Abstract
Three important antigenic sites involved in virus neutralization on polioviruses in mouse experiments have been identified. These sites are located at the surface of the virion and have been designated antigenic sites 1, 2, and 3. In mice, the antibody response to antigenic site 1 of serotype 3 poliovirus is considered to be immunodominant, but little is known about the immunogenicity of these sites in humans. In the present study, we developed inhibition enzyme-linked immunosorbent assays specific for antigenic sites 1 and 3 to measure antibody responses to these sites in fully vaccinated inactivated poliovirus vaccine (IPV) (n = 63) and oral live attenuated poliovirus vaccine (OPV) (n = 63) recipients and in naturally infected persons (n = 25). Similar levels of antibodies to site 1 in IPV and OPV vaccinees were detected. However, significantly more OPV recipients (88.7%) had detectable antibodies to antigenic site 3 (P < 0.01) than did IPV-vaccinated persons (63.1%). After an IPV booster vaccination, both previously IPV- and OPV-vaccinated persons responded with a significant increase in antibodies to sites 1 and 3 (P < 0.01). We conclude that the immune response to serotype 3 poliovirus in humans consists of both site 1- and site 3-specific antibodies and that these responses can be induced by either OPV or recent IPV vaccination.
The poliovirus capsid consists of 60 copies of each of the four structural virion proteins (VP1, VP2, VP3, and VP4) (10). The epitopes responsible for inducing poliovirus-neutralizing antibodies are located on surface-exposed loops in structural proteins VP1, VP2, and VP3 (4). VP4 is located completely inside the viral capsid, and it plays no known role in the induction of poliovirus-neutralizing antibodies. VP1 is the most-exposed surface protein and plays a major role in the induction of neutralizing antibodies for all three poliovirus serotypes (28).
Three important antigenic sites (epitopes) involved in virus neutralization on polioviruses, designated sites 1, 2, and 3, have been identified (10, 16). They have been identified by the isolation and characterization of Sabin mutant strains resistant to neutralization by poliovirus-specific antibodies and by epitope mapping with neutralizing monoclonal antibodies (16). Antigenic site 1, composed of amino acids 89 to 100 of VP1, is a major immunogenic site for serotype 2 and 3 polioviruses, as determined by neutralizing monoclonal antibodies induced in mice (16). This site is usually immunorecessive in serotype 1 poliovirus (22). Antigenic site 2 is a complex site including residues 220 to 222 of VP1 (site 2a) as well as residues 169 and 170 on VP2 (site 2b) (16). Sites 2a and 2b have both been detected in serotype 1 poliovirus, while only site 2b has been detected in serotype 3 poliovirus. Site 3 is also a complex site and includes residues 286 to 290 from VP1 (site 3a) as well as residues 58 and 59 and others on VP3 (site 3b). Sites 3a and 3b have both been detected in serotype 3 poliovirus, while as yet only neutralizing monoclonal antibodies to site 3b have been detected in serotype 1 poliovirus, suggesting that site 3a is not immunogenic in serotype 1 poliovirus (22). The location of the amino residues within the three-dimensional structure of the virion indicates that the majority of these amino acid residues are highly exposed and located within prominent structural features of the viral surface (21). A deep canyon or pit on the surface of the poliovirus has been identified as the receptor binding site (6). The neutralizing epitopes themselves are not involved in receptor binding, but binding of antibodies to these spots probably causes steric hindrance, with the actual receptor binding site located within the canyon (6). Whether all these sites are also antigenic for humans is not clear.
It has been reported that trypsin present in the gut lumen can cleave both serotype 1 and serotype 3 polioviruses at antigenic site 1 at residue 98 (arginine) (5, 12, 20, 25). While the poliovirus retains its infectivity in both cases, its antigenic properties are drastically altered, and the trypsin-cleaved viruses are not neutralized or immunoprecipitated by monoclonal antibodies to site 1 of nontreated virions (12). Trypsin cleavage of site 1 will occur in oral live attenuated poliovirus vaccine (OPV) recipients but will not occur in inactivated poliovirus vaccine (IPV) recipients, where the vaccine is given by intramuscular injection (23, 25). Therefore, if antigenic site 1 of poliovirus serotype 3 is also immunodominant in humans, in theory vaccination with IPV might also predominantly induce neutralizing antibodies to site 1, leaving a possible gap in the immune response to trypsin-cleaved serotype 3 poliovirus (10, 16).
In this study, we compared the site-specific humoral immune responses of naturally infected and IPV- or OPV-vaccinated persons to poliovirus serotype 3. The effect of an IPV booster vaccination on the site-specific antibody titers was also examined.
MATERIALS AND METHODS
Serum samples.
Negative control serum samples were used to test the specificity of antigenic site 1- and site 3-specific assays. These samples were obtained from nonvaccinated children (n = 20) from a religious group in The Netherlands that refuses vaccination. The sera had been prescreened by a neutralization test for the absence of neutralizing antibodies to poliovirus (log2 titer, <2) (7).
Serum samples from poliomyelitis patients (n = 25) from the 1992-1993 serotype 3 epidemic in The Netherlands were tested to determine antigenic site 1- and site 3-specific responses after natural infection (19). The serum samples of the poliomyelitis patients were all collected within 2.5 months after the onset of paralysis.
The seroprevalence of antigenic site 1- and site 3-specific antibodies after vaccination was determined for sera from IPV-vaccinated healthy blood donors (n = 63) and from age-matched OPV-vaccinated blood donors from Belgium (n = 63) who had received a complete series of vaccinations as children (7).
A separate group of IPV-vaccinated (n = 11) and OPV-vaccinated (n = 10) volunteers were given an IPV booster vaccination to determine the influence of IPV on the induction of antigenic site 1- and site 3-specific responses for the different vaccine backgrounds directly after booster vaccination. Blood samples were collected before booster vaccination and at 3, 7, and 28 days postvaccination. Full details of this study have been described elsewhere (9).
Monoclonal antibodies.
A panel of serotype 1-, 2-, and 3-specific monoclonal antibodies with specificity for antigenic site 1, 2, or 3 was used to test the newly developed assays for specificity. The serotype 1-specific (14D2E9), serotype 2-specific (5-15C6, 4-20D10, and 1-10C9E6), and serotype 3-specific (2-13D9 [site 1], 2-15E4 [site 1], and 4E5E9 [sites 2 and 3]) monoclonal antibodies had been produced at the National Institute of Public Health and the Environment (RIVM) (Bilthoven, The Netherlands) (13, 20). The serotype 3-specific monoclonal antibodies 204 (site 1), 877 (site 2), and 889 (site 3) were kindly donated by M. Ferguson (NIBSC, London, United Kingdom) (15, 16, 22). All monoclonal antibodies used were both Sabin- and wild-type-specific except for monoclonal antibodies 889 and 4E5E9, which reacted only with the Sabin strains.
Virus preparation.
Sabin mutant virus strains 335 (site 1) and 4021 (site 3) (kindly provided by M. Ferguson) were grown on HEp-2 cells in modified Eagle's medium supplemented with 10% fetal calf serum, until a full cytopathic effect developed (14). The culture supernatant was collected and centrifuged for 30 min at 2,000 × g to remove cell debris. The supernatant was extracted with 10% (vol/vol) arklone at 4°C for 45 min during constant shaking, followed by centrifugation (30 min at 2,000 × g). The supernatant was concentrated by direct ultrafiltration with membranes with a molecular weight cutoff of 10 kDa (type PM10; Amicon) (24). The optimal working dilution of the concentrated virus in the site-specific assay was determined by checkerboard titration.
Antigenic site 1-specific PoBI assay.
The site-specific poliovirus-binding inhibition (PoBI) assays were a modification of the previously described PoBI assay (7), except that monoclonal antibodies to specific sites were used as capture antibodies in the enzyme-linked immunosorbent assay (ELISA). The inhibition of the ELISA signal depends on both reduction of virus antigen binding to the capture antibody, due to the presence of competing (blocking) antibodies, and reduction of binding to the indicator monoclonal antibody by the same mechanism. Briefly, twofold dilutions of serum samples were made directly into the wells of microtiter cell culture plates (Greiner, Alphen aan de Rijn, The Netherlands) and poliovirus antigen was added to each well. The antigenic site 3 Sabin mutant strain 4021 (kindly provided by M. Ferguson) was used as antigen to prevent cross-reactivity with this antigenic site (15). Serum-virus mixtures were incubated for 2 h at 37°C. After the plates were washed, the preincubated serum-virus mixture was transferred to ELISA plates that had been coated overnight with antigenic site 1-specific monoclonal antibody 2-13D9 (immunoglobulin G [IgG] isotype) in a 1/8,000 dilution (13). After the incubation, the homologous monoclonal antibody (2-13D9), labelled with horseradish peroxidase, was used to detect bound poliovirus (18). Tetramethylbenzidine was used as a substrate, and color development was stopped after 15 min by the addition of 2 M H2SO4. The plates were read at 450 nm. Serum samples were considered positive if a reduction in extinction of ≥50% was reached. The reciprocal of the first serum dilution that was positive in the inhibition test was designated the titer of the test sample. For specificity testing, serum was replaced by serial dilutions of monoclonal antibodies to sites 1, 2, and 3. Optimal dilutions of reagents were determined by checkerboard titration.
Antigenic site 3-specific PoBI assay.
The antigenic site 3-specific assay was conducted in a similar manner to the site 1 assay described above, with site 3-specific IgM monoclonal antibody 889 as a capture antibody in the PoBI assay. These IgM molecules were first degraded into F(ab′)2 fragments by pepsin according to the manufacturer's instructions (Pierce, Rockford, Ill.), since IgM antibodies cannot coat efficiently (2). Sabin mutant strain 335 (kindly provided by M. Ferguson) was the antigen used to reduce cross-reactivity within antigenic site 1 (15). Biotin-labelled antigenic site 3-specific monoclonal antibody 889 was used to detect bound antigen. Avidin conjugated with alkaline phosphatase (Sigma, Zwijndrecht, The Netherlands) was added and incubated for 1 h at 37°C. The plates were washed, and 100 μl of p-nitrophenylphosphate at a concentration of 1 mg/ml in 0.1 M glycine buffer was added to each well. The plates were read at 405 nm after incubation at room temperature for 30 min. Results were analyzed as described for the site 1-specific assay.
Statistical methods.
An unpaired Student's t test was used to evaluate the differences in the titers of site-specific antibodies to poliovirus between the two groups. A chi-square test analysis was performed to determine the significance of the difference in seroprevalence between the two groups. P values of <0.01 were considered significant.
RESULTS
Specificity of the antigenic site 1- and site 3-specific PoBI serotype 3 assays.
None of the negative control serum samples tested (n = 20) showed any inhibition of the antigenic site 1 and site 3 PoBI signals (data not shown). A panel of monoclonal antibodies was used to test site specificity in the site 1 and site 3 PoBI assay. None of the serotype 1- and serotype 2-specific monoclonal antibodies showed any inhibition in the site 1- and site 3-specific assays, indicating no cross-reactivity with the other serotypes (titer, <1:100). The antigenic site 1-specific monoclonal antibodies 2-13D9 (titer, >12,800; homologous), 2-15E4 (titer, 12,800), and 204 (titer, >12,800) were able to inhibit antigenic site 1 PoBI signals, whereas none of the monoclonal antibodies (4E5E9, 877, and 889) to antigenic sites 2 and 3 of serotype 3 poliovirus did (titer, <100). In the site 3-specific assay, the homologous monoclonal antibody was able to inhibit the PoBI signal at a high level (titer, 6,400), and only low-level cross-reactivity was detected with site 1-specific monoclonal antibodies 204 (titer, 200), 2-15E4 (titer, 400), and 2-13D9 (titer, 400).
Antigenic site 1- and site 3-specific immune responses in poliomyelitis patients.
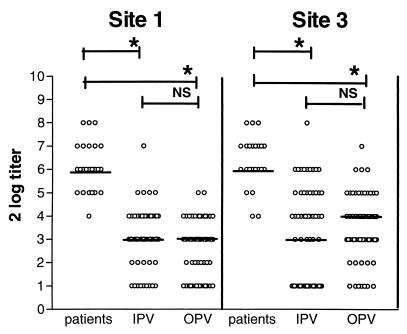
Twenty-five patients from the 1992-1993 serotype 3 outbreak in The Netherlands were tested with site-specific assays (19). Antibodies to antigenic sites 1 and 3 were detected in all patients, with a median log2 titer of 6 for both sites (Fig. 1).
FIG. 1.
Antigenic site 1- and site 3-specific antibody titers in patients with paralytic poliomyelitis (n = 25) and in IPV (n = 63)- and OPV (n = 63)- vaccinated blood donors. Results are expressed as log2 titers. Horizontal lines indicate median values. ∗, P < 0.01; NS, not significant.
Antigenic site 1- and site 3-specific immune responses in IPV- and OPV-vaccinated subjects.
There was no significant difference in the median titers to antigenic sites 1 and 3 of serotype 3 poliovirus between the IPV- and OPV-vaccinated groups, although the median titer for site 3 was 1 log step higher in OPV-vaccinated persons (Fig. 1). However, a significantly higher proportion of OPV-vaccinated persons (88.7%) had site 3-specific antibodies than did IPV-vaccinated persons (63.1%) (P < 0.01) (Table 1). No differences in the proportions of IPV and OPV recipients positive in the antigenic site 1-specific assay (72.3 versus 64.5%) (Table 1) were observed. A proportion of IPV and OPV recipients (19.0 and 7.9%) had antibodies to antigenic site 1 in the absence of site 3 antibodies. Significantly, more OPV recipients (27.0%; P < 0.01) had detectable levels of antibodies to antigenic site 3 in the absence of site 1-specific antibodies than did IPV recipients (4.8%).
TABLE 1.
Proportions of IPV- and OPV-vaccinated persons with detectable poliovirus-specific antibodies in site 1- and site 3-specific assays
| Assay result | % of recipients with detectable antibodies
|
|
|---|---|---|
| IPV | OPV | |
| Site 1 positive | 72.3 | 64.5 |
| Site 3 positive | 63.1 | 88.7a |
| Sites 1 and 3 positive | 57.1 | 52.4 |
| Site 1 positive and site 3 negative | 19.0 | 7.9 |
| Site 1 negative and site 3 positive | 4.8 | 27.0a |
| Sites 1 and 3 negative | 19.0 | 12.7 |
P < 0.01.
Antigenic site 1- and site 3-specific immune responses after IPV booster vaccination in IPV and OPV recipients.
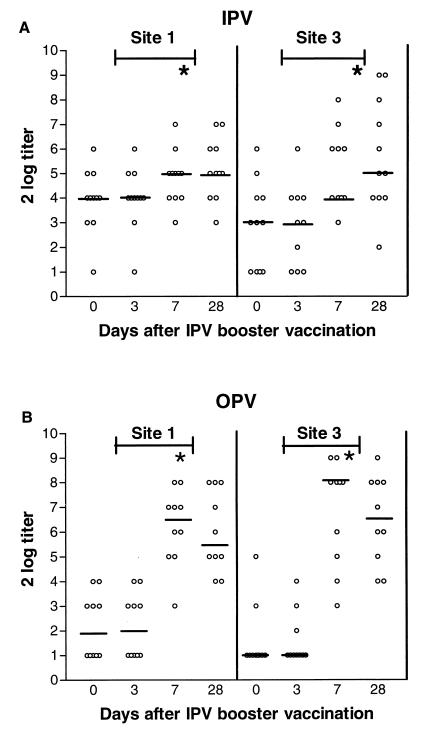
A significant increase in antigenic site 1- and 3-specific antibody titers was detected in both IPV and OPV recipients at days 7 and 28 compared to days 0 and 3 after booster vaccination with IPV (P < 0.01). However, the detected increase in titers was smaller in the IPV recipients than in the OPV recipients (P < 0.01) (Fig. 2).
FIG. 2.
Antigenic site 1- and site 3-specific responses after IPV booster vaccination of previously IPV (n = 11)- and OPV (n = 10)-vaccinated persons. Results are expressed as log2 titers. Horizontal lines indicate median values. ∗, P < 0.01; NS, not significant.
DISCUSSION
From the data presented in this paper, it is clear that in humans both antigenic sites 1 and 3 are immunogenic, as antibodies specific for these sites were detected after natural infection and in IPV- and OPV-vaccinated persons. Unlike the results from mice experiments (10, 16), site 1 appears less immunodominant in humans, since both site 1- and site 3-specific antibodies were readily detected in similar levels in both naturally exposed and OPV- and IPV-vaccinated persons.
We used site-specific inhibition ELISAs to measure antibody levels to antigenic sites 1 and 3. The inhibition levels reached in the site 1- and site 3-specific assays with the homologous antibodies were very high (log2 titers, >13.6 and 12.6, respectively). While the site 1 assay proved to be completely site specific, some cross-reactivity was detected in the site 3-specific assay with monoclonal antibodies to antigenic site 1, but with 16- to 32-fold-lower titers. Titers to poliovirus antigen as high as the levels found in the homologous reactions with monoclonal antibodies are not likely to be detected in the general population or in patients. In addition, a proportion of IPV and OPV recipients (4.8 and 27.0%, respectively), were only positive for site 3 antibodies in the absence of a site 1 response. Average titers for site 3 were not remarkably lower in the site 1-negative group (titer, 3.7; standard deviation, 0.7) than in the site 1-positive group (titer, 4.5; standard deviation, 1.1). For these reasons, this low-level cross-reactivity is not considered to be a problem in estimating the level of antibodies to site 3 when human serum is tested.
No significant difference in the immune response between OPV and IPV recipients with respect to antigenic site 1 of serotype 3 poliovirus was observed. However, significantly more OPV-vaccinated persons had detectable antibodies to antigenic site 3 (88.7%) than did IPV recipients (63.1%). In addition, more OPV recipients (27.0%) had site 3-specific antibodies detectable in the absence of site 1-specific antibodies than did the IPV recipients (4.8%). It is conceivable that the lower number of site 3 seropositive results in the IPV-vaccinated group than in the OPV-vaccinated group can be explained by trypsin cleavage of site 1 after passage of the OPV strains through the gut lumen (23, 25). In field trials with regular IPV or IPV made from trypsin-treated serotype 3 Saukett strains, it was demonstrated that both vaccines induced an increase in neutralizing antibody titers to intact and trypsin-treated virus, but the response was not studied at the level of individual antigenic sites (23). Alternatively, this difference between the number of positives in the OPV-vaccinated group compared to the IPV-vaccinated group might be explained by a longer-lasting induction of site 3-specific antibodies. After IPV booster vaccination, site 3-specific antibodies were induced in all persons receiving the IPV booster, but a lower percentage were positive when sera from people who had first been vaccinated less recently were tested.
In addition, T-cell immunity might play a role in the observed differences between IPV and OPV recipients. Immune lymphocytes were found to proliferate to purified capsid proteins VP1, VP2, and VP3 and, in some individuals, to VP4, indicating the presence of T-cell epitopes in each of the structural proteins (1, 26). T-cell epitopes adjacent to each of the B-cell antigenic sites in VP1 of poliovirus serotype 3 were identified (1). It is not unthinkable that trypsin cleavage of site 1 (within the B-cell epitope) also alters the response of the T cells to these sites. Further research is needed to understand the precise role of cellular immunity in antigenic site responses against polioviruses.
It is conceivable that in some of the IPV and OPV recipients, the site 3-specific antibodies are attributable to previous natural exposure to live poliovirus (wild-type or OPV strains). The high seroprevalence of IgA in the circulation of IPV-vaccinated persons probably indicates mucosal contact with poliovirus (8, 9). Poliovirus is no longer endemic in The Netherlands, but in 1992 a large outbreak of serotype 3 poliovirus occurred (19). While widespread circulation of virus during this epidemic was not demonstrated (3, 27), it cannot be excluded that part of the vaccinated population came into contact with the circulating wild-type strain or OPV strains that were used to control the epidemic. Similarly, the exposure of OPV to trypsin in the gut may result in reduced immunogenicity of antigenic site 1, favoring the development of site 3-specific antibodies. However, 49.0% of the IPV recipients in this study had site 3-specific antibodies in the absence of an IgA response (data not shown). These results might indicate that site 3 responses are longer lasting than the serotype 3-specific IgA in the circulation after mucosal contact. Alternatively, the site 3-specific antibodies may have been induced by IPV only.
Persons with no neutralizing antibodies to antigenic site 3 might not have an effective response to polioviruses with an altered antigenic site 1. The importance of site 3 for protection against poliomyelitis is illustrated by the Finland epidemic of 1983 (11). The serotype 3 endemic poliovirus strain was altered in antigenic site 1 (14). This outbreak was also influenced by low antibody titers to serotype 3 poliovirus in the vaccinated general population (14). In addition, the significantly lower antibody titers to the altered virus reflect the possible dangers of the reintroduction of antigenic site 1 variants or their generation in persons with no site 3-specific antibodies. Recently, we described a serotype 1 Sabin virus with a six-amino-acid deletion in antigenic site 1 (17). Neutralization of this mutant strain by an antigenic site 1 monoclonal antibody was altered. While this strain was still effectively neutralized by sera from IPV and OPV vaccinees, our present findings suggest that neutralizing activity should be investigated in persons with and without site 3-specific antibodies to estimate the risk of infection with mutant viruses. The role of site 2-specific antibodies also remains to be investigated.
In conclusion, we observed that the immune response following natural infection with serotype 3 poliovirus in humans consists of both site 1- and site 3-specific antibodies. No significant difference was observed in the immune response to antigenic site 1 between IPV and OPV recipients. Responses to antigenic site 3 of serotype 3 poliovirus were detected more frequently in persons who had been vaccinated by OPV in their childhood than in those who had received IPV. However, site 3-specific antibodies were increased after IPV booster vaccination in both groups.
ACKNOWLEDGMENTS
This work was supported by a grant from The Foundation for the Advancement of Public Health and Environment (SVM), Bilthoven, The Netherlands.
We especially thank all of the volunteers who have contributed to this study. We gratefully acknowledge Morag Ferguson from NIBSC for supplying the poliovirus-specific monoclonals and Sabin escape mutants used in these studies. We also thank the Utrecht blood bank for providing serum samples from IPV-vaccinated adults.
REFERENCES
- 1.Cello J, Strannegrad O, Svennerholm B. A study of the cellular immune response to enteroviruses in humans: identification of cross-reactive T cell epitopes on the structural proteins of enteroviruses. J Gen Virol. 1996;77:2097–2108. doi: 10.1099/0022-1317-77-9-2097. [DOI] [PubMed] [Google Scholar]
- 2.Chaplin H, Cohen S, Press E M. Preparation and properties of the peptide chains of normal human 19 s γ-globulin (IgM) Biochem J. 1965;95:256–261. doi: 10.1042/bj0950256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conyn-Spaendonck M A E, Oostvogel P M, van Loon A M, van Wijngaarden J K, Kromhout D. Circulation of poliovirus during the poliomyelitis outbreak in The Netherlands in 1992–1993. Am J Epidemiol. 1996;143:929–934. doi: 10.1093/oxfordjournals.aje.a008836. [DOI] [PubMed] [Google Scholar]
- 4.Emini E A, Jameson B A, Lewis A J, Larsen G R, Wimmer E. Poliovirus neutralizing epitopes: analysis and location with neutralizing monoclonal antibodies. J Virol. 1982;43:997–1005. doi: 10.1128/jvi.43.3.997-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fricks C E, Icenogle J P, Hogle J M. Trypsin sensitivity of the Sabin strain of type 1 poliovirus: cleavage sites in virions and related particles. J Virol. 1985;54:856–859. doi: 10.1128/jvi.54.3.856-859.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harber J, Bernhardt G, Lu H H, Sgro J Y, Wimmer E. Canyon rim residues, including antigenic determinants, modulate serotype-specific binding of polioviruses to mutants of the poliovirus receptor. Virology. 1995;20:559–570. doi: 10.1006/viro.1995.0067. [DOI] [PubMed] [Google Scholar]
- 7.Herremans M M P T, Reimerink J H R, Ras A, van der Avoort H G A M, Kimman T G, van Loon A M, Conyn-van Spaendonck M A E, Koopmans M P G. Evaluation of a poliovirus-binding inhibition assay as an alternative to the virus neutralization test. Clin Diagn Lab Immunol. 1997;4:659–664. doi: 10.1128/cdli.4.6.659-664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herremans M M P T, van Loon A M, Reimerink J H J, Rumke H C, van der Avoort H G A M, Kimman T G, Koopmans M P G. Poliovirus-specific immunoglobulin A in persons vaccinated with inactivated poliovirus vaccine in The Netherlands. Clin Diagn Lab Immunol. 1997;4:499–503. doi: 10.1128/cdli.4.5.499-503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herremans M M P T, Reimerink J H J, Buisman A M, Kimman T G, Koopmans M P G. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J Immunol. 1999;162:5011–5018. [PubMed] [Google Scholar]
- 10.Hogle J M, Chow M, Filman D J. The three-dimensional structure of poliovirus at 2.9A resolution. Science. 1985;229:1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- 11.Hovi T, Cantell K, Huovilainen A, Kinnunen E, Kuronen T, Lapinleimu K, Poyry T, Roivainen M, Salama N, Stenvik M. Outbreak of paralytic poliomyelitis in Finland: widespread circulation of antigenically altered poliovirus type 3 in a vaccinated population. Lancet. 1986;21:1427–1432. doi: 10.1016/s0140-6736(86)91566-7. [DOI] [PubMed] [Google Scholar]
- 12.Icenogle J P, Minor P D, Ferguson M, Hogle J M. Modulation of humoral responses to a 12-amino-acid site on the poliovirus. J Virol. 1986;60:297–301. doi: 10.1128/jvi.60.1.297-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kersten G F A, Lantinga M, Hazendonk T, Beuvery E C. Immunogenicity of trypsin treated type 2 and type 3 poliovirus in rats. Biologicals. 1995;23:179–183. doi: 10.1006/biol.1995.0029. [DOI] [PubMed] [Google Scholar]
- 14.Magrath D I, Evans D M, Ferguson M, Schild G C, Minor P D, Horaud F, Crainic R, Stenvik M, Hovi T. Antigenic and molecular properties of type 3 poliovirus responsible for an outbreak of poliomyelitis in a vaccinated population. J Gen Virol. 1986;67:899–905. doi: 10.1099/0022-1317-67-5-899. [DOI] [PubMed] [Google Scholar]
- 15.Minor P D, Evans D M, Ferguson M, Schild G C, Westrop G, Almond J W. Principal and subsidiary antigenic sites of VP1 involved in the neutralization of poliovirus type 3. J Gen Virol. 1985;66:1159–1165. doi: 10.1099/0022-1317-66-5-1159. [DOI] [PubMed] [Google Scholar]
- 16.Minor P D, Ferguson M, Evans D M A, Almond J W, Icenogle J P. Antigenic structure of poliovirus of serotypes 1, 2, and 3. J Gen Virol. 1986;67:1283–1291. doi: 10.1099/0022-1317-67-7-1283. [DOI] [PubMed] [Google Scholar]
- 17.Mulders M N, Reimerink J H J, Stenvik M, Alaeddinoglu I, van der Avoort H G A M, Hovi T, Koopmans M P G. A Sabin vaccine-derived field isolate of poliovirus type 1 displaying aberrant phenotypic and genetic features including a deletion in antigenic site 1. J Gen Virol. 1999;80:907–916. doi: 10.1099/0022-1317-80-4-907. [DOI] [PubMed] [Google Scholar]
- 18.Nibbeling R, Reimerink J H J, Agboatwala M, Naquid T, Ras A, Poelstra P, van der Avoort H A G M, van Loon A M. A poliovirus type-specific IgM antibody-capture enzyme-linked immunosorbent assay for the rapid diagnosis of poliomyelitis. Clin Diagn Virol. 1994;2:113–126. doi: 10.1016/0928-0197(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 19.Oostvogel P M, Wijngaarden J K, van der Avoort H G A M, Mulders M N, Conyn-van Spaendonck M A E, Rumke H C, van Steenis G, van Loon A M. Poliomyelitis outbreak in an unvaccinated community in The Netherlands, 1992–93. Lancet. 1994;344:665–670. doi: 10.1016/s0140-6736(94)92091-5. [DOI] [PubMed] [Google Scholar]
- 20.Osterhaus A D M E, van Wezel A L, Hazendonk T G, Uytdehaag F G C M, van Asten J A A M, van Steenis B. Monoclonal antibodies to polioviruses: comparison of intratypic strain differentiation of poliovirus type 1 using monoclonals versus cross-absorbed antisera. Intervirology. 1983;20:129–136. doi: 10.1159/000149381. [DOI] [PubMed] [Google Scholar]
- 21.Page G S, Mosser A G, Hogle J M, Filman D J, Rueckert R R, Chow M. Three-dimensional structure of poliovirus serotype 1 neutralizing determinants. J Virol. 1988;62:1781–1794. doi: 10.1128/jvi.62.5.1781-1794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel V, Ferguson M, Minor P D. Antigenic sites on type 2 poliovirus. Virology. 1993;192:361–364. doi: 10.1006/viro.1993.1044. [DOI] [PubMed] [Google Scholar]
- 23.Piirainen L, Roivainen M, Litmanen L, Eskola J, Beuvery C, Hovi T. Immunogenicity of a pilot inactivated poliovirus vaccine with trypsin-treated type 3-component. Vaccine. 1997;15:237–243. doi: 10.1016/s0264-410x(96)00119-3. [DOI] [PubMed] [Google Scholar]
- 24.Puig M, Jofre J, Lucena F, Allard A, Wadell G, Girones R. Detection of adenoviruses and enteroviruses in polluted waters by nested PCR amplification. Appl Environ Microbiol. 1994;60:2963–2970. doi: 10.1128/aem.60.8.2963-2970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roivainen M, Hovi T. Intestinal trypsin can significantly modify antigenic properties of polioviruses: implications for the use of inactivated poliovirus vaccine. J Virol. 1987;61:3749–3753. doi: 10.1128/jvi.61.12.3749-3753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simons J U, Kutubuddin M, Chow M. Characterization of poliovirus-specific T lymphocytes in the peripheral blood of Sabin-vaccinated humans. J Virol. 1993;67:1262–1268. doi: 10.1128/jvi.67.3.1262-1268.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Avoort H G A M, Reimerink J H J, Ras A, Mulders M N, van Loon A M. Isolation of epidemic poliovirus from sewage during the 1992-93 type 3 outbreak in The Netherlands. Epidemiol Infect. 1995;114:481–491. doi: 10.1017/s0950268800052195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Marel P, Hazendonk T G, Henneke M A, van Wezel A L. Induction of neutralizing antibodies by poliovirus capsid polypeptides VP1, VP2, and VP3. Vaccine. 1983;1:17–22. doi: 10.1016/0264-410x(83)90007-5. [DOI] [PubMed] [Google Scholar]