Abstract
Magnetic resonance imaging (MRI) studies have revealed positive associations between brain structure and physical activity, cardiorespiratory fitness, and exercise (referred to here as PACE). While a considerable body of research has investigated the effects of PACE on grey matter, much less is known about effects on white matter (WM). Hence, we conducted a systematic review of peer-reviewed literature published prior to 5th July 2021 using online databases (PubMed and Scopus) and PRISMA guidelines to synthesise what is currently known about the relationship between PACE and WM in healthy adults. A total of 60 studies met inclusion criteria and were included in the review. Heterogeneity across studies was calculated using Qochran’s q test, and publication bias was assessed for each meta-analysis using Begg and Mazumdar rank correlation test. A meta-regression was also conducted to explore factors contributing to any observed heterogeneity. Overall, we observed evidence of positive associations between PACE and global WM volume (effect size (Hedges’s g) = 0.137, p < 0.001), global WM anomalies (effect size = 0.182, p < 0.001), and local microstructure integrity (i.e., corpus callosum: effect size = 0.345, p < 0.001, and anterior limb of internal capsule: effect size = 0.198, p < 0.001). These findings suggest that higher levels of PACE are associated with improved global WM volume and local integrity. We appraise the quality of evidence, and discuss the implications of these findings for the preservation of WM across the lifespan. We conclude by providing recommendations for future research in order to advance our understanding of the specific PACE parameters and neurobiological mechanisms underlying these effects.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11682-022-00693-y.
Keywords: Exercise, Physical activity (PA), Physical fitness (PF), Cardiorespiratory fitness (CRF), White matter (WM), Magnetic resonance imaging (MRI)
Introduction
Engaging in regular physical activity is associated with numerous health benefits, including reduced incidence of certain cancers, cardiovascular disease, and type-2 diabetes (U.S. Department of Health and Human Services, 2019; Australian Department of Health, 2021). Remarkably, the positive effects of exercise also extend to the brain, with large scale epidemiological studies demonstrating that higher levels of physical activity, cardiorespiratory fitness, and exercise (referred to here as ‘PACE’) are associated with a significant reduction in the risk of mild cognitive impairment and dementia in later life (Mandolesi et al., 2018; Stigger et al., 2019). Underlying these effects, a considerable body of research has shown that exercise has profuse, broad effects on neuroplasticity – the brain’s intrinsic ability to modify its structure and function in line with changing internal or environmental factors (Voss et al., 2013). For example, engaging in cardiovascular exercise promotes the release of growth hormones and neurotrophic factors (such as brain-derived neurotrophic factor) that mediate neuroplasticity and are directly implicated in learning and memory (Alkadhi, 2018; Hendrikse et al., 2017).
Here, we use the acronym PACE to encompass any form of physical activity (PA), physical fitness (PF) (i.e., cardiorespiratory fitness; CRF), and exercise intervention. These terms are interrelated and are sometimes used interchangeably, but in fact have distinct definitions. PA can be defined as any bodily movements produced by skeletal muscles and requires energy expenditure, with exercise being a subset of physical activity that has planned, structured, and repetitive movements with a goal of maintaining or improving fitness (Caspersen et al., 1985). While PF is multi-factorial, cardiorespiratory and muscular components are the most commonly assessed, and can be quantified with health or performance measures that index the efficiency of the cardiovascular and respiratory systems. The gold standard method to assess cardiorespiratory fitness (CRF) is to measure the highest rate of oxygen consumption by muscles (known as V max) during exercise by maximal exercise test (Campbell et al., 2013; Bouchard et al., 2012).
Higher levels of physical activity, exercise, and cardiorespiratory fitness (i.e. PACE) have beneficial effects on brain volume and integrity (Firth et al., 2018; Sexton et al., 2016). For example, neuroimaging studies have reported positive associations between cardiorespiratory fitness (CRF) and gray matter volume in the hippocampus (Den Ouden et al., 2018), prefrontal cortex, anterior cingulate cortex, and striatum (Firth et al., 2018; Gujral et al., 2017). Similarly, exercise has been associated with improvements in white matter (WM), particularly in older adults (Sexton et al., 2016). WM is composed of myelinated axons, oligodendrocytes, and astrocytes and accounts for approximately half of total brain volume (Sampaio-Baptista & Johansen-Berg, 2017). The primary function of WM is to structurally connect cortical and subcortical regions into ensembles that support cognition. Therefore, optimal coordination, coherence, and conduction velocity of neural activities across different cortical regions are essential for proper cognitive function (Filley & Fields, 2016). WM health can be examined through structural MRI techniques by measuring WM volume (T1-weighted), WM anomalies (T2-weighted), and WM microstructure (e.g., diffusion weighted imaging).
WM anomalies observable as white matter hyperintensities (WMH) in T2-weighted (FLAIR) MRI scans indicate poor WM health. These WMH occur due to water accumulation, reflecting demyelination and axonal loss and are mainly caused by cerebral small vessel disease (Filley & Fields, 2016; Prins & Scheltens, 2015). Aging and poor cardiovascular health (e.g. chronic hypertension and high heart rate) are major risk factors for onset and severity of WMHs (Fuhrmann et al., 2019; Prins & Scheltens, 2015). Mounting evidence demonstrates that WMHs can increase the risk of cognitive impairment (Filley & Fields, 2016; Frey et al., 2019; Fuhrmann et al., 2019; Prins & Scheltens, 2015), dementia (Fuhrmann et al., 2019; Prins & Scheltens, 2015), stroke, and certain forms of mental illness, such as depression (Frey et al., 2019). Further, disruptions in WM integrity (e.g. white matter volume and plasticity) underlie a range of neurodevelopmental, psychiatric, and neurological conditions including autism, schizophrenia, obsessive compulsive disorder, depression, and Alzheimer’s disease (Filley & Fields, 2016). Hence, there is a critical need to investigate methods of maintaining/improving WM integrity throughout the lifespan. Increasing physical activity and/or exercise may provide a novel effective approach, though a comprehensive understanding of the corresponding effect on white matter is first required.
This review aims to provide a systematic review on MRI studies investigating the associations between WM and physical activity, cardiorespiratory fitness and exercise (PACE) in healthy populations. Again, to maintain a standard terminology throughout this review, we use the term PACE to encompass any form of physical activity (PA), physical fitness (PF) (i.e., cardiorespiratory fitness; CRF), and exercise intervention. A previous review by Sexton et al (2016) highlighted positive associations between higher CRF and WM volume and integrity in frontal and temporal brain regions. However, at the time of publication this review reported cautious support for a link between physical activity and WM outcomes due to the limited evidence base, and only included studies conducted on older adults above 60 years of age. Since then, many new studies featuring young and middle-aged adult samples have been published, warranting an updated review of this literature. Hence, we review the cross-sectional and longitudinal findings to date on each aspect of structural WM health, including WM volume, WM anomalies, and WM microstructure.
Methods
Data source and quality check
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework was used to extract data and report study outcomes (Page et al., 2021). Authors S.M and Y.C conducted a systematic search of the literature via PubMed and Scopus online databases. The search was conducted using the following keywords and operands: “exercise” OR “physical activity” OR “physical fitness” OR “cardiorespiratory fitness” AND “white matter” AND “MRI”. Reference lists of included studies were also screened. Searches were limited to human studies published prior to the 5th July 2021 in the English language. The search strategy is depicted in Fig. 1. The quality of evidence was assessed for each of the included studies by authors S.M and Y.C (and C.S in the case of inter-rater differences) using NIH study quality assessment tools (see supplementary materials). All eligible studies were deemed to have sufficient quality of evidence for inclusion in this review.
Fig. 1.
Flow chart depicting the search strategy and number of studies included in the systematic review
Study selection
Authors S.M. and Y.C. conducted independent title and abstract screening. Inter-rater differences were resolved through consult of author C.S. Studies were required to meet the following criteria for inclusion in this review: (1) published in a peer-reviewed academic journal; (2) utilised either a cross-sectional or longitudinal study design; (3) assessed PACE using objective/quantifiable methodology that could be generalised to wider population (e.g. VO2max for CRF, and actigraphy/accelerometry or self-report measures for PA); (4) included MRI assessment of WM (i.e. volume, anomalies, hyperintensities, and/or microstructural integrity); (5) conducted with healthy participants above 15 years of age. Studies that did not meet these criteria, and/or were conducted with N < 10, or utilised a multi-modal intervention without considering a separate exercise group (e.g., exercise combined with cognitive training) were excluded.
Data extraction
For each study, the following data were extracted: (1) sample demographics (N, age, biological sex), (2) WM assessment, i.e., volume (WMV), hyperintensities (WMH), and/or WM microstructure (fractional anisotropy (FA), mean diffusivity (MD)); (3) study design (i.e., cross-sectional or longitudinal); (4) PACE assessment (e.g., PA/CRF measure, and where applicable exercise intervention parameters including length, frequency and individual session duration). Studies that employed an exercise intervention but only assessed WM at a single timepoint (i.e., pre- or post-intervention were considered cross-sectional). All measures/results are reported as per original study definitions, with the exception of CRF, which for simplicity refers to both VO2max and other related exercise tests of cardiovascular/respiratory function.
Meta-analysis
Global WM volume and WM anomalies data form cross-sectional studies were analysed using Comprehensive Meta-Analysis (CMA, version 3) (Borenstein et al., 2013). The statistical outcomes included in the meta-analysis were calculated from the original reports. For studies not providing the statistical results of the regions of interest, we contacted the corresponding authors to retrieve these data to maximum our sample size. Given that this meta-analysis was conducted on correlational outcomes, we computed the correlation coefficients and Fisher’s z scale (based on sample size, and p-values of correlational outcomes) for individual studies. These estimates were then used to calculate effect size estimates (Hedges’s g), which provides an unbiased measure of standardised mean differences. We then applied a random-effects model to calculate the total effect size for all the meta-analyses (Borenstein et al., 2010). Similarly, FA measures of WM microstructure from cross-sectional studies were analysed within the most frequently reported regions, namely the corpus callosum and internal capsule (implicated in > 4 studies). Due to insufficient number of studies, a meta-analysis on longitudinal data and cross-sectional global WM microstructure was not performed.
We assessed evidence of heterogeneity across study outcomes using Cochran’s Q method (CMA software) for each performed meta-analysis. Similarly, CMA was used to explore evidence of publication bias using Begg and Mazumdar rank correlations test (Begg & Mazumdar, 1994). We also performed meta-regression analyses to assess the influence of primary subject characteristics (i.e. age and gender) on observed associations between PACE and WM (Hedges’s g). Mean Age and Biological Sex ratios (i.e. % Female) from each individual study sample were extracted and entered as covariates across each meta-analysis model.
Results
Overview on selected studies
According to the flow chart (Fig. 1), 60 studies out of 441 articles will be reviewed in this paper. 57 studies were deemed to have good evidence quality, and 3 were fair evidence quality (see supplementary Material). All studies provided a detailed description of their primary study aim(s) and sample demographics, and utilised reliable and valid measures of PACE. Across studies, there was considerable heterogeneity in sample size (longitudinal N = 21 – 352; cross-sectional N = 15—7148), and exercise intervention parameters. For example, studies utilised different exercise modalities (e.g., walking, cycling, resistance, etc.), intervention durations (i.e., 1 -13 months), frequencies (i.e., 1 – 4 sessions per week), and session durations (20—90 min). MRI scanner field strength also varied across studies (i.e., 1.5 or 3 Tesla). Also, studies varied in their reporting of experimenter blinding and statistical parameters (e.g., p-value specificity), and PACE methodology (e.g., objective vs subjective methods).
PACE and white matter volume
Narrative synthesis
Longitudinal studies
Seven studies measured the effects of PACE on WM volume. Five demonstrated significant positive influence of PACE on WM volume (Rehfeld et al., 2018; Tabei et al., 2017; Arnardottir et al., 2016; Best et al., 2015; Stanley J. Colcombe et al., 2006), and two studies did not observe significant effects (Sexton et al., 2020; Smith et al., 2014). Four out of five studies with positive results featured exercise interventions of a minimum six-month duration. However, different types of physical fitness measurements were utilised across studies (e.g., VO2max vs self-report measure), and thus it is difficult to conclude whether these effects were directly related to improvements in CRF (please refer to Table 1 for details of longitudinal studies).
Table 1.
Summary of studies with longitudinal designs
| Author | Age (female %) | Sample Size | Intervention Design |
Duration (months) |
Frequency (times pw) | Session (minutes) | Assessment (MRI and PACE) |
Results |
|---|---|---|---|---|---|---|---|---|
| Colmenares et al. (2021) | 60 – 79 (69.3) |
86 51 43 |
Walking Dance Active control |
6 | 3 | 60 |
TP1and TP2: T1/T2 ratio, DTI CRF (treadmill) |
WMH: ns Microstructural changes: Increase total WM (T1/T2 ratio) in genu and splenium of CC (p = 0.02), forceps minor (p = 0.04), and cingulum (p = 0.02) in walking group, relative to controls Increased total WM (T1/T2 ratio) and increased WM in the genu of CC (p = 0.05) CRF ~ T1/T2 ratio: ns ↑ FA in fornix and forceps minor in dance group relative to controls Cognition: increase in T1/T2 ratio was associated with higher cognitive function in the walking group |
| Sexton et al. (2020) | 60 – 85 (63) |
23 23 |
Cycling Control |
3 | 3 | 30 |
TP1and TP2: T1, DTI CRF PA (questionnaire) |
WMV: ns Microstructural changes: No significant group differences in FA, AD, and RD Cognition: ns |
| Lehmann et al. (2020) | 18 – 35 (64.5) |
15 16 |
Cycling Balance learning |
0.5 | 7 sessions in total | 20 |
TP1and TP2: T1, DTI CRF (bicycle ergometer) PA (questionnaire) |
Microstructural changes: FA: ns MD ↓ in exercise group (pFWE < 0.05) in the bilateral superior longitudinal fasciculus, bilateral anterior thalamic radiation, bilateral uncinate fasciculus, bilateral inferior fronto-occipital fasciculus, forceps minor, and right corticospinal tract RD: ↓ in exercise group (pFWE < 0.05) in the right superior longitudinal fasciculus, right inferior fronto-occipital fasciculus, right anterior thalamic radiation, right uncinate fasciculus, right corticospinal tract, and forceps minor Cognition: improved performance of complex motor tasks post intervention |
| Maltais et al. (2020) | > 70 (60) | 106 | Assessed exercise patterns associated with regular lifestyle | 60 | _ | _ |
TP1, TP-mid, TP2: T1, DTI PA (questionnaire) |
Microstructural changes: FA: ns MD: ↑ in participants with lower PA level in uncinate fasciculus Cognition: NA |
| Clark et al. (2019) | 57 – 86 (44) | 25 | Aerobic | 6 | 3 | 20—45 |
TP1and TP2: T1, DTI CRF |
Microstructural changes: FA: ↓ mean global FA across post intervention MD: ns Cognition: NA |
| Rehfeld et al. (2018) | 63 – 80 (51.9) |
20 18 |
Dance training Endurance training |
6 | 2 | 90 |
TP1and TP2: T1 CRF (bicycle ergometer) |
WMV: ↑ in both groups (p = 0.001 uncorrected). Specifically, higher WMV in the truncus and splenium of CC in the dance group, and higher WMV in the right occipital and temporal regions Cognition: ns |
| Moon et al. (2018) | > 70 (63.8) | 152 | Assessed exercise patterns associated with regular lifestyle | 36 | _ | _ |
TP1and TP2: T1, FLAIR PA (questionnaire) |
WMH: ns Reduced PA level over three years was associated with WMH progression Cognition: NA |
| Best et al. (2017) | 70 – 79 (60) | 141 | Assessed exercise patterns associated with regular lifestyle | 36 | _ | _ |
TP1and TP2 (after 10 and 13 years) T1, T2, DTI PA (questionnaire) |
Microstructural changes: FA: ns AD: Higher PA over 10-year period associated with smaller increase in AD in the inferior longitudinal fasciculus, and parahippocampal and dorsal regions of the cingulum Cognition: ↑ PA predicted better global cognitive performance (p = 0.01) |
| Tabei et al. (2017) | > 65 (86.3) |
61 51 32 |
Aerobic Aerobic + Music Control |
12 | 1 | 60 |
TP1and TP2: T1 PA (interviewed) |
WMV: ↑ in both exercise groups compared to controls in the right anterior corona radiata (p = 0.001 uncorrected) ↓ WMV in control group post intervention Cognition: Improved performance following exercise + music intervention, relative to exercise alone |
| Burzynska et al. (2017) | 60 – 79 (68.7) |
49 40 42 43 |
Dance Walking Walking + Nutrition active control |
6 | 3 | 60 |
TP1and TP2: DTI CRF PA (accelerometer) |
Microstructural changes: Significant changes reported in the Fornix, specifically: FA: ↑ in dance group, while ↓ in walking and control (p = 0.001) MD and RD: dance group showed smaller increase relative to other groups (p = 0.023 and p = 0.007 respectively) Cognition: ns |
| Arnardottir et al. (2016) | ~ 79.1 (61) | 352 | Assessed exercise patterns associated with regular lifestyle | 60 | _ | _ |
TP1and TP2: T1, T2 PA (accelerometer) |
WMV: At both time-points, ↑ WMV was associated with ↑ total PA (p = 0.03) Cognition: NA |
| Bolandzadeh et al. (2015) | 65 – 75 (100) |
18 13 15 |
Light resistance Moderate resistance Balance and toning |
12 |
1 2 2 |
60 |
TP1and TP2: T2, PD PA (questionnaire) |
WML: ↓ WML in moderate resistance group relative to the balance and toning condition (p = 0.03) ns difference between light resistance training and balance and toning condition Cognition: ns |
| Best et al. (2015) | 65 – 75 (100) |
41 37 41 |
Resistance training twice per week Resistance training once per week Balance and toning |
13 |
2 1 2 |
60 |
TP1, TP-mid, TP2: T1 PA (short physical performance battery) |
WMV: ↓ WMV over 2 years in all groups (p = 0.009): 0.8% in twice-weekly resistance training, 1.5% in weekly resistance training, 2% in balance and toning condition Cognition: improved memory and executive functions after 2-year follow-up |
| Smith et al. (2014) | 65 – 89 | 97 | Assessed exercise patterns associated with regular lifestyle | 18 | _ | _ |
TP1and TP2: T1 PA (questionnaire) |
WMV: ns Cognition: NA |
| Palmer et al. (2013) | 24 + -(2), (61) |
12 9 |
Unilateral strength training lower limb Control |
1 | 4 | _ |
TP1and TP2: T1, FLAIR, DTI PA (questionnaire) |
Microstructural changes: Significant changes in left cortico-spinal tract: MD: ↓ in strength training group (p = 0.02) FA: ↑ in strength training group relative to control (p = 0.01) Cognition: NA |
| Voss et al. (2013) | 55 – 80 (64) |
35 35 |
Walking Stretching |
12 | 3 | 40 |
TP1and TP2: T2, DTI CRF |
Microstructural changes: ↑ CRF was associated with ↑ FA only in walking group in prefrontal (p = 0.001), parietal (p = 0.005), and temporal regions (p = 0.03) AD: ns RD: ns Cognition: ↑ CRF ~ improved short-term memory |
| Colcombe et al. (2006) | 60 -79 (55) |
29 30 |
Aerobic Stretching |
6 | 3 | 60 |
TP1and TP2: T1 CRF |
WMV: ↑ WMV in aerobic compared to control group in anterior WM tracts including CC (p = 0.05) Cognition: NA |
PA Physical activity; CRF Cardiorespiratory fitness; WMV White matter volume; WMH White matter hyperintensity; WML White matter lesion. NA = not applicable (i.e. not measured or only assessed for screening), TP1 = time point at baseline, TP2 = time point at completion of intervention, TP-mid = middle time point (36 months for Maltais et al., 2020, and 12 months for Best et al., 2015). Symbol “ ~ ” means association/ correlation. All p-values are corrected unless otherwise stated. MRI Scanner field strength is 3 T, apart from two studies conducted at 1.5 T (Tabei et al., 2017 and Arnardottir et al., 2016). Regarding biological sex ratios, we averaged across sub-groups in five studies (Colmenares et al., 2021; Tabei et al., 2017; Burzynska et al., 2017; Palmer et al., 2013; Voss et al., 2013; and Colcombe et al., 2006). Non-significant outcomes are denoted as “ns”
Cross sectional studies
Seventeen studies have investigated the associations between PACE and WM volume. Nine studies reported significant positive associations (Balbim et al., 2021; Benedict et al., 2013; Demirakca et al., 2014; Erickson et al., 2007; Gow et al., 2012; Gu et al., 2020; Ho et al., 2011; Tian et al., 2015; Zhu et al., 2015), while the remaining eight did not observe any significant outcomes (Bugg & Head, 2011; Colcombe et al., 2003; Gordon et al., 2008; Jochem et al., 2017; Koblinsky et al., 2021; Pentikäinen et al., 2017; Tarumi et al., 2021; Wittfeld et al., 2020). Across studies, PACE was associated with increased WMV within particular regions including posterior cingulate gyrus (Balbim et al., 2021; Demirakca et al., 2014), temporal and parietal (Ho et al., 2011; Tian et al., 2015), corona radiata (Ho et al., 2011), and prefrontal and genu of corpus callosum (CC) (Erickson et al., 2007). Please refer to Table 2 for details of cross-sectional studies.
Table 2.
Summary of studies with cross-sectional designs
| Author | Sample Size | Age | Female Rate % |
MRI Protocol | Scanner Field Strength | PA/ CRF Assessment | Results |
|---|---|---|---|---|---|---|---|
| Palta et al. (2021) | 1604 | 45—64 | 61 |
T1 T2 DTI |
3 T | PA ( |
WMH: ns Microstructural changes: ↑ MVPA at midlife ~ ↑ FA (p = 0.021) and ↓ MD (p = 0.019) at late life (after 25 years) Cognition: NA |
| Balbim et al. (2021) | 34 | > 65 | 56 |
T1 T2 DTI |
3 T | PA (questionnaire) |
WMH: ns WMV: ↑ MVPA ~ ↑ WMV in posterior cingulate (p = 0.047) and isthmus cingulate (p = 0.044) Microstructural changes: PA ~ FA: ns Cognition: NA |
| d’Arbeloff et al. (2021) | 801 | At 45 | 48 |
T1 DTI |
3 T | CRF (cycle ergometer) |
Microstructural changes: FA ~ CRF: ns Cognition: NA |
| Koblinsky et al. (2021) | 66 | 65—85 | 62 | T1 | 3 T | PA (questionnaire) |
WMV: ns Cognition: ns |
| Tarumi et al. (2021) |
30 aerobic 30 sedentary |
45—64 | 50 |
T1 DTI |
3 T | CRF |
WMV: ns Microstructural changes: Global FA and AD was significantly higher in aerobic group. Regional TBSS results: ↑ FA in the genu of CC, cingulum, fornix, SLF, fronto-occipital fasciculus, uncinate fasciculus, anterior and superior corona radiata, anterior limb of internal capsule, (p = 0.042) ↑ CRF ~ ↑ FA and AD in same regions ↑ CRF ~ ↑ RD in brainstem; ↓ RD in the external capsule Cognition: NA |
| Gu et al. (2020) | 1443 | > 65 | 63.8 |
T1 T2 |
1.5 T | PA (questionnaire) |
WMH: ns WMV: ↑ leisure time PA ~ ↑ total WM and hippocampal volume (p = 0.03) Cognition: NA |
| Kim et al. (2020) |
35 super agers 55 typical agers |
> 60 | 83 |
T1 DTI |
3 T | PA (FitBit for a week) |
Microstructural changes: ↑ PA ~ ↑ FA in the body of CC (p = 0.04) and ↓ MD (p = 0.03) and RD (p = 0.01) in left inferior longitudinal fasciculus AD: ns Cognition: NA |
| Johnson et al. (2020) | 76 | 59—77 | 61.8 |
T1 T2 |
3 T | CRF |
WMH: ↑ CRF ~ ↓ WMH volume in older participants (p = 0.04) Cognition: NA |
| Strömmer et al. (2020) | 399 | 18—87 | 55.4 |
T1 DTI |
3 T | PA (questionnaire) |
Microstructural changes: ↑ PA ~ ↑ FA preservation in 4 out of 21 ROIs including the genu of CC, uncinate fasciculus, external capsule, anterior limb of internal capsule Cognition: ↑ FA in the genu of CC ~ less age-related slowing of cognitive processing |
| Wittfeld et al. (2020) | 2103 | 21 – 84 | 52.4 | T1 | 1.5 T | CRF |
WMV: ns Cognition: NA |
| Raichlen et al. (2019) | 7148 | 40 – 69 | 57.1 |
T1 T2 |
3 T |
CRF PA (accelerometer) |
WMH: ↑ CRF ~ ↓ WMH loads (p = 0.002) ↑ MV-PA ~ ↓ WMH loads (p = 0.02) MV: moderate-to-vigorous Cognition: NA |
| Opel et al. (2019) | 1050 | 28.8 | 54.5 |
T1 DTI |
3 T | PF (walking endurance test) |
Microstructural changes: ↑ PF ~ ↑ FA in widespread clusters including the genu of CC, bilateral SLF, bilateral uncinate fasciculus, bilateral internal and external capsule, CST, and cerebellar peduncles Cognition: ↑ PF ~ increased global cognitive function |
| Williamson et al. (2018) | 52 | 18—40 | 49 |
T1 T2 DTI |
3 T | PA (VO2) |
WMH: ↑ CRF associated with ↓ WMH anomalies and ↑ Blood flow & vessel density Cognition: NA |
| Vesperman et al. (2018) | 107 | 40 -65 | 65.4 |
T1 T2 |
3 T | CRF |
WMH: ↑ CRF ~ ↓ WMH volumes Cognition: NA |
| Gujral et al. (2018) | 105 | 60—80 | 63 |
T1 DTI |
3 T | PA (questionnaire) |
Microstructural changes: ↑ FA in CC, anterior thalamic radiation, and superior longitudinal fasciculus predicted higher adherence to PA over 12 months aerobic intervention (p = 0.05) Cognition: NA |
| Jochem et al. (2017) | 834 | 25—83 | 53.5 | T1 | 1.5 T |
PA (questionnaire) MRI after 6 years |
WMV: ns Cognition: NA |
| Pentikäinen et al. (2017) | 68 | 61 -75 | 42.6 | T1 | 1.5 T | CRF |
WMV: ns Cognition: NA |
| Bracht et al. (2016) | 30 | 25.5(4.2) | 57.5 |
T1 DTI mcDESPOT |
3 T | PA (actigraphy) |
Microstructural changes: FA: ns; MD: ns; AD: ns; RD: ns ↑ PA ~ ↑ MWF (p = 0.007) in the right parahippocampal cingulum (with positive ns trend in fornix) Cognition: NA |
| Smith et al. (2016) | 88 | 65—89 | 73.8 |
T1 DTI |
3 T |
PA (Questionnaire) DTI after 18 months |
Microstructural changes: ↓ PA ~ ↑ FA in the left fornix and stria terminals MD: ns; AD: ns; RD: ns Cognition: NA |
| Oberlin et al. (2016) |
113 (Group 1) 154 (Group 2) |
60—81 60—80 |
_ |
T2 DTI-G1 (12) DTI-G2 (30) |
3 T | CRF |
Microstructural changes: Group 1: ↑ CRF ~ ↑ FA in the CC, fornix, bilateral ACR and anterior internal capsule Group 2: ↑ CRF ~ ↑ FA in the CC, left Cingulum, bilateral internal capsule, anterior and superior corona radiata, bilateral superior longitudinal fasciculus ↑ CRF ~ ↓ FA in the bilateral posterior limb of internal capsule (all p < 0.05) Cognition: ↑ CRF associated with ↑ FA and this in turn was associated with better memory performance |
| Freudenberger et al. (2016) | 877 | 65 (7.7) | 55 |
T1 T2 |
1.5 T | CRF |
WML: ns Cognition: ↑ CRF ~ higher global cognitive function |
| Tian et al. (2015) | 146 | 69.6 | 58 | T1 | 1.5 T | CRF |
WMV: ↑ CRF ~ ↑ WMV in temporal and parietal at baseline (p = 0.05) Cognition: NA |
| Hayes et al. (2015) |
32 young 27 old |
18—31 55—82 |
53.1 55.5 |
T1 DTI |
3 T | CRF |
Microstructural changes: ↑ FA in young and higher fit old adults compared to lower fit old adults in splenium of CC, posterior corona radiata, sagittal stratum, and right superior parietal regions Cognition: NA |
| Zhu et al. (2015) | 565 | 18 -30 | 54.3 |
T1 T2 DTI |
_ |
CRF MRI after 5 years |
WMV: ↑ CRF ~ ↑ WMV Microstructural changes: ↑ CRF ~ ↑ FA Cognition: NA |
| Boots et al. (2015) | 315 | 40 -65 | 67.9 |
T1 T2 |
3 T |
CRF (questionnaire) 85 subsets (VO2) |
WMH: ↑ CRF ~ ↓ WMH volume, (p = 0.001) Cognition: ↑ CRF ~ better cognitive function |
| Frederiksen et al. (2015) | 282 | 64—85 | 58.1 |
T2 FLAIR |
_ | PA (questionnaire) |
WMH: ns Cognition: ↑ PA ~ higher executive function, but not memory |
| Fleischman et al. (2015) | 167 | 60 -96 | 79 |
T1 T2 |
1.5 T | PA (actigraphy) |
WMH: ns Cognition: NA |
| Burzynska et al. (2014) | 88 | 60—78 | 66.2 |
T2 DTI |
3 T | PA (accelerometer) |
WML: ↑ MV-PA ~ ↓ WML (p = 0.004) Microstructural changes: FA: ns ↑ light PA ~ ↑ FA in temporal lobe (p = 0.02) ↑ ~ ↓ FA in Parahippocampal (p = 0.03) Cognition: NA |
| Herting et al. (2014) | 34 | 15 – 18 | 0 |
T1 DTI |
3 T |
CRF PA (actigraphy) |
Microstructural changes: ↑ CRF ~ ↓ FA in left CST (p < 0.05) CRF ~ AD: ns CRF ~ RD: ns Cognition: NA |
| Tian et al. (2014a) |
39: Sedentary 148: Life activity 89: Exercise |
70—79 | 58.7 |
T1 DTI |
3 T | PA (self-report time spent walking) |
WMH: ns Microstructural changes: ↑ PA ~ ↓ MD in the medial temporal lobe (p = 0.023) and cingulate cortex (p = 0.006) PA ~ FA: ns Cognition: NA |
| Tian et al. (2014b) | 164 | > 80 | 51.8 |
T1 FLAIR DTI |
3 T | CRF based on 400 m walk as fast as possible |
Microstructural changes: ↑ CRF ~ ↑ FA in cingulum (p = 0.019), and ↓ MD in Hippocampus (p = 0.035) and entorhinal cortex (p = 0.006) Cognition: NA |
| Wirth et al. (2014) | 92 | 60 -90 | 63 |
T1 PET |
1.5 T | PA (questionnaire) |
WML: ↑ PA ~ ↓ WML volumes (p = 0.05) Cognition: ↑ PA ~ higher global cognitive performance |
| Demirakca et al. (2014) | 95 | 19 -82 | 53.6 | T1 | 3 T | PA (questionnaire) |
WMV: Only in subjects > 40 years old ↑ PA ~ ↑ WMV in the right posterior cingulate gyrus and precuneus (p = 0.004), and left posterior cingulate gyrus (p = 0.023) Cognition: NA |
| Liu et al. (2012) |
9 actives 6 control |
60—76 | 46.6 | DTI (21) | 3 T |
CRF PA (self-report time spent walking) |
Microstructural changes: ↑ CRF ~ ↑ FA, (voxel-wise correlation, p < 0.05) in the internal capsule, genu of CC, and brain stem Cognition: NA |
| Johnson et al. (2012) | 26 | 60—69 | 53.8 |
T1 DTI (36) |
3 T | CRF |
Microstructural changes: ↑ CRF ~ ↑ FA and ↓ RD in CC CRF ~ MD: ns CRF ~ AD: ns Cognition: NA |
| Benedict et al. (2013) | 331 | At 75 | 49.5 | T1 | 1.5 T | PA (questionnaire) |
WMV: ↑ PA ~ ↑ total WM volume Cognition: ↑ PA was associated with higher memory performance |
| Gow et al. (2012) | 638 | At 70 | 47.3 |
T1 T2, T2* DTI |
1.5 T | PA (questionnaire) |
WMV and WML: ↑ PA was associated with ↑ WMV and ↓ WML Microstructural changes: ↑ FA was associated with ↑ PA (p = 0.014) MD: ns Cognition: NA |
| Marks et al. (2011) |
8 actives 7 control |
60—76 | 46.6 |
T1 DTI |
3 T | CRF |
Microstructural changes: ↑ CRF ~ ↑ FA moderately in left middle cingulum (p = 0.04) CRF ~ MD: ns Cognition: NA |
| Bugg and Head (2011) | 52 | 55—79 | 71.1 | T1 | 1.5 T | PA (questionnaire) over the past 10 years |
WMV: ns Cognition: NA |
| Ho et al. (2011) | 226 | 77.9 (3.6) | 57.5 | T1 | 1.5 T | PA (questionnaire) |
WMV: ↑ PA ~ WMV in corona radiata extending into the parietal-occipital junction (p = 0.0002 uncorrected) Cognition: NA |
| Erickson et al. (2007) | 54 | 58—80 | 100 | T1 | 3 T | CRF |
WMV: ↑ CRF ~ WMV in prefrontal and genu of CC Cognition: ns |
| Gordon et al. (2008) |
20 young 40 old |
20 -28 60—81 |
50 57.5 |
T1 | 3 T | CRF |
WMV: ns Cognition: ↑ CRT predicted improved cognitive function |
| Colcombe et al. (2003) | 55 | 55—79 | 55.6 | T1 | 1.5 T | CRF |
WMV: ns Cognition: NA |
PA Physical activity; CRF Cardiorespiratory fitness. NA = not applicable (i.e. not assessed), WMV = white matter volume, WMH = white matter hyperintensity, WML = white matter lesion. Symbol “ ~ ” means association/ correlation. All p-values are corrected unless otherwise stated. Non-significant outcomes are denoted by “ns”. Footnotes: a) PA measured at baseline and after 25 years, but MRI only assessed at 25-year timepoint. b) individuals were divided into 3 categories of sedentary, light, and moderate-vigorous based on their PA
Meta-analysis
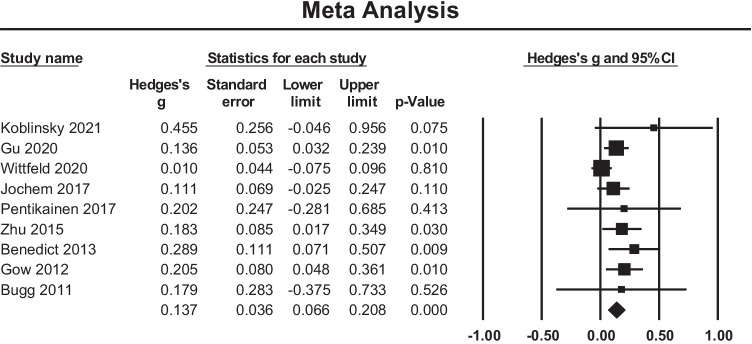
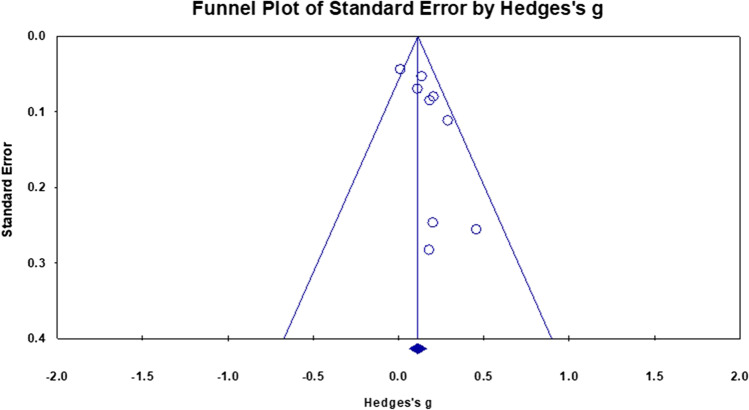
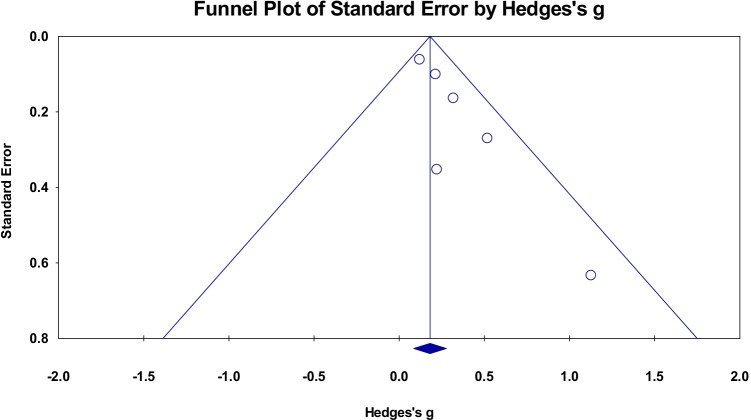
A meta-analysis of nine cross sectional studies examining the association between PACE and global WMV changes showed an overall small mean effect size of 0.137 (95% confidence interval (CI) = 0.066 to 0.208, p < 0.001) (Fig. 2). Studies were not significantly heterogeneous (Q = 12.199, p = 0.143, = 34.419). The possibility of publication bias was explored by inspecting a funnel plot (Fig. 3) and quantified by calculating Begg and Mazumdar rank correlation test. Qualitatively, there was some evidence of skew in the distribution, though this was not statistically significant (Tau = 0.25, two-tailed p = 0.348). There was also no evidence that the effect size (Hedges’s g) was influenced by sample characteristics (i.e.Age and Biological Sex) (Q = 2.78, df = 2, p = 0.24).
Fig. 2.
Effect sizes for global white matter volume within cross-sectional studies. Higher PACE is correlated with higher WM volumes
Fig. 3.
Funnel plot of standard errors plotted against effect sizes (Hedges’s g) for studies in Fig. 2 to visualise publication bias
PACE and white matter anomalies
Narrative synthesis
Longitudinal studies
Three studies examined the effect of PACE on WMH, with two non-significant results (Colmenares et al., 2021; Moon et al., 2018). One study reported significantly decreased WMH following moderate intensity resistance training (Bolandzadeh et al., 2015). Moon et al. (2018) did not observe significant positive associations, though higher WMH were observed in individuals who engaged in less PACE over a three year follow-up period (Moon et al., 2018).
Cross sectional studies
Fifteen studies examined associations between PACE and WMH (Table 2). Nine studies showed significantly reduced WMH in individuals with higher PACE (Boots et al., 2015; Burzynska et al., 2014; Freudenberger et al., 2016; Gow et al., 2012; Johnson et al., 2020; Raichlen et al., 2019; Vesperman et al., 2018; Williamson et al., 2018; Wirth et al., 2014). However, six studies did not observe any significant relation between PACE and WMH (Palta et al., 2021; Balbim et al., 2021; Gu et al., 2020; Frederiksen et al., 2015; Fleischman et al., 2015; Tian et al., 2014a). In summary, while longitudinal evidence is preliminary, the majority of existing cross-sectional studies suggest that greater PACE is associated with a reduced occurrence of WM anomalies.
Meta-analysis
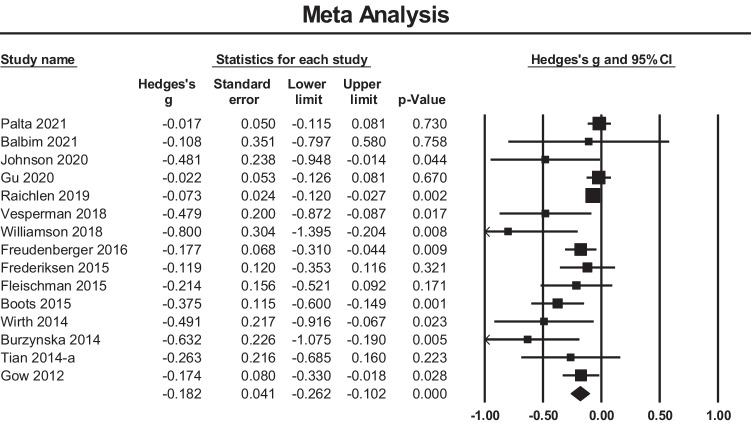
A meta-analysis of fifteen cross sectional studies examining the relationship between PACE and global WMH volume showed an overall small mean effect size of -0.182 (95% confidence interval (CI) = -0.262 to -0.102, p < 0.001) (Fig. 4). There was significant heterogeneity among the included studies (Q = 35.44, p = 0.001, = 60.50). The funnel plot (Fig. 5) was not symmetric and the Begg and Mazumdar rank correlation was non-significant (Tau = -0.36, two-tailed p = 0.06). The covariates of Age and Biological Sex were entered into the regression model to assess their influence on the observed heterogeneity, however, this was not significant (Q = 0.52, df = 2, p = 0.769).
Fig. 4.
Effect sizes for WM anomalies within cross sectional studies. Higher PACE is correlated with a reduced occurrence of WM anomalies (hyperintensities)
Fig. 5.
Funnel plot of standard errors plotted against effect sizes (Hedges’s g) for studies in Fig. 4 to visualise publication bias
PACE and white matter microstructural changes
Narrative synthesis
Longitudinal studies
Nine studies investigated the effect of PACE on WM microstructure, with the majority utilising DTI outcome measures (Table 1). Basic standard metrics of diffusion analysis are fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). The two most frequently reported metrics are FA and MD which generally reflect WM integrity and average diffusivity respectively (Curran et al., 2016). Also, increased AD (diffusivity along principal axis) has been linked to axonal damage and increased RD (average of diffusivity along perpendicular axes) has been associated with demyelination (Curran et al., 2016; Mayo et al., 2019).
Three studies found significant FA increase following exercise intervention, while four studies did not find significant effects (Best et al., 2017; Lehmann et al., 2020; Maltais et al., 2020; Sexton et al., 2020). Of those studies reporting positive effects, FA increases were observed across a number of brain regions including prefrontal, parietal, and temporal cortices (Voss et al., 2013), as well as specific WM tracts including the fornix (Burzynska et al., 2017), and left corticospinal tract (CST) (Palmer et al., 2013). Interestingly, one study reported a decrease in whole brain mean FA following 6 months aerobic exercise intervention, but these results may have been influenced by demographic differences which were not controlled for between groups (Clark et al., 2019).
The effect of PACE on MD has been analysed in five studies. Four studies reported significant associations between PACE and MD (Burzynska et al., 2017; Lehmann et al., 2020; Maltais et al., 2020; Palmer et al., 2013), while one study reported no significant effects (Clark et al., 2019). Three studies reported changes in MD following exercise interventions across a number of tracts including superior longitudinal fasciculus, anterior thalamic radiation, uncinate fasciculus, inferior fronto-occipital fasciculus, forceps minor, and the corticospinal tract (Burzynska et al., 2017; Lehmann et al., 2020; Palmer et al., 2013). However, the direction of MD change was inconsistent across studies, with studies reporting both increased (Burzynska et al., 2017) and decreased MD (Lehmann et al., 2020; Palmer et al., 2013) following exercise. One observational study reported greater decrease in MD over a five year period in individuals engaging in lower PACE (Maltais et al., 2020).
Four studies investigated the effect of PACE on RD. Two studies reported non-significant results (Sexton et al., 2020; Voss et al., 2013), and two studies reported significant outcomes (Burzynska et al., 2017; Lehmann et al., 2020), though the direction of these effects differed between studies. Specifically, one study reported decreased RD in right frontotemporal fiber tracts following exercise (Lehmann et al., 2020), while another study found that a dance-based intervention ameliorated the increase in RD observed over a 6-month period in older adults (Burzynska et al., 2017).
Three studies measured the effects of PACE on AD. One study found that higher PACE offset an increase in AD across inferior longitudinal fasciculus, parahippocampal and dorsal regions of the cingulum in individuals over a 10-year period (Best et al., 2017). Two other studies reported no change in AD following an exercise intervention (Sexton et al., 2020; Voss et al., 2013).
One study utilised the T1/T2 ratio (a measure of WM integrity derived by dividing the T1-weighted image by the T2-weighted image) to investigate the effect of a 6-month aerobic exercise on WM integrity. Significant differences in total WM were observed in a walking group relative to controls, with increases observed in the genu and splenium of CC, cingulum, and forceps minor in the walking group. Similarly, significant differences in total WM were also observed in a dance group relative to controls, with increases observed in the genu of CC following a dance intervention (Colmenares et al., 2021).
Cross sectional studies
The association between PACE and WM microstructure has been explored in 21 studies to date. Of these, fifteen studies reported significant associations between PACE and FA (Table 2), while four studies did not observe significant associations (Balbim et al., 2021; d’Arbeloff et al., 2021; Bracht et al., 2016; Tian et al., 2014a). Of the studies reporting significant associations, greater engagement in PACE was correlated with higher FA across numerous regions, particularly the CC (Hayes et al., 2015; Johnson et al., 2012; Kim et al., 2020; Liu, 2012; Oberlin et al., 2016; Opel et al., 2019; Strömmer et al., 2020; Tarumi et al., 2021), anterior limb of internal capsule (Liu et al., 2012; Oberlin et al., 2016; Opel et al., 2019; Strömmer et al., 2020; Tarumi et al., 2021), cingulum (Tarumi et al., 2021; Oberlin et al., 2016; Tian et al., 2014b; Marks et al., 2011), uncinate fasciculus (Opel et al., 2019; Strömmer et al., 2020; Tarumi et al., 2021), and superior longitudinal fasciculus (Oberlin et al., 2016; Opel et al., 2019; Tarumi et al., 2021). However, three studies have reported that higher PACE was associated with reduced FA across the bilateral posterior limb of internal capsule (Oberlin et al., 2016), left fornix and stria terminals (Smith et al., 2016), and left CST (Herting et al., 2014).
Correlation between PACE and MD have been reported in nine studies. Overall, four studies reported significant outcomes, while five studies did not observe any significant results (Bracht et al., 2016; Gow et al., 2012; Johnson et al., 2012; Marks et al., 2011; Smith et al., 2016). Of the studies reporting positive outcomes, one study reported significantly lower MD in middle-aged subjects engaging in moderate to vigorous PA, compared to controls (Palta et al., 2021). Two studies observed significant negative correlations between PACE and MD with associations observed across the hippocampus and entorhinal cortex (Tian et al., 2014b), and left inferior longitudinal fasciculus (Kim et al., 2020). One study compared MD between sedentary individuals and those engaging in regular exercise. Significant differences in MD were observed between groups, with lower MD observed in the medial temporal lobe and cingulate cortex in individuals engaging in regular exercise (Tian et al., 2014a).
The association between PACE and AD was examined in six studies. One study reported higher AD in a sample of physically active subjects, relative to controls, across several regions including CC, SLF, uncinate fasciculus, and fornix (Tarumi et al., 2021). However, five studies reported non-significant results (Bracht et al., 2016; Herting et al., 2014; Johnson et al., 2012; Kim et al., 2020; Smith et al., 2016).
Six studies examined the relationship between PACE and RD. Two studies reported significant negative correlations between PACE and RD in corpus callosum (Johnson et al., 2012), and left inferior longitudinal fasciculus (Kim et al., 2020). Of these, however, one study reported inconsistent effects, whereby increased RD was observed in the brainstem and decreased RD in external capsule in physically active subjects (Tarumi et al., 2021). Three studies reported non-significant findings (Bracht et al., 2016; Herting et al., 2014; Smith et al., 2016).
One study employed a multicomponent-driven equilibrium single pulse observation of T1 and T2 (mcDESPOT) sequence to investigate the relationship between PACE and WM integrity. They observed a positive association between PACE and myelin water fraction in right parahippocampal cingulum and a positive trend in the fornix (Bracht et al., 2016).
Meta-analysis
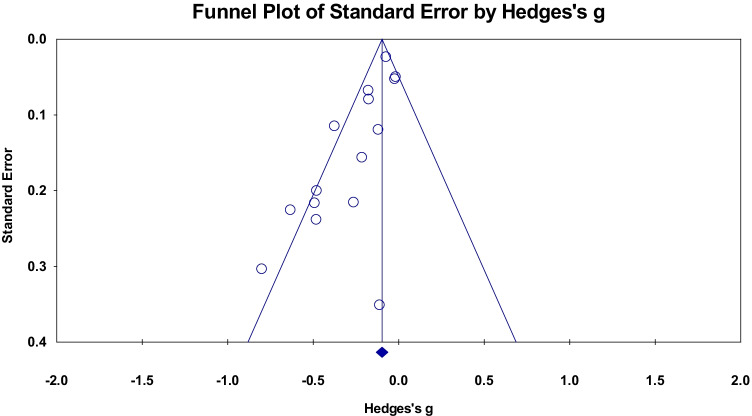
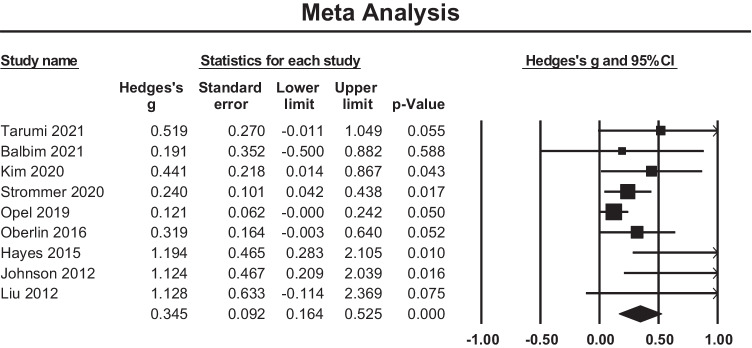
A meta-analysis of nine cross sectional studies reporting region-of-interest analysis of FA in corpus callosum was conducted. We observed a small positive effect size of 0.345 in the corpus callosum (95% confidence interval (CI) = 0.164 to 0.525, p < 0.001, Fig. 6). Studies were not significantly heterogeneous (Q = 15.399, p = 0.052, = 48.05). The funnel plot was partially asymmetric (Fig. 7) and the Begg and Mazumdar rank correlation was significant (Tau = 0.583, two-tailed p = 0.028). The meta-regression showed no significant relationship between Hedges’s g and both covariates (Q = 4.18, df = 2, p = 0.124).
Fig. 6.
Effect sizes for local WM microstructure reporting FA metric changes in corpus callosum. Higher FA values were positively associated with PACE level
Fig. 7.
Funnel plot of standard errors plotted against effect sizes (Hedges’s g) for studies in Fig. 6 to visualise publication bias
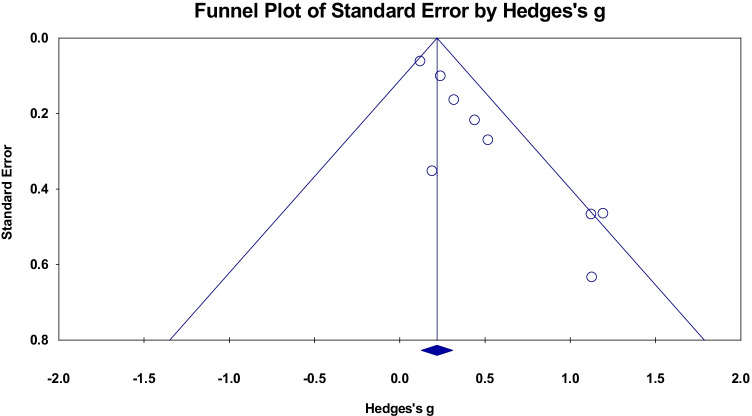
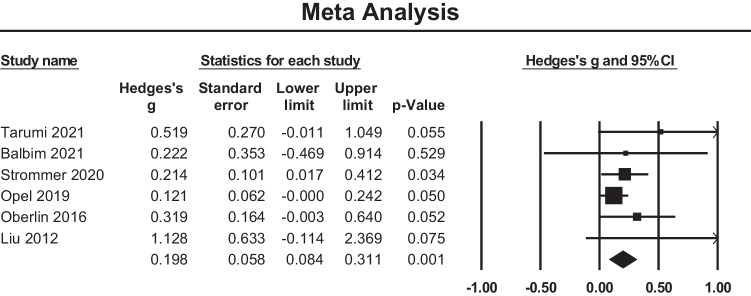
A meta-analysis of six cross sectional studies reporting FA changes at anterior limb of internal capsule was performed and a small positive effect size of 0.198 was observed (95% confidence interval (CI) = 0.084 to 0.311, p < 0.001) (Fig. 8). Studies were not significantly heterogeneous (Q = 5.562, p = 0.351, = 10.102). The funnel plot (Fig. 9) is symmetric and there was no evidence of significant bias (Tau = 0.53, two-tailed p = 0.132).
Fig. 8.
Effect sizes for local WM microstructural findings in anterior limb of internal capsule. Higher FA values were positively associated with PACE level
Fig. 9.
Funnel plot of standard errors plotted against effect sizes (Hedges’s g) for studies in Fig. 8 to visualise publication bias
Discussion
We conducted a systematic review of the literature investigating interactions between PACE and WM. We found that majority of cross-sectional and longitudinal studies reported that greater engagement in PACE was associated with greater WM volume and integrity. Similarly, across studies higher PACE was also associated with reduced WM anomalies. This pattern of results was also supported by meta-analysis of the data, which indicated a significant positive effect of PACE on WM volume and integrity, although the size of this effect was small. However, we note that within the sampled literature, several studies reported null results, suggesting that the effects of PACE on WM are likely variable, and quite plausibly influenced by certain methodological considerations and/or PACE parameters. Overall, despite considerable heterogeneity in study methodology and outcomes, we provide evidence of positive correlation between greater engagement in PACE and several aspects of WM. The following sections will provide a detailed discussion of our findings in relation to past evaluations of this evidence, and outline possible methodological variables that may moderate the effects of PACE on WM.
Evidence of regionally specific effects of PACE on WM
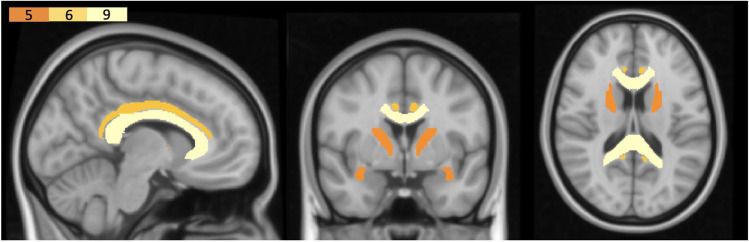
Across the sampled literature, there was some indication of regionally specific interactions between PACE and WM. Specifically, associations between PACE and WM integrity were observed primarily in the corpus callosum (Colmenares et al., 2021; Hayes et al., 2015; Johnson et al., 2012; Kim et al., 2020; Liu et al., 2012; Oberlin et al., 2016; Opel et al., 2019; Strömmer et al., 2020; Tarumi et al., 2021), uncinate fasciculus (Lehmann et al., 2020; Maltais et al., 2020; Opel et al., 2019; Strömmer et al., 2020; Tarumi et al., 2021), internal capsule (Liu et al., 2012; Oberlin et al., 2016; Opel et al., 2019; Strömmer et al., 2020; Tarumi et al., 2021), cingulum (Balbim et al., 2021; Tarumi et al., 2021; Oberlin et al., 2016; Tian et al. 2014a, 2014b; Marks et al., 2011), and fornix (Burzynska et al., 2017; Oberlin et al., 2016; Smith et al., 2016; Tarumi et al., 2021) (see Fig. 10). These findings are largely consistent with those of a previous review by Sexton et al. (2016), which reported some evidence of an association between PACE and WM volume and microstructure (particularly in frontal regions) in older adults. We extend on these findings by demonstrating that these effects are observed across all age cohorts, suggesting that the beneficial effects of PACE are observable across the lifespan.
Fig. 10.
Regions commonly reported in the outcomes of longitudinal and cross-sectional studies. The figure is only for visualisation purposes. The colour bar-plot on top left indicates the number of studies that reported associations between PACE and the WM region
We also note certain differences between our findings and those of Sexton et al. (2016). Our analysis indicates associations between PACE and WM in temporal regions. Comparatively, Sexton et al. provided some evidence of localised effects within frontal cortex. The cause of these topologically distinct findings is unclear, though it is possible that this may be partially attributable to differences in the age range of sampled literature. The previous review by Sexton et al. (2016) restricted their analysis to studies with a mean sample age > 60 years. Comparatively, we included all studies conducted with healthy adults (i.e. > 18 years of age). While our meta-regression analyses did not show clear evidence of associations between age and primary outcome measures, it remains possible that differences in our sampling approach and inclusion criteria have contributed to these inconsistencies. Frontal and temporal regions are particularly susceptible to age-related structural decline (Bennett & Madden, 2014; Sullivan & Pfefferbaum, 2006), and may therefore be differentially impacted by PACE at different life stages. We aimed to provide a comprehensive assessment of the associations between PACE and WM across the healthy adult lifespan, but future studies are required to elucidate the interactions between PACE, age, and white matter.
The relationship between exercise ‘dose’ and WM
The optimal exercise parameters for improving WM structure and integrity are yet to be elucidated. It is quite likely that associations between PACE and WM may vary according to the particular exercise parameters under investigation (i.e., modality, frequency, intensity, and duration). While the majority of the literature has investigated the effects of moderate intensity cardiovascular exercise (e.g., moderate intensity walking/cycling), it remains unclear whether these parameters are most effective for improving WM in the healthy population. Similarly, the ideal exercise frequency and duration (e.g., that balance potency and tolerability) are also yet to be established, and thus it is difficult to estimate an ideal ‘dose’ of exercise in this context. Differences in PACE measurement has also likely introduced the consistency in study outcomes. For instance, studies that measured PACE level based on subjective methods (e.g. self-report questionnaires) may have introduced systematic bias into their outcomes compared to studies that employed objective methods (e.g. VO2max test). However, given that the links between PACE and WM have been observed across a range of exercise protocols, it is possible that these associations may not relate to the specific nature of the activity per se, but rather depend on the individual’s physiological response to the regimen. In this sense, in developing effective exercise programs to improve WM it may be important to focus on parameters that elicit a certain physiological response (e.g., achieving a certain cardiorespiratory response), to modulate the relevant mechanisms (e.g., brain derived neurotrophic factor (BDNF)) that may mediate these effects. Interestingly, there is some evidence of a positive association between the heart rate response to exercise and BDNF circulation (Marquez et al., 2015). While speculative, focussing on the physiological response to exercise in this manner may offer a means of individualising exercise prescription to maximise associated benefits. Such a framework may also be beneficial in identifying factors which have contributed to the observed variability in study outcomes to date. Future studies are encouraged to report physiological outcomes in response to exercise (e.g., achieved heart rate, VO2max, perceived level of exertion) to assess the validity/utility of this perspective.
Mechanisms mediating the effects of PACE on WM
The underlying mechanisms mediating the effects of PACE on WM remain unclear (Sexton et al., 2016). One plausible hypothesis implicates the known effects of PACE on several cellular and molecular mechanisms mediating aspects of neuroplasticity. For example, both animal and human studies have shown that exercise influences the expression and circulation of key neurotrophins and growth factors (i.e. BDNF, vascular endothelial growth factor (VEGF), and insulin like growth factor (IGF-1)), which modulate a range of microscale structural and synaptic plasticity processes (e.g. synaptogenesis and angiogenesis) (Cotman et al., 2007; Maass et al., 2016). To date, few studies have evaluated the relationship between PACE, WM, and these mechanisms. However, there is preliminary evidence to suggest interactions between expression of these factors and WM (Weinstock-Guttman et al., 2007). Despite the limitations of available measurement techniques in vivo in humans (i.e., reliance on indirect peripheral estimates), future studies are encouraged to investigate the possible mediating role of these factors in the relationship between PACE and WM.
The effects of PACE on WM may also occur via activity-dependent myelination. There is evidence that action potentials trigger the sequence of events underlying myelination (Zatorre et al., 2012). Physical activity and exercise inherently rely upon movement and the distributed brain networks that underpin interlimb coordination (Byblow et al., 2007; Caeyenberghs et al., 2011; Coxon et al., 2010; Swinnen, 2002; Swinnen & Wenderoth, 2004). As such, it is plausible that the neural activity supporting interlimb coordination during physical activity/exercise stimulates myelination processes, which may over time manifest as an overall increase in WM volume and integrity across distributed networks. On a functional level, an increase in myelination in this manner may serve to increase conduction speed across networks supporting interlimb coordination to increase the efficiency/accuracy of movement intrinsic to specific forms of physical activity/exercise. This perspective may also help to explain the regionally specific effects of PACE on WM. Namely, the consistent relationships observed between PACE and certain tracts, such as the corpus collosum and anterior internal capsule, may reflect increased communication across brain networks involving these tracts to support motor coordination, or possibly other cognitive demands during exercise/physical activity (e.g., spatial memory, decision making). While speculative, future studies may also consider this potential relationship between the functional demands inherent to exercise, and associated influence on white matter.
Conclusion
In summary, following our systematic review of the literature, we report evidence of a significant positive association between PACE and WM within the healthy population. Interestingly, there was evidence of a regionally specific relationship between PACE and WM, with medial temporal regions/tracts commonly reported in study outcomes. Future studies are encouraged to consider/report the physiological response to exercise (e.g. heart rate, and BDNF) to help elucidate potential factors contributing to the heterogeneity in study outcomes and plausibly optimise the prescription of exercise. Future work in this field may also consider the relevance of particular neurotrophic growth factors in mediating neuroplasticity and the relationship between PACE and WM. In regard to MRI methodology, the majority of studies have employed diffusion imaging to investigate correlations between PACE and WM microstructure. Moving forward, studies are recommended to employ multi-modal methods to gain a more nuanced understanding of the specific WM components influenced by PACE. For example, future may employ MRI modalities that are sensitive to changes in myelination, such as the T1/T2 ratio, magnetisation transfer ratio, or mcDESPOT (Sampaio-Baptista & Johansen-Berg, 2017). It is hoped that improving our understanding of the influence of PACE on WM may yield novel, effective lifestyle-based interventions to optimise brain health across the lifespan.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Author contribution included study design (MY, KC, CS and SM), conducting systematic review (SM and YC), reviewing literature (SM, CS, and YC), performing meta-analysis (SM, CS, SO), interpreting the results (SM, CS, and JH), writing the manuscript (SM, JH, MY, KC, JC, CS, YC, and SO), and approval of final version to be published (all authors).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions There was no direct funding for the development of this systematic review. MY has received funding from Monash University, and Australian Government funding bodies such as the National Health and Medical Research Council (NHMRC; including Fellowship #APP1117188), the Australian Research Council (ARC), and the Department of Industry, Innovation and Science. He has also received philanthropic donations from the David Winston Turner Endowment Fund, Wilson Foundation, as well as payment from law firms in relation to court and/or expert witness reports. The funding sources had no role in the design, management, data analysis, or interpretation and write-up of the data. KC is supported by a National Health and Medical Research Council Career Development Fellowship (APP1143816). JH and JPC are supported by an ARC Discovery project grant (DP200100234). JH is supported by a Turner Institute for Brain and Mental Health Postdoctoral Bridging Fellowship.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
Not applicable.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chao Suo, Email: chao.suo@monash.edu.
Murat Yücel, Email: murat.yucel@monash.edu.
References
- Alkadhi KA. Exercise as a positive modulator of brain function. Molecular Neurobiology. 2018;55(4):3112–3130. doi: 10.1007/s12035-017-0516-4. [DOI] [PubMed] [Google Scholar]
- Arnardottir, N. Y., Koster, A., Van Domelen, D. R., Brychta, R. J., Caserotti, P., Eiriksdottir, G., … Sveinsson, T. (2016). Association of change in brain structure to objectively measured physical activity and sedentary behavior in older adults: Age, Gene/Environment Susceptibility-Reykjavik Study. Behavioural Brain Research, 296, 118–124. 10.1016/j.bbr.2015.09.005 [DOI] [PMC free article] [PubMed]
- Australian Department of Health. (2021). Australia’s Physical Activity Guidelines. Retrieved from https://www.health.gov.au/health-topics/physical-activity-and-exercise/physical-activity-and-exercise-guidelines-for-all-australians/for-adults-18-to-64-years
- Balbim GM, Erickson KI, Ajilore OA, Aguiñaga S, Bustamante EE, Lamar M, Marquez DX. Association of physical activity levels and brain white matter in older Latino adults. Ethnicity and Health. 2021 doi: 10.1080/13557858.2021.1913484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994 doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- Benedict, C., Brooks, S. J., Kullberg, J., Nordenskjöld, R., Burgos, J., Le Grevès, M., … Schiöth, H. B. (2013). Association between physical activity and brain health in older adults. Neurobiology of Aging, 34(1), 83–90. 10.1016/j.neurobiolaging.2012.04.013 [DOI] [PubMed]
- Bennett IJ, Madden DJ. Disconnected aging: Cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014;276:187–205. doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Chiu BK, Liang Hsu C, Nagamatsu LS, Liu-Ambrose T. Long-term effects of resistance exercise training on cognition and brain volume in older women: Results from a randomized controlled trial. Journal of the International Neuropsychological Society. 2015;21(10):745–756. doi: 10.1017/S1355617715000673. [DOI] [PubMed] [Google Scholar]
- Best, J. R., Rosano, C., Aizenstein, H. J., Tian, Q., Boudreau, R. M., Ayonayon, H. N., … Liu-Ambrose, T. (2017). Long-term changes in time spent walking and subsequent cognitive and structural brain changes in older adults. Neurobiology of Aging, 57, 153–161. 10.1016/j.neurobiolaging.2017.05.023 [DOI] [PMC free article] [PubMed]
- Bolandzadeh, N., Tam, R., Handy, T. C., Nagamatsu, L. S., Hsu, C. L., Davis, J. C., … Liu-Ambrose, T. (2015). Resistance training and white matter lesion progression in older women: Exploratory analysis of a 12-month randomized controlled trial. Journal of the American Geriatrics Society, 63(10), 2052–2060. 10.1111/jgs.13644 [DOI] [PubMed]
- Boots, E. A., Schultz, S. A., Oh, J. M., Larson, J., Edwards, D., Cook, D., … Okonkwo, O. C. (2015). Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer’s disease. Brain Imaging and Behavior, 9(3), 639–649. 10.1007/s11682-014-9325-9 [DOI] [PMC free article] [PubMed]
- Borenstein, M., Hedges, L., Higgins, J., & Rothstein, H. (2013). Comprehensive Meta-Analysis Version 3. Biostat, Englewood, NJ 2013.
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods. 2010 doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- Bracht T, Jones DK, Bells S, Walther S, Drakesmith M, Linden D. Myelination of the right parahippocampal cingulum is associated with physical activity in young healthy adults. Brain Structure and Function. 2016;221(9):4537–4548. doi: 10.1007/s00429-016-1183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiology of Aging. 2011;32(3):506–514. doi: 10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska, A. Z., Chaddock-Heyman, L., Voss, M. W., Wong, C. N., Gothe, N. P., Olson, E. A., … Kramer, A. F. (2014). Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS One, 9(9). 10.1371/journal.pone.0107413 [DOI] [PMC free article] [PubMed]
- Burzynska, A. Z., Jiao, Y., Knecht, A. M., Fanning, J., Awick, E. A., Chen, T., … Kramer, A. F. (2017). White matter integrity declined over 6-months, but dance intervention improved integrity of the Fornix of older adults. Frontiers in Aging Neuroscience, 9. 10.3389/fnagi.2017.00059 [DOI] [PMC free article] [PubMed]
- Byblow WD, Coxon JP, Stinear CM, Fleming MK, Williams G, Müller JFM, Ziemann U. Functional connectivity between secondary and primary motor areas underlying hand-foot coordination. Journal of Neurophysiology. 2007 doi: 10.1152/jn.00325.2007. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs, K., Leemans, A., Coxon, J., Leunissen, I., Drijkoningen, D., Geurts, M., … Swinnen, S. P. (2011). Bimanual coordination and corpus callosum microstructure in young adults with traumatic brain injury: A diffusion tensor imaging study. Journal of Neurotrauma. 10.1089/neu.2010.1721 [DOI] [PubMed]
- Campbell N., De Jesus S., P. H. (2013). Encyclopedia of Behavioral Medicine. In T. J. R. Gellman M.D. (Ed.), Encyclopedia of Behavioral Medicine (2013th ed.). New York: Springer. 10.1007/978-1-4419-1005-9_1167
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise and physical fitness definitions for health-related research. Public Health Reports. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- Clark CM, Guadagni V, Mazerolle EL, Hill M, Hogan DB, Pike GB, Poulin MJ. Effect of aerobic exercise on white matter microstructure in the aging brain. Behavioural Brain Research. 2019 doi: 10.1016/j.bbr.2019.112042. [DOI] [PubMed] [Google Scholar]
- Bouchard,C, Blair S. N., W. L. H. (2012). Physical Activity and Health (2nd ed.). Human Kinetics.
- Colcombe, S. J., Erickson, K. I., Raz, N., Webb, A. G., Cohen, N. J., McAuley, E., & Kramer, A. F. (2003). Aerobic fitness reduces brain tissue loss in aging humans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 58(2). 10.1093/gerona/58.2.m176 [DOI] [PubMed]
- Colcombe, S. J., Erickson, K. I., Scalf, P. E., Kim, J. S., Prakash, R., McAuley, E., … Kramer, A. F. (2006). Aerobic exercise training increases brain volume in aging humans. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 61(11), 1166–1170. 10.1093/gerona/61.11.1166 [DOI] [PubMed]
- Colmenares, A. M., Voss, M. W., Fanning, J., Salerno, E. A., Gothe, N. P., Thomas, M. L., … Burzynska, A. Z. (2021). White matter plasticity in healthy older adults: The effects of aerobic exercise. NeuroImage. 10.1016/j.neuroimage.2021.118305 [DOI] [PubMed]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences. 2007 doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Goble DJ, Van Impe A, De Vos J, Wenderoth N, Swinnen SP. Reduced basal ganglia function when elderly switch between coordinated movement patterns. Cerebral Cortex. 2010 doi: 10.1093/cercor/bhp306. [DOI] [PubMed] [Google Scholar]
- Curran, K. M., Emsell, L., & Leemans, A. (2016). Quantitative DTI measures. In Diffusion Tensor Imaging: A Practical Handbook. 10.1007/978-1-4939-3118-7_5
- d’Arbeloff, T., Elliott, M. L., Knodt, A. R., Sison, M., Melzer, T. R., Ireland, D., … Hariri, A. R. (2021). Midlife Cardiovascular Fitness Is Reflected in the Brain’s White Matter. Frontiers in Aging Neuroscience. 10.3389/fnagi.2021.652575 [DOI] [PMC free article] [PubMed]
- Demirakca, T., Brusniak, W., Tunc-Skarka, N., Wolf, I., Meier, S., Matthäus, F., … Diener, C. (2014). Does body shaping influence brain shape? Habitual physical activity is linked to brain morphology independent of age. World Journal of Biological Psychiatry, 15(5), 387–396. 10.3109/15622975.2013.803600 [DOI] [PubMed]
- Den Ouden, L., Kandola, A., Suo, C., Hendrikse, J., Costa, R. J. S., Watt, M. J., … Yücel, M. (2018). The influence of aerobic exercise on hippocampal integrity and function: Preliminary findings of a multi-modal imaging analysis. Brain Plasticity. 10.3233/bpl-170053 [DOI] [PMC free article] [PubMed]
- Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, Kramer AF. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiology of Aging. 2007;28(2):179–185. doi: 10.1016/j.neurobiolaging.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Filley CM, Fields RD. White matter and cognition: Making the connection. Journal of Neurophysiology. 2016;116(5):2093–2104. doi: 10.1152/jn.00221.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J, Stubbs B, Vancampfort D, Schuch F, Lagopoulos J, Rosenbaum S, Ward PB. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. NeuroImage. 2018;166:230–238. doi: 10.1016/j.neuroimage.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Fleischman, D. A., Yang, J., Arfanakis, K., Arvanitakis, Z., Leurgans, S. E., Turner, A. D., … Buchman, A. S. (2015). Physical activity, motor function, and white matter hyperintensity burden in healthy older adults. Neurology, 84(13), 1294–1300. 10.1212/WNL.0000000000001417 [DOI] [PMC free article] [PubMed]
- Frederiksen, K. S., Verdelho, A., Madureira, S., Bäzner, H., O’Brien, J. T., Fazekas, F., … Waldemar, G. (2015). Physical activity in the elderly is associated with improved executive function and processing speed: The LADIS Study. International Journal of Geriatric Psychiatry, 30(7), 744–750. 10.1002/gps.4220 [DOI] [PubMed]
- Freudenberger, P., Petrovic, K., Sen, A., Töglhofer, A. M., Fixa, A., Hofer, E., … Schmidt, H. (2016). Fitness and cognition in the elderly: The Austrian Stroke Prevention Study. Neurology, 86(5), 418–424. 10.1212/WNL.0000000000002329 [DOI] [PMC free article] [PubMed]
- Frey, B. M., Petersen, M., Mayer, C., Schulz, M., Cheng, B., & Thomalla, G. (2019). Characterization of white matter hyperintensities in large-scale MRI-studies. Frontiers in Neurology, 10. 10.3389/fneur.2019.00238 [DOI] [PMC free article] [PubMed]
- Fuhrmann, D., Nesbitt, D., Shafto, M., Rowe, J. B., Price, D., Gadie, A., … Kievit, R. A. (2019). Strong and specific associations between cardiovascular risk factors and white matter micro- and macrostructure in healthy aging. Neurobiology of Aging, 74, 46–55. 10.1016/j.neurobiolaging.2018.10.005 [DOI] [PMC free article] [PubMed]
- Gordon, B. A., Rykhlevskaia, E. I., Brumback, C. R., Lee, Y., Elavsky, S., Konopack, J. F., … Fabiani, M. (2008). Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology, 45(5), 825–838. 10.1111/j.1469-8986.2008.00676.x [DOI] [PMC free article] [PubMed]
- Gow, A. J., Bastin, M. E., Muñoz Maniega, S., Valdés Hernández, M. C., Morris, Z., Murray, C., … Wardlaw, J. M. (2012). Neuroprotective lifestyles and the aging brain. Neurology, 79(17), 1802–1808. 10.1212/WNL.0b013e3182703fd2 [DOI] [PubMed]
- Gu, Y., Beato, J. M., Amarante, E., Chesebro, A. G., Manly, J. J., Schupf, N., … Brickman, A. M. (2020). Assessment of leisure time physical activity and brain health in a multiethnic cohort of older adults. JAMA Network Open. 10.1001/jamanetworkopen.2020.26506 [DOI] [PMC free article] [PubMed]
- Gujral S, Aizenstein H, Reynolds CF, Butters MA, Erickson KI. Exercise effects on depression: Possible neural mechanisms. General Hospital Psychiatry. 2017 doi: 10.1016/j.genhosppsych.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral S, McAuley E, Oberlin LE, Kramer AF, Erickson KI. The role of brain structure in predicting adherence to a physical activity regimen. Psychosomatic Medicine. 2018;80(1):69–77. doi: 10.1097/PSY.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Salat DH, Forman DE, Sperling RA, Verfaellie M. Cardiorespiratory fitness is associated with white matter integrity in aging. Annals of Clinical and Translational Neurology. 2015;2(6):688–698. doi: 10.1002/acn3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrikse J, Kandola A, Coxon J, Rogasch N, Yücel M. Combining aerobic exercise and repetitive transcranial magnetic stimulation to improve brain function in health and disease. Neuroscience and Biobehavioral Reviews. 2017 doi: 10.1016/j.neubiorev.2017.09.023. [DOI] [PubMed] [Google Scholar]
- Herting MM, Colby JB, Sowell ER, Nagel BJ. White matter connectivity and aerobic fitness in male adolescents. Developmental Cognitive Neuroscience. 2014;7:65–75. doi: 10.1016/j.dcn.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, A. J., Raji, C. A., Becker, J. T., Lopez, O. L., Kuller, L. H., Hua, X., … Thompson, P. M. (2011). The effects of physical activity, education, and body mass index on the aging brain. Human Brain Mapping, 32(9), 1371–1382. 10.1002/hbm.21113 [DOI] [PMC free article] [PubMed]
- Jochem, C., Baumeister, S. E., Wittfeld, K., Leitzmann, M. F., Bahls, M., Schminke, U., … Jörgen Grabe, H. (2017). Domains of physical activity and brain volumes: A population-based study. NeuroImage, 156(101–108). 10.1016/j.neuroimage.2017.05.020 [DOI] [PubMed]
- Johnson NF, Bahrani AA, Powell DK, Jicha GA, Gold BT. Cardiorespiratory fitness diminishes the effects of age on white matter hyperintensity volume. PLoS ONE. 2020 doi: 10.1371/journal.pone.0236986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. NeuroImage. 2012;59(2):1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BR, Kwon H, Chun MY, Park KD, Lim SM, Jeong JH, Kim GH. White Matter Integrity Is Associated With the Amount of Physical Activity in Older Adults With Super-aging. Frontiers in Aging Neuroscience. 2020 doi: 10.3389/fnagi.2020.549983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblinsky ND, Meusel LAC, Greenwood CE, Anderson ND. Household physical activity is positively associated with gray matter volume in older adults. BMC Geriatrics. 2021 doi: 10.1186/s12877-021-02054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann N, Villringer A, Taubert M. Colocalized white matter plasticity and increased cerebral blood flow mediate the beneficial effect of cardiovascular exercise on long-term motor learning. Journal of Neuroscience. 2020;40(12):2416–2429. doi: 10.1523/JNEUROSCI.2310-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z. Automated voxel-wise brain DTI analysis of fitness and aging. The Open Medical Imaging Journal. 2012 doi: 10.2174/1874347101206010080. [DOI] [Google Scholar]
- Liu, Z., Farzinfar, M., Katz, L. M., Zhu, H., Goodlett, C. B., Gerig, G., … Marks, B. L. (2012). Automated voxel-wise brain DTI analysis of fitness and aging. Open Medical Imaging Journal. 10.2174/1874347101206010080
- Maass, A., Düzel, S., Brigadski, T., Goerke, M., Becke, A., Sobieray, U., … Düzel, E. (2016). Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage, 131, 142–154. 10.1016/j.neuroimage.2015.10.084 [DOI] [PubMed]
- Maltais, M., Rolland, Y., Boisvert-Vigneault, K., Perus, L., Mangin, J. F., Grigis, A., … de Souto Barreto, P. (2020). Prospective associations between physical activity levels and white matter integrity in older adults: results from the MAPT study. Maturitas. 10.1016/j.maturitas.2020.04.012 [DOI] [PubMed]
- Mandolesi, L., Polverino, A., Montuori, S., Foti, F., Ferraioli, G., Sorrentino, P., & Sorrentino, G. (2018). Effects of physical exercise on cognitive functioning and wellbeing: Biological and psychological benefits. Frontiers in Psychology, 9. 10.3389/fpsyg.2018.00509 [DOI] [PMC free article] [PubMed]
- Marks BL, Katz LM, Styner M, Smith JK. Aerobic fi tness and obesity: Relationship to cerebral white matter integrity in the brain of active and sedentary older adults. British Journal of Sports Medicine. 2011;45(15):1208–1215. doi: 10.1136/bjsm.2009.068114. [DOI] [PubMed] [Google Scholar]
- Marquez CMS, Vanaudenaerde B, Troosters T, Wenderoth N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. Journal of Applied Physiology. 2015 doi: 10.1152/japplphysiol.00126.2015. [DOI] [PubMed] [Google Scholar]
- Mayo CD, Garcia-Barrera MA, Mazerolle EL, Ritchie LJ, Fisk JD, Gawryluk JR. Relationship between DTI metrics and cognitive function in Alzheimer’s disease. Frontiers in Aging Neuroscience. 2019 doi: 10.3389/fnagi.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, S. Y., de Souto Barreto, P., Cesari, M., Chupin, M., Mangin, J. F., Bouyahia, A., … Vellas, B. (2018). Physical Activity and Changes in White Matter Hyperintensities over Three Years. Journal of Nutrition, Health and Aging, 22(3), 425–430. 10.1007/s12603-017-0959-3 [DOI] [PubMed]
- Oberlin, L. E., Verstynen, T. D., Burzynska, A. Z., Voss, M. W., Prakash, R. S., Chaddock-Heyman, L., … Erickson, K. I. (2016). White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults. NeuroImage, 131, 91–101. 10.1016/j.neuroimage.2015.09.053 [DOI] [PMC free article] [PubMed]
- Opel, N., Martin, S., Meinert, S., Redlich, R., Enneking, V., Richter, M., … Repple, J. (2019). White matter microstructure mediates the association between physical fitness and cognition in healthy, young adults. Scientific Reports. 10.1038/s41598-019-49301-y [DOI] [PMC free article] [PubMed]
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. The BMJ. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed]
- Palmer, H. S., Håberg, A. K., Fimland, M. S., Solstad, G. M., Moe Iversen, V., Hoff, J., … Eikenes, L. (2013). Structural brain changes after 4 wk of unilateral strength training of the lower limb. Journal of Applied Physiology, 115(2), 167–175. 10.1152/japplphysiol.00277.2012 [DOI] [PubMed]
- Palta, P., Sharrett, A. R., Gabriel, K. P., Gottesman, R. F., Folsom, A. R., Power, M. C., … Heiss, G. (2021). Prospective analysis of leisure-time physical activity in midlife and beyond and brain damage on MRI in older adults. Neurology. 10.1212/WNL.0000000000011375 [DOI] [PMC free article] [PubMed]
- Pentikäinen, H., Ngandu, T., Liu, Y., Savonen, K., Komulainen, P., Hallikainen, M., … Soininen, H. (2017). Cardiorespiratory fitness and brain volumes in men and women in the FINGER study. Age and Ageing, 46(2), 310–313. 10.1093/ageing/afw191 [DOI] [PubMed]
- Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: An update. Nature Reviews Neurology. 2015;11(3):157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- Raichlen DA, Klimentidis YC, Bharadwaj PK, Alexander GE. Differential associations of engagement in physical activity and estimated cardiorespiratory fitness with brain volume in middle-aged to older adults. Brain Imaging and Behavior. 2019 doi: 10.1007/s11682-019-00148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld, K., Lüders, A., Hökelmann, A., Lessmann, V., Kaufmann, J., Brigadski, T., … Müller, N. G. (2018). Dance training is superior to repetitive physical exercise in inducing brain plasticity in the elderly. PLoS One, 13(7). 10.1371/journal.pone.0196636 [DOI] [PMC free article] [PubMed]
- Sampaio-Baptista C, Johansen-Berg H. White Matter Plasticity in the Adult Brain. Neuron. 2017;96(6):1239–1251. doi: 10.1016/j.neuron.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, Johansen-Berg H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. NeuroImage. 2016;131:81–90. doi: 10.1016/j.neuroimage.2015.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton, C. E., Betts, J. F., Dennis, A., Doherty, A., Leeson, P., Holloway, C., … Johansen-Berg, H. (2020). The effects of an aerobic training intervention on cognition, grey matter volumes and white matter microstructure. Physiology and Behavior, 223. 10.1016/j.physbeh.2020.112923 [DOI] [PMC free article] [PubMed]
- Smith, J. C., Lancaster, M. A., Nielson, K. A., Woodard, J. L., Seidenberg, M., Durgerian, S., … Rao, S. M. (2016). Interactive effects of physical activity and APOE-ε4 on white matter tract diffusivity in healthy elders. NeuroImage, 131, 102–112. 10.1016/j.neuroimage.2015.08.007 [DOI] [PMC free article] [PubMed]
- Smith, J. C., Nielson, K. A., Woodard, J. L., Seidenberg, M., Durgerian, S., Hazlett, K. E., … Rao, S. M. (2014). Physical activity reduces hippocampal atrophy in elders at genetic risk for Alzheimer’s disease. Frontiers in Aging Neuroscience, 6. 10.3389/fnagi.2014.00061 [DOI] [PMC free article] [PubMed]
- Stigger FS, ZagoMarcolino MA, Portela KM, Della MéaPlentz R. Effects of exercise on inflammatory, oxidative, and neurotrophic biomarkers on cognitively impaired individuals diagnosed with dementia or mild cognitive impairment: A systematic review and meta-analysis. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2019 doi: 10.1093/gerona/gly173. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience & Biobehavioral Reviews. 2006;30(6):749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Strömmer JM, Davis SW, Henson RN, Tyler LK, Campbell KL. Physical activity predicts population-level age-related differences in frontal white matter. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2020 doi: 10.1093/gerona/gly220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen SP. Intermanual coordination: From behavioural principles to neural-network interactions. Nature Reviews Neuroscience. 2002 doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Wenderoth N. Two hands, one brain: Cognitive neuroscience of bimanual skill. Trends in Cognitive Sciences. 2004 doi: 10.1016/j.tics.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Tabei, K. I., Satoh, M., Ogawa, J. I., Tokita, T., Nakaguchi, N., Nakao, K., … Tomimoto, H. (2017). Physical exercise with music reduces gray and white matter loss in the frontal cortex of elderly people: The mihama-kiho scan project. Frontiers in Aging Neuroscience, 9. 10.3389/fnagi.2017.00174 [DOI] [PMC free article] [PubMed]
- Tarumi, T., Tomoto, T., Repshas, J., Wang, C., Hynan, L. S., Cullum, C. M., … Zhang, R. (2021). Midlife aerobic exercise and brain structural integrity: Associations with age and cardiorespiratory fitness. NeuroImage. 10.1016/j.neuroimage.2020.117512 [DOI] [PMC free article] [PubMed]
- Tian, Q., Erickson, K. I., Simonsick, E. M., Aizenstein, H. J., Glynn, N. W., Boudreau, R. M., … Rosano, C. (2014a). Physical activity predicts microstructural integrity in memory-related networks in very old adults. Journals of Gerontology - Series A Biological Sciences and Medical Sciences, 69(10), 1284–1290. 10.1093/gerona/glt287 [DOI] [PMC free article] [PubMed]
- Tian, Q., Simonsick, E. M., Erickson, K. I., Aizenstein, H. J., Glynn, N. W., Boudreau, R. M., … Rosano, C. (2014b). Cardiorespiratory fitness and brain diffusion tensor imaging in adults over 80 years of age. Brain Research, 1588, 63–72. 10.1016/j.brainres.2014.09.003 [DOI] [PMC free article] [PubMed]
- Tian Q, Studenski SA, Resnick SM, Davatzikos C, Ferrucci L. Midlife and Late-Life Cardiorespiratory Fitness and Brain Volume Changes in Late Adulthood: Results from the Baltimore Longitudinal Study of Aging. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2015;71(1):124–130. doi: 10.1093/gerona/glv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2019). Physical Activity Guidelines for Americans. Retrieved from https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
- Vesperman, C. J., Pozorski, V., Dougherty, R. J., Law, L. L., Boots, E., Oh, J. M., … Okonkwo, O. C. (2018). Cardiorespiratory fitness attenuates age-associated aggregation of white matter hyperintensities in an at-risk cohort. Alzheimer’s Research & Therapy, 10(1). 10.1186/s13195-018-0429-0 [DOI] [PMC free article] [PubMed]
- Voss, M. W., Heo, S., Prakash, R. S., Erickson, K. I., Alves, H., Chaddock, L., … Kramer, A. F. (2013a). The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Human Brain Mapping, 34(11), 2972–2985. 10.1002/hbm.22119 [DOI] [PMC free article] [PubMed]
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences. 2013;17(10):525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock-Guttman, B., Zivadinov, R., Tamaño-Blanco, M., Abdelrahman, N., Badgett, D., Durfee, J., … Ramanathan, M. (2007). Immune cell BDNF secretion is associated with white matter volume in multiple sclerosis. Journal of Neuroimmunology. 10.1016/j.jneuroim.2007.06.003 [DOI] [PubMed]
- Williamson, W., Lewandowski, A. J., Forkert, N. D., Griffanti, L., Okell, T. W., Betts, J., … Leeson, P. (2018). Association of cardiovascular risk factors with MRI indices of cerebrovascular structure and function and white matter hyperintensities in young adults. JAMA - Journal of the American Medical Association, 320(7), 665–673. 10.1001/jama.2018.11498 [DOI] [PMC free article] [PubMed]
- Wirth M, Haase CM, Villeneuve S, Vogel J, Jagust WJ. Neuroprotective pathways: Lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiology of Aging. 2014;35(8):1873–1882. doi: 10.1016/j.neurobiolaging.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittfeld, K., Jochem, C., Dörr, M., Schminke, U., Gläser, S., Bahls, M., … Grabe, H. J. (2020). Cardiorespiratory Fitness and Gray Matter Volume in the Temporal, Frontal, and Cerebellar Regions in the General Population. Mayo Clinic Proceedings, 95(1), 44–56. 10.1016/j.mayocp.2019.05.030 [DOI] [PubMed]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nature Neuroscience. 2012 doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, N., Jacobs, D. R., Schreiner, P. J., Launer, L. J., Whitmer, R. A., Sidney, S., … Bryan, R. N. (2015). Cardiorespiratory fitness and brain volume and white matter integrity: The CARDIA Study. Neurology, 84(23), 2347–2353. 10.1212/WNL.0000000000001658 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.