Abstract
Haemophilus parainfluenzae is often isolated from the sputa of patients with chronic obstructive lung disease. We have investigated the immune response to this organism in patients with chronic bronchitis (n = 3) and bronchiectasis (n = 10) and in healthy controls (n = 9). Outer membrane proteins (OMPs) of H. parainfluenzae were purified for use in enzyme-linked immunosorbent and immunoblot assays. Whole-cell H. parainfluenzae preparations were used to adsorb antibodies from serum samples, which were subsequently immunoblot assayed to investigate the antibody response to surface-exposed epitopes. Levels of H. parainfluenzae-specific immunoglobulin G (IgG), but not IgA or IgM, were increased in the sera of patients with chronic obstructive lung disease compared to levels in control subjects. The species specificity of the antibody response was confirmed, although a degree of cross-reactivity with H. influenzae antigens was observed. IgA and IgG specific for OMPs of H. parainfluenzae were demonstrated to be present in the sputa and sera of five patients with chronic obstructive lung disease. Variation in the pattern and intensity of antigen recognition was observed among patients and among immunoglobulin classes. OMPs of approximately 36, 22, and 15 kDa were confirmed to possess epitopes exposed on the surface of intact H. parainfluenzae. We have demonstrated the presence of a species-specific systemic immune response to H. parainfluenzae in colonized patients. A specific antibody response was also observed in sputum, and the antigen specificity of these responses in patients with chronic obstructive lung disease was investigated for the first time. The presence of a specific immune response suggests that H. parainfluenzae may have a pathogenic role in patients with chronic obstructive lung disease.
Haemophilus parainfluenzae, a common commensal organism of the normal oropharynx, is becoming recognized as an opportunistic pathogen causing systemic diseases with a spectrum similar to that of the closely related nontypeable Haemophilus influenzae (NTHI), including endocarditis, meningitis, and bacteremia (1, 4). This species of Haemophilus has also been isolated from the sputa of patients with chronic obstructive lung disease (20), but whereas the role of NTHI as a respiratory pathogen has become established, the role of H. parainfluenzae in both acute and chronic lung infections remains to be elucidated.
The presence of a specific antibody response, over and above that observed in healthy individuals, is often used as a marker of current or previous infection by a variety of infectious agents. Studies of the immune response to NTHI in patients with chronic obstructive lung disease have been important in establishing a pathogenic role for the organism (5, 7, 10, 13). In addition, research has focused on the antigenicity of outer membrane proteins (OMPs) of NTHI (6, 8, 12, 16–18), since naturally produced or vaccine-stimulated antibodies specific for surface-exposed epitopes of these proteins are important in immune-mediated bacterial clearance mechanisms. In contrast, few studies on either the presence or specificity of the immune response to H. parainfluenzae, particularly in patients with chronic obstructive lung disease, have been performed.
The outer membrane composition of H. parainfluenzae is similar to that of other gram-negative bacteria and includes a major heat-modifiable protein of approximately 37 kDa, peptidoglycan-associated proteins (15, 27), and lipopolysaccharides (21). H. parainfluenzae also appears to exhibit diversity in OMP profiles similar to that of NTHI (21); however, in contrast to the case of NTHI, little work has been published regarding the antigenic characteristics of the major OMPs of H. parainfluenzae. Work on H. influenzae has established that this species has OMPs which include a heat-modifiable protein, P5; a porin, P2 (26, 27); and a peptidoglycan-associated lipoprotein, P6 (2). Suzuki et al. (24) have shown that the outer membrane of H. parainfluenzae also contains proteins which display homology to P2, P5, and the P6 precursor of H. influenzae. These proteins have all been considered as vaccine candidates for protection against H. influenzae infection; however, their importance as targets for antibodies in patients with chronic lung disease who are infected with H. parainfluenzae has not yet been investigated.
In order to provide evidence for or against a role for H. parainfluenzae as a pathogen in chronic lung disease, we performed a pilot study of patients with chronic bronchitis or bronchiectasis, who are frequently infected with or colonized by this species. We investigated the systemic antibody response in 13 of these patients (3 with chronic bronchitis and 10 with bronchiectasis) using an enzyme-linked immunosorbent assay (ELISA) and compared their levels of specific antibody to those in healthy controls (n = 9). The species specificity of the response has been confirmed through adsorption of serum samples with either H. parainfluenzae or H. influenzae. We also investigated the patterns of OMP recognition of immunoglobulin G (IgG) and IgA in sputa and sera from five patients with chronic obstructive lung disease and the specificity of the systemic antibody response for epitopes exposed on the intact surface of H. parainfluenzae.
MATERIALS AND METHODS
Patients and controls.
Ten patients with idiopathic bronchiectasis proven by high-resolution computed tomography scanning (seven female and three male; mean age, 69 years; range, 51 to 83 years) and three patients with Medical Research Council-defined chronic bronchitis (two female and one male; mean age, 67 years; range, 63 to 70 years), all with cough and daily production of sputum from which H. parainfluenzae was isolated regularly, were studied. All patients had evidence of long-standing airflow obstruction (mean FEV1 as percentage of predicted was 56.9 [standard error, 9.2]). Patients provided sputum from which the sol phase was obtained by centrifugation at 50,000 × g for 90 min at 4°C and venous blood from which serum was obtained by low-speed centrifugation. Samples of sputum sol phase and serum were stored at −20°C. Aliquots of sputum from each patient were also subjected to quantitative bacterial culture (19), and H. parainfluenzae was present in the samples studied at a mean of 2 × 107 CFU/ml (range, 2 × 105 to 7 × 107 CFU/ml). The identity of H. parainfluenzae was confirmed by the API NH typing system (bioMerieux, Basingstoke, United Kingdom) and by requirements for NAD (V factor) and hemin (X factor). Isolates were stored in freezing broth (10% [vol/vol] glycerol in brain heart infusion [BHI] broth) at −70°C until required for study.
Nine healthy control subjects (five female and four male; mean age, 68 years; range, 61 to 78 years) provided venous blood samples from which serum was obtained for ELISA, and a further six healthy control subjects (all female; mean age, 45 years; range, 41 to 52 years) provided serum which was pooled for immunoblot assay. Two other healthy control subjects (both male, aged 23 and 26 years) underwent sputum induction. Briefly, subjects inhaled hypertonic saline (3.6% [wt/vol] NaCl) delivered from an ultrasonic nebulizer (Ultraneb; Devilbiss Healthcare Inc., Philadelphia, Pa.) for 20 min, followed by expectoration of secretions. The sol phase of these secretions was obtained by centrifugation as for sputum and pooled for use in immunoblot assay. Samples of sputum sol phase and sera were stored at −20°C until required.
All subjects gave oral consent to the study of their samples, and the regular study of samples from patients with bronchiectasis and chronic bronchitis (and suitable control subjects) had received approval from the local research ethics committee (South Birmingham Health Authority Local Research Ethical Committee).
ELISA for antibodies to H. parainfluenzae.
The capture antigen was prepared as follows. Eight distinct isolates of H. parainfluenzae (distinguished by differences in OMP profiles by sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE]) were grown overnight in BHI broth (75 ml per isolate). The bacteria were harvested by centrifugation (10,000 × g at 4°C for 10 min) and washed by resuspension in 10 ml of phosphate-buffered saline (PBS; pH 7.4), followed by centrifugation as before. Each isolate was then resuspended in 1 ml of PBS, and all isolates were combined. The bacteria were sonicated (four bursts of 15 s, setting 6; Soniprobe; Lucas Dawe Ultrasonics, Hayes, United Kingdom), and the sonicate was centrifuged as before to remove whole cells. Total protein content was measured by a modified Lowry method. The sonicate was aliquoted and stored at −20°C.
The ELISA was modified from the protocol of Suzuki et al. (25). Reagents were added to wells in volumes of 100 μl, the buffer was PBS containing 0.1% Tween 20 (PBS-Tw), and incubations were carried out for 1 h at 37°C unless specified otherwise. Plates were washed three times with PBS-Tw (100 μl/well) between each stage. Briefly, each well of a microtiter plate (Nunc Maxisorp Immunoplate; Life Technologies Ltd., Paisley, United Kingdom) was coated with sonicated H. parainfluenzae (10 μg/ml) in carbonate buffer (0.1 M, pH 9.6). The plate was incubated overnight at 4°C and washed, and nonspecific binding sites were blocked with PBS-Tw containing 0.5% bovine serum albumin. Serum samples from patients or healthy controls were diluted to 1/1,000 for IgG-specific assays or to 1/100 for IgA- and IgM-specific assays, added to the wells, and incubated. No-serum controls of buffer only were included in each assay. After washing, peroxidase-conjugated rabbit anti-IgG, anti-IgA, or anti-IgM (1/1,000 in PBS-Tw; Dako Ltd., High Wycombe, United Kingdom) was added to each well. Further incubation and washing were followed by the addition of O-phenylenediamine (0.4 mg/ml) in citrate-phosphate buffer (0.1 M, pH 5.0) with hydrogen peroxide (5 μl/ml of a 30% [vol/vol] solution). After incubation at room temperature for 30 min, the reaction was stopped by the addition of 50 μl of 2.5 M H2SO4. Absorbance was read at 490 nm. The background value (mean absorbance of no-serum controls) was subtracted, the data were expressed as absorbance units for IgG, IgA, and IgM in patients and controls, and results for the two groups of subjects were compared by using the Mann-Whitney U test.
The specificity of the antibody response was assessed by comparing results from the ELISA after serum samples (diluted to 1/500) had been adsorbed with an equal volume of either (i) a mixture of whole-cell preparations (prepared as for capture antigen) of eight different strains of NTHI (0.71 mg/ml) or (ii) whole-cell preparations of H. parainfluenzae, i.e., the capture antigen as described above (0.71 mg/ml). Differences between treatments were assessed by using Student's t test for paired data.
Preparation of OMPs from H. parainfluenzae.
OMPs were prepared, according to the method of Williams and Brown (28), from sonicated bacteria by using 2% (wt/vol) sodium N-lauroyl sarcosine (Sarkosyl; Sigma-Aldrich Company Ltd., Poole, United Kingdom) to remove cytoplasmic membranes. Protein content was estimated by a modified Lowry method, and confirmation of recovery of OMPs was provided by SDS-PAGE as described below. OMP preparations subjected to SDS-PAGE and stained with Fast Stain (Zoion Biotech, Worcester, Mass.) routinely had protein profiles similar to those observed by others (15, 28), i.e., three major proteins of approximately 15, 37, and 40 kDa and a number of minor proteins.
SDS-PAGE and immunoblot assay.
Immunoblot assays were used (i) to investigate the recognition of H. parainfluenzae OMPs by IgG and IgA in sputa and sera from patients with chronic obstructive lung disease who were regularly colonized with H. parainfluenzae and (ii) to measure the presence of H. parainfluenzae-specific antibodies in the products of adsorption and elution assays (as described below).
OMPs (25 μg) were subjected to SDS-PAGE on laneless modified Laemmli gels (containing 12.5% acrylamide) and either fixed (50% methanol, 10% glacial acetic acid) for total protein staining (Fast Stain; Zoion Biotech) or transferred to a Hybond nitrocellulose membrane (Amersham, Little Chalfont, United Kingdom) for immunoblot assay. The portion of the nitrocellulose containing the low-molecular-weight markers was separated and stained for total protein with colloidal gold (Aurodye forte; Amersham) or Fast Stain. The remainder of the nitrocellulose was immersed for 1 h at room temperature in Tris-buffered saline (0.05 M Tris-HCl, 0.15 M NaCl [pH 7.4]) with 0.1% Tween 20 (TTBS) containing 3% skim milk. The nitrocellulose was washed three times in TTBS and incubated in the presence of diluted serum, sputum sol phase, or buffer only for 1 h at room temperature and washed as before. The wash solution was replaced with peroxidase-conjugated sheep antibody specific for human IgG, IgA, and IgM in combination (diluted to 1/2,000 in TTBS; The Binding Site, Birmingham, United Kingdom) or for IgG or IgA alone (diluted to 1/2,000; Dako). After 1 h, the nitrocellulose was washed again and color was developed for 10 min with VIP development solution (Vector Laboratories Ltd., Petersborough, United Kingdom), after which the nitrocellulose was rinsed in distilled water and allowed to dry.
Adsorption and elution assay.
Adsorption of serum antibodies by whole H. parainfluenzae was modified from the protocols of Loeb (11) and Sethi et al. (22). Three different isolates of H. parainfluenzae were isolated from the sputa of bronchiectatic patients regularly colonized with this species and were used to adsorb antibodies from sera collected from the same patients. Sputa and sera were collected on the same day. The isolated strains of H. parainfluenzae were grown to stationary phase in 3 ml of BHI broth supplemented with NAD and hemin (10 μg/ml) and inoculated into 75 ml of supplemented BHI broth. These were incubated for 6 to 8 h at 37°C until the absorbance of the bacterial suspensions reached 0.3 at 600 nm. The bacteria were harvested by centrifugation (12,000 × g for 10 min at 4°C), washed at 4°C by resuspension in 10 ml of PCM buffer (0.01 M Na2PO4, 0.15 M NaCl, 0.15 mM CaCl2, 0.5 mM MgCl2 [pH 7.2]), and centrifuged as before. The pellets were resuspended in 1 ml of PCM buffer in a microcentrifuge tube and pelleted at high speed in a microcentrifuge for 5 min at 4°C. The supernatant was discarded and the bacterial pellet was gently resuspended in 1 ml of heat-inactivated serum (1/200). The suspensions were incubated on ice for 30 min with gentle mixing every 5 min, followed by centrifugation at maximum speed in a microcentrifuge for 15 min at 4°C. The supernatant was transferred to a fresh microcentrifuge tube and centrifuged as before, and the final supernatant was transferred to another microcentrifuge tube and stored at −20°C as adsorbed serum. A control organism, Escherichia coli, was subjected to the protocol simultaneously.
Antibodies were eluted from intact bacteria as follows. Briefly, strains of H. parainfluenzae or E. coli were subjected to the adsorption protocol described above, with the exception that serum was diluted to 1/10. The bacterial pellet was retained at the end of the procedure and the adsorbed serum was discarded. The pellet was washed three times with 1 ml of PCM buffer by resuspension and centrifugation at maximum speed in a microcentrifuge for 2 min at 4°C. The pellet was then resuspended in 1 ml of elution buffer (0.2 M NaCl, 0.2 M glycine [pH 2.8]) and incubated for 30 min at room temperature on an orbital shaker at 300 rpm. Native serum, adsorbed serum, and eluted antibodies were tested in immunoblot assays for the presence of antibodies which could recognize antigens in an OMP preparation of the isolate of H. parainfluenzae which was used to perform the adsorption and elution assay.
RESULTS
ELISA for antibodies to H. parainfluenzae.
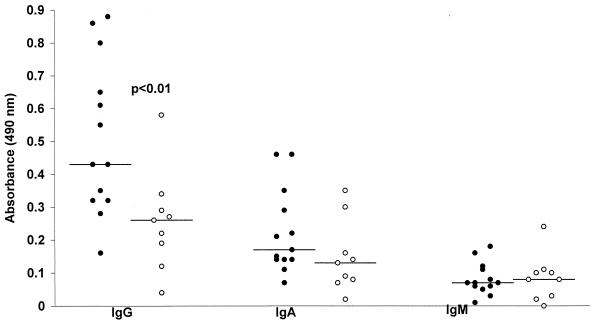
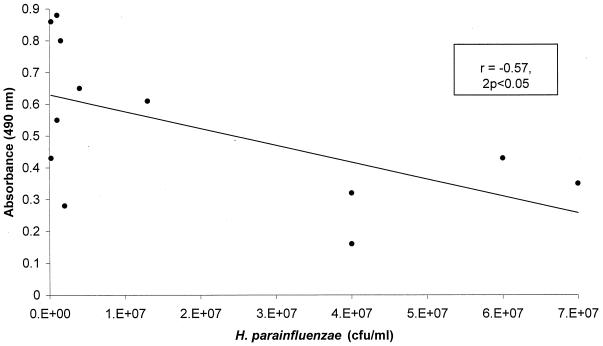
Levels of IgG, IgA, and IgM antibody against whole-cell H. parainfluenzae antigens in the sera of 13 patients with chronic obstructive lung disease (10 with bronchiectasis and 3 with chronic bronchitis) and 9 healthy control subjects are shown in Fig. 1. Antibody recognizing H. parainfluenzae was found to be present in all patients and control subjects studied, with the exception of a single control subject in whom only specific IgM was undetectable. Levels of H. parainfluenzae-reactive IgG were significantly higher in patients than in controls (mean absorbance in patients, 0.51; standard deviation [SD], 0.23; mean absorbance in controls, 0.26; SD, 0.15; P < 0.01). Mean levels of IgA and IgM recognizing H. parainfluenzae were lower than those of IgG in patients and controls, and no significant differences were observed between the two groups with regard to IgA (mean absorbance in patients, 0.22; SD, 0.15; mean absorbance in controls, 0.15; SD, 0.11) or IgM (mean absorbance in patients, 0.07; SD, 0.05; mean absorbance in controls, 0.08; SD, 0.07). There was a significant inverse correlation between bacterial load in sputum and levels of reactive IgG in serum (Spearman's rank correlation coefficient [r], −0.57; 2P < 0.05) (Fig. 2). There was no significant correlation between bacterial load and IgA or IgM.
FIG. 1.
Serum IgG, IgA, and IgM specific for H. parainfluenzae antigens in 13 patients with chronic obstructive lung disease (●) and in 9 healthy controls (○). Horizontal bars indicate means.
FIG. 2.
Numbers of H. parainfluenzae organisms isolated from sputum versus reactive IgG in serum (expressed as arbitrary absorbance units), with coefficient of correlation and statistical significance.
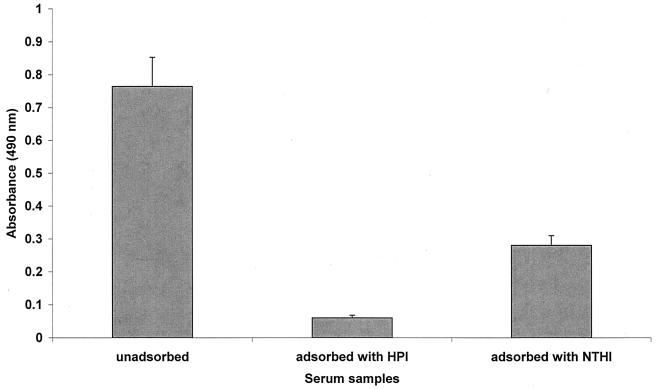
The species specificity of the antibody reacting with a whole-cell preparation of H. parainfluenzae was tested by comparing the results of the ELISA after serum samples from all of the patients had been adsorbed with either sonicated whole-cell preparations of H. parainfluenzae or similar preparations of H. influenzae. Results are shown in Fig. 3, along with the level of reactive antibody measured in unadsorbed serum. A reduction in H. parainfluenzae-reactive IgG was observed after adsorption of serum with H. influenzae (for unadsorbed sera, the mean absorbance was 0.79 and the SD was 0.33; for sera adsorbed with H. influenzae, the mean absorbance was 0.29 and the SD was 0.11 [P < 0.001]). However, a far greater reduction was observed when serum samples were adsorbed with H. parainfluenzae (mean absorbance, 0.06; SD, 0.03), resulting in levels which were significantly lower than those both in unadsorbed serum (P < 0.001) and in serum adsorbed with H. influenzae (P < 0.001).
FIG. 3.
Mean serum IgG (with standard errors) specific for H. parainfluenzae antigens in native serum, serum adsorbed with H. parainfluenzae (HPI) antigens, and serum adsorbed with NTHI antigens.
Immunoblot assay of serum and sputum sol phase.
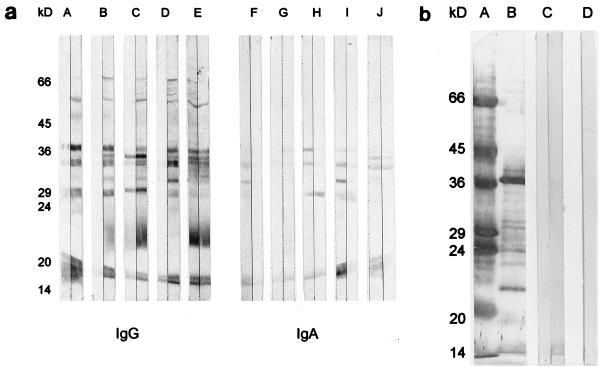
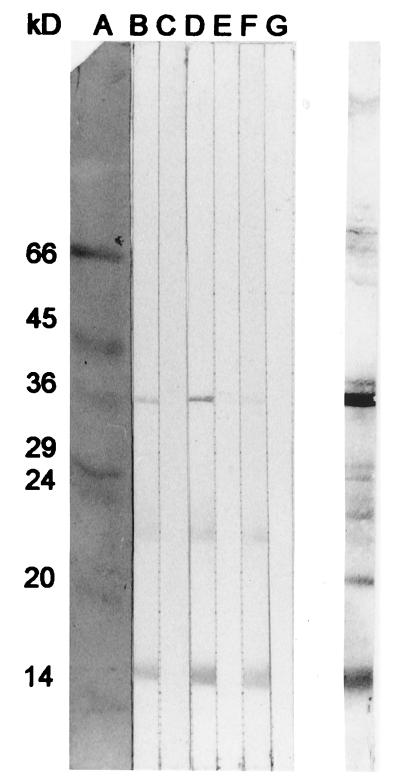
Sera (1/100) and sputum sol phase (1/10) from five patients (two with chronic bronchitis and three with bronchiectasis) were immunoblot assayed for the presence of IgG and IgA specific for OMPs prepared from a single strain (CF3115) of H. parainfluenzae (Fig. 4a). Staining by IgG-specific antibodies was more intense than that by IgA-specific antibodies. Similar patterns of recognition were observed when sputum and serum from the same patient were compared, although patterns did vary among individuals. Samples from two patients displayed low levels of staining of OMPs by sputum IgG (Fig. 4a, lanes B and D). Generally, staining was most intense in IgG-specific assays of serum, whereas for IgA, sputum provided more intense staining than serum. Overall, the specificities of IgG and IgA varied in that an antigen of approximately 22 kDa and several more between 45 and 66 kDa were recognized by IgG only. Two antigens consistently recognized by IgG and IgA had approximate molecular masses of 15 and 37 kDa.
FIG. 4.
Composite immunoblots of H. parainfluenzae (strain CF3115). (a) OMP-specific IgG and IgA in sputum sol phase (1/10) or sera (1/100) from each of five patients with chronic obstructive lung disease. Sputum sol phase is on the left of each pair of immunoassay strips. Samples assayed in pairs A and F and B and G were obtained from patients with chronic bronchitis; samples in pairs C and H, D and I, and E and J were obtained from patients with bronchiectasis. (b) Lanes A and B, molecular mass markers and OMPs, respectively, both stained for total protein with colloidal gold; lane C, a pair of immunoassay strips of OMP-specific IgG, IgA, and IgM in samples obtained from healthy controls, with pooled induced sputum sol phase (1/10) on the left and pooled serum (1/100) on the right; lane D, buffer-only control.
Pooled serum and induced sputum sol phase obtained from healthy controls were also immunoblot assayed for the presence of IgG, IgA, and IgM antibodies recognizing OMP antigens of strain CF3115 (Fig. 4b). Two antigens of approximately 15 and 37 kDa were recognized faintly by antibodies in serum (Fig. 4b, lane C). Staining by sputum antibodies was negligible.
No specific staining of the buffer-only control (Fig. 4b, lane D) was observed in either of the above assays.
Adsorption and elution assay.
Three adsorption assays were performed; the results of one are shown in Fig. 5. In two of the assays, three H. parainfluenzae OMPs (approximate molecular masses of 37, 22, and 15 kDa) were recognized by IgG, IgA, and IgM antibodies in heat-treated homologous serum, and two OMPs (22 and 15 kDa) were recognized by IgG, IgA, and IgM in heat-treated serum from the remaining patient. In all assays, adsorption of sera with the control organism, E. coli (Fig. 5, lane B), had no effect on the intensity of antibody recognition of H. parainfluenzae OMPs. Adsorption with intact H. parainfluenzae (Fig. 5, lane C), however, reduced the recognition of OMPs to negligible levels. After adsorption, antibodies specific for H. parainfluenzae OMPs could be eluted from the surface of H. parainfluenzae (Fig. 5, lane F) but not from the surface of similarly treated E. coli (Fig. 5, lane E).
FIG. 5.
Composite immunoblot demonstrating the results of an adsorption and elution assay specific for serum IgG, IgA, and IgM recognizing OMPs of H. parainfluenzae (strain CF3115) or E. coli. Lanes: A, low-molecular-mass markers stained with Fast Stain; B, serum adsorbed with E. coli; C, serum adsorbed with H. parainfluenzae; D, native serum (1/200); E, antibodies eluted from E. coli; F, antibodies eluted from H. parainfluenzae; G, buffer-only control. Unlabelled lane, stain for total protein with colloidal gold.
DISCUSSION
H. parainfluenzae is isolated from the sputa of patients with chronic lung disease in relatively large numbers and at a frequency similar to that of accepted respiratory pathogens such as NTHI (9). Little work, however, has been performed investigating the contribution of this species to the pathogenesis of chronic lung disease, and since, like other members of the genus including NTHI, H. parainfluenzae is frequently isolated from the oropharynx, it has been regarded as a contaminating commensal organism when isolated from sputa. In this pilot study we have therefore begun to investigate the antibody response to H. parainfluenzae as part of our studies of the role of this species in chronic lung infection.
Results from the ELISA described here demonstrated that patients who were regularly colonized with H. parainfluenzae had higher levels of serum IgG specific for this organism than did healthy control subjects (Fig. 1). Patients with chronic lung disease, however, are likely to have been colonized with NTHI, and H. parainfluenzae is known to possess antigens which cross-react with rabbit antibodies to H. influenzae (23). The specificity of the systemic immune response observed in our patients was therefore confirmed through adsorption of serum samples with whole-cell preparations of either NTHI or H. parainfluenzae prior to measurement of H. parainfluenzae-reactive IgG by ELISA. Adsorption with NTHI reduced the amount of IgG specific for H. parainfluenzae in serum samples (Fig. 3), though this was to be expected since a sonicated whole-cell preparation was used. This preparation is certain to contain antigens shared with a variety of bacterial species, including H. parainfluenzae (23), and will therefore adsorb cross-reactive antibody in serum samples tested. Adsorption of native serum with H. parainfluenzae, however, resulted in a reduction of reactive antibody to very low levels (P < 0.001; 13 times lower than that observed after adsorption with H. influenzae). This result, together with the data from the original ELISA, strongly indicates that there is an elevated systemic IgG response to H. parainfluenzae in patients from whom this organism is frequently isolated. Interestingly, there was an inverse correlation between bacterial load in the sputa of these patients and levels of H. parainfluenzae-reactive IgG in their sera. It is possible that in patients with lower levels of reactive IgG, the larger numbers of colonizing bacteria in their airways are a consequence of lower levels of opsonizing antibody transudating from serum to airway secretions. It is not possible, however, to draw any firm conclusions from the small number of patients studied here.
Immunoblot assays were performed in an effort to identify which OMPs of H. parainfluenzae were recognized by specific antibodies in sera and sputa from patients with chronic obstructive lung disease. IgG and IgA antibodies specific for OMPs were demonstrated to be present in both the sera and sputa of the patients studied. Up to 14 different OMPs of the single strain of H. parainfluenzae studied, ranging from 15 to 70 kDa, were recognized by serum IgG. Recognition by sputum IgG followed a similar pattern of staining but was generally of lower intensity. Fewer antigens were recognized by IgA in serum or sputum, but two antigens, of 37 and 15 kDa, were most consistently recognized by IgA and IgG. The exact identity of these OMPs is unknown, but it is possible that the 37-kDa OMP is the same as that described by Suzuki et al. (24) and possesses some homology to the porin protein, P2, of NTHI. The consistent antibody recognition of the 15-kDa OMP is similar to that previously observed for P6 (a 16-kDa, surface-exposed, antigenically conserved lipoprotein of NTHI) in patients with bronchiectasis (14). Bogdan and Apicella (3) have shown that of the Haemophilus species, H. parainfluenzae is the only one that does not contain the protein P6. Suzuki and colleagues (24), however, have identified an OMP which exhibits homology to the OMP P6 precursor of H. influenzae, and it is therefore possible that H. parainfluenzae possesses a closely related protein.
Since antibody-mediated clearance of infecting organisms can succeed only in the presence of antibodies recognizing epitopes exposed by live bacteria, the potential functional significance of the antibody response to H. parainfluenzae in these patients was investigated by using intact bacteria isolated from their sputa to adsorb antibodies from their sera. Antibodies reacting with surface-exposed epitopes were then eluted from the surface of the bacteria, and the results were visualized by immunoblot assay. The 36- and 15-kDa antigens described above, together with a 22-kDa antigen (recognized by serum IgG in three of the five patients' samples) initially studied by immunoassay, were demonstrated to possess surface-exposed epitopes, which suggests that they may be important in immune recognition and antibody-dependent clearance mechanisms.
These initial studies, therefore, have demonstrated the presence of a specific immune response in the sera and sputa of a group of patients with chronic obstructive lung disease who are colonized by or frequently infected with H. parainfluenzae. We have shown that a variety of OMPs are recognized by antibodies in these patients but that apparently only three of these possess epitopes exposed on the surface of the intact bacteria. Further studies are necessary to confirm the identity of these antigens and to measure the functional capabilities of antibodies recognizing H. parainfluenzae in these patients.
ACKNOWLEDGMENTS
This work was supported by a noncommercial educational grant from the Bayer Corporation.
REFERENCES
- 1.Albritton W L. Infections due to Haemophilus species other than H. influenzae. Annu Rev Microbiol. 1982;11:199–216. doi: 10.1146/annurev.mi.36.100182.001215. [DOI] [PubMed] [Google Scholar]
- 2.Barenkamp S J, Munson R S, Jr, Granoff D M. Outer membrane protein and biotype analysis of pathogenic nontypable Haemophilus influenzae. Infect Immun. 1982;36:535–540. doi: 10.1128/iai.36.2.535-540.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogdan J A, Jr, Apicella M A. Mapping of a surface-exposed, conformational epitope of the P6 protein of Haemophilus influenzae. Infect Immun. 1995;63:4395–4401. doi: 10.1128/iai.63.11.4395-4401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruun B, Christensen J J, Kilian M. Bacteraemia caused by a beta-lactamase producing H. parainfluenzae strain of a new biotype. Acta Pathol Microbiol Immunol Scand Sect B. 1984;92:135–138. doi: 10.1111/j.1699-0463.1984.tb02807.x. [DOI] [PubMed] [Google Scholar]
- 5.Burns M W, May J R. Haemophilus influenzae precipitins in the serum of patients with chronic bronchial disorders. Lancet. 1967;i:354–358. doi: 10.1016/s0140-6736(67)92895-4. [DOI] [PubMed] [Google Scholar]
- 6.Deich R A, Anilionis A, Fulginiti J, Metcalf B J, Quataert S, Quinn-Dey T, Zlotnick G W, Green B A. Antigenic conservation of the 15,000-dalton outer membrane lipoprotein PCP of Haemophilus influenzae and biologic activity of anti-PCP antisera. Infect Immun. 1990;58:3388–3393. doi: 10.1128/iai.58.10.3388-3393.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glynn A A. Antibodies to Haemophilus influenzae in chronic bronchitis. Br Med J Clin Res. 1959;2:911–914. doi: 10.1136/bmj.2.5157.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green B A, Vazquez M E, Zlotnick G W, Quigley-Reape G, Swarts J D, Green I, Cowell J L, Bluestone C D, Doyle W J. Evaluation of mixtures of purified Haemophilus influenzae outer membrane proteins in protection against challenge with nontypeable H. influenzae in the chinchilla otitis media model. Infect Immun. 1993;61:1950–1957. doi: 10.1128/iai.61.5.1950-1957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill S L, Pye A, Johnson M M, Munday C, Stockley R A. A role for Haemophilus parainfluenzae in chronic lung disease. Respir Crit Care Med. 1997;155:A105. [Google Scholar]
- 10.Korppi M, Katila M L, Jaaskelaine J, Leinonen M. Role of non-encapsulated Haemophilus influenzae as a respiratory pathogen in children. Acta Paediatr. 1992;81:989–992. doi: 10.1111/j.1651-2227.1992.tb12160.x. [DOI] [PubMed] [Google Scholar]
- 11.Loeb M R. Immunoblot method for identifying surface components, determining cross-reactivity, and investigating cell topology: results with Haemophilus influenzae type b. Anal Biochem. 1984;143:196–204. doi: 10.1016/0003-2697(84)90576-1. [DOI] [PubMed] [Google Scholar]
- 12.Loeb M R. Protection of infant rats from Haemophilus influenzae type b infection by antiserum to purified outer membrane protein a. Infect Immun. 1987;55:2612–2618. doi: 10.1128/iai.55.11.2612-2618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.May J R, Peto R, Tinker C M, Fletcher C M. A study of Haemophilus influenzae precipitins in the serum of working men in relation to smoking habits, bronchial infection, and airway obstruction. Am Rev Respir Dis. 1973;108:460–468. doi: 10.1164/arrd.1973.108.3.460. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell J L, Hill S L. Recognition of the P6 outer membrane protein of non-typeable Haemophilus influenzae by antibodies in sputum. Eur Respir J. 1995;8(Suppl. 19):2638. [Google Scholar]
- 15.Morton D J, Williams P. Characterisation of the outer membrane proteins of Haemophilus influenzae expressed under iron-sufficient and iron-restricted conditions. J Gen Microbiol. 1989;135:445–451. doi: 10.1099/00221287-135-2-445. [DOI] [PubMed] [Google Scholar]
- 16.Munson R S, Jr, Granoff D M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985;49:544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy T F, Bartos L C. Human bactericidal antibody response to outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1988;56:2673–2679. doi: 10.1128/iai.56.10.2673-2679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy T F, Bartos L C, Rice P A, Nelson M B, Dudas K C, Apicella M A. Identification of a 16,600-dalton outer membrane protein on nontypeable Haemophilus influenzae as a target for human serum bactericidal antibody. J Clin Investig. 1986;78:1020–1027. doi: 10.1172/JCI112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pye A, Stockley R A, Hill S L. Simple method for quantifying viable bacterial numbers in sputum. J Clin Pathol. 1995;48:719–724. doi: 10.1136/jcp.48.8.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhind G B, Gould G A, Ahmad F, Croughan M J, Calder M A. Haemophilus influenzae and H. parainfluenzae infections: comparison of clinical features. Br Med J. 1985;291:707–708. doi: 10.1136/bmj.291.6497.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts M C, Mintz C S, Morse S A. Characterisation of Haemophilus parainfluenzae strains with low-Mr or ladder-like LPS. J Gen Microbiol. 1986;132:611–616. doi: 10.1099/00221287-132-3-611. [DOI] [PubMed] [Google Scholar]
- 22.Sethi S, Hill S L, Murphy T F. Serum antibodies to outer membrane proteins (OMPs) of Moraxella (Branhamella) catarrhalis in patients with bronchiectasis: identification of OMP B1 as an important antigen. Infect Immun. 1995;63:1516–1520. doi: 10.1128/iai.63.4.1516-1520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoitz P O, Hoiby N, Hertz J B. Cross-reactions between Haemophilus influenzae and nineteen other bacterial species. Acta Pathol Microbiol Scand. 1979;87:337–344. doi: 10.1111/j.1699-0463.1979.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S, Nakatomi Y, Odami S, Sato H, Gejyo F, Arakawa M. Circulating IgA, IgG, and IgM class antibody against Haemophilus parainfluenzae antigens in patients with IgA nephropathy. Clin Exp Immunol. 1996;104:306–311. doi: 10.1046/j.1365-2249.1996.09703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki S, Nakatomi Y, Sato H. Haemophilus parainfluenzae antigen and antibody in renal biopsy samples and serum of patients with IgA nephropathy. Lancet. 1994;343:12–20. doi: 10.1016/s0140-6736(94)90875-3. [DOI] [PubMed] [Google Scholar]
- 26.Vachon V, Lyew D J, Coulton J W. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J Bacteriol. 1985;162:918–924. doi: 10.1128/jb.162.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Alphen L, Riemens T, Poolman J, Zanen H C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983;155:878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams P, Brown M R W. Influence of iron-restriction on growth and the expression of outer membrane proteins by Haemophilus influenzae and H. parainfluenzae. FEMS Microbiol Lett. 1986;33:153–157. [Google Scholar]