Abstract
Diabetes mellitus, associated with α-glucosidase, has been considered as a chronic metabolic disorder, seriously affecting human health. Thus, searching natural α-glucosidase inhibitors and investigating their inhibition mechanism are urgently important. In this study, sixty-two essential oils (EOs), derived from aromatic plants, were found to exert different inhibition on α-glucosidase. The further study revealed that the most potent EOs against α-glucosidase were chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger with IC50 values of 3.02, 2.88, 7.37, 5.06, 5.32 and 7.40 μg/mL. Moreover, the inhibitory mechanism and kinetics studies found that chuan-xiong and sacha inchi were reversible and mixed-type inhibitors. Fructus cnidii, aloe, ganoderma lucidum spore and ginger were reversible and uncompetitive-type inhibitors. It is suggested that EOs, being of natural origin, would be promising anti-α-glucosidase agents.
Keywords: Essential oil, α-Glucosidase, Inhibitory mechanism
Introduction
Diabetes mellitus, caused by deficiency in insulin secretion, was a common metabolic disease, severely affecting human health (Hu and Jia, 2019). Insulin secretion deficiency would lead to high blood glucose and serious complications, including high blood pressure, stroke, blindness and kidney disease (Matsui et al., 2007; Duckworth et al., 2009). It was well known that α-glucosidase was directly associated with postprandial hyperglycemia which played a key role in the development of type 2 diabetes mellitus (Priscilla et al., 2014). Thus, lowering postprandial hyperglycemia by inhibiting α-glucosidase was confirmed to be an effective strategy to treat type 2 diabetes mellitus (Bhandari et al., 2008; Kalra and Bhutani, 2014; Rhabasa-Lhoret and Chiasson, 2004). It was indicated that acarbose, metformin and sitagliptin had been used for glycemic control (Ali et al., 2006). However, long-term use of these synthetic drugs would lead to adverse effects, such as hypoglycemia, diarrhea and flatulence, which seriously affected the absorption of nutritious ingredient (Kumar and Sinha, 2012). Herein, there is an urgent need for searching safe α-glucosidase inhibitors.
Natural edible products, such as vegetables, fruits and Chinese traditional medicines, conducting various of bioactivities without possible adverse effects, were widely used to treat metabolic diseases (Ruxton, 2016). Essential oils (EOs), derived from natural edible products, were secondary metabolites comprising different bioactive components (Maffei et al., 2011). They were complex mixtures of different bioactive components, covering a broad range of functional properties, such as anti-oxidative, anti-diabetic, anti-microbial and anti-inflammatory properties (Raut and Karuppayil, 2014). Hence, EOs were considered an emerging repository of small molecules for drug discovery (Maffei et al., 2011). To date, several studies with plants essential oils were done to test their (and major components) inhibition on α-glucosidase (Yang et al., 2016; Zhao et al., 2017). Previous studies have found that the extract of tea could lower postprandial hyperglycemia through inhibiting the activities of α-amylase and α-glucosidase, which help to reduce the risk of diabetes mellitus (Goh et al., 2015). It has been reported that the EO of Origanum vulgare L. produced 57.7% α-glucosidase inhibition at concentration of 18 μg/mL (Salazar et al., 2020). In addition, the IC50 values of EO derived from Thymus species for α-amylase and α-glucosidase were found to be 7.19 ± 0.14 and 1.08 ± 3.34 mg/mL (Oboh et al., 2013). As described above, due to their inadequate potency, most of EOs have not yet used for anti-α-glucosidase applications.
Considering the relationship between the main bioactivity components in EOs and the α-glucosidase inhibition properties, and the lack of systematic studies about natural α-glucosidase inhibitors, the present study was aimed to develop more potent α-glucosidase inhibitors from natural source. Sixty-two commercial EOs which extracted from plants were evaluated for their anti-α-glucosidase activities. Furthermore, the main components of potent EOs were analyzed by gas chromatograph–mass spectrometer (GC–MS). The kinetics and the anti-α-glucosidase mechanism of potent EOs were discussed in this paper.
Materials and methods
Materials
α-Glucosidase (from Saccharomyces cerevisiae, EC 3.2.1.20) and acarbose were obtained from Sigma-Aldrich (St. Louis, MO). Sixty-two commercial EOs, pure without additive, were acquired from Jingjing Biotechnology Co. (Guangzhou, China). The information of these EOs was listed in Table 1. Other solvents and reagents which had analytical grade were obtained from Tansoole (Shanghai, China). α-Glucosidase solution was prepared as the following: α-glucosidase was dissolved with 0.05 M phosphate buffer solution (PBS, pH 6.81 ± 0.01), and then diluted to 0.3 U/mL. The final concentrations of α-glucosidase in PBS were kept in 15 U/L. Acarbose was additionally used as a standard compound.
Table 1.
The information of sixty-two EOs
| Name of EOs | Scientific Name | Family | Extracted Part | Solvent |
|---|---|---|---|---|
| Angelica | Angelica sinensis (Oliv.)Diels | Apiaceae | Root | CO2 |
| Angelica pubescence | Angelica pubescens Maxim.f. biserrata Shan et Yuan | Apiaceae | Root | CO2 |
| Bupleurum | Bupleurum scorzonerifolium Willd | Apiaceae | Root | CO2 |
| Celery seed | Apium graveolens L | Apiaceae | Seed | H2O |
| Chuan-xiong | Ligusticum chuanxiong hort | Apiaceae | Root | CO2 |
| Fructus cnidii | Cnidium monnieri (L.) Cuss | Apiaceae | Fruit | H2O |
| Ligusticum | Ligusticum sinense Oliv | Apiaceae | Root | EtOH |
| Notopterygium | Rhizoma et Radix Notopterygii | Apiaceae | Root | CO2 |
| Saposhnikovia divaricata | Saposhnikovia divaricata (Trucz.) Schischk | Apiaceae | Root | CO2 |
| Gallnut | Rhus chinensis Mill | Anacardiaceae | Leaf | H2O |
| Ginseng | Pannx ginseng C.A. Meyer | Araliaceae | Root | H2O |
| Sabal | Serenoarepens (Bartr.) J.K.Small | Arecaceae | Fruit | CO2 |
| Epimedium | Epimedium brevicornu Maxim | Berberidaceae | Whole | CO2 |
| Olive | Olea europaea | Burseraceae | Fruit | CO2 |
| Frankincense | Boswellia carterii Birdw | Burseraceae | Resin | CO2 |
| Honeysuckle | Lonicera japonica Thunb | Caprifoliaceae | Flower | EtOH |
| Papaya | Carica papaya L | Caricaceae | Fruit | EtOH |
| Carnation | Dianthus ‘Carnation' | Caryophyllaceae | Flower | EtOH |
| Tripterygium wilfordii | Tripterygium wilfordii Hook. f | Celastraceae | Leaf | H2O |
| Burdock | Arctium lappa L | Compositae | Fruit | H2O |
| Chrysanthemum | Chrysanthemum indicum L | Compositae | Flower | EtOH |
| Costus | Aucklandia lappa Decne | Compositae | Root | H2O |
| Mugwort | Artemisia argyi Levl.et Vant | Compositae | Leaf | CO2 |
| Cypevol | Cyperus rotundus L | Cyperaceae | Root | CO2 |
| Sseabuckthorn | Hippophae rhamnoides Linn | Elaeagnaceae | Fruit | EtOH |
| Seabuckthorn seed | Hippophae rhamnoides Linn | Elaeagnaceae | Seed | EtOH |
| Sacha inchi | Plukenetia volubilis Linneo | Euphorbiaceae | Fruit | CO2 |
| Oleum anisi stellati | Illicium verum | Illiciaceae | Fruit | H2O |
| Peppermint | Mentha canadensis Linnaeus | Labiatae | Whole | CO2 |
| Patchouli | Pogostemon cablin (Blanco) Benth | Labiatae | Leaf | H2O |
| Schizonepeta tenuifolia | Schizonepeta tenusfolia Briq | Labiatae | Flower | CO2 |
| Bay | Laurus nobilis | Lauraceae | Flower | EtOH |
| Cinnamon | Cinnamomum cassia Presl | Lauraceae | Skin | CO2 |
| Linseed | Linum usitatissimum L | Linaceae | Seed | H2O |
| Aloe | Aloe vera (Haw.) Berg | Liliaceae | Leaf | CO2 |
| Tulip | Tulipa gesneriana L | Liliaceae | Flower | H2O |
| Moringa leaf | Moringa oleifera Lam | Moringaceae | Leaf | CO2 |
| Clove | Eugenia caryophμllataThunb | Myrtaceae | Flower | H2O |
| Rosewood | Rhodamnia dumetorum (Poir.) Merr. et Perry | Myrtaceae | Stem | EtOH |
| Dendrobe | Dendrobium nobile Lindl | Orchidaceae | Stem | EtOH |
| Licorice | Glycyrrhiza uralensis Fisch | Papilionaceae | Leaf | EtOH |
| Black pepper | Piper nigrum | Piperaceae | Fruit | EtOH |
| Citronella | Mosla chinensis Maxim | Poaceae | Leaf | H2O |
| Lemongrass | Cymbopogon citratus (DC.) Stapf | Poaceae | Leaf | CO2 |
| Palmarosa | Cymbopogon martini | Poaceae | Leaf | EtOH |
| Ganoderma lucidum spore | Ganoderma Lucidum Karst | Polyporaceae | Spore | CO2 |
| Agrimonia | Agrimonia pilosa Ldb | Rosaceae | Whole | EtOH |
| Lavender | Rosa"Lavande | Rosaceae | Flower | EtOH |
| Rose | Rosa rugosa Thunb | Rosaceae | Flower | EtOH |
| Rosehip | Rosa rugosa Thunb | Rosaceae | Fruit | EtOH |
| Sweet almond | Amygdalus Communis Vas | Rosaceae | Seed | EtOH |
| Bergamot | Citrus medica L. var.sarcodactylis Swingle | Rutaceae | Fruit | EtOH |
| Lemon | Citrus limon (L.) Burm. f | Rutaceae | Leaf | CO2 |
| Pomelo | Citrus maxima (Burm) Merr | Rutaceae | Fruit | H2O |
| Zanthoxylum | Zanthoxylum bungeanum Maxim | Rutaceae | Fruit | EtOH |
| Moringa seed | Moringa oleifera Lam | Saxifragaceae | Seed | CO2 |
| Black currant | Ribes nigrum L | Saxifragaceae | Fruit | CO2 |
| Boxthorn seed | Lycium chinenseMill | Solanaceae | Seed | CO2 |
| Schizandrae fructus | Schisandra chinensis | Schisandraceae | Fruit | EtOH |
| Camellia seed | Camellia sinensis (L.) O.Ktze | Theaceae | Seed | H2O |
| Ginger | Zingiber officinale Rosc | Zingiberaceae | Root | CO2 |
| Zedoary turmeric | Curcuma phaeocaulis Valeton | Zingiberaceae | Root | EtOH |
Inhibition assay of EOs against α-glucosidase
All the EOs and acarbose were dissolved in acetone to obtain varying concentrations as required. As previously described (Chen et al., 2020), inhibition of EOs at different concentrations against α-glucosidase was tested. Typically, the solution containing 35 μL PBS, 10 μL α-glucosidase, and 5 μL EOs (acetone in blank) were firstly added in the 96-well plates at 0 °C and then pre-incubated for 10 min at 37 °C. Secondly, 50 μL p-Nitrophenol glucoside (PNPG) was immediately added in the 96-well plates to initiate the enzyme reaction and incubated in the microplate reader (Multiskan GO, Thermo Scientific, USA) at 37 °C for 30 min. Finally, the change in absorbance at 405 nm during the 30 min reaction was determined and recorded. The assays were respectively conducted as triplicate and the concentration required for 50% inhibition of each EO was determined as IC50 value. The inhibition rates of EOs were tested at more than 5 concentrations. The IC50 values were calculated by Origin 9.0 software. Inhibitory effect of EOs the α-glucosidase reaction was calculated as follows:
where A represents the absorbance of samples (30 min); B represents the absorbance of samples (30 min); C represents the absorbance of the blank (30 min); D represents the absorbance of the blank (30 min).
Kinetic analysis of α-glucosidase inhibition
As previously reported (Chen et al., 2020), the inhibition mechanism of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger was investigated. In brief, a series of diluted EO solutions were firstly prepared and the PNPG solution was kept in a constant concentration (1 mM). Secondly, the inhibitory effect of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger on α-glucosidase was determined with different concentrations of α-glucosidase (0, 5, 10 and 10 U/L), respctively. In addition, the inhibition kinetics of α-glucosidase was measured by Lineweaver–Burk plots. Typically, a series of diluted EO solutions were obtained and the α-glucosidase solution was kept in a constant concentration (10 U/L). The inhibition rates were tested with different concentrations of PNPG (0.75, 0.60, 0.45, 0.30, and 0.15 mM) by using the above method.
| Type | Mechanism | Characteristic |
|---|---|---|
| Competitive-type | Only bound free enzyme and not the enzyme–substrate complex |
1. Ki ≈ Kis; 2. The lines of Lineweaver–Burk are crossed on a 1/V axis |
| Noncompetitive-type | Only bound the enzyme–substrate complex |
1. Ki ≈ Kis; 2. The lines of Lineweaver–Burk crossed on a 1/[S] axis |
| Mixed-type | Bound free enzyme and the enzyme–substrate complex |
1. The lines of Lineweaver–Burk crossed on crossed on the second or third quadrant; 2. Ki < Kis, competitive-type; 3. Ki > Kis, noncompetitive-type |
The Inhibition kinetics, inhibition constants (Ki) and slope inhibition constant (Kis) were analyzed by Lineweaver–Burk plots. The specific type, mechanism and characteristic were show as following.
Analysis of the most potential EOs with GC–MS
EOs were analyzed with GC (TRACE 1300E, Thermo Scientific Corporation, USA) coupled to MS (ISQ Qd, Thermo Scientific Corporation, USA) using a TG-5 MS silica column (30 m × 0.25 mm; film thickness 0.25 μm). The procedure for analyzing GC–MS was showed as follow: helium was firstly used as the carrier gas, flowing with a rate of 1.0 mL/min. The oven temperature was secondly programmed at 60 °C, with an increase of 5 °C/min to 160 °C (isotherm at 2 min), and then 10 °C/min to 260 °C and held for 20 min. The mass spectra were recorded at 70 eV with a scanning range from 35 to 450 m/z. Composition (%) of EOs was calculated in software by using the peak normalization method. Comparing to Kovats retention indices and relative to a C8–C40 n-alkanes standard, the peak identification of different constituents in EOs was determined. Identification of the EOs constituents was performed by comparing the acquired mass spectrum with NIST mass spectral library.
Results and discussion
Inhibitory effect of sixty-two EOs against α-glucosidase
It has been indicated that EOs derived from natural products and their active constituents were promising α-glucosidase inhibitors (Zhao et al., 2017). However, systematic research in plant derived EOs as natural α-glucosidase inhibitors was still lacking. Hence, we determined the effect of sixty-two EOs on α-glucosidase in vitro. As shown in Table 2, all of EOs at 25 μg/mL showed stronger inhibitory activity against α-glucosidase than that of acarbose which was used as the positive control. Furthermore, most of EOs exhibited more effectively inhibition than acarbose (475.6 ± 24.1 μg/mL). The results indicated that the inhibitory effect of plant derived EOs from the family Apiaceae on α-glucosidase was in the descending order as follow: fructus cnidii > chuan-xiong > angelica > saposhnikovia divaricata = ligusticum > 50 μg/mL. In the family Burseraceae, olive EO (11.8 ± 0.1 μg/mL) showed the strongest inhibition against α-glucosidase. Moreover, costus EO with IC50 value of 16.3 ± 0.4 μg/mL showed most significant inhibition in the family Compositae. Aloe EO (5.06 ± 0.18 μg/mL) exhibited more effective inhibiton than that of other EO derive from the family Liliaceae. In the family Rosaceae, sweet almond EO (11.4 ± 3.7 μg/mL) showed the strongest inhibition against α-glucosidase activity. Among these EOs, the most potent EOs against α-glucosidase were chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger with IC50 values of 3.02, 2.88, 7.37, 5.06, 5.32 and 7.40 μg/mL, respectively. Taken together, chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger were selected for further investigation of the anti-α-glucosidase mechanism of action.
Table 2.
The half maximal inhibitory concentration of sixty-two EOs and acarbose
| NO | EOs | Inhibition rate at 50 μg/mL (%) | IC50 (μg/mL) |
|---|---|---|---|
| 1 | Angelica | 99.24 ± 0.17 | 11.0 ± 0.1 |
| 2 | Angelica pubescence | 26.99 ± 2.01 | 101.7 ± 0.1 |
| 3 | Bupleurum | 30.22 ± 3.42 | 67.6 ± 2.8 |
| 4 | Celery seed | 72.48 ± 6.66 | 37.3 ± 2.4 |
| 5 | Chuan-xiong | 99.87 ± 0.05 | 3.02 ± 0.06 |
| 6 | Fructus cnidii | 99.71 ± 0.15 | 2.88 ± 0.04 |
| 7 | Ligusticum | 89.80 ± 4.61 | 25.6 ± 1.1 |
| 8 | Notopterygium | 38.25 ± 6.74 | 157.0 ± 1.1 |
| 9 | Saposhnikovia divaricata | 98.33 ± 0.06 | 25.6 ± 0.3 |
| 10 | Gallnut | 63.62 ± 10.21 | 42.5 ± 2.4 |
| 11 | Ginseng | 53.39 ± 4.86 | 46.7 ± 10.7 |
| 12 | Sabal | 65.69 ± 3.19 | 26.2 ± 3.1 |
| 13 | Epimedium | 98.89 ± 0.11 | 22.5 ± 5.0 |
| 14 | Olive | 95.54 ± 0.12 | 11.8 ± 0.1 |
| 15 | Frankincense | 28.78 ± 4.73 | 301.5 ± 33.6 |
| 16 | Honeysuckle | 47.20 ± 2.34 | 61.4 ± 0.8 |
| 17 | Papaya | 46.70 ± 1.49 | 62.7 ± 0.7 |
| 18 | Carnation | 99.79 ± 0.08 | 10.1 ± 0.4 |
| 19 | Tripterygium wilfordii | 32.65 ± 5.49 | 198.8 ± 37.6 |
| 20 | Burdock | 92.71 ± 4.47 | 28.1 ± 1.1 |
| 21 | Chrysanthemum | 99.16 ± 0.93 | 17.8 ± 1.4 |
| 22 | Costus | 98.89 ± 0.38 | 16.3 ± 0.4 |
| 23 | Mugwort | 34.55 ± 2.74 | 91.4 ± 16.2 |
| 24 | Cypevol | 67.24 ± 0.49 | 18.9 ± 0.1 |
| 25 | Seabuckthorn | 98.79 ± 0.69 | 17.5 ± 1.1 |
| 26 | Seabuckthorn seed | 99.23 ± 0.54 | 10.6 ± 1.2 |
| 27 | Sacha inchi | 99.50 ± 0.21 | 7.37 ± 0.47 |
| 28 | Oleum anisi stellati | 31.48 ± 2.94 | 81.9 ± 9.7 |
| 29 | Peppermint | 46.95 ± 1.19 | 40.9 ± 4.1 |
| 30 | Patchouli | 98.56 ± 1.02 | 17.5 ± 0.4 |
| 31 | Schizonepeta tenuifolia | 57.91 ± 1.86 | 46.8 ± 1.0 |
| 32 | Bay | 97.95 ± 0.08 | 20.4 ± 2.4 |
| 33 | Cinnamon | 51.61 ± 1.54 | 30.6 ± 4.3 |
| 34 | Linseed | 24.22 ± 1.64 | 253.7 ± 22.5 |
| 35 | Aloe | 98.77 ± 1.08 | 5.06 ± 0.18 |
| 36 | Tulip | 22.44 ± 2.32 | 83.4 ± 0.3 |
| 37 | Moringa leaf | 70.65 ± 4.24 | 23.1 ± 0.4 |
| 38 | Clove | 49.80 ± 8.01 | 45.1 ± 13.0 |
| 39 | Rosewood | 63.73 ± 5.30 | 33.3 ± 7.6 |
| 40 | Dendrobe | 35.53 ± 6.13 | 105.8 ± 13.0 |
| 41 | Licorice | 83.32 ± 2.86 | 14.3 ± 1.1 |
| 42 | Black pepper | 84.27 ± 0.34 | 28.0 ± 0.5 |
| 43 | Citronella | 30.25 ± 1.81 | 230.4 ± 38.8 |
| 44 | Lemongrass | 54.70 ± 1.32 | 44.7 ± 3.7 |
| 45 | Palmarosa | 83.92 ± 1.14 | 17.6 ± 0.3 |
| 46 | Ganoderma lucidum spore | 99.31 ± 0.12 | 5.32 ± 0.13 |
| 47 | Agrimonia | 42.02 ± 0.12 | 85.0 ± 4.7 |
| 48 | Lavender | 57.85 ± 1.47 | 52.4 ± 28.3 |
| 49 | Rose | 99.16 ± 0.93 | 17.8 ± 0.6 |
| 50 | Rosehip | 99.62 ± 0.13 | 12.9 ± 1.6 |
| 51 | Sweet almond | 98.52 ± 1.05 | 11.4 ± 3.7 |
| 52 | Bergamot | 75.58 ± 2.34 | 31.0 ± 2.3 |
| 53 | Lemon | 53.56 ± 1.69 | 53.9 ± 3.4 |
| 54 | Pomelo | 36.42 ± 2.45 | 39.3 ± 16.7 |
| 55 | Zanthoxylum | 90.63 ± 1.38 | 21.9 ± 0.4 |
| 56 | Moringa seed | 10.63 ± 2.15 | 218.2 ± 82.7 |
| 57 | Black Currant | 9.47 ± 3.42 | 1074.7 ± 17.1 |
| 58 | Boxthorn seed | 26.70 ± 1.61 | 95.8 ± 1.4 |
| 59 | Schizandrae fructus | 98.99 ± 0.27 | 23.0 ± 0.1 |
| 60 | Camellia seed | 9.01 ± 2.11 | 1095.3 ± 45.0 |
| 61 | Ginger | 99.55 ± 0.04 | 7.40 ± 0.18 |
| 62 | Zedoary turmeric | 65.56 ± 1.73 | 32.1 ± 1.1 |
| 63 | Acarbose | 8.61 ± 0.21 | 475.6 ± 24.1 |
Mechanism study
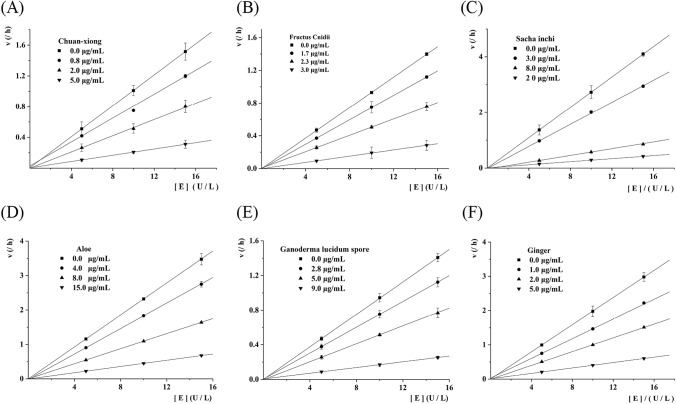
In order to investigate the inhibitory mechanism of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger on α-glucosidase, the inhibition constants and inhibition types of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger on α-glucosidase activity were tested by using PNPG as the substate. As described in Fig. 1, the four lines were obtained from three different concentrations of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger, respectively. The four lines were crossed on the origin. In addition, the slope of the line was gradually decreased with increasing EOs (chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger) concentrations. These results indicated that the effect of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger on α-glucosidase activity was reversible.
Fig. 1.
The inhibitory mechanism of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger against α-glucosidase
As shown in Fig. 2, the inhibition kinetics of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger were analyzed by Lineweaver–Burk plots. The four lines were obtained from five different concentrations of fructus cnidii, aloe, ganoderma lucidum spore and ginger (Fig. 2B, 2D, 2E and 2F). These lines of fructus cnidii, aloe, ganoderma lucidum spore and ginger were crossed on a 1/V axis, indicating that fructus cnidii, aloe, ganoderma lucidum spore and ginger exhibited competitive-type inhibition on α-glucosidase, respectively. These results indicated that fructus cnidii, aloe, ganoderma lucidum spore and ginger only bound free enzyme and not the enzyme–substrate complex. In addition, the four lines with different slopes were obtained from four different concentrations of chuan-xiong and sacha inchi (Fig. 2A and 2C). These lines were respectively crossed on the second or third quadrant, indicating that chuan-xiong and sacha inchi showed mixed-type inhibition.
Fig. 2.
Lineweaver-Burk plots for chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger against α-glucosidase. (A), (B), (C), (D), (E) and (F) represents the plot of slope versus the concentration of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger. (G) and (H) represents the plot of intercept versus the concentration of chuan-xiong and sacha inchi for the determination of Ki. (I), (J), (K), (L), (M) and (N) represents the plot of intercept versus the concentration of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger for the determination of Kis
To investigate whether chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger inhibited α-glucosidase activity by competitively forming enzyme-inhibitor (EI) complex or interrupting enzyme–substrate-inhibitor (ESI) complex in non-competitive manner, we determined EI dissociation constants Ki of chuan-xiong and sacha inchi, and the ESI dissociation constants Kis of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger. Inhibition type, Ki and Kis values of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger, were showed in Table 3. Ki values of chuan-xiong and sacha inchi were 3.90 ± 0.25 and 21.32 ± 2.36 μg/mL, respectively. Furthermore, a lower value of Ki in comparison with Kis indicated that there was stronger binding between enzyme and chuan-xiong, suggesting that it preferred competitive over noncompetitive manner. A lower value of Kis in comparison with Ki revealed that there was weaker binding between enzyme and sacha inchi, suggesting that it preferred noncompetitive over competitive manner. It was concluded that chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger had the significant inhibition on α-glucosidase activities, indicating that they might be potent α-glucosidase inhibitors.
Table 3.
Type of mechanism, Ki and Kis value of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger
| EOs | Inhibition mechanism | Ki value (μg/mL) | Kis value (μg/mL) |
|---|---|---|---|
| chuan-xiong | Mixed type | 3.90 ± 0.25 | 6.84 ± 0.55 |
| fructus cnidii | Uncompetitive type | – | 4.50 ± 0.60 |
| sacha inchi | Mixed type | 21.32 ± 2.36 | 14.25 ± 1.90 |
| aloe | Uncompetitive type | – | 7.87 ± 0.85 |
| ganoderma lucidum spore | Uncompetitive type | – | 12.68 ± 1.10 |
| ginger | Uncompetitive type | – | 7.28 ± 0.50 |
Main constituents of chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger
To characterize the constituents of the potential EOs that contribute to the anti-α-glucosidase activity, six EOs (chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger) were analyzed by GC–MS. As listed in Table 4, the main constituent of chuan-xiong EO was (E)-ligustilide (43.89%), followed by senkyunolide (20.78%), diacetone alcohol (12.0%), linoleic acid (4.05%) and neocnidilide (3.68%), etc. In fructus cnidii EO, osthole was the most abundant compound (40.56%), followed by oleic acid (6.70%), D, L-isobornylacetate (8.52%), myrtenyl isovalerate benzeneacetaldehyde (5.37%) and linoleic acid (3.35%), etc. Sacha inchi EO was characterized by the highest diacetone alcohol and (Z)-13-docosenamide content (67.84% and 16.06% of the total EO, respectively), followed by chlorbutanol (4.82%), o-phenyl carbamate (4.56%), dioctyl terephthalate (3.82%), methylcarbamic acid phenyl ester (2.90%). In addition, the main constituent of aloe EO was diacetone alcohol (68.25%), followed by (Z)-13-docosenamide (15.57%), chlorbutanol (4.89%), phenol (4.57%), linoleic acid (4.02%) and methylcarbamic acid phenyl ester (2.69%). In ganoderma lucidum spore EO, diacetone alcohol was reported to be the most abundant compound (70.74%), followed by (Z)-13-docosenamide (13.71%), chlorbutanol (4.94%), phenol (4.19%), bis(2-ethylhexyl)iosphthalate (3.60%) and methylcarbamic acid phenyl ester (2.82%). Finally, ginger EO was characterized by the highest α-zingiberene and diacetone alcohol content (25.62% and 11.50 of the total EO, respectively), followed by (-)-Sabinene (10.39%), β-Bisabolene (6.31%), 6-shogaol (6.27%), α-Curcumene (6.26%), etc.
Table 4.
Chemical components of chuan-xiong (CX), fructus cnidii (FC), sacha inchi (SI), aloe (A), ganoderma lucidum spore (GLS) and ginger (G)
| Chemical Composition a | Content/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CAS No | Name | RIb | RSIc | CX | FC | SI | A | GLS | G |
| 1943-79-9 | Methylcarbamic acid phenyl ester | 759 | 760 | 2.90 | 2.69 | 2.82 | |||
| 112-84-5 | (Z)-13-docosenamide | 803 | 809 | 3.62 | 16.06 | 15.57 | 13.71 | 3.88 | |
| 33900-84-4 | Myrtenyl isovalerate | 817 | 824 | 5.37 | |||||
| 137-89-3 | Bis(2-ethylhexyl)iosphthalate | 830 | 852 | 3.60 | |||||
| 484-12-8 | Osthole | 853 | 864 | 40.56 | |||||
| 108-95-2 | Phenol | 876 | 876 | 4.57 | 4.19 | ||||
| 4567-33-3 | Neocnidilide | 881 | 885 | 3.68 | |||||
| 57-15-8 | Chlorbutanol | 882 | 886 | 4.82 | 4.89 | 4.94 | |||
| 112-80-1 | Oleic acid | 882 | 897 | 6.70 | |||||
| 622-46-8 | o-phenyl carbamate | 883 | 891 | 4.56 | |||||
| 6422-86-2 | Dioctyl terephthalate | 883 | 893 | 3.82 | |||||
| 60-33-3 | Linoleic acid | 888 | 904 | 4.05 | 3.35 | 4.02 | |||
| 15111-96-3 | p-mentha-1, 8-dien-7-ylacetate | 902 | 942 | 3.27 | |||||
| 10408-16-9 | (−)-sabinene | 915 | 920 | 10.39 | |||||
| 555-66-8 | 6-shogaol | 916 | 924 | 6.27 | |||||
| 123-42-2 | Diacetone alcohol | 920 | 920 | 12.00 | 4.25 | 67.84 | 68.25 | 70.74 | 11.50 |
| 63038-10-8 | Senkyunolide | 927 | 933 | 20.78 | |||||
| 303187–89-5 | 4-(3-hydroxy-2-methoxyphenyl) -butan-2-one | 930 | 937 | 4.11 | |||||
| 495-62-5 | β-bisabolene | 930 | 937 | 6.31 | |||||
| 495-60-3 | α-zingiberene | 932 | 942 | 25.62 | |||||
| 92618-89-8 | d, l-isobornylacetate | 933 | 933 | 8.52 | |||||
| 644-30-4 | α-curcumene | 942 | 942 | 6.26 | |||||
| 81944-08-3 | (E)-ligustilide | 944 | 957 | 43.89 | |||||
| Others | 945 | 963 | 11.98 | 27.98 | 0.00 | 0.00 | 0.00 | 25.66 | |
aMajor components (content > 1%), listed in the order of RI value, are listed in the table
bLinear retention index is obtained from https://webbook.nist.gov/chemistry/
It has been indicated that α-glucosidase, a key digestive enzyme, was responsible for the hydrolysis of α(1–4) glucosidic bonds to control the amount of absorbable monosaccharides which released from oligosaccharides and glycoconjugates (Yue et al., 2017). Previous studies reported that α-glucosidase inhibitors could effectively control postprandial glucose level imbalance and caused serious side effects (Rosas-Ramírez et al., 2020; Kumar and Sinha, 2012). Therefore, the finding of safe α-glucosidase inhibitors would be urgently needed.
It was revealed that EO had a wild range of bioactivities (Raut and Karuppayil, 2014). Hence, we investigated whether the EOs derived from natural products had effect on α-glucosidase in present study. Our results showed that among these EOs, the most potent EOs against α-glucosidase were chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger with IC50 values of 3.02, 2.88, 7.37, 5.06, 5.32 and 7.40 μg/mL, respectively (Fig. 1). Interesting, our further study indicated that diacetone alcohol, a main constituent of sacha inchi, aloe and ganoderma lucidum spore EO, had an inhibitory effect on α-glucosidase with IC50 values of 22.89 mg/mL. Previous studies revealed that (E)-ligustilide, osthole and α-zingiberene had anti-α-glucosidase activity (Chen et al., 2021; Karakaya et al., 2018; Asraoui et al., 2021). Thus, chuan-xiong EO, fructus cnidii EO, and ginger EO might be responsible for anti-α-glucosidase activity of these compounds. These results indicated that EO and their main constituents may be valuable α-glucosidase inhibitor.
As shown in Fig. 2, our results suggested that there was stronger binding between enzyme and chuan-xiong, suggesting that it preferred competitive over noncompetitive manner. Furthermore, there was weaker binding between enzyme and sacha inchi, suggesting that it preferred noncompetitive over competitive manner. Above all, the results concluded that chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore and ginger had significantly anti-α-glucosidase activity, suggesting that they would be used as natural α-glucosidase inhibitors.
In summary, EOs (chuan-xiong, fructus cnidii, sacha inchi, aloe, ganoderma lucidum spore, and ginger), isolated from edible products, were safe and have potent anti-α-glucosidase activity. The in vitro experiments indicated that chuan-xiong and sacha inchi were reversible and mixed-type inhibitors, and fructus cnidii, aloe, ganoderma lucidum spore and ginger were reversible and uncompetitive-type inhibitors, revealing the mechanism of these EOs against α-glucosidase. Collectively, the results have provided new insights into potential application of EOs from natural products as α-glucosidase inhibitors to treat diabetes.
Acknowledgements
Financial support was provided by Natural Science Foundation of Guangdong Province (No. 2019A1515110941), the Basic and Theoretical Research Programs of Science and Technology Foundation of Jiangmen (No. 2020JC01013 and 2021A7), and the Joint Fund of Wuyi University-Macau (No. 2019WGALH01).
Author contributions
WL designed the experiments; ZY and YL conducted the experiments; WL and XZ analyzed the data; WL wrote the manuscript; while KZ, XZ and VKWW revised the manuscript.
Declarations
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zonglin You, Email: 1726107134@qq.com.
Yonglian Li, Email: liyli85@126.com.
Kun Zhang, Email: kzhang@gdut.edu.cn.
Xi Zheng, Email: xizhengch2006@163.com.
Vincent Kam Wai Wong, Email: bowaiwong@gmail.com.
Wenfeng Liu, Email: 254232528@qq.com.
References
- Ali H, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. Journal of Ethnopharmacology. 2006;107:449–455. doi: 10.1016/j.jep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Asraoui F, Kounnoun A, Cacciola F, El-Mansouri F, Kabach I, Oulad El Majdoub Y, Asraoui F, Kounnoun A, Cacciola F, El-Mansouri F, Kabach I, Oulad El Majdoub Y, Alibrando F, Arena K, Trovato E, Mondello L, Louajri A. Phytochemical profile, antioxidant capacity, α-amylase and α-glucosidase inhibitory potential of wild Moroccan inulaviscosa (L.) aiton leaves. Molecules. 2021;26:3134–3148. doi: 10.3390/molecules26113134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari MR, Nilubon JA, Gao H, Kawabata JA. Glucosidase and aamylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata Haw) Food Chemistry. 2008;106:247–252. doi: 10.1016/j.foodchem.2007.05.077. [DOI] [Google Scholar]
- Chen Q, Wang X, Yuan X, Shi J, Zhang C, Yan N, Jing C. Comparison of phenolic and flavonoid compound profiles and antioxidant and α-glucosidase inhibition properties of cultivated soybean (Glycine max) and wild Soybean (Glycine soja) Plants. 2021;10:813. doi: 10.3390/plants10040813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gao M, Jian R, Hong WD, Tang X, Li Y, Zhao D, Zhang K, Chen W, Zheng X. Sheng Z Design, synthesis and α-glucosidase inhibition study of novel embelin derivatives. Journal of Enzyme Inhibition and Medicinal Chemistry. 2020;35:565–573. doi: 10.1080/14756366.2020.1715386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R. Warren SR Glucose control and vascular complications in veterans with type 2 diabetes. The New England Journal of Medicine. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- Goh R, Gao J, Ananingsih VK, Ranawana V, Henry CJ, Zhou W. Green tea catechins reduced the glycaemic potential of bread: an in vitro digestibility study. Food Chemistry. 2015;180:203–210. doi: 10.1016/j.foodchem.2015.02.054. [DOI] [PubMed] [Google Scholar]
- Maffei ME, Gertsch J, Appendino G. Plant volatiles: production, function and pharmacology. Natural Product Reports. 2011;28:1359–1380. doi: 10.1039/c1np00021g. [DOI] [PubMed] [Google Scholar]
- Matsui T, Tanaka T, Tamura S, Toshima A, Tamaya K, Miyata Y, Tanaka K, Matsumoto K. Alpha-glucosidase inhibitory profile of catechins and the aflavins. Journal of Agricultural and Food Chemistry. 2007;55:99–105. doi: 10.1021/jf0627672. [DOI] [PubMed] [Google Scholar]
- Hu C, Jia W. Therapeutic medications against diabetes: what we have and what we expect. Advanced Drug Delivery Reviews. 2019;15:3–15. doi: 10.1016/j.addr.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Kalra, S., Bhutani, J. Alpha-glucosidase inhibitors. 1st. Diabetology: Type 2 Diabetes Mellitus (2014)
- Karakaya S, Ozbek H, Gözcü S, Güvenalp Z, Yuca H, Duman H, Kazaz C, Kiliç CS. α-Amylase and α-glucosidase inhibitory activities of the extracts and constituents of Ferulago blancheana, F. pachyloba and F. trachycarpa roots. Bangladesh Journal of Pharmacology. 2018;13:35–40. doi: 10.3329/bjp.v13i1.33668. [DOI] [Google Scholar]
- Kumar RV, Sinha VR. Newer insights into the drug delivery approaches of alpha-glucosidase inhibitors. Expert Opinion on Drug Delivery. 2012;9:403–416. doi: 10.1517/17425247.2012.663080. [DOI] [PubMed] [Google Scholar]
- Oboh G, Ademosun AO, Odubanjo OV, Akinbola IA. Antioxidative properties and inhibition of key enzymes relevant to type-2 diabetes and hypertension by essential oils from black pepper. Advances in Pharmacological Sciences. 2013;2013:1–6. doi: 10.1155/2013/926047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priscilla DH, Roy D, Suresh A, Kumar V, Thirumurugan K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chemico-Biological Interactions. 2014;210:77–85. doi: 10.1016/j.cbi.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Industrial Crops and Products. 2014;62:250–264. doi: 10.1016/j.indcrop.2014.05.055. [DOI] [Google Scholar]
- Ruxton C. Tea: hydration and other health benefits. Prim Health Care. 2016;26:34–42. doi: 10.7748/phc.2016.e1162. [DOI] [Google Scholar]
- Rhabasa-Lhoret R, Chiasson JL. Alpha-glucosidase inhibitors. 3. Weinheim: Wiley; 2004. [Google Scholar]
- Salazar MO, Osella MI, Arcusin DE, Lescano LE, Furlan RL. New α-glucosidase inhibitors from a chemically engineered essential oil of Origanum vulgare L. Industrial Crops and Products. 2020;156:112855. doi: 10.1016/j.indcrop.2020.112855. [DOI] [Google Scholar]
- Rosas-Ramírez D, Escandón-Rivera S, Pereda-Miranda R. Morning glory resin glycosides as α-glucosidase inhibitors: In vitro and in silico analysis. Phytochemistry. 2018;148:39–47. doi: 10.1016/j.phytochem.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Yang D, Xie HH, Jiang YM, Wei XY. Phenolics from strawberry cv. Falandi and their antioxidant and α-glucosidase inhibitory activities. Food Chemistry. 2016;194:857–863. doi: 10.1016/j.foodchem.2015.08.091. [DOI] [PubMed] [Google Scholar]
- Yue J, Xu J, Cao J, Zhang X, Zhao Y. Cucurbitane triterpenoids from Momordica charantia L. and their inhibitory activity against α-glucosidase, α-amylase and protein tyrosine phosphatase 1B (PTP1B) Journal of Functional Foods. 2017;37:624–631. doi: 10.1016/j.jff.2017.07.041. [DOI] [Google Scholar]
- Zhao M, Li D, Ye JH, Zheng XQ, Liang YR, Lu JL. Stop for tea? Enzyme inhibitors from tea-what good are they? International Journal of Food Science & Technology. 2017;52:586–594. doi: 10.1111/ijfs.13342. [DOI] [Google Scholar]