Abstract
The first step for a pathogenic bacterium to initiate infection is via attachment (i.e., through surface determinants) to a suitable receptor. An in vitro microbial artificial-mouth model was used to test the efficacy of polyclonal antibodies to Streptococcus mutans cell surface proteins (CsAb) and a cell surface 59-kDa protein (59Ab) in preventing S. mutans colonization and carious lesion formation. In study 1, groups of 12 human teeth specimens were inoculated with S. mutans, which were incubated with different concentrations of CsAb (A1 [positive control], sterile saline, no antibody; A2, 0.007 mg of antibody protein/ml; and A3, 0.7 mg of antibody protein/ml) for 1 h at 37°C. The negative control group (B1) was not infected and was incubated with Trypticase soy broth (TSB) without dextrose supplemented with 5% sucrose (TSBS). In study 2, the same study design was used except that 59Ab was used instead of CsAb, normal rabbit serum was used in the positive control group (A1), and TSB supplemented with 1% glucose was used as the nutrient to control for sucrose-dependent colonization. All groups were exposed for 4 days to circulating cycles of TSBS and TSB (study 1 and study 2, respectively; 30 min each, three times per day) and a mineral washing solution (21 h per day). Prior to each nutrient cycle, 1 ml of the appropriate CsAb or 59Ab solution was administered to each group and allowed to mix for 30 min before cycling was resumed. Data obtained by confocal laser scanning microscopy demonstrated the presence of a significantly smaller (P < 0.05) lesion area and a smaller total lesion fluorescence in group A3 than in group A1 for both studies. In study 1, group A2 had significantly smaller values than A1 for lesion depth and area. There were no significant differences between groups A2 and A3 for lesion area or between groups A1 and A2 for total lesion fluorescence. In study 2, there were no significant differences among groups A1 and A2 for lesion depth or between groups A2 and A3 for all of the parameters studied. In both studies, there were no significant differences between S. mutans plaque CFU numbers among any of the groups. These studies demonstrated the efficacy of CsAb and 59Ab in reducing primary caries development in this model, although the underlying mechanism remains unclear.
Streptococcus mutans has been identified as the major etiological agent in human dental caries and comprises a significant proportion of the oral streptococci in carious lesions (10). It has been suggested that surface antigens such as antigen I/II or P1 participate in sucrose-independent colonization of tooth surfaces (3, 8), while glucosyltransferase and glucan-binding proteins (GBP) may be responsible for the sucrose-dependent colonization of S. mutans (14, 17). An essential goal in the development of a vaccine for dental caries is to induce antibodies that block bacterial adhesion and, therefore, prevent lesion formation. A number of studies in experimental animals and humans have shown that active and passive immunization with S. mutans, either with whole cells or with different cellular components, inhibits S. mutans colonization and subsequent dental caries formation (7, 9, 11, 16). However, animal studies are very expensive and time-consuming. It would be desirable to have an in vitro system that would allow for easy, inexpensive, and fast screening of antibody or antimicrobial solutions that would be worthwhile to study with animals and/or humans. An in vitro microbial caries model (1) was modified to produce natural primary carious lesions and was used in this study to test the efficacy of antibodies in preventing S. mutans adhesion and carious lesion formation.
Fontana et al. (2) have recently reported that conventional Sprague-Dawley rats, infected with S. mutans and intranasally immunized with a mixture of S. mutans cell surface proteins conjugated with cholera toxin B subunit, developed statistically fewer smooth surface enamel caries lesions compared to control animals. Furthermore, Western blot results demonstrated that the protection was due to antibodies directed in saliva against two bands at approximately 59 to 65 kDa (termed 59 kDa here) and 190 kDa, while the immunoblot probed with the pooled serum from the immunized rats demonstrated only one band at 59 kDa. The band at 59 kDa is believed to be a cell surface component, one distinct from the 59-kDa GBP (14), since no reactivity was seen on Western blots with the 59-kDa GBP and polyclonal antibody developed against our 59-kDa protein (D. J. Smith, personal communication).
We have isolated a preparation of cell surface proteins from S. mutans, which very likely contained fimbrial components, as demonstrated by immunostaining and electron microscopy, and elicited antibodies (CsAb) in rabbits against this heterogeneous cell surface preparation (3). Furthermore, we purified the 59-kDa protein from this cell surface mixture and have raised polyclonal antibodies (59Ab) in rats to it.
The purpose of this study was to modify an in vitro, microbe-based “artificial mouth” model to test the efficacy of CsAb and 59Ab in the prevention of primary dental caries and to measure the extent of the developed primary carious lesions by using the quantifiable and reproducible method of confocal microscopy.
MATERIALS AND METHODS
CsAb and 59Ab preparation. (i) Cell surface protein preparation.
This procedure was previously described by Fontana et al. (3). Briefly, S. mutans TH16 (serotype c) was grown in 9 liters of Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) supplemented with 1% glucose at 37°C in 5% CO2 and 95% air for 24 h. Cells from 9 liters of culture were harvested by centrifuging at 16,000 × g for 15 min at 4°C, washed once in buffer (20 mM Tris, 1 mM MgCl2, 0.02% NaN3; pH 6.8), and frozen as a pellet at −20°C overnight. A mixture of surface proteins from S. mutans were isolated by using a shearing technique. Frozen cells were thawed, suspended in buffer, and blended in a Waring blender for two 1-min cycles at high speed. Intact cells and cell debris were removed by a slow centrifugation (16,000 × g, 4°C, 10 min), and the supernatant, containing the cell surface protein (Cs protein) preparation, was retained and centrifuged at 110,000 × g for 2 h. The resulting Cs protein pellet was resuspended in the same buffer and centrifuged a second time at 16,000 × g for 10 min to further remove cell debris and aggregated components. The supernatant containing the Cs preparation was divided into aliquots and frozen at −80°C until use.
(ii) 59-kDa protein isolation.
In order to separate cell surface protein fractions, preparative gel electrophoresis (Prep Cell model 491; Bio-Rad Laboratories, Richmond, Calif.) was utilized. The resolving and stacking gels were composed of 10 and 3% acrylamide (National Diagnostics, Atlanta, Ga.), respectively. A concentrated Cs protein preparation (2 ml, 1 mg/ml) from S. mutans A32-2 (serotype c) was added to an equal volume of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, boiled for 7 min, placed on a 6-cm column, and subjected to 12 W of continuous power. The protein of interest eluted after approximately 8 h of electrophoresis and was previously determined by immunoblots of Cs protein to have a molecular size of approximately 59 to 65 kDa (termed here the 59-kDa protein). The proteins were collected and analyzed for molecular size and purity by gel electrophoresis after staining with Coomassie brilliant blue. The fractions of interest were pooled, passed through an affinity column that removes SDS (Extracti-Gel; Pierce, Rockford, Ill.), and stored at −80°C.
(iii) CsAb preparation.
Three New Zealand White rabbits were immunized with the prepared S. mutans Cs protein preparation (0.377 mg of protein/ml) by using the RIBI adjuvant system as suggested by the manufacturer (RIBI ImmunoChem Research, Inc., Hamilton, Mont.). Injection of the surface protein preparation and RIBI adjuvant was done on day 0 and boosted on day 28. A total dose per animal of 1.0 ml (0.377 mg of protein/ml) was administered at each time period as follows: 0.3 ml intradermally (0.05 ml in each of six different sites), 0.4 ml intramuscularly (0.2 ml into each hind leg), 0.1 ml subcutaneously (in the neck region), and 0.2 ml intraperitoneally. Blood was collected by cardiac puncture on day 45, and serum was separated from the clot by centrifugation (5,000 × g, 10 min) and then stored at −20°C until use. The sera from the three rabbits were pooled and used as the antibody (CsAb) source for study 1. The ELISA absorbance values of the pooled sera were as follows: for a serum dilution of 1:1,000 the optical density at 490 nm (OD490) was 0.248 ± 0.043; for a serum dilution of 1:5,000 to OD490 was 0.069 ± 0.010; and for a serum dilution of 1:10,000 the OD490 was 0.056 ± 0.008. All animal studies received Institutional Animal Care and Use Committee approval.
(iv) 59Ab preparation.
Rat antisera to the 59-kDa surface protein were obtained from eight animals, each immunized with 5 mg of protein/ml incorporated into the RIBI adjuvant system (RIBI ImmunoChem Research). Preparations were injected with 0.2 ml subcutaneously in each of two sites (in the upper back of the animals) and 0.1 ml intraperitoneally twice, 21 days apart, and blood was collected 7 days after the last injection. The blood was allowed to clot, and serum was obtained and frozen at −20°C until used. The sera from the eight rats were pooled and used as the antibody (59Ab) source for study 2. The ELISA absorbance values of the pooled sera were as follows: for a serum dilution of 1:2 the OD490 was 1.038 ± 0.314; for a serum dilution of 1:4 the OD490 was 0.927 ± 0.002; and for a serum dilution of 1:8 the OD490 was 0.635 ± 0.042.
(v) Electrophoretic techniques.
To confirm CsAb and 59Ab antibody specificity to cell surface components, the Cs-enriched preparation and the 59-kDa protein were electrophoresed (150 V for approximately 1 h) by using reducing SDS–10% PAGE (National Diagnostics). Molecular size standards were included in the gel (Rainbow colored protein molecular size markers; Amersham, Arlington Heights, Ill.). Proteins were transferred electrophoretically (70 V for 2 h) to nitrocellulose paper for immunoblotting. The blots were blocked overnight with washing buffer (Trizma base; NaCl; Tween 20, pH 7.4) containing 0.5 ml of glutaraldehyde. The blots were probed with rabbit anti-S. mutans surface protein-enriched serum (CsAb; Fig. 1) and rat anti-59 kDa surface protein (59Ab) at a 1:1,000 dilution. Proteins which reacted with antibody were visualized on nitrocellulose by alkaline phosphatase-labeled anti-rabbit or anti-rat immunoglobulin G (IgG) heavy-chain-specific antibody (Sigma Chemical Company, St. Louis, Mo.) and nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP; Bio-Rad). Molecular sizes were determined by comparison to protein standards by using an UltroScan XL laser densitometer and GelScan XL software (Pharmacia LKB Biotechnology, Uppsala, Sweden).
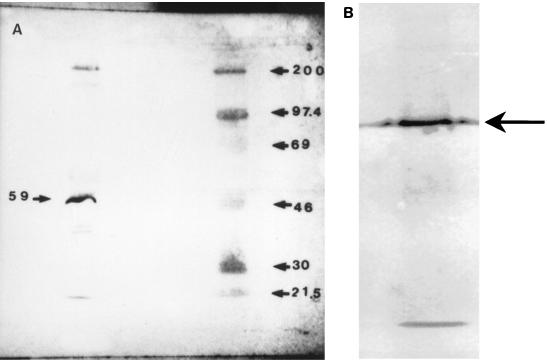
FIG. 1.
(A) Representative immunoblot of enriched S. mutans TH16 Cs protein preparation probed with the rabbit anti-CsAb used in study 1, followed by treatment with anti-rabbit IgG alkaline phosphatase-labeled antibody. An enriched S. mutans Cs protein preparation is in the left lane; molecular size standards (200, 97.4, 69, 46, 30, and 21.5 kDa) are also shown (right lane). (B) Representative immunoblot of enriched S. mutans A32-2 59-kDa protein probed with the rabbit anti-CsAb used in study 1, followed by treatment with anti-rabbit IgG alkaline phosphatase-labeled antibody.
The immunoblot of the S. mutans Cs protein preparation probed with CsAb demonstrated bands at approximately 190, 157, 65, 59, and 40 to 46 kDa (Fig. 1A), a result similar to that described previously (3). The major immunodominant band, whose role is currently being investigated, was at 59 kDa. This has been shown (unpublished data) to be distinct from the 59-kDa GBP (14). The minor bands seen at 190 and 157 kDa have been shown previously to be P1 and GTF, respectively (12). The immunoblot of the 59-kDa protein probed with CsAb demonstrated a single band at 59 to 65 kDa (Fig. 1B), supporting the purity of the isolated protein. Also, the 59Ab immunoblot demonstrated only one band at 59 to 65 kDa and has been identified to have amylase-binding properties (data not shown).
In vitro microbial caries model experiments. (i) General experimental design.
For each study, four groups of 12 human teeth specimens per group were treated for a 4-day test period in an in vitro microbial artificial-mouth caries model (1). The groups differed from each other in the presence (A1, A2, and A3) or absence (B1, negative control) of S. mutans (serotype c) and in the concentration of antibody used (Tables 1 and 2). Two studies were conducted. In study 1, the effect of CsAb on bacterial adhesion and caries development was tested. In study 2, the effect of 59Ab was assessed.
TABLE 1.
Confocal microscopy measurements of primary carious lesions after treatment with CsAb (study 1)a
 |
CsAb, rabibt antiserum to S. mutans TH16 Cs protein preparation (the dilution is shown in parentheses [Und, undiluted]). Group A1 received sterile saline. Values for area, total fluorescence, and depth are the mean ± the standard error of the mean. (n = 12 for groups A1, A2, A3, and B1; n = 8 for baseline values). Groups within brackets were not significantly different (P > 0.05), as determined by Tukey's procedure.
TABLE 2.
Confocal microscopy measurements of primary carious lesions after treatment with 59Ab (study 2)a
 |
59Ab, rat antiserum to S. mutans A32-2 59-kDa protein (the dilution is shown in parentheses [Und, undiluted]). Group A1 received rabbit normal sera. Values for area, total fluorescence, and depth are the mean ± the standard error of the mean (n = 7 for group A1; n = 9 for groups A2, A3, and B1). Groups within brackets were not significantly different (P > 0.05), as determined by Tukey's procedure.
(ii) Specimen preparation.
Enamel specimens (3 mm in diameter) were drilled from extracted, sound, human, lower permanent incisors which had been obtained from oral surgeons and sterilized by soaking in 3% buffered (neutral) formalin since the time of extraction. Each specimen was mounted on a polyacrylic rod by using methyl methacrylate resin. The specimens were first ground by using 600-grade silicon carbide paper to remove approximately 50 μm of the surface and then polished to a high luster with Gamma Alumina (0.05 μm) by standard methods. The specimens were then randomly assigned to test groups, with each group initially composed of 14 specimens. All specimens were sterilized with ethylene oxide gas. Two specimens from each group were randomly chosen before treatment and examined to obtain baseline confocal microscopy data. The 12 specimens that remained in each group were secured in caries-forming vessels by gluing the ends of their plexiglass rods to a round plexiglass base that fit in the bottom of the vessels.
(iii) Treatment regimen and circulating fluids.
Trypticase soy broth without dextrose (Difco Laboratories) supplemented with 5% sucrose (TSBS) was used as the bacterial nutrient broth for study 1. For each caries-forming vessel there was one 1-liter bottle of TSBS that dispensed the medium (0.7 ml/min) at three different times each day, for 30 min each (2), for a total of 63 ml/day by means of a peristaltic pump (Wiz Peristaltic Pump; ISCO, Inc., Lincoln, Nebr.). Study 2 used Trypticase soy broth without dextrose (Difco) supplemented with 1% glucose (TSB) as the bacterial nutrient broth to control for sucrose-dependent colonization.
A mineral wash (MW) solution (pH 7.0), modified from Stookey and Stahlman (15), was used to mimic the action of saliva. One liter of MW solution contained potassium chloride (624.6 mg), sodium chloride (866.6 mg), dipotassium hydrogen phosphate (33.8 mg), magnesium chloride (59.6 mg), and calcium chloride dihydrate (166.6 mg). Twenty-liter polypropylene bottles (Fisher Scientific, Pittsburgh, Pa.) were used to store the MW solution. There was one bottle for each of two groups. Each bottle dispensed approximately 882 ml of MW/day (0.7 ml/min) to each caries-forming vessel, intermittently over a period of 21 h, during the periods without TSBS or TSB flow, by a peristaltic pump.
Three times a day, immediately prior to each TSBS and TSB (study 1 and study 2, respectively) cycle, 1 ml of the appropriate antibody solution was administered to each group by injection, followed by flushing with 5 ml of sterile saline (8.78 g of NaCl/liter of deionized water). The antibody solutions were allowed to mix with fluid (MW) in the caries vessels by stirring for 30 min before the cycling was resumed.
(iv) Experimental setup.
All of the media and model components, except for the enamel specimens, were autoclaved at 121°C for 20 min prior to the initiation of each experiment. For both studies, each group of 12 specimens was placed in a caries-forming vessel (125-ml Pyrex slow speed stirring vessel; Fisher). All caries-forming vessels were placed on an electric stirrer inside an incubator at 37°C under aerobic conditions. Each caries vessel had three inlets, one for TSBS or TSB, one for MW, and one for injection of the antibody and one outlet for drainage tubing. The drainage tubing ended flush in a drainage container, which was also placed inside the incubator. Drainage of fluid from each caries vessel was maintained at 0.7 ml/min by a peristaltic pump.
(v) Preparation of bacterial inoculum and inoculation procedures.
Each specimen in groups A1, A2, and A3 was inoculated by use of a micropipette with 20 μl of washed, overnight (16 h), stationary-phase cells of S. mutans TH16 (serotype c) for study 1 and S. mutans A32-2 (serotype c) for study 2, resuspended in TSBS and TSB (study 1 and study 2, respectively) to an OD540 of 0.5. TSBS or TSB only was added to specimens in the negative control B1 groups. Prior to inoculation, filter (0.2 μm [pore size])-sterilized polyclonal antibodies prepared against S. mutans surface proteins (CsAb in study 1; 59Ab in study 2) were incubated in equal amounts (0.5 ml of each), at the appropriate concentration (Tables 1 and 2), with the S. mutans inoculum for 1 h at 37°C. Sterile saline (8.78 g of NaCl/liter of deionized water) was used instead of CsAb in the positive control A1 in study 1. Normal rabbit serum (undiluted) was used instead of 59Ab in the positive control A1 group in study 2. After inoculation, the specimens were incubated for 2 h at 37°C to allow the control bacteria or antibody-treated bacteria to implant on the teeth. Each group of specimens was then placed in a separate caries-forming vessel and attached to the MW, TSBS or TSB, and drainage container bottles.
(vi) Monitoring of specimens.
The following parameters were measured in the supply and drainage containers fluid at the beginning and at the end of the 4-day test periods to monitor the absence of contamination and the viability of the inoculum: (i) pH; (ii) S. mutans viability (by plating on mitis salivarius agar [Difco] supplemented with 20% sucrose and 200 IU of bacitracin per liter); and (iii) bacterial contamination (by plating on Trypticase soy agar [Difco]). Plates were incubated at 37°C in 5% CO2–95% air for 3 days.
In order to quantitate the bacteria adhered to the teeth at the end of the test periods, the specimens (two in study 1 and three in study 2) from each group were randomly selected and placed individually in 5 ml of sterile saline. Each specimen was vortexed (20 s) and sonicated (20 s) until all visible dental plaque was displaced from the surface of the tooth. All samples were then double plated on mitis salivarius and Trypticase soy agar.
For both studies, the two MW bottles and all four TSBS bottles maintained a neutral pH (approximately 7.0) at the beginning and at the end of the test period, indicating no contamination of the supply vessels. The pH of the negative control group caries vessel (B1) in both studies remained neutral throughout the experiment, also indicating a lack of contamination. In addition, the negative control caries-forming vessel, MW bottles, and TSBS bottles remained sterile throughout the treatment periods, and no contamination was observed in the experimental or control groups.
(vii) Evaluation of tooth samples.
After termination of each study, a casting resin (Meyer Plastics, Indianapolis, Ind.) was applied to the top of each specimen and allowed to polymerize. This was done in order to protect the surface of the specimen during the cutting procedure. Specimens were sectioned in half by using a Silverstone-Taylor hard-tissue microtome. One-half of each specimen was stained with a 0.1 mM solution of rhodamine B (Aldrich Chemical Co., Milwaukee, Wis.) overnight, with no further rinsing. The stained surface was analyzed by using a laser scanning confocal microscope (Odyssey; Noran Instruments, Inc., Middleton, Wis.) to determine the extent of the lesion (5). One area, 300 μm in length, was scanned for each specimen. The confocal microscope permits noninvasive imaging of subsurface tissue structures, including the enamel and dentin. Areas were scanned planoparallel to the transversal cut surface of the specimen and perpendicular to the natural surface of the tooth. The areas were not scanned directly at the cut surface of the specimen because of concern regarding the smear layer created during the cutting procedure. Areas were scanned at between 10 and 50 μm below the cut surface with the confocal microscope by using Image-1 (version 4.14.C) software (Universal Images Corp., West Chester, Pa.). After being brought into focus (with a 10× Nikon objective [numerical aperture, 0.25] and 10× eyepiece), the specimens were illuminated with an argon laser by using a 529-nm excitation wavelength. Confocal slits were set at 15 μm with a 550-nm long-pass filter, and the argon laser intensity was set at 100%. The argon laser used had a power intensity of 1.23 mW per scanned point in the specimen. After examination of all specimens, confocal settings (contrast and brightness) were maximized and held constant. For the collection of images, the samples were frame averaged by using 128 frames per image. The parameters that were measured included the area of the fluorescent lesion, the total lesion fluorescence, and the depth of the lesion.
Data analysis.
Means and variances were calculated for each measured parameter (area of the lesion, total lesion fluorescence, lesion depth, and numbers of plaque S. mutans CFU) and treatment group. These data were analyzed by using a single factor analysis of variance model. Where a significant effect was detected (α:0.05), multiple comparisons were conducted by using Tukey's procedure.
RESULTS
Baseline and uninoculated control group specimens for both studies did not exhibit carious lesions. Groups A1, A2, and A3 exhibited a similar decrease in pH for both studies, and by day 4 all had reached a pH of 4.51 to 4.55 after the sucrose or glucose cycles in the caries-forming vessels. In study 1, for all three parameters studied (lesion depth, lesion area, and total lesion fluorescence), data obtained by using confocal laser scanning microscopy (Table 1) demonstrated the presence of significantly smaller (P < 0.05) lesions in group A3 (Fig. 2C) than in group A1 (Fig. 2A). The lesion depth and lesion area parameters for group A2 (Fig. 2B) were significantly smaller than those for group A1. There was no statistically significant difference between A2 and A3 specimens in lesion area or between the A1 and A2 specimens in total lesion fluorescence. S. mutans plaque numbers were slightly, but not significantly, lower in A3 (5.5 × 104 total CFU ± 4.0 × 104 [mean ± the standard deviation]) than in the A2 (6.0 × 105 total CFU ± 3.0 × 105) and A1 (6.5 × 105 total CFU ± 2.0 × 105) groups.
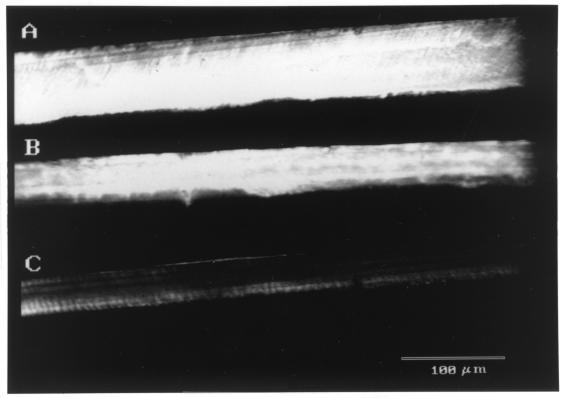
FIG. 2.
Representative confocal microscopy images of the primary carious lesions produced in study 1, groups A1 to A3. Only the fluorescent lesion image is shown in each case. The intensity of the gray scale (white to black) is indicative of the amount of dye and, therefore, the demineralization. Sound areas appear black, while more-demineralized areas appear progressively whiter (3). (A) Representative confocal microscopy image of a primary carious lesion produced in 4 days in the A1 group (S. mutans-inoculated positive control). Note the depth, area, and fluorescence (intensity) of the lesion compared to that in Fig. 2B and C. (B) Representative confocal microscopy image of a primary carious lesion produced in 4 days in the A2 group (1:100 CsAb). Note that the depth and area of the lesion are smaller than in Fig. 2A; however, the fluorescence (intensity) of the lesion is similar to that in Fig. 2A. (C) Representative confocal microscopy image of a primary carious lesion produced in 4 days in the A3 group (undiluted CsAb). Note that the depth, area, and fluorescence (intensity) of the lesion are significantly less than that in Fig. 2A.
In study 2, the area and total fluorescence data obtained by using confocal laser scanning microscopy (Table 2) demonstrated the presence of significantly smaller (P < 0.05) lesions in groups A3 and A2 than in group A1. The mean lesion depth for group A3 was not significantly different than that for groups A1 and A2. There was no statistically significant difference between group A3 and A2 specimens for all three parameters studied. S. mutans plaque numbers were not significantly different among the three groups studied (A1, 7.5 × 106 total CFU ± 1.8 × 106; A2, 2.8 × 107 ± 1.6 × 107; A3, 1.8 × 107 ± 6.8 × 106 [mean ± the standard deviation]).
DISCUSSION
Our laboratory has been extensively involved in establishing the role of S. mutans fimbrial components in the adherence and colonization of the tooth surface by this bacterium and in testing whether antibodies against S. mutans surface proteins, enriched in fimbria components, reduce the adherence of S. mutans to the tooth surface, thereby inhibiting the development of primary dental caries (3, 12, 13). Caries-free (CF) adult individuals have higher levels of salivary IgA antibodies to an enriched-fimbriae preparation of S. mutans than caries-active (CA) individuals (3). These results suggest that CF subjects may be protected immunologically from dental caries in part by salivary IgA antibody against S. mutans fimbrial-cell surface antigens. Perrone et al. (12) demonstrated by using immunoblot analysis and ELISA techniques with antibody to the cell surface, fimbria-enriched preparations, GTF, and P1 antigen that the levels of fimbria components, GTF, and P1 antigens were higher in cell surface fimbria-enriched preparations from S. mutans isolates from CA subjects than in preparations from CF individuals. These results suggest that the differences between the composition of S. mutans cell surface fimbria-enriched preparations in isolates from CA and CF subjects may play an important role in the virulence of this microorganism in dental caries. While S. mutans TH16 was the laboratory strain chosen for study 1, S. mutans A32-2 (a CA isolate) was chosen as the inoculum for study 2 based partly on the findings of Perrone et al. (12). Our laboratory has also reported that a 52-kDa salivary protein, identified as amylase, is the major S. mutans fimbria-enriched cell surface preparation binding protein in whole saliva (13). Preliminary studies were conducted to address the need to add a salivary pellicle to the teeth prior to bacterial inoculation in the microbial-caries model. Results showed no significant differences in bacterial attachment and caries scores between surfaces with and those without a salivary pellicle (4). Therefore, to minimize the number of variables in these initial studies, it was decided not to use a pellicle. Of course, the fact that we did not use a pure preparation in study 1, but rather a mixture of most major cell surface associated antigens of S. mutans, indicated that although most of the CsAb was directed against a cell surface component (59 kDa), there was some small reactivity with other surface antigens such as P1 and GTF that could in part be responsible for the elicited caries protection. Therefore, a follow-up study (study 2) was conducted with this same model and polyclonal antibodies elicited in rats to the 59-kDa protein to study the degree of protection obtained by targeting the 59-kDa surface protein, and the results demonstrated a similar degree of caries protection, supporting the importance of the 59-kDa protein in caries development. Studies to clone this protein are currently under way (6).
The goals in the prevention of colonization of specific pathogenic bacteria include long-lasting protection conferred by an appropriate vaccine. An intranasal vaccination study was conducted with the same cell surface preparation as the one used to prepare CsAb as the immunogen (2). The results demonstrated that the elicited antibodies were mainly elicited in both saliva and serum against a 59-kDa protein and protected against smooth surface caries. However, there were no differences in the ability of the antibody to inhibit bacterial colonization. The in vitro caries model described here is a fast, simple, economical, and novel approach for testing the effect of S. mutans anti-surface protein antibodies or other antimicrobials in caries prevention. Bacterial caries systems, where the flora is controlled by in vitro environmental and nutrient conditions, provide a controlled means for studying complex ecosystems, such as dental plaque and its effect on the development of dental caries. The use of a bacterial artificial caries system permits more clinically relevant in vitro investigations of primary caries etiology and prevention, since it links bacteria with the resulting demineralization of the tooth. By providing CsAb or 59Ab in the system, the production of salivary antibodies that occurs in caries-free subjects or that would result as a consequence of orally immunizing an animal or individual with S. mutans cell surface fimbria-enriched preparations was simulated. Since normal rabbit serum had no enzyme-linked immunosorbent assay reactivity to the enriched cell surface preparation, a saline solution was used as the negative control for study 1. However, the possibility that nonantibody components (i.e., albumin) may interfere with S. mutans colonization or metabolic activity was directly addressed in study 2, in which normal rabbit (preimmune) serum was used as a negative control. Unfortunately, CsAb and 59Ab could not be delivered continuously to the system as would occur by saliva in vivo. Also, since the drainage of liquid occurred from the top of the caries vessels and since the antibodies caused bacterial agglutination, the bacteria were not completely eliminated (they would be swallowed in vivo) but rather partially accumulated in the bottom of the vessel and may have maintained metabolic activity. This may have contributed to the low pH and relatively higher caries scores (compared to B1) observed in the A2 and A3 groups. This issue will be addressed in follow-up studies, since the caries vessels have been modified so that the outlet is located in the bottom of the vessel.
Our hypothesis was that the smaller lesions observed in the antibody-treated specimens might be due in part to a reduction in S. mutans adherence to the tooth surfaces and to reduced plaque formation. However, based on the data of these in vitro studies as well as results from the rat immunization study (2), the mechanism for the elicited caries protection is not clear. The data demonstrated that although CsAb-treated specimens in group A3 (study 1) showed a trend toward a decreased number of S. mutans adhered to the teeth surface, the results were not statistically significant, a result probably due to the small sample sizes used for bacterial analysis. However, these CsAb antibodies could have also affected bacterial metabolism, leading to a less-cariogenic S. mutans plaque. It must be noted that the results presented here for study 1 are representative of two identical experiments; therefore, the studies are reproducible. Also, the fact that sucrose was provided in the diet probably supported the action of GTF in inducing, mainly, a glucan-dependent plaque. This may partially explain why significant differences in bacterial numbers were not observed among the treatment groups in study 1, in which an effect of antibody on sucrose-independent attachment was anticipated. Therefore, study 2 was conducted without the use of sucrose in the nutrient medium. This change decreased the amount of caries obtained in study 2 compared to study 1, but it still did not result in significant differences in the numbers of plaque bacteria. Despite this, however, our results demonstrated the application of an in vitro caries model in testing the efficacy of antibodies to S. mutans surface proteins in decreasing the level of caries development.
ACKNOWLEDGMENTS
We are grateful to Carlos Gonzalez, Daniel Gomes, and Xiaochun Li for helpful discussions and critical comments on the manuscript.
REFERENCES
- 1.Fontana M, Dunipace A J, Gregory R L, Noblitt T W, Li Y, Park K K, Stookey G K. An in-vitro microbial model for studying secondary caries formation. Caries Res. 1996;30:112–118. doi: 10.1159/000262146. [DOI] [PubMed] [Google Scholar]
- 2.Fontana M, Dunipace A J, Stookey G K, Gregory R L. Intranasal immunization against dental caries with Streptococcus mutans enriched fimbrial preparation. Clin Diagn Lab Immunol. 1999;6:405–409. doi: 10.1128/cdli.6.3.405-409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontana M, Gfell L E, Gregory R L. Characterization of preparations enriched for Streptococcus mutans fimbriae: salivary IgA antibodies in caries-free and caries-active subjects. Clin Diagn Lab Immunol. 1995;2:719–725. doi: 10.1128/cdli.2.6.719-725.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontana M, Gregory R L, Dunipace A J, Wilson M E, Gonzalez-Cabezas C, Stookey G K. In vitro microbial caries model: role of salivary pellicle. J Dent Res. 1999;78:334. [Google Scholar]
- 5.Fontana M, Li Y, Dunipace A J, Noblitt T W, Fischer G M, Katz B P, Stookey G K. Measurement of demineralization of enamel using microradiography and confocal microscopy. Caries Res. 1996;30:317–325. doi: 10.1159/000262337. [DOI] [PubMed] [Google Scholar]
- 6.Gregory R L, Fontana M, Fives-Taylor P, LeBlanc D J, Li X C. Cloning of Streptococcus mutans saliva and amylase binding protein genes. J Dent Res. 1998;77:216. [Google Scholar]
- 7.Gregory R L, Michalek S M, Schechmeister I L, McGhee J R. Effective immunity to dental caries: protection of gnotobiotic rats by local immunization with a ribosomal preparation from Streptococcus mutans. Microbiol Immunol. 1983;27:787–800. doi: 10.1111/j.1348-0421.1983.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee S F, Progulske-Fox A, Erdos G W, Piacentini D A, Ayakawa G Y, Crowley P J, Bleiweis A S. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II) Infect Immun. 1989;57:3306–3313. doi: 10.1128/iai.57.11.3306-3313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehner T, Caldwell J, Smith R. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect Immun. 1985;50:796–799. doi: 10.1128/iai.50.3.796-799.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loesche W J, Straffon L H. Longitudinal investigation of the role of Streptococcus mutans in tissue decay. Infect Immun. 1979;26:498–507. doi: 10.1128/iai.26.2.498-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalek S M, McGhee J R, Mestecky J, Arnold R R, Bozzo L. Ingestion of Streptococcus mutans induces secretory immunoglobulin A and caries immunity. Science. 1976;192:1238–1240. doi: 10.1126/science.1273589. [DOI] [PubMed] [Google Scholar]
- 12.Perrone M, Gfell L E, Fontana M, Gregory R L. Antigenic characterization of fimbriae from Streptococcus mutans isolates from caries free and caries susceptible subjects. Clin Diagn Lab Immunol. 1997;4:291–296. doi: 10.1128/cdli.4.3.291-296.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray C A, Gfell L E, Buller T L, Gregory R L. Interactions of Streptococcus mutans fimbria-associated surface proteins with salivary components. Clin Diagn Lab Immunol. 1999;6:400–404. doi: 10.1128/cdli.6.3.400-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith D J, Taubman M A. Experimental immunization of rats with a Streptococcus mutans 59-kilodalton glucan-binding protein protects against dental caries. Infect Immun. 1996;64:3069–3073. doi: 10.1128/iai.64.8.3069-3073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stookey G K, Stahlman D B. Enhanced fluoride uptake in enamel with a fluoride-impregnated prophylactic cup. J Dent Res. 1976;55:333–341. doi: 10.1177/00220345760550030801. [DOI] [PubMed] [Google Scholar]
- 16.Taubman M A, Smith D J. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in rats and hamsters. J Immunol. 1976;118:710–716. [PubMed] [Google Scholar]
- 17.Yamashita Y, Bowen W H, Burne R A, Kuramitsu H K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]