBIs were 2.63 times higher among food handlers who had habits of eating raw meat and vegetables as compared to counterparts (OR: 2.63, 95%; CI: 0.92, 4.34, p=0.05) (S8).
Fourteen studies (70.0%) obtained high-quality scores, whereas six (30.0%) had medium-quality scores when it came to risk bias assessment (Table 1). The most common biases observed were representation and case definition. To know the effect of medium-quality studies, the pooled prevalence was calculated without involving it. The pooled prevalence estimates with and without these studies had confidence intervals that overlapped, indicating that there was no meaningful difference between them (Figure 4). Based on these findings, the majority of the primary study authors met high-quality standards (Figure 4). This gives the current study more credibility for healthcare providers, users, and policy makers.
Figure 4.
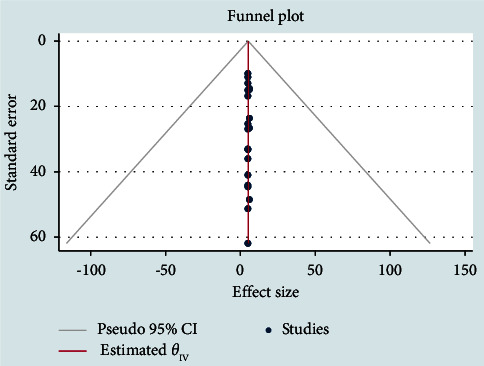
Meta funnel plot presentation, an indication of publication bias among studies in Ethiopia from 2014 to 2022.
4. Discussion
Food-borne parasitic and enteric bacterial illnesses are major public health concerns around the world, causing morbidity and mortality, especially in undeveloped countries like Ethiopia [59]. Personal sanitation and education of food handlers are as important as hygienic food preparation and delivery. This group of persons is involved in the handling, storage, transportation, processing, and preparation of food for users on a variety of levels. Knowing the exact pooled prevalence of intestinal parasites (IPs) and enteric bacterial infections (EBIs) among Ethiopian food handlers is useful as a guide for both governmental and nongovernmental policymakers and stakeholders to control food-related diseases.
The overall prevalence of IPs and EBIs among four region food handlers was 29.16% (95% CI: 22.61, 35.71) with covering (25.77%) and (3.39%) by IPs and EBIs, respectively. This 25.77% IPs prevalence was in line with findings in a previous study conducted in Ethiopia by Tegen et al. [35], who reported a prevalence of 25.01%. Similar studies by Liao et al. [60], Reh et al. [61], Harizanov et al. [62], and Staudacher et al. [63], who reported a prevalence of 28.6%, 28%, 25.53%, and 25.4% in the Democratic Republic of Congo, Spain, Bulgaria and Rwanda, respectively, were in close agreement with the current finding. However, the findings of this study was higher than those of studies conducted by Ismail, [64], 18.7% in Saudi Arabia, Coulibaly et al. [65], 18.7% in Côte d'Ivoire, Venkatajothi, [66], 17.4% in Tanzania and Abu-Madi et al. [67], 5.93% in Qatar. On the other hand, this result was lower than the reports by Sylla et al. [68], 32.6% in Senegal, Sanprasert et al. [69], 37.8% in Thailand, Al-Jawabreh et al. [70], 39.21% in Palestine, Tigabu et al. [71], 39.84% in Ethiopia, Walana et al. [72], 42.9% in Ghana, Suliman et al. [73], 54.2% in Sudan, Karshima, [74], 54.8% in Nigeria, Kimosop et al. [75], 56% in Kenya, Pasaribu et al. [76], 57.4% in Indonesia, Quihui et al. [77], 65% in Mexico, Yusuf et al. [78], 72.3% in Malaysia, Nsagha et al. [79], 74.3% in Cameroon, Erismann et al. [80], 84.7% in Burkina Faso, Osman et al. [81], 85% in Libya, and hamady obeid Al-Taei, [82], 98.8% in Iraq. This difference could be attributed to changes in hygiene and sanitation, environmental pollution, and the specificity and sensitivity of the diagnostic procedures used in various investigations.
In this study, the high pooled prevalence of IPs and EBIs among food handlers of the selected region was reported in the Oromia region at 38.56% (95% CI: 29.98, 47.14), followed by SNNPR 31.03% (95% CI: 19.66, 42.40), Amhara 24.36% (95% CI: 9.67, 29.05), and the Tigray region at 19.45% (95% CI: 6.08, 32.82). Ethiopia and Nepal both reported similar findings by Tegen et al. [35] and Chandrashekhar et al. [83], respectively. This variation within a country regions may be due to differences in study area, year of study, urbanization, food safety, and hygiene awareness and sociodemographic characteristics of the society.
In the current investigation, Ascaris lumbricoides was the most prevalent intestinal parasite with a pooled prevalence of 7.58%. This was consistent with the findings of a similar study conducted in South Asia [84], South Africa [85], Nigeria [86], and in different regions of Ethiopia [21, 25, 30, 87–89]. The current study found a high prevalence of A. lumbricoides, which could be attributed to the high level of environmental contamination caused by a number of infected people, the durability of Ascaris eggs under varying environmental conditions, the high fertility, and the sticky nature of the Ascaris egg shell, which aids in its attachment to human hands, fruits, and vegetables.
In this meta-analysis, the pooled prevalence of Entamoeba histolytica/dispar was 6.78%. It comparatively agrees with the reports from Mexico (5%) by Quihui et al. [77], Ethiopia (6.4%) by Yeshanew et al. [56], and Cameroon (7.3%) by Nsagha et al. [79]. However, the outcome of this investigation was higher than that of previous studies conducted by Walana et al. [72] in Ghana (0.21%), Bahrami et al. [90] in Iran (0.6%), Sanprasert et al. [69] in Thailand (0.73%), and Ismail [64] in Saudi Arabia (2%). In comparison, the findings of the current study were lower than those of previous investigations conducted in Sudan (31.2%) by Suliman et al. [73], in Côte d'Ivoire (56%) by Coulibaly et al. [65], in Burkina Faso (66.5%) by Erismann et al. [80], and in Iraq (88%) by Hamady obeid Al-Taei [82]. The variance could be attributed to the quality of the food and water of the various study locations, as well as their ambient conditions. Since E. histolytica/dispar is a pollutant in drinking water and food as such it can easily spread through drinking polluted water and eating contaminated vegetables and foods.
The pooled prevalence of G. lamblia in this meta-analysis was 3.67%, which is comparatively consistent with previous investigations conducted by Ismail [64] in Saudi Arabia (3%), Nsagha et al. [79] in Cameroon (3.3%), Rahi and Majeed, [91] in Iraq (4%), Sanprasert et al. [69] in Thailand (4.2%), Ghenghesh et al. [92] in Libya (4.9%), Okyay et al. [93] in Turkey (6.1%), and Kimosop et al. [75] in Kenya (6.5%). However, it was lower than in previous findings carried out in Ethiopia (10.03%) by Tegen et al. [35], Tanzania (10.6%) by Venkatajothi [66], Iraq (10.8%) by hamady obeid Al-Taei [82], Ghana (12.2%) by Walana et al. [72], Nepal (12.5%) by Erismann et al. [80], Côte d'Ivoire (13.1%) by Coulibaly et al. [65], Dhaka (17.6%) by Shahid et al. [94], Spain (18%) by Reh et al. [61], Philippines (19.2%) by Weerakoon et al. [95], Senegal (20.4%) by Sylla et al. [68], Sudan (22.9%) by Suliman et al. [73], Mexico (24%) by Quihui et al. [77], Burkina Faso (28.1%) by Erismann et al. [80], Libya (28.5%) by Osman et al. [81], Democratic Republic Congo (31.5%) by Liao et al. [60], Turkey (47.97%) by Doni et al. [96], and Bulgaria (62.05%) by Harizanov et al. [62]. However, the prevalence of G. lamblia (3.67%) in this study was significantly higher than that of Punsawad et al. [97] in Thailand (0.6%) and Bahrami et al. [90] in Iran (1.7%). The discrepancy might be attributed to educational status, insufficient sanitary surveillance by the regulatory team, and a lack of hand washing facilities in the workplace.
The pooled prevalence of hookworm was 2.70%, which is in agreement with other Ethiopian studies by Aklilu et al. [25] in Addis Ababa (2.1%) and Sahlemariam and Mekete [30] in Jimma (2.9%). The high frequency of such parasite could be attributed to poor personal hygiene and the parasite's simple mode of transmission, which is commonly found in contaminated soil, or surfaces contaminated with feces. Additionally, the disparity between studies could be explained by differences in eating habits, climate conditions, and other sociocultural variances between the location of the research and the year.
In the current study, the prevalence of Salmonella was (2.78%), which was comparable with a study from Motta town (2.5%) by Yesigat et al. [54], Sudan (3.8%) by Saeed and Hamid. [16], Debre Markos University (3.6%) by Mengist et al. [98] and Addis Ababa (3.8%) by Belhu et al. [34]. However, a higher prevalence of Salmonella was reported from Nigeria (5.5%) by Mobolaji and Olubunmi [99], Dire Dawa city (6%) by Tadesse et al. [100], Arbaminch (6.9%) by Mama and Alemu [41], and Wolyta Sodo (9.1%) by Solomon et al. [45] compared to the current finding. These differences in Salmonella prevalence may be related to differences in personal hygiene and geographic location.
In this finding, the pooled prevalence of Shigella was 0.61%, which is comparatively in line with the findings of Dire Dawa city (1.7%) by Tadesse et al. [100], Motta Town (1.6%) by Yesigat et al. [54], and Bahir Dar University (1.2%) by Abera et al. [42]. As compared to the current study, a higher prevalence of Shigella was reported from Gondar University (2.7%) by Dagnew et al. [101] and Gondar town (10.1%) by Getie et al. [102]. This difference could be attributed to the culture medium used and to the geographical distribution.
Food handlers who did not receive food hygiene training were 0.68 times more likely to have IPs and EBIs than those who had received food hygiene training. It is supported by other studies conducted elsewhere in Ethiopia by Abera et al. [103], Nigusse et al. [21], and Gizaw et al. [104]. This could be due to differences in the number of institutions engaged in safety, employers' proclivity to hire food handlers without considering a health certificate as a basic criterion, and low monthly wage (paid) for food handlers in other study locations.
The pooled results showed that food handlers with untrimmed fingernail and poor cleanness habits were 2.23 times more likely to be infected with IPs and EBIs than their counterparts. This finding is supported by studies conducted in Ethiopia by Tegen et al. [35], by Eshetu et al. [105] in Sri Lanka by Galgamuwa et al. [106] and in Nepal by Sah et al. [107]. Due to the difficulties of cleaning, untrimmed fingernails among food handlers could serve as a vehicle for transporting IPs and EBIs from source to food. Food handlers with untrimmed fingernails may also contaminate food while serving customers if they are infected, and they can be identified as possible public health threats. Furthermore, this may most likely owing to a lack of awareness, poor hygiene practices, and sociodemographic characteristics among the food handlers.
The probability of IPs and EBIs was 1.52 times higher among food handlers who did not attend a medical checkup than among their counterparts. It agrees with earlier studies conducted in Ethiopia by Marami et al. [29] and Gezehegn et al. [43]. Therefore, it is better Ethiopian food handlers to update their medical certificates every three months that reduces the prevalence of IPs and EBIs among them and the wide customers.
The odds of having IPs and EBIs were 1.97 times higher among food handlers who did not wash their hands before food handling with soap and water than among people who wash their hands. This finding was in line with the previous study conducted in Ethiopia by Tegen et al. [35], in Nigeria by Amuta et al. [108], in Indonesia by Pasaribu et al. [76], and in Cameroon by Tchakounté et al. [109]. This could be due to effective hand washing techniques interrupt the chain of transmission for IPs and EBIs.
The odds of having IPs and EBIs were 2.63 times higher among food handlers who had habits of eating raw meat and vegetables as compared with the counterparts. It agrees with the findings conducted in Ethiopia by Tolera and Dufera [110] and Alemu et al. [111] and outside in Sri Lanka by Galgamuwa et al. [106] and in Libya by Osman et al. [81]. This could be due to raw meat and unwashed vegetables can carry IPs and EBIs causing pathogens.
4.1. Limitation of the Study
A small number of published papers met the inclusion criteria and were involved in the current finding.
5. Conclusion
The pooled prevalence of IPs and EBIs was 29.16%. Ascaris lumbricoides, Entamoeba histolytica/dispar, Giardia lamblia, and hookworm were the most prevalent IPs among food handlers with a pooled prevalence of 7.58%, 6.78%, 3.67%, and 2.70%, respectively. Salmonella and Shigella spp. were the most prevalent EBIs among Ethiopian food handlers with a pooled prevalence of 2.78% and 0.61%, respectively. Food handlers who did not receive food hygiene training, untrimmed finger nail, eating raw vegetables and meat, lack of periodic medical checkup and hand washing habits were factors significantly associated with the prevalence of IPs and EBIs. Increasing food handler's knowledge about personal hygienic conditions and periodic medical checkup for IPs and EBIs could consider an appropriate intervention measure.
Abbreviations
- AOR:
Adjusted odds ratio
- CI:
Confidence interval
- EBIs:
Enteric bacterial infections
- GRADE:
Grading of Recommendations Assessment, Development and Evaluation
- IPs:
Intestinal parasites
- PRISMA:
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SNNPR:
Southern Nations, Nationalities, and Peoples' Region
- STATA:
Statistical software for data science.
Data Availability
All data sets have been presented within the manuscript and on supplementary data. The dataset supporting the conclusions of this article is available from the correspondence author upon a formal request.
Conflicts of Interest
The authors declare that theyhave no conflicts of interest regarding the publication of this paper.
Authors' Contributions
The authors designed the project and actively participated in study selection, quality evaluation, and data extraction, AG and AA analyzed the data, and AA wrote the manuscript and edited by AG. All the authors have read and approved the final manuscript.
Supplementary Materials
S1: pooled prevalence of IPs and EBIs among food handlers by sample size. S2: pooled prevalence of IPs and EBIs among food handlers from 2014 to 2022. S3: pooled prevalence of IPs and EBIs among food handlers by study area. S4: food hygiene training as an associated risk factor for IPs and EBIs among food handlers. S5: fingers nail status as an associated risk factor for IPs and EBIs among food handlers. S6: medical checkup as an associated risk factor for IPs and EBIs among food handlers. S7: hand washing habit before food handling as an associated risk factor for IPs and EBIs among food handlers. S8: eating raw vegetables and meat as an associated risk factor for IPs and EBIs among food handlers.
References
- 1.Girma A., Aemiro A. Evaluation of soil streptomyces isolates from north-western Ethiopia as potential inhibitors against spoilage and foodborne bacterial pathogens. Journal of Chemistry . 2022;2022:12. doi: 10.1155/2022/5547406.5547406 [DOI] [Google Scholar]
- 2.Girma A., Aemiro A. Antibacterial activity of lactic acid bacteria isolated from fermented Ethiopian traditional dairy products against food spoilage and pathogenic bacterial strains. Journal of Food Quality . 2021;2021:10. doi: 10.1155/2021/9978561.9978561 [DOI] [Google Scholar]
- 3.Who. The global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007-2015. 2015. https://repository.gheli.harvard.edu/repository/10998/
- 4.Scharff R. L. Economic burden from health losses due to foodborne illness in the United States. Journal of Food Protection . 2012;75(1):123–131. doi: 10.4315/0362-028x.jfp-11-058. [DOI] [PubMed] [Google Scholar]
- 5.Adane M., Teka B., Gismu Y., Halefom G., Ademe M. Food hygiene and safety measures among food handlers in street food shops and food establishments of Dessie town, Ethiopia: a community-based cross-sectional study. PLoS One . 2018;13(5) doi: 10.1371/journal.pone.0196919.e0196919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Who. National food safety systems in africa: a situation analysis. 2005. https://www.afro.who.int/publications/national-food-safety-systems-africa-situation-analysis .
- 7.Norhayati M., Fatmah M. S., Yusof S., Edariah A. B. Intestinal parasitic infections in man: a review. Medical Journal of Malaysia . 2003;58(2) [PubMed] [Google Scholar]
- 8.Ayeh-Kumi P. Prevalence of intestinal parasitic infections among food vendors in Accra, Ghana. Journal of Tropical Medicine and Parasitology . 2009;32(1):1–8. [Google Scholar]
- 9.Chan M. S. The global burden of intestinal nematode infections—fifty years on. Parasitology Today . 1997;13(11):438–443. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]
- 10.De Silva N. R., Brooker S., Hotez P. J., Montresor A., Engels D., Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends in Parasitology . 2003;19(12):547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Pham Duc P., Nguyen-Viet H., Hattendorf J., Zinsstag J., Dac Cam P., Odermatt P. Risk factors for Entamoeba histolytica infection in an agricultural community in Hanam province, Vietnam. Parasites and Vectors . 2011;4(1):102–109. doi: 10.1186/1756-3305-4-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yimam Y., Woreta A., Mohebali M. Intestinal parasites among food handlers of food service establishments in Ethiopia: a systematic review and meta-analysis. BMC Public Health . 2020;20(1):73–12. doi: 10.1186/s12889-020-8167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majowicz S. E., Musto J., Scallan E., et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clinical Infectious Diseases . 2010;50(6):882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 14.Crump J. A., Luby S. P., Mintz E. D. The global burden of typhoid fever. Bulletin of the World Health Organization . 2004;82(5):346–353. [PMC free article] [PubMed] [Google Scholar]
- 15.Wayne P. Clinical and laboratory standards institute. 2011. https://en.wikipedia.org/wiki/Clinical_and_Laboratory_Standards_Institute .
- 16.Saeed H. A., Hamid H. H. Bacteriological and parasitological assessment of food handlers in the Omdurman area of Sudan. Journal of Microbiology, Immunology, and Infection . 2010;43(1):70–73. doi: 10.1016/s1684-1182(10)60010-2. [DOI] [PubMed] [Google Scholar]
- 17.Tessema A. G., Gelaye K. A., Chercos D. H. Factors affecting food handling Practices among food handlers of Dangila town food and drink establishments, North West Ethiopia. BMC Public Health . 2014;14(1):571–575. doi: 10.1186/1471-2458-14-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudlová A., Juris P., Jurisova S., Jarcuska P., Krcmery V. Epidemiology and geographical distribution of gastrointestinal parasitic infection in humans in Slovakia. Helminthologia . 2016;53(4):309–317. doi: 10.1515/helmin-2016-0035. [DOI] [Google Scholar]
- 19.Faria C. P., Zanini G. M., Dias G. S., et al. Geospatial distribution of intestinal parasitic infections in Rio de Janeiro (Brazil) and its association with social determinants. PLoS Neglected Tropical Diseases . 2017;11(3) doi: 10.1371/journal.pntd.0005445.e0005445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adank M. Looking beyond headline indicators: water and sanitation services in small towns in Ethiopia. Journal of Water . 2016;6(3):435–446. [Google Scholar]
- 21.Nigusse D., Kumie A. Food hygiene practices and prevalence of intestinal parasites among food handlers working in Mekelle university student’s cafeteria, Mekelle. Global Advanced Research Journal of Social Science . 2012;1(4):65–71. [Google Scholar]
- 22.Wadilo F., Solomon F., Arota A., Abraham Y. Intestinal parasitic infection and associated factors among food handlers in South Ethiopia: a case of Wolaita Sodo town. Journal of Pharmacy and Alternative Medicine . 2016;12:5–10. [Google Scholar]
- 23.Garedew-Kifelew L., Wondafrash N., Feleke A. Identification of drug-resistant Salmonella from food handlers at the University of Gondar, Ethiopia. BMC Research Notes . 2014;7(1):545–546. doi: 10.1186/1756-0500-7-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiruneh M. Serodiversity and antimicrobial resistance pattern of Shigella isolates at Gondar University teaching hospital, Northwest Ethiopia. Japanese Journal of Infectious Diseases . 2009;62(2):93–97. [PubMed] [Google Scholar]
- 25.Aklilu A., Kahase D., Dessalegn M., et al. Prevalence of intestinal parasites, Salmonella and Shigella among apparently health food handlers of Addis Ababa University student’s cafeteria, Addis Ababa, Ethiopia. BMC Research Notes . 2015;8(1):1–6. doi: 10.1186/s13104-014-0967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moges D., Daniel A., Yimtubezinash W., Demiss N. Prevalence of intestinal parasites and Salmonella and Shigella among food handlers at food service establishments in the main campus and Health Sciences College of Hawassa University, Hawassa, Ethiopia. The Ethiopian Journal of Health Development . 2014;28(1) [Google Scholar]
- 27.Dagnew M., Tiruneh M., Moges F., Tekeste Z. Survey of nasal carriage of Staphylococcus aureus and intestinal parasites among food handlers working at Gondar University, Northwest Ethiopia. BMC Public Health . 2012;12(1) doi: 10.1186/1471-2458-12-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gebreyesus A., Adane K., Negash L., et al. Prevalence of Salmonella typhi and intestinal parasites among food handlers in Mekelle University student cafeteria, Mekelle, Ethiopia. Food Control . 2014;44:45–48. doi: 10.1016/j.foodcont.2014.03.040. [DOI] [Google Scholar]
- 29.Marami D., Hailu K., Tolera M. Prevalence and associated factors of intestinal parasitic infections among asymptomatic food handlers working at Haramaya University cafeterias, eastern Ethiopia. Annals of Occupational and Environmental Medicine . 2018;30(1):53–57. doi: 10.1186/s40557-018-0263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahlemariam Z., Mekete G. Examination of fingernail contents and stool for ova, cyst and larva of intestinal parasites from food handlers working in student cafeterias in three Higher Institutions in Jimma. Ethiopian Journal of Health Sciences . 2001;11(2) [Google Scholar]
- 31.Feleke D. G., Wage E. K., Getachew T., Gedefie A. Intestinal parasitic infections and associated factors among street dwellers’ in Dessie town, North-East Ethiopia: a cross sectional study. BMC Research Notes . 2019;12(1):262–265. doi: 10.1186/s13104-019-4302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ameya G., Zerdo Z., Tesfaye M., et al. Intestinal parasite infections and associated factors among inmates of Arba Minch prison, southern Ethiopia: cross sectional study. BMC Infectious Diseases . 2019;19(1):1086–1088. doi: 10.1186/s12879-019-4703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daba S. B. Prevalence of Intestinal Parasitic Infections Among Food Handlers and Microbial Safety of Ready-To-Eat Foods in Selected Orphanage Centers in Addis Ababa . Addis Ababa, Ethiopia: Addis Ababa University; 2010. [Google Scholar]
- 34.Belhu T., Fissehatsion K., Tesfaye A., Woldekidan D. Y., Desta K. Prevalence of intestinal parasites and gastrointestinal carriage of pathogenic gram negative enteric bacteria among apparently healthy food handlers of public hospitals, Addis Ababa, Ethiopia. International Journal of Microbiology . 2020;2020:9. doi: 10.1155/2020/8867033.8867033 [DOI] [Google Scholar]
- 35.Tegen D., Damtie D., Hailegebriel T. Prevalence and associated risk factors of human intestinal protozoan parasitic infections in Ethiopia: a systematic review and metaanalysis. Journal of Parasitology Research . 2020;2020:15. doi: 10.1155/2020/8884064.8884064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girma A., Tamir D. Prevalence of bovine mastitis and its associated risk factors among dairy cows in Ethiopia during 2005-2022: A Systematic Review and Meta-Analysis. Veterinary Medicine International . 2022;2022:19. doi: 10.1155/2022/7775197.7775197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D., Liberati A., Tetzlaff J., Altman D. G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine . 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 38.Atkins D., Eccles M., Flottorp S., et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE Working Group. BMC Health Services Research . 2004;4(1):38–47. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tefera T., Mebrie G. Prevalence and predictors of intestinal parasites among food handlers in Yebu town, southwest Ethiopia. PLoS One . 2014;9(10) doi: 10.1371/journal.pone.0110621.e110621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mama M., Alemu G. Prevalence and factors associated with intestinal parasitic infections among food handlers of Southern Ethiopia: cross sectional study. BMC Public Health . 2015;16(1):105–107. doi: 10.1186/s12889-016-2790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mama M., Alemu G. Prevalence, antimicrobial susceptibility patterns and associated risk factors of Shigella and Salmonella among food handlers in Arba Minch University, South Ethiopia. BMC Infectious Diseases . 2016;16(1):686–687. doi: 10.1186/s12879-016-2035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abera B., Yitayew G., Amare H. Salmonella serotypeTyphi, Shigella, and intestinal parasites among food handlers at Bahir Dar University, Ethiopia. The Journal of Infection in Developing Countries . 2016;10(2):121–126. doi: 10.3855/jidc.6890. [DOI] [PubMed] [Google Scholar]
- 43.Gezehegn D., Abay M., Tetemke D., et al. Prevalence and factors associated with intestinal parasites among food handlers of food and drinking establishments in Aksum Town, Northern Ethiopia. BMC Public Health . 2017;17(1):819–9. doi: 10.1186/s12889-017-4831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girma H., Beyene G., Mekonnen Z. Prevalence of intestinal parasites among food handlers at cafeteria of Jimma University specialized hospital, Southwest Ethiopia. Asian Pacific Journal of Tropical Disease . 2017;7(8):467–471. doi: 10.12980/apjtd.7.2017d7-20. [DOI] [Google Scholar]
- 45.Solomon F. B., Wada F. W., Anjulo A. A., Koyra H. C., Tufa E. G. Burden of intestinal pathogens and associated factors among asymptomatic food handlers in South Ethiopia: emphasis on salmonellosis. BMC Research Notes . 2018;11(1):502–506. doi: 10.1186/s13104-018-3610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asires A., Wubie M., Reta A. Prevalence and associated factors of intestinal parasitic infections among food handlers at prison, east and west Gojjam, Ethiopia. Advances in Medicine . 2019;8 doi: 10.1155/2019/2101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alemnew B., Belay Y., Demis A. Magnitude of intestinal parasitic infections and associated factors among food handlers working at Woldia University student’s cafeteria, Northeastern Ethiopia: an institution based cross-sectional study. BMC Research Notes . 2019;12(1):736–737. doi: 10.1186/s13104-019-4777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kebede E., Seid A., Akele S. Prevalence and associated risk factors of intestinal parasitic infections among asymptomatic food handlers in Wollo University student’s cafeteria, Northeastern Ethiopia. BMC Research Notes . 2019;12(1):1–6. doi: 10.1186/s13104-019-4182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumma W. P., Meskele W., Admasie A. Prevalence of intestinal parasitic infections and associated factors among food handlers in Wolaita Sodo University students caterings, Wolaita Sodo, Southern Ethiopia: a cross-sectional study. Frontiers in Public Health . 2019;7:p. 140. doi: 10.3389/fpubh.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alemu A. S., Baraki A. G., Alemayehu M., Yenit M. K. The prevalence of intestinal parasite infection and associated factors among food handlers in eating and drinking establishments in Chagni Town, Northwest Ethiopia. BMC Research Notes . 2019;12(1):302–306. doi: 10.1186/s13104-019-4338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuti K. A., Nur R. A., Donka G. M., Kerbo A. A., Roba A. E. Predictors of intestinal parasitic infection among food handlers working in Madda Walabu University, Ethiopia: a cross-sectional study. Interdisciplinary Perspectives on Infectious Diseases . 2020;2020:8. doi: 10.1155/2020/9321348.9321348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diriba K., Awulachew E., Ashuro Z. Prevalence and antimicrobial resistance pattern of Salmonella, Shigella, and intestinal parasites and associated factor among food handlers in Dilla University student cafeteria, Dilla, Ethiopia. International Journal of Microbiology . 2020;2020:10. doi: 10.1155/2020/3150539.3150539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Legese H., Kahsay T., Gebrewahd A., et al. Prevalence, antimicrobial susceptibility pattern, and associated factors of Salmonella and Shigella among food handlers in Adigrat University student’s cafeteria, northern Ethiopia, 2018. Tropical Diseases, Travel Medicine and Vaccines . 2020;6(1):19–9. doi: 10.1186/s40794-020-00119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yesigat T., Jemal M., Birhan W. Prevalence and associated risk factors of Salmonella, Shigella, and intestinal parasites among food handlers in Motta town, North West Ethiopia. The Canadian Journal of Infectious Diseases and Medical Microbiology . 2020;11 doi: 10.1155/2020/6425946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Regassa K., Tedla K., Bugssa G., Gebrekirstos G., Gebreyesus H., Shfare M. T. Prevalence and factors associated with intestinal parasites among food handlers in Medebay Zana District, north West Tigray, northern Ethiopia. Tropical Diseases, Travel Medicine and Vaccines . 2021;7(1):2–6. doi: 10.1186/s40794-020-00123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeshanew S., Tadege M., Abamecha A. Prevalence and associated factors of intestinal parasitic infections among food handlers in Mettu Town, Southwest Ethiopia. Journal of Tropical Medicine . 2021;2021:5. doi: 10.1155/2021/6669734.6669734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumalo A., Gambura E., Dodicho T., et al. Prevalence of intestinal parasites and Salmonella typhi among food handlers working in catering establishments of public institutes found in Dawuro Zone, South-Western Ethiopia. Journal of Parasitology Research . 2021;2021:10. doi: 10.1155/2021/8889302.8889302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gemechu T., Eshetu T., Kassa T., Jarso H. Assessment of intestinal parasites, enteric bacterial infections, and antimicrobial susceptibility among street food handlers in Jimma town, southwest Ethiopia. Journal of Tropical Medicine . 2022;2022:8. doi: 10.1155/2022/5483367.5483367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teferi S. C., Sebsibe I., Adibaru B. Food safety practices and associated factors among food handlers of fiche town, north shewa zone, Ethiopia. Journal of Environmental and Public Health . 2021;2021:7. doi: 10.1155/2021/6158769.6158769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao C.-W., Fu C. J., Kao C. Y., et al. Prevalence of intestinal parasitic infections among school children in capital areas of the Democratic Republic of São Tomé and Príncipe, West Africa. African Health Sciences . 2016;16(3):690–697. doi: 10.4314/ahs.v16i3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reh L., Muadica A. S., Koster P. C., et al. Substantial prevalence of enteroparasites cryptosporidium spp., Giardia duodenalis and blastocystis sp. in asymptomatic schoolchildren in madrid, Spain, november 2017 to June 2018. Euro Surveillance . 2019;24(43) doi: 10.2807/1560-7917.es.2019.24.43.1900241.1900241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harizanov R., Rainova I., Tsvetkova N., et al. Prevalence of intestinal parasitic infections among the Bulgarian population over a three year period (2015–2017) Helminthologia . 2020;57(1):12–18. doi: 10.2478/helm-2020-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staudacher O., Heimer J., Steiner F., et al. Soil‐transmitted helminths in southern highland R wanda: associated factors and effectiveness of school‐based preventive chemotherapy. Tropical Medicine and International Health . 2014;19(7):812–824. doi: 10.1111/tmi.12321. [DOI] [PubMed] [Google Scholar]
- 64.Ka I. Prevalence of intestinal parasitic infection among school children in Taif. Insights Biomed . 2018;3 doi: 10.21767/2572-5610.10045. [DOI] [Google Scholar]
- 65.Coulibaly G., Ouattara M., Dongo K., et al. Epidemiology of intestinal parasite infections in three departments of south-central Côte d’Ivoire before the implementation of a cluster-randomised trial. Parasite Epidemiology and Control . 2018;3(2):63–76. doi: 10.1016/j.parepi.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rvenkatajothi D. Incidence of intestinal protozoa infections among school going children. International Journal of Current Research in Medical Sciences . 2017;3(4):54–58. doi: 10.22192/ijcrms.2017.03.04.008. [DOI] [Google Scholar]
- 67.Abu-Madi M. A., Behnke J. M., Boughattas S., Al-Thani A., Doiphode S. H. A decade of intestinal protozoan epidemiology among settled immigrants in Qatar. BMC Infectious Diseases . 2016;16(1):370–379. doi: 10.1186/s12879-016-1728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sylla K., Tine R. K., Sow D., et al. Epidemiological profile of intestinal parasitic infection among preschool and school children living in a rural community in Senegal: a cross sectional survey. Journal of Bacteriology and Parasitology . 2018;9(4):1–7. [Google Scholar]
- 69.Sanprasert V., Srichaipon N., Bunkasem U., Srirungruang S., Nuchprayoon S. Prevalence of intestinal protozoan infections among children in Thailand: a large-scale screening and comparative study of three standard detection methods. Southeast Asian Journal of Tropical Medicine and Public Health . 2016;47(6):1123–1133. [PubMed] [Google Scholar]
- 70.Al-Jawabreh A., Ereqat S., Dumaidi K., Al-Jawabreh H., Abdeen Z., Nasereddin A. Prevalence of selected intestinal protozoan infections in marginalized rural communities in Palestine. BMC Public Health . 2019;19(1):1667–1711. doi: 10.1186/s12889-019-8024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tigabu A., Taye S., Aynalem M., Adane K. Prevalence and associated factors of intestinal parasitic infections among patients attending Shahura Health Center, Northwest Ethiopia. BMC Research Notes . 2019;12(1):333–338. doi: 10.1186/s13104-019-4377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walana W., Tay S. C. K., Tetteh P., Ziem J. B. Prevalence of Intestinal Protozoan Infestation Among Primary School Children in Urban and Peri-Urban Communities in Kumasi . 2014. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.920.5097&rep=rep1&type=pdf . [Google Scholar]
- 73.Suliman M. A., Magboul A. M., Mohammed H. Y., et al. Prevalence of intestinal parasitic infections and associated risk factors among school children in White Nile State, Sudan. Journal of Infectious Disease and Diagnosis . 2019;4(125):p. 2. [Google Scholar]
- 74.Karshima S. N. Prevalence and distribution of soil-transmitted helminth infections in Nigerian children: a systematic review and meta-analysis. Infectious Diseases of Poverty . 2018;7(1):69–14. doi: 10.1186/s40249-018-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kimosop R. J., Mulambalah C., Ngeiywa M. M. Prevalence of enteric parasitic diseases among patients referred at a teaching hospital in kenya. 2018. https://www.jhrr.org/article.asp?issn=2394-2010;year=2018;volume=5;issue=2;spage=78;epage=85;aulast=Kimosop;type=0 .
- 76.Pasaribu A. P., Alam A., Sembiring K., Pasaribu S., Setiabudi D. Prevalence and risk factors of soil-transmitted helminthiasis among school children living in an agricultural area of North Sumatera, Indonesia. BMC Public Health . 2019;19(1):1066–1068. doi: 10.1186/s12889-019-7397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quihui L., Valencia M. E., Crompton D. W., et al. Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health . 2006;6(1):225–228. doi: 10.1186/1471-2458-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yusuf N. A., Yusri Y. M., Ismail N., Vythilingam I. Prevalence of intestinal protozoa in an aborigine community in Pahang, Malaysia. Tropical Biomedicine . 2007;24(1):55–62. [PubMed] [Google Scholar]
- 79.Shey Nsagha D., Anna Njunda L., Jules Clement Assob N., et al. Prevalence and predisposing factors to intestinal parasitic infections in HIV/AIDS patients in Fako division of Cameroon. American Journal of Epidemiology and Infectious Disease . 2017;5(3):42–49. doi: 10.12691/ajeid-5-3-1. [DOI] [Google Scholar]
- 80.Erismann S., Diagbouga S., Odermatt P., et al. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the Plateau Central and Centre-Ouest regions of Burkina Faso. Parasites and Vectors . 2016;9(1):554–614. doi: 10.1186/s13071-016-1835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Osman M., El Safadi D., Cian A., et al. Prevalence and risk factors for intestinal protozoan infections with Cryptosporidium, Giardia, Blastocystis and Dientamoeba among schoolchildren in Tripoli, Lebanon. PLoS Neglected Tropical Diseases . 2016;10(3) doi: 10.1371/journal.pntd.0004496.e0004496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Taei A. The prevalence of intestinal parasite among the attending peoples to Al-Hashimyah hospitals for seven years, Babylon province, Iraq. Journal of Physics: Conference Series . 2019;1294(6) doi: 10.1088/1742-6596/1294/6/062022. [DOI] [Google Scholar]
- 83.Chandrashekhar T. S., Joshi H. S., Gurung M., Subba S. H., Rana M. S., Shivananda P. G. Prevalence and distribution of intestinal parasitic infestations among school children in kaski district. 2020. http://www.bioline.org.br/pdf?jm05011 .
- 84.Silver Z. A., Kaliappan S. P., Samuel P., et al. Geographical distribution of soil transmitted helminths and the effects of community type in South Asia and South East Asia–A systematic review. PLoS Neglected Tropical Diseases . 2018;12(1) doi: 10.1371/journal.pntd.0006153.e0006153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sacolo-Gwebu H., Chimbari M., Kalinda C. Prevalence and risk factors of schistosomiasis and soil-transmitted helminthiases among preschool aged children (1–5 years) in rural KwaZulu-Natal, South Africa: a cross-sectional study. Infectious Diseases of Poverty . 2019;8(1):47–12. doi: 10.1186/s40249-019-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Egbuobi R. C., Nwagbaraocha M. A., Dike-Ndudim J. N., et al. Incidence of intestinal parasites among food handlers (hawkers) around the University of Nigeria teaching hospital Enugu, Enugu state, Nigeria. Open Journal of Medical Microbiology . 2014;4(1):23–28. doi: 10.4236/ojmm.2014.41004. [DOI] [Google Scholar]
- 87.Andargie G., Kassu A., Moges F., Tiruneh M., Huruy K. Prevalence of bacteria and intestinal parasites among food-handlers in Gondar town, northwest Ethiopia. Journal of Health, Population and Nutrition . 2008;26(4):451–455. doi: 10.3329/jhpn.v26i4.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abate A., Kibret B., Bekalu E., et al. Cross-sectional study on the prevalence of intestinal parasites and associated risk factors in teda health centre, northwest ethiopia. 2013. https://pubmed.ncbi.nlm.nih.gov/27335860/ [DOI] [PMC free article] [PubMed]
- 89.Mariam S. T., Roma B., Sorsa S., Worku S., Erosie L. Assessment of sanitary and hygienic status of catering establishments of Awassa Town. The Ethiopian Journal of Health Development . 2000;14(1):91–98. doi: 10.4314/ejhd.v14i1.9934. [DOI] [Google Scholar]
- 90.Bahrami F., Haghighi A., Zamini G., Khadem-Erfan M. B., Azargashb E. Prevalence and associated risk factors of intestinal parasitic infections in Kurdistan province, northwest Iran. Cogent Medicine . 2018;5(1) doi: 10.1080/2331205x.2018.1503777.1503777 [DOI] [Google Scholar]
- 91.Rahi A. A., Majeed L. Epidemiological study of intestinal protozoa at Wasit province. Journal of Orthopaedic and Sports Physical Therapy . 2019;1(3):26–28. [Google Scholar]
- 92.Ghenghesh K. S., Ghanghish K., BenDarif E. T., Shembesh K., Franka E. Prevalence ofEntamoeba histolytica, Giardia lamblia, andCryptosporidiumspp. in Libya: 2000–2015. Libyan Journal of Medicine . 2016;11(1) doi: 10.3402/ljm.v11.32088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okyay P., Ertug S., Gultekin B., Onen O., Beser E. Intestinal parasites prevalence and related factors in school children, a western city sample-Turkey. BMC Public Health . 2004;4(1):64–66. doi: 10.1186/1471-2458-4-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shahid S. B., Wazib A., Chowdhury A., Shamsuzzaman S. M., Mamun K. Z. A study on different laboratory methods for diagnosis of intestinal protozoal infections. Bangladesh Medical Journal . 2014;40(2):47–49. doi: 10.3329/bmj.v40i2.18510. [DOI] [Google Scholar]
- 95.Weerakoon K. G., Gordon C. A., Williams G. M., et al. Co-parasitism of intestinal protozoa and Schistosoma japonicum in a rural community in the Philippines. Infectious Diseases of Poverty . 2018;7(1):121–134. doi: 10.1186/s40249-018-0504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doni N. Y., Gurses G., Simsek Z., Zeyrek F. Y. Prevalence and associated risk factors of intestial parasites among children of farm workers in the southeastern Anatolian region of Turkey. Annals of Agricultural and Environmental Medicine . 2015;22(3) doi: 10.5604/12321966.1167709. [DOI] [PubMed] [Google Scholar]
- 97.Punsawad C., Phasuk N., Bunratsami S., Thongtup K., Siripakonuaong N., Nongnaul S. Prevalence of intestinal parasitic infection and associated risk factors among village health volunteers in rural communities of southern Thailand. BMC Public Health . 2017;17(1):564–569. doi: 10.1186/s12889-017-4486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mengist A., Mengistu G., Reta A. Prevalence and antimicrobial susceptibility pattern of Salmonella and Shigella among food handlers in catering establishments at Debre Markos University, Northwest Ethiopia. International Journal of Infectious Diseases . 2018;75:74–79. doi: 10.1016/j.ijid.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 99.Mobolaji O., Olubunmi O. Assessment of the hygienic practices and the incidence of enteric bacteria in food handlers in small businesses in an urban area in Abeokuta. International Journal of Microbiology Reserch . 2014;5(3):41–49. [Google Scholar]
- 100.Tadesse G., Mitiku H., Teklemariam Z., Marami D. Salmonella and Shigella among asymptomatic street food vendors in the Dire Dawa city, Eastern Ethiopia: prevalence, antimicrobial susceptibility pattern, and associated factors. Environmental Health Insights . 2019;13 doi: 10.1177/1178630219853581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dagnew M., Tiruneh M., Moges F., Gizachew M. Bacterial profile and antimicrobial susceptibility pattern among food handlers at Gondar University Cafeteria, Northwest Ethiopia. Journal of Infectious Diseases and Therapy . 2013;6 [Google Scholar]
- 102.Getie M., Abebe W., Tessema B. Prevalence of enteric bacteria and their antimicrobial susceptibility patterns among food handlers in Gondar town, Northwest Ethiopia. Antimicrobial Resistance and Infection Control . 2019;8(1):111–116. doi: 10.1186/s13756-019-0566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abera B., Biadegelgen F., Bezabih B. Prevalence of Salmonella typhi and intestinal parasites among food handlers in Bahir Dar Town, Northwest Ethiopia. The Ethiopian Journal of Health Development . 2010;24(1) doi: 10.4314/ejhd.v24i1.62944. [DOI] [Google Scholar]
- 104.Gizaw Z., Gebrehiwot M., Teka Z. Food safety knowledge, attitude and associated factors of food handlers working in substandard food establishments in Gondar Town, Northwest Ethiopia. International Journal of Medical and Health Sciences Research . 2014;1(4):37–49. [Google Scholar]
- 105.Eshetu L., Dabsu R., Tadele G. Prevalence of intestinal parasites and its risk factors among food handlers in food services in Nekemte town, west Oromia, Ethiopia. Research and Reports in Tropical Medicine . 2019;10:25–30. doi: 10.2147/rrtm.s186723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galgamuwa L. S., Iddawela D., Dharmaratne S. D. Intestinal protozoa infections, associated risk factors and clinical features among children in a low-income tea plantation community in Sri Lanka. International Journal of Community Medicine and Public Health . 2016;3(9):2452–2458. doi: 10.18203/2394-6040.ijcmph20163053. [DOI] [Google Scholar]
- 107.Rb S., Paudel I. S., Baral R., Poudel P., Jha N., Pokharel P. K. A study of prevalence of intestinal protozoan infections and associated risk factors among the school children of Biratnagar Submetropolitan, eastern region of Nepal. Asian Pacific Journal of Health Sciences . 2016;3(1):181–197. doi: 10.21276/apjhs.2016.3.1.30. [DOI] [Google Scholar]
- 108.Eu A., Rs H., Sd M. Knowledge and risk factors of intestinal parasitic infections among women in Makurdi, Benue State. Asian Pacific Journal of Tropical Medicine . 2010;3(12):993–996. doi: 10.1016/s1995-7645(11)60016-3. [DOI] [Google Scholar]
- 109.Tchakounté B. N., Nkouayep V. R., Poné J. W. Soil contamination rate, prevalence, intensity of infection of geohelminths and associated risk factors among residents in Bazou (West Cameroon) Ethiopian Journal of Health Sciences . 2018;28(1):63–72. doi: 10.4314/ejhs.v28i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tolera A., Dufera M. The prevalence of soil-transmitted helminths and associated risk factors among school children at Sekela Primary School, Western Ethiopia. Journal of Parasitology Research . 2020;7 doi: 10.1155/2020/8885734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alemu G., Abossie A., Yohannes Z. Current status of intestinal parasitic infections and associated factors among primary school children in Birbir town, Southern Ethiopia. BMC Infectious Diseases . 2019;19(1):270–278. doi: 10.1186/s12879-019-3879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1: pooled prevalence of IPs and EBIs among food handlers by sample size. S2: pooled prevalence of IPs and EBIs among food handlers from 2014 to 2022. S3: pooled prevalence of IPs and EBIs among food handlers by study area. S4: food hygiene training as an associated risk factor for IPs and EBIs among food handlers. S5: fingers nail status as an associated risk factor for IPs and EBIs among food handlers. S6: medical checkup as an associated risk factor for IPs and EBIs among food handlers. S7: hand washing habit before food handling as an associated risk factor for IPs and EBIs among food handlers. S8: eating raw vegetables and meat as an associated risk factor for IPs and EBIs among food handlers.
Data Availability Statement
All data sets have been presented within the manuscript and on supplementary data. The dataset supporting the conclusions of this article is available from the correspondence author upon a formal request.


