Abstract
Certain bacteria, including overt pathogens as well as commensals, produce immunoglobulin A1 (IgA1) proteases. By cleaving IgA1, including secretory IgA1, in the hinge region, these enzymes may interfere with the barrier functions of mucosal IgA antibodies, as indicated by experiments in vitro. Previous studies have suggested that cleavage of IgA1 in nasal secretions may be associated with the development and perpetuation of atopic disease. To clarify the potential effect of IgA1 protease-producing bacteria in the nasal cavity, we have analyzed immunoglobulin isotypes in nasal secretions of 11 healthy humans, with a focus on IgA, and at the same time have characterized and quantified IgA1 protease-producing bacteria in the nasal flora of the subjects. Samples in the form of nasal wash were collected by using a washing liquid that contained lithium as an internal reference. Dilution factors and, subsequently, concentrations in undiluted secretions could thereby be calculated. IgA, mainly in the secretory form, was found by enzyme-linked immunosorbent assay to be the dominant isotype in all subjects, and the vast majority of IgA (median, 91%) was of the A1 subclass, corroborating results of previous analyses at the level of immunoglobulin-producing cells. Levels of serum-type immunoglobulins were low, except for four subjects in whom levels of IgG corresponded to 20 to 66% of total IgA. Cumulative levels of IgA, IgG, and IgM in undiluted secretions ranged from 260 to 2,494 (median, 777) μg ml−1. IgA1 protease-producing bacteria (Haemophilus influenzae, Streptococcus pneumoniae, or Streptococcus mitis biovar 1) were isolated from the nasal cavities of seven subjects at 2.1 × 103 to 7.2 × 106 CFU per ml of undiluted secretion, corresponding to 0.2 to 99.6% of the flora. Nevertheless, α-chain fragments characteristic of IgA1 protease activity were not detected in secretions from any subject by immunoblotting. Neutralizing antibodies to IgA1 proteases of autologous isolates were detected in secretions from five of the seven subjects but not in those from two subjects harboring IgA1 protease-producing S. mitis biovar 1. α-chain fragments different from Fcα and Fdα were detected in some samples, possibly reflecting nonspecific proteolytic activity of microbial or host origin. These results add to previous evidence for a role of secretory immunity in the defense of the nasal mucosa but do not help identify conditions under which bacterial IgA1 proteases may interfere with this defense.
The nasal mucosa is exposed to a large variety of inhaled substances, including microorganisms and potential allergens. For protection, the nasal cavity is lined by a ciliated pseudostratified epithelium, which is supplied continuously with mucous secretion and occasionally with inflammatory exudate of plasma origin (6, 16).
Nasal secretions contain immunoglobulins offering antibody-mediated defense. Previous studies indicate that a major part is in the form of secretory immunoglobulin A (S-IgA), but conflicting data exist regarding the contribution of serum-type immunoglobulins in the form of IgG and IgA (45). S-IgA antibodies mediate protection mainly by inhibiting microbial attachment and the absorption of molecular antigens, including potential allergens (43). The significance of serum-type antibodies in nasal secretions has not been clarified. The fact that parenteral immunization with antigens of mucosal pathogens may not only protect against infectious disease but also abrogate carriage of the causative organism (54) suggests that serum-type antibodies contribute to protection under some circumstances.
S-IgA antibodies are the effector molecules of the common mucosal immune system. In principle, this system provides for IgA antibodies induced at any mucosal site to be expressed as S-IgA in all secretions of the body by a particular mechanism of active secretion involving the polyimmunoglobulin receptor of secretory epithelial cells (4). Recent research, however, indicates a certain compartmentalization in the system. S-IgA antibodies in the secretions of the upper respiratory tract and in saliva appear to result primarily from antigenic stimulation of organized lymphoid follicles of the local mucosa, represented in humans by the pharyngeal, palatine, and lingual tonsils (also called Waldeyer's lymphoid ring) (38). Immunohistochemical studies of these follicles and the nasal mucosa have revealed a marked predominance of IgA1- over IgA2-producing cells (4). Based on these observations, S-IgA in nasal secretions is assumed to be mainly of the A1 subclass.
The subclass distribution of nasal S-IgA is of interest because several bacteria produce enzymes that selectively cleave IgA1, including S-IgA1, molecules in the hinge region, leaving them as intact Fabα and Fcα (or Fcα ·SC) fragments. Studies in vitro have indicated that such cleavage interferes with the protective functions of S-IgA antibodies, although the resulting Fabα fragments retain antigen-binding ability (25). IgA1 proteases are produced by several pathogens with the ability to colonize and potentially invade mucosal membranes, such as Haemophilus influenzae, Neisseria meningitidis, Neisseria gonorrhoeae, and Streptococcus pneumoniae. In addition, Streptococcus mitis biovar 1, Streptococcus oralis, and Streptococcus sanguis, which are numerically significant members of the oral commensal flora, produce such enzymes. Complete lists of organisms with documented IgA1 protease activity have been provided in reviews (25, 32).
IgA1 proteases have been shown to be targets of enzyme-neutralizing antibodies in serum and secretions (14), which may be induced in a state of bacterial carriage as well as during invasive infection (5). Accordingly, the effect of IgA1 proteases can be expected to depend on the balance of inhibiting antibodies and the amount of enzyme produced locally. Our previous detection of Fabα fragments on dental plaque bacteria (1) indicates that cleavage of IgA1 can occur under some circumstances in vivo. Moreover, as a point of relevance to nasal defense, Fabα and Fcα fragments have been detected in nasopharyngeal secretions collected from children under general anesthesia prior to adenoidectomy (46). Interestingly, cleavage of IgA was significantly more prevalent in children with a clinical history of atopic diseases than in controls, suggesting that IgA1 protease-induced deficiencies of secretory immunity may be a contributing factor in the development and perpetuation of these immunological dysfunctions (46). This hypothesis is further corroborated by the observation that in 18-month-old infants evidence of atopic disease was associated with elevated proportions of IgA1 protease-producing bacteria, mainly S. mitis biovar 1, in the oropharyngeal microflora (24).
Due to the scarcity of data on nasal microflora (57; T. T. Rasmussen, L. Kirkeby, J. Reinholdt, and M. Kilian, submitted for publication), it is not known to what extent oropharyngeal samples reflect the flora on the ciliated mucosa of the nasal cavity, which is presumably the more important site of atopic sensitization and reaction. To clarify the effect of IgA1 protease-producing bacteria on the mucosal immune barrier, we have characterized and quantified IgA1 protease-producing bacteria in the nasal flora of healthy humans and at the same time have analyzed immunoglobulin isotypes in nasal secretions of the subjects, with a focus on the concentration, subclass distribution, and molecular integrity of IgA. In addition, nasal secretions were examined for inhibiting activity towards IgA1 proteases of homologous bacterial isolates. Details of the comprehensive analyses of nasal flora are presented elsewhere (Rasmussen et al., submitted).
MATERIALS AND METHODS
Study population.
Ten adult volunteers (age range, 25 to 54 years) and a 9-year-old child were included in the study (the child was included after receipt of the informed consent of the parents). All subjects were healthy and did not experience symptoms of upper respiratory tract infection during the study. Although five adults experienced symptoms of atopic reactions to unidentified allergens during part of the year, they were all symptomless during the period of sampling. The study was approved by the ethics committee of the county of Aarhus, Denmark.
Sampling methods.
Three methods were selected on the basis of pilot experiments. The first method aimed at obtaining secretion from a localized area also accessible to microbiological sampling by swab. A piece of Whatman filter paper (0.5 by 1 cm) was placed on the inferior turbinate by use of sterile tweezers guided by a nasal speculum. When the filter paper was soaked (after 10 to 20 min), it was transferred to a microtube and immediately boiled in 100 μl of sample buffer for electrophoretic analysis.
The second method, which sampled from a larger area corresponding to the anterior part of the nasal cavity, used two inflatable urine catheters (code MA180003; Rüsch Manufacturing, Herfølge, Denmark), one for each nostril, placed with the balloon in the vestibule. The balloons were filled with water from a syringe until the nostrils were completely obstructed. Ten milliliters of phosphate-buffered saline, pH 7.4 (PBS), at room temperature was then introduced through each catheter, with the subject's head being leaned forward. After a few seconds, water was withdrawn from the balloons, allowing the nasal wash to escape through the nostrils into a beaker.
The third method sampled from the posterior part of the nasal cavity, including the nasopharynx. The subject was placed in a horizontal position. While the subject was holding his or her breath and pressing the soft palate upwards to close the nasal cavity, 10 ml of PBS was introduced through one of the nostrils with a syringe mounted on a central venous catheter without stiletto (Omehda, Swindon, Wiltshire, United Kingdom) that had been preintroduced into the nasal cavity to reach the posterior wall. After a few seconds, the washing fluid was withdrawn into the syringe and transferred to a sterile tube.
Each adult subject went through sampling by the three methods in one day. Sampling by method 1 was always first, followed by method 2, and then, with an interval of not less than 0.5 h, by method three. In addition, some adults were sampled repetitively by method 3 at intervals of 3 to 8 days.
The child was sampled exclusively by a simplified washing method. While she was sitting on a chair with a low back, she was instructed to lean her head maximally backwards and close her nasal cavity like for method 3. Eight milliliters of PBS was then introduced by using a syringe without cannula placed in one nostril. The fluid was recovered through the nostrils upon the child tilting her head forward.
Like the filter paper samples, parts of nasal wash samples were immediately processed for electrophoresis. Wash samples obtained by method 3 were also cultured on agar plates (see below) after vigorous vortexing. Remaining sample volumes were centrifuged at 10,000 × g for 10 min, and the supernatant was stored in aliquots at −20°C.
Determination of dilution factor.
To be able to determine the dilution factor of nasal secretion in the wash, the buffer was supplemented with 2 mM lithium in the form of LiOH as an inert marker. The concentration of lithium in the wash was measured by flame emission photometry at the Psychochemical Laboratory, Psychiatric Hospital, Risskov, Denmark, using the 2 mM lithium-containing buffer as standard. From the measured concentration, the dilution factor was calculated as described previously (26). The coefficient of variation of repetitive measurements with the photometer was 1.5%.
Quantification of immunoglobulin isotypes.
The enzyme-linked immunosorbent assay (ELISA) procedures used for the quantification of total IgA, IgA1 and IgA2 subclasses, IgG, and IgM in samples of nasal wash are presented in schematic form in Table 1. Polystyrene microplates with high coating efficiency (Maxisorp; Nunc, Roskilde, Denmark) were used as the solid phase. All reagents were added in volumes of 100 μl and all incubations were at room temperature for 2 h, except for coating (step 1), which was done overnight. For coating, antibody preparations were diluted to a protein concentration of ca. 5 μg ml−1 in PBS containing 4 mM sodium azide (PBSA). Two mouse monoclonal antibodies (MAbs) specific for IgA1 and IgA2, respectively, were obtained from Nordic, Tilburg, The Netherlands, in a form unsuitable for coating (ascites). Therefore, in the IgA subclass-specific assays, these MAbs were immobilized indirectly via a coating layer of polyclonal antibody [F(ab)2 fragments] specific for mouse immunoglobulin. This antibody as well as the biotinylated antibodies (step 3 or 4) were of affinity-purified grade. Polyclonal antibodies (all of rabbit origin) and alkaline phosphatase-conjugated streptavidin were the products of DAKO, Glostrup, Denmark. PBSA containing 0.15% Tween 20 was used for blocking and washing of wells between steps and as a diluent for all protein reactants, including samples and standards. Working dilutions for biotin conjugates (1:2,000), MAbs (1:2,000), and streptavidin (1:2,000) were selected on the basis of checkerboard titrations with immunoglobulin standards as samples. For all assays, the background signal obtained with diluent substituting for sample was less than 15% of the level corresponding to saturation of the assay. In addition, nonspecific binding of test sample immunoglobulins (2), standards, or subsequent reactants to the Maxisorp plate material was negligible as demonstrated by control titrations of nasal wash samples in uncoated, blocked wells.
TABLE 1.
ELISA procedures for quantification of total IgA, IgA1 and IgA2 subclasses, IgG, and IgM in human nasal wash
| Step | Quantification of isotype
|
||||
|---|---|---|---|---|---|
| IgG | IgM | Total IgA | IgA1 | IgA2 | |
| 1 | Anti-γ chain | Anti-μ chain | Anti-α chain | RaMb F(ab)2 | RaM F(ab)2 |
| 2 | Sample or standarda | Sample or standard | Sample or standard | Anti-A1 MAb | Anti-A2 MAb |
| 3 | Anti-γ chainbioc | Anti-μ chainbio | Anti-α chainbio | Sample or standard | Sample or standard |
| 4 | StreptavidinAPd | StreptavidinAP | StreptavidinAP | Anti-α chainbio | Anti-α chainbio |
| 5 | StreptavidinAP | StreptavidinAP | |||
Serial threefold dilutions starting at 1:75 (1:15 for IgA2 assay) for nasal wash and at 0.33 μg/ml (3.30 μg/ml for IgA2 assay) for immunoglobulin isotype standard. For a description of the standards, see the text.
RaM, rabbit anti-mouse immunoglobulin.
Biotin conjugate.
Alkaline phosphatase conjugate.
A human serum pool with immunoglobulin isotype contents calibrated against other commercial standards (Human Serum Protein Calibrator [code X908]; DAKO) was used as a standard in IgG and IgM assays. Standards in assays of total IgA, IgA1, and IgA2 were in the molecular form of S-IgA (dimeric), because this was the predominant form of IgA in the nasal wash samples (see Results). The S-IgA standards were purified as described previously (2). Briefly, S-IgA prepared from human colostrum was separated by affinity chromatography into S-IgA1 and S-IgA2 by using a column of agarose-bound Jacalin (Vector Laboratories, Burlingame, Calif.). For each subclass, the dimeric form (Mr of ∼400,000 [400K], corresponding to 11S [30]) was isolated by size exclusion chromatography. Concentrations of purified proteins were determined spectrophotometrically by using E (1 cm, 1%, 280 nm) = 13.4 for both subclasses (20). When the two subclass standards were titrated in parallel in the assay for total IgA (Table 1), almost-identical dose-response curves were obtained, with 1 μg of S-IgA2 being the immunochemical equivalent of 1.2 μg of S-IgA1 (results not shown). With this background, we used S-IgA1 as a standard in assays of total IgA.
Plates were developed with a solution of para-nitrophenyl phosphate (1 mg ml−1 in 0.5 M diethanolamine buffer [pH 9.8] containing 1 mM MgCl2) and read on a Titertek Multiscan ELISA reader (Flow Laboratories, Glasgow, Scotland) at 405 nm. Dose-response curves for standards were constructed by fitting a four-parameter asymmetrical sigmoid model (Fig P; Biosoft, Cambridge, United Kingdom). The level of an isotype in a sample was calculated as the mean of three determinations based on optical densities (ODs) for sample dilutions corresponding to the log-linear segment of the standard curve. Generally, the coefficient of variation for the three determinations was less than 5%.
Size exclusion chromatography.
To examine the molecular form of IgA in nasal secretions, samples (0.3 ml) of nasal wash obtained by method 3 were separated on a column (1.0 by 30 cm) of Superose 6 HR equilibrated in PBSA and attached to a Pharmacia-LKB high-pressure liquid chromatography system. Fractions of 0.5 ml were collected at a flow rate of 0.5 ml min−1, with the eluted protein being monitored at 280 nm. The column had been calibrated with Mr standards, including colostral S-IgA (11S) and monomeric (7S) IgA1 myeloma protein (our own product [1]).
Eluent fractions were examined with two different ELISAs. One was the assay for total IgA (Table 1). The other assay, detecting exclusively S-IgA, was similar except that biotinylated anti-α-chain antibody (step 3) was replaced by biotinylated anti-secretory component (SC). The latter conjugate was prepared by biotinylation of a commercial rabbit anti-secretory component antibody (code A187; DAKO) with N-hydroxysuccinimidobiotin according to the protocol suggested by the manufacturer (Sigma, St. Louis, Mo.). S-IgA, in fractions where it occurred, was quantified with the S-IgA-specific assay by using purified S-IgA1 as a standard. IgA in other fractions was quantified by using the assay for total IgA (Table 1) with Human Serum Protein Calibrator (DAKO) as the source of the standard.
Immunoblotting analysis of nasal IgA.
The integrity of IgA in samples of nasal secretion was examined essentially as described previously (1). Briefly, samples in filter paper or in the form of nasal wash were diluted approximately fivefold in reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer, boiled for 5 min, and centrifuged at 10,000 × g for 4 min. Ten-microliter volumes of the supernatant were electrophoresed in 8- by 10-cm, 1-mm-thick 4 to 20% polyacrylamide gradient gels, with lanes of Mr standards included in each gel, using a Mini Protean II Slab Cell (Bio-Rad, Richmond, Calif.). To provide for an optimal amount of sample protein in the analysis, lanes representing serial twofold dilutions of the sample were generally included. Separated proteins were electroblotted onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.) by using the Mini Protean II blotting cell operated at 120 mV for 2 h. Pilot experiments, including control staining of blotted gels with Coomassie brilliant blue, showed complete transfer of α heavy chains and fragments thereof under these conditions. Blots were developed by sequential incubation with biotinylated anti-α-chain antibody (DAKO) and alkaline phosphatase-conjugated streptavidin (DAKO) followed by a chromogenic substrate of 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium. Lanes of Mr standards were stained separately with amido black.
Microbiological analyses.
Studies of the nasal microflora employed two different types of samples. One sample was obtained from the mucosa of the inferior turbinate with a cotton swab immediately after sampling of the secretion from this site with filter paper. To prevent contamination by the vestibular flora, the swab was protected within a sterile plastic straw during introduction and withdrawal. The second sample was wash from the posterior part of the nasal cavity collected by method 3. Swabs and nasal wash were cultured on 5% horse blood agar, mitis salivarius agar, and chocolate agar with bacitracin, the last two media for selective recovery of streptococci and Haemophilus spp., respectively (Rasmussen et al., submitted). After incubation for 3 days at 37°C in an atmosphere of air plus 5% carbon dioxide, duplicate colonies of each morphotype detected on these media were subcultured for identification. Isolates were examined for IgA1 protease activity by immunoelectrophoresis (30).
Titration of IgA1 protease and IgA1 protease-inhibiting activity.
IgA1 proteases were prepared in the form of overnight culture supernatants by using appropriate media (40). Activity of preparations was titrated by an assay based on ELISA technology and recorded in terms of the C50 titer, i.e., the inverse of the dilution at which the preparation cleaved 50% of IgA1 under the conditions of the assay (39).
Nasal wash samples were titrated for inhibiting activity against IgA1 proteases of individual isolates by using a previously described assay (39). This assay is well suited for human test samples the titration of which may otherwise be problematic because of their content of inherent IgA1. Briefly, serial twofold dilutions of the inhibitor test sample were incubated with a fixed amount of IgA1 protease defined for the assay. For each dilution, uninhibited protease activity, if present, was subsequently quantified by its activity during continued incubation with cleavable IgA1. Essentially, the assessment of IgA1 cleavage in the assay is based on the inability of cleaved as opposed to intact IgA1 to cause an OD signal in an ELISA using Fcα-specific antibody for capture and enzyme-labeled anti-light-chain antibody for detection. The inhibition titer of a test sample was expressed as the CI50 titer, i.e., the sample dilution corresponding to 50% inhibition of IgA1 cleavage (39).
RESULTS
Evaluation of sampling methods.
The filter paper method collected 10 to 20 mg of secretion and was well accepted by the subjects. Method 2 caused only moderate discomfort to most subjects and provided ample wash which was, however, contaminated with bacteria and secretion residuals from the vestibulum. Accordingly, these samples were inadequate for microbiological analysis. Method 3 initially caused discomfort to most of the adults and was unacceptable to the child. Nevertheless, after training, all adults complied, and 5 to 7 ml of the original 10 ml of liquid could be recovered and was presumably free of vestibular and oral contaminants. Two of 28 samples in total collected by this method were discarded because of minute blood contamination, which was readily detected by inspection of the pellet after centrifugation. The modified method of washing without the use of a catheter was well accepted by the child.
Molecular forms of IgA in samples of nasal secretion.
Different forms of IgA have been found to perform differently in quantitative solid-phase immunoassays (8, 9). To select appropriate calibration standards for quantitative ELISA, therefore, it was necessary first to analyze the molecular form of IgA in the test samples. Five samples of nasal wash collected by method 3, all originating from subjects harboring IgA1 protease-producing nasal bacteria, were subjected to size exclusion chromatography and subsequent analysis of eluent fractions for total IgA.
In all five cases IgA was found to be distributed in two distinct peaks, a major and a minor peak, which appeared to represent S-IgA and monomeric serum IgA, respectively, by reference to column calibration standards (Fig. 1A). In support of this interpretation, the major but not the minor peak was reproducible with the ELISA specific for S-IgA (Fig. 1A). The merging curvatures of major peaks obtained with the two ELISAs (Fig. 1A) indicated that the dimeric form of serum IgA (Mr, ∼320K), if present, constituted a very small proportion of IgA in the samples. No signs of IgA1 protease-induced fragments were revealed, corroborating the results of immunoblotting analysis (see below).
FIG. 1.
Size exclusion chromatography profiles of IgA in a sample of nasal wash from subject B (sample B3,1 [Tables 2 and 3]). (A) OD profiles obtained by analysis of eluent fractions in ELISA for total IgA (▴) and S-IgA only (●). Distinct peaks of S-IgA and monomeric serum IgA were identified immunochemically and by reference to column calibration standards. Elution volumes for standards of 11S S-IgA and monomeric (7S) serum IgA are indicated. (B) Plot of IgA concentrations determined by titration of eluent fractions, using, within each peak, the relevant ELISA. By planimetry, S-IgA was found to account for 86% of total IgA in the sample.
From IgA concentration plots (Fig. 1B) generated by titration of eluent fractions, S-IgA was estimated to constitute the majority of IgA in the five samples (Table 2). Generally, the S-IgA peak in such plots was asymmetrical (Fig. 1B), indicating that part of the S-IgA was represented by molecules larger than the 11S calibration standard corresponding to the apex of the peak.
TABLE 2.
Immunoglobulin concentrations in 14 samples of nasal wash (obtained by method 3) from eight adults and 1 sample from a child as determined by isotype-specific ELISAa
| Subject and sampleb | Total IgA (μg ml−1) | S-IgA (%) | IgA1c (%) | IgG (μg ml−1) | IgM (μg ml−1) |
|---|---|---|---|---|---|
| A3,1 | 16.2 | 88 | 92 | 6.1 | 0.6 |
| B3,1 | 10.7 | 86 | 91 | 7.1 | 0.4 |
| B3,2 | 16.0 (458)d | NDe | 91 | 1.3 (37.2) | 0.4 (11.4) |
| C3,1 | 31.5 | 93 | 80 | 6.1 | 1.5 |
| D3,1 | 73.4 | 87 | 75 | 35.4 | 1.9 |
| E3,1 | 9.6 | 97 | 91 | 0.2 | 0.2 |
| E3,2 | 60.2 (1,336) | ND | 95 | 2.0 (44.4) | 1.1 (24.4) |
| E3,3 | 24.9 (553) | ND | 88 | 2.8 (62.2) | 0.4 (8.9) |
| F3,1 | 44.8 (896) | ND | 84 | 1.4 (28.0) | 0.3 (6.0) |
| F3,2 | 10.4 (231) | ND | 91 | 1.2 (26.6) | 0.1 (2.2) |
| G3,1 | 34.8 (870) | ND | 94 | 3.9 (97.5) | 0.4 (10.0) |
| G3,2 | 12.0 (343) | ND | 93 | 0.4 (11.4) | 0.1 (2.9) |
| G3,3 | 39.5 | ND | 91 | 1.0 | 0.3 |
| H3,1 | 116.3 (2,326) | ND | 93 | 5.5 (110) | 2.9 (58.4) |
| Iw,3f | 6.5 | ND | 89 | 0.2 | 0.05 |
| Median (range) | (711 [231–2,326]) | 88 [86–97] | 91 [75–95] | (40.8 [11.4–110]) | (9.5 [2.2–58.4]) |
IgA data include subclass distribution and, for five samples, estimates of S-IgA relative to total IgA. Calculated immunoglobulin levels in undiluted secretions are included for eight samples obtained with washing medium containing 2 mM lithium as an internal standard.
The first number indicates the method, and the second number indicates the sample.
Calculated as [IgA1] × ([IgA1] + [IgA2])−1 × 100%.
Numbers in parentheses indicate the concentration in the undiluted secretion (calculated for lithium-containing samples only).
ND, not determined.
Third sample of wash from the child (subject I).
Immunoglobulin levels according to isotypes and IgA subclasses.
Having found that S-IgA was the predominant molecular form of IgA in nasal wash samples, we used this form of standards for quantification of total IgA, IgA1, and IgA2 by ELISA. Total IgA levels measured in 15 samples of nasal wash, including one sample from the child, varied from 6.5 to 116.3 (median, 24.9) μg ml−1 (Table 2). For eight samples obtained from adults with lithium-containing washing medium, dilution factors of nasal secretion in wash were determined at a range of 20.0 to 28.6 (median, 23.2). By that means, total IgA levels in undiluted secretions were calculated to be in a wide range of 231 to 2,326 (median, 711) μg ml−1 (Table 2). Large differences in undiluted IgA were also observed between repetitive samples collected at an interval of 3 to 8 days (Table 2, subjects E, F, and G). By using subclass-specific assays, the vast majority (median, 91%; range, 75 to 95%) of IgA in the 14 samples was found to be IgA1. The sum of IgA1 and IgA2 levels determined for a sample deviated from the level of total IgA determined for that sample by a median of 8.6% (range, 0.2 to 13.4%), testifying to the validity of these analyses.
In addition to IgA, IgG and IgM were detected in all nasal wash samples. Levels of IgM and IgA were correlated (Spearman's r = 0.76; P < 0.01), but IgM levels generally did not exceed 5% of the IgA level (Table 2). IgG, however, varied considerably and was not significantly correlated to IgA (Spearman's r = 0.52; P > 0.05). Although IgG was low in 11 of the 15 samples, it amounted to between 20 and 66% of IgA levels in four samples (Table 2). For the eight lithium-containing samples, IgG levels in undiluted secretions were calculated to be in a range of 11.4 to 110 (median, 40.8) μg ml−1 (Table 2).
Total immunoglobulin levels, calculated as the sum of IgA, IgG, and IgM, in undiluted secretions (n = 8) ranged between 260 and 2,494 (median, 777) μg ml−1.
IgA fragmentation in relation to occurrence of IgA1 protease-producing bacteria.
A total of 46 secretion samples were examined for fragmentation of α chains by immunoblotting. These included sets of three samples from each of the 10 adults collected by methods 1, 2, and 3, respectively. Also included were seven additional samples from adults collected by method 3 and nine consecutive wash samples from the child.
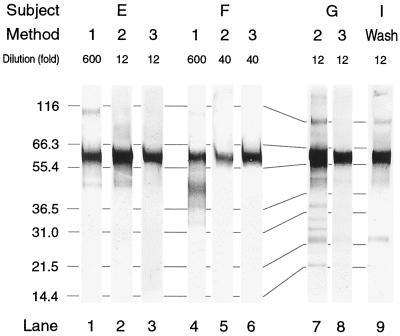
All samples displayed a strongly stained band identified as intact α heavy chain (Mr, ∼58K) by reference to Mr standards (Fig. 2). Many samples in addition showed a number of less prominent bands. Among these, a cluster of bands at 43 to 48K was particularly prevalent (Fig. 2, lanes 1, 2, 4, 7, and 9). Some of the samples showing this cluster also displayed other faint bands at lower Mrs, occasionally including two at ∼23 and ∼14K (Fig. 2, lanes 7 to 9). Bands at Mrs below that of intact α chains occurred predominantly in samples originating entirely or partly from the anterior part of the nasal cavity, i.e., in method 1 (filter paper) samples, in wash obtained by method 2, and in wash from the child (Fig. 2). Bands at Mrs above that of α chains occurred in some blots of abundantly loaded gel lanes (Fig. 2), possibly reflecting incomplete dissociation of IgA molecule components.
FIG. 2.
Selected immunoblots from three adults (subjects E, F, and G) and a child (subject I), illustrating the patterns of anti-α-chain antibody-reactive bands obtained from samples of nasal secretions. Samples obtained by different methods from individual adults were collected during one day. The dilutions of individual samples in reducing sample buffer prior to analysis are indicated. Note that all samples display a prominent band corresponding to intact α chain (Mr, ∼58K). For interpretation of other bands, see the text. Lane 9 represents sample Iw,3 from the child, from which H. influenzae was cultured (Table 3). Numbers on the left indicate molecular weight standards in kilodaltons.
Microbiological analyses, as described in detail elsewhere (T. T. Rasmussen et al., submitted for publication), revealed IgA1 protease-producing bacteria in one sample of wash from each of five adults and one sample from the child (Table 3). In addition, one filter paper sample could be assumed to originate from a site colonized by such bacteria based on the finding of 0.6% IgA1 protease-producing S. mitis biovar 1 in the corresponding swab (Table 3).
TABLE 3.
Cumultative data on IgA1 protease-producing bacteria and homologous IgA1 protease-inhibiting activity in samples (obtained by method 3) of nasal wash from six adults and a child
| Subject and samplea | IgA1 protease-producing bacteria
|
IgA1 protease-inhibiting activity
|
|||||
|---|---|---|---|---|---|---|---|
| Organism | Estimated density in undiluted secretion (CFU ml−1)b | % of total flora | IgA1 protease activity in vitro (C50)c | In nasal wash (CI50 titer) | Relative to total Ig conc (CI50 titer μg−1 ml) | Estimated for undiluted secretion (CI50 titer)d | |
| A3,1 | S. pneumoniae | 2.1 × 103 | 0.2 | 410 (275–5,066) | 4.2 | 0.18 | 97.5 |
| B3,1 | S. mitis biovar 1 | 6.8 × 104 | 22.3 | 1,621 (5.2–2,093) | 0.0 | 0 | 0 |
| C3,1 | H. influenzae | 7.2 × 106 | 99.6 | 4,760 (52–12,830) | 8.6 | 0.22 | 199 |
| D3,1 | H. influenzae | <1.5 × 104 | <0.01 | 639 (52–12,830) | 18.2 | 0.16 | 422 |
| E3,1 | S. mitis biovar 1 | 0e | 0 | 1,519 (5.2–2,093) | 0.0 | 0 | 0 |
| G3,3 | H. influenzae | >3 × 106 | NDf | 2,029 (52–12,830) | 51.0 | 1.26 | 1,183 |
| Iw,3 | H. influenzae | >3 × 106 | ND | 5,120 (52–12,830) | 10.4 | 1.58 | 241 |
Designations are as in Table 2.
Calculated as CFU per milliliter of wash × 23.2 because individual dilution factors for these samples were not available.
C50 titer of a stationary-phase liquid culture (range of previously recorded [40] C50 titers for strains of the species).
Calculated as CI50 for nasal wash × 23.2.
IgA1 protease-producing S. mitis biovar 1 was not detected in nasal wash but constituted 0.6% of the CFU in a swab corresponding to a filter paper sample.
ND, not determined.
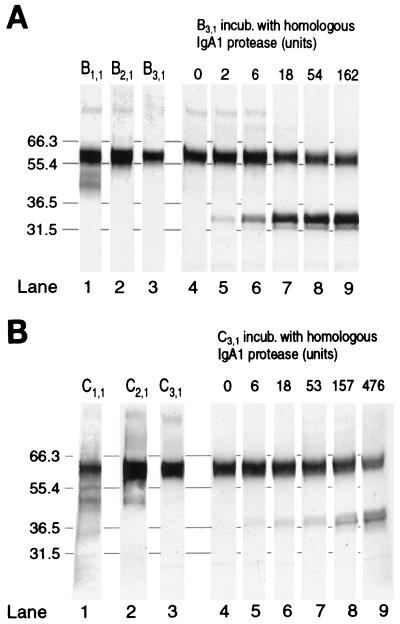
The occurrence of IgA1 protease-producing bacteria, however, was not reflected in immunoblots. Evidence of this in the case of one adult (subject B) is presented in Fig. 3A. IgA1 protease-producing S. mitis biovar 1 apparently constituted 22.3% of the bacteria in nasal wash from this subject, corresponding to a calculated S. mitis biovar 1 density of 6.8 × 104 CFU per ml of undiluted secretion (Table 3). The lack of IgA1 protease-induced α-chain fragments in all three types of samples became evident by comparison with control immunoblots of cleaved IgA in aliquots of the method 3 sample incubated in vitro with various amounts of IgA1 protease from the S. mitis biovar 1 isolated from the subject. By displaying bands corresponding to Fcα and Fdα fragments, the control blots showed that the natural IgA1 of the secretion was susceptible to cleavage in a dose-dependent manner (Fig. 3A). Notably, no α-chain fragments were revealed in control blots of sample incubated without added protease (Fig. 3A, lane 4), indicating that IgA1 protease possibly present in the wash after release from bacteria in vivo was insufficient to cause detectable cleavage.
FIG. 3.
Immunoblots providing evidence for the lack of detectable IgA1 protease-induced IgA1 fragments in nasal secretions from subject B (A) and subject C (B), harboring IgA1 protease-producing S. mitis biovar 1 and H. influenzae, respectively. Blots of synchronous samples obtained by the three methods (lanes 1, 2, 3) are shown together with blots of aliquots of the method 3 sample incubated (incub.) with various amounts of homologous IgA1 protease (lanes 5 to 9) or with buffer (lanes 4). Note that faint bands displayed by secretion samples do not correspond to IgA1 protease-induced α-chain fragments. Numbers on the left indicate molecular weight markers in kilodaltons.
In another subject, IgA1 protease-producing H. influenzae constituted 99.6% of the cultivable flora, corresponding to a calculated density of 7.2 × 106 CFU of H. influenzae per ml of undiluted nasal secretion (Table 3). Immunoblots demonstrating the lack of IgA1 protease-induced α-chain fragments in the secretion of that subject are presented in Fig. 3B.
Prompted by these results and our previous observation of large quantitative differences in IgA1 protease activity in vitro among species and strains producing this type of enzyme (40), we assessed the IgA1 protease activities of the isolates from the subjects by titration of activity in stationary-phase cultures. The titers measured, when compared with titers previously measured for strains of the relevant species, indicated that none of the isolates had distinctly low activity (Table 3).
IgA1 protease-inhibiting activity.
Nasal wash samples from which IgA1 protease-producing bacteria had been isolated were titrated for inhibiting activity against the IgA1 proteases produced by the relevant isolates. In the case of one subject where protease-producing bacteria were isolated from a swab sample (subject E) (Table 3), a concomitant sample of nasal wash was titrated. The secretions of five subjects harboring either H. influenzae or S. pneumoniae were all inhibitory (Table 3). In contrast, no inhibiting activity was observed in two subjects harboring IgA1 protease-producing S. mitis biovar 1, although in one of them (subject B), these bacteria apparently constituted 22.3% of the flora (Table 3).
To characterize the secretion components responsible for inhibiting activity, column eluent fractions of one inhibitory sample (sample D3,1) were tested in the inhibition assay. Inhibiting activity was restricted to fractions containing S-IgA or serum immunoglobulins, roughly reflecting the relative amounts of these immunoglobulin forms (results not shown). This observation indicates that in nasal secretion, like serum, colostrum, and saliva (14, 39), IgA1 protease-inhibiting activity is mediated by enzyme-neutralizing antibodies.
To compensate for influence of variable immunoglobulin contents of the samples, inhibition results (Table 3) are also recorded in the form of titer relative to total immunoglobulin concentration. Hypothetical inhibition titers for undiluted secretions (Table 3) were calculated by using the median dilution factor of 23.2 determined for lithium-containing samples because individual dilution factors were not available for the relevant samples.
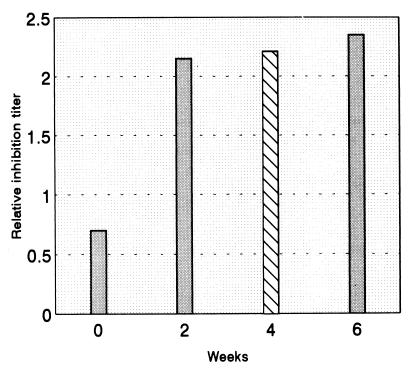
Presumably, cleavage of IgA1 can be expected in individuals who have acquired IgA1 protease-producing bacteria but have not yet responded with antibodies to the protease. As this situation is most likely to occur in early life (28), samples of nasal wash were collected from the child at regular intervals of 2 weeks. IgA1 protease-producing H. influenzae was isolated from one of nine samples (Table 3). However, considerable inhibiting activity to the protease of the isolate was found not only in this and subsequent samples but also in a sample collected 2 weeks before the isolation of the bacteria (Fig. 4). A sample collected 4 weeks in advance was also inhibitory, although at a lower titer (Fig. 4).
FIG. 4.
Inhibition titers against the IgA1 protease of an autologous H. influenzae isolate relative to total immunoglobulin concentration in sequential nasal wash samples from a 9-year-old child. H. influenzae was isolated from one sample (hatched bar) only.
DISCUSSION
Mucosal surfaces, including the nasal mucosa, are the principal sites of initial contact with a large variety of antigenic substances. A capacity for differentiation of the resulting immune responses seem to exist. Responses to pathogenic microorganisms and their harmful products usually include systemic immune reactions as well as secretory antibodies in the form of S-IgA. In contrast, nonthreatening antigens receive limited immunological attention, at least in the systemic compartment, in normal individuals. This low responsiveness, denoted mucosal tolerance, also affects IgE responses (36, 52) and is believed to be an important means of maintaining homeostasis of mucosal membranes and underlying tissues during the constant exposure to harmless environmental substances, including foodstuffs, the numerous antigens of an essentially beneficial commensal flora, and potential allergens (50, 51, 56). For poorly understood reasons (12, 51), the induction of mucosal tolerance to certain antigens may fail or be subsequently broken. In such cases, the prevention of atopic sensitization and subsequent atopic reaction may depend on mucosal barrier functions, including antibodies in secretions.
This study indicates that immunoglobulins in secretions covering the noninflamed nasal mucosae of healthy individuals are predominantly S-IgA in the ∼400-kDa (11S) form. The small amounts of S-IgA displaying an Mr of above 400K by size exclusion chromatography (Fig. 1) were not analyzed but might represent the 15S isoform of S-IgA (30) and potential immune complexes.
Serum-type immunoglobulins, mainly IgG and serum IgA, contributed significantly in a few cases (Table 2). Studies of nasal secretions from rhinitis patients have shown higher proportions of serum-type IgA relative to S-IgA and almost equal levels of the IgG and IgA isotypes (17, 34, 45). Presumably, this reflects an increased supply of IgG and monomeric serum IgA by way of inflammatory exudate, as these immunoglobulins, unlike S-IgA, reach secretions by passive diffusion (55). Nevertheless, IgG and serum IgA have also been found to account for large proportions of immunoglobulins in nasal secretions of individuals without nasal inflammation (34, 45). The discrepancy between these data and ours may reflect differences in the quantitative methods employed. Thus, several previous studies have used radial immunodiffusion, which is likely to underestimate S-IgA relative to monomeric immunoglobulin forms (10, 15). In one study using ELISAs comparable to ours, S-IgA was found to account for a median of 57% (range, 39 to 77%) of total IgA in nasal secretions of healthy children, but the analyses did not include separation prior to quantification of serum-type IgA and S-IgA (48).
The finding that levels of IgM, but not significantly those of IgG, were correlated with S-IgA levels corroborates previous observations (48) and may reflect the fact that only IgM shares the active secretion mechanism of S-IgA (4).
No attempts were made to quantify IgD and IgE, which have been found to be present at low levels in nasal secretions of healthy individuals (47, 49).
Analysis of repetitive samples from single individuals (Table 2) revealed considerable differences in immunoglobulin (mainly IgA) levels in undiluted nasal secretion, in accordance with previous observations (34, 45). Previous studies of nasal secretion (18, 33) as well as saliva (13) have indicated that such differences may be explained by variations in secretion rate in combination with the existence of an inverse relationship between the IgA concentration and the rate of secretion. Both of these parameters for nasal secretion have been found to undergo a certain diurnal variation (18, 33). Thus, the observed variation in IgA levels in undiluted secretions may be partly due to the fact that subjects were not sampled at fixed hours of the day.
It has long been assumed, on the basis of immunohistochemical studies of mucosal immune cells (23, 45), that S-IgA of the A1 subclass is the predominant immunoglobulin in nasal secretions. Nevertheless, to our knowledge, this study is the first to verify this.
The predominance of IgA1 is of interest in relation to antibody-mediated defense because of the exclusive susceptibility of this subclass to bacterial IgA1 proteases. However, evidence of IgA1 protease activity in vivo was not detected in any of 46 samples of nasal secretion from 11 subjects, 7 of whom harbored IgA1 protease-producing bacteria at the time of sampling as documented by culture. In five of the seven subjects, the presence of IgA1 protease-inhibiting antibodies in nasal secretions offered an explanation to the lack of detectable Fcα and Fabα fragments. However, such fragments were also not detected in two subjects with negative inhibition test results. These two harbored IgA1 protease-producing S. mitis biovar 1, one of them apparently in large numbers.
For an interpretation of this outcome, it may be relevant that oral S. mitis biovar 1 populations within a single host have been found to display considerable clonal heterogeneity (21), including serological diversity of IgA1 proteases as detected by enzyme-neutralizing antibodies (J. Reinholdt, unpublished data). Hypothetically, a similar clonal diversity might occur in nasal S. mitis biovar 1 populations. In addition, clones of oral streptococci may not always be distinguished by colony form (42). Thus, the negative results of the inhibition tests done with IgA1 proteases from only two colony-morphotypical isolates do not rule out the possibility that enzyme-neutralizing antibodies to IgA1 proteases of other S. mitis clones, if present, were partly responsible for the lack of IgA1 cleavage in the two subjects. Nevertheless, because the proteases of the isolates were uninhibited, the result for these subjects was unexpected in view of our previous detection of cleaved IgA1 in nasopharyngeal secretions collected from children prior to adenoidectomy (46).
On a speculative note, the contrasting results for nasal secretions in this study and the previous study (46) might relate to the efficiency of nasal mucociliary clearance in the two populations studied. In individuals free of any nasal disease, mucosally applied tracer substances are cleared to the pharynx in less than 30 min (35). Significant cleavage of IgA1 myeloma protein within such short periods has been obtained in vitro, but only with concentrated IgA1 protease preparations (31). Conversely, in patients with adenoid hyperplasia or polyposis, the highly viscous mixture of secretion and exudate can be expected to impair mucociliary transport (16). This, in turn, would lead to prolonged exposure of secreted IgA1 to bacteria accumulating under such conditions. Cleavage of IgA1 in the bacterial accumulations of dental plaque, as previously detected (1), probably occurs under similar favorable conditions.
In this context it should be mentioned that early studies indicating the presence of IgA1 protease activity in secretions from patients infected with H. influenzae (22) or N. gonorrhoeae (3) involved incubation with IgA1 substrate for periods of 10 to 18 h. In the present study, similar extensive incubation of nasal secretions from subjects harboring IgA1 protease-producing bacteria did not result in cleavage of IgA1 (Fig. 3, lanes 4). Possible explanations for this include the diluted state of the secretions and, in some cases, the effect of protease-inhibiting antibodies.
H. influenzae and S. pneumoniae are frequently carried by a high proportion of healthy children. For both species it has been found that a particular clone colonizes an individual only for a limited period, after which it disappears, presumably due to a clone-specific immune response (for a review, see reference 28). Considering that IgA1 proteases from these species display extensive antigenic diversity (28), IgA1 proteases of new clones are likely to be unaffected by preexisting antibodies. However, when IgA1 protease-producing H. influenzae was isolated from one of nine samples collected at biweekly intervals from the 9-year-old child, protease-inhibiting activity was present not only in this and subsequent samples but also in two preceding samples. Accordingly, no evidence of IgA1 protease activity was found. Hypothetically, the preexisting antibodies could have been induced by the isolate itself (or by a clone with a cross-reactive IgA1 protease) during potential colonization of other mucosal sites prior to its occurrence in the nasal cavity.
The complexity of factors regulating the effect of IgA1 proteases in vivo is also evident from a recent study of genital tract secretions from women infected with N. gonorrhoeae. Using an immunoblotting technique, Hedges and coworkers (19) failed to detect IgA1 protease-induced IgA1 fragments in any of 20 such secretions, irrespective of whether protease-inhibiting antibodies were present.
Some secretion samples, when analyzed by immunoblotting, did display α-chain fragments, but these differed from fragments induced by IgA1 proteases. Identification of the fragments was not attempted. Those of ∼43 to 48 and ∼14 kDa correspond by molecular mass to α-chain segments spanning three CHα domains and one CHα domain, respectively, possibly indicating that cleavage between domains had occurred. Cleavage at these sites is an early event in the degradation of immunoglobulins by several nonspecific proteinases of microbial and host origin under experimental conditions (29, 30, 37, 41). Other bands might reflect subsequent cleavage within domains (30).
S-IgA is known to be more resistant to nonspecific proteolytic degradation than other immunoglobulin isotypes, a property that can be ascribed to a stabilizing effect of the secretory component (7, 27, 30, 53). Serum IgA, being devoid of the secretory component, is more susceptible, particularly in its monomeric form (30). We suggest, therefore, that the α-chain fragments detected in some samples of nasal secretion resulted from nonspecific degradation of serum-type IgA. In support of this hypothesis, a host-derived nonspecific proteinase, neutrophil elastase, which is capable of degrading human IgA, has been detected in airway secretions in an active form (11). Conceivably, proteolytic activity could also originate from nasal microflora. The frequent occurrence of IgA degradation in samples exposed to the bacterial loads of the vestibulum supports the latter possibility. Nonspecific degradation of IgA was also detected in the study of genital tract secretions mentioned above (19).
The high levels of immunoglobulins in nasal secretions, as demonstrated in this study, indicate a large potential of the nasal mucosa for an immune response. This potential was reflected in the considerable titers of IgA1 protease-neutralizing antibodies observed in secretions of some subjects. In this view, our data hold promise for ongoing attempts to stimulate immune protection by way of the nasal mucosa (44). On the other hand, we failed to identify conditions under which IgA1 proteases of nasal bacteria might interfere with antibody-mediated defense. The methods for sampling and analysis of nasal secretions described here might be useful in future studies of this issue.
ACKNOWLEDGMENTS
We are grateful to Niels Mygind for theoretical discussions, instruction in the sampling of nasal secretions, and critical reading of the manuscript.
This study is part of a multilateral allergy research program supported by the Danish Allergy Research Center (DARC).
REFERENCES
- 1.Ahl T, Reinholdt J. Detection of immunoglobulin A1 protease-induced Fabα fragments in dental plaque bacteria. Infect Immun. 1991;59:563–569. doi: 10.1128/iai.59.2.563-569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahl T, Reinholdt J. Subclass distribution of salivary secretory immunoglobulin A antibodies to oral streptococci. Infect Immun. 1991;59:3619–3625. doi: 10.1128/iai.59.10.3619-3625.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blake M, Holmes K K, Swanson J. Studies on gonococcus infection. XVII. IgA1-cleaving protease in vaginal washings from women with gonorrhoea. J Infect Dis. 1979;139:89–92. doi: 10.1093/infdis/139.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P, Farstad I N. The human mucosal B-cell system. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. New York, N.Y: Academic Press; 1999. pp. 439–468. [Google Scholar]
- 5.Brooks G F, Lammel C J, Blake H S, Kusecek B, Achtman M. Antibodies against IgA1 proteases are stimulated both by clinical disease and asymptomatic carriage of serogroup A Neisseria meningitidis. J Infect Dis. 1992;166:1316–1321. doi: 10.1093/infdis/166.6.1316. [DOI] [PubMed] [Google Scholar]
- 6.Calderón M A, Devalia J L, Davier R J. Biology of nasal epithelium. In: Mygind N, Lildholdt T, editors. Nasal polyposis, an inflammatory disease and its treatment. Copenhagen, Denmark: Munksgaard; 1997. pp. 31–43. [Google Scholar]
- 7.Crottet P, Corthésy B. Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab′)2: a possible implication for mucosal defense. J Immunol. 1998;161:5445–5453. [PubMed] [Google Scholar]
- 8.de Fijter J W, van den Wall Bake A W L, Braam C A, van Es L A, Daha M R. Immunoglobulin A subclass measurement in serum and saliva: sensitivity of detection of dimeric IgA2 in ELISA depends on the antibody used. J Immunol Methods. 1995;187:221–232. doi: 10.1016/0022-1759(95)00188-8. [DOI] [PubMed] [Google Scholar]
- 9.Delacroix D L, Dehennin J P, Vaerman J P. Influence of molecular size of IgA on its immunoassay by various techniques. II. Solid phase radioimmunoassays. J Immunol Methods. 1982;48:327–332. doi: 10.1016/0022-1759(82)90333-7. [DOI] [PubMed] [Google Scholar]
- 10.Delacroix D L, Meykens R, Vaerman J P. Influence of molecular size of IgA on its immunoassay by various techniques. I. Direct and reverse single radial immunodiffusion. Mol Immunol. 1982;19:297–303. doi: 10.1016/0161-5890(82)90343-1. [DOI] [PubMed] [Google Scholar]
- 11.Döring G, Goldstein W, Botzenhart K, Kharazmi A, Schiøtz P O, Høiby N, Dasgupta N. Elastase from polymorphonuclear leucocytes: a regulatory enzyme in immune complex disease. Clin Exp Immunol. 1986;64:597–605. [PMC free article] [PubMed] [Google Scholar]
- 12.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Büschenfelde K-H. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease. Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gahnberg L, Krasse B. Salivary immunoglobulin A antibodies reacting with antigens from oral streptococci: longitudinal study in humans. Infect Immun. 1981;33:697–703. doi: 10.1128/iai.33.3.697-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert J V, Plaut A G, Longmaid B, Lamm M E. Inhibition of bacterial IgA proteases by human secretory IgA and serum. Ann NY Acad Sci. 1983;409:625–634. doi: 10.1111/j.1749-6632.1983.tb26904.x. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg S B, Rossen R D, Six H R, Baxter B D, Couch R B. Determination of immunoglobulin A concentrations in human nasal secretions with a serum immunoglobulin A standard. J Clin Microbiol. 1978;8:465–467. doi: 10.1128/jcm.8.4.465-467.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greiff L, Erjefält J S, Anderson M, Svensson C, Persson C G A. Microvascular exudation of plasma and epithelial shedding-restitution processes as causative events in inflammatory airway diseases. In: Mygind N, Lildholdt T, editors. Nasal polyposis, an inflammatory disease and its treatment. Copenhagen, Denmark: Munksgaard; 1997. pp. 50–60. [Google Scholar]
- 17.Hamaguchi Y, Ohi M, Sakakura Y, Mukojima T. Quantitation of nasal secretory IgA by enzyme-linked immunosorbent assay. Int Arch Allergy Appl Immun. 1982;69:1–6. doi: 10.1159/000233136. [DOI] [PubMed] [Google Scholar]
- 18.Harada T, Hamaguchi Y, Sakakura Y, Miyoshi Y. Circadian variation of secretory IgA in nasal secretions from normal subjects. Acta Otolaryngol. 1984;97:359–362. doi: 10.3109/00016488409131000. [DOI] [PubMed] [Google Scholar]
- 19.Hedges S R, Mayo M S, Kallman L, Mestecky J, Hook III E W, Russell M W. Evaluation of immunoglobulin A1 (IgA1) protease and IgA1 protease-inhibitory activity in human female genital infection with Neisseria gonorrhoeae. Infect Immun. 1998;66:5826–5832. doi: 10.1128/iai.66.12.5826-5832.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heremans J F. Immunoglobulin A. In: Sela M, editor. The antigens. Vol. 2. New York, N.Y: Academic Press; 1974. pp. 365–522. [Google Scholar]
- 21.Hohwy J, Kilian M. Clonal diversity of the Streptococcus mitis biovar 1 population in the human oral cavity and pharynx. Oral Microbiol Immunol. 1995;10:19–25. doi: 10.1111/j.1399-302x.1995.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 22.Insel R A, Allen P Z, Berkowitz I D. Types and frequency of Haemophilus influenzae IgA1 proteases. In: Robbins J B, editor. Bacterial vaccines. New York, N.Y: Thieme-Stratton Inc.; 1982. pp. 225–231. [Google Scholar]
- 23.Kett K, Brandtzaeg P, Radl J, Haaijman J F. Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J Immunol. 1986;136:3631–3635. [PubMed] [Google Scholar]
- 24.Kilian M, Husby S, Høst A, Halken S. Increased proportions of bacteria capable of cleaving IgA1 in the pharynx of infants with atopic disease. Pediatr Res. 1995;38:182–186. doi: 10.1203/00006450-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen E V G. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996;104:321–338. doi: 10.1111/j.1699-0463.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 26.Linder A, Ronquist G, Deuschl H. Random distribution of exogenous lithium in nasal secretion and its application in substance determination. Acta Otolaryngol. 1983;96:287–293. doi: 10.3109/00016488309132901. [DOI] [PubMed] [Google Scholar]
- 27.Lindh E. Increased resistence of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J Immunol. 1975;114:284–289. [PubMed] [Google Scholar]
- 28.Lomholt H. Molecular biology and vaccine aspects of bacterial immunoglobulin A1 proteases. APMIS Suppl. 1996;104:5–28. doi: 10.1111/j.1600-0463.1996.tb05580.x. [DOI] [PubMed] [Google Scholar]
- 29.Loomes L M, Senior B W, Kerr M A. A proteolytic enzyme secreted by Proteus mirabilis degrades immunoglobulins of the immunoglobulin A1 (IgA1), IgA2, and IgG isotypes. Infect Immun. 1990;58:1979–1985. doi: 10.1128/iai.58.6.1979-1985.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mestecky J, Kilian M. Immunoglobulin A. Methods Enzymol. 1985;116:37–75. doi: 10.1016/s0076-6879(85)16005-2. [DOI] [PubMed] [Google Scholar]
- 31.Mortensen S B, Kilian M. Purification and characterization of an IgA1 protease from Bacteroides melaninogenicus. Infect Immun. 1984;45:550–557. doi: 10.1128/iai.45.3.550-557.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulks M H. Microbial IgA proteases. In: Holder I A, editor. Bacterial enzymes and virulence. Boca Raton, Fla: CRC Press, Inc.; 1985. pp. 81–140. [Google Scholar]
- 33.Mygind N, Thomsen J. Diurnal variation of nasal protein concentration. Acta Otolaryngol. 1976;82:219–21. doi: 10.3109/00016487609120888. [DOI] [PubMed] [Google Scholar]
- 34.Mygind N, Wihl J-Å. Concentration of immunoglobulins in nasal secretion from children with recurrent infections in the upper airways. Acta Otolaryngol. 1976;82:216–218. doi: 10.3109/00016487609120887. [DOI] [PubMed] [Google Scholar]
- 35.Passali D, Bellussi L, Lauwiello M. Diurnal activity of the nasal mucosa. Acta Otolaryngol. 1990;110:437–442. doi: 10.3109/00016489009107466. [DOI] [PubMed] [Google Scholar]
- 36.Prescott S L, Macaubas C, Smallacombe T, Holt B J, Sly P D, Holt P G. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 37.Prince H E, Folds J D, Spitznagel J K. Proteolysis of human IgG by human polymorphonuclear leucocyte elastase produces an Fc fragment with in vitro biological activity. Clin Exp Immunol. 1979;37:162–168. [PMC free article] [PubMed] [Google Scholar]
- 38.Quiding-Järbrink M, Granström G, Nordström I, Holmgren J, Czerkinsky C. Induction of compartmentalized B-cell responses in human tonsils. Infect Immun. 1995;63:853–857. doi: 10.1128/iai.63.3.853-857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinholdt J. A method for titration of inhibiting antibodies to bacterial immunoglobulin A1 proteases in human serum and secretions. J Immunol Methods. 1996;191:39–48. doi: 10.1016/0022-1759(95)00286-3. [DOI] [PubMed] [Google Scholar]
- 40.Reinholdt J, Kilian M. Comparative analysis of immunoglobulin A1 protease activity among bacteria representing different genera, species, and strains. Infect Immun. 1997;65:4452–4459. doi: 10.1128/iai.65.11.4452-4459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reinholdt J, Krogh P, Holmstrup P. Degradation of IgA1, IgA2, and S-IgA by Candida and Torulopsis species. Acta Pathol Microbiol Immunol Scand Sect C. 1987;95:265–274. doi: 10.1111/j.1699-0463.1987.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 42.Rudney J D, Larson C J. Species identification of oral viridans streptococci by restriction fragment polymorphism analysis of rRNA genes. J Clin Microbiol. 1993;31:2467–2473. doi: 10.1128/jcm.31.9.2467-2473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell M W, Kilian M, Lamm M E. Biological activities of IgA. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. New York, N.Y: Academic Press; 1999. pp. 225–240. [Google Scholar]
- 44.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sørensen C H. Quantitative aspects of the mucosal immunity and bacteriology of the nasopharynx and middle ear cavity. APMIS Suppl. 1990;16:7–41. [PubMed] [Google Scholar]
- 46.Sørensen C H, Kilian M. Bacterium-induced cleavage of IgA in nasopharyngeal secretions from atopic children. Acta Pathol Microbiol Immunol Scand Sect C. 1984;92:85–87. doi: 10.1111/j.1699-0463.1984.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 47.Sørensen C H, Larsen P L. IgD in nasopharyngeal secretions and tonsils from otitis-prone children. Clin Exp Immunol. 1988;73:149–154. [PMC free article] [PubMed] [Google Scholar]
- 48.Sørensen C H, Nielsen L K. Nasopharyngeal secretory immunoglobulins in children with recurrent acute otitis media and secretory otitis media. APMIS. 1988;96:199–205. doi: 10.1111/j.1699-0463.1988.tb05291.x. [DOI] [PubMed] [Google Scholar]
- 49.Sørensen H, Mygind N, Pedersen C B, Prytz S. Long-term treatment of nasal polyps with beclomethasone dipropionate aerosol. Acta Otolaryngol. 1976;82:260–262. doi: 10.3109/00016487609120899. [DOI] [PubMed] [Google Scholar]
- 50.Strobel S, McMowat A. Immune responses to dietary antigens: oral tolerance. Immunol Today. 1998;19:173–181. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 51.Strober W, Kensall B, Marth T. Oral tolerance. J Clin Immunol. 1998;18:1–30. doi: 10.1023/a:1023222003039. [DOI] [PubMed] [Google Scholar]
- 52.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–1745. [PubMed] [Google Scholar]
- 53.Tax A, Korngold L. Comparison of the effect of elastase on human secretory IgA and serum IgA. J Immunol. 1971;107:1189–1191. [PubMed] [Google Scholar]
- 54.Underdown B J, Plotkin S A. The induction of mucosal protection by parenteral immunization, a challenge to the mucosal immunity paradigm. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. New York, N.Y: Academic Press; 1999. pp. 719–728. [Google Scholar]
- 55.Wagner D K, Clements M L, Reimer M L C B, Snyder M, Nelson D L, Murphy B R. Analysis of immunoglobulin G antibody responses after administration of live and inactivated influenza A vaccine indicates that nasal wash immunoglobulin G is a transudate from serum. J Clin Microbiol. 1987;25:559–562. doi: 10.1128/jcm.25.3.559-562.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waldo F B, van den Wall Bake A W L, Mestecky J, Husby S. Suppression of the immune response by nasal immunization. Clin Immunol Immunopathol. 1994;72:30–34. doi: 10.1006/clin.1994.1103. [DOI] [PubMed] [Google Scholar]
- 57.Winther B, Brofeldt S, Grønborg H, Mygind N, Petersen M, Vejlsgaard R. Study of bacteria in the nasal cavity and nasopharynx during naturally acquired common colds. Acta Otolaryngol. 1984;98:315–320. doi: 10.3109/00016488409107569. [DOI] [PubMed] [Google Scholar]