Abstract
The SARS-CoV-2 infection causes COVID-19 disease, characterized by acute respiratory distress syndrome, bilateral pneumonia, and organ failure. The consequences of maternal SARS-CoV-2 infection for the pregnant woman, fetus, and neonate are controversial. Thus, it is required to determine whether there is viral and non-viral vertical transmission in COVID-19. The disease caused by SARS-CoV-2 leads to functional alterations in asymptomatic and symptomatic pregnant women, the fetoplacental unit and the neonate. Several diseases of pregnancy, including COVID-19, affect the fetoplacental function, which causes in utero programming for young and adult diseases. A generalized inflammatory state and a higher risk of infection are seen in pregnant women with COVID-19. Obesity, diabetes mellitus, and hypertension may increase the vulnerability of pregnant women to infection by SARS-CoV-2. Alpha, Delta, and Omicron variants of SARS-CoV-2 show specific mutations that seem to increase the capacity of the virus to infect the pregnant woman, likely due to increasing its interaction via the virus S protein and angiotensin-converting enzyme 2 receptors. This review shows the literature addressing to what extent COVID-19 in pregnancy affects the pregnant woman, fetoplacental unit, and neonate. Prospective studies that are key in managing SARS-CoV-2 infection in pregnancy are discussed.
Keywords: Virus, Pregnancy, Placenta, Fetus, Neonate, Covid-19
1. Introduction
Viral infections can be devastating to maternal and perinatal health. Many viruses cross the placental barrier and affect the fetus, such as Zika [1], rubella [2], and parvovirus B19 [3], among others [4], [5], [6]. Also, influenza is more likely to present a severe form and has a higher death risk in pregnant women than in non-pregnant women [7], [8]. Since the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–induced coronavirus disease 2019 (COVID-19) pandemic, the possibility of increased maternal and perinatal risk secondary to this disease has been kept in mind [9]. At present, more knowledge is available about the effects of SARS-CoV-2 on pregnant women, fetuses, and newborns, although much remains to unveil.
Physiological changes in pregnant women include alterations in the immune system and anatomic and functional changes in the cardiovascular and respiratory systems [10], [11]. These changes, along with other adaptive modifications to maternal homeostasis, have led to consider pregnant women as high-risk and susceptible to viral infections [12]. Viral and respiratory diseases have a poorer prognosis and more severe clinical presentation in pregnant women when compared to non-pregnant women [9]. It is well described that immune cells such as the helper T cells (Th) play a crucial role in the modulation of the immune responses in pregnancy, a mechanism required for pregnancy success [13]. Thus, dysregulation of T-cells as other immune cells in pregnant women infected with SARS-CoV-2 may result in poor pregnancy outcomes. The latter might explain why pregnant women are more susceptible to severe illness from COVID-19 than non-pregnant women [14].
Studies performed early in the COVID-19 pandemic showed that pregnant women with this disease had a higher risk of severe illness and adverse perinatal outcomes than non-pregnant women [14]. The latter is complemented by the more recent identification of different variants of SARS-CoV-2, such as Alpha (B.1.1.7) and Delta (B.1.617.2) [15]. Pregnant women infected with the Alpha or Delta variants present with a higher probability of severe disease and adverse perinatal outcomes compared to pregnant women infected by SARS-CoV-2 [16], [17], [18]. On the other hand, after the recent increase in COVID-19 cases by the Omicron variant (B.1.1.529), it seems that this variant's infection is associated with a lower probability of severe illness in pregnant women [19].
The SARS-CoV-2 infection has a broad spectrum of clinical manifestations. The disease usually presents with mild symptoms. It can range from asymptomatic to severe illness with pneumonia and acute respiratory distress syndrome requiring ICU admission and assisted ventilation. The National Institutes of Health divided infected adults into five severity of illness categories [20], and the Chinese Center for Disease Control and Prevention categorized the severity of symptoms as mild, severe, or critical [21].
The reported prevalence of asymptomatic infections among the confirmed population is ~41 % [22]. The data from most cohorts with longitudinal reports suggest that only a small percentage of asymptomatic patients will become symptomatic [23]. One of the first sources of information about asymptomatic patients comes from the analysis of SARS-CoV-2 infection among the “Diamond Princess” cruise ship passengers. A retrospective analysis found that 32 % of these subjects were classified as asymptomatic until the complete follow-up of 107 patients admitted to a hospital in Japan [24]. Another report showed statistical modelling developed to calculate the delay-adjusted asymptomatic proportion of infections among the 634 confirmed cases from the cruise [25]. The latter was due to the lack of available information on the clinical progression of the patients that did not allow to differentiate the asymptomatic infections from the pre-symptomatic ones [25]. The results of the later study estimated an asymptomatic proportion of ~18 %, with a mean incubation period between 5.5 and 9.5 days [25].
The prevalence of asymptomatic infection in pregnant women was reported as 54.1 % (95 % CI: 39.16–69.05) [22]. Other reports show that universal screening for SARS-CoV-2 in pregnant women resulted in different proportions of asymptomatic patients depending on the studied area and the pandemic period, which could vary between 73 % and 87 % during COVID-19 breakthroughs [26], [27], [28]. However, most of the studies did not report the complete follow-up of the women to distinguish the real asymptomatic from the pre-symptomatic ones. Given the prevalence of asymptomatic infection in pregnant women, in areas with a high prevalence of COVID-19 infection, implementing a universal testing policy among pregnant women presenting to labour and admission units may be cost-effective in helping curb disease transmission [29].
Symptomatic infection ranges from mild to critical cases, with most patients managed on an outpatient basis. Extensive reports from the Chinese Center for Disease Control and Prevention show that during the first COVID-19 breakthrough, the leading presentation was a mild disease in ~81 % of cases. The severe illness appears in ~14 % and critical condition in ~5 % of patients requiring hospital and ICU admission [21]. Nevertheless, COVID-19 variants have different clinical manifestations. After adjusting for age and sex, the Delta variant is associated with increased oxygen requirement, ICU admission, and death compared with the Alpha variant [30], [31]. In contrast, the presence of the Delta variant in various parts of the United States of America did not result in increased hospitalizations, ICU admission, or death in adults [30]. Partly, the impact of this variant on transmission rates and fatal outcomes was associated with people who had not yet been vaccinated [30]. Conversely, the Omicron variant was associated with less death and UCI admissions than earlier breakthroughs for Alpha and Delta variants (4.5 versus 21.3 % for death, and 1 versus 4.3 % for UCI admission) [32]. Pregnant women suffer a similar rate of clinical complications; however, women with mild infection experience the same outcomes as uninfected pregnant women [33]. Pregnant women with severe and critical diseases have a higher risk of perinatal infection, morbidity, and maternal death [33], [34].
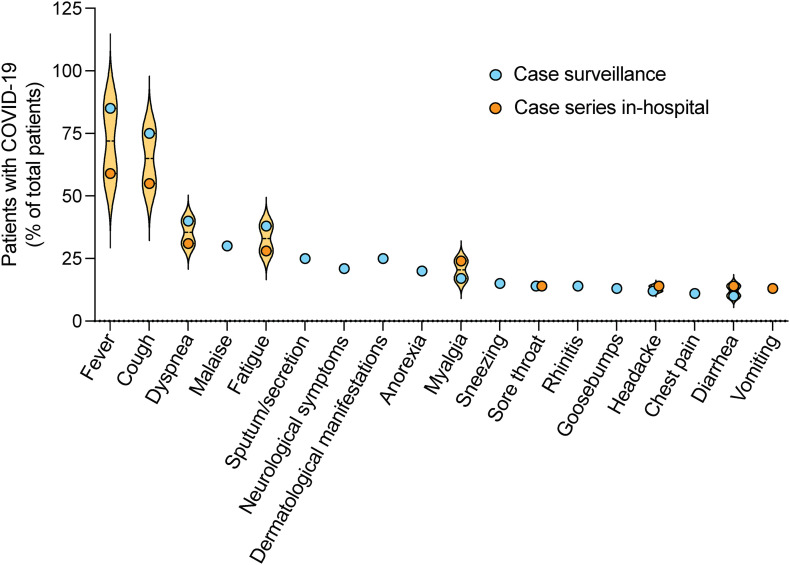
Reports of COVID-19 case surveillance show that the most frequent symptoms are fever, cough, and dyspnea, with lower patients showing malaise, fatigue, and sputum/secretion (Fig. 1 ). Other patients also report neurological symptoms, dermatological manifestations, anorexia, myalgia, sneezing, sore throat, rhinitis, goosebumps, headache, and chest pain [35], [36]. According to published case series among in-hospital patients, the principal described symptoms are fever and cough. Gastrointestinal symptoms such as vomiting and diarrhoea were also reported [37], [38]. For significant symptoms, such as cough and fever, patients infected by the Delta variant had been found in much lower incidence. Additionally, sore throat that was not common in patients with wild-type COVID-19 is a large portion of patients with the Delta variant [36]. Interestingly, pregnant women are at a lower risk of experiencing fever, headache, myalgia, diarrhoea, chest tightness, and expectoration compared with non-pregnant women [14]. Also, the relative risk of being asymptomatic (RR 3.94, 95 % CI 1.69–9.20) was higher among pregnant women than non-pregnant women [14].
Fig. 1.
Symptoms in patients with COVID-19. The distribution of most frequent symptoms in reports of patients with coronavirus disease 2019 (COVID-19) in case surveillance and in-hospital patients with fever and cough are predominant, with few cases of gastrointestinal complications. Circles show the mean values reported in [35], [36], [37], [38].
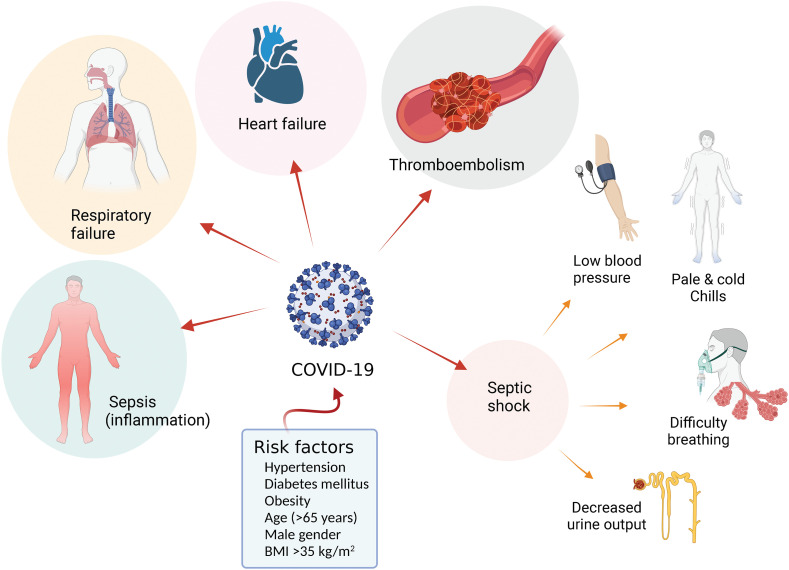
COVID-19 courses with, at present well-identified complications (Fig. 2 ). The most frequent described complications of the disease are sepsis, respiratory failure, acute respiratory distress syndrome, heart failure and cardiac difficulties, septic shock, and thromboembolic complications [39]. It is also reported that COVID-19 in pregnant women increases the risk of thromboembolism [40]. These complications seem associated with a variety of risk factors for developing COVID-19. Among admitted patients, 48 % had co-morbidities, mainly hypertension, diabetes mellitus, and obesity [41]. Older age (≥65 years old), male sex, high body mass index value (>35 kg/m2), and the presence of co-morbidities, including hypertension, and diabetes mellitus, were also risk factors for developing severe illness [42].
Fig. 2.
Complications and risk factors in patients with COVID-19. Infection with SARS-CoV-2 causes coronavirus disease 2019 (COVID-19), which may result in generalized inflammation configuring sepsis (Sepsis), respiratory and heart failure, and thromboembolism. COVID-19 may also lead to septic shock associated with low blood pressure, pale and cold arms and legs, chills, difficulty breathing, and decreased urine output. Risk factors for COVID-19 include chronic diseases (hypertension, diabetes mellitus, obesity), body mass index (BMI) >35 kg/m2, male gender and age > 65 years. From data in [39], [40], [41], [42].
Patients with COVID-19 at admission show lymphopenia, thrombocytopenia, or elevated levels of lactate dehydrogenase (LDH), interleukin 6 (IL-6), creatine kinase, C-reactive protein (CRP), and serum ferritin [43], [44], [45]. The patients also show decreased plasma albumin. Also, altered liver and kidney function markers and coagulation (D-dimer) were reported. Among laboratory findings, elevated CRP, lymphopenia, and elevated LDH are associated with illness severity. In general, biomarkers of inflammation, cardiac and muscle injury, liver and kidney function, and coagulation are higher in severe and fatal COVID-19 patients [42], [43], [46].
Pregnant women with COVID-19 have similar findings with lymphopenia, neutropenia, thrombocytopenia, and altered liver and kidney function [43]. On the other hand, critically ill pregnant women with COVID-19 have elevated D-dimer, neutrophil count and CRP, and decreased lymphocyte count from the first to the third trimester as the most frequent abnormalities [43]. These laboratory findings suggest obstetric pathologies such as preeclampsia and haemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome. Due to these similarities, preeclampsia- and HELLP syndrome-like have been described in these patients [47], [48]. Therefore, other markers than those for angiogenesis can help rule out preeclampsia or HELLP syndrome in pregnant women with COVID-19 [49].
The most common features found by chest computed tomography (CCT) in patients with COVID-19 include vascular enlargement and ground-glass opacities (GGO), mixed GGO with consolidation, and adjacent pleura thickening [50], [51]. Consolidations were diagnosed in ~27 % of patients with COVID-19 pneumonia [50], [51]. Other reports described alterations in the CCT include bronchogram sign, crazy-paving pattern, patchy, and spider web sign [50], [51], [52]. Most patients with COVID-19 had bilateral lung involvement, peripheral distribution, and multilobe involvement [50], [51], [52]. Interestingly, CCT abnormalities were found in ~63 % of asymptomatic patients at diagnosis [53]. Similarly, pregnant women with COVID-19 show comparable CCT features [54]. Noteworthy, the American College of Obstetrics and Gynaecology (ACOG), the Royal College of Obstetricians and Gynaecologists (RCOG), and the International Society of Ultrasound in Obstetrics and Gynaecology (ISUOG) recommend that CCT should not be withheld in pregnant women if it is clinically indicated [54], [55], [56].
Unfortunately, several long-term effects of COVID-19, i.e. post-covid alterations, have been reported [57], [58], [59], [60], [61], [62]. The most common symptoms in adult subjects are fatigue, cough, dyspnea, chest tightness, post-traumatic stress disorder, anxiety, depression, and concentration and sleep abnormalities [57], [58], [59], [60]. Interestingly, post-covid effects are more frequent in women and seem to be reduced in the vaccinated population [59], [62]. Moreover, infants born during the COVID-19 pandemic show neurodevelopmental delay compared to infants born before the pandemic, regardless of whether they were exposed to maternal SARS-CoV-2 infection during pregnancy or not [63]. The latter suggests a potential underlying mechanism and the importance of long-term follow-up of children born during the COVID-19 pandemic.
This narrative review summarizes critical aspects of COVID-19 during pregnancy, explaining the clinical characteristics of the disease, the potential mechanism(s) of infection and placental passage of the virus, the fetal and neonatal consequences of the infection, the suggested management for pregnant women affected by COVID-19, and recommendations for post-COVID-19 management.
2. SARS-CoV-2 and infection mechanisms
SARS-CoV-2 strongly interacts with the angiotensin-converting enzyme 2 (ACE2), a cell protein attached to the plasma membrane [64], [65]. ACE2 is addressed as the receptor for SARS-CoV-2, facilitating the infection by this virus. While ACE2 expression is predominant in the lungs within type II alveolar cells, this enzyme is also present at several extrapulmonary sites such as the oral cavity and gastrointestinal system [64], [65]. The expression pattern of ACE2 across most human tissues also includes the human placenta [66]. It is nowadays better known that cell entry and the spread of SARS-CoV-2 depend on the ACE2 receptors [67] and, potentially, the proteases transmembrane protein serine 2 (TMPRSS2), cathepsins B/L7, and furin [68]. Interestingly, ACE2 receptor expression is associated with adverse outcomes in viral-affected pregnancies such as miscarriages, preeclampsia, and ectopic pregnancy [69]. Therefore, the ACE2 receptor's role in SARS-CoV-2 infection of the human placenta involves altered foetoplacental function.
The SARS-CoV-2 virus is formed by structural proteins referred to as envelope (E), spike (S), membrane (M), and nucleocapsid (N) proteins. The S, M, and E proteins form the virus envelope covering the N protein and the virus RNA. The S protein gets embedded in the plasma membrane [67]. The latter mechanism helps viral entry into target cells via binding the surface unit S1 of the S protein to the cellular receptor, allowing the viral attachment to the cell surface. The viral entry requires S protein priming by cellular proteases, which entails S protein cleavage at the S1/S2 and the S2 site, thus allowing the fusion of viral and cellular membranes [64]. Therefore, SARS-CoV-2 infects cells by binding its S protein to cell surface receptors. The latter is followed by S protein cleavage by TMPRSS2 or cathepsins L or B, the fusion of viral and cellular membranes, and viral RNA entry into the cytoplasm [70].
The immune system response is vital in the SARS-CoV-2 infection. Th lymphocytes release cytokines that help regulate immune responses and inflammation. Th1 cells release cytokines that are microbicides and pro-inflammatory. These cytokines include interferon gamma (IFN-γ), IL-1, IL-1β, IL-6, and IL-12. Conversely, Th2 cells release anti-inflammatory cytokines, including IL-4, IL-10, IL-13, and transforming growth factor β (TGF-β). Further, Th1/Th2 imbalance affected outcomes of patients with COVID-19 [71]. Increased levels of IL-6 are associated with a higher risk of lung damage and overall mortality in patients with COVID-19 [72]. These cytokines are generated by highly inflammatory macrophages, which play a crucial role in the cytokine storm. Total lymphocyte count, specifically CD4+ and CD8+ T cells, was slightly decreased in moderate cases and significantly reduced in severe cases of COVID-19. The latter shows that the cytokine storm is associated with the severity of COVID-19, through increased lung damage and T cells depletion and dysfunction [73].
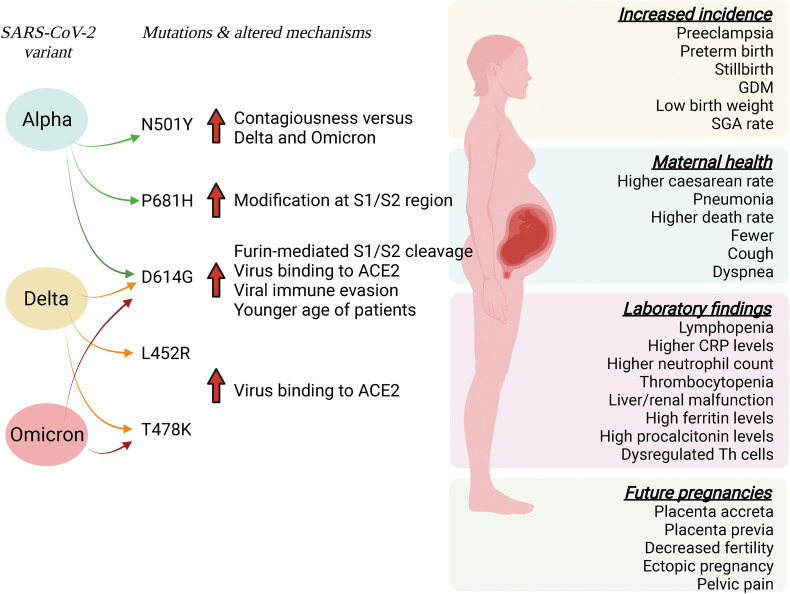
The Alpha, Delta, and Omicron variants of SARS-CoV-2 have a similar mutation on the replacement of the aspartic acid by glycine at position 614 (D614G) of the S protein of the virus, limiting S protein binding to ACE2 and its cell entry. D614G mutation can increase the receptor binding affinity and infectivity, and viral immune evasion [74]. The latter study also shows that D614G associated with higher viral load and younger age of patients but unaltered COVID-19 mortality or clinical severity in the United Kingdom. Interestingly, D614G mutation in the S protein increases the efficiency of S1/S2 cleavage by furin facilitating the virus binding to ACE2 [75] (Fig. 3 ).
Fig. 3.
SARS-CoV-2 variants and effects in pregnant women. SARS-CoV-2 infection of pregnant women may result from exposure to the Alpha, Delta, or Omicron variants. Mutations in these variants lead to several alterations in maternal health, including complications in future pregnancies and a higher incidence of diseases during pregnancy. These alterations are associated with various laboratory parameters in patients with mild or severe infections. Gene mutations result in modifications of the mechanisms by which the virus enters the target cells by predominantly modifying the S1/S2 regions of the S protein of the virus leading to higher cleavage of this protein S1/S2 region by furin. These alterations increase virus binding to their membrane receptor angiotensin-converting enzyme 2 (ACE2). Alternatively, some mutations may increase the virus immune evasion or affect predominantly young patients. Mutations: N501Y, asparagine by tyrosine at position 501; P681H, proline by histidine at position 681; D614G, aspartic acid by glycine at position 614; L452R, leucine by arginine at position 452; T478K, threonine by lysine at position 478. CRP, C reactive protein; GDM, gestational diabetes mellitus; SGA, small for gestational age; Th, helper T cells. Solid red arrows show an increase. Information is taken from references quoted in the body text. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The Alpha variant has an asparagine replaced by tyrosine at position 501 (N501Y), a mutation directly affecting the interaction with ACE2 receptors making it more contagious than other strains. The Alpha variant also has a proline by histidine mutation at position 681 (P681H) in the S1/S2 site of the S gene [76]. Further, the Delta variant shows leucine by arginine mutation at position 452 (L452R) and threonine by lysine at position 478 (T478K). These mutations are in positions that are shown to increase the interaction of SARS-CoV-2 with ACE2 receptors [75]. The Omicron variant carries more than thirty amino acid mutations in the S protein, including the shared T478K with Delta and D614G present in the other VOCs for COVID-19 [76].
3. The pregnant women
Pregnant women had a higher risk of severe COVID-19 than non-pregnant women at comparable ages [14]. Evidence supports the adverse perinatal impact of maternal SARS-CoV-2 infection, including a higher risk of preeclampsia [47.48,77], preterm birth, and stillbirth [78] compared with pregnant women without SARS-CoV-2 infection (Fig. 3). Compared with mild COVID-19, severe COVID-19 was strongly associated with preeclampsia, gestational diabetes mellitus (GDM), preterm birth, and low birth weight [78]. Also, pregnant women with severe COVID-19 show an elevated risk of caesarean delivery [79].
Several studies show a higher risk of severe disease, pneumonia, admission to the ICU, mechanical ventilation, and the need for extracorporeal membrane oxygenation in pregnant women in any trimester of pregnancy, compared to non-pregnant women of the same age [33], [34], [80]. Nevertheless, pregnant women with mild COVID-19 have similar obstetric outcomes as uninfected pregnant women [33]. Furthermore, the death rate in pregnant women with symptomatic COVID-19 is higher (OR: 1.84, 95 % CI: 1.26–2.69) than non-pregnant women [33], [34].
The reports of two registries of pregnant women with COVID-19, the UK and Global Pregnancy and Neonatal outcomes in COVID-19 (PAN-COVID) in the United Kingdom and the American Academy of Pediatrics Section on Neonatal-Perinatal Medicine (AAP-SONPM) in the United States of America include 4005 women with suspected or confirmed SARS-CoV-2 infection [81]. These reports show that pregnant women with confirmed SARS-CoV-2 had a maternal death rate of 0.5 % and 0.2 % in PAN-COVID and AAP-SONPM, respectively. Moreover, the early neonatal death rate was 0.3 % in both registries and the stillbirth rate was 0.6 % and 0.4 % for PAN-COVID and AAP-SOPM, respectively. Further information addresses ~16 % preterm delivery (<37 weeks of gestation), ~0.5 % extreme premature delivery (<27 weeks of gestation), 2 % neonatal infection rate, and ~ 10 % small-for-gestational-age rate [80]. A higher maternal and perinatal risk of adverse outcomes is expected in pregnant women due to COVID-19, especially those with severe disease [77], [78], [79], [80].
3.1. Symptomatology
Several studies have intended to describe the clinical presentation of SARS-CoV-2 in pregnant women. Regular physiological changes in pregnancy could mask or modify early SARS-CoV2 symptoms leading to a delayed diagnosis or misdiagnosis. As mentioned, around half of pregnant women present asymptomatic [22]. Moreover, in preprocedural asymptomatic infection, i.e., before surgery or delivery, pregnant women have a higher odds ratio for SARS-CoV-2 infection than non-pregnant women [82]. Thus, a detailed and precise description of the clinical presentation of SARS-CoV2 is mandatory. Regarding pregnant women with symptomatic COVID-19, the most reported symptoms are fever (~60 %), cough (~45 %), and dyspnea (~20 %) compared with non-pregnant women [14], [16], [83], [84] (Fig. 3). Other symptoms in symptomatic pregnant women, such as myalgia, fatigue, rhinorrhea, chills, nausea and vomiting, rash, abdominal pain, dizziness, sore throat, nasal congestion, and loss of appetite, are like non-pregnant women [14], [83], [84].
Laboratory findings show that in pregnant women with COVID-19, lymphopenia and elevated CRP are the most consistently reported alterations with estimated incidences of ~42 % [85], [86], [87], [88], [89], [90], [91], [92] and ~ 51 % [83], [84], [85], respectively, in pregnant women with COVID-19. Other laboratory alterations often reported in severe and critically ill patients include elevated neutrophil count, thrombocytopenia, abnormal liver and renal function, elevated D-dimer, ferritin and procalcitonin levels [43].
Radiological pneumonia is diagnosed in pregnant women with COVID-19, while severe disease requiring ICU admission ranges 4–13 % [83], [84]. Among severely ill patients, 2.5 % and 0.4 % needed invasive mechanical ventilation and extracorporeal membrane oxygenation, respectively [83], [86]. Maternal risk factors commonly associated with severe presentation of COVID-19 are advancing age, pre-gestational diabetes mellitus, GDM, chronic hypertension, asthma, high BMI, and pre-existing cardiovascular disease [83]. Adverse pregnancy outcomes are also increased in pregnancy complications in patients with COVID-19. The most reported complications are preterm birth [78], [85], preterm premature rupture of membranes [87], [88], preeclampsia [77], non-reassuring fetal testing or fetal distress [81], [87], [88], and stillbirth [81], [85], [87].
3.2. Systemic alterations
Pregnancy is a unique immunological state creating an immunotolerant environment to avoid the rejection of a semi-allogenic fetus while protecting the mother from infections. The physiological changes of the maternal immune system associate with anatomic and functional changes in the cardiovascular and respiratory systems, which have led to consider pregnant women as high-risk and susceptible to viral infections [9], [12]. Several cells from the immunological system play a role in this adaptive mechanism with Th cells playing a preponderant role on keeping this balance by stimulating killer T cells, macrophages, and B cells to result in the immune response [66].
Patients with severe SARS-CoV2 infection show dysregulation of the immune system with fewer B, T, and natural killer T cells (NK) [66]. Besides Th cells level being below average values in patients with severe COVID-19, there is a significant decrease of regulatory T cells (Treg) and an increase in Th17 number. Therefore, the Treg/Th17 cell ratio is lower than expected. Since Treg cells have an anti-inflammatory role [66], keeping a Treg/Th17 balance is crucial to controlling excessive lung inflammation and injury. The Th cells are also involved in a successful pregnancy; thus, in SARS-CoV-2 infected patients, dysregulation of Th cells balance may result in poor pregnancy outcomes such as preeclampsia, preterm birth, or miscarriage [13], [66].
Another mechanism that might play a role in pregnancy outcomes is the renin-angiotensin-aldosterone system. As mentioned, viral entry occurs by binding to the ACE2 receptor. ACE2 handles converting angiotensin II to Ang-(1–7), which have vasodilatory, prothrombotic and anti-inflammatory properties [91]. Since the levels of ACE2 rise during pregnancy, pregnant women may be more susceptible to SARS-CoV2 infection [90]. So, cases of preeclampsia-like symptoms in SARS-CoV2 infected patients have been reported [47], including one case in which resolution of preeclampsia-like symptoms was achieved after recovery of SARS-CoV2 pneumonia [47]. This may also worsen the already prothrombotic state of pregnant women, increasing their risk of venous or arterial thromboembolism. However, the current evidence is limited and does not support an increased likelihood of thrombosis in pregnant women compared with non-pregnant women [91], [92].
3.3. Consequences in future pregnancies
The impact of SARS-CoV-2 infection during pregnancy is reported unless still uncertain. However, few reports show consequences of COVID-19 in future pregnancies. Pregnant women with severe COVID-19 have a higher risk of caesarean delivery (RR: 1.57, 95 % CI 1.30–1.90) than asymptomatic pregnant women [79]. Furthermore, the rate was higher at the beginning of the pandemic. A meta-analysis showed that 88 % of pregnant women with COVID-19 had a caesarean delivery compared to non-infected women [79]. This difference in the caesarean delivery rate has long-term consequences such as higher risk in later pregnancies for placenta accreta spectrum disorder [93] (Fig. 3). Moreover, pregnancies following caesarean delivery also have an increased risk for abnormal placentation (placenta previa and abruption), small-for-gestational-age fetuses, preterm birth, and in some cases, stillbirth [93]. Also, adverse reproductive effects may include decreased fertility and increased risk of spontaneous abortion and ectopic pregnancy, and chronic maternal morbidities associated with caesarean delivery include pelvic pain and adhesions [93].
On the other hand, the human ovarian tissue also expresses ACE2 [94]. Therefore, evaluating the effect of SARS-CoV-2 infection on ovarian function and fertility is essential. The anti-Müllerian hormone and basal follicle-stimulating hormone are markers of ovarian reserve. A decrease in the level of anti-Müllerian hormone and a rise in follicle-stimulating hormone are associated with lower ovarian reserve [95]. Nevertheless, it is also reported that the anti-Müllerian hormone level was significantly lower, and basal follicle-stimulating hormone levels were increased in women with COVID-19 [96]. Interestingly, the ovarian reserve was unaltered in patients recovering from mild and severe SARS-CoV-2 infection [97].
Also, SARS-CoV-2 infection is associated with an increased prevalence of major depressive and anxiety disorders [98]. As a result, we could have women who begin pregnancy under these conditions and have an impact on pregnancy outcomes. For instance, depression during pregnancy is a significant risk factor for preterm birth and small-for-gestational-age fetuses [99]. Thus, recognizing and treating these women is a strategy to reduce these adverse pregnancy outcomes. Therefore, it is necessary to carry out studies that address the outcomes in future pregnancies in women who previously had COVID-19.
4. The placenta, fetus, and neonate
Viral infection in pregnant women and vertical transplacental transmission remain unclear in the literature [100], [101], [102]. It is reported that vertical transmission may happen in ~3 % of pregnant women infected in the third trimester [100]. However, no congenital anomalies due to SARS-CoV-2 infection have been reported [101]. The few available evidence for potential vertical transmission also includes a report showing that three out of 11 placentas were positive for SARS-CoV-2 RNA [102]. In 19 positive pregnancies for COVID-19, only two placentas have been reported positive for SARS-CoV-2 RNA in the syncytiotrophoblast and cytotrophoblast [103]. It is also shown that out of 75 pregnant women with SARS-CoV-2 positive at the time of delivery, one patient had symptoms of COVID-19, six had symptoms before delivery, and 68 patients were asymptomatic [104]. In the asymptomatic patients, only 25 placentas were positive for SARS-CoV-2 and eight were negative [104]. This low frequency of infection shows that although the placenta can become infected, these events are rare and may be due to polarized expression of ACE2 away from maternal blood [103]. In the case of the Delta variant, placental infection with transmission to the fetus was reported [105]. Viral infections in the placenta have been associated with morphological changes, including some pathological changes due to placenta fibrin accumulation [106].
4.1. Placenta structural and functional changes
Placental infections with SARS-CoV-2 are not associated with any specific histopathology [103]. Consistent with these results, Suhren and colleagues published a meta-analysis in which 30 publications from 2019 to 2021 with 1452 cases were analysed. In this study, no specific placental changes were found for COVID-19, and the incidence of inflammatory and vascular lesions was comparable to that of pregnancies without COVID-19 [107]. On the contrary, a prospective cohort study found that SARS-CoV-2 infection at 24–44 weeks of gestation was associated with a higher incidence of low-weight placentas and a higher birthweight/placental weight (b/p) ratio [108]. It is worth noting that the latter study was carried out after the study by Suhren and colleagues [107]. The temporal difference between these two studies may imply a different type of VOC to which the patients were exposed. It is likely that the VOCs that emerged later, such as Delta and Omicron, have more significant effects at the placental level.
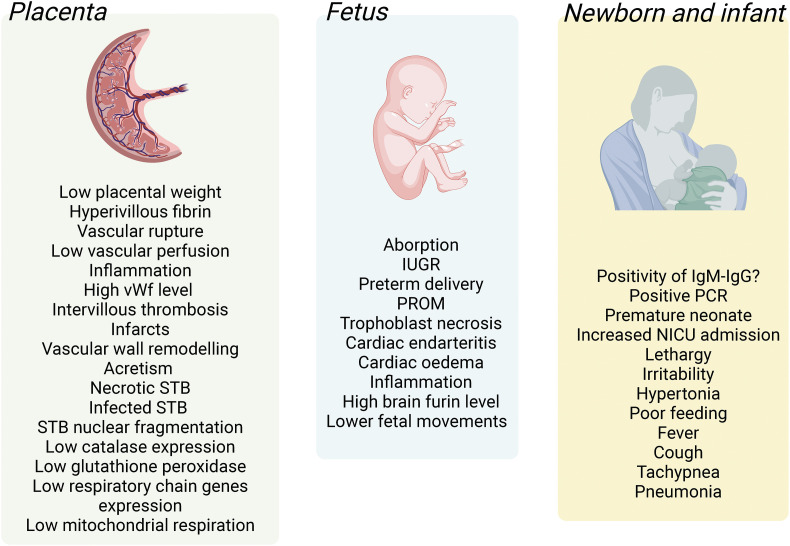
In a case study that suggested vertical transmission of the virus to the newborn, the placenta showed greater perivillous fibrin, vascular rupture on the placental fetal side, infiltration of CD68-positive Hoffbauer cells in the villous stroma, as well as the presence of SARS-CoV-2 RNA in the villous of the syncytiotrophoblast [109] (Fig. 4 ). In a systematic review including 20 studies with infected placentas in the third trimester of pregnancy, abnormal fetal (35 % of cases) and maternal (46 % of cases) placental vascular perfusion was described [110]. The latter study also showed villitis, intervillitis, and chorioamnionitis, which are inflammatory reactions like those reported for SARS-CoV-1 infection.
Fig. 4.
Reported alterations in the placenta, fetus, and neonate/infant in pregnancies with coronavirus disease 2019. The human placenta (Placenta) in pregnancies where the mother was positive for SARS-CoV-2 show several alterations, including increased inflammation and reduced vascular perfusion, expression of antioxidant enzymes, and mitochondrial respiration. Several alterations are also described in the growing fetus (Fetus), including restricted growth, cardiac alterations, and reduced fetal movements. Pregnancies with SARS-CoV-2 infection are associated with prematurity and increased admission to the intensive care unit after birth (Neonate and infant). IUGR, intrauterine growth restriction; IgM, immunoglobulin M; IgG, immunoglobulin G; PCR, polymerase chain reaction; PROM, premature rupture of membranes; NICU, neonatal intensive care unit; STB, syncytiotrophoblast; vWf, von Willebrand factor. Information is taken from references quoted in the body text.
Apart from the above-mentioned findings, placental vasculopathy, specifically in the decidua, has also been reported [111], [112]. Another study in which SARS-CoV-2 positive placentas were compared with placentas from caesarean sections showed only chronic villitis and accelerated villous maturation [113]. In placentas from patients positive for SARS-CoV-2 with severe symptoms, the expression of von Willebrand factor in the endothelium of decidua and chorionic villi has been reported [114]. The placentas also showed signs of thrombosis, infarcts, and vascular wall remodelling [114]. The latter findings are also consistent with the report of fetal vascular thrombosis [115] and the presence of decidual arteriopathy [116] in SARS-CoV-2 positive placentas.
SARS-CoV-2 positive placentas also showed intervillous thrombosis, myometrial fibrillar attached to the basal plate, oedema in the villi, haemorrhage in the membranes, and microscopic acretism [117]. In contrast, no abnormalities were found in a reduced proportion (17 %) of these infected placentas [117]. The alterations mentioned above and the higher incidence of inflammatory processes in infected placentas are indicative of systemic inflammation and a state of hypercoagulability [106]. On the other hand, at the time of delivery, it has been reported that of 25 placentas positive for SARS-CoV-2 RNA, 12 placentas presented normal histology compared to placentas from uninfected pregnancies [104]. Only one placenta from an asymptomatic mother had SARS-CoV-2 detected by viral staining. The placenta showed fibrin deposits on the apical surface of the syncytiotrophoblast, causing a rupture of the placental barrier [104].
Also, in photomicrographs of SARS-CoV-2 positive placenta, infiltration of inflammatory cells in the intervillous space has been reported, with signs of necrosis in the syncytiotrophoblast layer and nuclear fragmentation of syncytiotrophoblast [118]. Inflammatory processes and viral infections are accompanied by the generation of reactive oxygen species, generating oxidative stress that actively causes pathological alterations [119]. Infection with SARS-CoV-2 resulted in decreased expression of genes with antioxidant function (v.g. catalase and glutathione peroxidase) in the placenta, lower expression of respiratory chain genes (NDUFA9, SDHA, COX4I1), and genes that regulate mitochondrial dynamics (DNM1L, FIS1) [120]. These alterations favour the development of oxidative stress and decrease mitochondrial efficiency in the placenta.
It seems contradictory that although infected placentas exist, some present with drastic morphological changes, while in other patients, the morphology is similar to that of non-infected patients. The degree of severity of SARS-CoV-2 may play a role in these changes. A higher prevalence of necrotic trophoblasts has been found in the villous of women requiring respiratory support, showing that the severity of the disease is associated with the histopathological changes observed in the placenta after infection with SARS-CoV-2 [121]. This could mean less oxygenation in the placental tissue that favours infection of the placenta [122]. Another factor that could be involved in the variant that infects placentas was proposed in a case study where after infection with the Alpha variant, placentitis with loss of the intervillous space, fibrin deposition, and infiltration of inflammatory cells (CD68 positive) was seen [123]. Among the structural changes reported in a case study of the placenta infected with the Delta variant, intervillositis increased fibrin deposition, and syncytiotrophoblast necrosis stands out. The placental cells also presented apoptosis, senescence, and ferroptosis [105]. The frequency of placenta alterations such as maternal vascular abnormal perfusion and decidual artery disease changes with the VOC to which they were exposed [124]. Interestingly, these placental alterations had a higher incidence in cases infected with the Delta variant [124].
Another factor that may influence the appearance of malformations in the placenta is the gestational age at which the infection is acquired. In a prospective cohort study, it was shown that women with infection in the acute SARS-CoV-2 phase (<14 days after delivery) have a higher frequency of lesions due to fetal vascular malperfusion than women with non-acute phase (>14 days after delivery). In contrast, maternal vascular malperfusion lesions did not show differences. The latter results might indicate that the time of infection to delivery can alter the placental pathology [125].
It is also proposed that the poles of specific genes for each breed may also influence the appearance of the mentioned alterations since the histopathological alterations in placentas SARS-CoV-2 positives to vary depending on different environmental factors, including the geographical location. A fibrin excess is presented transversally in samples from other regions where placentas from Canada present deciduitis. At the same time, those from the United Kingdom show calcifications, and in samples collected in the French population, agglutinations and chorangiosis are more common [126]. All these lesions and others may be associated with adverse perinatal outcomes.
4.2. The fetus
Although vertical SARS-CoV-2 transmission in pregnancy is rare, it could occur when the virus enters the placenta through the uterine arteries, passing to the intervillous space. Through the chorionic villous tree, it could enter fetal circulation [127]. Fetal infection with SARS-CoV-2 due to placental alterations could generate alterations in fetal development that would favour the outcome of pregnancy complications such as abortions, growth restrictions, or preterm deliveries (Fig. 4). In a group of 79 pregnant women with SARS-CoV-2 It is reported that ~39 % had abortions, ~24 % had preterm deliveries, ~21 % had premature rupture of the membrane, and ~ 12 % had fetal restriction [128].
Fetal death in SARS-CoV-2 infection is shown to associate with trophoblast necrosis which affects the chorionic villous tissue collapsing the intervillous space to varying degrees with swelling of inflammatory cells (histocytes, neutrophils, and CD3+ T lymphocytes) in the intervillous and fibrinoid deposition in the perivillous [129]. Also, infected fetal tissue has been reported during the first trimester of pregnancy associated with endarteritis in small arteries with inflammatory mononuclear cells (CD68+) in the interstitial space of the fetal heart and oedema between cardiomyocytes [130]. Fetal lungs presented reactive bronchial epithelium and hypercellularity composed of CD68+ macrophages, myositis in the striated muscle of the neck, extremities, and diaphragm and kidneys infiltrated with these macrophages. Thus, SARS-CoV-2 infection might associate with a massive inflammatory response induced by the release of cytokines from the mother in response to the infection [130].
Another fetal tissue that may be affected by SARS-CoV-2 is the brain. ACE2 and TMPRSS2 show with low expression but furin is highly expressed in the fetal brain. Thus, these molecules may play a role in the pathogenic infection of the fetal brain during the second and third trimester of pregnancy [131]. It is also reported that SARS-CoV-2 infection may result in lower fetal movements seen as bilateral fronto-parieto-occipital cystic peri-ventricular leukomalacia on day 25 postnatally, suggesting that infection may cause damage to the newborn brain [132].
Not only abnormalities in the placenta after infection with SARS-CoV-2 may affect fetal development, but the mother's pre-existing condition may also contribute to these changes. To date, the pattern of histiocytic intervillositis and massive perivillous fibrin deposition, currently known as SARS-CoV-2 placentitis, in obese women is associated with a higher frequency of pregnancy loss compared to non-obese women SARS-CoV-2 positive [133]. Pre-pregnancy maternal obesity has increased in the last decades and keeps rising worldwide [134]. It is now more recognized that pre-pregnancy obesity increases the risk or susceptibility to developing other pregnancy diseases such as hypertension in pregnancy, GDM and gestational diabesity [135], [136]. However, it is still unclear whether pre-pregnancy obesity is a risk factor for more drastic and detrimental effects following SARS-CoV-2 on the fetus.
4.3. The neonate
There has been great concern about the impact that SARS-CoV-2 infection could have on the neonate. The latter is due to the vulnerability of the neonates and their immune immaturity, especially in extremely ill patients, very low birth weight infants, and children with major congenital malformations [137]. The primary concerns are the uncertainty of vertical transmission, postnatal contact, and breastfeeding. The positivity of IgM-IgG in neonates and its interpretation is still controversial, and further studies are required to answer this question [138], [139], [140], [141], [142], [143]. Although it has not been possible to clearly define whether the severity of the maternal disease is associated with a potential vertical transmission, it could influence child symptomatology [144], [145]. Given the data currently available, the source of infection of the newborn is mainly postnatal, so care practices are fundamental and should be maintained after postnatal hospital discharge with strict outpatient monitoring.
Several clinical guidelines have been generated in different countries for managing the suspected/infected SARS-CoV-2 mother and her newborn in the delivery room and postnatal care. Based on earlier experience with SARS-CoV-1 and MERS-CoV, stringent guidelines were made without clarifying their risks and benefits [69], [146]. The pandemics' evolution showed repercussions on the neonate and the effectiveness of care practices. Fortunately, the impact on the neonatal population has been much lower than expected, although severe cases and even some fatal cases have been reported. The risk of mother-to‑neonate transmission is low, at least for developing severe infections in healthy newborns [144].
4.3.1. Neonatal infection
Different series have reported newborn infection rates and their characteristics during the pandemic (Fig. 4). In one first case review, 836 women with SARS-CoV-2 were analysed [144]. The results show that ~4 % of neonates showed PCR positive for the virus in the first hours of life. In a publication of the Spanish Society of Neonatology [147], 497 pregnant women were SARS-CoV-2 positive at the time of delivery. In this group of patients, ~33 % of deliveries were by a caesarean section, and ~ 16 % were premature birth, both primarily attributed to the maternal status. Another report shows 3 % PCR positive during the first hours of life (1−12h) and 4 % PCR positive in the second sample (30–48 h) [147]. Another consequence is that ~20 % of the neonates were hospitalized in the NICU due to symptomatology, including premature neonates. The rest of the neonates were in-room with their mothers without seeing adverse effects from receiving breastfeeding, skin-to-skin care, and in-room stay [147]. The reported findings suggest that the transmission rate may be low and has a less severe impact on the neonate.
4.3.2. Neonatal symptomatology and systemic alterations
Standard prevention practices have shown to be effective in reducing a potential vertical transmission and avoiding strict measures that may have later adverse effects. In cases where the infection has been detected, the symptoms are variable. Protocolized management of the delivery room ensures minimal risk to the neonate. No more significant need for neonatal resuscitation is reported, and the Apgar score of the reported cases is like the normal population. In some reports, lower scores are associated with a higher incidence of preterm birth [148], [149], [150], [151].
Neonates to SARS-CoV-2 positive pregnancies may be asymptomatic or have mild to moderate symptoms. The most described symptoms are lethargy, irritability, hypotonia, poor feeding, diarrhoea, vomiting, cough, rhinorrhoea, fever, tachycardia, tachypnoea and respiratory distress [152]. The most described symptoms are lethargy, irritability, hypertonia, poor feeding, cough, fever, tachypnoea, and pneumonia. A French series reports fever, respiratory, gastrointestinal, and neurological symptoms [153]. Clinical manifestation tends to resolve in one to two weeks with complete recovery [154], [155], [156], [157], [158], [159], [160], [161], [162]. Severe cases in the neonate have been associated with mothers with severe SARS-CoV-2 disease, related to perinatal hypoxia and a higher risk of preterm birth [78]. The severity of maternal symptoms has also been associated with a higher rate of caesarean section and the need for neonatal phototherapy [149], [158].
Severe cases may also present as multisystemic inflammatory syndrome in newborns born to mothers with SARS-CoV2 infection (MIS-N) and in newborns or children with acquired SARS-CoV2 infection (MIS-C) [163], [164]. Multisystem inflammatory involvement manifests mainly with cardiovascular involvement, shock, myocardial dysfunction, arrhythmias and coronary dilatation. It also presents with respiratory distress, fever, gastrointestinal and hematologic alterations. Thus, in patients with signs of multisystem inflammatory involvement, cardiovascular evaluation should be included.
The most severe cases of SARS-CoV2 infection have been described in extremely premature neonates or patients with comorbidities, attributing their final severity to numerous factors and not only to viral infection [143], [150], [157]. Although the symptoms may appear early, cases with later symptoms are reported. In these cases, it has been considered that the form of transmission is horizontal and not vertical [154]. For this reason, patients discharged with their mothers should be checked for one or two weeks after discharge for symptoms [155]. Since there is concern about cardiorespiratory and neurological complications in the medium and long term and these neonates [165], they should be followed up.
4.3.3. Prevention and general care practices
Care practices that should be taken to avoid transmission are using a face mask by the mother, hand washing and hygiene before and after breastfeeding, and physical distance (2 m) between the mother's bed and crib. Practices such as early cord clamping, avoiding initial skin-to-skin contact, early bathing, restriction/suspension of breastfeeding, or separation from the mother in the first days of life have not shown efficacy. The benefits of these practices are more significant than the risks of contracting the infection taking general care practices [144], [147], [149], [166]. In cases of moderate to severe maternal illness, it may be advisable to expand care to reduce transmission and improve the care and comfort of the mother. The analysis should be case-to-case [147]. Thus, modification of the clinical care guidelines should depend on the evidence and experience seen in the pandemic. Guidelines and protocols should be evaluated and modified according to the context of each unit considering resources, the prevalence of infection, the evolution of the different VOCs and the vaccination status of the population [167].
Breastfeeding has undoubtedly been a subject of great controversy during the pandemic. Although it has been possible to detect the presence of SARS-CoV2 in breast milk, it has not been possible to prove a clear link between the infection of a newborn and the consumption of breast milk, especially if the standard care practices are taken [78]. On the other hand, breastfeeding represents many benefits for the mother and the neonate, so the risk is less than the benefit [144], [147], [166]. In summary, if the mother's and the neonate's health allow it, breastfeeding should be recommended [168]. Measures should be taken to avoid droplet transmission during breastfeeding [169].
5. Concluding thoughts and future directions
After around two years of the COVID-19 pandemic, it is gratifying to conclude that there is greater certainty about the routes of transmission, symptoms, and acute repercussions of maternal SARS-CoV-2 infection in neonates. Also, there is greater clarity about effective prevention and monitoring practices. The generation of new information has been essential in optimizing resources and avoiding suspending interventions beneficial for the mother and child (e.g. breastfeeding, skin-to-skin contact). Looking to the future, we still must determine how exposure to the viral infection by SARS-CoV-2 during pregnancy may affect the newborn, child, or adult (Fig. 5 ). The search for specific mechanisms is essential to generate protocols protecting the mother, fetus, and neonate. Maternal immune activation, the inflammatory storm, and the effect on in utero programming of adult diseases due to fetal and perinatal environment are aspects on which we must focus our attention. An example of a more specific mechanism is the ACE2 expression and the components of its signalling cascade(s) at various levels in the fetus [141]. The impact of these factors is still unclear and will require further research and follow-up of these patients in the medium and long term to figure out their value.
Fig. 5.
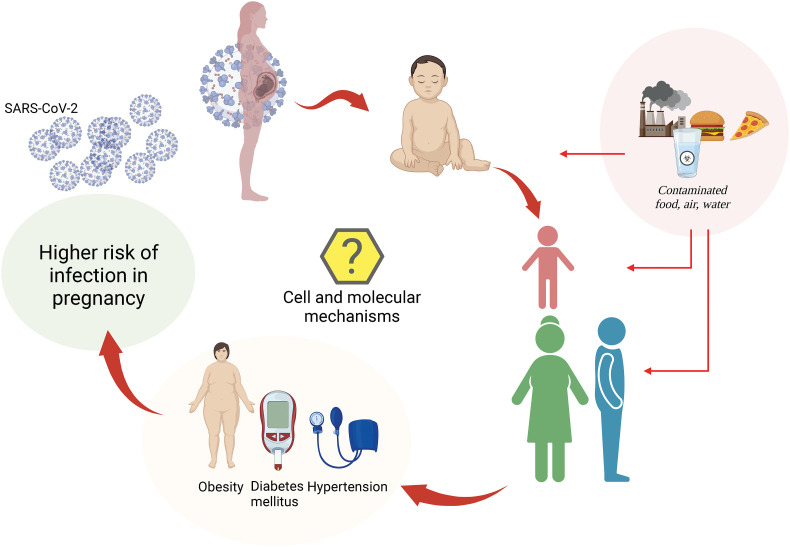
Proposed SARS-CoV-2 infection cycle in the generation of women from COVID-19 pregnancies. Pregnancy is a physiological condition where the mother can get infected with SARS-CoV-2 developing coronavirus disease 2019 (COVID-19). The intrauterine and perinatal environments are stages in life where the programming of human diseases may happen, and this may get worse when the neonate comes from a mother with COVID-19. The exposure of the mother to breastfeeding, neonate, children, and adults (women and men) from COVID-9 mothers may determine high susceptibility to developing young and adult diseases. The latter is triggered by ecto-exposome factors that are part of the environmental contaminants, mainly in the form of contaminated food, air, and water. Unhealthy conditions such as obesity, diabetes mellitus, and hypertension will increase the risk of infection in pregnancy. However, the cell and molecular mechanisms accounting for SARS-CoV-2 infection in the COVID-19 pregnancies generation of women of reproductive age are not yet available.
Environmental factors may increase the susceptibility of pregnant women to be infected by SARS-CoV-2, including pre-pregnancy obesity —expected as non-stop increasing for 2030 according to the World Obesity Federation 2022 report [170], diabetes mellitus, hypertension, nutrition habits, air, food, and water contaminants. These factors are considered part of the ecto-exposome and endo-exposome [136], [171] to which pregnant women, their fetuses, and newborns are exposed. Also, it will be necessary to explore the effect of mass vaccination on infection during pregnancy and the newborn. Vaccines are shown to be safe in the short term for the mother and fetus/neonate during both pregnancy and breastfeeding [172]. Vaccination may reduce maternal complications derived from infection, reduce stillbirth and transfer of antibodies through the placenta and breastfeeding. However, more studies are needed to see the effect of vaccination and vaccine immunogenicity in this population in the long term. The latter will surely change the dynamics of infection and manifestations of COVID-19 in the way it is know so far.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) [grant number 1190316], Chile, and International Sabbaticals (PC, LS) (University Medical Centre Groningen, University of Groningen, The Netherlands) from the Vicerectorate of Academic Affairs, Academic Development Office of the Pontificia Universidad Católica de Chile. PV holds PhD fellowships from Agencia Nacional de Investigación y Desarrollo (ANID) [grant number 21221870] and Universidad de Talca, Chile. KA holds a PhD fellowship from ANID [grant number 21221138].
Data availability
No data was used for the research described in the article.
References
- 1.Martins M.M., Alves da Cunha A.J.L., Robaina J.R., Raymundo C.E., Barbosa A.P., Medronho R.A. Fetal, neonatal, and infant outcomes associated with maternal zika virus infection during pregnancy: a systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0246643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouthry E., Picone O., Hamdi G., Grangeot-Keros L., Ayoubi J.M., Vauloup-Fellous C. Rubella and pregnancy: diagnosis, management and outcomes. Prenat. Diagn. 2014;34:1246–1253. doi: 10.1002/pd.4467. [DOI] [PubMed] [Google Scholar]
- 3.Ornoy A., Ergaz Z. Parvovirus B19 infection during pregnancy and risks to the fetus. Birth Defects Res. 2017;109:311–323. doi: 10.1002/bdra.23588. [DOI] [PubMed] [Google Scholar]
- 4.Shi T.L., Huang L.J., Xiong Y.Q., Zhong Y.Y., Yang J.J., Fu T., Lei X.F., Chen Q. The risk of herpes simplex virus and human cytomegalovirus infection during pregnancy upon adverse pregnancy outcomes: a meta-analysis. J. Clin. Virol. 2018;104:48–55. doi: 10.1016/j.jcv.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Bebell L.M., Oduyebo T., Riley L.E. Ebola virus disease and pregnancy: a review of the current knowledge of ebola virus pathogenesis, maternal, and neonatal outcomes. Birth Defects Res. 2017;109:353–362. doi: 10.1002/bdra.23558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson-Famy A., Gardella C. Herpes simplex virus infection during pregnancy. Obstet. Gynecol. Clin. N. Am. 2014;41:601–614. doi: 10.1016/j.ogc.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Abdullahi H., Elnahas A., Konje J.C. Seasonal influenza during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021;258:235–239. doi: 10.1016/j.ejogrb.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Vousden N Bunch K., Knight M, UKOSS Influenza Co-Investigators Group Incidence, risk factors and impact of seasonal influenza in pregnancy: a national cohort study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0244986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narang K., Enninga E.A.L., Gunaratne M.D.S.K., Ibirogba E.R., Trad A.T.A., Elrefaei A., Theiler R.N., Ruano R., Szymanski L.M., Chakraborty R., Garovic V.D. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin. Proc. 2020;95:1750–1765. doi: 10.1016/j.mayocp.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soma-Pillay P., Nelson-Piercy C., Tolppanen H., Mebazaa A. Physiological changes in pregnancy. Cardiovasc J. Afr. 2016;27:89–94. doi: 10.5830/CVJA-2016-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Raya B., Michalski C., Sadarangani M., Lavoie P.M. Maternal immunological adaptation during normal pregnancy. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kucirka L.M., Norton A., Sheffield J.S. Severity of COVID-19 in pregnancy: a review of current evidence. Am. J. Reprod. Immunol. 2020;84 doi: 10.1111/aji.13332. [DOI] [PubMed] [Google Scholar]
- 13.Kieffer T.E.C., Laskewitz A., Scherjon S.A., Faas M.M., Prins J.R. Memory T cells in pregnancy. Front. Immunol. 2019;10:625. doi: 10.3389/fimmu.2019.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan D.S.A., Pirzada A.N., Ali A., Salam R.A., Das J.K., Lassi Z.S. The differences in clinical presentation, management, and prognosis of laboratory-confirmed COVID-19 between pregnant and non-pregnant women: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2021;18:5613. doi: 10.3390/ijerph18115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. SARS-CoV-2 variant classifications and definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html. (Accessed July 19, 2022).
- 16.Sahin D., Tanacan A., Anuk A.T., Sinaci S., Besimoglu B., Oluklu D., Hendem D.U., Beser D.M., Yildirim M., Sakcak B., Erol S.A., Colakoglu Y., Ayhan S.G., Turgut E., Unlu S., Canpolat F.E., Izdes S., Turan S., Surel A.A., Tekin O.M. Comparison of clinical features and perinatal outcomes between pre-variant and post-variant periods in pregnant women with SARS-CoV-2: analysis of Cases. Arch. Gynecol. Obstet. 1935;2022:1–10. doi: 10.1007/s00404-022-06493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seasely AR Blanchard C.T., Arora N, CWRH COVID-19 Working Group Maternal and perinatal outcomes associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) delta (B.1.617.2) variant. Obstet. Gynecol. 2021;138:842–844. doi: 10.1097/AOG.0000000000004607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vousden N., Ramakrishnan R., Bunch K., Morris E., Simpson N., Gale C., O’Brien P., Quiley M., Brocklehurst P., Kurinczuk J., Knight M. Severity of maternal infection and perinatal outcomes during periods of SARS-CoV-2 wildtype, alpha, and delta variant dominance in the UK: prospective cohort study. BMJ Medicine. 2022;1 doi: 10.1136/bmjmed-2021-000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adhikari E.H., MacDonald L., SoRelle J.A., Morse J., Pruszynski J., Spong C.Y. COVID-19 cases and disease severity in pregnancy and neonatal positivity associated with delta (B.1.617.2) and omicron (B.1.1.529) variant predominance. JAMA. 2022;327:1500–1502. doi: 10.1001/jama.2022.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institutes of Health . 2019. Coronavirus disease.https://www.covid19treatmentguidelines.nih.gov/ (COVID-19) treatment guidelines. [Google Scholar]
- 21.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 22.Ma Q., Liu J., Liu Q., Kang L., Liu R., Jing W., Wu Y., Liu M. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.37257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann. Intern. Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabata S., Imai K., Kawano S., Ikeda M., Kodama T., Miyoshi K., Obinata H., Mimura S., Kodera T., Kitagaki M., Sato M., Suzuki S., Ito T., Uwabe Y., Tamura K. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the diamond princess cruise ship: a retrospective analysis. Lancet Infect. Dis. 2020;20:1043–1050. doi: 10.1016/S1473-3099(20)30482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutton D., Fuchs K., D'Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N. Engl. J. Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vintzileos W.S., Muscat J., Hoffmann E., John N.S., Vertichio R., Vintzileos A.M., Vo D. Screening all pregnant women admitted to labor and delivery for the virus responsible for coronavirus disease 2019. Am. J. Obstet. Gynecol. 2020;223:284–286. doi: 10.1016/j.ajog.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalil A., Hill R., Ladhani S., Pattisson K., O'Brien P. Severe acute respiratory syndrome coronavirus 2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg. Am. J. Obstet. Gynecol. 2020;223:296–297. doi: 10.1016/j.ajog.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashim N.A.F., Mahdy Z.A., Abdul Rahman R., Kalok A.H.M., Sutan R. Universal testing policy for COVID-19 in pregnancy: a systematic review. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.588269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendiola-Pastrana I.R., López-Ortiz E., Río de la Loza-Zamora J.G., González J., Gómez-García A., López-Ortiz G. SARS-CoV-2 variants and clinical outcomes: a systematic review. Life (Basel) 2022;12:170. doi: 10.3390/life12020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong SWX, Chiew CJ, Ang LW, Mak TM, Cui L, Toh MPHS, Lim YD, Lee PH, Lee TH, Chia PY, Maurer-Stroh S, Lin RTP, Leo YS, Lee VJ, Lye DC, Young BE. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Clin Infect Dis. 2021:ciab721. doi:10.1093/cid/ciab721. [DOI] [PMC free article] [PubMed]
- 32.Abdullah F., Myers J., Basu D., Tintinger G., Ueckermann V., Mathebula M., Ramlall R., Spoor S., de Villiers T., Van der Walt Z., Cloete J., Soma-Pillay P., Rheeder P., Paruk F., Engelbrecht A., Lalloo V., Myburg M., Kistan J., van Hougenhouck-Tulleken W., Boswell M.T., Gray G., Welch R., Blumberg L., Jassat W. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane. South Africa. Int. J. Infect. Dis. 2022;116:38–42. doi: 10.1016/j.ijid.2021.12.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Portilla R.J., Sotiriadis A., Chatzakis C., Torres-Torres J., Sosa Espino Y., Sandoval-Mandujano K., Castro-Bernabe D.A., Medina-Jimenez V., Monarrez-Martin J.C., Figueras F., Poon L.C. Pregnant women with SARS-CoV-2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx) Ultrasound Obstet. Gynecol. 2021;57:224–231. doi: 10.1002/uog.23575. [DOI] [PubMed] [Google Scholar]
- 34.Meyyazhagan A., Pushparaj K., Balasubramanian B., Kuchi Bhotla H., Pappusamy M., Arumugam V.A., Easwaran M., Pottail L., Mani P., Tsibizova V., Di Renzo G.C. COVID-19 in pregnant women and children: insights on clinical manifestations, complexities, and pathogenesis. Int. J. Gynaecol. Obstet. 2022;156:216–224. doi: 10.1002/ijgo.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.da Rosa Mesquita R., Francelino Silva Junior L.C., Santos Santana F.M., Farias de Oliveira T., Campos Alcântara R., Monteiro Arnozo G., da Silva Rodrigues, Filho E., Galdino Dos Santos A.G., Oliveira da Cunha E.J., Salgueiro de Aquino S.H., Freire de Souza C.D. Clinical manifestations of COVID-19 in the general population: systematic review. Wien. Klin. Wochenschr. 2021;133:377–382. doi: 10.1007/s00508-020-01760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Z., Huang X., Zhang J., Fu S., Ding D., Tao Z. Differences in clinical characteristics between delta variant and wild-type SARS-cov-2 infected patients. Front. Med. 2022;8 doi: 10.3389/fmed.2021.792135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., Satlin M.J., Campion T.R., Nahid M., Ringel J.B., Hoffman K.L., Alshak M.N., Li H.A., Wehmeyer G.T., Rajan M., Reshetnyak E., Hupert N., Horn E.M., Martinez F.J., Gulick R.M., Safford M.M. Clinical characteristics of covid-19 in New York city. New. Engl. J. Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan W.J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., DSC Hui, Du B., Li L.-J., Zeng G., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-L., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease. n ChinaN. Engl. J. Med. 2019;2020(382):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Servante J., Swallow G., Thornton J.G., Myers B., Munireddy S., Malinowski A.K., Othman M., Li W., O'Donoghue K., Walker K.F. Haemostatic and thrombo-embolic complications in pregnant women with COVID-19: a systematic review and critical analysis. BMC Pregnancy Childbirth. 2021;21:108. doi: 10.1186/s12884-021-03568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dessie Z.G., Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021;21:855. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolff D., Nee S., Hickey N.S., Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. 2021;49:15–28. doi: 10.1007/s15010-020-01509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Saadi E., Abdulnabi M.A. Hematological changes associated with COVID-19 infection. J. Clin. Lab. Anal. 2022;36 doi: 10.1002/jcla.24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Y., Wang Z., Liao H., Marley G., Wu D., Tang W. Epidemiologic, clinical, and laboratory findings of the COVID-19 in the current pandemic: systematic review and meta-analysis. BMC Infect. Dis. 2020;20:640. doi: 10.1186/s12879-020-05371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z.L., Hou Y.L., Li D.T., Li F.Z. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand. J. Clin. Lab. Invest. 2020;80:441–447. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henry B.M., de Oliveira M., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 47.Mendoza M., Garcia-Ruiz I., Maiz N., Rodo C., Garcia-Manau P., Serrano B., Lopez-Martinez R.M., Balcells J., Fernandez-Hidalgo N., Carreras E., Suy A. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127:1374–1380. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Čivrná J., Skanderová D., Ehrmann J., Pilka R. HELLP syndrome and HELLP-like syndrome in pregnancies with covid-19-case reports. Ceska Gynekol. 2021;86:236–241. doi: 10.48095/cccg2021236. [DOI] [PubMed] [Google Scholar]
- 49.Palomo M., Youssef L., Ramos A., Torramade-Moix S., Belen Moreno-Castaño A., Martinez-Sanchez J., Bonastre L., Pino M., Gomez-Ramirez P., Martin L., Mateos E.G., Sanchez P., Fernandez S., Crovetto F., Escolar G., Carreras E., Castro P., Gratacos E., Crispi F., Diaz-Ricart M. Differences and similarities in endothelial and angiogenic profiles of preeclampsia and COVID-19 in pregnancy. Am. J. Obstet. Gynecol. 2022;S0002–9378(22) doi: 10.1016/j.ajog.2022.03.048. 002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Awulachew E., Diriba K., Anja A., Getu E., Belayneh F. Computed tomography (CT) imaging features of patients with COVID-19: systematic review and meta-analysis. Radiol. Res. Pract. 2020;2020:1023506. doi: 10.1155/2020/1023506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Y., Wang L., Ben S. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J. Med. Virol. 2021;93:241–249. doi: 10.1002/jmv.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bao C., Liu X., Zhang H., Li Y., Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J. Am. Coll. Radiol. 2020;17:701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair A.V., Ramanathan S., Venugopalan P. Chest imaging in pregnant patients with COVID-19: recommendations, justification, and optimization. Acta. Radiol. Open. 2022;11 doi: 10.1177/20584601221077394. 20584601221077394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am. J. Obstet. Gynecol. 2020;222:415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Royal College of Obstetricians and Gynaecologists (RCOG) Quick reference summary of acute COVID-19 care. 2022. https://www.rcog.org.uk/media/xsubnsma/2022-03-07-coronavirus-covid-19-infection-in-pregnancy-v15.pdf Accessed July 19, 2022.
- 56.Committee Opinion No 723: guidelines for diagnostic imaging during pregnancy and lactation. Obstet. Gynecol. 2017;130:e210–e216. doi: 10.1097/AOG.0000000000002355. [DOI] [PubMed] [Google Scholar]
- 57.Vehar S., Boushra M., Ntiamoah P., Biehl M. Post-acute sequelae of SARS-CoV-2 infection: caring for the 'long-haulers'. Cleve. Clin. J. Med. 2021;88:267–272. doi: 10.3949/ccjm.88a.21010. [DOI] [PubMed] [Google Scholar]
- 58.Barr E., Whitaker D., Stratton P. Pregnancy and SARS-CoV-2: an opportunity to systematically study the complexity of maternal health. Lancet Digit Health. 2022;4:e76–e77. doi: 10.1016/S2589-7500(21)00277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borch L., Holm M., Knudsen M., Ellermann-Eriksen S., Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur. J. Pediatr. 2022;181:1597–1607. doi: 10.1007/s00431-021-04345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P.A., Cuapio A., Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci. Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nehme M., Braillard O., Salamun J., Jacquerioz F., Courvoisier D.S., Spechbach H., Guessous I. Symptoms after COVID-19 vaccination in patients with post-acute sequelae of SARS-CoV-2. J. Gen. Intern. Med. 2022;37:1585–1588. doi: 10.1007/s11606-022-07443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shuffrey L.C., Firestein M.R., Kyle M.H., Fields A., Alcántara C., Amso D., Austin J., Bain J.M., Barbosa J., Bence M., Bianco C., Fernández C.R., Goldman S., Gyamfi-Bannerman C., Hott V., Hu Y., Hussain M., Factor-Litvak P., Lucchini M., Mandel A., Marsh R., McBrian D., Mourad M., Muhle R., Noble K.G., Penn A.A., Rodriguez C., Sania A., Silver W.G., O'Reilly K.C., Stockwell M., Tottenham N., Welch M.G., Zork N., Fifer W.P., Monk C., Dumitriu D. Association of birth during the COVID-19 pandemic with neurodevelopmental status at 6 months in infants with and without in utero exposure to maternal SARS-CoV-2 infection. JAMA Pediatr. 2022;176 doi: 10.1001/jamapediatrics.2021.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral. Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Penninger J.M., Grant M.B., Sung J. The role of angiotensin converting enzyme 2 in modulating gut microbiota, intestinal inflammation, and coronavirus infection. Gastroenterology. 2021;160:39–46. doi: 10.1053/j.gastro.2020.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muyayalo K.P., Huang D.H., Zhao S.J., Xie T., Mor G., Liao A.H. COVID-19 and Treg/Th17 imbalance: potential relationship to pregnancy outcomes. Am. J. Reprod. Immunol. 2020;84 doi: 10.1111/aji.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Araújo J., Menezes D., de Aguiar R.S., de Souza R.P. IFITM3, FURIN, ACE1, and TNF-α genetic association with COVID-19 outcomes: systematic review and meta-analysis. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.775246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:194. doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muhl L., He L., Sun Y., Andaloussi Mäe M., Pietilä R., Liu J., Genové G., Zhang L., Xie Y., Leptidis S., Mocci G., Stritt S., Osman A., Anisimov A., Hemanthakumar K.A., Räsänen M., Hansson E.M., Björkegren J., Vanlandewijck M., Blomgren K., Betsholtz C. The SARS-CoV-2 receptor ACE2 is expressed in mouse pericytes but not endothelial cells: implications for COVID-19 vascular research. Stem. Cell. Reports. 2022;17:1089–1104. doi: 10.1016/j.stemcr.2022.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pavel A.B., Glickman J.W., Michels J.R., Kim-Schulze S., Miller R.L., Guttman-Yassky E. Th2/Th1 cytokine imbalance is associated with higher COVID-19 risk mortality. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.706902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santa Cruz A., Mendes-Frias A., Oliveira A.I., Dias L., Matos A.R., Carvalho A., Capela C., Pedrosa J., Castro A.G., Silvestre R. Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pedersen S.F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Volz E., Hill V., McCrone J.T., Price A., Jorgensen D., O'Toole Á., Southgate J., Johnson R., Jackson B., Nascimento F.F., Rey S.M., Nicholls S.M., Colquhoun R.M., da Silva F.A., Shepherd J., Pascall D.J., Shah R., Jesudason N., Li K., Jarrett R., Connor T.R. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell. 2021;184:64–75.e11. doi: 10.1016/j.cell.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takeda M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 2022;66:15–23. doi: 10.1111/1348-0421.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X., Wu S., Wu B., Yang Q., Chen A., Li Y., Zhang Y., Pan T., Zhang H., He X. SARS-CoV-2 omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal Transduct Target Ther. 2021;6:430. doi: 10.1038/s41392-021-00852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conde-Agudelo A., Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2022;226:68–89.e3. doi: 10.1016/j.ajog.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei S.Q., Bilodeau-Bertrand M., Liu S., Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193:E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flaherman V.J., Afshar Y., Boscardin W.J., Keller R.L., Mardy A.H., Prahl M.K., Phillips C., Asiodu I.V., Berghella V., Chambers B.D., Crear-Perry J., Jamieson D.J., Jacoby V.L., Gaw S.L. Infant outcomes following maternal infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): first report from the pregnancy coronavirus outcomes registry (PRIORITY) study. Clin. Infect. Dis. 2021;73:e2810–e2813. doi: 10.1093/cid/ciaa1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez-Portilla R.J., Smith E.R., He S., Torres-Torres J., Espino-Y-Sosa S., Solis-Paredes J.M., Poon L.C. Young pregnant women are also at an increased risk of mortality and severe illness due to coronavirus disease 2019: analysis of the mexican National Surveillance Program. Am. J. Obstet. Gynecol. 2021;224:404–407. doi: 10.1016/j.ajog.2020.12.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mullins E., Hudak M.L., Banerjee J., Getzlaff T., Townson J., Barnette K., Playle R., Perry A., Bourne T., Lees C.C. AN-COVID investigators and the National Perinatal COVID-19 Registry Study Group. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet. Gynecol. 2021;57:573–581. doi: 10.1002/uog.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelly J.C., Raghuraman N., Carter E.B., Palanisamy A., Stout M.J. Preprocedural asymptomatic coronavirus disease 2019 cases in obstetrical and surgical units. Am. J. Obstet. Gynecol. 2021;224:114–116. doi: 10.1016/j.ajog.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., Debenham L., Llavall A.C., Dixit A., Zhou D., Balaji R., Lee S.I., Qiu X., Yuan M., Coomar D., Sheikh J., Lawson H., Ansari K., van Wely M., van Leeuwen E., Kostova E., Kunst H., Khalil A., Tiberi S., Brizuela V., Broutet N., Kara E., Kim C.R., Thorson A., Oladapo O.T., Mofenson L., Zamora J., Thangaratinam S. for PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease, In pregnancy: living systematic review and meta-analysis. BMJ. 2019;2020(370) doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao Y.J., Ye L., Zhang J.S., Yin Y.X., Liu M., Yu H.B., Zhou R. Clinical features and outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. BMC Infect. Dis. 2020;20:564. doi: 10.1186/s12879-020-05274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dubey P., Reddy S.Y., Manuel S., Dwivedi A.K. Maternal and neonatal characteristics and outcomes among COVID-19 infected women: an updated systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;252:490–501. doi: 10.1016/j.ejogrb.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet. Gynecol. 2020;56:15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akhtar H., Patel C., Abuelgasim E., Harky A. COVID-19 (SARS-CoV-2) infection in pregnancy: a systematic review. Gynecol. Obstet. Investig. 2020;85:295–306. doi: 10.1159/000509290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Capobianco G., Saderi L., Aliberti S., Mondoni M., Piana A., Dessole F., Dessole M., Cherchi P.L., Dessole S., Sotgiu G. COVID-19 in pregnant women: a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;252:543–558. doi: 10.1016/j.ejogrb.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cohen J.B., Hanff T.C., Bress A.P., South A.M. Relationship between ACE2 and other components of the renin-angiotensin system. Curr. Hypertens. Rep. 2020;22:44. doi: 10.1007/s11906-020-01048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tamanna S., Clifton V.L., Rae K., van Helden D.F., Lumbers E.R., Pringle K.G. Angiotensin converting enzyme 2 (ACE2) in pregnancy: preeclampsia and small for gestational age. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.590787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levy J.H., Iba T., Olson L.B., Corey K.M., Ghadimi K., Connors J.M. COVID-19: thrombosis, thromboinflammation, and anticoagulation considerations. Int. J. Lab. Hematol. 2021;43:29–35. doi: 10.1111/ijlh.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.D'Souza R., Malhamé I., Teshler L., Acharya G., Hunt B.J., McLintock C. A critical review of the pathophysiology of thrombotic complications and clinical practice recommendations for thromboprophylaxis in pregnant patients with COVID-19. Acta Obstet. Gynecol. Scand. 2020;99:1110–1120. doi: 10.1111/aogs.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clark E.A., Silver R.M. Long-term maternal morbidity associated with repeat cesarean delivery. Am. J. Obstet. Gynecol. 2011;205:S2–S10. doi: 10.1016/j.ajog.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 94.Reis F.M., Bouissou D.R., Pereira V.M., Camargos A.F., dos Reis A.M., Santos R.A. Angiotensin-(1–7), its receptor mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil. Steril. 2011;95:176–181. doi: 10.1016/j.fertnstert.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 95.Fertil. Steril. 2015;103:e9–e17. doi: 10.1016/j.fertnstert.2014.12.093. [DOI] [PubMed] [Google Scholar]
- 96.Ding T., Wang T., Zhang J., Cui P., Chen Z., Zhou S., Yuan S., Ma W., Zhang M., Rong Y., Chang J., Miao X., Ma X., Wang S. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational study. Front Med. 2021;8 doi: 10.3389/fmed.2021.635255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carp-Veliscu A., Mehedintu C., Frincu F., Bratila E., Rasu S., Iordache I., Bordea A., Braga M. The effects of SARS-CoV-2 infection on female fertility: a review of the literature. Int. J. Environ. Res. Public Health. 2022;19:84. doi: 10.3390/ijerph19020984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in Due to the COVID-19 pandemic. Lancet. 2020;2021(398):1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Szegda K., Markenson G., Bertone-Johnson E.R., Chasan-Taber L. Depression during pregnancy: a risk factor for adverse neonatal outcomes? A critical review of the literature. J. Matern. Fetal Neonatal Med. 2014;27:960–967. doi: 10.3109/14767058.2013.845157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Metz T.D., Clifton R.G., Hughes B.L., Sandoval G., Saade G.R., Grobman W.A., Manuck T.A., Miodovnik M., Sowles A., Clark K., Gyamfi-Bannerman C., Mendez-Figueroa H., Sehdev H.M., Rouse D.J., Tita A., Bailit J., Costantine M.M., Simhan H.N., Macones G.A. Institute, Eunice Kennedy Shriver National, of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease, ,, COVID-19. Obstet. Gynecol. 2019;2021(137):571–580. doi: 10.1097/AOG.0000000000004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kotlyar A.M., Grechukhina O., Chen A., Popkhadze S., Grimshaw A., Tal O., Taylor H.S., Tal R. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021;224:35–53.e3. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Penfield C.A., Brubaker S.G., Limaye M.A., Lighter J., Ratner A.J., Thomas K.M., Meyer J.A., Roman A.S. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hecht J.L., Quade B., Deshpande V., Mino-Kenudson M., Ting D.T., Desai N., Dygulska B., Heyman T., Salafia C., Shen D., Bates S.V., Roberts D.J. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Debelenko L., Katsyv I., Chong A.M., Peruyero L., Szabolcs M., Uhlemann A.-C. Trophoblast damage with acute and chronic intervillositis: disruption of the placental barrier by severe acute respiratory syndrome coronavirus 2. Hum. Pathol. 2021;109:69–79. doi: 10.1016/j.humpath.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shen W.-B., Turan S., Wang B., Cojocaru L., Harman C., Logue J., Reece E.A., Frieman M.B., Yang P. A SARS-CoV-2 Delta variant case manifesting as extensive placental infection and fetal transmission. Gynecol. Obstet. Investig. 2022;87:165–172. doi: 10.1159/000524905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bahadur G., Homburg R., Yoong W., Singh C., Bhat M., Kotabagi P., Acharya S., Huirne J., Doreski P.A., Lukaszuk M. Adverse outcomes in SAR-CoV-2 (COVID-19) and SARS virus related pregnancies with probable vertical transmission. JBRA Assist Reprod. 2020;24:351–357. doi: 10.5935/1518-0557.20200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suhren J.T., Meinardus A., Hussein K., Schaumann N. Meta-analysis on COVID-19-pregnancy-related placental pathologies shows no specific pattern. Placenta. 2022;117:72–77. doi: 10.1016/j.placenta.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Radan A.P., Baud D., Favre G., Papadia A., Surbek D., Baumann M., Raio L. Low placental weight and altered metabolic scaling after severe acute respiratory syndrome coronavirus type 2 infection during pregnancy: a prospective multicentric study. Clin. Microbiol. Infect. 2022;28:718–722. doi: 10.1016/j.cmi.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alamar I., Abu-Arja M.H., Heyman T., Roberts D.J., Desai N., Narula P., Dygulska B. A possible case of vertical transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a newborn with positive placental in situ hybridization of SARS-CoV-2 RNA. J. Pediatr. Infect. Dis .Soc. 2020;9:636–639. doi: 10.1093/jpids/piaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharps M.C., Hayes D.J.L., Lee S., Zou Z., Brady C.A., Almoghrabi Y., Kerby A., Tamber K.K., Jones C.J., Adams Waldorf K.M., Heazell A.E.P. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13–29. doi: 10.1016/j.placenta.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hsu A.L., Guan M., Johannesen E., Stephens A.J., Khaleel N., Kagan N., Tuhlei B.C., Wan X.F. Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease. J. Med. Virol. 2021;93:1038–1044. doi: 10.1002/jmv.26386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Menter T., Tzankov A., Bruder E. Impact of SARS-CoV-2/COVID-19 on the placenta. Pathologe. 2021;42:591–597. doi: 10.1007/s00292-021-00952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bertero L., Borella F., Botta G., Carosso A., Cosma S., Bovetti M., Carosso M., Abbona G., Collemi G., Papotti M., Cassoni P., Benedetto C. Placenta histopathology in SARS-CoV-2 infection: analysis of a consecutive series and comparison with control cohorts. Virchows Arch. 2021;479:715–728. doi: 10.1007/s00428-021-03097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Flores-Pliego A., Miranda J., Vega-Torreblanca S., Valdespino-Vázquez Y., Helguera-Repetto C., Espejel-Nuñez A., Borboa-Olivares H., Espino Y., Sosa S., Mateu-Rogell P., León-Juárez M., Ramírez-Santes V., Cardona-Pérez A., Villegas-Mota I., Torres-Torres J., Juárez-Reyes Á., Rizo-Pica T., González R.O., González-Mariscal L., Estrada-Gutierrez G. Molecular insights into the thrombotic and microvascular injury in placental endothelium of women with mild or severe COVID-19. Cells. 2021;10:364. doi: 10.3390/cells10020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baergen R.N., Heller D.S. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr. Dev. Pathol. 2020;23:177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Di Girolamo R., Khalil A., Alameddine S., D'Angelo E., Galliani C., Matarrelli B., Buca D., Liberati M., Rizzo G., D'Antonio F. Placental histopathology after SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kirtsman M., Diambomba Y., Poutanen S.M., Malinowski A.K., Vlachodimitropoulou E., Parks W.T., Erdman L., Morris S.K., Shah P.S. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. 2020;192:E647 -EE50. doi: 10.1503/cmaj.200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beltrán-García J., Osca-Verdegal R., Pallardó F.V., Ferreres J., Rodríguez M., Mulet S., Sanchis-Gomar F., Carbonell N., García-Giménez J.L. Oxidative stress and inflammation in COVID-19-associated sepsis: the potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants (Basel) 2020;9:936. doi: 10.3390/antiox9100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mandò C., Savasi V.M., Anelli G.M., Corti S., Serati A., Lisso F., Tasca C., Novielli C., Cetin I. Mitochondrial and oxidative unbalance in placentas from mothers with SARS-CoV-2 infection. Antioxidants (Basel) 2021;10:1517. doi: 10.3390/antiox10101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meyer J.A., Roman A.S., Limaye M., Grossman T.B., Flaifel A., Vaz M.J., Thomas K.M., Penfield C.A. Association of SARS-CoV-2 placental histopathology findings with maternal-fetal comorbidities and severity of COVID-19 hypoxia. J. Matern. Fetal. Neonatal. Med. 2021:1–7. doi: 10.1080/14767058.2021.1977791. [DOI] [PubMed] [Google Scholar]
- 122.Mao Q., Chu S., Shapiro S., Young L., Russo M., De Paepe M.E. Placental SARS-CoV-2 distribution correlates with level of tissue oxygenation in COVID-19-associated necrotizing histiocytic intervillositis/perivillous fibrin deposition. Placenta. 2022;117:187–193. doi: 10.1016/j.placenta.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fleming P., Pereira S., Kapellou O., Claxton A., Bamford A., Aladangady N. Possible in utero transmission of SARS-CoV-2 and severe respiratory disease in a preterm infant. Pediatrics. 2022;150 doi: 10.1542/peds.2021-054557. [DOI] [PubMed] [Google Scholar]
- 124.Shanes E.D., Miller E.S., Otero S., Ebbott R., Aggarwal R., Willnow A.S., Ozer E.A., Mithal L.B., Goldstein J.A. Placental pathology after SARS-CoV-2 infection in the pre-variant of concern, Alpha/Gamma, Delta, or omicron eras. Int. J. Surg. Pathol. 2022 doi: 10.1177/10668969221102534. 10668969221102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Glynn S.M., Yang Y.J., Thomas C., Friedlander R.L., Cagino K.A., Matthews K.C., Riley L.E., Baergen R.N., Prabhu M. SARS-CoV-2 and placental pathology: malperfusion patterns are dependent on timing of infection during pregnancy. Am. J. Surg. Pathol. 2022;46:51–57. doi: 10.1097/PAS.0000000000001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brien M.E., Bouron-Dal Soglio D., Dal Soglio S., Couture C., Boucoiran I., Nasr Y., Widdows K., Sharps M.C., El Demellawy D., Ep Heazell A., Menzies D., Girard S. Pandemic stress and SARS-CoV-2 infection are associated with pathological changes at the maternal-fetal interface. Placenta. 2021;115:37–44. doi: 10.1016/j.placenta.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schwartz D.A., Dhaliwal A. Infections in pregnancy with Covid-19 and other respiratory RNA virus diseases are rarely, if ever, transmitted to the fetus: experiences with coronaviruses, HPIV, hMPV RSV, and influenza. Arch. Pathol. Lab. Med. 2020 doi: 10.5858/arpa.2020-0211-SA. [DOI] [PubMed] [Google Scholar]
- 128.Di Mascio D., Khalil A., Saccone G., Rizzo G., Buca D., Liberati M., Vecchiet J., Nappi L., Scambia G., Berghella V., D'Antonio F. Outcome of coronavirus spectrum infections (SARS, MERS, COVID 1–19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bouachba A., Allias F., Nadaud B., Massardier J., Mekki Y., Bouscambert Duchamp M., Fourniere B., Huissoud C., Trecourt A., Collardeau-Frachon S. Placental lesions and SARS-Cov-2 infection: diffuse placenta damage associated to poor fetal outcome. Placenta. 2021;112:97–104. doi: 10.1016/j.placenta.2021.07.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Garrido-Pontnou M., Navarro A., Camacho J., Crispi F., Alguacil-Guillén M., Moreno-Baró A., Hernandez-Losa J., Sesé M., Cajal S.R., Garcia Ruíz I., Serrano B., Garcia-Aguilar P., Suy A., Ferreres J.C., Nadal A. Diffuse trophoblast damage is the hallmark of SARS-CoV-2-associated fetal demise. Mod. Pathol. 2021;34:1704–1709. doi: 10.1038/s41379-021-00827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Valdespino-Vázquez M.Y., Helguera-Repetto C.A., León-Juárez M., Villavicencio-Carrisoza O., Flores-Pliego A., Moreno-Verduzco E.R., Díaz-Pérez D.L., Villegas-Mota I., Carrasco-Ramírez E., López-Martínez I.E., Giraldo-Gómez D.M., Lira R., Yocupicio-Monroy M., Rodríguez-Bosch M., Sevilla-Reyes E.E., Cortés-Bonilla M., Acevedo-Gallegos S., Merchant-Larios H., Cardona-Pérez J.A., Irles C. Fetal and placental infection with SARS-CoV-2 in early pregnancy. J. Med. Virol. 2021;93:4480–4487. doi: 10.1002/jmv.26965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Favre G., Mazzetti S., Gengler C., Bertelli C., Schneider J., Laubscher B., Capoccia R., Pakniyat F., Ben Jazia I., Eggel-Hort B., de Leval L., Pomar L., Greub G., Baud D., Giannoni E. Decreased fetal movements: a sign of placental SARS-CoV-2 infection with perinatal brain injury. Viruses. 2021;13:2517. doi: 10.3390/v13122517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stenton S., McPartland J., Shukla R., Turner K., Marton T., Hargitai B., Bamber A., Pryce J., Peres C.L., Burguess N., Wagner B., Ciolka B., Simmons W., Hurrell D., Sekar T., Moldovan C., Trayers C., Bryant V., Palm L., Cohen M.C. SARS-COV2 placentitis and pregnancy outcome: a multicentre experience during the alpha and early Delta waves of coronavirus pandemic in England. EClinicalMedicine. 2022;47 doi: 10.1016/j.eclinm.2022.101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cornejo M., Fuentes G., Valero P., Vega S., Grismaldo A., Toledo F., Pardo F., Moore-Carrasco R., Subiabre M., Casanello P., Faas M.M., van Goor H., Sobrevia L. Gestational diabesity and foetoplacental vascular dysfunction. Acta. Physiol. (Oxf). 2021;232 doi: 10.1111/apha.13671. [DOI] [PubMed] [Google Scholar]
- 135.World Health Organization. Coronavirus (COVID-19) dashboard https://covid19.who.int/ (Accessed July 19, 2022).
- 136.Valero P., Salas R., Pardo F., Cornejo M., Fuentes G., Vega S., Grismaldo A., Hillebrands J.L., van der Beek E.M., van Goor H., Sobrevia L. Glycaemia dynamics in gestational diabetes mellitus. Biochim. Biophys. Acta Gen. Subj. 2022;1866 doi: 10.1016/j.bbagen.2022.130134. [DOI] [PubMed] [Google Scholar]
- 137.Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C., O'Brien P., Quigley M., Brocklehurst P., Kurinczuk J.J. UK obstetric surveillance system SARS-CoV-2 infection in pregnancy collaborative group. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ashraf M.A., Keshavarz P., Hosseinpour P., Erfani A., Roshanshad A., Pourdast A., Nowrouzi-Sohrabi P., Chaichian S., Poordast T. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J. Reprod. Infertil. 2020;21:157–168. PMID: 32685412. [PMC free article] [PubMed] [Google Scholar]
- 140.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Forestieri S., Pintus R., Marcialis M.A., Pintus M.C., Fanos V. COVID-19 and developmental origins of health and disease. Early Hum. Dev. 2021;155 doi: 10.1016/j.earlhumdev.2021.105322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Patanè L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L., Mangili G., Arosio M., Cornolti G. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kyle M.H., Glassman M.E., Khan A., Fernández C.R., Hanft E., Emeruwa U.N., Scripps T., Walzer L., Liao G.V., Saslaw M., Rubenstein D., Hirsch D.S., Keown M.K., Stephens A., Mollicone I., Bence M.L., Gupta A., Sultan S., Sibblies C., Whittier S., Abreu W., Akita F., Penn A., Orange J.S., Saiman L., Welch M.G., Gyamfi-Bannerman C., Stockwell M.S., Dumitriu D. A review of newborn outcomes during the COVID-19 pandemic. Semin. Perinatol. 2020;44 doi: 10.1016/j.semperi.2020.151286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bellos I., Pandita A., Panza R. Maternal and perinatal outcomes in pregnant women infected by SARS-CoV-2: a meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021;256:194–204. doi: 10.1016/j.ejogrb.2020.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maxwell C., KFY Tai, Sermer M. MATERNAL FETAL MEDICINE COMMITTEE; INFECTIOUS DISEASE COMMITTEE. Management guidelines for obstetric patients and neonates born to mothers with suspected or probable severe acute respiratory syndrome (SARS). J. Obstet. Gynaecol. Can. 2009;31:358–364. doi: 10.1016/S1701-2163(16)34155-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sánchez-Luna M Fernández Colomer B., de Alba Romero C Neonates born to mothers with COVID-19: data from the spanish Society of Neonatology Registry. Pediatrics. 2021;147 doi: 10.1542/peds.2020-015065. [DOI] [PubMed] [Google Scholar]
- 148.Chen H.Y., Chauhan S.P. Apgar score at 10 minutes and adverse outcomes among low-risk pregnancies. J. Matern. Fetal Neonatal Med. 2021:1–10. doi: 10.1080/14767058.2021. [DOI] [PubMed] [Google Scholar]
- 149.Dumitriu D., Emeruwa U.N., Hanft E., Liao G.V., Ludwig E., Walzer L., Arditi B., Saslaw M., Andrikopoulou M., Scripps T., Baptiste C., Khan A., Breslin N., Rubenstein D., Simpson L.L., Kyle M.H., Friedman A.M., Hirsch D.S., Miller R.S., Fernández C.R., Fuchs K.M., Keown M.K., Glassman M.E., Stephens A., Gupta A., Sultan S., Sibblies C., Whittier S., Abreu W., Akita F., Penn A., D'Alton M.E., Orange J.S., Goffman D., Saiman L., Stockwell M.S., Gyamfi-Bannerman C. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York City. JAMA Pediatr. 2021;175:157–167. doi: 10.1001/jamapediatrics.2020.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., Seferovic M.D., Aski S.K., Arian S.E., Pooransari P., Ghotbizadeh F., Aalipour S., Soleimani Z., Naemi M., Molaei B., Ahangari R., Salehi M., Oskoei A.D., Pirozan P., Darkhaneh R.F., Laki M.G., Farani A.K., Atrak S., Miri M.M., Kouchek M., Shojaei S., Hadavand F., Keikha F., Hosseini M.S., Borna S., Ariana S., Shariat M., Fatemi A., Nouri B., Nekooghadam S.M., Aagaard K. Maternal death due to COVID-19. Am. J. Obstet. Gynecol. 2020;223:109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yan J., Guo J., Fan C., Juan J., Yu X., Li J., Feng L., Li C., Chen H., Qiao Y., Lei D., Wang C., Xiong G., Xiao F., He W., Pang Q., Hu X., Wang S., Chen D., Zhang Y., Poon L.C., Yang H. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am. J. Obstet. Gynecol. 2020;223:111.e1–111.e14. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.García H., Allende-López A., Morales-Ruíz P., Miranda-Novales G., Villasis-Keever M.Á. COVID-19 in neonates with positive RT-PCR test. Systematic review. Arch. Med. Res. 2022;53:252–262. doi: 10.1016/j.arcmed.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Raschetti R., Vivanti A.J., Vauloup-Fellous C., Loi B., Benachi A., De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat. Commun. 2020;11:5164. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Buonsenso D., Costa S., Sanguinetti M., Cattani P., Posteraro B., Marchetti S., Carducci B., Lanzone A., Tamburrini E., Vento G., Valentini P. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am. J. Perinatol. 2020;37:869–872. doi: 10.1055/s-0040-1710541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Farghaly M.A.A., Kupferman F., Castillo F., Kim R.M. Characteristics of newborns born to SARS-CoV-2-positive mothers: a retrospective cohort study. Am. J. Perinatol. 2020;37:1310–1316. doi: 10.1055/s-0040-1715862. [DOI] [PubMed] [Google Scholar]
- 156.Malhotra Y., Rossberg M.C., Bajaj K., Shtern A., Moore R.M. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID-19) pneumonia. Obstet. Gynecol. 2020;136:632–633. doi: 10.1097/AOG.0000000000004073. [DOI] [PubMed] [Google Scholar]
- 157.Mehta H., Ivanovic S., Cronin A., VanBrunt L., Mistry N., Miller R., Yodice P., Rezai F. Novel coronavirus-related acute respiratory distress syndrome in a patient with twin pregnancy: a case report. Case Rep. Womens Health. 2020;27 doi: 10.1016/j.crwh.2020.e00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pierce-Williams R.A.M., Burd J., Felder L., Khoury R., Bernstein P.S., Avila K., Penfield C.A., Roman A.S., DeBolt C.A., Stone J.L., Bianco A., Kern-Goldberger A.R., Hirshberg A., Srinivas S.K., Jayakumaran J.S., Brandt J.S., Anastasio H., Birsner M., O'Brien D.S., Sedev H.M., Dolin C.D., Schnettler W.T., Suhag A., Ahluwalia S., Navathe R.S., Khalifeh A., Anderson K., Berghella V. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sharma K.A., Kumari R., Kachhawa G., Chhabra A., Agarwal R., Sharma A., Kumar S., Bhatla N. Management of the first patient with confirmed COVID-19 in pregnancy in India: from guidelines to frontlines. Int. J. Gynaecol. Obstet. 2020;150:116–118. doi: 10.1002/ijgo.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., Benachi A., De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J., Feng L. A case report of neonatal 2019 coronavirus disease in China. Clin. Infect. Dis. 2020;71:853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X., Liu Y., Xiao J., Liu H., Deng D., Chen S., Zeng W., Feng L., Wu J. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-Centre, descriptive study. Lancet Infect. Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.De Rose D.U., Pugnaloni F., Calì M., Ronci S., Caoci S., Maddaloni C., Martini L., Santisi A., Dotta A., Auriti C. Multisystem inflammatory syndrome in neonates born to mothers with SARS-CoV-2 infection (MIS-N) and in neonates and infants younger than 6 months with acquired COVID-19 (MIS-C): a systematic review. Viruses. 2022;14:750. doi: 10.3390/v14040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shaiba L.A., More K., Hadid A., Almaghrabi R., Al Marri M., Alnamnakani M., Shah P. Multisystemic inflammatory syndrome in neonates: a systematic review. Neonatology. 2022;119:405–417. doi: 10.1159/000524202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.de Moraes F.M., de Souza J.W.P.S., Alves L.P., de Siqueira M.F.R., Dos Santos A.P.A., de Carvalho Berardo M.M., Granja M.G., de Castro-Faria-Neto H.C. SARS-CoV-2 infection and possible neonatal neurological outcomes: a literature review. Viruses. 2022;14:1037. doi: 10.3390/v14051037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Caparros-Gonzalez R.A., Pérez-Morente M.A., Hueso-Montoro C., Álvarez-Serrano M.A., de la Torre-Luque A. Congenital, intrapartum and postnatal maternal-fetal-neonatal SARS-CoV-2 infections: a narrative review. Nutrients. 2020;12:3570. doi: 10.3390/nu12113570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gold S., Clarfield L., Johnstone J., Diambomba Y., Shah P.S., Whittle W., Abbasi N., Arzola C., Ashraf R., Biringer A., Chitayat D., Czikk M., Forte M., Franklin T., Jacobson M., Keunen J., Kingdom J., Lapinsky S., MacKenzie J., Maxwell C., Preisman M., Ryan G., Selk A., Sermer M., Silversides C., Snelgrove J., Watts N., Young B., De Castro C., D'Souza R. Adapting obstetric and neonatal services during the COVID-19 pandemic: a scoping review. BMC Pregnancy Childbirth. 2022;22:119. doi: 10.1186/s12884-022-04409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Centeno-Tablante E., Medina-Rivera M., Finkelstein J.L., Rayco-Solon P., Garcia-Casal M.N., Rogers L., Ghezzi-Kopel K., Ridwan P., Peña-Rosas J.P., Mehta S. Transmission of SARS-CoV-2 through breast milk and breastfeeding: a living systematic review. Ann. N. Y. Acad. Sci. 2021;1484:32–54. doi: 10.1111/nyas.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bhatt H. Should COVID-19 mother breastfeed her newborn child? A literature review on the safety of breastfeeding for pregnant women with COVID-19. Curr. Nutr. Rep. 2021;10:71–75. doi: 10.1007/s13668-020-00343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Federation World Obesity. 2022. World obesity atlas.https://s3-eu-west-1.amazonaws.com/wof-files/World_Obesity_Atlas_2022.pdf Accessed July 19, 2022. [Google Scholar]
- 171.Sobrevia L. Glycaemia dynamics concepts before and after insulin. Biochem. Pharmacol. 2022;201 doi: 10.1016/j.bcp.2022.115092. [DOI] [PubMed] [Google Scholar]
- 172.Prasad S., Kalafat E., Blakeway H., Townsend R., O'Brien P., Morris E., Draycott T., Thangaratinam S., Le Doare K., Ladhani S., von Dadelszen P., Magee L.A., Heath P., Khalil A. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat. Commun. 2022;13:2414. doi: 10.1038/s41467-022-30052-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.