Abstract
The filamentous fungus Aspergillus fumigatus is an ubiquitous mold that can cause invasive pulmonary infections in immunocompromised patients. Within the lung, A. fumigatus forms biofilms that can enhance resistance to antifungals and immune defenses, highlighting the importance of defining the mechanisms underlying biofilm development and associated emergent properties. A. fumigatus biofilms display a morphology and architecture that is distinct from bacterial and yeast biofilms. Moreover, A. fumigatus biofilms display unique characteristics in the composition of their extracellular matrix (ECM) and the regulatory networks governing biofilm formation. This review will discuss our current understanding of the form and function of A. fumigatus biofilms, including the unique components of ECM matrix, potential drug resistance mechanisms, the regulatory networks governing A. fumigatus biofilm formation, and potential therapeutics targeting these structures.
Subject terms: Biofilms, Pathogens
Introduction
Biofilms are structured microbial communities surrounded by an extracellular matrix (ECM). Distinct from their free-living counterparts, the formation of biofilms increased resistance to anti-microbial drugs and the host immune system, which making them very difficult to combat1–3. Biofilms have been best studied in bacteria, and more recently in Candida species, however, emerging evidence suggests that filamentous fungi also make biofilms. Aspergillus fumigatus is the most common airborne filamentous human fungal pathogen, and causes a spectrum of different symptoms including invasive aspergillosis (IA) in immunocompromised hosts and chronic pulmonary aspergillosis and in patients with chronic lung disease4. A. fumigatus forms biofilms in both acute and chronic infections although the structure and composition of biofilms can vary between these two sets of conditions5. A. fumigatus can also colonize the airways of patients with cystic fibrosis (CF) patients, but biofilm formation in this condition has not been studied6,7.
Like biofilms in bacteria and yeast, the biofilms of A. fumigatus provide protection from antifungal therapy and host immune defenses8,9. Recent clinical trials highlight that the mortality of invasive aspergillosis remains as high as 30%, although treatment with the current antifungal agents10,11. Biofilm-mediated antifungal resistance is likely a contributing factor to the antifungal treatment failures observed in vivo with A. fumigatus isolates that are susceptible to antifungal agents by in vitro antifungal susceptibility testing12–14.
The composition of A. fumigatus biofilms is distinct from yeast biofilms. The Candida biofilms consisted of a dense network of mixture morphological forms, including yeast cells, hyphae and pseudohyphae15. In comparison, interconnected, branched multinucleate vegetative hyphae are the main type of cells within A. fumigatus biofilms8. Three-dimensional surface plot analysis has revealed that spatially ordered hyphae, well-structured hyphal channels and vertical hyphal growth are characteristics of the A. fumigatus biofilms16,17. Recent studies revealed that these specific features of filamentous fungal biofilms morphologies might play a role in fungal drug resistance and the virulence of A. fumigatus17,18. In addition, the components of ECM, the mechanisms of drug resistance and the regulatory network governing A. fumigatus biofilms are also likely different than in bacteria and yeast. This review will summarize the current state of our knowledge on A. fumigatus biofilms architecture, formation, function, and the potential for the development of therapeutics targeting A. fumigatus biofilms.
GAG, a unique glycan within the extracellular matrix in A. fumigatus biofilms
One of the hallmarks of all biofilms is the presence of extracellular matrix. This extracellular matrix has diverse functions, including mediating surface adherence, as well as enhancing resistance to antifungal agents and host defenses9,19,20. The matrix of A. fumigatus biofilms is mainly composed of extracellular DNA, polyols, proteins, lipids, and exopolysaccharides including α-glucans, galactomannan, and galactosaminogalactan (GAG)21,22. Among them, GAG is a critical structural and functional component of the ECM produced both in vitro and in vivo5,23. GAG-mediated adherence is crucial for A. fumigatus biofilm formation, strains deficient in GAG production are unable to produce extracellular matrix and fail to form adherent biofilms24–27.
Heteropolysaccharide GAG is composed of α-1,4-linked of galactose and partially deacetylated N-acetyl galactosamine (GalNAc)28,29. The biosynthesis of GAG is mediated by a cluster of five genes on chromosome 3. The uge3 gene encodes a glucose 4-epimerase mediating production of the nucleotide monosaccharides uridine diphosphate (UDP)-galactopyranose and UDP-GalNAc24. Next, putative transmembrane glycosyltransferase encoded by gtb3, polymerizes and exports these substrate sugars26. Loss of either uge3 or gtb3 is associated with a complete loss of GAG synthesis24,26. Two glycoside hydrolases, encoded by ega3 and sph3, exhibit specificity for different regions within the GAG polymer25,27. The phenotype of an Ega3-deficient mutant has not been reported, however, Sph3 is required for GAG synthesis25. The agd3 gene encodes a secreted polysaccharide deacetylase mediating deacetylation of GalNAc residues within GAG, rendering the polysaccharide polycationic30,31. Agd3-deficient mutants of A. fumigatus produce fully acetylated, non-adherent GAG that cannot support biofilm formation30. The GAG biosynthetic gene cluster exists on the genomes of some plant and human fungal pathogens, but is absent in Saccharomyces cerevisiae and fungal pathogens C. albicans30.
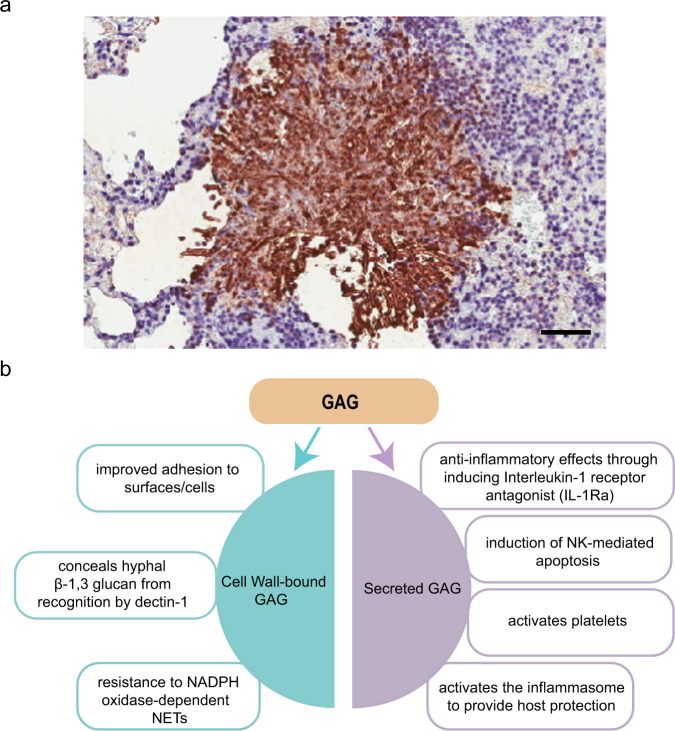
As GAG is both covered on the surface of A. fumigatus hyphae and secreted as component of ECM, it is therefore at the frontline of the interaction between A. fumigatus and the host immune system (Fig. 1a, b). A. fumigatus cell wall β-1,3 glucans are recognized as fungal pathogen-associated molecular patterns (PAMPs) by the C-type lectin dectin-132. The cell wall-bound GAG conceals hyphal β-1,3 glucan from recognition by dectin-1. A GAG-deficient ∆uge3 mutant is associated with increased β-1,3 glucan exposure, enhanced binding of dectin-1 to cell wall β-1,3 glucans, and induced hyperinflammatory response24,33. Cell wall-bound GAG also enhances resistance to NADPH oxidase-dependent neutrophil extracellular traps (NETs) which contributes to virulence34. In addition, secreted GAG has anti-inflammatory effects through inducing interleukin-1 receptor antagonist (IL-1Ra), which blocks IL-1 signaling35, and has been associated with neutrophil apoptosis both in vitro and in vivo35,36. GAG is also a direct activator of platelets, which play a key role in the innate immune response37,38. More recently, it was reported that GAG activates the NLRP3 inflammasome by binding to ribosome proteins through charge-charge interactions and inhibiting cellular translation mechanisms26. Given the multiple effects of GAG in modulating immune responses, GAG is an important fungal virulence factor. GAG-deficient strains showed attenuated virulence in mouse and invertebrate models of invasive aspergillosis24,30. A correlation between the ability to produce cell wall GAG and pathogenicity of different Aspergillus species has also been observed, underlining the important role of GAG in virulence34.
Fig. 1. A. fumigatus produce GAG in vivo and the multiple roles of GAG in pathogenesis.
a Immunohistochemistry of pulmonary tissue from an immunocompromised mouse infected with A. fumigatus and stained with an anti-galactosaminogalactan antibody. Brown indicates accumulation of galactosaminogalactan-containing biofilm matrix surrounding hyphae growing within pulmonary tissues. b Both cell wall-bound GAG and secreted GAG play multiple roles in Aspergillus pathogenesis. Scale bar: 150 μm.
Besides GAG, the roles of other polysaccharides within the ECM of A. fumigatus biofilms has not been well determined. Galactomannan, consisting of a mannan core decorated with β-1,5-linked galactofuranose, is dispensable for biofilm formation, although deletion of genes within the galactomannan biosynthetic pathway has been linked to alterations in expression of GAG39,40. The A. fumigatus biofilm matrix also contains abundant α-glucans, which are also one of the main cell wall polysaccharides of A. fumigatus41,42. It has been reported that α-1,3-glucans contributes to the aggregation of germinating conidia of A. fumigatus43. In addition, an α-1,3-glucan deficient mutant agsB in Aspergillus nidulans formed dispersed mycelial cells under liquid conditions indicated that α-glucans played crucial role in the agglutination of hyphae44. However, the role of α-glucans in biofilm formation has not been well defined. Further studies are required to better understand the roles of individual components of the ECM in A. fumigatus biofilm development and drug resistance.
A transcriptional network controls A. fumigatus biofilm formation
Studies in C. albicans have revealed that at least 54 transcriptional regulators are involved in biofilm formation in this organism45,46. Considering the differences in biofilm structure, adhesion factors (GPI proteins vs polysaccharides) and cell types in biofilm (mixture of yeast cells, hyphae and pseudohyphae vs hyphae)47–49, it has been hypothesized that the regulators of biofilm formation between C. albicans and A. fumigatus might not well conserved. Using the Reciprocal Best Hits (RBH)50, we identified three orthologs of the 54 C. albicans biofilms regulators (Flo8, Ada2, and Efg1) have a role in governing A. fumigatus biofilms formation. The 28 out of 54 C. albicans biofilms regulators have orthologs in A. fumigatus but with no reported roles on A. fumigatus adhesion and biofilm formation. No orthologs in the genome of A. fumigatus were detected for the 23 remaining C. albicans biofilms regulators (Supplementary Table 1).
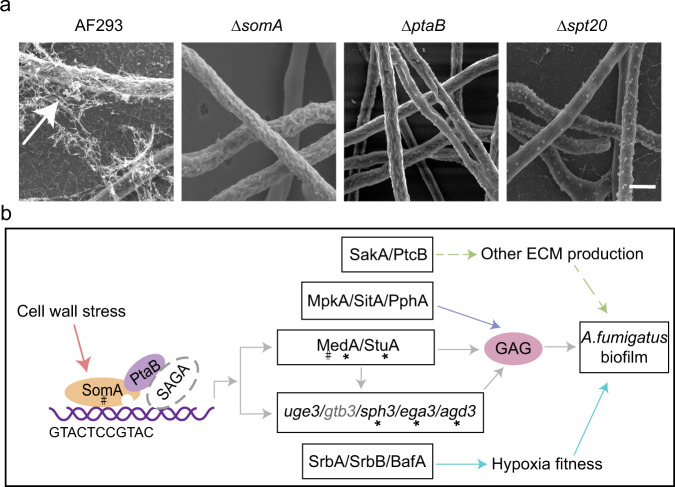
In A. fumigatus, several proteins have been identified that play a role in the regulation of adhesion, ECM production, and biofilm formation (Fig. 2a, b). The developmental regulators StuA (the ortholog of Efg1) and MedA positively regulate gene expression in GAG biosynthesis cluster24,51,52. The Lim-binding protein PtaB forms a complex with sequence-specific transcription factor SomA (the ortholog of Flo8) which can directly bind to conserved motifs in the medA and stuA as well as the GAG biosynthesis-related genes agd3 and sph3 promoter regions to activate transcription53. Recently, some subunits of the transcriptional co-activator Spt-Ada-Gcn5-acetyltransferase (SAGA) complex, including Spt20, Gcn5, AdaB (the ortholog of Ada2), Spt3 and Spt8 were identified as regulators of GAG biosynthesis and biofilm formation54,55. Among them, Spt20, a structural subunit of the SAGA complex was found to immunoprecipitate with PtaB, suggesting cooperation between SAGA complex and ptaB/SomA in activating GAG biosynthesis and biofilm formation55. Interestingly, the orthologs of C. albicans biofilms regulators Flo8, Ada2, and Efg1 are all GAG regulators in A. fumigatus. Considering the lack of GAG in C. albicans, this observation suggests that fungi can utilize conserved regulators of adhesion and biofilm formation, although the downstream effectors of these pathways are markedly different. In addition to these transcriptional factors, mitogen-activated protein kinases (MAPK) MpkA and SakA as well as phosphatases SitA, PtcB and PphA have been reported to play a role in regulating cell wall compositions, ECM production and biofilm formation in A. fumigatus56–58. These findings suggest that post-transcriptional pathways are involved in the regulation of A. fumigatus biofilms.
Fig. 2. Morphology and regulatory network of A. fumigatus biofilms.
a Scanning electron micrographs of the A. fumigatus biofilms. Extensive matrix material, indicated by the arrow, is present on biofilms of A. fumigatus wild-type AF293, but is absent in the deletion mutants of somA, ptaB and spt20. Scale bars: 2 μm. b Regulatory network model of A. fumigatus biofilms. The asterisks indicated direct binding by SomA via to a conserved “GTACTCCGTAC” motif. The # indicated regulators were also required in the GAG production under cell wall stresses condition.
As with bacteria, the formation of A. fumigatus biofilm can be influenced by environmental factors. In bacteria, sub-lethal concentrations of antibiotics commonly induce biofilm formation59–61. Antifungal drug caspofungin (inhibiting 1,3-β-glucan synthase) can induce GAG-dependent biofilm formation in A. fumigatus53. This process is dependent on SomA and MedA, while PtaB and StuA play only a minor role53. Recently, it has been reported that light signal regulates the formation of Aspergillus niger biofilm by affecting the biosynthesis of melanin and extracellular polysaccharide, although it is not known if this is also the case in A. fumigatus62, Oxygen tension also significantly affects A. fumigatus biofilm development, structure, and function18. Hypoxic microenvironments arise during A. fumigatus biofilm development. As such, several proteins required for hypoxic fitness can play a role in A. fumigatus biofilm development and maturation. Biofilm architecture factor A (BafA), encoded by a small open reading frame within a subtelomeric gene cluster, was found to modify A. fumigatus biofilm architecture by increasing the hypoxic fitness17,63. Hypoxia-responsive transcription factors SrbA and SrbB, required for A. fumigatus growth in low oxygen, can also influence biofilm formation64. The loss of SrbA results in an inability to develop a mature biofilm, while the loss of SrbB caused a reduction in overall biofilm biomass and abnormal biofilm structure, albeit to a lesser extent than SrbA disruption18. SrbA also plays a role in hyphal polarity and microtubule dynamics, which may be also required for biofilm structure and maturation65. Extrapolating from studies in other fungi such as C. albicans, it is highly likely that many other regulatory factors involved in A. fumigatus biofilm formation remain undiscovered. The availability of a genome-wide collection of A. fumigatus transcription-factor-deficient strains provides an opportunity to expand our understanding of regulatory networks for biofilm formation under a range of environmental conditions66.
A. fumigatus biofilms and drug resistance
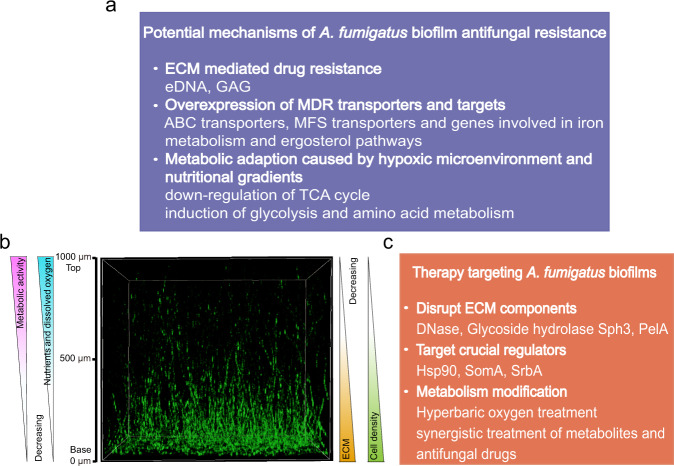
A. fumigatus biofilm exhibit greatly increased resistance to all current antifungal drug classes, including azoles, echinocandins, and polyenes when compared to growth under planktonic conditions19. As an example, the concentration of voriconazole required to reduce the metabolic activity by 90% (MIC90) exceeds 256 mg/mL in mature A. fumigatus biofilms67. A. fumigatus biofilm-associated antifungal resistance is thought to be a consequence of several interrelated factors, including elevated efflux pump activity, ECM production, and altered metabolic states (Fig. 3a).
Fig. 3. Potential mechanisms of A. fumigatus biofilm antifungal resistance and therapeutic approaches to overcome them.
a Potential antifungal resistance mechanisms within A. fumigatus biofilms. b 3D view of 24-hour A. fumigatus submerged biofilms. Gradients of oxygen, nutrients, metabolic activity, ECM, and cell density result in environmental heterogeneity within A. fumigatus biofilms. c Approaches to therapies targeting A. fumigatus biofilms.
Several lines of evidence implicate multidrug resistance (MDR) efflux pumps are one of the major contributors to azole resistance of A. fumigatus biofilm. An alanine-β-naphthylamine (Ala-Nap) fluorescence assay demonstrated a significant increase in efflux pump activity during the mature of A. fumigatus biofilm. Inhibition of efflux pump activity by using an efflux pump inhibitor increases A. fumigatus biofilm sensitivity to voriconazole68. The A. fumigatus genome contains 278 predicted major facilitator superfamily (MFS) transporters and 49 predicted ABC transporters, of which 35 are putative multidrug permeases69. Only a few of these transporters have been experimentally linked A. fumigatus drug resistance. An increased transcript level of mdr4, which encodes an ABC transporter, was observed during the development of A. fumigatus biofilm. This increase in mdr4 transcription was coincidental with a significant increased drug resistance in biofilm mature68. Additionally, the expression of ABC transporters cdr1B and mdr1 was significantly higher in some azole-resistant strains. The lack of cdr1B and mdr1 largely reduced drug resistance in both wild type A. fumigatus and their respective azole-resistant strains70,71. However, whether Mdr1 and Cdr1B contributes to A. fumigatus biofilm drug resistance remains to be defined. Future experiments assessing the expression patterns and the biofilm drug resistance of those MDR efflux pump mutants individually are crucial to better understanding the contribution of MDR efflux pumps on A. fumigatus biofilm drug resistance.
ECM production is a fundamental feature of biofilms, providing protection from antimicrobial agents. ECM-mediated drug resistance is common in both bacterial and yeast biofilms72,73. The exopolysaccharide GAG is a key component of A. fumigatus biofilm ECM. The addition of the GAG specific hydrolase Sph3 significantly increased the activity of the antifungals caspofungin, posaconazole and amphotericin B against 9 h A. fumigatus biofilms74. While this result suggests a role for GAG in antifungal resistance, these data contrast with the observation that GAG-deficient hyphae of the Δuge3 null mutant did not exhibit increased susceptibility to antifungals when grown under biofilm-forming conditions18. The mechanisms underlying these seemingly contradictory results remain undefined, however it is possible deletion of uge3 may result in compensatory upregulation of other matrix or cell wall components that enhance antifungal resistance. Extracellular DNA (eDNA) is an important component of biofilm ECM of both fungal and bacterial biofilms75–77. In C. albicans, eDNA contributes to maintenance and stability of mature biofilms and enhances biofilm antifungal resistance78. eDNA also plays an important functional role in maintaining biofilm structural and architectural integrity in A. fumigatus20. As with anti-GAG hydrolases, DNase treatment enhanced A. fumigatus biofilm susceptibility to caspofungin and amphotericin B20. Taken as a whole, these results suggest that ECM-mediated drug resistance occurs within A. fumigatus biofilms, however, the role of individual ECM components in A. fumigatus biofilm antifungal resistance needs to be better defined.
The complex structure of the mature A. fumigatus biofilm, composed of spatially ordered mycelium, results in the production of gradients of oxygen, nutrients, metabolic activity, ECM and cell density (Fig. 3b). These nutrients and oxygen gradients generate physiological heterogeneity within the biofilms, a phenomenon associated with antimicrobial resistance in bacterial biofilms79,80. The occurrence of hypoxic microenvironment is a canonical feature of many bacterial and yeast biofilms81–83, and have been observed in A. fumigatus biofilms despite the abundant space between hyphae in the biofilm18. Low-oxygen stress can result in increased expression of genes associated with iron and sterol metabolism which has been hypothesized to contribute to the azole drug resistance84–86. The transcription factor SrbA, which shares common features with the mammalian sterol regulatory element-binding proteins (SREBPs), can coordinate ergosterol biosynthesis and iron metabolism to mediate both the hypoxic response and azole resistance in A. fumigatus64,65. Interestingly, hypoxic adaptation, sterol metabolism, and azole drug resistance are instead regulated by zinc finger transcriptional factor Upc2 in C. albicans87,88, which highlights the differences in regulation of drug resistance between A. fumigatus and C. albicans. In addition, low-oxygen stress can modify primary metabolic pathways, including the down-regulation of the TCA cycle, induction of glycolysis as well as alanine, aspartate, glutamate metabolism84. Consistent with this hypothesis, it was recently reported that an alanine aminotransferase, AlaA, was involved in the resistance of A. fumigatus biofilms to echinocandin treatment89. However, growth of A. fumigatus in a low-oxygen environment is not sufficient to promote antifungal drug resistance, which indicated that other features of filamentous fungal biofilms may also be required to contribute to antimicrobial drug resistance90,91. Further investigation is required to explore the signals that guide polar hyphal growth in biofilms. Elucidating the role of oxygen gradients, nutrients, and secondary metabolites in biofilm development and how fungi sense those signals are promising areas of future study. Additionally, more work is required to identify and characterize genes and metabolic pathways that confer biofilm antifungal resistance in A. fumigatus.
Therapeutics targeting Aspergillus fungal biofilms
The biofilm lifestyle affords fungi with greater resistance to antifungal agents, an improved ability to evade host immune responses and survive in the in vivo environment92. Although antifungal drugs treatment is currently the most important and effective measure for the control of fungal infections, biofilm formation can compromise their efficacy67. Therefore, there is a critical need to identify antifungals active against fungal biofilms, or develop novel therapeutics that target the process of biofilm formation itself (Fig. 3c).
A number of studies in bacterial biofilms have suggested that disruption of the ECM in combination with antimicrobial therapy can be an effective strategy to combat biofilm-forming organisms93,94. Consistent with this strategy, enzymatic degradation of A. fumigatus biofilms ECM components eDNA and GAG have been successfully employed to disrupt biofilms, reduce fungal growth and increase antifungal efficacy in vitro and in vivo. The combination of DNase and antifungal drugs can improve the effect of polyenes and echinoctins against mature A. fumigatus biofilms in vitro20. These data suggest that DNase therapy may be effective in the management of A. fumigatus infections. Importantly, DNase is currently used as an adjunct to antibiotic treatment for cystic fibrosis, supporting the potentials of this agent for the clinical development95. Two glycoside hydrolases (GH) Sph3 and Ega3 that can cleave GAG were found by studying the GAG biosynthesis pathway. Treatment with Sph3 and Ega3 soluble recombinant GH domains can hydrolyze GAG and disrupt A. fumigatus biofilms in vitro25,27,74. GH enzymes can also exhibit cross-kingdom activity. Pseudomonas aeruginosa can produce a biofilm exopolysaccharide Pel, which is structurally similar to GAG. The soluble recombinant GH domain of PelA (a protein within the Pel biosynthetic pathway) can cleave GAG and disrupt A. fumigatus biofilms in vitro96. Intratracheal GH prophylaxis improved survival in neutropenic mice, possibly by increasing pulmonary inflammatory responses97. Prophylactic Sph3h combined with posaconazole therapy also enhanced the antifungal activity in a neutropenic mouse model of invasive pulmonary aspergillosis97. The activity of these agents against established fungal biofilms in animal models have not yet been reported. Collectively these studies suggest that therapies targeting ECM may hold promise as novel therapeutics for invasive aspergillosis.
Modulating the regulators of biofilm formation is another attractive target for the development of anti-biofilm therapies. Molecular chaperone Hsp90 is a key regulator of fungal drug resistance in multiple fungal species98–101. Genetic or pharmacologic inhibition of Hsp90 function significantly increased the efficacy of fluconazole in eradicating the biofilm of C. albicans in a rat venous catheter infection model102. This finding is consistent with the result that Hsp90 positively regulates matrix glucan production, an important carbohydrate for drug resistance of C. albicans biofilms102. Inhibition of Hsp90 also reduced the resistance of A. fumigatus biofilms to echinocandins and azoles in vitro, although, whether Hsp90 regulates A. fumigatus biofilms matrix production remains unknown102. Other regulators of A. fumigatus biofilm development are potential valuable anti-biofilms targets. SomA is a master transcriptional factor required for both GAG production and cell wall stress responses, and lacks an identifiable ortholog in humans, suggesting it might serve as an attractive target for anti-biofilm drug development53.
The hypoxic microenvironment within the A. fumigatus biofilm is critical for antifungal resistance18. Increasing levels of oxygen within biofilms, therefore, has the potential to reduce biofilm-mediated drug resistance. Hyperbaric oxygen treatment (HBOT) has been successfully to enhance the effect of tobramycin against biofilms formed by the bacterial pathogens Staphylococcus aureus and P. aeruginosa103,104. In A. fumigatus, HBOT markedly reduced biofilm proliferation in vitro and increased survival time in a chemotherapy murine model of invasive pulmonary aspergillosis, but this treatment failed to synergize with voriconazole or amphotericin B both in vitro and in vivo105. In addition to oxygen, other factors such as specific nutrients, secondary metabolites, or host-produced molecules may have the potential to alter metabolic adaptations of biofilm lifestyles, leading to drug resistance. A better understanding of these pathways may open up novel approaches for treating biofilm-associated infections.
Conclusions
In the last two decades, we have made significant progress on understanding the mechanisms underlying A. fumigatus biofilm formation and regulation. However, there are many unanswered questions about Aspergillus biofilm development and mechanisms of drug resistance. The knowledge derived from bacteria and yeast biofilms cannot all be directly extrapolated to filamentous fungi. An improved understanding of the unique aspects of filamentous fungal biofilm architecture may help open new therapeutic avenues to combat these deadly infections.
Supplementary information
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC32170040), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Author contributions
S.Z., D.C.S., and S.L. conceived the review. S.Z., D.C.S., S.L., and F.L.M. contributed to the writing and editing.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Donald C. Sheppard, Email: don.sheppard@mcgill.ca
Shizhu Zhang, Email: szzhang@njnu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41522-022-00347-3.
References
- 1.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11:30–36. doi: 10.1016/S0966-842X(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 2.Donlan RM. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donlan RM, Costerton JW. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chabi ML, et al. Pulmonary aspergillosis. Diagn. Inter. Imaging. 2015;96:435–442. doi: 10.1016/j.diii.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Loussert C, et al. In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol. 2010;12:405–410. doi: 10.1111/j.1462-5822.2009.01409.x. [DOI] [PubMed] [Google Scholar]
- 6.Nelson LA, Callerame ML, Schwartz RH. Aspergillosis and atopy in cystic fibrosis. Am. Rev. Respiratory Dis. 1979;120:863–873. doi: 10.1164/arrd.1979.120.4.863. [DOI] [PubMed] [Google Scholar]
- 7.Al Shakirchi M, et al. The effects of Aspergillus fumigatus colonization on lung function in patients with cystic fibrosis. J. Fungi. 2021;7:944. doi: 10.3390/jof7110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morelli KA, Kerkaert JD, Cramer RA. Aspergillus fumigatus biofilms: Toward understanding how growth as a multicellular network increases antifungal resistance and disease progression. PLoS Pathog. 2021;17:e1009794. doi: 10.1371/journal.ppat.1009794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauvais A, et al. Aspergillus biofilm in vitro and in vivo. Microbiol. Spectr. 2015;3:4.08. doi: 10.1128/microbiolspec.MB-0017-2015. [DOI] [PubMed] [Google Scholar]
- 10.Maertens JA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 11.Maertens JA, et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: A phase 3, randomised, controlled, non-inferiority trial. Lancet. 2021;397:499–509. doi: 10.1016/S0140-6736(21)00219-1. [DOI] [PubMed] [Google Scholar]
- 12.Pierce CG, Thomas DP, Lopez-Ribot JL. Effect of tunicamycin on Candida albicans biofilm formation and maintenance. J. Antimicrob. Chemother. 2009;63:473–479. doi: 10.1093/jac/dkn515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding MW, Marques LLR, Howard RJ, Olson ME. Can filamentous fungi form biofilms? Trends Microbiol. 2009;17:475–480. doi: 10.1016/j.tim.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Paterson PJ, Seaton S, Prentice HG, Kibbler CC. Treatment failure in invasive aspergillosis: Susceptibility of deep tissue isolates following treatment with amphotericin B. J. Antimicrob. Chemother. 2003;52:873–876. doi: 10.1093/jac/dkg434. [DOI] [PubMed] [Google Scholar]
- 15.Lohse MB, Gulati M, Johnson AD, Nobile CJ. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018;16:19–31. doi: 10.1038/nrmicro.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villena GK, Fujikawa T, Tsuyumu S, Gutierrez-Correa M. Structural analysis of biofilms and pellets of Aspergillus niger by confocal laser scanning microscopy and cryo scanning electron microscopy. Bioresour. Technol. 2010;101:1920–1926. doi: 10.1016/j.biortech.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Kowalski CH, et al. Fungal biofilm morphology impacts hypoxia fitness and disease progression. Nat. Microbiol. 2019;4:2430–2441. doi: 10.1038/s41564-019-0558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowalski CH, Morelli KA, Schultz D, Nadell CD, Cramer RA. Fungal biofilm architecture produces hypoxic microenvironments that drive antifungal resistance. Proc Natl Acad. Sci. USA. 2020;117:22473–22483. doi: 10.1073/pnas.2003700117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seidler MJ, Salvenmoser S, Müller FM. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob. Agents Chemother. 2008;52:4130–4136. doi: 10.1128/AAC.00234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajendran R, et al. Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot. Cell. 2013;12:420–429. doi: 10.1128/EC.00287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheppard DC, Howell PL. Biofilm exopolysaccharides of pathogenic fungi: Lessons from bacteria. J. Biol. Chem. 2016;291:12529–12537. doi: 10.1074/jbc.R116.720995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reichhardt C, Joubert LM, Clemons KV, Stevens DA, Cegelski L. Integration of electron microscopy and solid-state NMR analysis for new views and compositional parameters of Aspergillus fumigatus biofilms. Med. Mycol. 2019;57:S239–S244. doi: 10.1093/mmy/myy140. [DOI] [PubMed] [Google Scholar]
- 23.Beauvais A, et al. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell Microbiol. 2007;9:1588–1600. doi: 10.1111/j.1462-5822.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- 24.Gravelat FN, et al. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal beta-glucan from the immune system. PLoS Pathog. 2013;9:e1003575. doi: 10.1371/journal.ppat.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bamford NC, et al. Sph3 is a glycoside hydrolase required for the biosynthesis of galactosaminogalactan in Aspergillus fumigatus. J. Biol. Chem. 2015;290:27438–27450. doi: 10.1074/jbc.M115.679050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briard B, et al. Galactosaminogalactan activates the inflammasome to provide host protection. Nature. 2020;588:688–692. doi: 10.1038/s41586-020-2996-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bamford NC, et al. Ega3 from the fungal pathogen Aspergillus fumigatus is an endo-alpha-1,4-galactosaminidase that disrupts microbial biofilms. J. Biol. Chem. 2019;294:13833–13849. doi: 10.1074/jbc.RA119.009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontaine T, et al. Galactosaminogalactan, a new immunosuppressive polysaccharide of Aspergillus fumigatus. PLoS Pathog. 2011;7:e1002372. doi: 10.1371/journal.ppat.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briard B, Muszkieta L, Latge JP, Fontaine T. Galactosaminogalactan of Aspergillus fumigatus, a bioactive fungal polymer. Mycologia. 2016;108:572–580. doi: 10.3852/15-312. [DOI] [PubMed] [Google Scholar]
- 30.Lee MJ, et al. Deacetylation of fungal exopolysaccharide mediates adhesion and biofilm formation. mBio. 2016;7:e00252–00216. doi: 10.1128/mBio.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bamford NC, et al. Structural and biochemical characterization of the exopolysaccharide deacetylase Agd3 required for Aspergillus fumigatus biofilm formation. Nat. Commun. 2020;11:2450. doi: 10.1038/s41467-020-16144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steele C, et al. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005;1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaussart A, El-Kirat-Chatel S, Fontaine T, Latge JP, Dufrene YF. Nanoscale biophysical properties of the cell surface galactosaminogalactan from the fungal pathogen Aspergillus fumigatus. Nanoscale. 2015;7:14996–15004. doi: 10.1039/C5NR04399A. [DOI] [PubMed] [Google Scholar]
- 34.Lee MJ, et al. The fungal exopolysaccharide galactosaminogalactan mediates virulence by enhancing resistance to neutrophil extracellular traps. PLoS Pathog. 2015;11:e1005187. doi: 10.1371/journal.ppat.1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gresnigt MS, et al. A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist. PLoS Pathog. 2014;10:e1003936. doi: 10.1371/journal.ppat.1003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinet P, et al. A polysaccharide virulence factor of a human fungal pathogen induces neutrophil apoptosis via NK cells. J. Immunol. 2014;192:5332–5342. doi: 10.4049/jimmunol.1303180. [DOI] [PubMed] [Google Scholar]
- 37.Deshmukh H, et al. Aspergillus-derived galactosaminogalactan triggers complement activation on human platelets. Front. Immunol. 2020;11:550827. doi: 10.3389/fimmu.2020.550827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshmukh H, et al. Galactosaminogalactan secreted from Aspergillus fumigatus and Aspergillus flavus induces platelet activation. Microbes Infect. 2020;22:331–339. doi: 10.1016/j.micinf.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Lee MJ, et al. Overlapping and distinct roles of Aspergillus fumigatus UDP-glucose 4-epimerases in galactose metabolism and the synthesis of galactose-containing cell wall polysaccharides. J. Biol. Chem. 2014;289:1243–1256. doi: 10.1074/jbc.M113.522516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamarre C, et al. Galactofuranose attenuates cellular adhesion of Aspergillus fumigatus. Cell Microbiol. 2009;11:1612–1623. doi: 10.1111/j.1462-5822.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- 41.Chakraborty A, et al. A molecular vision of fungal cell wall organization by functional genomics and solid-state NMR. Nat. Commun. 2021;12:6346. doi: 10.1038/s41467-021-26749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latge JP, Beauvais A, Chamilos G. The cell wall of the human fungal pathogen Aspergillus fumigatus: Biosynthesis, organization, immune response, and virulence. Annu. Rev. Microbiol. 2017;71:99–116. doi: 10.1146/annurev-micro-030117-020406. [DOI] [PubMed] [Google Scholar]
- 43.Fontaine T, et al. Cell wall alpha1-3glucans induce the aggregation of germinating conidia of Aspergillus fumigatus. Fungal Genet. Biol. 2010;47:707–712. doi: 10.1016/j.fgb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimi A, et al. Functional analysis of the α-1,3-glucan synthase genes agsA and agsB in Aspergillus nidulans: AgsB is the major α-1,3-glucan synthase in this fungus. PLoS One. 2013;8:e54893. doi: 10.1371/journal.pone.0054893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox EP, et al. An expanded regulatory network temporally controls Candida albicans biofilm formation. Mol. Microbiol. 2015;96:1226–1239. doi: 10.1111/mmi.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nobile CJ, et al. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–138. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheppard DC. Molecular mechanism of Aspergillus fumigatus adherence to host constituents. Curr. Opin. Microbiol. 2011;14:375–379. doi: 10.1016/j.mib.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoyer LL, Cota E. Candida albicans agglutinin-like sequence (Als) family vignettes: A review of Als protein structure and function. Front. Microbiol. 2016;7:280. doi: 10.3389/fmicb.2016.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- 50.Moreno-Hagelsieb G, Latimer K. Choosing BLAST options for better detection of orthologs as reciprocal best hits. Bioinformatics. 2008;24:319–324. doi: 10.1093/bioinformatics/btm585. [DOI] [PubMed] [Google Scholar]
- 51.Gravelat FN, et al. Aspergillus fumigatus MedA governs adherence, host cell interactions, and virulence. Cell Microbiol. 2010;12:473–488. doi: 10.1111/j.1462-5822.2009.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheppard DC, et al. The Aspergillus fumigatus StuA protein governs the up-regulation of a discrete transcriptional program during the acquisition of developmental competence. Mol. Biol. Cell. 2005;16:5866–5879. doi: 10.1091/mbc.e05-07-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, et al. The transcription factor SomA synchronously regulates biofilm formation and cell wall homeostasis in Aspergillus fumigatus. mBio. 2020;11:e02329–02320. doi: 10.1128/mBio.02329-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin CJ, Hou YH, Chen YL. The histone acetyltransferase GcnE regulates conidiation and biofilm formation in Aspergillus fumigatus. Med. Mycol. 2020;58:248–259. doi: 10.1093/mmy/myz043. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, et al. Spt20, a structural subunit of the SAGA complex, regulates Aspergillus fumigatus biofilm formation, asexual development, and virulence. Appl. Environ. Microbiol. 2022;88:e0153521. doi: 10.1128/AEM.01535-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bom VL, et al. The Aspergillus fumigatus SitA phosphatase homologue is important for adhesion, cell wall integrity, biofilm formation, and virulence. Eukaryot. Cell. 2015;14:728–744. doi: 10.1128/EC.00008-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winkelstroter LK, et al. High osmolarity glycerol response PtcB phosphatase is important for Aspergillus fumigatus virulence. Mol. Microbiol. 2015;96:42–54. doi: 10.1111/mmi.12919. [DOI] [PubMed] [Google Scholar]
- 58.Manfiolli AO, et al. Mitogen activated protein kinases (MAPK) and protein phosphatases are involved in Aspergillus fumigatus adhesion and biofilm formation. Cell Surf. 2018;1:43–56. doi: 10.1016/j.tcsw.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haddadin H, et al. The effect of subminimal inhibitory concentrations of antibiotics on virulence factors expressed by Staphylococcus aureus biofilms. J. Appl. Microbiol. 2010;108:1281–1291. doi: 10.1111/j.1365-2672.2009.04529.x. [DOI] [PubMed] [Google Scholar]
- 60.Hoffman LR, et al. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 61.Oliveira NM, et al. Biofilm formation as a response to ecological competition. PLoS Biol. 2015;13:e1002191. doi: 10.1371/journal.pbio.1002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun W, et al. Light signaling regulates Aspergillus niger biofilm formation by affecting melanin and extracellular polysaccharide biosynthesis. mBio. 2021;12:e03434–03420. doi: 10.1128/mBio.03434-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kowalski CH, et al. A heterogeneously expressed gene family modulates the biofilm architecture and hypoxic growth of Aspergillus fumigatus. mBio. 2021;12:e03579–03520. doi: 10.1128/mBio.03579-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung D, et al. ChIP-seq and in vivo transcriptome analyses of the Aspergillus fumigatus SREBP SrbA reveals a new regulator of the fungal hypoxia response and virulence. PLoS Pathog. 2014;10:e1004487. doi: 10.1371/journal.ppat.1004487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willger SD, et al. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008;4:e1000200. doi: 10.1371/journal.ppat.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Furukawa T, et al. The negative cofactor 2 complex is a key regulator of drug resistance in Aspergillus fumigatus. Nat. Commun. 2020;11:427. doi: 10.1038/s41467-019-14191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mowat E, et al. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J. Antimicrob. Chemother. 2008;62:1281–1284. doi: 10.1093/jac/dkn402. [DOI] [PubMed] [Google Scholar]
- 68.Rajendran R, et al. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob. Agents Chemother. 2011;55:2092–2097. doi: 10.1128/AAC.01189-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferreira MED, et al. The ergosterol biosynthesis pathway, transporter genes, and azole resistance in Aspergillus fumigatus. Med. Mycol. 2005;43:S313–S319. doi: 10.1080/13693780400029114. [DOI] [PubMed] [Google Scholar]
- 70.Fraczek MG, et al. The Cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 2013;68:1486–1496. doi: 10.1093/jac/dkt075. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, et al. Mitochondrial dysfunctions trigger the calcium signaling-dependent fungal multidrug resistance. Proc. Natl Acad. Sci. USA. 2020;117:1711–1721. doi: 10.1073/pnas.1911560116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 73.Ramage G, Rajendran R, Sherry L, Williams C. Fungal biofilm resistance. Int. J. Microbiol. 2012;2012:528521. doi: 10.1155/2012/528521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Snarr BD, et al. Microbial glycoside hydrolases as antibiofilm agents with cross-kingdom activity. Proc. Natl Acad. Sci. USA. 2017;114:7124–7129. doi: 10.1073/pnas.1702798114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martins M, et al. Presence of extracellular DNA in the Candida albicans biofilm matrix and its contribution to biofilms. Mycopathologia. 2010;169:323–331. doi: 10.1007/s11046-009-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 77.Ibáñez de Aldecoa AL, Zafra O, González-Pastor JE. Mechanisms and regulation of extracellular DNA release and its biological roles in microbial communities. Front. Microbiol. 2017;8:1390. doi: 10.3389/fmicb.2017.01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martins M, Henriques M, Lopez-Ribot JL, Oliveira R. Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses. 2012;55:80–85. doi: 10.1111/j.1439-0507.2011.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crabbe A, Jensen PO, Bjarnsholt T, Coenye T. Antimicrobial tolerance and metabolic adaptations in microbial biofilms. Trends Microbiol. 2019;27:850–863. doi: 10.1016/j.tim.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Uruén C, Chopo-Escuin G, Tommassen J, Mainar-Jaime RC, Arenas J. Biofilms as promoters of bacterial antibiotic resistance and tolerance. Antibiotics. 2021;10:3. doi: 10.3390/antibiotics10010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rossignol T, et al. Correlation between biofilm formation and the hypoxic response in Candida parapsilosis. Eukaryot. Cell. 2009;8:550–559. doi: 10.1128/EC.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas VC, Fey PD. Take my breath away. eLife. 2017;6:e25739. doi: 10.7554/eLife.25739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stewart PS, et al. Reaction-diffusion theory explains hypoxia and heterogeneous growth within microbial biofilms associated with chronic infections. NPJ Biofilms Microbiomes. 2016;2:16012. doi: 10.1038/npjbiofilms.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barker BM, et al. Transcriptomic and proteomic analyses of the Aspergillus fumigatus hypoxia response using an oxygen-controlled fermenter. BMC Genomics. 2012;13:62. doi: 10.1186/1471-2164-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blatzer M, et al. SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen Aspergillus fumigatus. PLoS Genet. 2011;7:e1002374. doi: 10.1371/journal.pgen.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prasad T, Chandra A, Mukhopadhyay CK, Prasad R. Unexpected link between iron and drug resistance of Candida spp.: Iron depletion enhances membrane fluidity and drug diffusion, leading to drug-susceptible cells. Antimicrob. Agents Chemother. 2006;50:3597–3606. doi: 10.1128/AAC.00653-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang H, et al. Structural mechanism of ergosterol regulation by fungal sterol transcription factor Upc2. Nat. Commun. 2015;6:6129. doi: 10.1038/ncomms7129. [DOI] [PubMed] [Google Scholar]
- 88.Schubert S, et al. Regulation of efflux pump expression and drug resistance by the transcription factors Mrr1, Upc2, and Cap1 in Candida albicans. Antimicrob. Agents Chemother. 2011;55:2212–2223. doi: 10.1128/AAC.01343-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kerkaert JD, et al. An alanine aminotransferase is required for biofilm-specific resistance of Aspergillus fumigatus to echinocandin treatment. mBio. 2022;13:e0293321. doi: 10.1128/mbio.02933-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Binder U, Maurer E, Lackner M, Lass-Florl C. Effect of reduced oxygen on the antifungal susceptibility of clinically relevant aspergilli. Antimicrob. Agents Chemother. 2015;59:1806–1810. doi: 10.1128/AAC.04204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Warn PA, Sharp A, Guinea J, Denning DW. Effect of hypoxic conditions on in vitro susceptibility testing of amphotericin B, itraconazole and micafungin against Aspergillus and Candida. J. Antimicrob. Chemother. 2004;53:743–749. doi: 10.1093/jac/dkh153. [DOI] [PubMed] [Google Scholar]
- 92.Kaur S, Singh S. Biofilm formation by Aspergillus fumigatus. Med. Mycol. 2014;52:2–9. doi: 10.3109/13693786.2013.819592. [DOI] [PubMed] [Google Scholar]
- 93.Wu H, Moser C, Wang HZ, Hoiby N, Song ZJ. Strategies for combating bacterial biofilm infections. Int. J. Oral. Sci. 2015;7:1–7. doi: 10.1038/ijos.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Srinivasan R, et al. Bacterial biofilm inhibition: A focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol. 2021;12:676458. doi: 10.3389/fmicb.2021.676458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frederiksen B, Pressler T, Hansen A, Koch C, Hoiby N. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatr. 2006;95:1070–1074. doi: 10.1080/08035250600752466. [DOI] [PubMed] [Google Scholar]
- 96.Le Mauff F, et al. Molecular mechanism of Aspergillus fumigatus biofilm disruption by fungal and bacterial glycoside hydrolases. J. Biol. Chem. 2019;294:10760–10772. doi: 10.1074/jbc.RA119.008511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ostapska H, et al. Preclinical evaluation of recombinant microbial glycoside hydrolases in the prevention of experimental invasive Aspergillosis. mBio. 2021;12:e0244621. doi: 10.1128/mBio.02446-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lamoth F, Juvvadi PR, Steinbach WJ. Heat shock protein 90 (Hsp90): A novel antifungal target against Aspergillus fumigatus. Crit. Rev. Microbiol. 2016;42:310–321. doi: 10.3109/1040841X.2014.947239. [DOI] [PubMed] [Google Scholar]
- 99.Cowen LE. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathog. 2009;5:e1000471. doi: 10.1371/journal.ppat.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cowen LE, et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl Acad. Sci. USA. 2009;106:2818–2823. doi: 10.1073/pnas.0813394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lamoth F, Juvvadi PR, Gehrke C, Steinbach WJ. In vitro activity of calcineurin and heat shock protein 90 inhibitors against Aspergillus fumigatus azole- and echinocandin-resistant strains. Antimicrob. Agents Chemother. 2013;57:1035–1039. doi: 10.1128/AAC.01857-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Robbins N, et al. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 2011;7:e1002257. doi: 10.1371/journal.ppat.1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kolpen M, et al. Reinforcement of the bactericidal effect of ciprofloxacin on Pseudomonas aeruginosa biofilm by hyperbaric oxygen treatment. Int. J. Antimicrob. Agents. 2016;47:163–167. doi: 10.1016/j.ijantimicag.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 104.Lerche CJ, et al. Hyperbaric oxygen therapy augments tobramycin efficacy in experimental Staphylococcus aureus endocarditis. Int. J. Antimicrob. Agents. 2017;50:406–412. doi: 10.1016/j.ijantimicag.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 105.Dhingra S, Buckey JC, Cramer RA. Hyperbaric oxygen reduces Aspergillus fumigatus proliferation in vitro and influences in vivo disease outcomes. Antimicrob. Agents Chemother. 2018;62:e01953–01917. doi: 10.1128/AAC.01953-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).