Abstract
Aim
Describe community consultation and surrogate consent rates for two Exception From Informed Consent (EFIC) trials for out-of-hospital cardiac arrest (OOHCA) - before and during the COVID-19 pandemic.
Methods
The PEARL study (2016–2018) randomized OOHCA patients without ST-elevation to early cardiac catheterization or not. Community consultation included flyers, radio announcements, newspaper advertisements, mailings, and in-person surveys at basketball games and ED waiting rooms. The PROTECT trial (2021-present) randomizes OOHCA survivors to prophylactic ceftriaxone or placebo; the community consultation plan during the pandemic included city council presentations, social media posts, outpatient flyers, but no in-person encounters. Demographics for PROTECT community consultation were compared to PEARL and INTCAR registry data, with p-value < 0.05 considered significant.
Results
PEARL surveyed 1,362 adults, including 64 % ≥60 years old, 96 % high school graduates or beyond; research acceptance rate was 92 % for the community and 76 % for personal level. PROTECT initially obtained 221 surveys from electronic media – including fewer males (28 % vs 72 %,p < 0.001) and those > 60 years old (14 % vs 53 %;p < 0.001) compared to INTCAR. These differences prompted a revised community consultation plan, targeting 79 adult in-patients with cardiac disease which better matched PEARL and INTCAR data: the majority were ≥ 60 years old (66 %) and male (54 %). Both PEARL and PROTECT enrolled more patients using surrogate consent vs EFIC (57 %, 61 %), including 71 % as remote electronic consents during PROTECT.
Conclusions
Community consultation for EFIC studies changed with the COVID-19 pandemic, resulting in different demographic patterns. We describe effective adaptations to community consultation and surrogate consent during the pandemic.
Keywords: Cardiac arrest, Exception From Informed Consent (EFIC), Ethics, Research ethics, Community Consultation, Research in emergency settings
Introduction
With limited exceptions, federal and international regulations require investigators to obtain consent from prospective subjects or legally authorized representatives prior to medical research,1, 2 but patients experiencing events such as cardiac arrest, stroke, or trauma are often unable to engage in these discussions, and surrogates may not be available. To enable research in these situations, the Food and Drug Administration developed Exception from Informed Consent (EFIC) rules for subjects in a life-threatening situation with unsatisfactory available treatments, when informed consent is not feasible.3 The EFIC regulations require investigators to seek surrogate consent from legally authorized representatives prior to proceeding without obtaining consent.3
The EFIC regulations also require discussions with communities where the research will take place and from which the subjects will be drawn about potentially being enrolled without their consent.3 Community consultation methods have varied, with differing approaches to define the community of interest.4, 5, 6, 7, 8 Several studies have surveyed crowds (e.g. at state fairs) as a less expensive and more time-efficient method of community consultation.4, 7
The COVID-19 pandemic reduced opportunities to engage large gatherings or waiting rooms, but the impact on EFIC studies has not been reported. The FDA research guidance regarding the pandemic did not address EFIC or community consultation.9
The objective of this study was to compare community consultation processes and results for two EFIC trials conducted at the same center - one before and one during the COVID-19 pandemic.
Methods
PEARL trial
The PEARL study was a randomized multicenter trial evaluating early coronary angiography after out-of-hospital cardiac arrest (OOHCA) conducted from 2016-201810 and approved by the Maine Medical Center Institutional Review Board (MMCIRB - #4592, NCT02387398). The EFIC community consultation conducted from April-December 2015 utilized flyers in town buildings, radio announcements, newspaper advertisements, Facebook posting, and community mailings – all linked to an on-line survey. In-person surveys were performed with 223 attendees at a professional basketball game or an Emergency Department waiting room (Supplement 1).
INTCAR
The International Cardiac Arrest Registry (INTCAR) is a multicenter database enrolling cardiac arrest patients treated with targeted temperature management.11 We accessed center–specific data for 2018–2019 as a demographic benchmark for the community from which subjects would be drawn for the PROTECT trial.
PROTECT trial
The PROTECT trial, a randomized, blinded study comparing prophylactic ceftriaxone and placebo to reduce early-onset pneumonia, was approved by the MMCIRB (1661144-2, NCT04999592). The community consultation plan conducted from December 2020 - May 2021 included city council presentations, social media posts, and flyers at cardiology offices - all linked to an on-line survey (Supplement 2). Though not identical, the surveys for the two studies asked similar questions with similar language and provided an opportunity for potential subjects to opt-out of the research by adding their name to a list which was reviewed before enrolling each subject.
The COVID-19 pandemic limited in-person access, including large gatherings and waiting rooms. A planned community consultation interim review identified a large demographic difference compared to PEARL and INTCAR data, prompting community consultation plan revisions. In-person interviews of hospitalized cardiac patients were approved by the MMCIRB and completed from April-May 2021.
Statistical analysis
Continuous data were reported as median (IQR) and proportions as number (%). Our primary objective was to compare the characteristics suggested in the FDA guidance3 (e.g., sex, ethnicity, age) of the PROTECT community consultation cohort to INTCAR and PEARL data using chi-square or Fisher exact tests, and secondarily to assess opt-out rates and approval to conduct the research in the community or on the responder. A p-value < 0.05 was considered significant.
Results
PEARL study
Among 1,362 community members completing the PEARL survey (Table 1), the majority were ≥ 60 years of age (64 %), white (95 %), non-Hispanic/Latino (99 %), with high school education or beyond (96 %). The research acceptance rate was 92 % for the community and 76 % personally. In contrast, the 14 subjects enrolled in the PEARL trial at MMC10 were less likely to be ≥ 60 years old (29 % vs 64 %,p = 0.002) with similar race and ethnicity (Table 1).
Table 1.
PEARL trial community consultation results compared to the trial enrollees.
| PEARL MMC COMMUNITY CONSULTATION |
PEARL MMC Enrolled (n = 14) |
p-value | |
|---|---|---|---|
| Age, ≥60 years | 885/1262 (65) | 4/14 (29) | 0.002 |
| Sex, male | NR | 11/14 (79 %) | NR |
| Race, white | 1235/1297 (95) | 14/14 (100) | 1 |
| Non-Hispanic/Latino | 1287/1297 (99) | 14/14 (100) | 1 |
| Education, ≥ HS diploma | 1228/1281 (96) | NR | NR |
| Approve, community | 1252/1363 (92) | NR | NR |
| Approve, personal | 1016/1345 (76) | NR | NR |
| Opt-outs | 329/1354 (24) | NR | NR |
HS = high school; MMC = Maine Medical Center; NR = not reported; PEARL = Pilot Randomized Clinical Trial of Early Coronary Angiography versus No Early Coronary Angiography for Post-Cardiac Arrest Patients without ECG ST Segment Elevation.
Data presented as number (%). Data for PEARL at MMC only. Denominator for PEARL is based on number of respondents completing that question.
p-value = PEARL trial community consultation vs PEARL trial enrollments.
PROTECT study
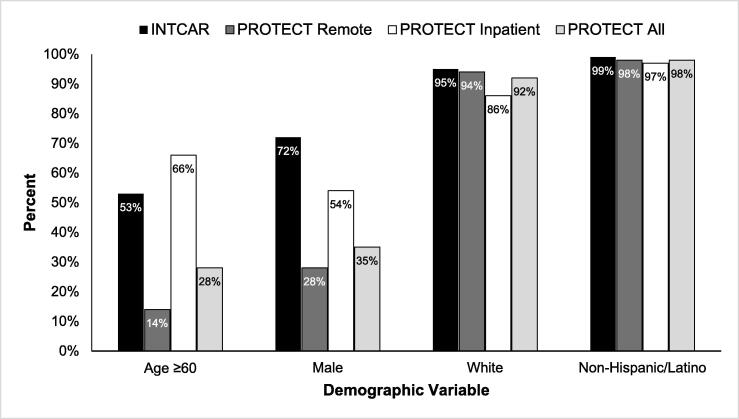
Among 221 community members remotely completing the PROTECT survey (Table 2), the majority were < 60 years of age (86 %), female (72 %), white (94 %), with high school education or beyond (99 %). The research acceptance rate was 98 % for the community and 96 % personally. These remote PROTECT respondents were less frequently ≥ 60 years old compared to PEARL respondents (14 % vs 64 %; p < 0.001) or INTCAR data (14 % vs 53 %; p < 0.001), and were less often male compared to INTCAR (28 % vs 72 %; p < 0.001, Fig. 1). The 79 in-patient interviews better matched INTCAR data, PEARL respondents, and PEARL enrolled patients (Table 2). The majority were ≥ 60 years of age (66 %), male (54 %), with a high school education or beyond (92 %).
Table 2.
PROTECT trial Community Consultation results stratified by cardiology inpatient or not and compared to INTCAR data at MMC.
| PROTECT All | PROTECT Remote |
PROTECT In-patient |
INTCAR | p-value | |
|---|---|---|---|---|---|
| N | 300 | 221 | 79 | 392 | |
| Age, ≥60 years | 84 (28) | 32 (14) | 52 (66) | 207 (53) | <0.001 |
| Sex, male | 105 (35) | 62 (28) | 43 (54) | 283 (72) | <0.001 |
| Race, white | 276 (92) | 208 (94) | 68 (86) | 371 (95) | 0.21 |
| Non-Hispanic/Latino | 294 (98) | 217 (98) | 77 (97) | 378 (99) | 0.32 |
| Education, ≥ HS diploma | 291 (97) | 218 (99) | 73 (92) | NR | NR |
| Approve, community* | 288 (96) | 217 (98) | 71 (90) | NA | 0.003 |
| Approve, personal* | 276 (92) | 213 (96) | 63 (80) | NA | <0.001 |
| Opt-out* | 24 (8) | 8 (4) | 16 (20) | NA | <0.001 |
HS = high school; INTCAR = International Cardiac Arrest Registry; NA = not applicable; NR = not reported; PROTECT = Ceftriaxone to Prevent Pneumonia and Inflammation after Cardiac Arrest Trial.
Data presented as number (%). Denominator for PROTECT community consultation is based on number of respondents completing that question.
*p-value = PROTECT All vs center INTCAR data, except approval and opt-out data compared remote surveys to inpatient interviews.
Fig. 1.
Comparison of demographic data between Maine Medical Center cardiac arrest patients enrolled in INTCAR during 2018–2019 and community consultation data for the PROTECT trial.
Most patients enrolled in the PEARL study (8/14; 57 %) and the PROTECT trial to date (14/23; 61 %) were enrolled using surrogate consent rather than the EFIC pathway without initial consent. All 8 PEARL consents were obtained in-person, while in PROTECT, 10/14 (71 % vs 0 % PEARL, p = 0.002) were consented remotely.
Discussion
This study describes challenges to the EFIC community consultation process emerging during the COVID-19 pandemic, and the adaptive response at a single medical center. Without large in-person gatherings, community consultation responders in our initial PROTECT EFIC plan were different than the PEARL trial and the INTCAR registry data. Revising our community consultation plan by interviewing in-patients with cardiac disease produced a cohort more similar to the “community from which subjects will be drawn” as recommended by the FDA.3.
FDA guidance states that “respect for subjects’ autonomy” may be demonstrated by this similarity between the community consultation and eventual study populations.3 Relevant similarities include medical profile (addressing the likelihood of experiencing the medical issue being studied) and demographic characteristics (residence, age, sex, educational and socioeconomic status, race, and ethnicity). A close match between community consultation and study participants has not always been achieved.12 Statistical representation alone is not adequate, and must be interpreted cautiously; seeking meaningful input and identifying community-level concerns are also important. The guidance identifies the importance of open-ended input among different groups during community consultation,3 since clinical trials should conform with broader community norms and priorities; 13differing perspectives exist regarding the proper balance between these competing targets.
Another adaptation to the consenting process emerging from the COVID pandemic has been greater familiarity and reliance on remote electronic consent to contact legally authorized representatives. Feldman reviewed 41 EFIC trials conducted from 1997-2017, enrolling 46,964 patients, of which 96 % were enrolled without consent.14 In the hospital-based PEARL study, our center enrolled a majority of patients using surrogate consent; all were in-person consents. The ongoing PROTECT study has also enrolled a majority with surrogate consent, and most were obtained remotely (71 %). As electronic consent evolves, the impact on EFIC enrollments without consent is uncertain, but could potentially be reduced, especially for hospital-based studies when the therapeutic window may be longer and legally authorized representatives can be identified and contacted.
The community acceptance rates in the PEARL (92 %) and PROTECT (96 %) trials were higher than reported in a review of EFIC trials (78 %, 95 % confidence interval 75–79 %), and the personal acceptance rates in PROTECT were higher than previously reported, especially for the remote online cohort which was younger and predominantly educated and female. These differences may reflect variation in diseases and severity, study designs, survey question phrasing, approaches to community consultation, and population demographics.15 Community meetings and individual interviews are generally associated with higher rates of acceptance than remote surveys,15 but may include a different demographic group relative to age, sex, educational level, and familiarity with electronic technology.8 Our PROTECT in-patient interviews yielded lower acceptance rates and higher opt-out rates than our remote surveys, perhaps reflecting hospitalization for a medical problem or demographic differences between the groups. Hsu et al found a similar lower acceptance rate with individuals in cardiology clinic waiting rooms before the pandemic compared to surveys returned electronically.8 Though commonly reported as part of the community consultation process, closed-ended surveys designed to provide a percent “acceptance” may fail to capture and explore distinct views of sub-groups within the population; opportunities for open-ended comments and two-way communication should also be included.
This report has several limitations. The data are from a single medical center and compare two EFIC trials for the same diagnosis – cardiac arrest. We targeted in-patients with cardiac disease in our adaptive community consultation; we did not sample other patient cohorts which also suffer cardiac arrests. Whether our observations apply to other centers, other diseases, and other trials is unknown.
Conclusion
Community consultation was feasible during the COVID-19 pandemic, but restricted public gatherings eliminated an effective approach to engagement. To address population inequities resulting from a remote electronic-based approach to consultation, targeted interviews with in-patients were demographically more similar to our study population. Future strategies to address these issues may include more direction from the FDA and feedback to local IRBs regarding the role of remote electronic consent, caution with the wording and interpretation of EFIC research acceptance questions, and adaptive strategies during community consultation such as we describe in this report.
CRediT authorship contribution statement
David J. Gagnon: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Funding acquisition. Richard R. Riker: Conceptualization, Methodology, Formal analysis, Writing – original draft. Frank Chessa: Conceptualization, Writing – original draft. Christine Lord: Investigation. Ashley Eldridge: Investigation. Meghan Searight: Investigation. Sarah Bockian: Investigation. Barbara McCrum: Methodology, Investigation, Project administration. Teresa L. May: Writing – review & editing. Douglas Sawyer: Resources, Funding acquisition. David B. Seder: Conceptualization, Methodology, Investigation, Resources, Funding acquisition, Writing – review & editing.
Acknowledgements
The funding sources provided financial support to complete the EFIC process as part of the PEARL and PROTECT trials. They did not contribute to study or community consultation design, the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. We thank the members of the southern Maine (USA) community for their participation and comments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2022.100322.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.https://www.ecfr.gov/on/2018-07-19/title-45/subtitle-A/subchapter-A/part-46.
- 2.World Medical Association Declaration of Helsinki ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–4. [DOI] [PubMed]
- 3.FDA. Guidance for institutional review boards, clinical investigators, and sponsors: exception from informed consent requirements for emergency research. Silver Spring, MD: Food and Drug Administration, Center for Drug Evaluation and Research: Division of Drug Information; 2013.
- 4.Dickert N.W., Metz K., Fetters M.D., et al. Meeting unique requirements: Community consultation and public disclosure for research in emergency setting using exception from informed consent. Acad Emerg Med. 2021;28:1183–1194. doi: 10.1111/acem.14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichol G., Zhuang R., Russell R., et al. Variation in time to notification of enrollment and rates of withdrawal in resuscitation trials conducted under exception from informed consent. Resuscitation. 2021;168:160–166. doi: 10.1016/j.resuscitation.2021.07.039. [DOI] [PubMed] [Google Scholar]
- 6.Carlson J.N., Zive D., Griffiths D., et al. Variations in the application of exception from informed consent in a multicenter clinical trial. Resuscitation. 2019;135:1–5. doi: 10.1016/j.resuscitation.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eubank L., Lee K.S., Seder D.B., et al. Approaches to community consultation in Exception from Informed Consent: Analysis of scope, efficiency, and cost at two centers. Resuscitation. 2018;130:81–87. doi: 10.1016/j.resuscitation.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Hsu C.H., Fowler J., Cranford J.A., Thomas M.P., Neumar R.W. Integration of social media with targeted emails and in-person outreach for exception from informed consent community consultation. Acad Emerg Med. 2022;29:217–227. doi: 10.1111/acem.14377. [DOI] [PubMed] [Google Scholar]
- 9.FDA. COVID-19-Related Guidance Documents for Industry, FDA Staff, and Other Stakeholders. Silver Spring, MD: Food and Drug Administration, Center for Drug Evaluation and Research: Division of Drug Information; 2021.
- 10.Kern K.B., Radsel P., Jentzer J.C., et al. Randomized pilot clinical trial of early coronary angiography versus no early coronary angiography after cardiac arrest without ST-segment elevation: the PEARL study. Circulation. 2020;142:2002–2012. doi: 10.1161/CIRCULATIONAHA.120.049569. [DOI] [PubMed] [Google Scholar]
- 11.International Cardiac Arrest Registry (INTCAR): https://mhir.org/?page_id=15952.
- 12.Feldman W.B., Hey S.P., Franklin J.M., Kesselheim A.S. Public approval of Exception From Informed Consent in emergency clinical trials: A systematic review of community consultation surveys. JAMA Netw Open. 2019;2:e197591. doi: 10.1001/jamanetworkopen.2019.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organizing Committee for Assessing Meaningful Community Engagement in Health & Health Care Programs & Policies. 2022. Assessing Meaningful Community Engagement: A Conceptual Model to Advance Health Equity through Transformed Systems for Health. NAM Perspectives. Commentary, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/202202c. [DOI] [PMC free article] [PubMed]
- 14.Feldman W.B., Hey S.P., Kesselheim A.S. A systematic review of the Food and Drug Administration’s ‘Exception From Informed Consent’ Pathway. Health Affairs. 2018;37:1605–1614. doi: 10.1377/hlthaff.2018.0501. [DOI] [PubMed] [Google Scholar]
- 15.Fehr A.E., Pentz R.D., Dickert N.W. Learning From experience: A systematic review of community consultation acceptance data. Ann Emerg Med. 2015;65:162–171. doi: 10.1016/j.annemergmed.2014.06.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.