Abstract
Purpose
Stroke is one of the most prevalent vascular diseases. Non-invasive molecular imaging methods have the potential to provide critical insights into the temporal dynamics and follow alterations of receptor expression and metabolism in ischemic stroke. The aim of this study was to assess the cannabinoid type 2 receptor (CB2R) levels in transient middle cerebral artery occlusion (tMCAO) mouse models at subacute stage using positron emission tomography (PET) with our novel tracer [18F]RoSMA-18-d6 and structural imaging by magnetic resonance imaging (MRI).
Procedures
Our recently developed CB2R PET tracer [18F]RoSMA-18-d6 was used for imaging neuroinflammation at 24 h after reperfusion in tMCAO mice. The RNA expression levels of CB2R and other inflammatory markers were analyzed by quantitative real-time polymerase chain reaction using brain tissues from tMCAO (1 h occlusion) and sham-operated mice. [18F]fluorodeoxyglucose (FDG) was included for evaluation of the cerebral metabolic rate of glucose (CMRglc). In addition, diffusion-weighted imaging and T2-weighted imaging were performed for anatomical reference and delineating the lesion in tMCAO mice.
Results
mRNA expressions of inflammatory markers TNF-α, Iba1, MMP9 and GFAP, CNR2 were increased to 1.3–2.5 fold at 24 h after reperfusion in the ipsilateral compared to contralateral hemisphere of tMCAO mice, while mRNA expression of the neuronal marker MAP-2 was markedly reduced to ca. 50 %. Reduced [18F]FDG uptake was observed in the ischemic striatum of tMCAO mouse brain at 24 h after reperfusion. Although higher activity of [18F]RoSMA-18-d6 in ex vivo biodistribution studies and higher standard uptake value ratio (SUVR) were detected in the ischemic ipsilateral compared to contralateral striatum in tMCAO mice, the in vivo specificity of [18F]RoSMA-18-d6 was confirmed only in the CB2R-rich spleen.
Conclusions
This study revealed an increased [18F]RoSMA-18-d6 measure of CB2R and a reduced [18F]FDG measure of CMRglc in the ischemic striatum of tMCAO mice at subacute stage. [18F]RoSMA-18-d6 might be a promising PET tracer for detecting CB2R alterations in animal models of neuroinflammation without neuronal loss.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11307-021-01655-4.
Key words: Cannabinoid type 2 receptor, [18F]RoSMA-18-d6, Ischemic stroke, Neuroinflammation, Magnetic resonance imaging, Positron emission tomography
Introduction
The pathophysiology of ischemic stroke is complex and associated with a myriad of cellular and molecular pathways. The severe reduction in cerebral blood flow (CBF) initiates a cascade of hemodynamic, vascular and inflammatory processes in a time-dependent manner in the supplied brain territory, and subsequent defensive response for repair related to lesion expansion and containment. Irreversible tissue damage occurs in the core of the ischemic area, while neurons in the ischemic penumbra face excitotoxicity, peri-infarct polarizations, inflammation, and apoptosis, leading to a secondary tissue damage and expansion of the lesion if reperfusion cannot be restored within an early time frame [1–3]. Neuroinflammation post stroke has been an important therapeutic target. Anti-inflammatory, immunomodulatory treatments and microglia-targeted therapy were evaluated in clinical stroke trials [4–6]. Thus, there is a need for imaging the regional neuroinflammatory pattern for understanding disease mechanism and for therapeutic monitoring.
Positron emission tomography (PET) using [18F]fluorodeoxyglucose ([18F]FDG) for cerebral metabolic rate of glucose (CMRglc), [15O]H2O for perfusion imaging, and diffusion weighted (DW) magnetic resonance imaging (MRI) are valuable tools to support understanding of the pathophysiology in patients with ischemic stroke [2, 7–13]. However, in vivo imaging of neuroinflammation and gliosis is challenging [11, 12, 14]. One reason is that the astrocytes and microglia are highly dynamic and heterogeneous in their subtypes, locations, and activation status [15]. Additionally, the identification of an ideal target for neuroinflammation imaging is highly demanding. Translocator protein (TSPO) is the most widely used neuroinflammation target for PET imaging. [11C]PK-11195, the first-generation TSPO PET tracer, and several second-generation tracers such as [11C]DAA1106, [11C]PBR06, [11C]PBR28, and [18F]DPA-713, [18F]DPA-714, and third-generation tracers [11C]GE180, and [11C]ER176 [16–27], have been evaluated in (pre-)clinical studies. PET imaging studies using TSPO tracers have shown increased brain uptake in post-stroke, although different time course and distribution were detected depending on the animal models and procedures. A faster time-course is observed in permanent compared to temporary ischemic stroke, and a higher increase of TSPO ligand uptake in the infarct core in temporary stroke than in peri-infarct areas in permanent stroke was reported [12, 15, 20, 28]. Thus, the development of novel PET probes for visualizing alternative targets in neuroinflammation have received great attention in recent years [29–32].
Cannabinoid type 2 receptors (CB2R) are mainly expressed by immune cells including monocytes and macrophages. In the brain, CB2Rs are primarily found on microglia and have low expression levels under physiological conditions [1, 3, 33]. Upregulation of brain CB2R expression is reported under acute inflammation such as ischemic stroke, and related to lesion extension in the penumbra and subsequent functional recovery [34]. Treatment with CB2R agonists has been shown to be neuroprotective and attenuates macrophage/microglial activation in mouse models of cerebral ischemia [34–38]. CB2R was also upregulated in other brain diseases with involvement of inflammation/microglia under chronic inflammation in neurodegenerative diseases such as Alzheimer’s disease [39–42] and senescence-accelerated models [43] associated with amyloid-β deposits [33, 44–50].
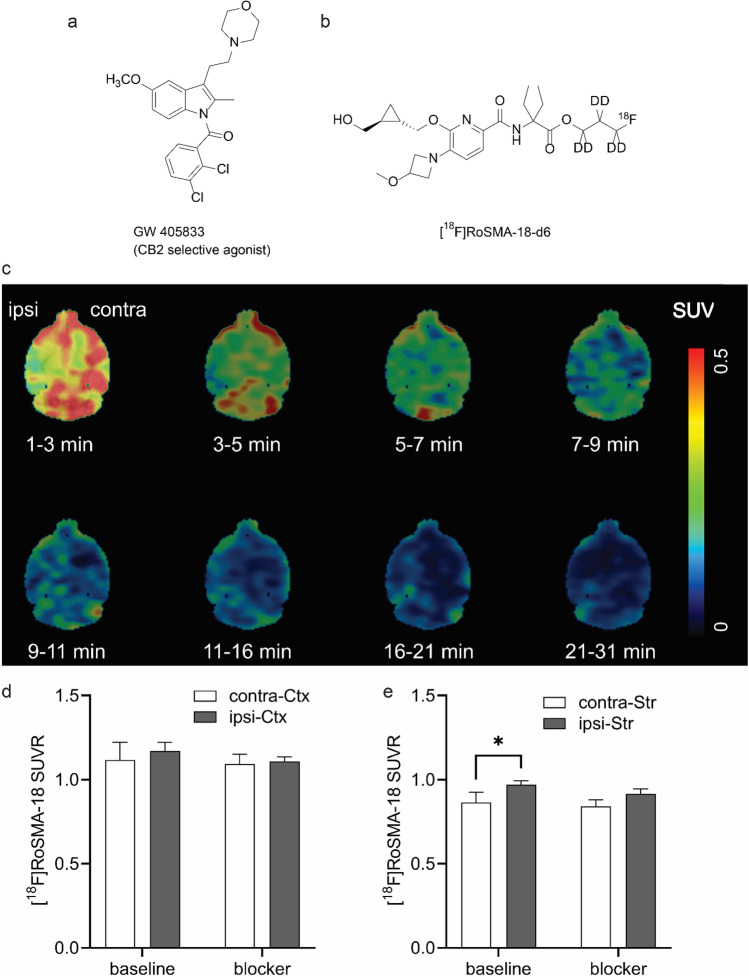
Several structural scaffolds of CB2R PET tracers have recently been developed [51–55] including pyridine derivatives, oxoquinoline derivatives, thiazole derivatives [56, 57], oxadiazole derivatives [58], carbazole derivatives [59], imidazole derivative [60], and thiophene derivatives [61, 62]. In this study, our newly developed pyridine derivative [18F]RoSMA-18-d6 (Fig. 3b), which exhibited sub-nanomolar affinity and high selectivity towards CB2R (Ki: 0.8 nM, CB2R/CB1R > 12′000) [63], was selected for CB2R-targeted neuroinflammation imaging.
Fig. 3.
In vivo microPET imaging of tMCAO mouse brain using [18F]RoSMA-18-d6. a,b Chemical structures of blocker GW405833 and [18F]RoSMA-18-d6; c representative PET images of horizontal mouse brain sections at different time frames after intravenous injection of [18F]RoSMA-18-d6; SUV: 0–0.5; d,e ratios of [18F]RoSMA-18-d6 uptake under baseline and blockade conditions in cortex and striatum. Significantly higher [18F]RoSMA-18-d6 standard uptake value ratio (SUVR) was observed in the ischemic ipsilateral striatum under baseline conditions, but not in the ipsilateral cortex. Midbrain was used as reference brain region for SUVR calculation. *p < 0.05.
The aim of the current study was to evaluate the novel CB2R tracer, [18F]RoSMA-18-d6, in the transient middle cerebral artery occlusion (tMCAO) mouse models of focal cerebral ischemia [64–70] using microPET. In addition, [18F]FDG was included for evaluation of the cerebral metabolic rate of glucose (CMRglc) and as a readily available radiotracer benchmark for neuroinflammation [71, 72]. Diffusion-weighted imaging (DWI) and T2-weighted imaging were performed for anatomical reference and for delineating the lesion in tMCAO mice.
Methods
Radiosynthesis
[18F]RoSMA-18-d6 was synthesized by nucleophilic substitution of the tosylate precursor with [18F]KF/Kryptofix222 in acetonitrile [63]. The crude product was purified by reverse phase semi-preparative high-performance liquid chromatography and formulated with 5 % ethanol in water for intravenous injection and for biological evaluations. In a typical experiment, a moderate radiochemical yield of ~ 12 % (decay corrected) was achieved with a radiochemical purity > 99 %. The molar activities ranged from 156 to 194 GBq/μmol at the end of synthesis. The identity of the final product was confirmed by comparison with the HPLC retention time of the non-radioactive reference compound by co-injection. [18F]FDG was obtained from a routine clinical production from the University Hospital Zurich, Switzerland.
Animals
Twenty-four male C57BL/6 J mice were obtained from Janvier Labs (Le Genest-Saint-Isle, France). The mice were scanned at 8–10 weeks of age (20–25 g body weight). Mice were randomly allocated to sham-operation (n = 10) or tMCAO (n = 14). Mice underwent MRI, µPET/computed tomography (CT), and 2,3,5-triphenyltetrazolium chloride (TTC) histology staining for validation 24 h or 48 h after reperfusion. Animals were housed in ventilated cages inside a temperature-controlled room, under a 12-h dark/light cycle. Pelleted food (3437PXL15, CARGILL) and water were provided ad libitum. Paper tissue and red Tecniplast mouse house® (Tecniplast, Milan, Italy) shelters were placed in cages as environmental enrichments. All experiments were performed in accordance with the Swiss Federal Act on Animal Protection and were approved by the Cantonal Veterinary Office Zurich (permit number: ZH018/14 and ZH264/16).
Surgeries for tMCAO and sham-operation were performed using standard-operating procedures as described before [73, 74]. Anesthesia was initiated by using 3 % isoflurane (Abbott, Cham, Switzerland) in a 1:4 oxygen/air mixture and maintained at 2 %. Before the surgical procedure, a local analgesic (Lidocaine, 0.5 %, 7 mg/kg, Sintectica S.A., Switzerland) was administered subcutaneously (s.c.). Temperature was kept constant at 36.5 ± 0.5 °C with a feedback controlled warming pad system. All surgical procedures were performed in 15–30 min. After surgery, buprenorphine was administered as s.c. injection (Temgesic, 0.1 mg/kg b.w.), and at 4 h after reperfusion and supplied thereafter via drinking water (1 mL/32 mL of drinking water) until 24 h or 48 h. Animals received softened chow in a weighing boat on the cage floor to encourage eating. tMCAO animals were excluded from the study if they met one of the following criteria: Bederson testing was performed 2 h post-reperfusion. Bederson score of 0, no reflow after filament removal, and premature death.
mRNA Isolation, Reverse-Transcription Reaction, and Real-Time Polymerase Chain Reaction
Brain hemispheres of C57BL/6 mouse and tMCAO mice at 24 h and 48 h post reperfusion were used for total mRNA isolation according to the protocols of the Isol-RNA Lysis Reagent (5 PRIME, Gaithersburg, USA) and the bead-milling TissueLyser system (Qiagen, Hilden, Germany). QuantiTect® Reverse Transcription Kit (Qiagen, Hilden, Germany) was used to generate cDNA. The primers (Microsynth, Balgach, Switzerland) used for the quantitative polymerase chain reaction (qPCR) are summarized in Supplementary Table 1. Quantitation of CNR2, ionized calcium binding adaptor molecule 1 (Iba1), tumor necrosis factor (TNF-a), matrix metallopeptidase 9 (MMP9), glial fibrillary acidic protein (GFAP), and microtubule-associated protein 2 (MAP-2) mRNA expression was performed with the DyNAmo™ Flash SYBR® Green qPCR Kit (Thermo Scientific, Runcorn, UK) using a 7900 HT Fast Real-Time PCR System (Applied Biosystems, Carlsbad, USA). The amplification signals were detected in real-time, which permitted accurate quantification of the amounts of the initial RNA template during 40 cycles according to the manufacturer’s protocol. All reactions were performed in duplicates and in two independent runs. Quantitative analysis was performed using the SDS Software (v2.4) and a previously described 2− ΔΔCt quantification method [75]. The specificity of the PCR products of each run was determined and verified with the SDS dissociation curve analysis feature.
In vivo MRI
Data were acquired at 24 h after reperfusion on a 7 T Bruker Pharmascan (Bruker BioSpin GmbH, Germany), equipped with a volume resonator operating in quadrature mode for excitation and a four-element phased-array surface coil for signal reception and operated by Paravision 6.0 (Bruker BioSpin) [73, 76–78]. Mice were anesthetized with an initial dose of 4 % isoflurane in oxygen/air (200:800 ml/min) and maintained at 1.5 % isoflurane in oxygen/air (100:400 ml/min). Body temperature was monitored with a rectal temperature probe (MLT415, ADInstruments) and kept at 36.5 ± 0.5 °C using a warm water circuit integrated into the animal support (Bruker BioSpin GmbH, Germany). T2-weighted MR images were obtained using a spin echo sequence (TurboRARE) with an echo time 3 ms, repetition time 6 ms, 100 averages, slice thickness 1 mm, field-of-view 2.56 cm × 1.28 cm, matrix size 256 × 128, giving an in-plane resolution of 100 μm × 100 μm. For DWI, a four-shot spin echo–echo planar imaging sequence with an echo time = 28 ms, repetition time = 3000 [76, 77], acquired with a field-of-view of 3.3 cm × 2 cm and a matrix size of 128 × 128, resulting in a nominal voxel size of 258 μm × 156 μm. Diffusion-encoding was applied in the x-, y-, and z-directions with b values of 100, 200, 400, 600, 800, and 1000 s/mm2, respectively, acquisition time 3 min 48 s. The ischemic lesion was determined as an area of significant reduction of the apparent diffusion coefficient (ADC) value compared with the unaffected contralateral side [79]. On T2-weighted images, the lesion was determined as an area of hyperintensities compared with the contralateral side.
In vivo microPET Studies
MicroPET/CT scans were performed at 24 h after reperfusion with a calibrated SuperArgus µPET/CT scanner (Sedecal, Madrid, Spain) with an axial field-of-view of 4.8 cm and a spatial resolution of 1.6–1.7 mm (full width at half maximum). tMCAO and the sham-operated C57BL/6 J mice were anesthetized with ca. 2.5 % isoflurane in oxygen/air (1:1) during tracer injection and the whole scan time period. The formulated radioligand solution ([18F]FDG: 9.9–11 MBq or [18F]RoSMA-18-d6: 7.2–13 MBq) was administered via tail vein injection, and mice were dynamically scanned for 60 min. For blocking experiments, 1.5 mg/kg GW405833 (Fig. 3a) was dissolved in a vehicle of 2 % Cremophor (v/v), 10 % ethanol (v/v), and 88 % water for injection (v/v) and injected together with [18F]RoSMA-18-d6. Body temperature was monitored by a rectal probe and kept at 37 °C by a heated air stream (37 °C). The anesthesia depth was measured by the respiratory frequency (SA Instruments, Inc., Stony Brook, USA). µPET acquisitions were combined with CT for anatomical orientation and attenuation correction. The obtained data were reconstructed in user-defined time frames with a voxel size of 0.3875 × 0.3875 × 0.775 mm3 as previously described [80].
Triphenyltetrazolium Chloride (TTC) Staining
To assess the ischemic lesion severity in the brain of tMCAO mice and to validate the absence of lesion in the sham-operated mice, staining with TTC staining was performed. After measurements mice were euthanized, their brains were removed and 1-mm thick brain slices were obtained with a brain matrix. Slices were incubated in a 2.5 % TTC solution (Sigma-Aldrich, Switzerland) in PBS at 37 °C for 3 min. Photographs of the brain sections were taken. Edema-corrected lesion volumes were quantified as described [81].
Biodistribution Studies in the Mouse Brain
After PET/CT scanning of tMCAO mice at 24 h after reperfusion with [18F]RoSMA-18-d6, animals were sacrificed at 70 min post injection by decapitation. The spleen and brain regions of ischemic ipsilateral area and contralateral hemisphere were collected for analysis with a gamma counter. The accumulated radioactivities in the different tissues were expressed as percent normalized injected dose per gram of tissue normalized to 20 g body weight of the animals (norm. percentage injected dose per gram tissue ( % ID/g tissue)).
Data Analysis and Statistics
Images were processed and analyzed using PMOD 4.2 software (PMOD Technologies Ltd., Zurich, Switzerland). The time − activity curves were deduced from specific volume-of-interest that were defined based on a mouse MRI T2-weighted image template [82]. Radioactivity is presented as standardized uptake value (SUV) (decay-corrected radioactivity per cm3 divided by the injected dose per gram body weight). [18F]RoSMA-18-d6 SUVR was calculated by using the midbrain in the corresponding hemisphere as reference brain region. For [18F]FDG PET, regional SUV was calculated. Two-way ANOVA with Sidak post hoc analysis was used for comparison between groups (Graphpad Prism 9.0, CA, USA).
Results
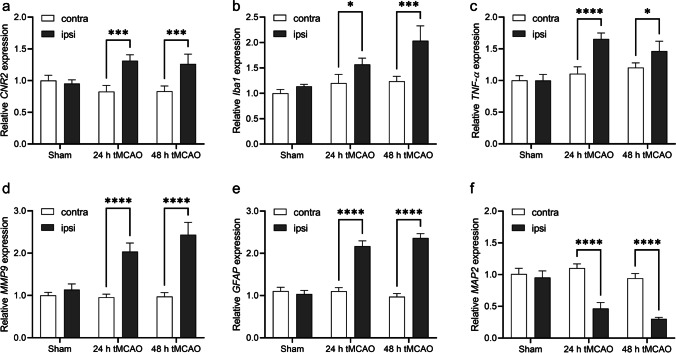
Increased expression of inflammation makers and neuronal damage after focal cerebral ischemia in tMCAO mice mRNA levels were measured to address the question whether mouse non-ischemic and ischemic hemispheres differ in their expression levels of CNR2 and other inflammatory genes. CNR2 mRNA expression was increased to around 1.3-fold after 24 h reperfusion and at 48 h in the ipsilateral comparing to contralateral hemisphere (Fig. 1a). Similarly, 1.5–2.5-fold increases were observed in the mRNA expression of inflammatory markers including TNF-α, Iba1, MMP9, and GFAP at 24 h and 48 h after reperfusion in the ipsilateral compared to contralateral brain region (Fig. 1b–e). MAP-2 expression has been shown to be a reliable marker of neurons that undergo cell death [83, 84]. The neuron-specific MAP-2 expression was markedly reduced in the ipsilateral compared to contralateral hemisphere at 24 h and 48 h after reperfusion (Fig. 1f). As similar CNR2 mRNA expression was observed at 24 h and 48 h, our studies were performed at early time point of 24 h after reperfusion for investigating the functional, structural, and molecular changes in the following experiments.
Fig. 1.
Relative mRNA levels of inflammatory markers and neuronal damage in sham-operated and tMCAO mouse brain in contra-and ipsilateral brain hemisphere at 24 h and 48 h after reperfusion. a CNR2, b Iba1, c TNF-α, d MMP9, e GFAP, and f MAP-2. Values represent mean ± standard deviation. Expression levels were quantified by qPCR relative to β-actin. *p < 0.05, ***p < 0.001, ****p < 0.0001.
Reduced Cerebral Glucose Metabolism and Structural MRI Lesion Following tMCAO
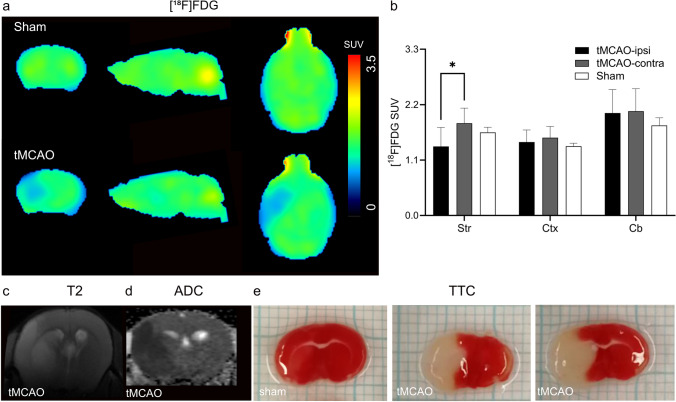
Reduced [18F]FDG uptake was observed in the presumed MCA territory of the ipsilateral hemisphere in tMCAO mice, while there was no difference in [18F]FDG uptake between hemispheres in sham-operated mice (Fig. 2a). SUVs were significantly lower in the ipsilateral striatum in tMCAO compared to the contralateral side and compared to the same region in sham-operated mice 1.8 vs 1.4 (Fig. 2b). There were no differences in [18F]FDG uptake in the cortex and cerebellum between the ipsilateral and contralateral hemisphere in tMCAO mice and sham-operated mice. T2-weighted MRI and DWI imaging were performed in tMCAO and sham-operated animals at 24 h after reperfusion (Fig. 2c). The lesions in the ipsilateral side in the striatum and cortex were visible as areas of decreased values on the ADC maps calculated from DWI and as areas of increased intensities on the T2-weighted MR images at 24 h after reperfusion following 1 h tMCAO (Figs. 2c–d). Ischemic lesions in the tMCAO were also seen ex vivo as white areas, while viable tissue appeared red in TTC stained brain sections (Fig. 2e). Homogenous deep red color was observed across both hemispheres in sham-operated mice, verifying the absence of any lesion. The hemispheric lesion volumes in tMCAO mice were 42.8 ± 10.2 % (mean ± standard deviation).
Fig. 2.
In vivo MRI and [18F]FDG PET in tMCAO mouse brain. a Representative PET images of coronal, sagittal and horizontal mouse brain sections after intravenous injection of [18F]FDG in sham-operated and tMCAO mice. The radiosignals were averaged from 21 to 61 min; b [18F]FDG accumulation (SUV) at different mouse brain regions (Str: striatum; Ctx: cortex; Cb: cerebellum) in sham and tMCAO mice. Significantly reduced [18F]FDG accumulation was observed in the ipsilateral striatum compared to contralateral side in tMCAO mice; c–e in vivo T2-weighted image, ADC map, and ex vivo TTC stained brain sections, indicating the delineation in tMCAO mice. TTC, 2,3,5-triphenyltetrazolium chloride; ADC, apparent diffusion coefficient; SUV, standard uptake value. *p < 0.05.
Increased [18F]RoSMA-18-d6 Retention in the Striatum After tMCAO
To analyze the distribution of [18F]RoSMA-18-d6 in tMCAO mice brain, dynamic µPET/CT scans were performed at 24 h after reperfusion. The standard uptake values (SUVs) of [18F]RoSMA-18-d6 did not reveal significant difference in various brain regions of tMCAO mice (Supplementary Fig. 1). However, we found a reduced uptake at early time frame (1–3 min) and a similar uptake after 7 min in the ipsilateral side compared to that of the contralateral side (Fig. 3c, and whole head images in Supplementary Fig. 2). Thus, to exclude the perfusion influence, we averaged the brain signals from 21–61 min and selected the midbrain as the reference region. Higher [18F]RoSMA-18-d6 SUVR was observed in the ischemic ipsilateral striatum compared to the contralateral striatum (two-way ANOVA with Sidak multiple comparison correction, 0.97 ± 0.02 vs 0.87 ± 0.06, p = 0.0274), but not in other brain regions such as cortex (Figs. 3d, e). The increased signals at ischemic ipsilateral striatum, however, could not be blocked by the selective CB2R agonist GW405833 (Fig. 3e).
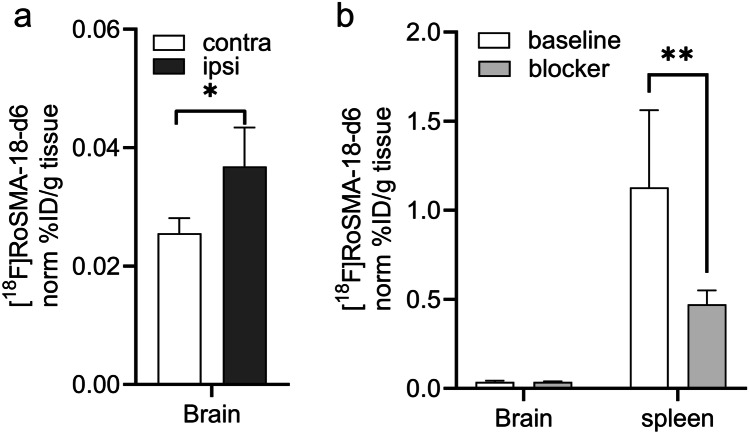
At the end of the in vivo experiments, we dissected the mice to verify the activity accumulation and specificity of [18F]RoSMA-18-d6 in the spleen and different brain regions with a gamma counter. In line with the results obtained from the averaged SUVRs in the tMCAO mouse brain, the radioactivity in the ipsilateral side was indeed significantly higher than that of the contralateral hemisphere (0.037 ± 0.007 vs 0.026 ± 0.003, n = 5 each group), but no blockade effect was seen under blocking conditions (Fig. 4a). As expected, radioactivity in the CB2R-rich spleen was much higher than the brain and 58 % of the signals was blocked by co-injection of CB2R specific ligand GW405833, demonstrating specific target engagement of [18F]RoSMA-18-d6 in vivo (Fig. 4b).
Fig. 4.
Ex vivo biodistribution of [18F]RoSMA-18-d6 in the brain and spleen of tMCAO mouse. Animals (n = 4) were sacrificed at 70 min post-injection, and the spleen and brain regions were dissected and analyzed with a gamma counter. a Higher [18F]RoSMA-18-d6 binding (norm % ID/g tissue) was detected in the ipsilateral vs contralateral hemisphere under baseline conditions. b In the spleen about 58 % of the [18F]RoSMA-18-d6 binding (norm % ID/g tissue) was blocked. No significant blocking was observed in the brain. Data are presented as the mean of the percentage of injected dose per gram tissue normalized to 20 g body weight; mean ± standard deviation. % ID/g: percentage injected dose per gram. *p < 0.05, **p < 0.01.
Discussion
This study assessed the utility of CB2R PET tracer [18F]RoSMA-18-d6 for imaging tMCAO mouse at subacute stage, concomitant with decreased CMRglc levels and formation of a structural lesion. Previous PET imaging of stroke animal models led to inconclusive results. In a rat model of photothrombotic stroke at 24 h after surgery, increased [11C]NE40 (CB2R tracer) uptake and unvaried [11C]PK11195 (TSPO tracer) uptake were reported [85]. In another study, [11C]NE40 uptake did not show any difference in the same rat model of photothrombotic stroke [86]. Moreover, reduced [11C]A836339 (CB2R tracer) uptake was reported in a focal tMCAO rat model over 1–28 days after occlusion [56]. Possible reasons for these different observations include the time point of assessment, different methods for inducing acute stroke (transient or permanent ischemia) resulting in variations of ischemic severity and levels of inflammatory cell expression [36].
CB2R has negligible expression in the mouse brain and is mainly expressed in the spleen under physiological conditions [39, 45, 64–69, 87]. Under neuroinflammatory conditions, CB2R is upregulated in activated microglial cells. In this study, we used quantitative real-time polymerase chain reaction to measure gene expression levels of CNR2, TNF-α, Iba1, MMP9, GFAP, and MAP-2 at 24 h and 48 h. All tested inflammatory markers displayed increased mRNA levels in the ipsilateral brain hemisphere, in agreement with the reported findings in tMCAO mouse model [34, 38, 88, 89]. In line with the increased CNR2 gene expression levels, significantly higher [18F]RoSMA-18-d6 SUVR (standard uptake value ratio) was observed in striatum at ipsilateral vs contralateral under baseline conditions in our PET studies. The 50 % reduction of the neuronal marker MAP-2 indicated neuronal damage.
The dynamic µPET scan using [18F]RoSMA-18-d6 indicated a reduced perfusion in the lesion brain regions at the first time frame of 1–3 min. This is probably due to the changes of microvascular response (no-reflow phenomenon) and the reduction in neuronal activity. Taking the midbrain as the reference region, the ratios of SUV averaged from 21 to 61 min revealed increased [18F]RoSMA-18-d6 SUVR in the ipsilateral ischemic striatum compared to that of the contralateral side. Our ex vivo bio-distribution studies confirmed the difference of the radioactivity distribution in the left and right brain hemisphere. The in vivo specificity of [18F]RoSMA-18-d6 towards CB2R is evidenced by a 58 % reduction in radioactivity in the mouse spleen under blockade conditions in ex vivo biodistribution studies. Underlying reasons for the lack of specificity of [18F]RoSMA-18-d6 in the mouse brain may include (1) the increased tracer availability in the blood induced by blocking the CB2R peripheral targets in the presence of the blocker GW405833; and (2) the relatively low brain uptake of our CB2R-selective radioligand [18F]RoSMA-18-d6 in the mouse brain resulted in undetectable changes of radiosignals under baseline and blockade conditions. Notably, the time-activity curves of [18F]RoSMA-18-d6 in tMCAO mouse brain showed remarkably higher initial brain uptake under blockade conditions than the baseline in both sides of the mouse brain (Supplementary Fig. 1), indicating the influence of blocking CB2R target in the peripheral organs on the availability of radiotracer concentrations in the blood. In our previous studies with Wistar rats, the spleen uptake of [18F]RoSMA-18-d6 was blocked by nearly 90 %. The higher specific binding of [18F]RoSMA-18-d6 in rat spleen is likely due to the increased available targets. Govaerts et al. reported higher binding sites Bmax in the rat spleen (0.71 ± 0.02 pmol/mg protein) compared to that in the mouse spleen (0.31 ± 0.03 pmol/mg protein) by using [3H]CP55940 binding assay [90]. In the mouse P-glycoprotein assay, compound RoSMA-18 exhibits an efflux ratio (ER) of 4.6, which partly explains the low initial brain uptake in mice. Compared to other CB2R radioligand developed in our group, [18F]ROSMA-18-d6 has the highest specificity (86 %) towards CB2R based on the ex vivo biodistribution results in the rat spleen and was therefore selected for in vivo studies.
We observed that [18F]FDG measure of CMRglc was reduced in the ischemic areas, i.e., ipsilateral striatum of the tMCAO mice at 24 h after reperfusion. The reduced CMRglc was reported in many earlier studies in disease animal models and in stroke patients, [91–94], masking CMRglc reduction of neuronal tissue in the brain. At an extended time points of the recovery stage from days 4 to 40, an increased CMRglc level was reported in the ischemic regions due to the increased consumption from inflammatory cells along with microglial activation [91, 95, 96].
There are several limitations in the current study. (1) As there is no reliable specific CB2R antibody, we did not include immunohistochemical staining for CB2R protein distribution in the mouse brain. The qPCR measures of CNR2 mRNA level provided an alternative readout, but do not provide spatial distribution of cerebral CB2R expression. (2) Due to the logistic barrier, MRI and µPET/CT scans were performed with different cohorts of animals. Nevertheless, standard operating procedures for the surgery were used. (3) Our in vivo data with tMCAO mice were collected at 24 h after surgery, longitudinal imaging of tMCAO mice with [18F]RoSMA-18-d6 along with structural and functional readout will provide further insight into the spatio–temporal dynamics of CB2R expression in the brain. Moreover, [18F]FDG-PET was used as a benchmark for neuroinflammation in the current study. Further study using TSPO tracer to assess the glial activation along with CB2R tracer will provide valuable insights.
Conclusion
Our newly developed CB2R PET tracer, [18F]RoSMA-18-d6, revealed limited utility to image neuroinflammation in the ischemic ipsilateral of the tMCAO mice at 24 h after reperfusion. Although lesion regions in tMCAO mouse brain could be followed by the ratios of averaged SUVs from 21 to 61 min with midbrain as the reference region, the in vivo specificity of [18F]RoSMA-18-d6 was confirmed only in the CB2R-rich spleen. Different neuroinflammatory animal models which have comparable neuronal numbers in the lesion regions are recommended for evaluation of CB2R in further PET imaging studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Yingfang He and Annette Krämer at Center for Radiopharmaceutical Sciences, Department of Chemistry and Applied Biosciences, ETH Zurich, and Dr Mark Augath, at the Institute for Biomedical Engineering, ETH Zurich, and University of Zurich, for technical assistance.
Author Contributions
RN, JK, LM, and SMA designed the study; RN, KC, AH, AMH, GL, and LM performed the experiment; RN and LM performed data analysis; RN, JK, and LM wrote the initial manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Swiss Federal Institute of Technology Zurich. JK received funding from the Swiss National Science Foundation (320030_179277), in the framework of ERA-NET NEURON (32NE30_173678/1), the Synapsis Foundation, the Olga Mayenfisch Stiftung, and the Vontobel foundation. RN received funding from Forschungskredit, Synapsis foundation career development award (2017 CDA-03), and the Vontobel foundation.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Astrup J (1982) Energy-requiring cell functions in the ischemic brain. Their critical supply and possible inhibition in protective therapy. J Neurosurg 56:482–497 [DOI] [PubMed]
- 2.Heiss W-D. Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab. 2000;20:1276–1293. doi: 10.1097/00004647-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/S0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 4.Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflamm. 2019;16:178. doi: 10.1186/s12974-019-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13:661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambertsen KL, Finsen B, Clausen BH. Post-stroke inflammation—target or tool for therapy? Acta Neuropathol. 2019;137:693–714. doi: 10.1007/s00401-018-1930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans NR, et al. PET imaging of the neurovascular interface in cerebrovascular disease. Nat Rev Neurol. 2017;13:676–688. doi: 10.1038/nrneurol.2017.129. [DOI] [PubMed] [Google Scholar]
- 8.Marchal G, et al. PET imaging of cerebral perfusion and oxygen consumption in acute ischaemic stroke: relation to outcome. Lancet. 1993;341:925–927. doi: 10.1016/0140-6736(93)91214-7. [DOI] [PubMed] [Google Scholar]
- 9.Muir KW, Buchan A, von Kummer R, Rother J, Baron J-C. Imaging of acute stroke. Lancet Neurol. 2006;5:755–768. doi: 10.1016/S1474-4422(06)70545-2. [DOI] [PubMed] [Google Scholar]
- 10.Carter SF, et al. Astrocyte biomarkers in Alzheimer’s disease. Trends Mol Med. 2019;25:77–95. doi: 10.1016/j.molmed.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Jain P, et al. Neuroinflammation PET imaging: current opinion and future directions. J Nucl Med. 2020;61:1107–1112. doi: 10.2967/jnumed.119.229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zinnhardt B, et al. in vivo imaging biomarkers of neuroinflammation in the development and assessment of stroke therapies - towards clinical translation. Theranostics. 2018;8:2603–2620. doi: 10.7150/thno.24128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martín A, et al. Imaging of perfusion, angiogenesis, and tissue elasticity after stroke. J Cereb Blood Flow Metab. 2012;32:1496–1507. doi: 10.1038/jcbfm.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venneti S, Lopresti BJ, Wiley CA. Molecular imaging of microglia/macrophages in the brain. Glia. 2013;61:10–23. doi: 10.1002/glia.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreisl WC, et al. PET imaging of neuroinflammation in neurological disorders. The Lancet Neurology. 2020;19:940–950. doi: 10.1016/S1474-4422(20)30346-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiel A, Heiss WD. Imaging of microglia activation in stroke. Stroke. 2011;42:507–512. doi: 10.1161/STROKEAHA.110.598821. [DOI] [PubMed] [Google Scholar]
- 17.Boutin H, et al. 18F-GE-180: a novel TSPO radiotracer compared to 11C-R-PK11195 in a preclinical model of stroke. Eur J Nucl Med Mol Imag. 2015;42:503–511. doi: 10.1007/s00259-014-2939-8. [DOI] [PubMed] [Google Scholar]
- 18.Lartey FM, et al. PET Imaging of stroke-induced neuroinflammation in mice using [18F]PBR06. Mol Imag Biol. 2014;16:109–117. doi: 10.1007/s11307-013-0664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tóth M, et al. Acute neuroinflammation in a clinically relevant focal cortical ischemic stroke model in rat: longitudinal positron emission tomography and immunofluorescent tracking. Brain Struct Funct. 2016;221:1279–1290. doi: 10.1007/s00429-014-0970-y. [DOI] [PubMed] [Google Scholar]
- 20.Chaney A, et al (2018) 11C-DPA-713 versus 18F-GE-180: a preclinical comparison of TSPO-PET tracers to visualize acute and chronic neuroinflammation in a mouse model of ischemic stroke. J Nucl Med [DOI] [PMC free article] [PubMed]
- 21.Martín A, et al. Evaluation of the PBR/TSPO radioligand [18F]DPA-714 in a rat model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2009;30:230–241. doi: 10.1038/jcbfm.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiel A, et al. The temporal dynamics of poststroke neuroinflammation: a longitudinal diffusion tensor imaging–guided pet study with 11C-PK11195 in acute subcortical stroke. J Nucl Med. 2010;51:1404–1412. doi: 10.2967/jnumed.110.076612. [DOI] [PubMed] [Google Scholar]
- 23.Zinnhardt B, et al. Multimodal imaging reveals temporal and spatial microglia and matrix metalloproteinase activity after experimental stroke. J Cereb Blood Flow Metab. 2015;35:1711–1721. doi: 10.1038/jcbfm.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishikawa A, et al. in vivo visualization of Tau accumulation, microglial activation, and brain atrophy in a mouse model of tauopathy rTg4510. J Alzheimer's Dis. 2018;61:1037–1052. doi: 10.3233/JAD-170509. [DOI] [PubMed] [Google Scholar]
- 25.Zhou R et al (2021) PET imaging of neuroinflammation in Alzheimer’s disease. Front Immunol 12:739130 [DOI] [PMC free article] [PubMed]
- 26.Zanotti-Fregonara P, et al. Head-to-head comparison of (11)C-PBR28 and (11)C-ER176 for quantification of the translocator protein in the human brain. Eur J Nucl Med Mol Imaging. 2019;46:1822–1829. doi: 10.1007/s00259-019-04349-w. [DOI] [PubMed] [Google Scholar]
- 27.Ni R, et al. In vitro characterization of the regional binding distribution of amyloid PET tracer florbetaben and the glia tracers deprenyl and PK11195 in autopsy Alzheimer’s brain tissue. J Alzheimers Dis. 2021;80:1723–1737. doi: 10.3233/JAD-201344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boutin H, Pinborg LH. TSPO imaging in stroke: from animal models to human subjects. Clin Transl Imaging. 2015;3:423–435. doi: 10.1007/s40336-015-0146-7. [DOI] [Google Scholar]
- 29.Razansky D, Klohs J, Ni R (2021) Multi-scale optoacoustic molecular imaging of brain diseases. Eur J Nucl Med Mol Imaging [DOI] [PMC free article] [PubMed]
- 30.Ling X, et al. A novel near-infrared fluorescence imaging probe that preferentially binds to cannabinoid receptors CB2R over CB1R. Biomaterials. 2015;57:169–178. doi: 10.1016/j.biomaterials.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarott RC, et al. Development of high-specificity fluorescent probes to enable cannabinoid type 2 receptor studies in living cells. J Am Chem Soc. 2020;142:16953–16964. doi: 10.1021/jacs.0c05587. [DOI] [PubMed] [Google Scholar]
- 32.Shi X-F et al (2021) Multimodal contrast agents for optoacoustic brain imaging in small animals. Front Bioeng Biotechnol 9:746815 [DOI] [PMC free article] [PubMed]
- 33.Stempel AV, et al. Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron. 2016;90:795–809. doi: 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarruk JG, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke. 2012;43:211–219. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- 35.Ronca RD, et al. A selective cannabinoid CB2 agonist attenuates damage and improves memory retention following stroke in mice. Life Sci. 2015;138:72–77. doi: 10.1016/j.lfs.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bravo-Ferrer I, et al. Cannabinoid type-2 receptor drives neurogenesis and improves functional outcome after stroke. Stroke. 2017;48:204–212. doi: 10.1161/STROKEAHA.116.014793. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, et al. Cannabinoid CB(2) receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb Blood Flow Metab. 2007;27:1387–1396. doi: 10.1038/sj.jcbfm.9600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu SJ, et al (2015) Time-dependent protection of CB2 receptor agonist in stroke. PLoS ONE 10:e0132487 [DOI] [PMC free article] [PubMed]
- 39.López A, et al. Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. J Neuroinflamm. 2018;15:158. doi: 10.1186/s12974-018-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Vieitez E, et al. Astrocytosis precedes amyloid plaque deposition in Alzheimer APPswe transgenic mouse brain: a correlative positron emission tomography and in vitro imaging study. Eur J Nucl Med Mol Imaging. 2015;42:1119–1132. doi: 10.1007/s00259-015-3047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heneka MT, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rapic S, et al. Imaging microglial activation and glucose consumption in a mouse model of Alzheimer's disease. Neurobiol Aging. 2013;34:351–354. doi: 10.1016/j.neurobiolaging.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Yamagishi S, et al. Upregulation of cannabinoid receptor type 2, but not TSPO, in senescence-accelerated neuroinflammation in mice: a positron emission tomography study. J Neuroinflamm. 2019;16:208. doi: 10.1186/s12974-019-1604-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci. 2015;16:30–42. doi: 10.1038/nrn3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Sickle MD, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 46.Xi ZX, et al. Brain cannabinoid CB(2) receptors modulate cocaine's actions in mice. Nat Neurosci. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benito C, Tolón RM, Pazos MR (2008) Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol 153 [DOI] [PMC free article] [PubMed]
- 48.Koppel J, et al (2014) CB2 receptor deficiency increases amyloid pathology and alters Tau processing in a transgenic mouse model of Alzheimer’s disease. 20 [DOI] [PMC free article] [PubMed]
- 49.Aso E, Ferrer I (2016) CB2 cannabinoid receptor as potential target against Alzheimer’s disease. Front Neurosci 10 [DOI] [PMC free article] [PubMed]
- 50.Parbo P, et al. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer’s disease. Brain. 2017;140:2002–2011. doi: 10.1093/brain/awx120. [DOI] [PubMed] [Google Scholar]
- 51.Spinelli F, Capparelli E, Abate C, Colabufo NA, Contino M (2017) Perspectives of cannabinoid type 2 receptor (CB2R) ligands in neurodegenerative disorders: structure-affinity relationship (SAfiR) and structure-activity relationship (SAR) studies. J Med Chem [DOI] [PubMed]
- 52.Ni R, Mu L, Ametamey S. Positron emission tomography of type 2 cannabinoid receptors for detecting inflammation in the central nervous system. Acta Pharmacol Sin. 2019;40:351–357. doi: 10.1038/s41401-018-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spinelli F, Mu L, Ametamey SM. Radioligands for positron emission tomography imaging of cannabinoid type 2 receptor. J Labelled Comp Radiopharm. 2018;61:299–308. doi: 10.1002/jlcr.3579. [DOI] [PubMed] [Google Scholar]
- 54.Hou L, et al. Positron emission tomography imaging of the endocannabinoid system: opportunities and challenges in radiotracer development. J Med Chem. 2021;64:123–149. doi: 10.1021/acs.jmedchem.0c01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varlow C, Boileau I, Wey HY, Liang SH, Vasdev N. Classics in neuroimaging: imaging the endocannabinoid pathway with PET. ACS Chem Neurosci. 2020;11:1855–1862. doi: 10.1021/acschemneuro.0c00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pottier G, et al. PET imaging of cannabinoid type 2 receptors with [11C]A-836339 did not evidence changes following neuroinflammation in rats. J Cereb Blood Flow Metab. 2017;37:1163–1178. doi: 10.1177/0271678X16685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moldovan R-P, et al. 18F-JHU94620, a high affinity PET radioligand for imaging of cannabinoid subtype 2 receptors (CB2R) J Nucl Med. 2015;56:1048–1048. doi: 10.1021/acs.jmedchem.6b00554. [DOI] [PubMed] [Google Scholar]
- 58.Attili B, et al. Preclinical evaluation of [(18) F]MA3: a CB2 receptor agonist radiotracer for PET. Br J Pharmacol. 2019;176:1481–1491. doi: 10.1111/bph.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heimann D, et al. Optimization of pharmacokinetic properties by modification of a carbazole-based cannabinoid receptor subtype 2 (CB(2)) ligand. Eur J Med Chem. 2018;143:1436–1447. doi: 10.1016/j.ejmech.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 60.Kallinen A, et al. Synthesis and in vitro evaluation of fluorine-18 benzimidazole sulfones as CB2 PET-radioligands. Org Biomol Chem. 2019;17:5086–5098. doi: 10.1039/C9OB00656G. [DOI] [PubMed] [Google Scholar]
- 61.Haider A, et al. Synthesis and biological evaluation of thiophene-based cannabinoid receptor type 2 radiotracers for PET imaging. Front Neurosci. 2016;10:350. doi: 10.3389/fnins.2016.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caillà F, et al. Biodistribution of [18F] FC0324, a new PET tracer targeting CB2 receptors. J Nucl Med. 2017;58:855–855. [Google Scholar]
- 63.Haider A, et al. Identification and preclinical development of a 2,5,6-trisubstituted fluorinated pyridine derivative as a radioligand for the positron emission tomography imaging of cannabinoid type 2 receptors. J Med Chem. 2020;63:10287–10306. doi: 10.1021/acs.jmedchem.0c00778. [DOI] [PubMed] [Google Scholar]
- 64.Offner H, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 65.Garbuzova-Davis S, et al. Blood-spinal cord barrier alterations in subacute and chronic stages of a rat model of focal cerebral ischemia. J Neuropathol Exp Neurol. 2016;75:673–688. doi: 10.1093/jnen/nlw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang B, et al. GlyT1 Inhibitor NFPS Exerts Neuroprotection via GlyR alpha1 subunit in the rat Model of transient focal cerebral ischaemia and reperfusion. Cell Physiol Biochem. 2016;38:1952–1962. doi: 10.1159/000445556. [DOI] [PubMed] [Google Scholar]
- 67.Dang G, et al. Dynamic secondary degeneration in the spinal cord and ventral root after a focal cerebral infarction among hypertensive rats. Sci Rep. 2016;6:22655. doi: 10.1038/srep22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moisse K, Welch I, Hill T, Volkening K, Strong MJ. Transient middle cerebral artery occlusion induces microglial priming in the lumbar spinal cord: a novel model of neuroinflammation. J Neuroinflamm. 2008;5:29. doi: 10.1186/1742-2094-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dang G, et al. Alterations in the spinal cord and ventral root after cerebral infarction in non-human primates. Restor Neurol Neurosci. 2018;36:729–740. doi: 10.3233/RNN-180854. [DOI] [PubMed] [Google Scholar]
- 70.Saleh A, et al. in vivo MRI of brain inflammation in human ischaemic stroke. Brain. 2004;127:1670–1677. doi: 10.1093/brain/awh191. [DOI] [PubMed] [Google Scholar]
- 71.Love C, Tomas MB, Tronco GG, Palestro CJ. FDG PET of infection and inflammation. Radiographics. 2005;25:1357–1368. doi: 10.1148/rg.255045122. [DOI] [PubMed] [Google Scholar]
- 72.Probasco JC, et al (2017) Abnormal brain metabolism on FDG-PET/CT is a common early finding in autoimmune encephalitis. Neurology(R) 4:e352 [DOI] [PMC free article] [PubMed]
- 73.Ni R, Vaas M, Ren W, Klohs J (2018) Non-invasive detection of acute cerebral hypoxia and subsequent matrix-metalloproteinase activity in a mouse model of cerebral ischemia using multispectral-optoacoustic-tomography. Neurophotonics 5:015005 [DOI] [PMC free article] [PubMed]
- 74.Vaas M, Ni R, Rudin M, Kipar A, Klohs J. Extracerebral tissue damage in the intraluminal filament mouse model of middle cerebral artery occlusion. Front Neurol. 2017;8:85. doi: 10.3389/fneur.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 76.Ni R, et al. fMRI reveals mitigation of cerebrovascular dysfunction by bradykinin receptors 1 and 2 inhibitor noscapine in a mouse model of cerebral amyloidosis. Front Aging Neurosci. 2019;11:27–27. doi: 10.3389/fnagi.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ni R, Rudin M, Klohs J. Cortical hypoperfusion and reduced cerebral metabolic rate of oxygen in the arcAbeta mouse model of Alzheimer's disease. Photoacoustics. 2018;10:38–47. doi: 10.1016/j.pacs.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ren W, et al (2019) Automated registration of magnetic resonance imaging and optoacoustic tomography data for experimental studies. Neurophotonics 6:1–10 [DOI] [PMC free article] [PubMed]
- 79.Vaas M, et al. Vascular and tissue changes of magnetic susceptibility in the mouse brain after transient cerebral ischemia. Transl Stroke Res. 2018;9:426–435. doi: 10.1007/s12975-017-0591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krämer SD, et al. Evaluation of (11)C-Me-NB1 as a potential PET radioligand for measuring GluN2B-containing NMDA receptors, drug occupancy, and receptor cross talk. J Nucl Med. 2018;59:698–703. doi: 10.2967/jnumed.117.200451. [DOI] [PubMed] [Google Scholar]
- 81.Lin TN, He YY, Wu G, Khan M, Hsu CY. Effect of brain edema on infarct volume in a focal cerebral ischemia model in rats. Stroke. 1993;24:117–121. doi: 10.1161/01.STR.24.1.117. [DOI] [PubMed] [Google Scholar]
- 82.Ma Y, et al. A three-dimensional digital atlas database of the adult C57BL/6J mouse brain by magnetic resonance microscopy. Neuroscience. 2005;135:1203–1215. doi: 10.1016/j.neuroscience.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 83.Pettigrew LC, et al. Microtubular proteolysis in focal cerebral ischemia. J Cereb Blood Flow Metab. 1996;16:1189–1202. doi: 10.1097/00004647-199611000-00013. [DOI] [PubMed] [Google Scholar]
- 84.Dawson DA, Hallenbeck JM. Acute focal ischemia-induced alterations in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab. 1996;16:170–174. doi: 10.1097/00004647-199601000-00020. [DOI] [PubMed] [Google Scholar]
- 85.Hosoya T, et al. in vivo TSPO and cannabinoid receptor type 2 availability early in post-stroke neuroinflammation in rats: a positron emission tomography study. J Neuroinflamm. 2017;14:69. doi: 10.1186/s12974-017-0851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vandeputte C, et al. Small-animal PET imaging of the type 1 and type 2 cannabinoid receptors in a photothrombotic stroke model. Eur J Nucl Med Mol Imag. 2012;39:1796–1806. doi: 10.1007/s00259-012-2209-6. [DOI] [PubMed] [Google Scholar]
- 87.Jordan CJ, Xi Z-X. Progress in brain cannabinoid CB(2) receptor research: from genes to behavior. Neurosci Biobehav Rev. 2019;98:208–220. doi: 10.1016/j.neubiorev.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fernández-López D, et al. Reduced infarct size and accumulation of microglia in rats treated with WIN 55,212–2 after neonatal stroke. Neuroscience. 2012;207:307–315. doi: 10.1016/j.neuroscience.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang M, et al. Modulation of the balance between cannabinoid CB(1) and CB(2) receptor activation during cerebral ischemic/reperfusion injury. Neuroscience. 2008;152:753–760. doi: 10.1016/j.neuroscience.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Govaerts SJ, Hermans E, Lambert DM. Comparison of cannabinoid ligands affinities and efficacies in murine tissues and in transfected cells expressing human recombinant cannabinoid receptors. Eur J Pharm Sci. 2004;23:233–243. doi: 10.1016/j.ejps.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 91.Yuan H, et al. Spatiotemporal uptake characteristics of [18]F-2-fluoro-2-deoxy-d-glucose in a Rat middle cerebral artery occlusion model. Stroke. 2013;44:2292–2299. doi: 10.1161/STROKEAHA.113.000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walberer M, et al. Potential of early [(18)F]-2-fluoro-2-deoxy-D-glucose positron emission tomography for identifying hypoperfusion and predicting fate of tissue in a rat embolic stroke model. Stroke. 2012;43:193–198. doi: 10.1161/STROKEAHA.111.624551. [DOI] [PubMed] [Google Scholar]
- 93.Jin T, Mehrens H, Wang P, Kim S-G (2017) Chemical exchange-sensitive spin-lock MRI of glucose analog 3-O-methyl- d -glucose in normal and ischemic brain. J Cerebral Blood Flow Metab 38: 0271678X1770741 [DOI] [PMC free article] [PubMed]
- 94.Liu N-W, et al (2017) Evolutional characterization of photochemically induced stroke in rats: a multimodality imaging and molecular biological study. Transl Stroke Res 8 [DOI] [PMC free article] [PubMed]
- 95.Backes H, et al. Glucose consumption of inflammatory cells masks metabolic deficits in the brain. Neuroimage. 2016;128:54–62. doi: 10.1016/j.neuroimage.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sobrado M, et al. Longitudinal studies of ischemic penumbra by using 18F-FDG PET and MRI techniques in permanent and transient focal cerebral ischemia in rats. Neuroimage. 2011;57:45–54. doi: 10.1016/j.neuroimage.2011.04.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.