Abstract
Background
In recent years, the resurgence of pertussis has posed a public health challenge in many countries. This study aimed to evaluate the immunity levels against pertussis among populations of different ages in China.
Methods
We conducted a cross-sectional serological survey in Zhejiang Province, China in 2020. Serum IgG antibodies against pertussis toxin (anti-PT), filamentous hemagglutinin (anti-FHA), and pertactin (anti-PRN) were quantitatively measured. The geometric mean concentration (GMC) of three antibodies was calculated. An anti-PT level < 5 IU/mL was considered undetectable, ≥20 IU/mL as seropositive and ≥80 IU/mL as an indicator of recent infection. Mathematical models were fitted for anti-PT concentrations over time in children after four doses of the pertussis vaccination.
Results
A total of 4459 participants aged 0–59 years were included in the analyses. The overall positivity rate of anti-PT was 29.80% with the highest (81.44%) rate in the 1–2 years old and the lowest (4.72%) in 10–14 years old. The GMCs of anti-PT, anti-FHA and anti-PRN for the whole participants were 9.67 (95%CI: 9.25–10.10),18.93 (18.24–19.67), and 8.99 (8.61–9.38) IU/mL, respectively. Over 50% of subjects aged ≥ 7 years had undetectable anti-PT IgG antibodies (<5IU/mL). The proportions of the populations with anti-PT IgG ≥ 80 IU/mL were approximately 0.9%, 0.3% and 1.1% among the 10–14, 15–29, and 40–59 years old groups, respectively. The power regression equation of the attenuation model after last dose of pertussis vaccine was y = 41.088x-1.238 (R2 = 0.935, p < 0.001). The fitted anti-PT concentrations was only 5.60 IU/mL at 5 years following the last vaccination dose.
Conclusion
The prevalence of pertussis decreased during the study period in the COVID-19 pandemic; however, there was still a certain proportion of adolescents and adults with evidence of recent infection. The decline in antibody levels after pertussis vaccination was observed, and booster doses are in urgent need in China.
Keywords: Pertussis, Vaccine, Seroprevalence, Antibody
1. Introduction
Pertussis, commonly known as whooping cough, is a highly contagious infectious disease caused by Bordetella pertussis [1]. It is not only an important cause of mortality in infants and young children but also aggravates health risks of individuals with underlying chronic diseases such as asthma and chronic obstructive pulmonary disease [2], [3]. Nowadays, pertussis continues to be a global public health problem in the context of even at least 90% coverage of three-dose primary series diphtheria-tetanus-pertussis (DTP3) (-containing) vaccines worldwide [4]. In China, the incidence of pertussis has also increased in recent years. According to the Chinese National Notifiable Diseases Surveillance System (NNDSS), the reported cases of pertussis exceeded 30,000 nationwide in 2019, which is comparable to that reported in the late 1980s [5]. Furthermore, the disease burden of pertussis in China may be underestimated, especially in older children, adolescents, and adults, due to limitations in laboratory testing techniques and lack of awareness of clinician to diagnose and report pertussis cases [6].
Serosurvey, which is generally performed to detect the serum antibodies that derived from infection or vaccination, is useful for evaluating the incidence or susceptibility to infection in the population. Pertussis toxin (PT), filamentous hemagglutinin (FHA), and pertactin (PRN) are the key virulence factors of Bordetella pertussis, playing an important role in the induction of clinical immunity [7], and thus to be the main components of many acellular pertussis vaccines. Several studies have reported the seroprevalence of anti-PT IgG in China [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], but until now, only one study has evaluated anti-FHA IgG [14] and none have evaluated anti-PRN IgG. A previous study carried out in six counties of Zhejiang Province in 2014 reported anti-PT IgG levels among the general population but without data of anti-FHA and anti-PRN [20].
In response to the COVID-19 pandemic, China has implemented strict public health intervention measures since early 2020. The epidemiological profile of many other respiratory infectious diseases has been reported to change due to non-pharmaceutical interventions (NPI) [21], [22], [23], [24]. Furthermore, COVID-19 pandemic has also influenced DTP vaccination coverage. A decrease of 5% of DTP3 coverage in children was observed from 2019 to 2021 according to WHO data, with the DTP3 coverage decreased to be 81% globally, sounding a red alarm for child health [25]. Meanwhile, the DTP vaccination coverage of Zhejiang province maintained a level of above 95%. In this context, reevaluating the level of antibodies against pertussis can help to comprehend immunity status, estimate the prevalence, and optimize public health interventions.
Zhejiang, a province located in eastern China, is a highly socioeconomically developed province, with a high-level performance of an immunization program since 1978. The replacement of DTwP (combined diphtheria, tetanus, and whole-cell pertussis vaccine) with DTaP (diphtheria, tetanus toxoid, and acellular pertussis vaccine) was completed through over Zhejiang province in 2010. The 4-dose series of DTP administered at 3, 4, 5, and 18 months of age was applied since 1978. Although maintaining a high DTP vaccination rate among the population for several decades, the incidence of pertussis in Zhejiang Province has been increasing over the past 20 years, and reached a new high of 1.36/10 0000 in 2018 [26].
Herein, we conducted a cross-sectional serological investigation of anti-PT, anti-FHA, and anti-PRN antibodies, including participants from all 11 cities in Zhejiang in 2020, aiming to provide further insight into the disease burden of pertussis and corresponding effective immunization strategies.
2. Method
2.1. Study design and participants
This cross-sectional seroepidemiological study was carried out across Zhejiang Province in 2020. Subjects were randomly selected from each city according to the following age groups: <1 year, 1–2 years, 3–4 years, 5–6 years, 7–9 years, 10–14 years, 15–19 years, 20–29 years, 30–39 years, and 40–59 years, based on an age-stratified sampling method with a sample size of at least 30 subjects per age group. A 5 mL blood sample and a self-response questionnaire were collected from each participants. All sera were transported using a cold chain system and stored at −70 °C until processing. Vaccination information of the electronic accurate records were obtained from Zhejiang Provincial Immunization Information System established in 2005.
The study protocol was approved by the Ethics Committee of Zhejiang Provincial Center for Disease Control and Prevention (T-043-R-2020-014). Written informed consent form was obtained from all participants or their legal guardians before enrollment.
2.2. Laboratory testing
IgG antibodies against pertussis toxin (anti-PT IgG), filamentous hemagglutinin (anti-FHA IgG), and pertactin (anti-PRN IgG) were measured quantitatively using an in-house enzyme-linked immunosorbent assay (ELISA) kit developed by Whuan institute of biological products CO.,LTD. Briefly, according to WHO Human International Standard for Pertussis Antiserum (NIBSC 06/140), 96-well ELISA plates were coated with purified PT, FHA, or PRN, and blocked with skim milk solution. The serum was diluted from 50 to 6400-fold, 100 to 12800-fold and 100 to 12800-fold for testing PT, FHA, and PRN, respectively. The antibody concentration was generated using a standard curve obtained from point-to-point plotting (linear/linear) of the optical density values.
According to the laboratory testing method [27] and the instructions of the ELISA kit used in our study [28], a cut-off of 20 IU/ml was defined as seropositive for anti-PT and anti-FHA IgG, and a level of <5 IU/ml was considered as undetectable. An anti-PT IgG concentration ≥80 IU/mL was considered to indicate a recent infection if the subject had not received the pertussis-containing vaccine within the previous year. Given that some studies consider cutoff of ≥100 IU/mL as recent infection using the different ELISA detection kits (Institut Virion/Serion GmbH, Würzburg; and Euroimmun Medizinische Labordiagnostika AG, Germany) [8], [29], the corresponding percentages for both cutoffs were calculated.
2.3. Statistical analysis
Descriptive statistics were performed using geometric mean concentration (GMC, 95% confidence interval) and frequency (percentage) for continuous and categorical variables, respectively. The Spearman’s rank test was used to evaluate the correlation between anti-PT and anti-FHA or anti-PRN IgG concentrations. The Wilcoxon rank sum test was used to compare the concentrations of these three IgG antibodies for different previous vaccination histories.
Modeling of attenuation after pertussis immunization: A scatter plot was created using the years since last pertussis-containing vaccination as the independent variable and GMC of anti-PT as the dependent variable. After linear and several curve models were fitted, power curve fitting for the largest coefficient of determination was chosen.
Statistical significance was set at a two-tailed p-value of 0.05. Data entry and checks were conducted using EpiData (version 3.1). Data analysis was performed using SPSS for Windows (IBM SPSS Statistics 19) and R software (version 3.6.1; R Foundation, Vienna, Austria).
3. Results
3.1. Demographic characteristics of study population
4474 subjects aged from 0 to 59 years were primarily enrolled in the study. 15 were excluded due to poor quality of blood samples or absence of demographic data. Therefore, a total of 4459 subjects were included in the analyses. The ratio of males to female was 1:1.05 (2167:2292), and the ratio of local residents to migrant residents was 9.25:1 (4024:435). Of the children aged 1–14 years old, 98.93% (2680/2709) had confirmed records of three primary doses of pertussis-containing vaccine. Of the children aged 2–14, 97.32% (2435/2502) had a history of four doses of pertussis vaccine.
3.2. Seroprevalence of anti-PT IgG antibodies
The GMC of anti-PT IgG antibodies for 4459 subjects was 9.67 (9.25–10.10) IU/ml. As shown in Table 1 , seropositive rate (≥20 IU/ml) was the highest (81.44%) in the age group of 1–2 years and the lowest (4.72%) in the age group of 10–14 years. The seropositive rate dropped sharply from 67.70% at the age of 3–4 years to 9.81% at the age of 5–6 years, and continued to be at low level (4–6%) in subsequent age groups.
Table 1.
Demographic data and distribution of anti-PT IgG concentration in the study population.
| GMC(95%CI) | <5 IU/mL |
≥20 IU/mL |
≥80 IU/mL |
≥100 IU/mL |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | (IU/mL) | n | (%) | n | (%) | n | (%) | n | (%) |
| Age (years) | ||||||||||
| <1 | 343 | 45.2(39.02–52.37) | 30 | 8.73 | 248 | 72.30 | 145 | 42.27 | 119 | 34.69 |
| 1–2 | 529 | 49.01(44.92–53.47) | 10 | 1.89 | 431 | 81.44 | 158 | 29.87 | 123 | 23.25 |
| 3–4 | 675 | 30.67(28.47–33.05) | 27 | 4.00 | 457 | 67.70 | 109 | 16.15 | 66 | 9.78 |
| 5–6 | 673 | 5.88(5.43–6.37) | 275 | 40.86 | 66 | 9.81 | 6 | 0.89 | 3 | 0.45 |
| 7–9 | 472 | 4.34(3.93–4.78) | 248 | 52.54 | 23 | 4.87 | 0 | 0.00 | 0 | 0 |
| 10–14 | 360 | 4.19(3.77–4.66) | 212 | 58.89 | 17 | 4.72 | 3 | 0.83 | 3 | 0.83 |
| 15–19 | 340 | 4.18(3.68–4.76) | 184 | 54.12 | 21 | 6.18 | 1 | 0.29 | 0 | 0 |
| 20–29 | 356 | 3.99(3.59–4.44) | 221 | 62.08 | 23 | 6.46 | 1 | 0.28 | 1 | 0.28 |
| 30–39 | 355 | 3.8(3.39–4.26) | 202 | 56.90 | 20 | 5.63 | 0 | 0.00 | 0 | 0 |
| 40–59 | 356 | 5.2(4.69–5.77) | 169 | 47.47 | 24 | 6.74 | 4 | 1.12 | 4 | 1.12 |
| Sex | ||||||||||
| Male | 2167 | 11.05(10.37–11.76) | 717 | 33.09 | 734 | 33.87 | 243 | 11.21 | 186 | 8.58 |
| Female | 2292 | 8.53(8.03–9.07) | 816 | 35.59 | 595 | 25.96 | 184 | 8.03 | 133 | 5.81 |
| Registered population | ||||||||||
| Native | 4024 | 9.59(9.15–10.05) | 1440 | 35.78 | 1193 | 29.64 | 393 | 9.77 | 295 | 7.33 |
| migrant | 435 | 10.46(9.19–11.91) | 138 | 31.72 | 136 | 31.26 | 34 | 7.81 | 24 | 5.52 |
| Total | 4459 | 9.67(9.26–10.11) | 1578 | 35.39 | 1329 | 29.80 | 427 | 9.57 | 319 | 7.15 |
To explore more details, we further divided participants under 2 years of age into more specific groups according to the vaccination schedule. As shown in Fig. 1 , the GMC of anti-PT IgG increased significantly from the age group of 0–2 months, reaching a peak of 108.73 IU/mL at 6 months, and subsequently decreased to 40.39 IU/mL in 12–17 months age group. An increase of 20 IU/mL was observed in the ages 12–17 months to 18–23 months; however, the anti-PT IgG GMC declined continuously afterwards, with only 5.88, 4.34 and 4.19 IU/mL at ages 5–6, 7–9, and 10–14 years, respectively. Furthermore, over 50% of subjects had undetectable anti-PT IgG antibodies (<5 IU/mL) in each age group from 7 to 39 years.
Fig. 1.
Anti-PT, anti-FHA and anti-PRN IgG among children aged 0–14 years. m: month; y: years.
The proportion of subjects with anti-PT IgG antibodies ≥ 80 IU/mL was around 0.9% for those aged 5–6 and 10–14 years, and nearly 0.3% for those aged 15–19 and 20–29 years. Participants aged 40–59 years had the highest proportion (1.12%) on anti-PT IgG antibodies ≥ 80 IU/mL. The overall proportion of all study subjects with anti-PT IgG antibodies ≥ 80 IU/mL was 9.57%. This proportion became 7.15% when the cutoff value switched to 100 IU/mL, and the trend across different age groups was almost the same (Table 1).
3.3. Seroprevalence of anti-FHA and anti-PRN IgG antibodies
For anti-FHA IgG antibodies, the GMC of all subjects was 18.93 (18.24–19.67) IU/mL, and the seropositive rate was 47.30%. The changes of FHA across the different age groups were roughly parallel to those of PT but still had slight differences. The GMCs showed an upward trend between the ages of 3 and 6 months, from 9.78 IU/mL at 3 months to a maximum of 72.64 IU/mL at 6 months. A decrease was observed in subsequent age groups, and the GMC was 21.15 IU/mL at 12–17 months. A second peak appeared at 2 years (57.65 IU/mL), after then the GMC decreased again (Fig. 1).
For anti-PRN IgG antibodies, the GMC for all subjects was 8.99 (8.61–9.38) IU/mL. For subjects under 5 years of age, the trend of anti-PRN IgG across age groups was similar to that of anti-PT while the GMC level was relatively lower than that of anti-PT and anti-FHA. However, after the age of 5 years, the anti-PRN GMCs exhibited an upward trend (Fig. 1).
In the total population, the concentrations of anti-PT and anti-FHA were significantly correlated (r = 0.631, p < 0.001), so were the correlation between the concentrations of anti-PT and anti-PRN (r = 0.451, p < 0.001). The scatter plots of serum anti-PT vs. anti-FHA or anti-PRN by age groups are shown in the supplementary materials (Supplementary Figure I).
3.4. Model of attenuation after pertussis immunization
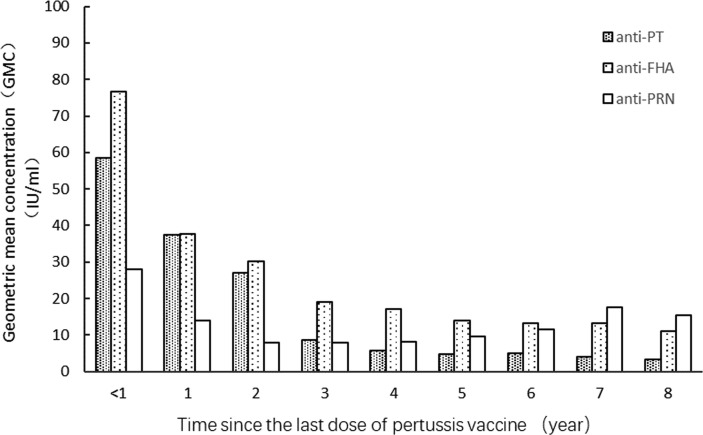
A total of 2435 children had confirmed records of four doses of the pertussis-containing vaccine. We calculated the time interval between the date of the final pertussis vaccination and the date at which the blood sample was obtained. Within the first year of receiving the fourth dose of the pertussis vaccine, the GMCs of anti-PT, anti-FHA, and anti-PRN antibodies were 58.41 IU/mL, 76.69 IU/ml and 28.1 IU/mL, respectively. A downward trend was observed in the levels of all three antibodies. However, the decline in anti-PRN antibody was not as significant as those of anti-PT and anti-FHA. A slight increasing trend for anti-PRN antibody was observed since 5–8 years after the last dose (Fig. 2 ).
Fig. 2.
The GMC of anti-PT, anti-FHA, anti-PRN over time from the last pertussis vaccine dose.
Considering antigen specificity[7], [27], we only fitted mathematical models for anti-PT antibody concentrations over time after vaccination. The persistence and attenuation of anti-PT IgG antibodies were reflected in power curve fitting (Fig. 3 ). The power regression equation was y = 41.088x−1.238 (R2 = 0.935, p < 0.001). The fitted anti-PT antibody concentrations were 10.54, 7.38 and 5.60 IU/mL at 3, 4, and 5 years after the last vaccination dose, respectively. A scatter plot of the concentration of anti-PT antibody with time since last dose of pertussis vaccine was provided in the supplementary materials (Supplementary Figure III). In addition, the decline of anti-FHA antibody over time was similar to anti-PT antibody (Supplementary Figure IV).
Fig. 3.
The concentration of anti-PT with time since last dose of pertussis vaccine. Dot circle: real data; Solid line: fitted power curve; dotted line: fitted exponential curve.
4. Discussion
In this study, we evaluated the levels of serum anti-PT, anti-FHA, and anti-PRN antibodies during the COVID-19 pandemic in 2020, and estimated the susceptibility and recent infection rate of B. pertussis among different age groups, mainly based on the anti-PT antibody level. We also fitted an exponential regression model for the attenuation of anti-PT IgG antibody levels.
Pertussis toxin (PT) is a specific antigen of B. pertussis, and anti-PT IgG antibodies are generally used as serological measures for recent infections or immune response after vaccinations [5], [8]. The overall GMCs of anti-PT antibodies among infants and children in our study were slightly higher than those reported in previous studies in other regions of China [8], [10], [11], [12], [14]. Studies conducted in central [14] and southwestern China [12] showed that the levels of anti-PT IgG antibodies remained low throughout all age groups, and there were no obvious immune responses after primary vaccination and booster. In the current study, the GMCs of anti-PT increased significantly after the primary (at 3, 4, and 5 months of age) and booster immunization schedule (at 18–24 months), indicating that vaccination induced humoral immunity, and a high vaccination rate is helpful for the prevention of pertussis in these age groups. This discrepancy between our results and others may be explained by differences in laboratory techniques, coverage of the pertussis vaccine, and different study periods.
Despite the relatively high level of anti-PT induced at the sixth month and at 18–23 months, a continuous decline over time was observed afterwards in our study. At 5–6 years of age, the GMC of anti-PT dropped to approximately 5 IU/mL, and a large proportion of children aged 7–14 years had undetectable levels of anti-PT IgG antibodies. A Japanese sero-epidemiology study showed that children aged 3–6 years exhibited much lower levels of anti-PT IgG than younger children aged 1–2 years [30], which was in consist with our findings. In addition, our mathematical model revealed that the waning of anti-PT IgG after vaccination followed a power curve. It was estimated that the concentration would decline to 5.60 IU/mL at 5 years after the last vaccination, approaching an undetectable level. This finding echoes several case-control studies conducted in Canada, the United States, and Australia that assessed waning immunity by calculating the odds of developing pertussis per year since the last dose. Schwartz et al. observed high early effectiveness of the pertussis vaccine that rapidly declined over time since the last dose surpassed 4 years [31]. Another study used meta-regression models to evaluate the duration of immunity with DTaP, and estimated that the odds of pertussis infection increased by 1.33 times (95% CI: 1.23–1.43), for every additional year after the last dose of DTaP [32].
In view of the decline in antibodies induced by vaccines, the re-increase in anti-PT IgG antibodies in older children or adults mainly reflect pertussis infection. We considered anti-PT IgG ≥ 80 IU/mL as an indicator of recent infection in participants aged ≥5 years and found that a substantial minority of preschool children, adolescents, and adults might have had recent infection. The trend was almost the same when cutoff value switched to 100 IU/mL, and the sero-estimated infection rate of pertussis infection were still high. While the estimated rate of recent infection in this study was lower than that in a previous study in Zhejiang during September and October 2014 [20]. This discrepancy might be influenced by the pandemic COVID‐19 as many strict prevention and control measures were implemented in 2020, which may reduce the circulation of pertussis bacteria [33]. In addition, since pertussis was a cyclic disease, the downward trend always come after the peak of the disease. Indeed, the reported incidence of pertussis declined dramatically nationwide in 2020. Nevertheless, the estimated infection rates were still much higher than the notification incidence rates of surveillance systems, particularly in adolescents and adults. The notification incidence rates in Zhejiang were 1.28/100000 per year for the entire population in 2019 and 0.10/100000 in 2020, and the vast majority (∼90%) of cases was reported in infants and young children aged <5 years [34]. The neglected prevalence of pertussis infection in order children, adolescents, and adults needs more attention, since the population are more active and play an important role in transmission, leading to possible infection sources of infant pertussis [35]. These results indicate that the immunity to pertussis is low, and a certain proportion of infections occur even with strict implementation of NPI against the COVID-19 pandemic in Zhejiang, China.
To better understand immunity against pertussis, serum anti-FHA and anti-PRN antibodies were also evaluated in this study. FHA and PRN are not unique to B. pertussis, but they are also highly immunogenic and the corresponding antibodies could somehow reflect the immune response to vaccination. Mice immunized with FHA or PRN are protected against lethal respiratory challenge with pertussis [7]. In this study, we observed that anti-FHA and anti-PRN followed a similar trend to anti-PT, reaching a peak at sixth month and two years of age, which implies that vaccination had elicited effective antibodies against them. Although the anti-FHA levels of infants and children (0–14 years) in our study were higher than those in a previous study conducted in Henan, China, both studies demonstrated a significant correlation between anti-FHA and anti-PT [14]. Our results also showed that anti-PRN was statistically correlated with anti-PT, although the correlation was weaker than that between anti-FHA and anti-PT. These phenomena can be explained by the different specificities of antigens [7] and components contained in pertussis vaccines [31]. As of 2020, three types of pertussis vaccines are available in Zhejiang, China: DTaP, DTaP/Hib, and DTaP-IPV/Hib. The former two vaccines were domestic-manufactured based on co-purification technology with three pertussis-related antigen components (PT, FHA and PRN). The latter was based on component purification technology, and the vaccine instructions was declared to contain two pertussis-related antigen components (PT and FHA). Our results implied that the levels of anti-PRN increase between age 6–14 years was an indication of pertussis or other bacteria infection in the population [36].
Accordingly, we identified distinct levels of these three types of antibodies in children vaccinated with different types of vaccines (Supplementary Figure II). Children who received component purified vaccine exhibited higher anti-PT IgG and anti-FHA IgG levels than those who received the co-purified vaccine, but their anti-PRN IgG were at lower levels. Manufacturing techniques could be an important factor influencing post-immunization antibody levels [37]. It should also be noted that the relationship between serological level and protection has not yet been well established [7], and the levels of antibodies induced by different vaccines might not fully represent their effectiveness. Therefore, further investigations such as cohort or case-control studies are still required to compare the effectiveness of different vaccine.
In response to the resurgence of pertussis and to reduce its hazards to children, some developed countries have administered pertussis vaccine not only for toddlers but also for preschool children, adolescents and adults. For example, the Advisory Committee on Immunization Practices (ACIP) of the United States recommends a 5-dose series of DTaP vaccines for infants and young children (2, 4, 6, 15–18 months and 4–6 years), with one adolescent booster dose of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine for adolescents aged 11–18-years. Adults who have never received Tdap are also recommended to get a booster dose of Tdap [38]. Unfortunately, so far, pertussis-containing vaccines targeted to preschool children, adolescents, or adults have not yet been approved for use in China. However, our findings, which demonstrating an obvious waning of antibody levels at approximately 5 years of age and the potentially high rates of infections among adolescents and adults emphasizes the need for booster doses. Studies in Germany and England have demonstrated that a booster dose at 4–6 years old can reduce the incidence of pertussis in the entire population and prevent household transmission [39], [40]. In addition, recent studies have showed that macrolide resistant B. pertussis is growing more common in China, which brings challenges to clinic treatment [41], [42].There is a booster dose for diphtheria and tetanus at 6 years of age with DT (combined diphtheria and tetanus vaccine) according to the Chinese national immunization program. In order to address the decline in immunity and circulation of pertussis among adolescents, therefore, it may be much more practical and protective to switch DT into DTaP for the 6-year-old group.
Our study has several strengths, including the relatively large sample size of subjects from all municipalities in Zhejiang province, covering 10 age groups ranged from 0 to 59 years, measurements of three kinds of antibodies against PT, FHA, and PRN, combination of vaccine history, and fitting with a mathematical model. However, this study also has some limitations. First, antibodies induced by vaccines or natural infection cannot be distinguished in young infants and children, making it impossible to accurately estimate pertussis infection in these populations. Second, this study did not observe antibody level declines after immunization in the real world prospective cohort, but we conducted a model analysis of antibody attenuation for children at different time points after immunization, which can also provide reference information for the assessment of vaccine-induced antibody decay. Third, the immunization records for subjects > 15 years old were unclear due to restrictions of information system development, but they should have not received any vaccination because no booster dose is given after two years of age in China. Therefore, the anti-PT antibodies of them were most likely due to infection rather than vaccination [29]. Last, the sex distribution was not balanced in each age group and created the illusion that males possessed a higher anti-PT GMC than females. However, after adjusting for age, there was no significant difference in antibody concentrations between males and females.
5. Conclusion
The prevalence of pertussis decreased during the study period of the COVID-19 pandemic, but a certain proportion of adolescents and adults exhibited evidence of recent pertussis infection, who were not identified in routine diagnosis and surveillance. The proportion of population susceptible to pertussis were high, and more than half may not retain immunity after the age of 7 years. This study also indicated that the pertussis vaccine induced humoral immunity after the primary and booster immunization schedules, but antibodies wane to a quite low level at approximately 5 years after the last dose of DTaP. An extra booster dose for older children and adolescents is highly recommended to protect the susceptible population, and the optimal operational strategy is to change the 6-year-old group inoculated with DT to DTaP in the immunization program.
Funding/Support
This work was supported by the Science and Technology Talent Incubation Project of Zhejiang Provincial Center for Disease Control and Prevention.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all participants who participated in the study and provided the samples. We are grateful for the efforts of the staff of municipal centers for disease control and prevention in Zhejiang involved in this study, including Hangzhou CDC, Jiaxing CDC, Huzhou CDC, Ningbo CDC, Shaoxing CDC, Wenzhou CDC, Jinhua CDC, Taizhou CDC, Zhoushan CDC, Lishui CDC, and Quzhou CDC. We would also like to thank Dr. Dewu Zhu and his colleagues at the Wuhan Institute of Biological Products Co. Ltd. for their laboratory tests.
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.10.020.
Appendix A. Supplementary material
The following are the Supplementary material to this article:
Data availability
Data will be made available on request.
References
- 1.Nieves D.J., Heininger U. Bordetella pertussis. Microbiol Spectrum. 2016;4(3) doi: 10.1128/microbiolspec.EI10-0008-2015. [DOI] [PubMed] [Google Scholar]
- 2.Bhavsar A., Aris E., Harrington L., Simeone J.C., Ramond A., Lambrelli D., et al. Burden of Pertussis in Individuals with a Diagnosis of Asthma: A Retrospective Database Study in England. J Asthma Allergy. 2022;15:35–51. doi: 10.2147/JAA.S335960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aris E., Harrington L., Bhavsar A., Simeone J.C., Ramond A., Papi A., et al. Burden of Pertussis in COPD: A Retrospective Database Study in England. COPD. 2021;18(2):157–169. doi: 10.1080/15412555.2021.1899155. [DOI] [PubMed] [Google Scholar]
- 4.Pertussis[EB/OL]. [2022-7-23]. https://www.who.int/health-topics/pertussis#tab=tab_2.
- 5.Zhang J., Deng J., Yang Y. Pertussis vaccination in Chinese children with increasing reported pertussis cases. Lancet Infect Dis. 2022;22(1):21–22. doi: 10.1016/S1473-3099(21)00752-0. [DOI] [PubMed] [Google Scholar]
- 6.Yu J., He H., Zhang Y., Gao Y., Chen C., Xu J., et al. Burden of whooping cough in China (PertussisChina): study protocol of a prospective, population-based case-control study. BMJ Open. 2022;12(3):e053316. doi: 10.1136/bmjopen-2021-053316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plotkin S.A., Orenstein W.A., Offit P.A. 7th ed. Elsevier; Philadelphia, PA: 2018. Vaccines. [Google Scholar]
- 8.Zhang Z., Pan J., Chen M., Zhang T., Li J., Lu L. Seroepidemiology of pertussis in China: A population-based, cross-sectional study. Vaccine. 2021;39(12):1687–1692. doi: 10.1016/j.vaccine.2021.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z., Zhang J., Cao L., Zhang N., Zhu J., Ping G., et al. Seroprevalence of pertussis among adults in China where whole cell vaccines have been used for 50 years. J Infect. 2016;73(1):38–44. doi: 10.1016/j.jinf.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Li X., Chen M., Zhang T., Li J., Zeng Y., Lu L. Seroepidemiology of diphtheria and pertussis in Beijing, China: A cross-sectional study. Hum Vacc Immunotherapeut. 2015;11(10):2434–2439. doi: 10.1080/21645515.2015.1062954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y.T., Luo X.Q., Zhong X.B., Cai L.M., Zhu L.P., Chen X.Q., et al. Seroprevalences of antibodies against pertussis, diphtheria, tetanus, measles, mumps and rubella: A cross-sectional study in children following vaccination procedure in Guangzhou, China. Vaccine. 2020;38(23):3960–3967. doi: 10.1016/j.vaccine.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 12.Liu D., Cheng X., Wei S., Yuan L., Chen C., Yao K. Decline of serologic immunity to diphtheria, tetanus and pertussis with age suggested a full life vaccination in mainland China. Hum Vacc Immunotherapeut. 2021;17(6):1757–1762. doi: 10.1080/21645515.2020.1840253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y., Zhu B., Gao Y., Shi Z., Wang J., Wang H., et al. Clustered cases of Bordetella pertussis infection cause high levels of IgG antibodies against pertussis toxin in adolescents in Gaobeidian city, China. Epidemiol Infect. 2014;142(4):738–743. doi: 10.1017/S0950268813003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y., Wang L., Xu J., Wang X., Wei C., Luo P., et al. Seroprevalence of pertussis in China: need to improve vaccination strategies. Hum Vacc Immunotherapeut. 2014;10(1):192–198. doi: 10.4161/hv.26335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y., Xu E., Liu S., Zheng W., Zhang X., Du J., et al. Seroepidemiology of pertussis in Hangzhou, China, during 2009–2017. Hum Vacc Immunotherapeut. 2019;15(11):2564–2570. doi: 10.1080/21645515.2019.1608130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao N., Zeng Q., Wang Q. Seroepidemiology of diphtheria and pertussis in Chongqing, China: serology-based evidence of Bordetella pertussis infection. Public Health. 2018;156:60–66. doi: 10.1016/j.puhe.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q., Han F., Nie Q., Ren H., Zhang B., Liu Q., et al. Seroprevalence of antibodies to pertussis and diphtheria among healthy adults in China. J Infect. 2011;63(6):441–446. doi: 10.1016/j.jinf.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q., Zheng H., Liu M., Han K., Shu J., Wu C., et al. The seroepidemiology of immunoglobulin G antibodies against pertussis toxin in China: a cross sectional study. BMC Infect Dis. 2012;12:138. doi: 10.1186/1471-2334-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y., Huang H., Gao Z., Liu Y., Liu P., Ding Y., et al. A sera-epidemiological study on pertussis immunity levels among community populations and an analysis of the underlying factors in Tianjin China. Vaccine. 2015;33(51):7183–7187. doi: 10.1016/j.vaccine.2015.10.133. [DOI] [PubMed] [Google Scholar]
- 20.He H., Yao P., Zhou Y., Deng X., Pan J. Is Pertussis Infection Neglected in China? Evidence from a Seroepidemiology Survey in Zhejiang, an Eastern Province of China. PLoS ONE. 2016;11(5):e0155965. doi: 10.1371/journal.pone.0155965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W., Luan R. Estimating the impact of non-pharmaceutical interventions against COVID-19 on mumps incidence in Sichuan, China. BMC Infect Dis. 2021;21(1):886. doi: 10.1186/s12879-021-06584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin C.F., Huang Y.H., Cheng C.Y., Wu K.H., Tang K.S., et al. Public Health Interventions for the COVID-19 Pandemic Reduce Respiratory Tract Infection-Related Visits at Pediatric Emergency Departments in Taiwan. Front Public Health. 2020 Dec;16(8) doi: 10.3389/fpubh.2020.604089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X., Xu Y., Zhu Y., Tang F. Impact of non-pharmaceutical interventions on the incidences of vaccine-preventable diseases during the COVID-19 pandemic in the eastern of China. Hum Vacc Immunotherapeut. 2021;17(11):4083–4089. doi: 10.1080/21645515.2021.1956227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaabna K., Doraiswamy S., Mamtani R., Cheema S. Facemask use in community settings to prevent respiratory infection transmission: A rapid review and meta-analysis. Int J Infect Dis. 2021;104:198–206. doi: 10.1016/j.ijid.2020.09.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.COVID-19 pandemic fuels largest continued backslide in vaccinations in three decades[EB/OL]. [2022-9-4]. https://www.who.int/news/item/15-07-2022-covid-19-pandemic-fuels-largest-continued-backslide-in-vaccinations-in-three-decades.
- 26.Tang X., Zhou Y., Wang Y., Yan R., Deng X., He H., et al. Epidemiological characteristics of pertussis in Zhejiang Province. Prev Med (in Chinese) 2020;32(7):712–714. [Google Scholar]
- 27.Zou G.R., Lan H., Xiang M.J., Yang R.P., Yany X.M., Hou Q.M., et al. Immunogenicity and immune persistence of adsorbed diphtheria, tetanus and acellular pertussis combined vaccine. Chin J Biol (in Chinese) 2013;26(12):1805–1811. [Google Scholar]
- 28.Chen W., Zheng M.T., Hu Y., Lei N., Bai Y., Li P., et al. Detection and analysis of antibodies against pertussis toxin and filamentous hemagglutinin among healthy adults. Chin J Vacc Immuniz (in Chinese) 2020;26(02):169–172. [Google Scholar]
- 29.Chen Z., Pang J., Zhang Y., Ding Y., Chen N., Zhang N., et al. Seroprevalence of Pertussis in Adults at Childbearing Age Pre- and Post- COVID-19 in Beijing, China. Vaccines (Basel) 2022;10(6):872. doi: 10.3390/vaccines10060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fumimoto R., Otsuka N., Sunagawa T., Tanaka-Taya K., Kamiya H., Kamachi K. Age-related differences in antibody avidities to pertussis toxin and filamentous hemagglutinin in a healthy Japanese population. Vaccine. 2019;37(18):2463–2469. doi: 10.1016/j.vaccine.2019.03.055. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz K.L., Kwong J.C., Deeks S.L., Campitelli M.A., Jamieson F.B., Marchand-Austin A., et al. Effectiveness of pertussis vaccination and duration of immunity. CMAJ. 2016;188(16):E399–E406. doi: 10.1503/cmaj.160193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGirr A., Fisman D.N. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics. 2015;135(2):331–343. doi: 10.1542/peds.2014-1729. [DOI] [PubMed] [Google Scholar]
- 33.Tessier E., Campbell H., Ribeiro S., Rai Y., Burton S., Roy P., et al. Impact of the COVID-19 pandemic on Bordetella pertussis infections in England. BMC Public Health. 2022;22(1):405. doi: 10.1186/s12889-022-12830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Z., Wu H., Lu Q., Wu C., Lin J. Epidemiological characteristics of the notifiable infectious diseases reported in Zhejiang Province, 2020. Prevent Med (in Chinese) 2021;33(4):325–331. [Google Scholar]
- 35.Skoff T.H., Kenyon C., Cocoros N., Liko J., Miller L., Kudish K., et al. Sources of Infant Pertussis Infection in the United States. Pediatrics. 2015;136(4):635–641. doi: 10.1542/peds.2015-1120. [DOI] [PubMed] [Google Scholar]
- 36.Poolman J.T., Hallander H.O. Acellular pertussis vaccines and the role of pertactin and fimbriae. Expert Rev Vacc. 2007;6(1):47–56. doi: 10.1586/14760584.6.1.47. [DOI] [PubMed] [Google Scholar]
- 37.Edwards K.M., Meade B.D., Decker M.D., Reed G.F., Rennels M.B., Steinhoff M.C., et al. Comparison of 13 acellular pertussis vaccines: overview and serologic response. Pediatrics. 1995;96(3 Pt 2):548–557. [PubMed] [Google Scholar]
- 38.Liang J.L., Tiwari T., Moro P., Messonnier N.E., Reingold A., Sawyer M., et al. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2018;67(2):1–44. doi: 10.15585/mmwr.rr6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward J.I., Cherry J.D., Chang S.J., Keitel W., Barenkamp S., Bernstein D.I., et al. Efficacy of an acellular pertussis vaccine among adolescents and adults. N Engl J Med. 2005;353(15):1555–1563. doi: 10.1056/NEJMoa050824. [DOI] [PubMed] [Google Scholar]
- 40.Edmunds W.J., Brisson M., Melegaro A., Gay N.J. The potential cost-effectiveness of acellular pertussis booster vaccination in England and Wales. Vaccine. 2002;20(9–10):1316–1330. doi: 10.1016/s0264-410x(01)00473-x. [DOI] [PubMed] [Google Scholar]
- 41.Li L., Deng J., Ma X., Zhou K., Meng Q., Yuan L., et al. High Prevalence of Macrolide-Resistant Bordetella pertussis and ptxP1 Genotype, Mainland China, 2014–2016. Emerg Infect Dis. 2019;25(12):2205–2214. doi: 10.3201/eid2512.181836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Z., Wang Z., Luan Y., Li Y., Liu X., Peng X., et al. Genomic epidemiology of erythromycin-resistant Bordetella pertussis in China. Emerging Microbes Infect. 2019;8(1):461–470. doi: 10.1080/22221751.2019.1587315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.