Abstract
Introduction
Childhood cancer is a leading cause of death. It is unclear whether the COVID-19 pandemic has impacted childhood cancer mortality. In this study, we aimed to establish all-cause mortality rates for childhood cancers during the COVID-19 pandemic and determine the factors associated with mortality.
Methods
Prospective cohort study in 109 institutions in 41 countries. Inclusion criteria: children <18 years who were newly diagnosed with or undergoing active treatment for acute lymphoblastic leukaemia, non-Hodgkin's lymphoma, Hodgkin lymphoma, retinoblastoma, Wilms tumour, glioma, osteosarcoma, Ewing sarcoma, rhabdomyosarcoma, medulloblastoma and neuroblastoma. Of 2327 cases, 2118 patients were included in the study. The primary outcome measure was all-cause mortality at 30 days, 90 days and 12 months.
Results
All-cause mortality was 3.4% (n=71/2084) at 30-day follow-up, 5.7% (n=113/1969) at 90-day follow-up and 13.0% (n=206/1581) at 12-month follow-up. The median time from diagnosis to multidisciplinary team (MDT) plan was longest in low-income countries (7 days, IQR 3–11). Multivariable analysis revealed several factors associated with 12-month mortality, including low-income (OR 6.99 (95% CI 2.49 to 19.68); p<0.001), lower middle income (OR 3.32 (95% CI 1.96 to 5.61); p<0.001) and upper middle income (OR 3.49 (95% CI 2.02 to 6.03); p<0.001) country status and chemotherapy (OR 0.55 (95% CI 0.36 to 0.86); p=0.008) and immunotherapy (OR 0.27 (95% CI 0.08 to 0.91); p=0.035) within 30 days from MDT plan. Multivariable analysis revealed laboratory-confirmed SARS-CoV-2 infection (OR 5.33 (95% CI 1.19 to 23.84); p=0.029) was associated with 30-day mortality.
Conclusions
Children with cancer are more likely to die within 30 days if infected with SARS-CoV-2. However, timely treatment reduced odds of death. This report provides crucial information to balance the benefits of providing anticancer therapy against the risks of SARS-CoV-2 infection in children with cancer.
Keywords: Paediatrics, Cancer, Health systems, COVID-19
WHAT IS ALREADY KNOWN ON THIS TOPIC
Cancer is the leading cause of death by disease in children. Preliminary data, mostly in high-income countries, suggested that children with cancer and SARS-CoV-2 infection were not at increased risk of death compared with the general paediatric population.
WHAT THIS STUDY ADDS
This is the largest international cohort study to date to report COVID-19 and oncological outcomes for childhood cancers; the majority of participants were from lower income countries: a neglected group in existing studies. SARS-CoV-2 infection increased odds of death by 30 days, but not after 30 days. Participants in lower income countries had more overall complications, higher odds of starting palliative care and higher odds of death at all time points.
HOW THUS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Given the longer term consequences of delaying treatment, it would be prudent to prioritise timely therapy where feasible. Reducing treatment delays has health and economic benefits and could save countless lives during the pandemic.
Introduction
In the 1960s, 5-year survival for childhood cancer globally was as low as 20%.1 The introduction of chemotherapy tripled 5-year survival for childhood cancer in high-income countries (HICs).2 Further advances in chemotherapy, radiotherapy, immunotherapy and surgery and the personalisation of treatment increased 5-year survival for childhood cancer in HICs to 80%.3–5 Despite this, cancer remains the leading cause of death by disease in children in HICs.6 The situation is bleaker in low and middle-income countries (LMICs). The 5-year survival for childhood cancer in LMICs collectively lies between 20% and 30%.5 7 8 Even when considering LMICs with the highest 5-year survival for childhood cancer, survival is higher in HICs.7 8 Absence of or inaccessibility to both effective diagnostics9–11 and optimal care12–15 account for this inequity in childhood cancer outcomes and derive primarily from inadequate healthcare infrastructure and service delivery networks.16–18 In recognition of this, the WHO launched the Global Initiative for Childhood Cancer (GICC) in 2018.19 The GICC laid out a framework for all countries to reach at least 60% 5-year survival for children with the six most common childhood cancers globally by 2030: acute lymphoblastic leukaemia (ALL), Burkitt lymphoma, Hodgkin lymphoma, retinoblastoma, Wilms tumour and low-grade glioma.19
The year after the launch of the GICC, the SARS-CoV-2 responsible for the COVID-19 pandemic was detected.20 21 Preliminary data from HICs suggested that children with cancer and SARS-CoV-2 infection were not at increased risk of mortality compared with the general paediatric population.22 However, children from HICs are not representative of the majority of childhood cancers. More than 90% of children at risk of developing childhood cancer each year live in LMICs,23 and they account for 95% of the mortality from cancer in this age group worldwide.3 16 In the only global cohort study published to date including children with cancer and SARS-CoV-2 infection, the authors detected increased mortality and morbidity in children residing in LMICs.24 However, it was unclear whether this increase in morbidity and mortality was related to infection status or changes in the standard of oncological care provided. Several cross-sectional studies have identified that the COVID-19 pandemic has substantially affected childhood cancer diagnosis and management worldwide, with its effect being more prominent in LMICs than HICs.25 26
In order to support frontline clinicians and governments in making data-driven decisions about the management of childhood cancers, it is critical to determine whether the increased morbidity and mortality documented in the recent cohort study24 are due to SARS-CoV-2 infection or changes from normal standards of care. One of the challenges of paediatric cancer research is that it is a relatively small disease population.2 To overcome this obstacle, multicentre studies are essential to generate statistically significant results. Given global studies published to date on childhood cancers during the COVID-19 pandemic have predominantly collected data from HICs, it is also critical to increase data collection from LMICs. However, given the extra pressures on clinicians currently—especially in LMICs—it is also imperative to reduce data collection burden by focussing on a subset of cancers; principally, those espoused by GICC. This study primarily aims to determine all-cause mortality rates for childhood cancers during the COVID-19 pandemic across LMICs and HICs. The secondary aim of the study is to determine the factors that influenced these outcomes including tumour-specific data, patient-specific demographics and changes to health system frameworks as outlined in the study protocol.27
Methods
Study design and participants
This is a prospective cohort study with cases reported from 109 institutions in 41 countries (online supplemental appendix S2). Data was collected in the REDCap application hosted at the University of Oxford (Oxford, UK). Data were voluntarily reported for all children under the age of 18 years who were newly diagnosed with or undergoing active treatment for an eligible cancer between 12 March 2020—the date that the WHO declared the start of the COVID-19 pandemic—and 12 December 2020 inclusive. Eligible cancers were those identified by the GICC19 and those deemed significant by LMIC collaborators: ALL, non-Hodgkin's lymphoma (including Burkitt lymphoma), Hodgkin lymphoma, retinoblastoma, Wilms tumour, glioma, sarcoma (osteosarcoma, Ewing sarcoma and rhabdomyosarcoma), medulloblastoma and neuroblastoma.28 Participants who were 18 years or older were excluded to reduce confounding by care provided by adult cancer services. There were no centre-specific exclusion criteria. This study was reviewed by the University of Oxford institutional review board as not involving human participants, and no identifiable private information or biospecimens being provided. This study was subjected to approvals by local ethics committees according to local policy. Individual investigators (listed in online supplemental appendix S1) were responsible for assuring that participation was compliant with local regulations.
bmjgh-2022-008797supp001.pdf (53.9KB, pdf)
bmjgh-2022-008797supp002.pdf (127.8KB, pdf)
Procedures
Deidentified data were requested on a maximum of 112 variables (10 required responses) contained on eight forms. Baseline information was collected regarding the patient’s age, weight, sex, American Society of Anaesthesiologists (ASA) grade and whether the patient was newly diagnosed with or undergoing active treatment for an eligible cancer between 12 March 2020 and 12 December 2020 inclusive. Participants 18 years or older and participant data outside of this data range were excluded. Where date of birth was unknown, the contributor of that data set was contacted to verify the patient was born in 2003 or later. Tumour-specific data were collected regarding diagnosis,28 date of diagnosis, staging,29 multidisciplinary team (MDT) decision date, what the MDT management plan was during the pandemic, and what the MDT plan would have been prior to the pandemic. The time from diagnosis to MDT decision date was calculated for each patient. Data were collected regarding the chemotherapy, radiotherapy, immunotherapy, surgery, and palliative care that patients received, any deviations from the MDT plan made during the pandemic, and any specific factors related to the COVID-19 pandemic that had driven these deviations. Outcomes collected included laboratory and clinical status of SARS-CoV-2 infection, complications within 30 days from anticancer treatment, interruptions in cancer-directed treatment and vital status. All terminology used were selected to be globally applicable and clinically relevant. Participant data could be collected prospectively or retrospectively provided 30-day, 90-day and 12-month follow-up data were collected prospectively. Each institution that had reported at least a single case was requested to confirm that all institutional cases had been entered in an unbiased fashion as of the time of the final case entry by the institution. All cases corresponding to institutions that did not confirm unbiased data entry were excluded from analysis. Data validation was performed on a randomly selected subset (10%) of participating centres. A research checklist has been used to report the study (online supplemental appendix S3).
bmjgh-2022-008797supp003.pdf (50KB, pdf)
Statistical analysis
Descriptive statistics were used to summarise demographic and clinical characteristics and outcomes. 2021 World Bank designations for income groups—HICs, upper middle-income countries (UMICs), lower middle-income countries (LoMICs) and low-income countries (LICs)—were used to describe economic context.30 The primary outcome measures were all-cause mortality at 30 days, 90 days and 12 months from MDT plan. Secondary outcomes were treatment modification, complications within 30 days of first anticancer treatment and SARS-CoV-2 infection status: cases were those confirmed by laboratory testing; cases without laboratory confirmation were classified as ‘probably SARS-CoV-2’. Another secondary outcome was the health system framework factors affected by the COVID-19 pandemic, defined as one or more of the following: decision-making, infrastructure, workforce, service delivery, financing and patient factors. Comparison of proportions between groups was made with χ2. The Kruskal-Wallis test and Dunn’s post hoc test were used to compare medians between groups. Univariate logistic regression was used to examine the association between each outcome and patient characteristics. Multivariable logistic regression was used to explore the effect of factors that were significant (p<0·05) in univariate analyses for each outcome. All data analyses were done using STATA/IC V.16.1.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Results
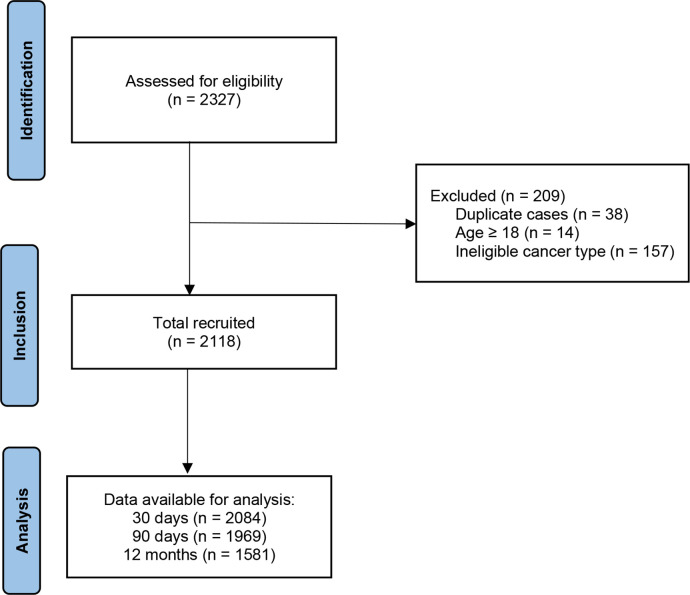
Between 12 March 2020 and 12 December 2020, 2327 cases were identified. Of these 2327 cases, 209 were excluded from analyses (figure 1). Thirty-eight were duplicates of cases that had already been included into the study, 14 were 18 years or older and 157 were excluded based on cancer diagnosis eligibility. Of the remaining 2118 qualifying cases, 2084 (98.4%) completed 30-day follow-up, 1969 (93.0%) completed 90-day follow-up and 1581 (74.6%) completed 12-month follow-up. Clinical characteristics of the included patients by World Bank country income level are summarised in table 1. Most patients (n=1450/2118, 68.5%) were from LMICs, principally LoMICs (n=766/2118, 36.2%) (online supplemental appendix S4). The median age of patients included was 6 years (IQR 3–11), with 7.6% (n=161/2107) younger than 1 year and 8.7% (n=183/2107) aged 15–17 years, with age missing for 11 patients. 1244 (59.0%) of 2108 patients were men, with sex missing for 10 patients and one participant being inter-sex.
Figure 1.
STROBE flowchart of participants in this study. STROBE, STrengthening the Reporting of OBservational studies in Epidemiology.
Table 1.
Baseline characteristics by World Bank income group
| Low-income countries (N=36), n (%) | Lower-middle income countries (N=766), n (%) |
Upper-middle income countries (N=648), n (%) |
High-income countries (N=668), n (%) |
|
| Age (years), median (IQR) | 4 (2.5, 7), 36 (100) | 5 (3, 10), 761 (99.3) | 6 (2, 11), 647 (99.8) | 7 (3, 12), 663 (99.3) |
| Sex | ||||
| Female | 15 (41.7) | 277 (36.2) | 296 (45.7) | 275 (41.2) |
| Male | 21 (58.3) | 485 (63.3) | 351 (54.2) | 387 (57.9) |
| Intersex | 0 (0) | 0 (0) | 0 (0) | 1 (0.1) |
| Missing | 0 (0) | 4 (0.5) | 1 (0.1) | 5 (0.7) |
| Weight (kg), median (range) | 17.7 (15.0, 27.75), 36 (100) | 17.7 (12.0, 26.0), 741 (96.7) | 20.3 (14.1, 35), 632 (97.5) | 25 (16, 46.4), 648 (97.0) |
| American Society of Anesthesiologists (ASA) grade | ||||
| 1—A normal healthy patient | 2 (5.6) | 198 (25.8) | 169 (26.1) | 107 (16.0) |
| 2—A patient with mild systemic disease | 21 (58.3) | 218 (28.5) | 377 (58.2) | 259 (38.8) |
| 3—A patient with severe systemic disease | 8 (22.2) | 132 (17.2) | 90 (13.9) | 267 (40.0) |
| 4—A patient with severe systemic disease that is a constant threat to life | 3 (8.3) | 39 (5.1) | 9 (1.4) | 25 (3.7) |
| 5—A moribund patient who is not expected to survive without an operation | 2 (5.6) | 6 (0.8) | 2 (0.3) | 1 (0.1) |
| Missing | 0 (0) | 173 (22.6) | 1 (0.2) | 9 (1.3) |
| Tumour type | ||||
| Acute lymphoblastic leukaemia | 11 (30.6) | 292 (38.1) | 251 (38.7) | 291 (43.6) |
| Ewing sarcoma | 0 (0) | 30 (3.9) | 21 (3.2) | 33 (4.9) |
| Glioma | 1 (2.8) | 30 (3.9) | 57 (8.8) | 79 (11.8) |
| Hodgkin lymphoma | 3 (8.3) | 48 (6.3) | 30 (4.6) | 43 (6.4) |
| Medulloblastoma | 4 (11.1) | 29 (3.8) | 47 (7.3) | 33 (4.9) |
| Neuroblastoma | 4 (11.1) | 48 (6.3) | 45 (6.9) | 61 (9.1) |
| Non-Hodgkin lymphoma | 4 (11.1) | 53 (6.9) | 45 (6.9) | 36 (5.4) |
| Osteosarcoma | 0 (0.0) | 24 (3.1) | 37 (5.7) | 28 (4.2) |
| Retinoblastoma | 6 (16.7) | 47 (6.1) | 40 (6.2) | 6 (0.9) |
| Rhabdomyosarcoma | 1 (2.8) | 47 (6.1) | 25 (3.9) | 30 (4.5) |
| Wilms tumour | 2 (5.6) | 118 (15.4) | 50 (7.7) | 28 (4.2) |
bmjgh-2022-008797supp004.pdf (69.8KB, pdf)
Data were available for 1215 patients who were newly diagnosed with cancer during the COVID-19 pandemic concerning the time from diagnosis to the time the initial MDT management plan was made. The median time from diagnosis to MDT plan varied across LICs (7 days, IQR 3–11; n=23), LoMICs (2 days, IQR 0–8; n=524), UMICs (1 day, IQR 0–9; n=348) and HICs (2 days, IQR 0–7; n=320). The Kruskal-Wallist test revealed a statistically significant difference in median time from diagnosis to MDT plan (χ2=10.688, p=0.0135) between two or more of the income groups. Pairwise comparisons using Dunn’s test indicated that the median time from diagnosis to MDT plan among LIC patients was significantly different from those of LoMIC (p=0.009), UMIC (p=0.002) and HIC (p=0.003) patients. Dunn’s test also indicated that the median time from diagnosis to MDT plan among LoMIC patients was significantly different from those of UMIC (p=0.030) patients. No other differences were statistically significant at a significance level of 0.05.
The MDT plans made for all the participants are summarised in table 2. MDTs in HICs were observed to be significantly more likely to opt for chemotherapy (χ2=7.462, p=0.006) and immunotherapy (χ2=32.019, p<0.001) over no treatment compared with MDTs in LMICs. No other differences between HICs and LMICs were statistically significant at a significance level of 0.05. Among patients planned for chemotherapy by the MDT, there was a significant difference between the proportion of patients who had a central venous catheter inserted in two or more of the income groups (χ2=479.287, p<0.001): 84.0% (n=489/582) in HICs, 61.1% (n=275/450) in UMICs, 24.9% (n=163/654) in LoMICs and 0.0% (n=0/31) in LICs. Of the 2118 participants, 20 (0.9%) would reportedly have had a different management plan if the MDT meeting had been held prior to the pandemic: 17 (85.0%) were based in LoMICs and 3 (15.0%) in UMICs.
Table 2.
Treatments planned and received during the COVID-19 pandemic by World Bank income group
| Low-income countries % (n/N) |
Lower-middle income countries % (n/N) |
Upper-middle income countries % (n/N) |
High-income countries % (n/N) |
|
| Multi-disciplinary team (MDT) management plan during the pandemic | ||||
| Chemotherapy | 86.1 (31/36) | 89.4 (685/766) | 84.0 (544/648) | 91.0 (608/668) |
| Radiotherapy | 16.7 (6/36) | 16.7 (128/766) | 12.7 (82/648) | 16.8 (112/668) |
| Immunotherapy | 0.0 (0/36) | 0.3 (2/766) | 2.9 (19/648) | 5.8 (39/668) |
| Surgery | 30.6 (11/36) | 31.9 (244/766) | 33.0 (214/648) | 28.6 (191/668) |
| Palliative care | 8.3 (3/36) | 0.8 (6/766) | 0.5 (3/648) | 0.6 (4/668) |
| MDT plan that would have been proposed prior to the pandemic | ||||
| Chemotherapy | 86.1 (31/36) | 90.2 (691/766) | 83.8 (543/648) | 91.0 (608/668) |
| Radiotherapy | 16.7 (6/36) | 17.1 (131/766) | 12.8 (83/648) | 16.8 (112/668) |
| Immunotherapy | 0.0 (0/36) | 0.5 (4/766) | 2.9 (19/648) | 5.8 (39/668) |
| Surgery | 30.6 (11/36) | 31.1 (238/766) | 32.9 (213/648) | 28.6 (191/668) |
| Palliative care | 8.3 (3/36) | 0.5 (4/766) | 0.5 (3/648) | 0.6 (4/668) |
| Treatment provided within 30 days for patients planned to have that treatment at MDT | ||||
| Chemotherapy | 80.6 (25/31) | 84.7 (559/660) | 86.4 (389/450) | 87.8 (496/565) |
| Radiotherapy | 0.0 (0/6) | 22.0 (28/127) | 44.4 (20/45) | 42.2 (43/102) |
| Immunotherapy | – | 100.0 (2/2) | 75.0 (12/16) | 55.9 (19/34) |
| Surgery | 18.2 (2/11) | 27.6 (62/225) | 36.4 (60/165) | 36.4 (63/173) |
| Treatment provided within 90 days for patients planned to have that treatment at MDT | ||||
| Chemotherapy | 80.6 (25/31) | 88.9 (601/676) | 88.7 (481/542) | 91.3 (553/606) |
| Radiotherapy | 0.0 (0/6) | 48.0 (60/125) | 39.0 (32/82) | 46.8 (52/111) |
| Immunotherapy | – | 100.0 (2/2) | 89.5 (17/19) | 69.2 (27/39) |
| Surgery | 18.2 (2/11) | 84.0 (110/131) | 38.8 (83/214) | 47.1 (89/189) |
| Treatment provided within 12 months for patients planned to have that treatment at MDT | ||||
| Chemotherapy | 90.0 (27/30) | 96.6 (625/647) | 95.1 (504/530) | 93.7 (562/600) |
| Radiotherapy | 0.0 (0/6) | 62.7 (69/110) | 49.4 (40/81) | 54.7 (58/106) |
| Immunotherapy | – | 100.0 (2/2) | 89.5 (17/19) | 83.3 (30/36) |
| Surgery | 45.5 (5/11) | 75.7 (159/210) | 66.0 (132/200) | 64.0 (119/186) |
| Treatment provided within 12 months for all patients | ||||
| Chemotherapy | 84.9 (28/33) | 92.5 (649/702) | 88.9 (552/621) | 88.4 (577/653) |
| Radiotherapy | 0.0 (0/30) | 16.6 (93/560) | 20.8 (119/571) | 12.7 (76/599) |
| Immunotherapy | 0.0 (0/30) | 1.5 (8/547) | 9.8 (54/551) | 10.7 (64/597) |
| Surgery | 22.6 (7/31) | 37.2 (226/607) | 34.6 (202/584) | 33.7 (207/615) |
There was a significant difference in laboratory testing for SARS-CoV-2 infection (χ2=213.606, p<0.001) between HICs (n=362/635, 57.0%) and LMICs (n=318/1347, 23.6%). In LMICs, most patients were not screened for SARS-CoV-2 infection (n=719/1347, 53.4%). A minority of participants in LMICs underwent symptomatic screening (n=300/1347, 22.3%) or CT testing (n=10/1347, 0.7%). In HICs, 192 patients (30.2%) were not screened for SARS-CoV-2 infection. The remaining 81 patients in HICs (12.8%) underwent symptomatic screening only. Thirty-five patients were confirmed to be infected with SARS-CoV-2 following laboratory testing (HICs: 7; LMICS: 28), and 27 patients were suspected to probably have SARS-CoV-2 infection (HICs: 2; LMICs: 25).
A total of 212 patients (10.0%), 42 patients (2.0%), 5 patients (0.2%) and 98 patients (4.6%) were, respectively, reported to have had their chemotherapy, radiotherapy, immunotherapy and surgery care affected by the COVID-19 pandemic. In multivariable analysis, residing in an LMIC (OR 3.72 (95% CI 2.52 to 5.50)) was associated with increased odds of oncological care being affected by the COVID-19 pandemic. Online supplemental appendix S5 summarises the specific factors related to the COVID-19 pandemic that were reported to have affected cancer care. Of 2075 patients, 238 patients (11.5%) were started on palliative care. 232 of these patients originally had treatment plans made at the initial MDT: 206 (14.6%) of 1413 participants in LMICs and 26 (3.9%) of 662 participants in HICs (χ2=51.502, p<0.001) (online supplemental appendix S6). Income group, age, sex, ASA grade, chemotherapy and radiotherapy within 30 days of the MDT, change in radiotherapy and surgery treatment due to the COVID-19 pandemic and tumour type were significantly associated with palliative treatment in univariate logistic regression analyses, whereas immunotherapy within 30 days of the MDT, surgery within 30 days of the MDT and SARS-CoV-2 infection status were not. In multivariable analysis, residing in an LIC (OR 27.13 (95% CI 12.7 to 58.6); p<0.001), an LoMIC (OR 3.29 (95% CI 2.10 to 5.17); p<0.001), and an UMIC (OR 4.82 (95% CI 3.09 to 7.51); p<0.001) were associated with increased odds of palliative care. Increasing age, male sex, ASA grade, radiotherapy within 30 days of the MDT and tumour type were also associated with increased odds of palliative care (table 3).
Table 3.
Results of univariate and multivariable analysis for palliative care
| Univariate analysis | Multivariable analysis (N=1706) | |||
| P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | |
| Income group | ||||
| High-income countries | Reference | 1.0 | Reference | 1.0 |
| Upper-middle income countries | <0.001 | 4.82 (3.09 to 7.51) | <0.001 | 7.52 (4.45 to 12.69) |
| Lower-middle income countries | <0.001 | 3.29 (2.10 to 5.17) | <0.001 | 5.09 (3.03 to 8.56) |
| Low-income countries | <0.001 | 27.3 (12.7 to 58.6) | <0.001 | 44.46 (19.15 to 103.23) |
| Age (for every year older) | 0.004 | 1.04 (1.01 to 1.07) | 0.001 | 1.06 (1.02 to 1.10) |
| Sex | ||||
| Female | Reference | 1.0 | Reference | 1.0 |
| Male | 0.046 | 1.34 (1.01 to 1.77) | 0.026 | 1.45 (1.04 to 2.00) |
| American Society of Anaesthesiologists (ASA) grade | ||||
| 1 | Reference | 1.0 | Reference | 1.0 |
| 2 | 0.005 | 1.73 (1.18 to 2.55) | 0.001 | 2.09 (1.34 to 3.26) |
| 3 | 0.307 | 1.26 (0.81 to 1.96) | 0.001 | 2.54 (1.48 to 4.36) |
| 4 | <0.001 | 4.27 (2.34 to 7.80) | <0.001 | 4.95 (2.43 to 10.09) |
| 5 | <0.001 | 9.32 (2.72 to 31.96) | 0.011 | 5.99 (1.50 to 23.99) |
| Treatment provided within 30 days | ||||
| Chemotherapy | <0.001 | 0.43 (0.31 to 0.57) | 0.119 | 0.74 (0.51 to 1.08) |
| Radiotherapy | <0.001 | 2.87 (1.93 to 4.28) | 0.013 | 1.83 (1.14 to 2.96) |
| Immunotherapy | 0.083 | 0.45 (0.18 to 1.11) | NA | NA |
| Surgery | 0.683 | 1.09 (0.73 to 1.61) | NA | NA |
| Changes to treatment due to the COVID-19 pandemic | ||||
| Change to chemotherapy | 0.123 | 1.38 (0.92 to 2.07) | NA | NA |
| Change to radiotherapy | 0.038 | 2.22 (1.04 to 4.70) | 0.808 | 0.90 (0.37 to 2.17) |
| Change to immunotherapy | NA | NA | NA | NA |
| Change to surgery | 0.009 | 2.01 (1.19 to 3.39) | 0.092 | 1.82 (0.91 to 3.66) |
| Tumour type | ||||
| Acute lymphoblastic leukaemia | Reference | 1.0 | Reference | 1.0 |
| Ewing sarcoma | 0.473 | 1.35 (0.59 to 3.07) | 0.559 | 1.31 (0.53 to 3.19) |
| Glioma | <0.001 | 3.97 (2.55 to 6.20) | <0.001 | 4.28 (2.40 to 7.62) |
| Hodgkin lymphoma | 0.839 | 0.92 (0.43 to 1.98) | 0.435 | 0.70 (0.29 to 1.70) |
| Medulloblastoma | <0.001 | 4.42 (2.67 to 7.30) | 0.002 | 2.59 (1.40 to 4.79) |
| Neuroblastoma | 0.010 | 2.00 (1.18 to 3.39) | 0.013 | 2.22 (1.18 to 4.17) |
| Non-Hodgkin lymphoma | 0.482 | 1.26 (0.66 to 2.41) | 0.910 | 0.96 (0.46 to 2.00) |
| Osteosarcoma | <0.001 | 3.47 (1.94 to 6.22) | 0.008 | 2.57 (1.28 to 5.13) |
| Retinoblastoma | 0.474 | 1.31 (0.63 to 2.73) | 0.933 | 0.96 (0.41 to 2.26) |
| Rhabdomyosarcoma | <0.001 | 2.77 (1.56 to 4.91) | 0.002 | 2.84 (1.46 to 5.51) |
| Wilms tumour | 0.301 | 1.34 (0.77 to 2.32) | 0.237 | 1.48 (0.77 to 2.85) |
| SARS-CoV-2 infection status | ||||
| Not suspected or detected | Reference | 1.0 | NA | NA |
| Probable SARS-CoV-2 infection | 0.534 | 1.40 (0.48 to 4.10) | NA | NA |
| Laboratory confirmed SARS-CoV-2 infection | 0.938 | 1.04 (0.36 to 2.98) | NA | NA |
bmjgh-2022-008797supp005.pdf (78KB, pdf)
bmjgh-2022-008797supp006.pdf (480KB, pdf)
At 30 days, there were 71 (3.4%) deaths in this cohort with 66 deaths (4.6%) among 1427 participants in LMICs, and five deaths (0.8%) among 657 participants in HICs (χ2=20.411, p<0.001). Income group, ASA grade, chemotherapy within 30 days of the MDT, SARS-CoV-2 infection status and tumour type were significantly associated with death at 30 days (table 4). No other factors were statistically significant at a significance level of 0.05. Thirty-day complications from the various anticancer therapies are described in online supplemental appendix S7. At 90 days, there were 113 (5.7%) deaths in this cohort with 105 deaths (7.9%) among 1321 participants in LMICs, and 8 deaths (1.2%) among 648 participants in HICs (χ2=36.226, p<0.001). Income group, ASA grade, chemotherapy within 90 days of the MDT, surgery within 90 days of the MDT, and tumour type were significantly associated with death at 90 days. At 12 months, there were 206 (13.0%) deaths in this cohort with 174 deaths (15.7%) among 1107 participants in LMICs, and 32 deaths (6.8%) among 474 participants in HICs (χ2=23.550, p<0.001). Income group, ASA grade, chemotherapy within 30 days of the MDT, immunotherapy within 30 days of the MDT and tumour type were significantly associated with death at 12 months.
Table 4.
Univariate analysis and multivariable analysis of patient vital status at 30 days, 90 days and 12 months
| Univariate analysis | Multivariable analysis | |||
| P value | OR (95% CI) | P value | OR (95% CI) | |
| Factors associated with death at 30 days | ||||
| Income group | ||||
| High-income countries | Reference | 1.0 | Reference | 1.0 |
| Upper-middle income countries | 0.090 | 2.48 (0.87 to 7.07) | 0.150 | 2.43 (0.72 to 8.15) |
| Lower-middle income countries | <0.001 | 8.36 (3.30 to 21.19) | <0.001 | 9.52 (3.46 to 26.20) |
| Low-income countries | <0.001 | 43.47 (13.64 to 138.52) | <0.001 | 39.79 (9.65 to 164.16) |
| Age (for every year older) | 0.858 | 1.00 (0.95 to 1.05) | NA | NA |
| Sex | ||||
| Female | Reference | 1.0 | NA | NA |
| Male | 0.961 | 0.99 (0.61 to 1.60) | NA | NA |
| Weight (for every kg heavier) | 0.247 | 0.99 (0.98 to 1.01) | NA | NA |
| American Society of Anaesthesiologists (ASA) grade | ||||
| 1 | Reference | 1.0 | Reference | 1.0 |
| 2 | 0.722 | 0.87 (0.39 to 1.92) | 0.471 | 0.72 (0.29 to 1.77) |
| 3 | 0.125 | 1.84 0.84 to 3.99) | 0.077 | 2.27 (0.91 to 5.64) |
| 4 | <0.001 | 13.69 (5.98 to 31.34) | <0.001 | 8.93 (3.34 to 23.85) |
| 5 | <0.001 | 55.08 (14.39 to 210.78) | <0.001 | 30.75 (4.70 to 200.98) |
| Tumour type | ||||
| Acute lymphoblastic leukaemia | Reference | 1.0 | Reference | 1.0 |
| Ewing sarcoma | 0.708 | 0.68 (0.09 to 5.19) | 0.791 | 1.34 (0.15 to 11.65) |
| Glioma | 0.003 | 3.52 (1.56 to 7.97) | 0.106 | 2.55 (0.82 to 7.92) |
| Hodgkin lymphoma | NA (no deaths) | NA (no deaths) | NA (no deaths) | NA (no deaths) |
| Medulloblastoma | <0.001 | 7.92 (3.71 to 16.90) | 0.001 | 6.33 (2.22 to 18.04) |
| Neuroblastoma | 0.044 | 2.56 (1.03 to 6.39) | 0.560 | 1.43 (0.43 to 4.81) |
| Non-Hodgkin lymphoma | 0.001 | 3.95 (1.69 to 9.22) | 0.047 | 3.12 (1.01 to 9.62) |
| Osteosarcoma | 0.657 | 0.63 (0.08 to 4.83) | 0.703 | 1.51 (0.18 to 12.80) |
| Retinoblastoma | 0.040 | 2.95 (1.05 to 8.30) | 0.563 | 1.54 (0.36 to 6.69) |
| Rhabdomyosarcoma | 0.044 | 2.89 (1.03 to 8.12) | 0.069 | 3.01 (0.92 to 9.89) |
| Wilms tumour | 0.764 | 1.19 (0.39 to 3.62) | 0.807 | 0.85 (0.24 to 3.07) |
| Treatment provided within 30 days | ||||
| Chemotherapy | <0.001 | 0.20 (0.12 to 0.32) | 0.002 | 0.35 (0.18 to 0.69) |
| Radiotherapy | 0.466 | 1.35 (0.60 to 3.00) | NA | NA |
| Immunotherapy | NA (no deaths) | NA (no deaths) | NA (no deaths) | NA (no deaths) |
| Surgery | 0.644 | 0.84 (0.40 to 1.77) | NA | NA |
| Changes to treatment due to the COVID-19 pandemic | ||||
| Change to chemotherapy | 0.961 | 0.98 (0.44 to 2.17) | NA | NA |
| Change to radiotherapy | 0.149 | 2.42 (0.73 to 8.06) | NA | NA |
| Change to immunotherapy | NA (no deaths) | NA (no deaths) | NA (no deaths) | NA (no deaths) |
| Change to surgery | 0.278 | 1.68 (0.66 to 4.27) | NA | NA |
| SARS-CoV-2 infection status | ||||
| Not suspected or detected | Reference | 1.0 | Reference | 1.0 |
| Probable SARS-CoV-2 infection | <0.001 | 7.40 (2.70 to 20.25) | 0.002 | 8.40 (2.23 to 31.58) |
| Laboratory confirmed SARS-CoV-2 infection | 0.071 | 3.05 (0.91 to 10.26) | 0.029 | 5.33 (1.19 to 23.84) |
| Factors associated with death at 90 days | ||||
| Income group | ||||
| High-income countries | Reference | 1.0 | Reference | 1.0 |
| Upper-middle income countries | <0.001 | 5.43 (2.52 to 11.70) | <0.001 | 6.12 (2.71 to 13.81) |
| Lower-middle income countries | <0.001 | 7.42 (3.51 to 15.69) | <0.001 | 10.19 (4.52 to 22.97) |
| Low-income countries | <0.001 | 31.30 (11.07 to 88.50) | <0.001 | 23.94 (7.06 to 81.21) |
| Age (for every year older) | 0.870 | 1.00 (0.97 to 1.04) | NA | NA |
| Sex | ||||
| Female | Reference | 1.0 | Reference | 1.0 |
| Male | 0.595 | 0.90 (0.61 to 1.32) | NA | NA |
| Weight (for every kg heavier) | 0.284 | 0.99 (0.98 to 1.01) | NA | NA |
| American Society of Anaesthesiologists (ASA) grade | ||||
| 1 | Reference | 1.0 | Reference | 1.0 |
| 2 | 0.013 | 2.34 (1.20 to 4.55) | 0.021 | 2.32 (1.14 to 4.73) |
| 3 | 0.023 | 2.29 (1.12 to 4.67) | 0.002 | 3.36 (1.56 to 7.21) |
| 4 | <0.001 | 12.61 (5.60 to 28.40) | <0.001 | 9.59 (3.78 to 24.32) |
| 5 | <0.001 | 75.64 (16.71 to 342.28) | <0.001 | 29.39 (5.02 to 172.07) |
| Tumour type | ||||
| Acute lymphoblastic leukaemia | Reference | 1.0 | Reference | 1.0 |
| Ewing sarcoma | 0.580 | 0.66 (0.16 to 2.83) | 0.519 | 1.65 (0.36 to 7.47) |
| Glioma | 0.015 | 2.31 (1.17 to 4.53) | 0.029 | 2.76 (1.11 to 6.86) |
| Hodgkin lymphoma | NA (no deaths) | NA (no deaths) | NA (no deaths) | NA (no deaths) |
| Medulloblastoma | <0.001 | 5.30 (2.84 to 9.89) | <0.001 | 4.30 (1.92 to 9.65) |
| Neuroblastoma | 0.003 | 2.77 (1.43 to 5.36) | 0.002 | 3.37 (1.57 to 7.23) |
| Non-Hodgkin lymphoma | 0.013 | 2.48 (1.21 to 5.08) | 0.091 | 2.11 (0.89 to 5.02) |
| Osteosarcoma | 0.320 | 1.64 (0.62 to 4.34) | 0.042 | 3.04 (1.04 to 8.87) |
| Retinoblastoma | 0.183 | 1.85 (0.75 to 4.57) | 0.988 | 1.01 (0.32 to 3.13) |
| Rhabdomyosarcoma | 0.053 | 2.32 (0.99 to 5.47) | 0.087 | 2.30 (0.89 to 5.95) |
| Wilms tumour | 0.837 | 1.09 (0.47 to 2.53) | 0.911 | 1.05 (0.42 to 2.61) |
| Treatment provided within 90 days | ||||
| Chemotherapy | <0.001 | 0.26 (0.17 to 0.39) | 0.002 | 0.42 (0.24 to 0.73) |
| Radiotherapy | 0.642 | 0.86 (0.47 to 1.60) | NA | NA |
| Immunotherapy | NA (no deaths) | NA (no deaths) | NA (no deaths) | NA (no deaths) |
| Surgery | 0.015 | 0.48 (0.27 to 0.87) | 0.001 | 0.32 (0.16 to 0.65) |
| Changes to treatment due to the COVID-19 pandemic | ||||
| Change to chemotherapy | 0.467 | 1.25 (0.69 to 2.27) | NA | NA |
| Change to radiotherapy | 0.026 | 3.02 (1.14 to 7.98) | 0.728 | 1.23 (0.39 to 3.90) |
| Change to immunotherapy | NA (no deaths) | NA (no deaths) | NA (no deaths) | NA (no deaths) |
| Change to surgery | 0.059 | 2.00 (0.97 to 4.10) | NA | NA |
| SARS-CoV-2 infection status | ||||
| Not suspected or detected | Reference | 1.0 | Reference | 1.0 |
| Probable SARS-CoV-2 infection | 0.002 | 4.95 (1.80 to 13.60) | 0.018 | 3.90 (1.26 to 12.09) |
| Laboratory confirmed SARS-CoV-2 infection | 0.403 | 1.67 (0.50 to 5.55) | 0.467 | 1.64 (0.43 to 6.28) |
| Factors associated with death at 12 months | ||||
| Income group | ||||
| High-income countries | Reference | 1.0 | Reference | 1.0 |
| Upper-middle income countries | <0.001 | 2.22 (1.44 to 3.41) | <0.001 | 3.49 (2.02 to 6.03) |
| Lower-middle income countries | <0.001 | 2.78 (1.82 to 4.26) | <0.001 | 3.32(1.96 to 5.61) |
| Low-income countries | <0.001 | 6.58 (2.86 to 15.15) | <0.001 | 6.99 (2.49 to 19.68) |
| Age (for every year older) | 0.226 | 1.02 (0.99 to 1.05) | NA | NA |
| Sex | ||||
| Female | Reference | 1.0 | Reference | 1.0 |
| Male | 0.694 | 1.06 (0.79 to 1.43) | NA | NA |
| Weight (for every kg heavier) | 0.874 | 1.00 (0.99 to 1.01) | NA | NA |
| American Society of Anaesthesiologists (ASA) grade | ||||
| 1 | Reference | 1.0 | Reference | 1.0 |
| 2 | 0.632 | 1.11 (0.72 to 1.71) | 0.211 | 1.37 (0.83 to 2.26) |
| 3 | 0.044 | 1.62 (1.01 to 2.59) | <0.001 | 3.24 (1.81 to 5.79) |
| 4 | <0.001 | 5.34 (2.78 to 10.27) | <0.001 | 6.58 (3.03 to 14.26) |
| 5 | <0.001 | 26.72 (5.18 to 137.95) | 0.002 | 17.51 (2.87 to 106.80) |
| Tumour type | ||||
| Acute lymphoblastic leukaemia | Reference | 1.0 | Reference | 1.0 |
| Ewing sarcoma | 0.106 | 1.93 (0.87 to 4.28) | 0.133 | 2.01 (0.81 to 4.99) |
| Glioma | <0.001 | 3.71 (2.21 to 6.24) | 0.003 | 2.87 (1.45 to 5.70) |
| Hodgkin lymphoma | 0.067 | 0.26 (0.06 to 1.10) | – | – |
| Medulloblastoma | <0.001 | 3.97 (2.30 to 6.86) | 0.040 | 2.12 (1.03 to 4.33) |
| Neuroblastoma | <0.001 | 3.42 (2.04 to 5.73) | <0.001 | 3.26 (1.80 to 5.90) |
| Non-Hodgkin lymphoma | 0.015 | 2.10 (1.16 to 3.83) | 0.224 | 1.58 (0.76 to 3.32) |
| Osteosarcoma | <0.001 | 3.69 (1.94 to 7.02) | <0.001 | 4.54 (2.18 to 9.47) |
| Retinoblastoma | 0.356 | 1.45 (0.66 to 3.17) | 0.724 | 0.84 (0.31 to 2.25) |
| Rhabdomyosarcoma | <0.001 | 3.38 (1.75 to 6.50) | 0.054 | 2.18 (0.99 to 4.80) |
| Wilms tumour | 0.453 | 1.29 (0.67 to 2.48) | 0.942 | 1.03 (0.47 to 2.25) |
| Treatment provided within 30 days | ||||
| Chemotherapy | <0.001 | 0.36 (0.25 to 0.49) | 0.008 | 0.55 (0.36 to 0.86) |
| Radiotherapy | 0.002 | 2.03 (1.29 to 3.21) | 0.069 | 1.68 (0.96 to 2.94) |
| Immunotherapy | 0.036 | 0.29 (0.09 to 0.92) | 0.035 | 0.27 (0.08 to 0.91) |
| Surgery | 0.589 | 0.88 (0.55 to 1.40) | NA | NA |
| Treatment provided within 90 days | ||||
| Chemotherapy | <0.001 | 0.32 (0.23 to 0.45) | NA | NA (co-linearity) |
| Radiotherapy | 0.020 | 1.60 (1.08 to 2.37) | NA | NA (co-linearity) |
| Immunotherapy | 0.082 | 0.47 (0.20 to 1.10) | NA | NA |
| Surgery | 0.777 | 0.95 (0.65 to 1.38) | NA | NA |
| Changes to treatment due to the COVID-19 pandemic | ||||
| Change to chemotherapy | 0.555 | 1.15 (0.72 to 1.86) | NA | NA |
| Change to radiotherapy | 0.001 | 4.08 (1.84 to 9.03) | 0.403 | 1.50 (0.58 to 3.92) |
| Change to immunotherapy | NA (no deaths) | NA (no deaths) | NA (no deaths) | NA (no deaths) |
| Change to surgery | 0.343 | 1.38 (0.71 to 2.68) | NA | NA |
| SARS-CoV-2 infection status | ||||
| Not suspected or detected | Reference | 1.0 | Reference | 1.0 |
| Probable SARS-CoV-2 infection | 0.033 | 2.84 (1.09 to 7.42) | 0.110 | 2.35 (0.82 to 6.72) |
| Laboratory confirmed SARS-CoV-2 infection | 0.248 | 1.71 (0.69 to 4.21) | 0.449 | 1.51 (0.52 to 4.36) |
bmjgh-2022-008797supp007.pdf (64.1KB, pdf)
Discussion
This is the largest international cohort study to date to report COVID-19 outcomes for childhood cancers. We have shown that during the COVID-19 pandemic, children with cancer are more likely to die within 30 days if infected with SARS-CoV-2, even when adjusting for changes from normal standards of oncological care. However, the timely administration of chemotherapy is significantly associated with reduced odds of death at 30 days, 90 days and 12 months. Similar significant associations exist between timely surgery and reduced odds of death at 90 days, and timely administration of immunotherapy and reduced odds of death at 12 months. This report provides crucial information for public health policymakers to balance the benefits of providing anticancer therapy against the risks of SARS-CoV-2 infection in children with cancer.
During the COVID-19 pandemic, studies investigating COVID-19 outcomes for cancer patients have typically used 30-day mortality.24 31–34 The 30-day mortality from our study was 3.4% (n=71/2084). This is similar to the 30-day mortality reported by the Mukkada et al study: 3.8%. Their population of interest was children with cancer and SARS-CoV-2 infection. Their marginally higher death rate may reflect the impact of SARS-CoV-2 infection. Equally the high death rate observed in our study may be due to the impact of having a majority of participants from LMICs: to our knowledge, a first for global cohort studies on childhood cancers. Children with cancer in LMICs have historically had lower 5-year survival compared with their HIC counterparts.,5 7 8 and residence in LMICs is a factor that has been shown in our study to be associated with an increased odds of death at 30 days (4.6%), 90 days (7.9%) and 12 months (15.7%).
Of note, the 30-day mortality figure in our study is substantially lower than the 24% to 30.6% 30-day mortality reported among adult cancer patients during the COVID-19 pandemic.31–34 This may reflect the lower risk of death in children compared with adults infected with SARS-CoV-2.35 However, 30-day mortality figures for adults with cancer prior to the pandemic ranged from 3% to 10.6%.36–38 Therefore, there may have been a higher mortality rate at baseline in adults compared with children; comparable 30-day mortality figures for childhood cancers prior to the pandemic have not—to our knowledge—been published. A high 30-day mortality rate may reflect the aggressiveness of certain adult cancers; however, studies to date have highlighted lack of timely anticancer treatment to be a significant causative factor in driving the high rate of mortality in adult cancers.36–38 The use of 30-day mortality is increasingly recognised as a novel indicator to monitor quality of care in adult cancer treatment.36 37 39 40 However, this transition has yet to be made for childhood cancers, where there is a focus on using 5-year survival data.2 Gathering a sufficient sample from one centre or even one country can take up to 5 years,2 with an estimated additional 6 years required to formally publish mature 5-year survival data,41–43 and an average delay of 17 years before the findings is translated into clinical practice.44 Utilising 30-day mortality may reduce the time taken to recruit, conduct and disseminate the findings from a study. Our study shows that 30-day mortality is significantly different between LMICs and HICs, and this metric can identify patient-specific and system-specific factors associated with mortality. Therefore, the utility of 30-day mortality may extend beyond that of the pandemic as a useful indicator of quality of care in childhood cancer treatment internationally. This comes with a caveat, however, that using 30-day mortality figures that are focused on only one setting may not give a true reflection of the quality of childhood cancer treatment, for example, a hospital-focused 30-day mortality figure may increase if children who would otherwise die at home (with no palliative care) start to be brought in to hospital.
In addition, our study showed that 90-day mortality and 12-month mortality are also significantly different between LMICs and HICs. Therefore, they may prove to be other useful indicators of quality of care for WHO and GICC to monitor the progress of cancer care in LMICs. Of note, SARS-CoV-2 infection was not significantly associated with mortality at 90 days or 12 months. Given the association with 30-day mortality, this could suggest that SARS-CoV-2 infection accelerates mortality among vulnerable children with cancer, but ultimately does not change long-term mortality trends in childhood cancers. However, it is important to note that only a minority of patients underwent laboratory testing for SARS-CoV-2 infection, especially in LMICs, and, therefore, there may be an under-reporting of SARS-CoV-2 infection in our cohort, which could be leading to a misclassification bias affecting the results. Since laboratory testing only occurred for a minority of participants in LMICs, the higher odds of death among patients designated to have ‘Probable SARS-CoV-2 infection’ may be both a reflection of the impact of SARS-CoV-2 infection and infrastructural issues. Furthermore, SARS-CoV-2 infection was only recorded if it occurred within 30 days of starting anticancer therapy. Patients may have gone onto become infected with SARS-CoV-2 after 30 days, which may account in part for the increase in mortality seen in 90 days and 12 months. The absence of published 90-day and 12-month mortality figures for childhood cancers prior to the pandemic renders this difficult to ascertain. With increasing international attention on scaling-up testing for SARS-CoV-2 infection,45 future studies can address these uncertainties.
A significant strength of this study is that it is the first international study that has been designed and powered to detect if changes from normal standards of care due to the COVID-19 pandemic have impacted outcomes in childhood cancers. A total of 201 patients (9.5%) reportedly had their care affected because of the COVID-19 pandemic. However, changes to treatment due to the COVID-19 pandemic were not significantly associated with mortality at 30 days, 90 days or 12 months. Yet, delays in treatment—regardless of the underlying reason behind them—were associated with an increased odd of death. Patients in HICs had the fastest average time from diagnosis to initial MDT management plan being made, were more likely to have a central venous catheter inserted on a chemotherapy plan being made and were more likely to have planned anticancer therapy treatment within 30 days and 90 days. These factors may be playing a role in patients in HICs having lower odds of mortality at 30 days, 90 days and 12 months. Tackling the time-lags between diagnosis and treatment are cost-effective interventions for childhood cancers.18 It is critical that interventions here focus on the unique challenges posed by each LMIC, as extrapolation of cancer control programme experiences in HICs to LMICs is inappropriate without considering local resources and cultures.46 47
There are several potential limitations to consider when interpreting our results. First, clinicians were tasked with determining whether a change to treatment was due to the COVID-19 pandemic. It is possible that response bias might have led to clinicians accounting any change to the COVID-19 pandemic. To mitigate against this bias, we requested that all data collectors attest they have only submitted new issues brought about by the pandemic. Therefore, although we are not aware of a bias towards baseline gaps in service delivery, we cannot confirm that pre-existing issues with service provision and supply chains did not contribute to the disparity in care showcased by this study. Second, participating LMIC sites tended to be tertiary hospitals, while HIC sites included a larger mix of general hospitals, paediatric hospitals and paediatric oncology hospitals (online supplemental appendix S2). There is an inherent variability in capacity for cancer care between these hospital types.48 The inclusion of hospitals in HICs that were not specialised for the care of children with cancer may have resulted in an underestimation of the effect of the COVID-19 pandemic—including the disruptions to care—on this population in LMICs relative to HICs. Third, due to the inability to capture socioeconomic status and ethnicity consistently across a global cohort, we were unable to ascertain the effects of these factors on outcomes. Fourth, due to global data privacy rules and the need to collect this data urgently due to a new infectious threat, no patient reported outcome measures were collected. Fifth, there may have been selection bias from the type of participants lost to follow-up. Sixth, given the small number of children in the study who had laboratory-confirmed SARS-CoV-2 infection or who were started on immunotherapy, the effect sizes for these variables may not reflect a true effect (ie, the findings may be false positives). Finally, this study was unable to collect data from patients who failed to reach a healthcare service provider, and, therefore, it might not reflect the true impact of the pandemic on oncological outcomes.
Childhood cancer is a highly curable disease when healthcare systems provide timely, accurate diagnoses and appropriate therapy. In our study, we have shown for the first time that paediatric cancer survival rate is significantly lower in the short term in LMICs than in HICs. This disparity may be due to health system challenges such as limited access to early detection and lack of effective treatment and care.
bmjgh-2022-008797supp008.pdf (88.6KB, pdf)
Acknowledgments
Thank you to the University of Oxford Medical Sciences Division IT Services Systems Team (MSDIT Systems Team) for the REDCap administration and management. Thank you to the representatives from the College of Surgeons of Southern, East and Central Africa (COSECSA), the Global Children’s Initiative for Surgery (GICS), the Pan-African Paediatric Surgical Association (PAPSA), the International Society of Paediatric Oncology (SIOP), and the International Society of Paediatric Surgical Oncology (IPSO) who made this study possible. Thank you to all our collaborators from our country leads to the members of our local mini-teams for driving this study forward.
Footnotes
Handling editor: Seye Abimbola
Collaborators: Global Children’s NCDs Collaborative: Steering committee: Soham Bandyopadhyay [UK], Noel Peter [UK] (Asia Lead), Kokila Lakhoo [UK], Simone de Campos Vieira Abib [Brazil] (South America Lead), Hafeez Abdelhafeez [Sudan] (Africa and Middle East Lead), Shaun Wilson [UK] (Australasia Lead), Max Pachl [UK] (Europe and North America Lead), Benjamin Martin [UK] (Europe Lead), Sonal Nagras [Australia] (Australasia Lead), and Mihir Sheth [India]. Operational committee: Catherine Dominic [UK], Suraj Gandhi [UK], Divya Parwani [India], Rhea Raj [UAE], Diella Munezero [Burundi], Rohini Dutta [India], Nsimire Mulanga Roseline [DRC], Kellie McClafferty [UK], Armin Nazari [UK], Smrithi Sriram [UK], Sai Pillarisetti [UK], King-David Nweze [UK], Aishwarya Ashwinee [Grenada], Gul Kalra [India], Poorvaprabha Patil [India], Priyansh Nathani [India], Khushman Kaur Bhullar [India], Muhammed Elhadi [Libya], Maryam Khan [Pakistan], Nehal Rahim [Pakistan], Shweta Madhusudanan [UK], Joshua Erhabor [UK], Manasi Shirke [UK], Aishah Mughal [UK], Darica Au [UK], Mahan Salehi [UK], Sravani Royyuru [UK], Mohamed Ahmed [Egypt], Syeda Namayah Fatima Hussain [Pakistan], Daniel Robinson [UK], Anna Casey [UK], Mehdi Khan [UK], Alexandre Dukundane [Rwanda], Kwizera Festus [Rwanda], Vaishnavi Govind [Grenada], Rohan Pancharatnam [UK], Lorraine Ochieng [UK], Elliott H Taylor [UK], Hritik Nautiyal [UK], Marta de Andres Crespo [UK], Somy Charuvila [UK], and Alexandra Valetopoulou [UK]. Writing committee: Soham Bandyopadhyay [UK], Amanpreet Brar [Canada], Hira Zuberi [Pakistan], Imane Ammouze [Morocco], Dhruva Ghosh [India], Nitin James Peters [India], Noel Peter [UK], and Kokila Lakhoo [UK]. Statistics committee: Soham Bandyopadhyay [UK] and Mihir Sheth [India]. Local teams: Abubakar Tafawa Balewa University Teaching Hospital, Nigeria: Kefas John Bwala, AM Umar, Abdurahaman Aremu, Dauda E. Suleiman, Tybat Aliyu. Aga Khan University Hospital, Pakistan: Ayesha Saleem, Muhammad Arshad, Kashaf Turk, Sadaf Altaf. Ahmadu Bello University Teaching Hospital, Nigeria: Oluseyi Oyebode Ogunsua, Tunde Talib Sholadoye, Musliu Adetola Tolani, Yakubu Alfa, Keffi Mubarak Musa. AIC Kijabe Hospital, Kenya: Eric Mwangi Irungu, Ken Muma, Sarah Muma, Mitchelle Obat. Ain Shams Hospitals "El-Demerdash", Egypt: Youssef Sameh Badran. Al-Basheer Hospital, Jordan: Abdulrahman Ghassan Qasem, Faris Ayasra, Reema Alnajjar. Al-Hussein University Hospital, Egypt: Mohamed Abdel-Maboud, Abdelrahman Bahaa, Ayat M. Saadeldin, Mohamed Adwi, Mahmoud Adly, Abdallah Elshenawy. Alder Hey Children Hospital, UK: Amer Harky, Leanne Gentle, Kirstie Wright, Jessica Luyt, Olivia White, Charlotte Smith, Nathan Thompson, Thomas Smith, Imogen Harrison. All India Institute of Medical Sciences (AIIMS), Bhubaneshwar, India: Santosh Kumar Mahalik. All India Institute of Medical Sciences (AIIMS), Rishikesh, India: Rajat Piplani, Enono Yhoshu, Manoj Gupta, Uttam Kumar Nath, Amit Sehrawat, Rajkumar K S, Vivek Singh. Augusta Victoria Hospital, Palestine: Sadi A. Abukhalaf. Bangladesh Shishu Hospital & Institute, Bangladesh: Ashrarur Rahman Mitul, Sabbir Karim, Nazmul Islam. Benghazi pediatric hospital, Libya: Sara Kader Alsaeiti, Fatma Saleh Benkhial, Mohammed Miftah Faraj Almihashhish, Eman Salem Muftah Burzeiza, Hend Mohammed Masoud, Mabroukah Saeid Alshamikh, Raja Mari Mohammed Nasef, Fatma Mohammed Masoud. Birmingham Children's Hospital, UK: William B Lo, Nyararai Togarepi, Elaine Carrolan, Benjamin Martin, Max Pachl, Benjamin J O'Sullivan. Borg El Arab University Hospital, Egypt: Mohamed Hassanin, Ahmed Saleh, Mahmoud Bassiony, Mostafa Qatora, Mohamed Bahaaeldin, Shady Fadel, Yasmine El Chazli. Centre Anti-Cancer, Batna, Algeria: Anfel Bouderbala, Kamel Hamizi, Safia Lorabi, Mehdi Anouar Zekkour, Rima Rahmoun, Boutheyna Drid, Salma Naje Abu Teir. Centre hospitalier universitaire de Batna, Algeria: Safia Lorabi, Mohamed Yazid Kadir, Yassine Zerizer, Nacer Khernane, Brahim Saada. Centre Hospitalo-Universitaire Ibn Sina de Rabat (CHIS), Morocco: Imane Ammouze, Yahya Elkaoune, Hajar Moujtahid, Ghita Chaoui, Hajar Benaouda, Meryem Gounni, Narjiss Aji, Laila Hessissen. Centro Hospitalar Universitário de São João, Portugal: Joana Mafalda Monteiro, Susana Nunes, Maria do Bom-Sucesso. Children's Hospital of Wisconsin, United States of America: Dave R. Lal, Brian T. Craig, Kerri Becktell. Chittagong Research Institute For Children Surgery, Bangladesh: Tahmina Banu, Md Afruzul Alam, Orindom Shing Pulock, Tasmiah Tahera Aziz. Christian Medical College & Hospital, Ludhiana, India: Vishal Michael, M Joseph John, William Bhatti, Bobby John, Swati Daniel, Jyoti Dhiman, Hunar Mahal, Atul Suroy. Clinic for Neurosurgery, Clinical Center of Serbia, Serbia: Rosanda Ilic, Danica Grujicic, Tijana Nastasovic, Igor Lazic, Mihailo Milicevic, Vladimir Bascarevic, Radovan Mijalcic, Vuk Scepanovic, Aleksandar Stanimirovic, Aleksandra Paunovic, Ivan Bogdanovic. Dayanand Medical College & Hospital Ludhiana, India: Shruti Kakkar, Shaina Kamboj, Suraj Singh. Dhaka Medical College Hospital, Bangladesh: Shahnoor Islam, AKM Amirul Morshed, A. K. M. Khairul Basher, Mehnaz Akter, S. M. Rezanur Rahman, Zannat Ara, Mohammed Tanvir Ahammed, Tania Akter, Kamrun Nahar, Fatema Sayed, Ashfaque Nabi, Md. Asif Iqbal, Md. Masud Rana, Md. Asaduzzaman, Md. Hasanuzzaman. Dr. Lutfi Kirdar Kartal Training and Research Hospital, Turkey: Kemal Tolga Saracoglu, Elif Akova, Evren Aydogmus, Bekir Can Kendirlioglu, Tufan Hicdonmez. Dubai Hospital, United Arab Emirates: Arshiya Adhnon, Asim Noor Rana, Hani Humad, Anjan Madasu. El Safa Hospital, Egypt: Ahmed Y Azzam, Mohammed A Azab. El Sheikh Zayed Specialized Hospital, Egypt: Sherief Ghozy, Alzhraa Salah Abbas. El-Salam Hospital, Egypt: Monica Dobs, Mohamed Atef Mohamed Ghamry, Mohammed Alhendy. Faculty of Medicine, University of Porto, Portugal: Joana Monteiro. Federal Medical Center, Abeokuta, Nigeria: Olanrewaju Moses. Federal Medical Center, Lokoja, Nigeria: Ibiyeye Taiye Taibat, Taiwo Jones, Kalu Ukoha, Olagundoye Goke, Okorie Ikechukwu. Federal Teaching Hospital Ido-Ekiti, Nigeria: Abiodun Idowu Okunlola. Frere Hospital, South Africa: Milind Chitnis, Helga Nauhaus, Danelle Erwee. Gloucestershire Hospitals NHS Foundation Trust, United Kingdom: Robyn Brown, Agata Chylinska, Robin Simpson, Prasanna Gomes, Noel Peter. GPACI - Grupo de Pesquisa e Assistência ao Câncer Infantil, Brazil: Marco Aurelio Ciriaco Padilha, Elvercio Pereira de Oliveira Junior, Lucas Garschagen de Carvalho, Fabiola Leonelli Diz. Helwan University Hospital, Egypt: Mohamed El Kassas, Usama Eldaly, Ahmed Tawheed, Mohamed Abdelwahab. Hôpital des Spécialités ONO, Morocco: Oudrhiri Mohammed Yassaad, Bechri Hajar, El Ouahabi Abdessamad, Arkha Yasser, Hessissen Laila. Ibn-Al-Atheer Teaching Hospital, Mosul, Iraq: Farah Sameer Yahya (Department of Pediatrics, College of Medicine, University of Mosul, Mosul, Iraq), Yasir Al-Agele. Indira Gandhi Institute of Medical Sciences (IGIMS), India: Sandip Kumar Rahul, Vijayendra Kumar, Digamber Chaubey. Instituto Nacional de Enfermedades Neoplásicas, Peru: Maria Teresa Peña Gallardo, Jacqueline Elizabeth Montoya Vásquez, Juan Luis García León, Sebastián Shu Yip. Ipswich Hospital NHS Trust, UK: Georgios Karagiannidis. Istanbul University, Turkey: Rejin Kebudi, Sema Bay Buyukkapu. Jawaharlal Institute of Postgraduate Medical Education and Research, India: Krishna Kumar Govindarajan, Kumaravel Sambandan, Smita Kayal, Gunaseelan Karunanithi, Bikash Kumar Naredi, Bibekanand Jindal. John Radcliffe Hospital, United Kingdom: Mariam Lami, Matthew H V Byrne, Duha Jasim, Harmit Ghattaura, Soham Bandyopadhyay, Kokila Lakhoo. Johns Hopkins Hospital Bloomberg Children's Hospital, United States of America: Eric W Etchill, Daniel Rhee, Stacy Cooper, Kevin Crow, Morgan Drucker, Megan Murphy, Benjamin Shou, Alan Siegel. Kanuni Sultan Süleyman Research and Training Hospital, Turkey: Yasin Kara, Gül Nihal Özdemir. Kasr Al Ainy Hospital, Egypt: Mahmoud Elfiky, Ehab El Refaee. Khoula Hospital, Oman: John George Massoud. King Abdullah University Hospital, Jordan: Ayah Bassam Ibrahim, Ruaa Bassam Ibrahim, Faris Abu Za'nouneh, Ranya M. Baddourah, Toqa Fahmawee, Ayah Al_Shraideh. King Fahd Central Hospital, Saudi Arabia: Ghazwani Salman, Ehab Alameer (Jazan University), Al-Mudeer Ali, Ghazwani Yahia, Khozairi Waleed. King George's Medical University, India: Ahmad Ozair, Ankur Bajaj, Bal Krishna Ojha, Kaushal Kishor Singh, Atique Anwar, Vinay Suresh. King Hussein Cancer Center, Jordan: Mohamad K. Abou Chaar, Iyad Sultan, Khalil Ghandour, Shaima' Al-Dabaibeh, Ammar Al-Basiti, Hazim Ababneh, Omaima El-Qurneh. King Salman Armed Forces Hospital, Saudi Arabia: Yousef Alalawi, Ahmad Al Ayed, Ehab Hanafy, Naif Al Bolowi. Women's and Children's Hospital, Singapore: Amos HP Loh, Anette S Jacobsen Heidi Barola Aubrey L Pagaduan Jingdan Fan. Lagos University Teaching Hospital, Nigeria: Olumide Abiodun Elebute, Adesoji O. Ademuyiwa Christopher O. Bode Justina O. Seyi-Olajide Oluwaseun Ladipo-Ajayi Felix M. Alakaloko George C. Ihediwa Kareem O. Musa, Edamisan O. Temiye, Olufemi Oni, Adeseye M. Akinsete. Lahore General Hospital, Pakistan: Janita Zarrish, Ramsha Saleem, Soha Zahid, Atiqa Amirali, Ahsan Nadeem, Sameer Saleem Tebha, Zonaira Qayyum, Sana Tahir, Anneqa Tahir, Rabbey Raza Khan, Ayesha Mehmood, Iqra Effendi. Liaquat National Hospital and Medical College, Pakistan: Muhammad Arshad, Taimur Iftikhar Qureshi, Pooja Kumari. Mansoura University Hospitals, Egypt: Mohamed Bonna, Khaled Mamdouh, Mohamed Atef, Mohamed Faried. Mater Dei Hospital, Sir Anthony Mamo Oncology Centre, Malta: Victor Calvagna, Nathalie Galea, Ariana Axiaq. Mayo Clinic, United States of America: Matthew R Schuelke, Jake A. Kloeber, Robert L. Owen, Alexander S. Roth, Catherine Yang, J. Hudson Barnett, Lucien P. Jay, Kirk David Wyatt, Paul J. Galardy, Mbeya Zonal Referral Hospital, University of Dar es Salaam, Tanzania: Bernard Mbwele, Irene Nguma, Moshi Moshi Shabani, Amani Twaha, Bilal Matola. Medical University of Pecs, Department of Paediatrics, Hungary: Agnes Vojcek. Menoufia University Hospital, Egypt: Mahmoud Maher Abdelnaby Alrahawy, Seham M Ragab, Abdallah R Allam, Eman Ibrahim Hager, Abdelrahman Azzam, Ammar Ayman. Ministry of Health Marmara University Pendik Research and Application Hospital, Turkey: Kıvılcım Karadeniz Cerit, Adnan Dağçınar, Tümay Umuroğlu, Ayten Saraçoğlu, Mustafa Sakar, Can Kıvrak, Gül Çakmak. MISR Cancer Centre, Egypt: Ibrahim Sallam, Gamal Amira, Mohamed Sherief, Ahmed Sherif. National Cancer Institute, Brazil: Simone de Oliveira Coelho, Arissa Ikeda, Licia Portela, Marianne Monteiro Garrigo, Ricardo Vianna de Carvalho, Fernanda Lobo, Sima Ester Ferman, Fernanda Ferreira da Silva Lima. National Cancer Institute, Sudan: Moawia Mohammed Ali Elhassan, Nada Osman Yousif Elhaj, Hytham K. S. Hamid. National Hospital, Nigeria: Emmanuel A. Ameh, Vincent E. Nwatah, Adewumi B. Oyesakin. Nnamdi Azikiwe University Teaching Hospital, Nigeria: Andrew Nwankwo Osuigwe, Okechukwu Hyginus Ekwunife, Chisom Adaobi Nri-Ezedi, Eric Okechukwu Umeh. Ola During Children's Hospital, Sierra Leone: Nellie Bell. Olabisi Onabanjo University Teaching Hospital, Nigeria: Ibukunolu Olufemi Ogundele, Abiodun Folashade Adekanmbi, Olubunmi Motunrayo Fatungase, Olubunmi Obafemi Obadaini. Ondokuz Mayıs Üniversitesi, Turkey: Sarah Al-Furais, Humaida Hemlae, Sreylis Nay. Pantai Jerudong Specialist Centre, Brunei: John Mathew, R M Jeffri Ismail. Pediatric Oncology Institute – GRAACC, Brazil: Simone de Campos Vieira Abib, Fabianne Altruda de Moraes Costa Carlesse, Mayara Caroline Amorim Fanelli, Fernanda Kelly Marques de Souza. Policlinico Umberto I, Sapienza University of Rome, Italy: Pierfrancesco Lapolla, Andrea Mingoli, Denis Cozzi, Anna Maria Testi, Paolo Musiu, Paolo Sapienza, Gioia Brachini, Martina Zambon, Simona Meneghini, Pierfranco Cicerchia, Bruno Cirillo. Post Graduate Institute of Medical Education and Research, Chandigarh, India: Manjul Tripathi, Nitin James Peters, Sandeep Mohindra, Vishal Kumar, Ninad R Patil, Richa Jain, Renu Madan, Madhivanan Karthigeyan, Pravin Salunke. Prince Mohammed bin Nasser Hospital, Jazan, Saudi Arabia: Ghazwani Salman. Prince Sattam Bin Abdulaziz University, Saudi Arabia Gopal Nambi. Raparin Pediatric Teaching Hospital, Iraq: Abdulrahman Omar Taha. RIPAS Hospital, Brunei: Janice Hui Ling Wong, Norehan Johari, Anas Shikha, Win Sabai Phyu Han, Zahidah Ahmad, Yen Yan Lim, Roserahayu Idros, Noorainun Mohd Yusof, David Nelson Jaisingh. Saadna Mohamed Abdenour, Algeria: Aouabed Nesrine, Bouaoud Souad, Mebarki Malika, Bioud Belkacem. Salmaniya Medical Complex, Bahrain: Fayza Haider, Fatema Naser Al Fayez. Shahid Baghaei Hospital, Iran: Fakher Rahim. Sheba Medical Center/Tel HaShomer Hospital, Israel: Elana Kleinman, Taylor Ibelli, Emily Hamilton, Rochelle Fayngor, Tzvi Najman, Gideon Karplus, Etai Adam, Daniella Melamed, Cecilia Paasche. St. George's Hospital, UK: Amir Labib. Sultan Qaboos University Hospital, Muscat Sultanate of Oman: Farman Ali Laghari, Dhruva Ghosh, Zainab Al Balushi, Abdulhakim Awadh Salim Al-Rawas, Ali Al Sharqi, Ammar Saif Al Shabibi, Ismail Al Bulushi, Muna Alshahri, Abdulrahman AlMirza, Ola Al Hamadani, Jawaher Al Sharqi, Anisa Al Shamsi, Bashar Dawud, Sareya Al Sibai. Tamale Teaching Hospital, Ghana: Alhassan Abdul-Mumin, Halwani Yaninga Fuseini, Peter Gyamfi Kwarteng, Abubakari Bawa Abdulai, Sheba Mary Pognaa Kunfah, Gilbert B. Bonsaana, Stephanie Ajinkpang, Edmund M. Der, Francis A. Abantanga, Mary Joan Kpiniong, Kingsley Aseye Hattor, Kingsley Appiah Bimpong. Tanta University Hospital, Egypt: Mohamed Elbahnasawy, Sherief Abdelsalam, Ahmed Samir. The Hospital for Sick Children, Canada: Reto M. Baertschiger, Amanpreet Brar, Andreea C, Matei, Augusto Zani. The Indus Hospital, Pakistan: Lubna Samad, Hira Khalid Zuberi, Kishwer Nadeem, Naema Khayyam, Fatima Ambreen Imran, Nida Zia, Sadia Muhammad, Muhammad Rafie Raza, Muhammad Rahil Khan. Tishreen University Hospital, Syria: Alaa Hamdan, Ammar Omran, Ahmed Moussa, Bardisan Gawrieh, Hassan Salloum, Alaa Ahmed, Abdeljawad Mazloum, Ali Abodest, Nisreen Ali, Munawar Hraib, Victor Khoury, Abdulrahman Almjersah, Mohammad Ali Deeb, Mohammad Ahmad Almahmod Alkhalil, Akram Ahmed, Waseem Shater, Ali Farid Alelayan, Alaa Guzlan. Tobruk Medical Centre, Libya: Ahmad Bouhuwaish, Alqasim Abdulkarim. Tripoli University Hospital, Libya: Eman Abdulwahed, Marwa Biala, Reem Ghamgh, Amani Alamre, Marwa Shelft, Asmaa A. M. Albanna, Hoda Tawel. Unit of Paediatric and Adolescent Haematology and Oncology, 2nd Department of Peadiatrics, Aristotle University of Thessaloniki, University General Hospital AHEPA, Greece: Emmanuel Hatzipantelis, Athanasios Tragiannidis, Eleni Tsotridou, Assimina Galli-Tsinopoulou. Universiti Kebangsaan Malaysia Medical Centre, Malaysia: Dayang Anita Abdul Aziz, Zarina Abdul Latiff, Hamidah Alias, C-Khai Loh, Doris Lau, Azrina Syarizad Khutubul Zaman, University College Hospital (UCH), Nigeria: Taiwo Akeem Lawal, Kelvin Ifeanyichukwu Egbuchulem, Olakayode Olaolu Ogundoyin, Isaac Dare Olulana, Biobele J. Brown, Oluwasegun Joshua Afolaranmi, AbdulBasit Fehintola. University Hospital Hamburg-Eppendorf, Germany: Annika Heuer, Christine Nitschke, Michael Boettcher, Matthias Priemel, Lennart Viezens, Martin Stangenberg, Marc Dreimann, Alonja Reiter, Jasmin Meyer, Leon Köpke, Karl-Heinz Frosch. University of Abuja Teaching Hospital, Nigeria: Samson Olori, Uduak Offiong, Philip Mari Mshelbwala, Fashie Andrew Patrick, Aminu Muhammed Umar, Otene ThankGod N. University of Ilorin Teaching Hospital, Nigeria: Abdulrasheed A Nasir, Kazeem O. O. Ibrahim, Dupe S. Ademola-Popoola, Olayinka T. Sayomi, Alege Abdurrzzaq, Ademola A. Adeyeye, Khadijah O. Omokanye, Lukman O Abdur-Rahman, Olubisi Olutosin Bamidele, Shakirullah AbdulAzeez, Aminat Akinoso, Michael O. Adegboye. University of Malaya Medical Centre, Malaysia: Shireen Anne Nah, Yuki Julius Ng, Syukri Ahmad Zubaidi, University of Texas Medical Branch, United States of America: Murad Almasri, Sara Ali, Rasaq Olaosebikan, Akila Muthukumar. University Teaching Hospital, Zambia: Patricia Shinondo, Amon Ngongola, Bruce Bvulani, Azad Patel, Usman Danfodiyo University Teaching Hospital, Nigeria: Abdullahi Nuhu-Koko, Baba Jibrin, Ajiboye L. Olalekan, Christopher S. Lukong, Ezekiel I. Ajayi. Vall d'Hebron University Hospital, Spain: Gabriela Guillén, Sergio López, José Andrés Molino, Pablo Velasco, Wingat Royal Hospital, Egypt: Omar Elmandouh, Omar Hamam, Rim Elmandouh, Yale New Haven Hospital, United States of America: Nensi Melissa Ruzgar, Rachel Levinson, Shashwat Kala, Sarah Ullrich, Emily Christison-Lagay. Zagazig University Hospital, Egypt: Aya Sabry Mortada, Mahmoud Ahmed Ebada, Eman Seif Alnaser Solimam, Khaled Abualkher, Amr Mohammed Elsayed Yousf, Mohamed Mohamed Holail, Reem Mohamed Almowafy. National/Regional Leads: Algeria: Salah Eddine Oussama Kacimi, Bahrain: Fayza Haider, Bangladesh: Tahmina Banu, Ashrarur Rahman Mitul, Brazil: Simone de Campos Vieira Abib, Brunei: Janice Hui Ling Wong, Canada: Reto Baertschiger, Egypt: Essam Elhalaby, Muath Alser, Mahmoud M. Saad, France: Luca Pio, Germany: Guido Seitz, Judith Lindbert, Ghana: Francis Abantanga, Greece: Georgios Tsoulfas, Asimina Galli-Tsinopoulou, Hungary: Agnes Vojcek, India: Dhruva Ghosh, Nitin James Peter, Ankur Bajaj, Vrisha Madhuri, Ravi Kishore, Iran: Maryam Ghavami Adel, Iraq: Abdulrahman Omar Taha, Italy: Calogero Virgone, Francesco Pata, Gaetano Gallo, Jordan: Mohammad K. Abou Chaar, Faris Ayasra, Israel: Elana Kleinman, Taylor Ibelli, Kenya: Eric Mwangi Irungu, Libya: Muhammed Elhadi, Malaysia: Shireen Anne Nah, Dayang Anita Abdul Aziz, Malta: Victor Calvagna, Morocco: Outani Oumaima, Zineb Bentounsi, Hajar Moujtahid, Nigeria: Adesoji Ademuyiwa, Oman: Dhruva Nath Ghosh, Pakistan: Muhammad Arshad, Lubna Samad, Peru: Lily Saldana, Portugal: Jan Godzinsky, Saudi Arabia: Abdelbasit Ali, Ehab Alameer, Serbia: Dragana Janic, Sierra Leone: Mohamed Bella Jalloh, Nellie Bell, Singapore: Annette Jacobsen, Chan Hon Chui, South Africa: Milind Chitnis, Spain: Israel Fernandez Pineda, Lucas Krauel, Maricarmen Olivos, Sudan: Waha Rahama, Hazim Elfatih, Switzerland: Raphael N. Vuille-dit-Bille, Syria: Alaa Hamdan, Turkey: Arda Isik, United Arab Emirates: Asim Noor Rana, United Kingdom: Kokila Lakhoo, Kate Cross, Max Pachl, United States of America: Andrea Hayes-Jordan, Roshni Dasgupta, Zambia: Patricia Shinondo, Amon Ngongola, Middle-East and North Africa: Mohamedraed Elshami.
Contributors: This is a paper produced under a collaborative authorship model: Global Health Research Group on Children’s Non-Communicable Diseases Collaborative. All authors are solely listed under the collaborative authorship. A full authorship list can be found in Appendix S2. KL is the author acting as gurantor.
Funding: Donation from the Kids Operating Room Research Fund.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: The reflexivity statement for this paper is linked as an online supplemental file 1.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: Soham Bandyopadhyay, Noel Peter, Kokila Lakhoo, Simone de Campos Vieira Abib, Hafeez Abdelhafeez, Shaun Wilson, Max Pachl, Benjamin Martin, Sonal Nagras, Mihir Sheth, Catherine Dominic, Suraj Gandhi, Divya Parwani, Rhea Raj, Diella Munezero, Rohini Dutta, Nsimire Mulanga Roseline, Kellie McClafferty, Armin Nazari, Smrithi Sriram, Sai Pillarisetti, King-David Nweze, Aishwarya Ashwinee, Gul Kalra, Poorvaprabha Patil, Priyansh Nathani, Khushman Kaur Bhullar, Muhammed Elhadi, Maryam Khan, Nehal Rahim, Shweta Madhusudanan, Joshua Erhabor, Manasi Shirke, Aishah Mughal, Darica Au, Mahan Salehi, Sravani Royyuru, Mohamed Ahmed, Syeda Namayah Fatima Hussain, Daniel Robinson, Anna Casey, Mehdi Khan, Alexandre Dukundane, Kwizera Festus, Vaishnavi Govind, Rohan Pancharatnam, Lorraine Ochieng, Elliott H Taylor, Hritik Nautiyal, Marta deAndres Crespo, Somy Charuvila, Alexandra Valetopoulou, Soham Bandyopadhyay, Amanpreet Brar, Hira Zuberi, Imane Ammouze, Dhruva Ghosh, Nitin James Peters, Noel Peter, Kokila Lakhoo, Soham Bandyopadhyay, Mihir Sheth, Kefas John Bwala, AM Umar, Abdurahaman Aremu, Dauda E Suleiman, Tybat Aliyu, Ayesha Saleem, Muhammad Arshad, Kashaf Turk, Sadaf Altaf, Oluseyi Oyebode Ogunsua, Tunde Talib Sholadoye, Musliu Adetola Tolani, Yakubu Alfa, Keffi Mubarak Musa, Eric Mwangi Irungu, Ken Muma, Sarah Muma, Mitchelle Obat, Youssef Sameh Badran, Abdulrahman Ghassan Qasem, Faris Ayasra, Reema Alnajjar, Mohamed Abdel-Maboud, Abdelrahman Bahaa, Ayat M Saadeldin, Mohamed Adwi, Mahmoud Adly, Abdallah Elshenawy, Amer Harky, Leanne Gentle, Kirstie Wright, Jessica Luyt, Olivia White, Charlotte Smith, Nathan Thompson, Thomas Smith, Imogen Harrison, Santosh Kumar Mahalik, Rajat Piplani, Enono Yhoshu, Manoj Gupta, Uttam Kumar Nath, Amit Sehrawat, K S Rajkumar, Vivek Singh, Sadi A Abukhalaf, Ashrarur Rahman Mitul, Sabbir Karim, Nazmul Islam, Sara Kader Alsaeiti, Fatma Saleh Benkhial, Mohammed Miftah Faraj Almihashhish, Eman Salem Muftah Burzeiza, Hend Mohammed Masoud, Mabroukah Saeid Alshamikh, Raja Mari Mohammed Nasef, Fatma Mohammed Masoud, William B Lo, Nyararai Togarepi, Elaine Carrolan, Benjamin Martin, Max Pachl, Benjamin J O'Sullivan, Mohamed Hassanin, Ahmed Saleh, Mahmoud Bassiony, Mostafa Qatora, Mohamed Bahaaeldin, Shady Fadel, Yasmine El Chazli, Anfel Bouderbala, Kamel Hamizi, Safia Lorabi, Mehdi Anouar Zekkour, Rima Rahmoun, Boutheyna Drid, Salma Naje Abu Teir, Safia Lorabi, Mohamed Yazid Kadir, Yassine Zerizer, Nacer Khernane, Brahim Saada, Imane Ammouze, Yahya Elkaoune, Hajar Moujtahid, Ghita Chaoui, Hajar Benaouda, Meryem Gounni, Narjiss Aji, Laila Hessissen, Joana Mafalda Monteiro, Susana Nunes, Maria do Bom-Sucesso, Dave R Lal, Brian T Craig, Kerri Becktell, Tahmina Banu, Md Afruzul Alam, Orindom Shing Pulock, Tasmiah Tahera Aziz, Vishal Michael, M Joseph John, William Bhatti, Bobby John, Swati Daniel, Jyoti Dhiman, Hunar Mahal, Atul Suroy, Rosanda Ilic, Danica Grujicic, Tijana Nastasovic, Igor Lazic, Mihailo Milicevic, Vladimir Bascarevic, Radovan Mijalcic, Vuk Scepanovic, Aleksandar Stanimirovic, Aleksandra Paunovic, Ivan Bogdanovic, Shruti Kakkar, Shaina Kamboj, Suraj Singh, Shahnoor Islam, AKM Amirul Morshed, AKM Khairul Basher, Mehnaz Akter, SM Rezanur Rahman, Zannat Ara, Mohammed Tanvir Ahammed, Tania Akter, Kamrun Nahar, Fatema Sayed, Ashfaque Nabi, Md Asif Iqbal, Md Masud Rana, Md Asaduzzaman, Md Hasanuzzaman, Kemal Tolga Saracoglu, Elif Akova, Evren Aydogmus, Bekir Can Kendirlioglu, Tufan Hicdonmez, Kemal Tolga Saracoglu, Elif Akova, Evren Aydogmus, Bekir Can Kendirlioglu, Tufan Hicdonmez, Ahmed Y Azzam, Mohammed A Azab, Sherief Ghozy, Alzhraa Salah Abbas, Monica Dobs, Mohamed Atef Mohamed Ghamry, Mohammed Alhendy, Joana Monteiro, Olanrewaju Moses, Ibiyeye Taiye Taibat, Taiwo Jones, Kalu Ukoha, Olagundoye Goke, Okorie Ikechukwu, Abiodun Idowu Okunlola, Milind Chitnis, Helga Nauhaus, Danelle Erwee, Robyn Brown, Agata Chylinska, Robin Simpson, Prasanna Gomes, Noel Peter, Marco Aurelio Ciriaco Padilha, Elvercio Pereira de Oliveira Junior, Lucas Garschagen deCarvalho, Fabiola Leonelli Diz, Mohamed El Kassas, Usama Eldaly, Ahmed Tawheed, Mohamed Abdelwahab, Oudrhiri Mohammed Yassaad, Bechri Hajar, El Ouahabi Abdessamad, Arkha Yasser, Hessissen Laila, Farah Sameer Yahya, Farah Sameer Yahya, Sandip Kumar Rahul, Vijayendra Kumar, Digamber Chaubey, Maria Teresa Peña Gallardo, Jacqueline Elizabeth Montoya Vásquez, Juan Luis García León, Sebastián Shu Yip, Georgios Karagiannidis, Rejin Kebudi, Sema Bay Buyukkapu, Krishna Kumar Govindarajan, Kumaravel Sambandan, Smita Kayal, Gunaseelan Karunanithi, Bikash Kumar Naredi, Bibekanand Jindal, Mariam Lami, Matthew HV Byrne, Duha Jasim, Harmit Ghattaura, Soham Bandyopadhyay, Kokila Lakhoo, Eric W Etchill, Daniel Rhee, Stacy Cooper, Kevin Crow, Morgan Drucker, Megan Murphy, Benjamin Shou, Alan Siegel, Yasin Kara, Gül Nihal Özdemir, Mahmoud Elfiky, Ehab El Refaee, John George Massoud, Ayah Bassam Ibrahim, Ruaa Bassam Ibrahim, Faris Abu Za'nouneh, Ranya M Baddourah, Toqa Fahmawee, Ayah Al Shraideh, Ghazwani Salman, Ehab Alameer, Al-Mudeer Ali, Ghazwani Yahia, Khozairi Waleed, Ahmad Ozair, Ankur Bajaj, Bal Krishna Ojha, Kaushal Kishor Singh, Atique Anwar, Vinay Suresh, Mohamad K Abou Chaar, Iyad Sultan, Khalil Ghandour, Shaima' Al-Dabaibeh, Ammar Al-Basiti, Hazim Ababneh, Omaima El-Qurneh, Yousef Alalawi, Ahmad Al Ayed, Ehab Hanafy, Naif Al Bolowi, Anette S Jacobsen, Heidi Barola, Aubrey L Pagaduan, Jingdan Fan, Olumide Abiodun Elebute, Adesoji O Ademuyiwa, Christopher O Bode, Justina O Seyi-Olajide, Oluwaseun Ladipo-Ajayi, Felix M Alakaloko, George C Ihediwa, Kareem O Musa, Edamisan O Temiye, Olufemi Oni, Adeseye M Akinsete, Janita Zarrish, Ramsha Saleem, Soha Zahid, Atiqa Amirali, Ahsan Nadeem, Sameer Saleem Tebha, Zonaira Qayyum, Sana Tahir, Anneqa Tahir, Rabbey Raza Khan, Ayesha Mehmood, Iqra Effendi, Muhammad Arshad, Taimur Iftikhar Qureshi, Pooja Kumari, Mohamed Bonna, Khaled Mamdouh, Mohamed Atef, Mohamed Faried, Victor Calvagna, Nathalie Galea, Ariana Axiaq, Matthew R Schuelke, Jake A Kloeber, Robert L Owen, Alexander S Roth, Catherine Yang, J Hudson Barnett, Lucien P Jay, Kirk David Wyatt, Paul J Galardy, Bernard Mbwele, Irene Nguma, Moshi Moshi Shabani, Amani Twaha, Bilal Matola, Agnes Vojcek, Mahmoud Maher Abdelnaby Alrahawy, Seham M Ragab, Abdallah R Allam, Eman Ibrahim Hager, Abdelrahman Azzam, Ammar Ayman, Kıvılcım Karadeniz Cerit, Adnan Dağçınar, Tümay Umuroğlu, Ayten Saraçoğlu, Mustafa Sakar, Can Kıvrak, Gül Çakmak, Ibrahim Sallam, Gamal Amira, Mohamed Sherief, Ahmed Sherif, Simone deOliveira Coelho, Arissa Ikeda, Licia Portela, Marianne Monteiro Garrigo, Ricardo Vianna deCarvalho, Fernanda Lobo, Sima Ester Ferman, FernandaFerreira daSilva Lima, Moawia Mohammed AliElhassan, Nada Osman Yousif Elhaj, Hytham KS Hamid, Emmanuel A Ameh, Vincent E Nwatah, Adewumi B Oyesakin, Andrew Nwankwo Osuigwe, Okechukwu Hyginus Ekwunife, Chisom Adaobi Nri-Ezedi, Eric Okechukwu Umeh, Nellie Bell, Ibukunolu Olufemi Ogundele, Abiodun Folashade Adekanmbi, Olubunmi Motunrayo Fatungase, Olubunmi Obafemi Obadaini, Sarah Al-Furais, Humaida Hemlae, Sreylis Nay, John Mathew, RM Jeffri Ismail, Simone deCamposVieira Abib, Fabianne Altruda de Moraes Costa Carlesse, Mayara Caroline Amorim Fanelli, Fernanda Kelly Marques de Souza, Pierfrancesco Lapolla, Andrea Mingoli, Denis Cozzi, Anna Maria Testi, Paolo Musiu, Paolo Sapienza, Gioia Brachini, Martina Zambon, Simona Meneghini, Pierfranco Cicerchia, Bruno Cirillo, Manjul Tripathi, Nitin James Peters, Sandeep Mohindra, Vishal Kumar, Ninad R Patil, Richa Jain, Renu Madan, Madhivanan Karthigeyan, Pravin Salunke, Ghazwani Salman, Gopal Nambi, Abdulrahman Omar Taha, Janice Hui Ling Wong, Norehan Johari, Anas Shikha, Win SabaiPhyu Han, Zahidah Ahmad, Yen Yan Lim, Roserahayu Idros, Noorainun Mohd Yusof, David Nelson Jaisingh, Aouabed Nesrine, Bouaoud Souad, Mebarki Malika, Bioud Belkacem, Fayza Haider, Fatema Naser AlFayez, Fakher Rahim, Elana Kleinman, Taylor Ibelli, Emily Hamilton, Rochelle Fayngor, Tzvi Najman, Gideon Karplus, Etai Adam, Daniella Melamed, Cecilia Paasche, Amir Labib, Farman Ali Laghari, Dhruva Ghosh, Zainab Al Balushi, Abdulhakim Awadh SalimAl-Rawas, Ali Al Sharqi, Ammar Saif AlShabibi, Ismail Al Bulushi, Muna Alshahri, Abdulrahman AlMirza, Ola Al Hamadani, Jawaher Al Sharqi, Anisa Al Shamsi, Bashar Dawud, Sareya Al Sibai, Alhassan Abdul-Mumin, Halwani Yaninga Fuseini, Peter Gyamfi Kwarteng, Abubakari Bawa Abdulai, Sheba Mary Pognaa Kunfah, Gilbert B Bonsaana, Stephanie Ajinkpang, Edmund M Der, Francis A Abantanga, Mary Joan Kpiniong, Kingsley Aseye Hattor, Kingsley Appiah Bimpong, Mohamed Elbahnasawy, Sherief Abdelsalam, Ahmed Samir, Reto M Baertschiger, Amanpreet Brar, Andreea C Matei, Augusto Zani, Lubna Samad, Hira Khalid Zuberi, Kishwer Nadeem, Naema Khayyam, Fatima Ambreen Imran, Nida Zia, Sadia Muhammad, Muhammad Rafie Raza, Muhammad Rahil Khan, Alaa Hamdan, Ammar Omran, Ahmed Moussa, Bardisan Gawrieh, Hassan Salloum, Alaa Ahmed, Abdeljawad Mazloum, Ali Abodest, Nisreen Ali, Munawar Hraib, Victor Khoury, Abdulrahman Almjersah, Mohammad Ali Deeb, Mohammad Ahmad Almahmod Alkhalil, Akram Ahmed, Waseem Shater, Ali Farid Alelayan, Alaa Guzlan, Ahmad Bouhuwaish, Alqasim Abdulkarim, Eman Abdulwahed, Marwa Biala, Reem Ghamgh, Amani Alamre, Marwa Shelft, Asmaa AM Albanna, Hoda Tawel, Emmanuel Hatzipantelis, Athanasios Tragiannidis, Eleni Tsotridou, Assimina Galli-Tsinopoulou, Dayang AnitaAbdul Aziz, Zarina Abdul Latiff, Hamidah Alias, C-Khai Loh, Doris Lau, Azrina Syarizad Khutubul Zaman, Taiwo Akeem Lawal, Kelvin Ifeanyichukwu Egbuchulem, Olakayode Olaolu Ogundoyin, Isaac Dare Olulana, Biobele J Brown, Oluwasegun Joshua Afolaranmi, AbdulBasit Fehintola, Annika Heuer, Christine Nitschke, Michael Boettcher, Matthias Priemel, Lennart Viezens, Martin Stangenberg, Marc Dreimann, Alonja Reiter, Jasmin Meyer, Leon Köpke, Karl-Heinz Frosch, Samson Olori, Uduak Offiong, Philip Mari Mshelbwala, Fashie Andrew Patrick, Aminu Muhammed Umar, N Otene ThankGod, Shireen Anne Nah, Yuki Julius Ng, Syukri Ahmad Zubaidi, Murad Almasri, Sara Ali, Rasaq Olaosebikan, Akila Muthukumar, Patricia Shinondo, Amon Ngongola, Bruce Bvulani, Azad Patel, Abdullahi Nuhu-Koko, Baba Jibrin, Ajiboye L Olalekan, Christopher S Lukong, Ezekiel I Ajayi, Gabriela Guillén, Sergio López, José Andrés Molino, Pablo Velasco, Omar Elmandouh, Omar Hamam, Rim Elmandouh, Nensi Melissa Ruzgar, Rachel Levinson, Shashwat Kala, Sarah Ullrich, Emily Christison-Lagay, Reto Baertschiger, Essam Elhalaby, Muath Alser, Mahmoud M Saad, Luca Pio, Guido Seitz, Judith Lindbert, Francis Abantanga, Georgios Tsoulfas, Asimina Galli-Tsinopoulou, Agnes Vojcek, Dhruva Ghosh, Nitin James Peter, Ankur Bajaj, Vrisha Madhuri, Ravi Kishore, Maryam Ghavami Adel, Abdulrahman Omar Taha, Calogero Virgone, Francesco Pata, Gaetano Gallo, Mohammad K Abou Chaar, Faris Ayasra, Eric Mwangi Irungu, Muhammed Elhadi, Shireen Anne Nah, Dayang Anita Abdul Aziz, Victor Calvagna, Outani Oumaima, Zineb Bentounsi, Hajar Moujtahid, Adesoji Ademuyiwa, Dhruva Nath Ghosh, Muhammad Arshad, Lubna Samad, Lily Saldana, Jan Godzinsky, Abdelbasit Ali, Ehab Alameer, Dragana Janic, Mohamed Bella Jalloh, Nellie Bell, Annette Jacobsen, Chan Hon Chui, Milind Chitnis, Israel Fernandez Pineda, Lucas Krauel, Maricarmen Olivos, Waha Rahama, Hazim Elfatih, Raphael N Vuille-dit-Bille, Alaa Hamdan, Arda Isik, Asim Noor Rana, Kokila Lakhoo, Kate Cross, Max Pachl, Andrea Hayes-Jordan, Roshni Dasgupta, Patricia Shinondo, Amon Ngongola, and Mohamedraed Elshami
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All participating centres have gained study approval to participate according to their local institutional ethical regulations. Examples of ethical approval: Brunei Medical and Health Research & Ethics Committee (MHREC/MOH/2020/14(2); Yale Human Research Protection Program Institutional Review Board (2000028852); and Tanta University Research Ethics Committee (33965/7/20).
References
- 1.Birch JM, Marsden HB, Jones PH, et al. Improvements in survival from childhood cancer: results of a population based survey over 30 years. Br Med J 1988;296:1372. 10.1136/bmj.296.6633.1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saletta F, Seng MS, Lau LMS. Advances in paediatric cancer treatment. Transl Pediatr 2014;3:156–82. 10.3978/j.issn.2224-4336.2014.02.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pritchard-Jones K, Pieters R, Reaman GH, et al. Sustaining innovation and improvement in the treatment of childhood cancer: lessons from high-income countries. Lancet Oncol 2013;14:e95–103. 10.1016/S1470-2045(13)70010-X [DOI] [PubMed] [Google Scholar]
- 4.SEER Statistics . Cancer statistics review, 1975-2015. Available: https://seer.cancer.gov/archive/csr/1975_2015/ [Accessed 02 Oct 2020].
- 5.Bhakta N, Force LM, Allemani C, et al. Childhood cancer burden: a review of global estimates. Lancet Oncol 2019;20:e42–53. 10.1016/S1470-2045(18)30761-7 [DOI] [PubMed] [Google Scholar]
- 6.The Lancet Child Adolescent Health . Fighting childhood cancer with data. Lancet Child Adolesc Health 2019;3:585. 10.1016/S2352-4642(19)30238-X [DOI] [PubMed] [Google Scholar]
- 7.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023–75. 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam CG, Howard SC, Bouffet E, et al. Science and health for all children with cancer. Science 2019;363:1182–6. 10.1126/science.aaw4892 [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Howard SC, Hunger SP. Treating Childhood Cancer in Low- and Middle-Income Countries. In: Disease Control Priorities, Third Edition (Volume 3): Cancer. The World Bank, 2015: 121–46. [PubMed] [Google Scholar]
- 10.Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer 2008;112:461–72. 10.1002/cncr.23205 [DOI] [PubMed] [Google Scholar]
- 11.Wilson ML, Atun R, DeStigter K, et al. The Lancet Commission on diagnostics: advancing equitable access to diagnostics. Lancet 2019;393:2018–20. 10.1016/S0140-6736(19)31052-9 [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro RC, Steliarova-Foucher E, Magrath I, et al. Baseline status of paediatric oncology care in ten low-income or mid-income countries receiving my child matters support: a descriptive study. Lancet Oncol 2008;9:721–9. 10.1016/S1470-2045(08)70194-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen P, Friedrich P, Lam C, et al. Global access to essential medicines for childhood cancer: a cross-sectional survey. J Glob Oncol 2018;4:1–11. 10.1200/JGO.18.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan R, Alatise OI, Anderson BO, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol 2015;16:1193–224. 10.1016/S1470-2045(15)00223-5 [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Romo L, Olaya Vargas A, Gupta S, et al. Delivery of pediatric cancer care in Mexico: a national survey. J Glob Oncol 2018;4:1–12. 10.1200/JGO.17.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magrath I, Steliarova-Foucher E, Epelman S, et al. Paediatric cancer in low-income and middle-income countries. Lancet Oncol 2013;14:e104–16. 10.1016/S1470-2045(13)70008-1 [DOI] [PubMed] [Google Scholar]
- 17.Silbermann M, Al-Hadad S, Ashraf S, et al. Mecc regional initiative in pediatric palliative care. J Pediatr Hematol Oncol 2012;34:S1–11. 10.1097/MPH.0b013e318249aa98 [DOI] [PubMed] [Google Scholar]
- 18.Atun R, Bhakta N, Denburg A, et al. Sustainable care for children with cancer: a Lancet oncology Commission. Lancet Oncol 2020;21:e185–224. 10.1016/S1470-2045(20)30022-X [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . WHO global initiative for childhood cancer: an overview; 2018.
- 20.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. Available: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 [Accessed 03 May 2020].
- 22.Millen GC, Arnold R, Cazier J-B, et al. Severity of COVID-19 in children with cancer: report from the United Kingdom paediatric coronavirus cancer monitoring project. Br J Cancer 2021;124:754–9. 10.1038/s41416-020-01181-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GBD 2017 Childhood Cancer Collaborators . The global burden of childhood and adolescent cancer in 2017: an analysis of the global burden of disease study 2017. Lancet Oncol 2019;20:1211–25. 10.1016/S1470-2045(19)30339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukkada S, Bhakta N, Chantada GL, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol 2021;22:1416–26. 10.1016/S1470-2045(21)00454-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graetz D, Agulnik A, Ranadive R, et al. Global effect of the COVID-19 pandemic on paediatric cancer care: a cross-sectional study. Lancet Child Adolesc Health 2021;5:332–40 http://www.thelancet.com/article/S2352464221000316/fulltext 10.1016/S2352-4642(21)00031-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasquez L, Sampor C, Villanueva G, et al. Early impact of the COVID-19 pandemic on paediatric cancer care in Latin America. Lancet Oncol 2020;21:753–5. 10.1016/S1470-2045(20)30280-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peter N, Bandyopadhyay S, Lakhoo K, et al. Impact of the COVID-19 pandemic on paediatric patients with cancer in low-income, middle-income and high-income countries: protocol for a multicentre, international, observational cohort study. BMJ Open 2021;11:e045679. 10.1136/bmjopen-2020-045679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steliarova-Foucher E, Stiller C, Lacour B, et al. International classification of childhood cancer, third edition. Cancer 2005;103:1457–67. 10.1002/cncr.20910 [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Aitken JF, Bartels U, et al. Paediatric cancer stage in population-based cancer registries: the Toronto consensus principles and guidelines. Lancet Oncol 2016;17:e163–72. 10.1016/S1470-2045(15)00539-2 [DOI] [PubMed] [Google Scholar]
- 30.World Bank . World bank country and lending groups, 2021. Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [Accessed 10 Jul 2021].
- 31.Lee LYW, Cazier J-B, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol 2020;21:1309–16. 10.1016/S1470-2045(20)30442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee LY, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020;395:1919–26. 10.1016/S0140-6736(20)31173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell B, Moss CL, Shah V, et al. Risk of COVID-19 death in cancer patients: an analysis from Guy's cancer centre and King's College hospital in London. Br J Cancer 2021;125:939–47. 10.1038/s41416-021-01500-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assaad S, Zrounba P, Cropet C, et al. Mortality of patients with solid and haematological cancers presenting with symptoms of COVID-19 with vs without detectable SARS-COV-2: a French nationwide prospective cohort study. Br J Cancer 2021;125:658–71. 10.1038/s41416-021-01452-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhopal SS, Bagaria J, Olabi B, et al. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc Health 2021;5:e12–13. 10.1016/S2352-4642(21)00066-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khoja L, McGurk A, O'Hara C, et al. Mortality within 30 days following systemic anti-cancer therapy, a review of all cases over a 4 year period in a tertiary cancer centre. Eur J Cancer 2015;51:233–40. 10.1016/j.ejca.2014.11.011 [DOI] [PubMed] [Google Scholar]
- 37.Wallington M, Saxon EB, Bomb M, et al. 30-Day mortality after systemic anticancer treatment for breast and lung cancer in England: a population-based, observational study. Lancet Oncol 2016;17:1203–16. 10.1016/S1470-2045(16)30383-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgers JA, Damhuis RA. 30-Day mortality after the start of systemic anticancer therapy for lung cancer: is it really a useful performance indicator? ERJ Open Res 2018;4. 10.1183/23120541.00030-2018. [Epub ahead of print: 05 11 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Department of Health . Improving Outcomes: A Strategy for Cancer; 2011.
- 40.Mort D, Lansdown M, Smith N, et al. For better, for worse? 2008. [Google Scholar]
- 41.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the children's Oncology Group. Blood 2011;118:243. 10.1182/blood-2010-12-322909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woessmann W, Seidemann K, Mann G, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM group study NHL-BFM95. Blood 2005;105:948–58. 10.1182/blood-2004-03-0973 [DOI] [PubMed] [Google Scholar]
- 43.Lannering B, Rutkowski S, Doz F, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol 2012;30:3187–93. 10.1200/JCO.2011.39.8719 [DOI] [PubMed] [Google Scholar]
- 44.Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med 2011;104:510. 10.1258/jrsm.2011.110180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercer TR, Salit M. Testing at scale during the COVID-19 pandemic. Nat Rev Genet 2021;22:415–26. 10.1038/s41576-021-00360-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belkacemi Y, Grellier N, Ghith S, et al. A review of the International early recommendations for departments organization and cancer management priorities during the global COVID-19 pandemic: applicability in low- and middle-income countries. Eur J Cancer 2020;135:130–46. 10.1016/j.ejca.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adegboyega G, Ozair A, Kanmounye US, et al. Letter: is the Stupp protocol an expensive and Unsustainable standard of care for glioblastoma in low- and middle-income country settings? A call to action! Neurosurgery 2021;89:E249–51. 10.1093/neuros/nyab273 [DOI] [PubMed] [Google Scholar]
- 48.Richards M. Children’s Cancer Services: A review on behalf of NHS England 2019/20, 2020. NHS England. Available: https://www.england.nhs.uk/wp-content/uploads/2020/01/board-meeting-item-9-update-on-specialised-services-c-appendix-2.pdf [Accessed 08 Dec 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2022-008797supp001.pdf (53.9KB, pdf)
bmjgh-2022-008797supp002.pdf (127.8KB, pdf)
bmjgh-2022-008797supp003.pdf (50KB, pdf)
bmjgh-2022-008797supp004.pdf (69.8KB, pdf)
bmjgh-2022-008797supp005.pdf (78KB, pdf)
bmjgh-2022-008797supp006.pdf (480KB, pdf)
bmjgh-2022-008797supp007.pdf (64.1KB, pdf)
bmjgh-2022-008797supp008.pdf (88.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request.