Abstract
Autistic children have differences in their movements which impact their functional performance. Virtual-reality enables researchers to study movement in safe, engaging environments. We used motion-capture to measure how 7–13-year-old autistic and neurotypical children make whole-body movements in a virtual-reality task. Although children in both groups were successful, we observed differences in their movements. Autistic children were less efficient moving to the target. Autistic children did not appear to use a movement strategy. While neurotypical children were more likely to overshoot near targets and undershoot far targets, autistic children did not modulate their strategy. Using kinematic data from tasks in virtual-reality, we can begin to understand the pattern of movement challenges experienced by autistic children.
Keywords: Autism spectrum disorder, Motor skills, Movement, Postural control, Balance, Virtual reality, Kinematics
Autism is diagnosed based on two core symptom domains–differences in social communication and restricted, repetitive patterns of behavior, interests, or activities–but differences in movements are also associated features of autism (American Psychiatric Association, 2013). Although movement differences are listed as associated features of autism, there has been less research in this domain than on the core symptoms used for diagnosis. To begin shedding light on the specific nature of movement differences in autistic children, we examined how autistic children perform goal-directed, whole-body movements.
As many as 79–97% of autistic children are at risk of or have motor difficulties compared to general population norms (Bhat, 2020; Green et al., 2009; Miller, et al., 2021; Hilton et al., 2012). These differences begin early in development and persist throughout childhood (Bhat et al., 2011; Fournier et al., 2010; Lloyd et al., 2013; Perin et al., 2020; Van Waelvelde et al., 2010). In particular, autistic individuals often have gross motor differences, beginning with delays in meeting motor milestones (Davidovitch et al., 2018; Fulceri et al., 2019), continuing through the school years with differences in performance on standardized motor assessments (Fisher et al., 2018; Green et al., 2009; Jansiewicz et al., 2006; Liu & Breslin, 2013; Miller et al., 2021; Perin et al., 2020; Purpura et al., 2020), and persisting into adulthood as evidenced by decreased postural control and stability (Doumas et al., 2016; Lim et al., 2017). These motor differences are often related to the functional outcomes of autistic people (Fears et al., 2022; Licari et al., 2019; Travers et al., 2017). Although these studies have documented the presence of motor problems in autism using motor milestone checklists, standardized assessments of motor ability, and measures of postural stability, there has been little attention paid to how movements of autistic children may differ from those of neurotypical children in terms of their efficiency, variability, and accuracy.
Mastery of early motor skills is necessary to access many early developmental opportunities (Adolph, 2008), and motor skills are foundational to the development of skills in many other domains (e.g. affect, perception, social behavior, tool-use, additional motor skills; Adolph & Robinson, 2013). In autism, motor difficulties may impede children’s abilities to engage in many activities of daily living (ADLs; Fisher et al., 2018; Licari et al., 2019). Delayed sitting and crawling in infants at higher likelihood of autism have been linked to delays in the development of communication skills (LeBarton & Iverson, 2016). The development of self-care behaviors (e.g., eating, bathing, toileting, and dressing) in autistic children is related to locomotion, grasping, and visuomotor integration (Jasmin et al., 2009). While many autistic children eventually learn to perform most of these activities at some level of competency, underlying motor control differences likely limit their efficiency, accuracy, or level of independence.
Researchers have begun to examine how movements made by autistic individuals may differ from neurotypical individuals. Autistic individuals exhibit differences during a wide array of movement types including quiet standing (for review, see Lim et al., 2017), leaning (Miller et al., 2019; Wang et al., 2016), stepping (Bojanek et al., 2020), reaching (Glazebrook et al., 2006, 2008; Rodgers et al., 2019), grasping (Carment et al., 2020; Mosconi et al., 2015), catching (Chen et al., 2019), tool-use (Mostofsky et al., 2006), and handwriting (Grace et al., 2017). To date, postural control (e.g., standing, leaning, stepping) and upper extremity movements (e.g., reaching, grasping, catching, tool-use, handwriting) have been the primary focus of many studies. However, these studies have not provided information about how autistic individuals make whole-body movements to accomplish a task.
Autistic individuals consistently demonstrate less stable posture (Lim et al., 2017) and less efficient movements across a wide range of tasks (Cook, 2016). These differences may put autistic individuals at an elevated risk for fatigue and falls (Maki & McIlroy, 1996; Zwicker et al., 2018). Specifically, medial–lateral postural control is a strong predictor of fall risk in community-dwelling elderly individuals (Swanenburg et al., 2010) and individuals with multiple sclerosis (Sun et al., 2019), but has not been studied in autistic individuals during goal-directed whole-body movements. Additionally, most studies of postural stability and control with autistic individuals have either used static tasks (for review, see Lim et al., 2017), or dynamic tasks that constrain a participant’s foot placement and movements (e.g., Miller et al., 2019). These limitations constrain our ability to generalize the results of static postural control studies in autism to dynamic postural control ability, and to generalize the results of both static and dynamic postural control studies in neurotypical development to autistic individuals. The approach used in prior studies also limits our ability to evaluate the efficiency of goal-directed whole-body movements in autism.
In this study, we used a medial–lateral dynamic postural control task with no constraints on the types of movements that participants use to complete the task. This allowed us to investigate the efficiency, variability, and accuracy of autistic individuals’ movements in the medial–lateral direction, as well as the postural control strategies that autistic individuals use to complete whole-body goal-directed tasks. By focusing on these measures, we can begin to ascertain which sensorimotor mechanisms are driving performance differences. For example, low efficiency in the path taken to a target implicates differences in motor planning and execution (Chern et al., 2010), high variability in movements implicates differences in on-line modification of movements in response to sensory feedback mechanisms (Adamovich, et al., 2001; Zheng et al., 2019), and low accuracy of an overall movement implicates differences in visuomotor integration of information about spatial locations of targets (Zheng et al., 2019). Consequently, these movement differences may indicate difficulty maintaining postural control during dynamic tasks, leading to increased risk of falls or fatigue (Maki & McIlroy, 1996; Swanenburg, et al., 2010; Zwicker et al., 2018) and decreased participation in activities (Cairney et al., 2010; Izadi-Najafabadi et al., 2019).
New developments in the fields of virtual reality and gaming have provided a wide range of opportunities for researchers to measure the efficiency of movements and movement strategies in highly-controlled, safe, and engaging tasks for children (Malihi et al., 2020). Prior work with autistic children has focused primarily on the use of virtual reality for the assessment and teaching of social skills (for review, see Miller & Bugnariu, 2016). Conversely, work with neurotypical and other neurodivergent populations (e.g., developmental coordination disorder, cerebral palsy, Parkinson’s disease) have used virtual reality as a tool for the assessment of movements (Canning et al., 2020; Gonsalves et al., 2015; Levac et al., 2010; Li et al., 2009; Prasertsakul et al., 2018; Ravi et al., 2017). In related work, researchers have begun using video games to distinguish between autistic and neurotypical children by examining body movement (Ardalan et al., 2019) and imitation (Tunçgenç et al., 2020) but virtual reality has not been widely utilized in this way, with a few notable exceptions (e.g., Greffou et al., 2012; Miller et al., 2019).
We have extended the use of virtual reality technology in autistic children by integrating it with motion capture, which has enabled us to precisely quantify and characterize differences in how autistic children make goal-directed, whole-body movements (Miller, Bugnariu, Patterson, Wijayasinghe, & Popa, 2017). Specifically, we use this method to examine goal-directed movement efficiency and variability, such as trial duration and path length (i.e., end-point trajectory). These indices of movement efficiency and variability have been used to measure differences in movements in pediatric and adult populations with neurotypical development (Levac et al., 2010; Sveistrup et al., 2008), cerebral palsy (Schneiberg et al., 2010), stroke (Longhi et al., 2016), Parkinson’s disease (Stylianou et al., 2011), and autism (Glazebrook et al., 2006). Using this innovative approach to measuring movement efficiency and variability differences between autistic and neurotypical children, we can begin to understand the unique pattern of movement challenges experienced by autistic children.
Current Study
In autism, there has been work on general gross motor skills and static postural control, but there is less work on dynamic, goal-directed, whole-body movements. In this study, we examined (1) accuracy, (2) spatial efficiency, and (3) variability of whole-body movements to a static target in autistic and neurotypical children using immersive virtual reality and motion-capture technology. We examined whether (1) autistic children could successfully complete whole-body medial–lateral movements to a static target. We hypothesized that (1) autistic children would be as successful as neurotypical children in completing this task. We examined (2) how spatially efficient autistic children’s movements and the amount of time autistic children used to complete the task compared to neurotypical children. We hypothesized that (2) autistic children would be less spatially efficient and use more time to complete the task than neurotypical children. We also examined the (3) variability in autistic children’s path length and how children adjusted their movements to the target based on the distance from their starting position to the target location. We hypothesized that (3) autistic children’s movements would be more variable than neurotypical children’s movements.
Method
Participants
Potential autistic or neurotypical participants were recruited via recruitment flyers and word of mouth for this study from a major metropolitan area in the state of Texas in the United States. Potential participants with a current or prior diagnosis of a genetic or neurological disorder (not including autism), brain injury, meningitis, structural brain abnormality, motion sickness, neurofibromatosis, seizure disorder, head injury or concussion with loss of consciousness, psychiatric diagnosis (not including anxiety or depression), movement disorder (e.g., cerebral palsy), or oculomotor disorder were excluded from participation, as well as those who were currently taking benzodiazepines or antipsychotics. Participants who scored < 70 in the nonverbal domain of the WASI-II (Wechsler, 2011) were also excluded. Neurotypical individuals that met any of the exclusion criteria for the autistic group, had a score of > 7 on the Social Communication Questionnaire (Rutter et al., 2003a), or had any first-degree relatives with a diagnosis of Autism Spectrum Disorder, Asperger’s Syndrome, or related developmental disorder were excluded from participation. For the autistic group, the participant’s guardian confirmed that their child had a prior diagnosis of Autism Spectrum Disorder or Asperger’s syndrome from an educational or healthcare professional according to DSM-IV or DSM-V criteria. This community diagnosis was confirmed by the research team using the ADOS-2 (Lord et al., 2012) and ADI-R (Rutter et al., 2003b) prior to experimental testing. This study was approved by the North Texas Regional Institutional Review Board in accordance with U.S. Federal Policy for the Protection of Human Subjects. Prior to participation, all guardians gave informed consent, and all minor participants gave assent.
Safe Zone Task
The Safe Zone task involves a blue ball (29.21 cm in diameter) controlled by the participant and a green safe zone (a green rectangle, 31.75 cm across) projected onto an immersive virtual reality screen (Fig. 1). The task requires a participant to move their blue ball (controlled by a marker placed on the participant’s 7th cervical vertebrae, C7) in the mediolateral direction to a safe zone within 3 s. Participants’ movements were amplified 3.65 times that of the screen distance (e.g., a participant moving from the center of the screen to the safe zone at 97.85 cm would need to move their body 26.8 cm to overlap the safe zone). The safe zone appeared on the left or right side of the screen at either 48.93 cm (requiring the participant to move 13.4 cm from center) or 97.85 cm (requiring the participant to move 26.8 cm) from the center of screen in a random order. Each trial began with the participant standing in the middle of the screen and a fixation cross at the center of the screen for 600 ms. Following the cross, the trial began when the user-controlled ball and the safe zone appeared simultaneously on the screen (Fig. 1A). Participants were instructed to move their body to get their ball into the safe zone (For full task instructions, see Appendix 1). The participant could lean and/or step to move their ball to the safe zone. Participants were also instructed to return to the middle of the screen and stand on red foot markers taped to the floor approximately shoulder-width apart. To successfully complete the task, or score a “hit”, participants needed to move at least 70% of their ball into the safe zone for 200 ms continuously (Fig. 1D). If participants did not reach the safe zone or were unable to keep their ball in the safe zone for 200 ms continuously within 3 s, the safe zone disappeared, and the trial was scored as unsuccessful. Participants completed for 16 trials of this task.
Fig. 1.
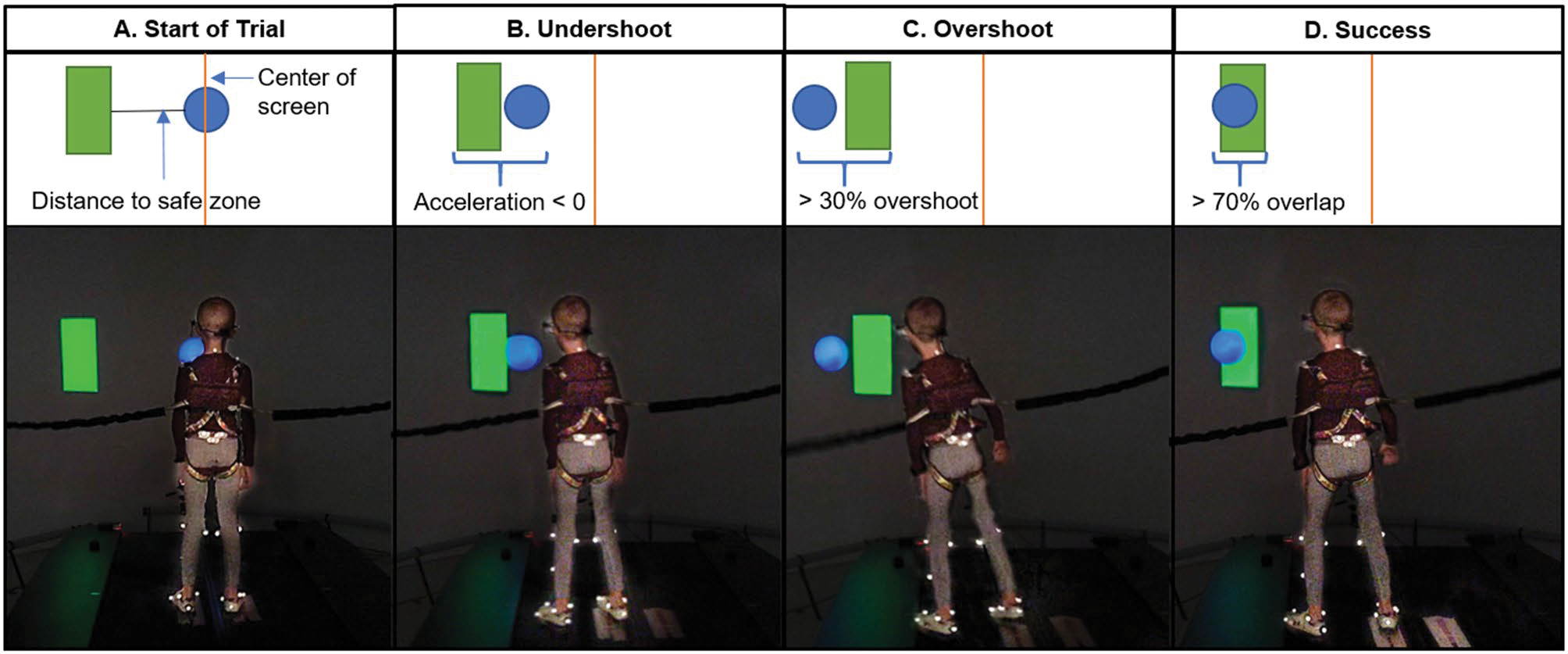
Participant moving their user-controlled blue ball into the safe zone (green rectangle). A The participant starts in the center of the screen and distance is calculated as the distance from the participant’s location to the center of the safe zone. B An undershoot is counted when a participant begins moving and their acceleration drops below 0 before reaching the safe zone. C An overshoot is counted if the participant passes the safe zone by more than 30% of the blue ball diameter. D Success is counted if the participant moves 70% of their blue ball into safe zone for 200 ms within 3 s
Although the safe zones always appeared at a specific distance from the center of the screen, the participants did not always start on the red feet markers or exactly at the center of the screen creating variable distances from the participants’ starting positions and the locations of the safe zone. To ensure that movements on trials were comparable, trials were only included in the analysis if the participant started a minimum of 6.7 cm and a maximum of 35 cm from the safe zone location and were moving less than 50% of their maximum velocity for that trial. Of the 448 possible trials, 318 trials (70.98%) met the inclusion criteria (Autistic: 63.7%, Neurotypical: 80.7%).
The Safe Zone task was part of a battery of tasks implemented in the V-Gait Computer-Assisted Rehabilitation Environment (CAREN; Motek Medical, Amsterdam, Netherlands). The data were collected using a 12-camera 120 Hz infrared motion-capture system (Motion Analysis Corporation, Santa Rosa, CA, USA). The motion-capture system records the three-dimensional position of reflective markers placed on anatomical locations (e.g., C7) on a participant’s body within 0.5 mm of accuracy. The position data for the C7 marker was initially processed in Cortex Software Suite (Motion Analysis Corporation, Santa Rosa, CA, USA) and a 6 Hz low pass Butterworth filter was applied to this data. Variables of interest were calculated using a custom MATLAB script (The Mathworks, Inc., USA). For a full list of marker placement locations, see Supplementary Table 1. For more details on the apparatus, see Miller et al. (2017).
Variables of Interest
Trial duration was calculated as the duration of time between when the safe zone appeared to the time that the participant scored a hit. Trial duration was only calculated for successful trials with a possible range of 0.2 to 2.9 s because failed trials were fixed at 3 s.
Path efficiency is the ratio of the actual path length to the optimal path length. Actual path length was calculated by summing the three-dimensional Euclidean distance (i.e., mediolateral, anteroposterior, longitudinal axis) between the position of the marker on the 7th cervical vertebrae (C7) at each consecutive frame within a single trial. Optimal path length was calculated by measuring the nearest distance between the three-dimensional position of the participant’s C7 marker at the start of the trial and the three-dimensional center of the safe zone (Fig. 1A). Smaller ratios are indicative of better path efficiency. Path efficiency indicates the overall spatial efficiency of the movement during the task.
Path variability is the ratio of the standard deviation of each participant’s three-dimensional path length to near and far safe zones (determined via median split) to the mean three-dimensional path length distance of each participant’s movements to near and far safe zones multiplied by 100. Path variability is a standardized measure of variation of three-dimensional path length distances.
Trials were coded as overshoots when a participant moved their ball through the safe zone with more than 30% of the ball extended beyond the opposite side of the safe zone (Fig. 1C; e.g., moving from the left side, passing through the safe zone, and continuing to the right). Overshoots indicate an overestimate of the amount of movement necessary to reach the safe zone.
Trials were coded as undershoots when a participant’s movement acceleration dropped below 0 and then increased above 0 again prior to reaching the safe zone (Fig. 1B). Undershoots indicate an underestimate of the amount of movement necessary to reach the safe zone. Undershoots that resulted from the change in direction required to come back to the safe zone following an overshoot were excluded from analysis.
Statistical Analyses
Generalized linear and linear mixed effects models were used to regress success, trial duration, overshoots, undershoots, path efficiency, and path variability on group (Autistic, Neurotypical), age (continuous), distance from the safe zone (continuous), screen side (left, right), and/or trial number (1–16) with a random intercept of participant (R Core Team, 2021; Bates et al., 2015). For the linear mixed effects model, model assumptions of linearity and homoskedasticity of the residuals were visually evaluated using residual plots and the model assumption of normality of residuals was visually evaluated using Q-Q plots. If these assumptions were violated using a linear model, the data was modeled with binomial, poisson, or gamma distributions with logit or log links. For count data, (e.g., overshoots, undershoots), the models used poisson distributions which assume a lack of overdispersion. The tests for overdispersion were not significant for either model (ps > .05). We conducted χ2-tests for fixed effects of generalized linear and linear mixed effects models (Fox & Weisberg, 2019). Alpha was set at p < .05. Estimated marginal means were reported in response scale, β weights and 95% confidence intervals (CI) were reported in their link scale or odds ratio, if applicable. For analyses of trial duration, path efficiency, path variability, overshoots, and undershoots, only trials that ended in a successful hit were included (282 trials, 62.95% of all possible trials).
Results
Participant Characteristics
We tested 16 autistic children (Male = 14, Female = 2, Mage = 10.63 years, SDage = 1.82 years, Rangeage 7–13 years) and 12 neurotypical children (Male = 7, Female = 5, Mage = 9.25 years, SDage = 1.91 years, Rangeage 7–13 years). Participants were Asian (n = 3), Black (n = 4), and White (n = 21). Three participants were Hispanic. See Table 1 for clinical characteristics of the sample.
Table 1.
Clinical characteristics of the participants
| Variable | Group | Count | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Age | Autistic | 16 | 10.63 | 1.82 | 11 | 7 | 13 |
| Neurotypical | 12 | 9.25 | 1.91 | 9 | 7 | 13 | |
| MABC-2 total | Autistic | 16 | 35.19 | 12.94 | 32 | 20 | 69 |
| Neurotypical | 12 | 72.25 | 18.52 | 79 | 34 | 90 | |
| WASI-II non-verbal IQ | Autistic | 15 | 98.40 | 15.22 | 99 | 71 | 128 |
| Neurotypical | 12 | 113.50 | 9.09 | 114 | 99 | 127 | |
| SCQ | Autistic | 16 | 21.38 | 7.83 | 23 | 5 | 30 |
| Neurotypical | 12 | 2.75 | 1.71 | 2.5 | 1 | 7 | |
| ADOS-2 comparison | Autistic | 16 | 8.63 | 1.36 | 9 | 6 | 10 |
ADOS-2 Autism Diagnostic Observation Schedule, MABC-2 Movement Assessment Battery for Children, SCQ social communication questionnaire, WASI-II Weschler Abbreviated Scale of Intelligence
Success
As expected, both autistic and neurotypical participants were capable of performing the task, successfully scoring hits on most valid trials (Autistic: 89.96% success rate, Neurotypical: 88.39% success rate). Differences in success were due to more difficulty at increased distance and participants learning to move to the safe zone more successfully in later trials. A generalized linear mixed effects model using a binomial distribution with a logit link was used to regress success onto fixed factors of group, age, distance, screen side, and trial number with a random intercept by participant. This analysis showed main effects of distance to the safe zone (Wald χ21 = 7.13, p = .008; Odds Ratio 0.93, 95% CI 0.88, 0.98; Supplementary Fig. 1) and trial number (Wald χ21 = 12.98, p < .001; Odds Ratio 1.18, 95% CI 1.08, 1.29; Supplementary Fig. 2). Participants were less successful as the distance to the safe zone increased. Participants were more successful as the trial number increased (i.e., later trials compared to earlier trials). There were no group differences in success (p > .05).
Trial Duration
Autistic participants took longer to complete the task on successful trials. A linear mixed effects model was used to regress trial duration of successful trials onto group, age, and distance to the safe zone with a random intercept by participant. This analysis showed main effects of group (Wald χ21 = 6.98, p = .008; β = 0.24, 95% CI 0.06, 0.42; Fig. 2), age (Wald χ21 = 10.31, p = .001; β = − 0.08, 95% CI − 0.12, − 0.03), and distance to the safe zone (Wald χ21 = 59.75, p < .001; β = 0.03, 95% CI 0.02, 0.04; Supplementary Fig. 3). Autistic participants (MAutistic = 1.94 s, SEAutistic = 0.06 s) used more time to get to the safe zone compared to neurotypical participants (MNeurotypical = 1.70 s, SENeurotypical = 0.07 s). Both autistic and neurotypical participants used more time to get to the safe zone as the distance to the safe zone increased. Older participants in both groups used less time to get to the safe zones than younger participants.
Fig. 2.
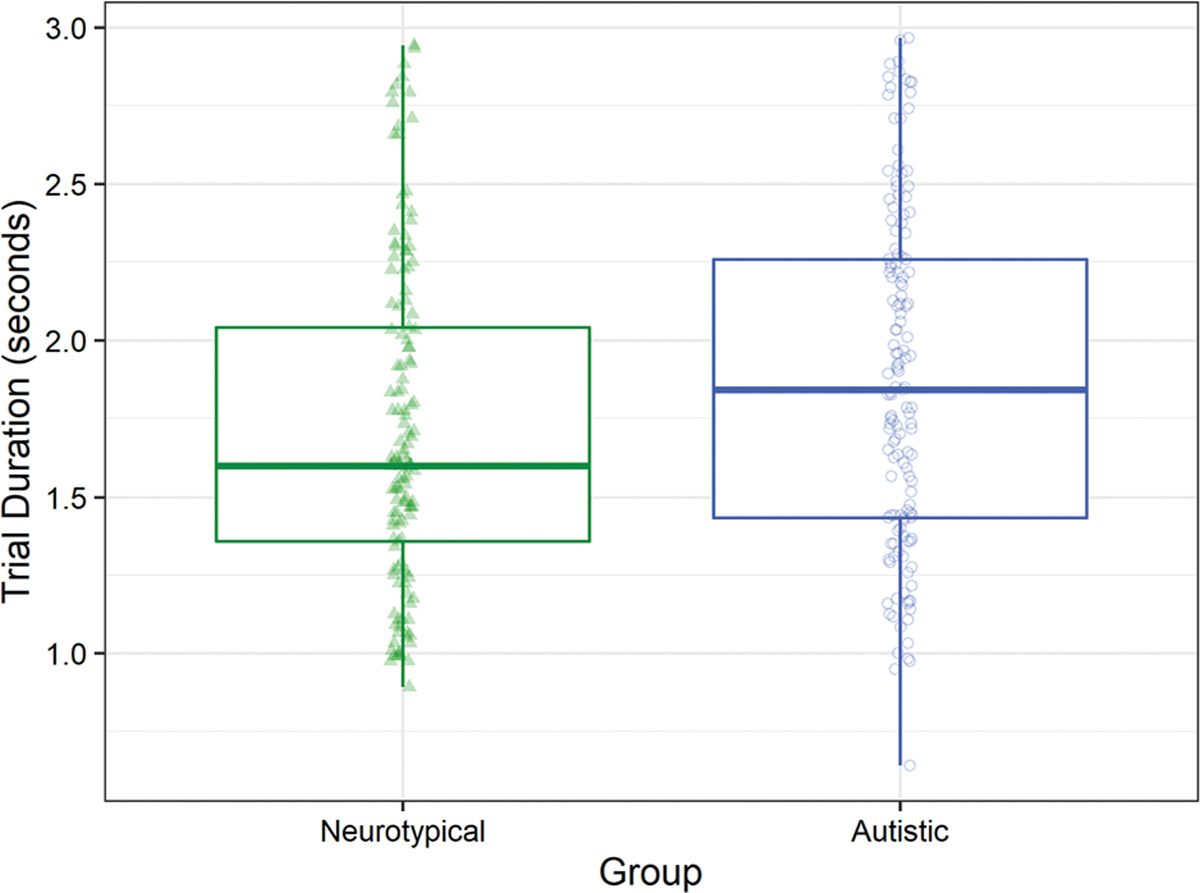
Autistic children used more time to move to the safe zone than neurotypical children
Path Efficiency
Autistic participants made less efficient movements compared to neurotypical participants. Additionally, participants in both groups made more efficient movements to the safe zone as distance increased. A generalized linear mixed effects model using a Gamma distribution with a log link was used to regress the path efficiency of successful trials onto fixed factors of group, age, and distance with a random intercept by participant. This analysis showed a main effect of group (Wald χ21 = 3.89, p = .048; β = 0.23, 95% CI 0.001, 0.47; Fig. 3), such that autistic participants (MAutistic = 1.79, SEAutistic = 0.13) used less efficient paths than neurotypical participants (MNeurotypical = 1.42, SENeurotypical = 0.13) as indicated by higher path efficiency scores. There was also a main effect of distance to the safe zone (Wald χ21 = 86.64, p < .001; β = − 0.02, 95% CI − 0.03, − 0.02; Supplementary Fig. 4), such that both groups took more efficient paths to the safe zone as the distance to the safe zone increased as indicated by lower path efficiency scores.
Fig. 3.
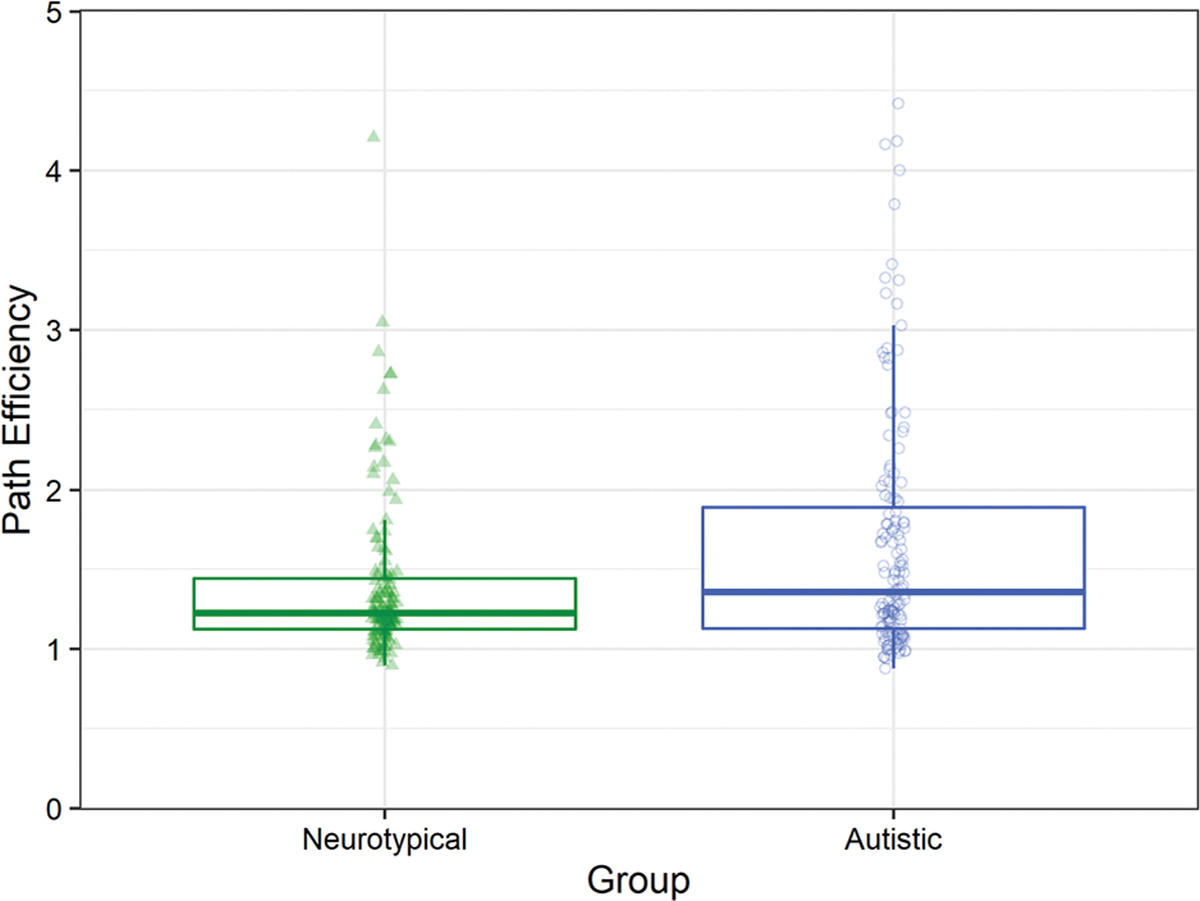
Autistic children were less efficient than neurotypical children. 5 (1.8% of trials, 3 Neurotypical, 2 Autistic) outlier points were removed to enhance data visualization. See Supplementary Fig. 6 to view the full data
Path Variability
Both autistic and neurotypical participants made more variable movements to the near safe zones compared to far safe zones. A generalized linear mixed effects model using a Gamma distribution and a log link was used to regress the coefficient of variability of the movement path of successful trials onto fixed factors of group, distance, and age with a random intercept by participant. This analysis showed a main effect of distance to the safe zone (Wald χ21 = 18.25, p < .001; β = − 0.45, 95% CI − 0.65, − 0.24; Fig. 4), such that participants in both groups had larger coefficients of variability for the movements to near safe zones (MNear = 35.00, SENear = 3.03) compared to movements to far safe zones (MFar = 22.40, SEFar = 2.17).
Fig. 4.
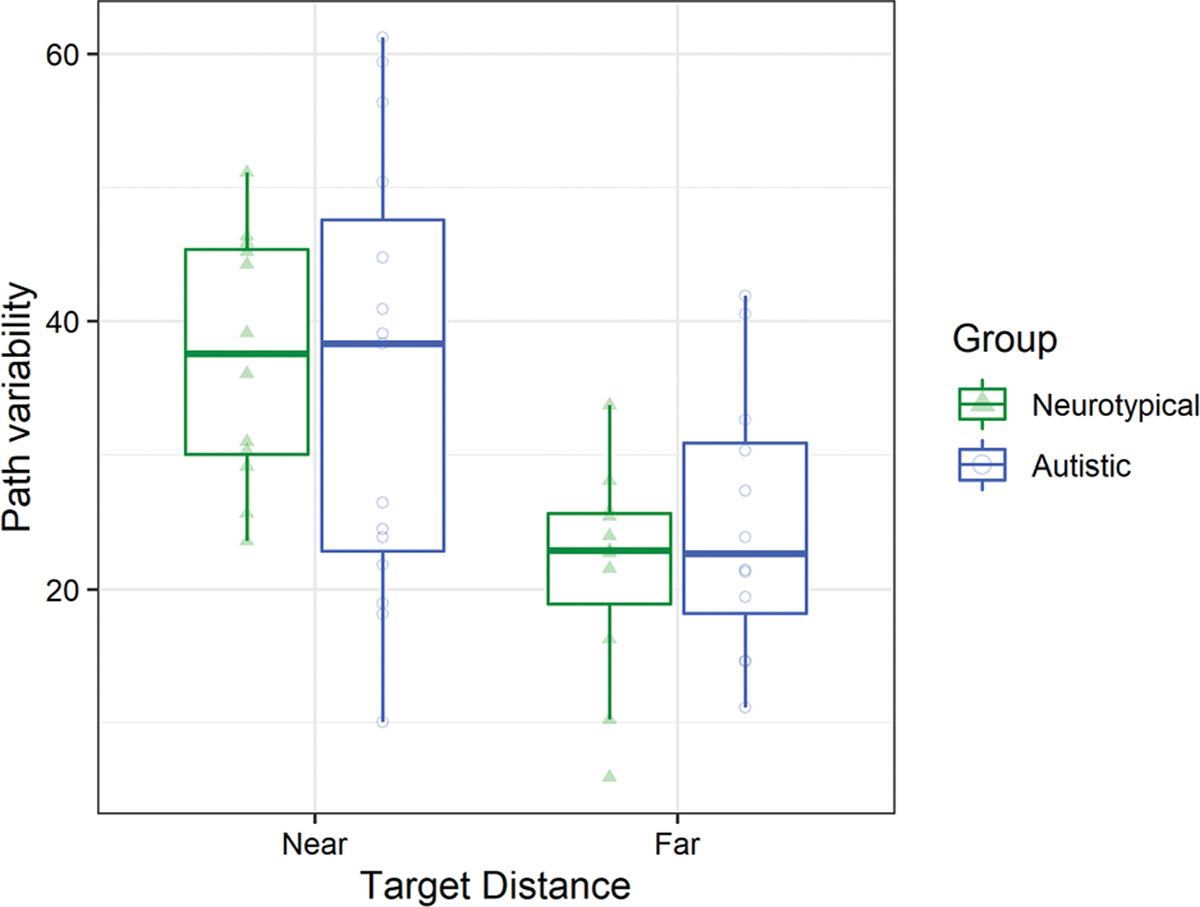
In both groups, children had more variable paths to the safe zone at near distances compared to far distances. Hinges correspond to the first and third quartiles. Whiskers extend to the largest value within 1.5 times the interquartile range
Overshoots
Neurotypical participants made fewer overshoots to safe zones that were further away than autistic participants. A generalized linear mixed effects model using a Poisson distribution with a log link was used to regress number of overshoots on successful trials onto fixed factors of group, age and distance with a random intercept by participant. This analysis showed main effects of group (Wald χ21 = 6.71, p = .010; β = 0.65, 95% CI 0.16, 1.14; MAutistic = 0.48, SEAutistic = 0.07; MNeurotypical = 0.26, SENeurotypical = 0.05) and distance to the safe zone (Wald χ21 = 16.47, p < .001; β = − 0.06, 95% CI − 0.09, − 0.03). These main effects were qualified by a Group X Distance interaction (Wald χ21 = 7.23, p = .007; β = 0.08, 95% CI 0.2, 0.13; Supplementary Fig. 5). Neurotypical participants made fewer overshoots than autistic participants as distance increased.
Undershoots
Neurotypical participants made more undershoots to safe zones compared to autistic participants. A generalized linear mixed effects model using a Poisson distribution with a log link was used to regress number of undershoots on successful trials onto fixed factors of group, age, and distance with a random intercept by participant. This analysis showed a main effect of distance to the safe zone (Wald χ21 = 26.11, p < .001; β = 0.06, 95% CI 0.04, 0.08), but there was not a significant main effect of Group (Wald χ21 = 0.34, p = .561; β = 0.11, 95% CI − 0.25, 0.46; MAutistic = 0.60, SEAutistic = 0.07, MNeurotypical = 0.53, SENeurotypical = 0.07). These main effects were qualified by a Group X Distance interaction (Wald χ21 = 6.57, p = .010; β = − 0.06, 95% CI − 0.10, − 0.01; Supplementary Fig. 6). Neurotypical participants made more undershoots as distance increased compared to autistic participants.
Discussion
In this study of goal-directed whole-body movements to a static target, we observed that the movements of autistic children were markedly less efficient than those of their neurotypical peers, both in terms of timing and path length. Although children in both groups were able to complete this task (> 88% of trials scored as a hit), autistic children took an average of 240 ms longer to complete each trial compared to neurotypical children. At first glance, this increase in trial duration may seem negligible. However, when considered in the context of how many and how quickly a child performs goal-directed movements in their daily lives, a 10–20% increase in time-to-completion could contribute, at least in part, to physical and mental health difficulties seen in similar neurodevelopmental conditions with motor symptoms, such as developmental coordination disorder (Hendrix et al., 2014; Tamplain & Miller, 2021). This difference may also reflect signaling delays in visual processing and visuomotor integration (Neufeld et al., 2020; Wang et al., 2019), or in activation of muscle groups needed to initiate or modify movement as found in other neurodevelopmental conditions (e.g., DCD, Fong et al., 2013).
Autistic children also exhibited differences in the path efficiency of their movements compared to neurotypical children. Autistic children moved toward the safe zone via paths that diverged more from the optimal path (i.e., a straight line) compared to neurotypical children. Combined with increased trial durations, autistic children not only took longer to reach the safe zone, but also moved more to reach the same location compared to neurotypical children. These within-movement inefficiencies in autism implicate motor planning and motor execution difficulties, perhaps again driven by differences in visuomotor integration. These results extend similar findings from movement planning and execution during reaching in autistic adults. Across a series of studies, Glazebrook and colleagues have demonstrated that autistic adults make less efficient arm movements and show differences in motor planning (Glazebrook et al, 2006, 2008; Zheng et al., 2019). In terms of activities of daily living, this suggests that autistic children may not only take longer to complete individual tasks, but may also expend additional energy in doing so. This may, in turn, lead to a higher level of exertion and fatigue for autistic children than their neurotypical peers when completing the same tasks. Higher levels of exertion and fatigue could discourage autistic children from trying similar activities in the future.
To identify potential factors influencing path inefficiency, we examined the path variability in children’s movements to near and far safe zone locations. Interestingly, there were not significant differences in the path variability of movements between autistic and neurotypical children. This finding adds to the mixed findings indicating more variable movements in certain contexts in autistic individuals (Dufek et al., 2018; Parma & de Marchena, 2016) and more research needs to be done to determine whether and when the variability of autistic individuals movements may differ from neurotypical individuals. This indicates that the inefficiencies in children’s movements to a safe zone are not due entirely to inconsistencies from movement to movement, but also differences that occur within each movement. Furthermore, although autistic children did not show significant differences in their path variability, they had a higher rate of excluded trials compared to neurotypical children, likely due to difficulty getting their bodies back to the center of the screen before the next trial began. This indicates that autistic children are not only less efficient within a single trial, but also across a series of trials. In other words, performing consecutive tasks without sufficient time between them may also be more difficult for autistic children compared to neurotypical children.
There were, however, differences in path variability for near and far safe zone distances. Children’s movements to safe zones at near distances were significantly more variable than their movements to safe zones at far distances. This difference in variability may be due to the amount of time and distance available to update an inaccurate motor plan. If a child executes a poor motor plan to a near target, there is less time and distance for that child to adjust their movement to reach the target compared to a far target. This limited time and distance to adjust their movement may cause them to make more adjustments after the execution of a movement to a near target causing greater variability overall. Further research is needed to delineate the effects of motor planning and execution in mediolateral whole body movements to far and near targets.
We also considered strategies used to reach the safe zone as a potential factor influencing efficiency. Examining the number of overshoots and undershoots that autistic and neurotypical children used to reach the safe zone, a distinct pattern emerged. Neurotypical children made more undershoots to further safe zone distances and more overshoots to nearer safe zones distances. Conversely, autistic children made similar numbers of overshoots and undershoots to safe zones at all distances. This modulation of strategy seen in neurotypical children may reflect a prioritization of postural control for the purpose of maintaining balance. Given that children started in the center for trials included in the analyses with their feet roughly shoulder width apart, overshooting safe zones at far distances would potentially place the body’s center of mass outside of the limits of postural stability, meaning an undershoot of the safe zones at further distances is a safer strategy. For safe zones at a near distance, however, there is likely no potential for a negative postural stability consequence for overshooting a safe zone as the body’s center of mass would still be within children’s limits of stability. The lack of consistent modulation observed in autistic children may indicate either an inability to accurately estimate limits of their postural stability or an inability to change strategy based on perceived challenge to their postural stability. These findings are aligned with work by Mosconi and colleagues, in which autistic individuals did not effectively use feedforward information when executing and modulating grip force, making less accurate initial movements and often overshooting targets (Mosconi et al., 2015; Wang et al., 2015). Our results add to prior work, which generally implicated differences in visuomotor integration, by also highlighting reduced learning and refinement of motor strategies in autism across trials where targets recurred in the same locations multiple times.
The current study adds to the growing literature indicating that immersive virtual reality can be used effectively for research with autistic children (Malihi et al., 2020; Miller & Bugnariu, 2016). Further, the precise measurements of movements in immersive virtual reality environments can be leveraged to not only determine whether the motor skills of autistic children are different from those of neurotypical children, but how their individual movements are different. This opens the door for a wide array of potential questions that can be answered regarding how differences in the movements of autistic children impact their daily life in varying contexts.
Implications
Overall, autistic children used more time to complete a goal-directed whole-body movement and were less efficient when making these movements compared to neurotypical children. The increased time and energy necessary to complete goal-directed whole body movements may have a negative impact on the functional abilities of autistic children. Considering our results in conjunction with prior findings of upper-extremity motor inefficiencies (Glazebrook et al., 2006), it is possible that complex ADLs requiring both whole-body and upper-extremity movements (e.g., many self-care tasks) may be even more taxing for autistic individuals. The additive impact of these inefficiencies across a self-care routine that includes dressing, eating, brushing teeth, and gathering personal items would be substantial in terms of both time and energy.
Given these differences, it is imperative that autistic children receive adequate therapeutic intervention to improve their motor skills. Unfortunately, few autistic people receive intervention that would improve whole-body motor skills, with only 5% of autistic individuals receiving physical therapy and only 37.5% receiving occupational therapy (Zablotsky et al., 2015). Further, many of these services are limited in scope. In the case of physical therapy, the focus is often on simply meeting early motor milestones (e.g., crawling, standing, walking). In the case of occupational therapy, goals are typically focused on sensory processing or handwriting. To help improve functional outcomes and quality of life for autistic children, policy-makers need to substantially increase access to physical and occupational therapy for autistic individuals, and researchers and clinicians need to develop interventions tailored specifically to the unique movement challenges experienced by this population.
Supplementary Material
Acknowledgments
Out of respect for preferences expressed by many autistic self-advocates in our studies and in the community, we have chosen to use identity-first (rather than person-first) language throughout this manuscript. In doing so, it is not our intention to diminish or invalidate the preferences or perspectives of those who prefer person-first language. We continue to welcome feedback on ways that we can effectively partner with the autistic community to advocate for respect, acceptance, inclusion, and representation in research. The authors would also like to acknowledge our funding sources, National Science Foundation (SMA-1514495), National Institute of Clinical and Translational Sciences (KL2-TR001103, UL1-TR001105), and National Institute of Mental Health (K01-MH107774).
Appendix 1
Participant Task Instructions
Participants heard a recording read the following instructions as they were projected on to the screen with images of the objects they describe:
Look down at your feet. Do you see the red footprints? They look like this < → Image of red footprints>. Put your feet on the red footprints
If you move during the game, put your feet back on the footprints when you see this < → Image of the cross >.
Try to always keep your feet on the footprints!
In this game, you will see a blue ball on the screen. This is your ball. <Image of blue ball >. When you move your body, your ball will move too.
This is a green safe zone. <Image of green safe zone>. Move your body to get your ball in the green safe zone. Move as fast as you can! Sometimes the safe zone is in a different place. Try to stay in the safe zone!
If participants did not understand the instructions, they could ask the proctor any questions before the task began.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10803-022-05523-0.
References
- Adamovich SV, Archambault PS, Ghafouri M, Levin MF, Poizner H, & Feldman AG (2001). Hand trajectory invariance in reaching movements involving the trunk. Experimental Brain Research, 138(3), 288–303. 10.1007/s002210100694 [DOI] [PubMed] [Google Scholar]
- Adolph KE (2008). Learning to move. Current Directions in Psychological Science, 17, 213–218. 10.1111/j.1467-8721.2008.00577.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph KE, & Robinson SR (2013). The road to walking: what learning to walk tells us about development. In Zelazo PD (Ed.), The Oxford Handbook of Developmental Psychology (Vol. 1, pp. 403–443). Oxford University Press. 10.1093/oxfordhb/9780199958450.013.0015 [DOI] [Google Scholar]
- American Psychiatric Association. (2013). Autism Spectrum Disorder. In Diagnostic and Statistical Manual of Mental Disorders. (5 ed.). American Psychiatric Association. 10.1176/appi.books.9780890425596. [DOI] [Google Scholar]
- Ardalan A, Assadi AH, Surgent OJ, & Travers BG (2019). Whole-body movement during videogame play distinguishes youth with autism from youth with typical development. Scientific Reports, 9(1), 20094. 10.1038/s41598-019-56362-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bhat AN (2020). Is motor impairment in Autism Spectrum Disorder (ASD) distinct from Developmental Coordination Disorder (DCD)? A report from the SPARK study. Physical Therapy, 100(4), 633–644. 10.1093/ptj/pzz190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AN, Landa RJ, & Galloway JC (2011). Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Physical Therapy, 91(7), 1116–1129. 10.2522/ptj.20100294 [DOI] [PubMed] [Google Scholar]
- Bojanek EK, Wang Z, White SP, & Mosconi MW (2020). Postural control processes during standing and step initiation in autism spectrum disorder. Journal of Neurodevelopmental Disorders, 12(1), 1–13. 10.1186/s11689-019-9305-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney J, Hay JA, Veldhuizen S, Missiuna C, & Faught BE (2010). Developmental coordination disorder, sex, and activity deficit over time: A longitudinal analysis of participation trajectories in children with and without coordination difficulties. Developmental Medicine & Child Neurology, 52(3), e67–e72. 10.1111/j.1469-8749.2009.03520.x [DOI] [PubMed] [Google Scholar]
- Canning CG, Allen NE, Nackaerts E, Paul SS, Nieuwboer A, & Gilat M (2020). Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nature Reviews Neurology. 10.1038/s41582-020-0370-2 [DOI] [PubMed] [Google Scholar]
- Carment L, Khoury E, Dupin L, Guedj L, Bendjemaa N, Cuenca M, & Amado I (2020). Common vs. distinct visuomotor control deficits in Autism Spectrum Disorder and Schizophrenia. Autism Research. 10.1002/aur.2287 [DOI] [PubMed] [Google Scholar]
- Chen LC, Su WC, Ho TL, Lu L, Tsai WC, Chiu YN, & Jeng SF (2019). Postural control and interceptive skills in children with Autism Spectrum Disorder. Physical Therapy. 10.1093/ptj/pzz084 [DOI] [PubMed] [Google Scholar]
- Chern JS, Lo CY, Wu CY, Chen CL, Yang S, & Tang FT (2010). Dynamic postural control during trunk bending and reaching in healthy adults and stroke patients. American Journal of Physical Medicine & Rehabilitation, 89(3), 186–197. 10.1097/PHM.0b013e3181c56287 [DOI] [PubMed] [Google Scholar]
- Cook J (2016). From movement kinematics to social cognition: The case of autism. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1693), 20150372. 10.1098/rstb.2015.0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovitch M, Stein N, Koren G, & Friedman BC (2018). Deviations from typical developmental trajectories detectable at 9 months of age in low risk children later diagnosed with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 48(8), 2854–2869. 10.1007/s10803-018-3549-2 [DOI] [PubMed] [Google Scholar]
- Doumas M, McKenna R, & Murphy B (2016). Postural control deficits in Autism Spectrum Disorder: The role of sensory integration. Journal of Autism and Developmental Disorders, 46(3), 853–861. 10.1007/s10803-015-2621-4 [DOI] [PubMed] [Google Scholar]
- Dufek JS, Harry JR, Eggleston JD, & Hickman RA (2018). Walking mechanics and movement pattern variability in monozygotic twins with autism Spectrum disorder. Journal of Developmental and Physical Disabilities, 30(6), 793–805. 10.1152/jn.00647.2015 [DOI] [Google Scholar]
- Fears NE, Palmer SA, & Miller HL (2022). Motor skills predict adaptive behavior in autistic children and adolescents. Autism Research. 10.1002/aur.2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A, Engel C, Geist R, Lillie K, Lutman S, & Travers BG (2018). Brief report: Postural balance and daily living skills in children and adolescents with autism. Journal of Autism and Developmental Disorders, 48(9), 3210–3215. 10.1007/s10803-018-3558-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong SS, Ng SS, & Yiu BP (2013). Slowed muscle force production and sensory organization deficits contribute to altered postural control strategies in children with developmental coordination disorder. Research in Developmental Disabilities, 34(9), 3040–3048. 10.1016/j.ridd.2013.05.035 [DOI] [PubMed] [Google Scholar]
- Fournier KA, Hass CJ, Naik SK, Lodha N, & Cauraugh JH (2010). Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders, 40(10), 1227–1240. 10.1007/s10803-010-0981-3 [DOI] [PubMed] [Google Scholar]
- Fox J, & Weisberg S (2019). An R companion to applied regression (3rd ed.). Sage. [Google Scholar]
- Fulceri F, Grossi E, Contaldo A, Narzisi A, Apicella F, Parrini I, & Muratori F (2019). Motor skills as moderators of core symptoms in Autism Spectrum Disorders: Preliminary data from an exploratory analysis With artificial neural networks. Frontiers in Psychology, 9, 2683. 10.3389/fpsyg.2018.02683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook CM, Elliott D, & Lyons J (2006). A kinematic analysis of how young adults with and without autism plan and control goal-directed movements. Motor Control, 10, 244–264. 10.1123/mcj.10.3.244 [DOI] [PubMed] [Google Scholar]
- Glazebrook CM, Elliott D, & Szatmari P (2008). How do individuals with autism plan their movements? Journal of Autism and Developmental Disorders, 38(1), 114–126. 10.1007/s10803-007-0369-1 [DOI] [PubMed] [Google Scholar]
- Gonsalves L, Campbell A, Jensen L, & Straker L (2015). Children with Developmental Coordination Disorder play active virtual reality games differently than children with typical development. Physical Therapy, 95(3), 360–368. 10.2522/ptj.20140116 [DOI] [PubMed] [Google Scholar]
- Grace N, Enticott PG, Johnson BP, & Rinehart NJ (2017). Do handwriting difficulties correlate with core symptomology, motor proficiency and attentional behaviours? Journal of Autism and Developmental Disorders, 47(4), 1006–1017. 10.1007/s10803-016-3019-7 [DOI] [PubMed] [Google Scholar]
- Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, & Baird G (2009). Impairment in movement skills of children with autistic spectrum disorders. Developmental Medicine & Child Neurology, 51(4), 311–316. 10.1111/j.1469-8749.2008.03242.x [DOI] [PubMed] [Google Scholar]
- Greffou S, Bertone A, Hahler EM, Hanssens JM, Mottron L, & Faubert J (2012). Postural hypo-reactivityin autism is contengent on development and visual environment: A fully immersive virtual reality study. Journal of Autism and Developmental Disorders, 42, 961–970. 10.1007/s10803-011-1326-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix CG, Prins MR, & Dekkers H (2014). Developmental coordination disorder and overweight and obesity in children: A systematic review. Obesity Reviews, 15(5), 408–423. 10.1111/obr.12137 [DOI] [PubMed] [Google Scholar]
- Hilton CL, Zhang Y, Whilte MR, Klohr CL, & Constantino J (2012). Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism, 16(4), 430–441. 10.1177/1362361311423018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadi-Najafabadi S, Ryan N, Ghafooripoor G, Gill K, & Zwicker JG (2019). Participation of children with developmental coordination disorder. Research in Developmental Disabilities, 84, 75–84. 10.1016/j.ridd.2018.05.011 [DOI] [PubMed] [Google Scholar]
- Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, & Mostofsky SH (2006). Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. Journal of Autism and Developmental Disorders, 36(5), 613–621. 10.1007/s10803-006-0109-y [DOI] [PubMed] [Google Scholar]
- Jasmin E, Couture M, McKinley P, Reid G, Fombonne E, & Gisel E (2009). Sensori-motor and daily living skills of preschool children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39, 231–241. 10.1007/s10803-008-0617-z [DOI] [PubMed] [Google Scholar]
- LeBarton ES, & Iverson JM (2016). Associations between gross motor and communicative development in at-risk infants. Infant Behavior and Development, 44, 59–67. 10.1016/j.infbeh.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levac D, Pierrynowski MR, Canestraro M, Gurr L, Leonard L, & Neeley C (2010). Exploring children’s movement characteristics during virtual reality video game play. Human Movement Science, 29(6), 1023–1038. 10.1016/j.humov.2010.06.006 [DOI] [PubMed] [Google Scholar]
- Li W, Lam-Damji S, Chau T, & Fehlings D (2009). The development of a home-based virtual reality therapy system to promote upper extremity movement for children with hemiplegic cerebral palsy. Technology and Disability, 21(3), 107–113. 10.3233/tad-2009-0277 [DOI] [Google Scholar]
- Licari MK, Alvares GA, Varcin K, Evans KL, Cleary D, Reid SL, & Whitehouse AJO (2019). Prevalence of motor difficulties in Autism Spectrum Disorder: Analysis of a population-based cohort. Autism Research. 10.1002/aur.2230 [DOI] [PubMed] [Google Scholar]
- Lim YH, Partridge K, Girdler S, & Morris SL (2017). Standing postural control in individuals with Autism Spectrum Disorder: Systematic review and meta-analysis. Journal of Autism and Developmental Disorders, 47(7), 2238–2253. 10.1007/s10803-017-3144-y [DOI] [PubMed] [Google Scholar]
- Liu T, & Breslin CM (2013). Fine and gross motor performance of the MABC-2 by children with autism spectrum disorder and typically developing children. Research in Autism Spectrum Disorders, 7(10), 1244–1249. 10.1016/j.rasd.2013.07.002 [DOI] [Google Scholar]
- Lloyd M, MacDonald M, & Lord C (2013). Motor skills of toddlers with autism spectrum disorders. Autism, 17(2), 133–146. 10.1177/1362361311402230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhi M, Merlo A, Prati P, Giacobbi M, & Mazzoli D (2016). Instrumental indices for upper limb function assessment in stroke patients: A validation study. Journal of Neuroengineering and Rehabilitation, 13(1), 1–11. 10.1186/s12984-016-0163-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part I): Modules (pp. 1–4). Western Psychological Services. [Google Scholar]
- Maki BE, & McIlroy WE (1996). Postural control in the older adult. Clinics in Geriatric Medicine, 12(4), 635–658. 10.1016/S0749-0690(18)30193-9 [DOI] [PubMed] [Google Scholar]
- Malihi M, Nguyen J, Cardy RE, Eldon S, Petta C, & Kushki A (2020). Short report: Evaluating the safety and usability of head-mounted virtual reality compared to monitor-displayed video for children with autism spectrum disorder. Autism. 10.1177/1362361320934214 [DOI] [PubMed] [Google Scholar]
- Miller HL, Bugnariu N, Patterson RM, Wijayasinghe I, & Popa DO (2017). Development of a novel visuomotor integration paradigm by integrating a virtual environment with mobile eye-tracking and motion-capture systems. In 2017 International Conference on Virtual Rehabilitation (ICVR). 10.1109/ICVR.2017.8007481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HL, & Bugnariu NL (2016). Level of immersion in virtual environments impacts the ability to assess and teach social skills in Autism Spectrum Disorder. Cyberpsychology, Behavior, and Social Networking, 19, 246–256. 10.1089/cyber.2014.0682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HL, Caçola P, Sherrod G, Patterson RM, & Bugnariu NL (2019). Children with Autism Spectrum Disorder, Developmental Coordination Disorder, and typical development differ in characteristics of dynamic postural control: A preliminary study. Gait & Posture, 67, 9–11. 10.1016/j.gaitpost.2018.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HL, Sherrod GM, Mauk JE, Fears NE, Hynan LS, & Tamplain PM (2021). Shared features or co-occurrence? Evaluating symptoms of Developmental Coordination Disorder in children and adolescents with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 10.1007/s10803-020-04766-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Mohanty S, Greene RK, Cook EH, Vaillancourt DE, & Sweeney JA (2015). Feedforward and feedback motor control abnormalities implicate cerebellar dysfunctions in Autism Spectrum Disorder. Journal of Neuroscience, 35(5), 2015–2025. 10.1523/jneurosci.2731-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Dubey P, Jerath VK, Jansiewicz EM, Goldberg MC, & Denckla MB (2006). Developmental dyspraxia is not limited to imitation in children with autism spectrum disorders. Journal of the International Neuropsychological Society, 12(03), 314–326. 10.1017/s1355617706060437 [DOI] [PubMed] [Google Scholar]
- Neufeld J, Hagström A, Van’tWesteinde A, Lundin K, Cauvet É, Willfors C, & Bölte S (2020). Global and local visual processing in autism–a co-twin-control study. Journal of Child Psychology and Psychiatry, 61(4), 470–479. 10.1111/jcpp.13120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, & de Marchena AB (2016). Motor signatures in autism spectrum disorder: The importance of variability. Journal of Neurophysiology. 10.1152/jn.00647.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin C, Valagussa G, Mazzucchelli M, Gariboldi V, Cerri CG, Meroni R, & Piscitelli D (2020). Physiological profile assessment of posture in children and adolescents with autism spectrum disorder and typically developing peers. Brain Sciences, 10(10), 681. 10.3390/brainsci10100681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasertsakul T, Kaimuk P, Chinjenpradit W, Limroongreungrat W, & Charoensuk W (2018). The effect of virtual reality-based balance training on motor learning and postural control in healthy adults: A randomized preliminary study. Biomedical Engineering Online, 17(1), 124. 10.1186/s12938-018-0550-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura G, Fulceri F, Puglisi V, Masoni P, & Contaldo A (2020). Motor coordination impairment in children with autism spectrum disorder: A pilot study using Movement assessment Battery for children-2 checklist. Minerva Pediatrica, 72(1), 22–29. 10.23736/S0026-4946.16.04633-8 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Ravi DK, Kumar N, & Singhi P (2017). Effectiveness of virtual reality rehabilitation for children and adolescents with cerebral palsy: An updated evidence-based systematic review. Physiotherapy, 103(3), 245–258. 10.1016/j.physio.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Rodgers RA, Travers BG, & Mason AH (2019). Bimanual reach to grasp movements in youth with and without autism spectrum disorder. Frontiers in Psychology, 9, 2720. 10.3389/fpsyg.2018.02720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003a). The social communication questionnaire: Manual. Western Psychological Services. [Google Scholar]
- Rutter M, Le Couteur A, & Lord C (2003b). Autism diagnostic interview-revised. Western Psychological Services. [Google Scholar]
- Schneiberg S, McKinley P, Gisel E, Sveistrup H, & Levin MF (2010). Reliability of kinematic measures of functional reaching in children with cerebral palsy. Developmental Medicine & Child Neurology, 52(7), e167–e173. 10.1111/j.1469-8749.2010.03635.x [DOI] [PubMed] [Google Scholar]
- Stylianou AP, McVey MA, Lyons KE, Pahwa R, & Luchies CW (2011). Postural sway in patients with mild to moderate Parkinson’s disease. International Journal of Neuroscience, 121(11), 614–621. 10.3109/00207454.2011.602807 [DOI] [PubMed] [Google Scholar]
- Sun R, Hsieh KL, & Sosnoff JJ (2019). Fall risk prediction in multiple sclerosis using postural sway measures: A machine learning approach. Scientific Reports, 9(1), 1–7. 10.1038/s41598-019-52697-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveistrup H, Schneiberg S, McKinley PA, McFadyen BJ, & Levin MF (2008). Head, arm and trunk coordination during reaching in children. Experimental Brain Research, 188(2), 237–247. 10.1007/s00221-008-1357-1 [DOI] [PubMed] [Google Scholar]
- Swanenburg J, de Bruin ED, Uebelhart D, & Mulder T (2010). Falls prediction in elderly people: A 1-year prospective study. Gait & Posture, 31(3), 317–321. 10.1016/j.gaitpost.2009.11.013 [DOI] [PubMed] [Google Scholar]
- Tamplain P, & Miller HL (2021). What can we do to promote mental health among individuals with developmental coordination disorder? Current Developmental Disorders Reports, 8(1), 24–31. 10.1007/s40474-020-00209-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers BG, Bigler ED, Duffield TC, Prigge MDB, Froehlich AL, Lange N, & Lainhart JE (2017). Longitudinal development of manual motor ability in autism spectrum disorder from childhood to mid-adulthood relates to adaptive daily living skills. Developmental Science, 20, 1–15. 10.1111/desc.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunçgenç B, Pacheco C, Rochowiak R, Nicholas R, Rengarajan S, Zou E, & Mostofsky SH (2020). Computerised Assessment of Motor Imitation (CAMI) as a scalable method for distinguishing children with autism. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 10.1016/j.bpsc.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waelvelde H, Oostra A, Dewitte G, Van Den Broeck C, & Jongmans MJ (2010). Stability of motor problems in young children with or at risk of autism spectrum disorders, ADHD, and or developmental coordination disorder. Developmental Medicine and Child Neurology, 52(8), e174–178. 10.1111/j.1469-8749.2009.03606.x [DOI] [PubMed] [Google Scholar]
- Wang Z, Hallac RR, Conroy KC, White SP, Kane AA, Collinsworth AL, & Mosconi MW (2016). Postural orientation and equilibrium processes associated with increased postural sway in autism spectrum disorder (ASD). Journal of Neurodevelopmental Disorders, 8, 43. 10.1186/s11689-016-9178-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Magnon GC, White SP, Greene RK, Vaillancourt DE, & Mosconi MW (2015). Individuals with autism spectrum disorder show abnormalities during initial and subsequent phases of precision gripping. Journal of Neurophysiology, 113(7), 1989–2001. 10.1152/jn.00661.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang Y, Sweeney JA, Gong Q, Lui S, & Mosconi MW (2019). Resting-state brain network dysfunctions associated with visuomotor impairments in autism spectrum disorder. Frontiers in Integrative Neuroscience, 13, 17. 10.3389/fnint.2019.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2011). WASI-II: Wechsler abbreviated scale of intelligence. Pearson. [Google Scholar]
- Zablotsky B, Pringle BA, Colpe LJ, Kogan MD, Rice C, & Blumberg SJ (2015). Service and treatment use among children diagnosed with Autism Spectrum Disorders. Journal of Developmental & Behavioral Pediatrics, 36(2), 98–105. 10.1097/DBP.0000000000000127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Naiman ID, Skultety J, Passmore SR, Lyons J, & Glazebrook CM (2019). The impact of different movement types on motor planning and execution in individuals with autism spectrum disorder. Motor Control, 23(3), 398–417. 10.1123/mc.2017-0084 [DOI] [PubMed] [Google Scholar]
- Zwicker JG, Suto M, Harris SR, Vlasakova N, & Missiuna C (2018). Developmental coordination disorder is more than a motor problem: Children describe the impact of daily struggles on their quality of life. British Journal of Occupational Therapy, 81(2), 65–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


