Abstract
Objectives
We sought to perform a network meta-analysis to compare the safety and efficacy of the systemic administration of corticosteroids for the treatment of COVID-19.
Methods
A Bayesian network meta-analysis was performed to combine the direct and indirect evidence. The surface under the cumulative ranking curve was obtained to estimate the ranking probability of the treatment agents for each outcome. The efficacy outcome was 28-day all-cause mortality. The safety outcome was serious adverse events.
Results
A total of 16 trials with 2992 patients comparing four treatments (dexamethasone, hydrocortisone, methylprednisolone, and placebo) were identified. Direct analysis showed that corticosteroids were associated with a reduced risk of 28-day mortality compared with usual care (risk ratio [RR] 0.83; 95% confidence interval [CrI] 0.70-0.99). Network analysis showed that the pooled RR was 0.63 (95% CrI 0.39-0.93) for all-cause mortality at 28 days comparing methylprednisolone with usual care or placebo (surface under the cumulative ranking curve: 91%). Our analysis demonstrated that patients who received a low dose of corticosteroids (RR 0.80; 95% CrI 0.70-0.91) and a long course of treatment (RR 0.81; 95% CrI 0.71-0.91) had higher survival rates than patients in the placebo group.
Conclusion
Administration of corticosteroids was associated with a reduced all-cause mortality at 28 days compared with placebo or usual care. Our analysis also confirmed the mortality benefit associated with low-dose and long-term treatment with corticosteroids.
Keywords: Corticosteroids, Mortality, COVID-19, Dexamethasone, Indirect comparisons, SARS-CoV-2
1. Introduction
As of August 5, 2022, nearly 600 million persons have been diagnosed with COVID-19, and more than 6 million individuals have died because of this disease (World Health Organization, 2022). Evidence has shown that a severely dysregulated immune response plays a critical role in patients with COVID-19 (Prete et al., 2020; Vabret et al., 2020).
Corticosteroids are nonspecific immunosuppressants and have been proposed as a potential treatment agent for acute respiratory distress syndrome. Corticosteroid treatment may reduce pulmonary and systemic injury in this group of patients by improving tissue damage caused by excessive generation of inflammatory mediators (Steinberg et al., 2006; Tomashefski, 2000; Villar et al., 2020). However, uncertainty exists as to whether other types of corticosteroids, such as methylprednisolone or hydrocortisone, differ from dexamethasone in efficacy for treating patients with COVID-19 (El Mezzeoui et al., 2021).
A recently published meta-analysis pooled results from randomized controlled trials (RCTs) in patients with COVID-19. This study showed that the use of systemic corticosteroids reduced all-cause mortality at 28 days (WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020). These results provide a strong recommendation for corticosteroids in critically ill patients with COVID-19. However, the conclusions were based mostly on the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, the largest trial included in the analysis, and the only trial that showed a significant association between corticosteroids and mortality (RECOVERY Collaborative Group et al., 2021). Without this largest trial, the result of the study turned out to be insignificant. Therefore, there is still an urgent need to evaluate the effectiveness of corticosteroids in this group of patients. Ongoing questions mainly related to the type of corticosteroids, optimal dosage, and duration (Confalonieri et al., 2021; Du Plessis et al., 2021; Zhang et al., 2021).
Hence, we conducted a network meta-analysis of RCTs to investigate the efficacy and safety of three different types of corticosteroids (i.e., dexamethasone, hydrocortisone, and methylprednisolone), as well as different doses of corticosteroids (i.e., high and low doses), in patients with COVID-19. This study aimed to provide robust evidence for the clinical application of these corticosteroids.
2. Materials and methods
2.1. Protocol and guidance
This study was registered in the international prospective register of systematic reviews database (CRD42022325173) and the Open Science Framework platform (https://osf.io/7s8md). The methods and reporting of the systematic review with network meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses Extension Statement for Network Meta-analyses (Hutton et al., 2015).
2.2. Search strategy and data sources
We searched the Ovid MEDLINE, Ovid Embase, https://www.clinicaltrials.gov/, and Cochrane Central Register of Controlled Trials from inception to August 10, 2021. There were no language restrictions for the search. The search was updated on July 15, 2022. We also manually searched the reference lists of the included articles and previously published systematic reviews on this topic to identify any additional eligible studies. The search strategy was designed and performed by an experienced researcher (Supplemental Table 1).
2.3. Selection criteria
The eligibility of studies was determined based on the participants, interventions, comparators, outcomes, and study design criteria, as follows: (i) population: patients with COVID-19; (ii) intervention: any type of corticosteroid agent including dexamethasone, hydrocortisone, and methylprednisolone; a predefined cut-off was used to determine whether the study used low or high doses of corticosteroids, that is 15 mg/d dexamethasone, 400 mg/d hydrocortisone, and 1 mg/kg/d methylprednisolone (Annane et al., 2017); (iii) comparison: placebo, usual care, or a different type of corticosteroid; (iv) outcome: efficacy outcome was all-cause mortality at 28 days (if mortality at this time point was not reported, we assessed the time point nearest 28 days), and the safety outcome was serious adverse events (opportunistic infections, muscle weakness, gastrointestinal bleeding, and hyperglycemia); and (v) study design: RCTs.
In addition, we excluded studies with observational design, nonrandomized trials, single-arm trials, and trials that compared corticosteroids with other active substances.
2.4. Selection process
Based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses Extension Statement for Network Meta-analyses guidelines, we excluded duplicate publications, and then, we screened the titles and abstracts to assess eligibility. Then, we excluded studies based on the participants, interventions, comparators, outcomes, and study design criteria after screening the full texts of the articles.
Two authors reviewed the publications and completed this process together. Disagreements were resolved by consulting an independent author.
2.5. Data extraction
Data related to the following categories were extracted onto a standardized form: (i) study characteristics: primary author, geographical location, publication year, and the number of centers in each study; (ii) treatment characteristics: type, dosage, duration of treatment, and administration of corticosteroids; and (iii) patient characteristics: age and sex. We classified the included trials according to dosage and duration of therapy, and the cut-offs we used were based on the previous studies in the same field (Chaudhuri et al., 2021; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020).
Two authors independently extracted data and completed this process. Disagreements were resolved by consulting an independent author.
2.6. Quality of evidence and assessment of risk of bias
The Cochrane Collaboration Risk of Bias tool was used to evaluate the risk of bias for all RCTs across seven domains: random sequence generation; incomplete outcome data, blinding of study participants, allocation concealment, selective reporting, blinding of outcome assessment, and other potential bias (Higgins et al., 2011). Each domain was assessed as having either a low, unclear, or high risk of bias. We contacted the original study investigators for more information if necessary.
The Grading of Recommendations Assessment, Development, and Evaluation framework was used to assess the quality of evidence for each outcome estimate to rank the evidence quality (Guyatt et al., 2011). Our confidence assessment addressed publication bias, indirectness, limitations in design, inconsistency, and imprecision.
2.7. Statistical analysis
To incorporate direct and indirect comparisons, we performed Bayesian network meta-analyses with a consistency model in the R environment. The comparative safety and efficacy of any two treatment regimens were modeled for each treatment agent relative to the reference treatment agent. We conducted random effects and fixed effects models to pool the network results and selected the preferred model by comparing the deviance information criteria (McGavock et al., 2020; Spiegelhalter et al., 2002). The models were based on 30,000 iterations after a burn-in of 10,000 iterations. The risk ratio (RR) and the corresponding 95% credible interval (CrI) were obtained from the 97.5th and 2.5th percentiles of the posterior distribution. We also reported the RR and relevant 95% confidence interval (CI) from direct comparisons. The surface under the cumulative ranking curve (SUCRA) was obtained to estimate the ranking probability of the treatment agents for each outcome (Salanti et al., 2011). The SUCRA values range from 0-100% and summarize treatment rankings. A larger area under the curve means a higher ranking of therapy effectiveness. The heterogeneity of treatment effects among the included studies was examined using the I2 statistic. An I2 value of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively. We also performed sensitivity analysis by excluding trials that were assessed as having a high risk of bias.
All analyses were performed in R software (release version 4.0.3, gemtc package) and RevMan (5.4.0; The Cochrane Collaboration). A two-sided P-value of <0.05 was considered to indicate statistical significance.
3. Results
3.1. Eligible studies and study characteristics
Our search generated 4178 publications. Finally, 16 trials were deemed eligible and included in the network meta-analysis (Angus et al., 2020; Corral-Gudino et al., 2021; Dastenae et al., 2022; Dequin et al., 2020; Edalatifard et al., 2020; Jamaati et al., 2021; Jeronimo et al., 2021; Munch et al., 2021; Ranjbar et al., 2021; RECOVERY Collaborative Group et al., 2021; Salvarani et al., 2022; Soliman et al., 2022; Tang et al., 2021; Tomazini et al., 2020; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020). The publication screening process and a list of excluded studies with reasons for exclusion are summarized in Figure 1 .
Figure 1.
Summary of study selection process.
RCT: randomized clinical trial.
Table 1 summarizes the characteristics of each trial. We included seven RCTs from a previously published meta-analysis addressing the topic. The updated search generated nine additional RCTs. Finally, a total of 16 RCTs met the eligibility criteria and were included in the present analysis, which involved a total of 2992 patients. There was one study each conducted in the United Kingdom, Denmark, Italy, Egypt, and France. Two studies were conducted in Brazil, two were conducted in China, four were conducted in Iran, and two were conducted in Spain. One trial was conducted in multiple countries. Population sizes ranged from 19 to 1007 patients, and the mean age was 62 years. In most included studies, the majority of patients were male. A total of four trials compared dexamethasone with placebo or usual care, three trials compared hydrocortisone with control, six trials compared methylprednisolone with placebo or usual care, and three trials compared dexamethasone with methylprednisolone. Eight RCTs provided usual care to their control group, whereas five trials administered a placebo.
Table 1.
Characteristics of studies included in the systematic review and meta-analysis.
| Study | Registration | No. of patients | Country | Mean age (% male)a | Intervention | Dosage and administration | Dose classification | Control | Mortality outcomes |
|---|---|---|---|---|---|---|---|---|---|
| DEXA-COVID 19 (WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020)c | NCT04325061 | 19 | Spain | 62 (57.1) | Dexamethasone | 20 mg/d for 5 days and then 10 mg/d for 5 days | High | Usual care | 28-day |
| CoDEX (Tomazini et al., 2020) | NCT04327401 | 299 | Brazil | 62.7 (65.6) | Dexamethasone | 20 mg/d for 5 days and then 10 mg/d for 5 days | High | Usual care | 28-day |
| RECOVERY (RECOVERY Collaborative Group et al., 2021) | NCT04381936 | 1007 | UK | 65.8 (64) | Dexamethasone | 6 mg/d for up to 10 days | Low | Usual care | 28-day |
| Jamaati (2021) (Jamaati et al., 2021) | IRCT20151227025726N17 | 50 | Iran | 62 (72) | Dexamethasone | 20 mg/d for 5 days and then 10 mg/d for 5 days | High | Usual care | 28-day |
| CAPE COVID (Dequin et al., 2020) | NCT02517489 | 149 | France | 66.3 (68.5) | Hydrocortisone | 200 mg/d for 4 d or 7 d, then 100 mg/d for 2 days or 4 days and 50 mg/d for 2 days or 3 days | Low | Placebo | 21-day |
| COVID STEROID (Munch et al., 2021)c | NCT04348305 | 29 | Denmark | Not applicable | Hydrocortisone | 200 mg/d for 7 days | Low | Placebo | 28-day |
| REMAP-CAP (Angus et al., 2020) | NCT02735707 | 197 | Multiple countries b | 59.9 (71.3) | Hydrocortisone | 50 mg every 6 h for 7 days | Low | Usual care | 28-day |
| Steroids-SARI (WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020)c | NCT04244591 | 47 | China | 62 (78) | Methylprednisolone | 40 mg twice daily for 5 days | High | Usual care | 30-day |
| Ranjbar (2021) (Ranjbar et al., 2021) | IRCT20200204046369N1 | 86 | Iran | 61.3 (52.4) | Methylprednisolone | 2 mg/kg per day for 10 days | High | Dexamethasone (6 mg per day for 10 days); Low | 28-day |
| Jeronimo (2021) (Jeronimo et al., 2021) | NCT04343729 | 393 | Brazil | 57 (64.3) | Methylprednisolone | 0.5 mg/kg twice daily for 5 days | Low | Placebo | 28-day |
| Tang (2021) (Tang et al., 2021) | NCT04273321 | 86 | China | 55 (46.5) | Methylprednisolone | 1 mg/kg per day for 7 days | Low | Placebo | In-hospital |
| GLUCOCOVID (Corral-Gudino et al., 2021) | 2020-001934-37 | 64 | Spain | 66 (55) | Methylprednisolone | 40 mg twice daily for 3 days, followed by 20 mg twice daily for another 3 days | High | Usual care | 28-day |
| Edalatifard 2020 (Edalatifard et al., 2020) | IRCT20200404046947N1 | 62 | Iran | 61.7 (53.6) | Methylprednisolone | 250 mg per day for 3 days | High | Usual care | In-hospital |
| Salvarani (2022) (Salvarani et al., 2022) | NCT04673162 | 301 | Italy | 64.0 (70.7) | Methylprednisolone | 1 g per day for 3 days | High | Placebo | 28-day |
| Soliman (2022) (Soliman et al., 2022) | NCT04909918 | 60 | Egypt | 58.1 (46.7) | Methylprednisolone | 1 mg/kg/ per day for 7 days | Low | Dexamethasone (8 mg per day for 7 days); Low | 7-day |
| Dastenae 2022 (Dastenae et al., 2022) | IRCT20210223050466N1 | 143 | Iran | 64.5 (58.6) | Methylprednisolone | 60 mg/ per day for 7 days | Low | Dexamethasone (8 mg per day for 10 days); Low | 28-day |
This data was extracted from control group.
Australia, Canada, European Union, New Zealand, UK, US.
Age was presented in median.
Of these studies, eight studies were rated as having an overall “low risk of bias” (Angus et al., 2020; Dequin et al., 2020; Jeronimo et al., 2021; Munch et al., 2021; Ranjbar et al., 2021; RECOVERY Collaborative Group et al., 2021; Salvarani et al., 2022; Tomazini et al., 2020). The remaining studies were assessed as having an overall “high risk of bias” (Corral-Gudino et al., 2021; Dastenae et al., 2022; Edalatifard et al., 2020; Jamaati et al., 2021; Soliman et al., 2022; Tang et al., 2021; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020). The high risk of bias was mainly due to the lack of blinding of outcome assessment. The risk of bias is shown in Figure S1 and Figure S2 in the Supporting Information.
3.2. 28-day all-cause mortality
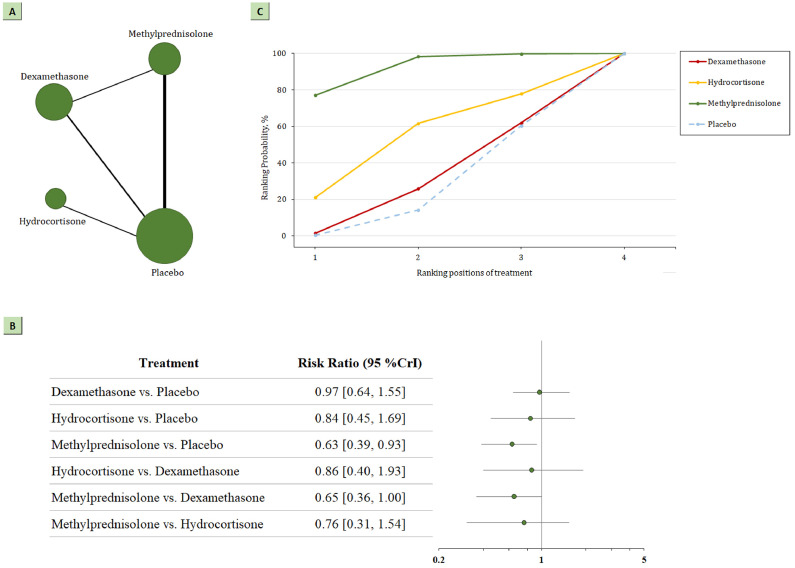
The network plot for head-to-head comparisons between the different management strategies for 28-day all-cause mortality is presented in Figure 2 a. There were 410 deaths among 1457 patients randomized to receive corticosteroids, and 566 deaths among 1498 patients randomized to receive placebo or standard care. This corresponds to an absolute mortality risk of 28% for corticosteroids compared with an absolute risk of 38% for placebo or standard care. According to the direct analysis, corticosteroids showed better efficacy in reducing all-cause mortality than placebo or standard care (RR 0.83; 95% CI 0.70-0.99; Figure A3). Network analysis (Figure 2b; Table A2) showed that the pooled RR was 0.63 (95% CrI 0.39-0.93) for all-cause mortality at 28 days comparing methylprednisolone with usual care or placebo (nine trials, 613 participants). The analysis also demonstrated that methylprednisolone showed better efficacy in reducing 28-day mortality than dexamethasone (RR 0.65; 95% CrI 0.36-1.00; RR 0.53; 95% CI 0.33-0.84; Figure A4). However, the summary RR did not indicate statistically significant differences between dexamethasone and usual care or placebo (seven trials, 647 participants; RR 0.97; 95% CrI 0.64-1.55) or between hydrocortisone and usual care or placebo (three trials, 197 participants; RR 0.84; 95% CrI 0.45-1.69) for patients with COVID-19.
Figure 2.
(a) Network plot of all-cause mortality. The width of the lines is proportional to the number of studies comparing every pair of treatments, and the size of each circle is proportional to the number of participants. (b) SUCRA-based ranking probabilities graph of each medication. The SUCRA values for each treatment were as follows: 92% for methylprednisolone; 53% for hydrocortisone; 30% for dexamethasone. (c) The forest plot shows the risk ratio and CrI.
CrI, credible interval; SUCRA, surface under the cumulative ranking curve.
For the outcome of mortality, methylprednisolone had the highest probability of being the best management strategy in patients with COVID-19, with a SUCRA value of 0.92; this result was statistically significant. The second-best strategy for mortality was hydrocortisone (SUCRA 0.53). The least beneficial intervention was dexamethasone (SUCRA 0.30). SUCRA values for mortality are shown in Figure 2c. SUCRA values in the sensitivity analysis remained consistent after excluding specified studies (Supplementary Table 3).
3.3. Serious adverse events
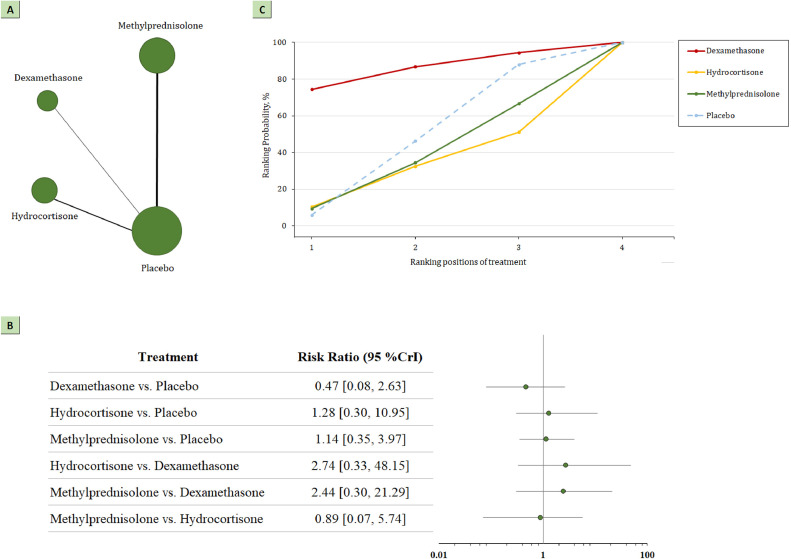
The network plot for head-to-head comparisons between the different management strategies for serious adverse events is presented in Figure 3 a. The associations between corticosteroids vs placebo or standard care and serious adverse events are presented in Figure 3b and Figure A5. A total of 10 trials, including 1216 participants reported serious adverse events. Among them, 109 events occurred among 626 patients randomized to the treatment group, and 102 events occurred among 590 patients randomized to placebo or standard care (17% vs 17%). The serious events reported by each trial are summarized in Table 2 . The summary RR did not show statistically significant differences in any of the comparisons.
Figure 3.
(a) Network plot of serious adverse events. The width of the lines is proportional to the number of studies comparing every pair of treatments, and the size of each circle is proportional to the number of participants. (b) SUCRA-based ranking probabilities graph of each medication. The SUCRA values for each treatment were as follows: 86% for dexamethasone; 35% for methylprednisolone; 30% for hydrocortisone. (c) The forest plot shows the risk ratio and CrI.
CrI, credible interval; SUCRA, surface under the cumulative ranking curve.
Table 2.
Serious adverse events in intervention group.
| Trial | Intervention | Dose classification | Treatment-related adverse events |
|---|---|---|---|
| DEXA-COVID 19 (WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020) | Dexamethasone | High | Secondary pneumonia, sepsis, pulmonary embolism |
| CoDEX (Tomazini et al., 2020) | Dexamethasone | High | Acute myocardial infarction, deep vein thrombosis, gastrointestinal perforation, unspecified hyperglycemia, and pneumothorax |
| RECOVERY (RECOVERY Collaborative Group et al., 2021) | Dexamethasone | Low | NA |
| Jamaati (2021) (Jamaati et al., 2021) | Dexamethasone | High | NA |
| CAPE COVID (Dequin et al., 2020) | Hydrocortisone | Low | Cerebral vasculitis, pulmonary embolism |
| COVID STEROID (Munch et al., 2021) | Hydrocortisone | Low | Septic shock, invasive fungal infection, clinically important gastrointestinal bleeding, or anaphylactic reaction |
| REMAP-CAP (Angus et al., 2020) | Hydrocortisone | Low | Severe neuromyopathy, fungemia |
| Steroids-SARI (WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020) | Methylprednisolone | High | Secondary bacterial infections, barotrauma, severe hyperglycemia, gastrointestinal bleeding requiring transfusion, acquired weakness |
| Ranjbar (2021) (Ranjbar et al., 2021) | Methylprednisolone | High | NA |
| Jeronimo (2021) (Jeronimo et al., 2021) | Methylprednisolone | Low | NA |
| Tang (2021) (Tang et al., 2021) | Methylprednisolone | Low | Hyperglycemia, secondary pneumonia |
| GLUCOCOVID 2020 (Corral-Gudino et al., 2021) | Methylprednisolone | High | Hyperglycemia, nosocomial infection |
| Edalatifard 2020 (Edalatifard et al., 2020) | Methylprednisolone | High | Infection, edema |
| Salvarani (2022) (Salvarani et al., 2022) | Methylprednisolone | High | Cardiac disorders, gastrointestinal disorders, infections and infestations, respiratory, thoracic and mediastinal disorders, surgical and medical procedures, vascular disorders |
| Soliman (2022) (Soliman et al., 2022) | Methylprednisolone | Low | NA |
| Dastenae 2022 (Dastenae et al., 2022) | Methylprednisolone | Low | NA |
NA: not applicable.
SUCRA values for serious adverse events are shown in Figure 3c. For prespecified safety outcomes, SUCRA values ranked dexamethasone (RR 0.47; 95% CrI 0.08-2.63; SUCRA 0.84) as the most beneficial intervention for the prevention of serious adverse events. Methylprednisolone (RR 1.14; 95% CrI 0.35-3.97; SUCRA 0.37) and hydrocortisone (RR 1.28; 95% CrI 0.30-10.95; SUCRA 0.32) were ranked as the second and third most beneficial interventions for this outcome.
3.4. Dosage and duration of therapy
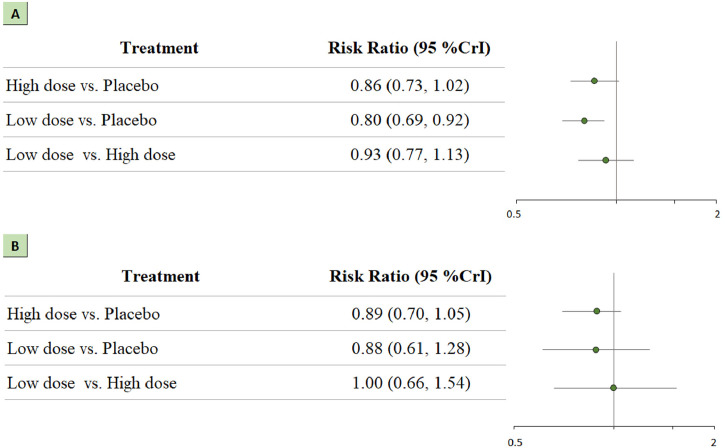
A total of 1862 patients were administered low-dose treatment, and 807 patients were administered high-dose treatment. Patients who received a low dose of corticosteroids had higher rates of survival than those who received a placebo (RR 0.80; 95% CrI 0.70-0.92). However, we did not observe the same favorable effect of a high dose of corticosteroids with respect to 28-day mortality (RR 0.87; 95% CrI 0.73-1.02). The summary RR did not show significant differences regarding serious adverse events. These results are shown in Figure 4 a and 4b.
Figure 4.
Network analysis for high-dose vs low-dose of corticosteroids. (a) The forest plot for all-cause mortality. (b) The forest plot for serious adverse events.
CrI: credible interval.
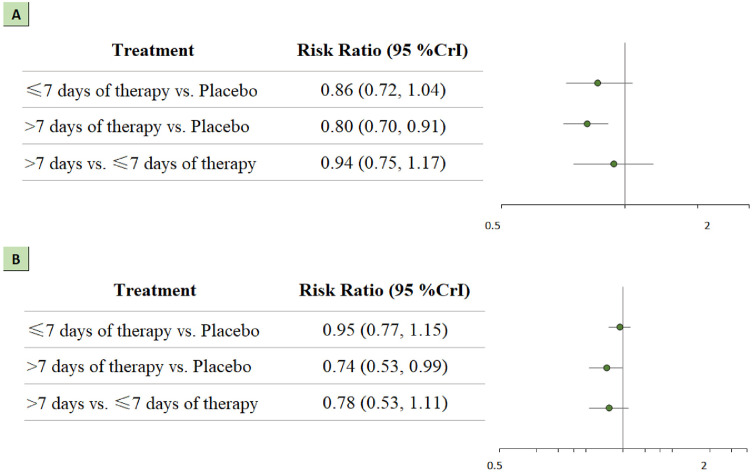
A total of 1145 patients were administered a short course of treatment (≤7 days), and 1524 patients were administered a long course of treatment (>7 days). Patients in the long course of treatment group had higher rates of survival than those in the placebo group (RR 0.80; 95% CrI 0.70-0.91). This treatment regimen also showed a significant association with serious adverse events (RR 0.74; 95% CrI 0.53-0.99). These results are presented in Figure 5 a and 5b.
Figure 5.
Network analysis for long course treatment (>7 days) vs short course treatment (≤7 days). (a) The forest plot for all-cause mortality. (b) The forest plot for serious adverse events. CrI: credible interval.
4. Discussion
In this Bayesian network meta-analysis of 16 randomized clinical trials with 2992 patients with COVID-19, we aimed to explore the optimal treatment agent for this group of patients. Compared with usual care or placebo, administration of methylprednisolone was associated with a lower 28-day all-cause mortality. The quality of evidence for these findings was rated as “moderate” due to inconsistency. In addition, our findings suggested that patients might benefit more from low-dose corticosteroids and a long course of treatment.
4.1. Comparison with other studies
Previous direct meta-analyses also explored the association between the administration of corticosteroids and all-cause mortality at 28 days in patients with COVID-19. In 2020, WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al. (2020) concluded that the use of systemic corticosteroids was associated with lower 28-day mortality (odds ratio [OR] 0.66; 95% CI 0.53-0.82). They conducted a subgroup analysis for different drugs and found that only dexamethasone was associated with decreased all-cause mortality (OR 0.64; 95% CI 0.50-0.82). In 2021, Chaudhuri et al. (2021) included 18 RCTs with 2826 patients with COVID-19 or non-COVID-19 acute respiratory distress syndrome (ARDS). They came to a similar conclusion that patients with ARDS, either derived from COVID-19 or not, could benefit from the use of corticosteroids because the drug probably reduces mortality (RR 0.82; 95% CI 0.72-0.95). However, only patients with non-COVID-19-related ARDS showed significant results (RR 0.71; 95% CI 0.54-0.92). Moreover, this study included patients with both COVID-19-related and non-COVID-19-related ARDS, leading to potential clinical heterogeneity. Similar conclusions were drawn by Li et al. (2021) in their study. They included both RCTs and observational studies, thereby extending the sample size on the one hand but also downgrading the quality of evidence due to selection bias.
Most of the previous studies were designed as direct meta-analyses, which provided only partial information in this case and therefore did not optimally inform decision making on the comparative effectiveness of different treatment agents. The present study used network analysis, which can help evaluate the comparative effectiveness of various treatment regimens. This method is useful for improving the precision of the outcome estimate and allows the estimation of the comparative effectiveness of different types of corticosteroids.
4.2. Study implications
Although the guidelines of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine recommend applying corticosteroids in patients with moderate to severe ARDS within 14 days after disease onset, the evidence of the administration of corticosteroids in patients with COVID-19 is heavily complex and paradoxical (Annane et al., 2017). The RECOVERY trial provided evidence in favor of systemic corticosteroid use, where there was a significantly lower risk of mortality with the administration of dexamethasone than usual care (RR 0.83; 95% CI 0.75-0.93) in critically ill patients with COVID-19 (RECOVERY Collaborative Group et al., 2021). Nevertheless, it remains unclear which types, doses, and courses of corticosteroid treatment are more effective. In this study, we assessed the differences between high doses and low doses of corticosteroids in terms of all-cause mortality and serious adverse events (Annane et al., 2017), and the pooled results indicated that low doses of corticosteroids were beneficial for 28-day mortality. In addition, the RECOVERY trial confirmed the mortality benefit associated with low-dose dexamethasone treatment (6 mg per day orally or intravenously for up to 10 days). To obtain a robust conclusion, more RCTs should be included.
The timing of glucocorticoid treatment is another issue that should be considered. For example, Tsai et al. (2020) conducted a multicenter, retrospective cohort study to assess the effectiveness of corticosteroids in patients presenting with influenzaassociated ARDS in Taiwan. This study included 241 patients overall and found that patients who received corticosteroids early had a significantly higher in-hospital mortality rate than those who did not (43.5% vs 19.2%; P <0.001). The study also revealed that early corticosteroid treatment was an independent factor associated with an increased overall rate of inhospital mortality (adjusted OR 5.02; 95% CI 2.39-10.54; P <0.001). In addition, according to their findings, earlier treatment was related to a significantly increased OR of subsequent bacteremia (adjusted OR 2.37; 95% CI 1.01-5.56). An ongoing trial (NCT04530409) may provide straight views on early versus late administration of corticosteroid treatment on mortality in patients with COVID-19.
4.3. Strength and limitations
Given the limited comparative effectiveness of different types of corticosteroids in patients with COVID-19, a Bayesian network meta-analysis was established. To determine the best approach benefiting the patients most, we used 28-day all-cause mortality to evaluate the efficacy and serious adverse events to evaluate the safety. In addition, our method included explicit eligibility criteria and a comprehensive search strategy. Thus, our analysis is strong and extends and integrates the recent guidelines in a novel way.
Although SUCRA scores and ranking scales provide a convenient approach to compare the effects of different outcomes in network meta-analysis, caution is necessary when interpreting the SUCRA values (Mbuagbaw et al., 2017). The values should not be interpreted in isolation because they do not capture the extent of differences in outcomes among different treatment regimens; the value needs to be interpreted in combination with the certainty of evidence. Furthermore, SUCRA values may differ for one intervention across outcomes. Although an intervention may be ranked higher for its significant effectiveness, it might be ranked down for safety concerns. Therefore, treatment rankings should be interpreted with other outputs from a network analysis that display the magnitude of effect sizes. Clinicians need to consider these factors before interpreting the SUCRA and adapting any intervention in their practice.
The last consideration is the limitations of the study. First, the definitions and reporting of serious adverse events varied across different trials. These serious adverse events mainly focused on secondary infections and sepsis. Second, one trial reported mortality at 30 days after randomization, two trials reported in-hospital mortality in-hospital, and one trial reported mortality at 21 days, potentially leading to inconsistency. Third, the optimal dose of each corticosteroid agent could not be determined due to the limited the number of eligible studies. Therefore, we compared the differences between high-dose treatment and low-dose treatment, and the results showed that a low dose of corticosteroids was more favorable. Regarding the duration of therapy, we did not detect any difference between long-course treatment and short-course treatment because both treatment strategies showed better efficacy than placebo. Further studies should conduct direct comparisons to validate the current findings. Fourth, nearly all the studies reported mortality at 28 days; however, it is also important to report on longer-term mortality. Future research should pay attention to this problem.
5. Conclusion
Among patients with COVID-19, administration of corticosteroids was associated with a reduced 28-day mortality compared with usual care. The analysis suggested a potential superiority of methylprednisolone over dexamethasone. However, the level of evidence regarding this comparison was downgraded due to imprecision and indirectness. Large trials with an adequate number of patients are necessary to validate this finding. Moreover, our analysis confirmed the mortality benefit associated with a low dose and a long course of treatment with corticosteroids.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
The study was supported by the National Natural Science Foundation of China (82201450). The funders had no role in the study design, data analysis, and manuscript writing.
Ethical approval
Not required.
Acknowledgments
None.
Author contributions
XW, LM, and CY designed the meta-analysis; XW and DW searched for relevant studies; JY, QH and CT selected the studies and extracted the relevant information; XW, CT, and LM synthesized the data; and XW wrote the first draft of the paper. All authors revised the manuscript and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.10.021.
Appendix. Supplementary materials
References
- Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2017;43:2078–2088. doi: 10.1097/CCM.0000000000002737. [DOI] [PubMed] [Google Scholar]
- Chaudhuri D, Sasaki K, Karkar A, Sharif S, Lewis K, Mammen MJ, et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47:521–537. doi: 10.1007/s00134-021-06394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confalonieri M, Salton F, Confalonieri P, Rochwerg B, Meduri GU. Caution when comparing the impact of corticosteroids in COVID-19. Chest. 2021;160:e243–e244. doi: 10.1016/j.chest.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, Gómez-Barquero J, Abadía-Otero J, García-Ibarbia C, et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia: an open-label randomized trial (GLUCOCOVID) Wien Klin Wochenschr. 2021;133:303–311. doi: 10.1007/s00508-020-01805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastenae ZH, Bahadori A, Dehghani M, Asadi-Samani M, Izadi I, Shahraki HR. Comparison of the effect of intravenous dexamethasone and methylprednisolone on the treatment of hospitalized patients with COVID-19: a randomized clinical trial. Int J Infect Dis. 2022;122:659–664. doi: 10.1016/j.ijid.2022.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: A randomized clinical trial. JAMA. 2020;324:1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Plessis EM, Lalla U, Allwood BW, Louw EH, Nortje A, Parker A, et al. Corticosteroids in critical COVID-19: are all corticosteroids equal? S Afr Med J. 2021;111:550–553. [PubMed] [Google Scholar]
- Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56 doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mezzeoui S, El Aidouni G, Merbouh M, El Kaouini A, Aftiss FZ, Berrichi S, et al. Dexamethasone or methylprednisolone therapy in covid-19 pneumonia: a retrospective and comparative study of 513 cases. Ann Med Surg (Lond) 2021;70 doi: 10.1016/j.amsu.2021.102858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897 doi: 10.1016/j.ejphar.2021.173947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): a randomized, double-blind, Phase IIb, placebo-controlled trial. Clin Infect Dis. 2021;72:e373–e381. doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liao X, Zhou Y, Wang L, Yang H, Zhang W, et al. Comparison of associations between glucocorticoids treatment and mortality in COVID-19 patients and SARS patients: a systematic review and meta-analysis. Shock. 2021;56:215–228. doi: 10.1097/SHK.0000000000001738. [DOI] [PubMed] [Google Scholar]
- Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. 2017;6:79. doi: 10.1186/s13643-017-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavock J, Chauhan BF, Rabbani R, Dias S, Klaprat N, Boissoneault S, et al. Layperson-led vs professional-led behavioral interventions for weight loss in pediatric obesity: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch MW, Meyhoff TS, Helleberg M, Kjaer MN, Granholm A, Hjortsø CJS, et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: the COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiol Scand. 2021;65:1421–1430. doi: 10.1111/aas.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prete M, Favoino E, Catacchio G, Racanelli V, Perosa F. SARS-CoV-2 inflammatory syndrome. Clinical features and rationale for immunological treatment. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21093377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021;21:337. doi: 10.1186/s12879-021-06045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Salvarani C, Massari M, Costantini M, Franco Merlo D, Lucia Mariani G, Viale P, et al. Intravenous methylprednisolone pulses in hospitalised patients with severe COVID-19 pneumonia, a double-blind, randomised, placebo-controlled trial. Eur Respir J. 2022 doi: 10.1183/13993003.00025-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman OM, Moeen SM, Abbas YA, Kamel EZ. The impact of dexamethasone versus methylprednisolone upon neutrophil/lymphocyte ratio in COVID-19 patients admitted to ICU and its implication upon mortality. Egypt J Anaesth. 2022;38:78–84. [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde AVD. Bayesian measures of model complexity and fit. J Royal Statistical Soc B. 2002;64:583–639. [Google Scholar]
- Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- Tang X, Feng YM, Ni JX, Zhang JY, Liu LM, Hu K, et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration. 2021;100:116–126. doi: 10.1159/000512063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomashefski JF., Jr Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–466. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, Yang KY, Chan MC, Kao KC, Wang HC, Perng WC, et al. Impact of corticosteroid treatment on clinical outcomes of influenza-associated ARDS: a nationwide multicenter study. Ann Intensive Care. 2020;10:26. doi: 10.1186/s13613-020-0642-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Jüni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Møller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Coronavirus disease (COVID-19) dashboard. https://www.who.int/emergencies/diseases/novel-coronavirus-2019, 2022 (accessed 7 August 2022).
- Zhang M, Bai X, Cao W, Ji J, Wang L, Yang Y, et al. The influence of corticosteroids, immunosuppressants and biologics on patients with inflammatory bowel diseases, psoriasis and rheumatic diseases in the era of COVID-19: a review of current evidence. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.677957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.