Abstract
This study aimed to establish and validate a novel evaluation method using digital tomosynthesis to quantify bone formation in the gap after opening wedge high tibial osteotomy (OW-HTO). We retrospectively analyzed bone formation in the gap in 22 patients who underwent OW-HTO using digital tomosynthesis at 1, 2, 3, 6, 9, and 12 months postoperatively. Bone formation was semi-quantitatively assessed using the modified van Hemert’s score and density measurements on digital tomosynthesis images. The gap filling value (GFV) was calculated as the ratio of the intensities of the opening gap and the tibial shaft. In addition, the relationship between the modified van Hemert’s score and GFV was evaluated. The reproducibility of GFV had an interclass correlation coefficient (ICC [1,2]) of 0.958 for intraobserver reliability and an ICC (2,1) of 0.975 for interobserver reliability. The GFV increased in a time-dependent manner and was moderately correlated with the modified van Hemert’s score (r = 0.630, p < 0.001). The GFV plateaued at 6 months postoperatively. In addition, the GFV was higher in patients with a modified van Hemert’s score of 2 than in patients with a modified van Hemert’s score of 3 (p = 0.008). The GFVs obtained using digital tomosynthesis can be used to assess postoperative bone formation in the opening gap after OW-HTO with high accuracy and reproducibility.
Keywords: High tibial osteotomy, Bone formation, Gap filling value, Digital tomosynthesis, Fiji, ImageJ
Introduction
High tibial osteotomy (HTO) is an established technique to treat medial unicompartmental knee osteoarthritis (OA). This procedure changes the mechanical axis from medial to lateral to delay the progression of OA [1, 2]. HTO has good outcomes with time to revision of over 15 years [3–5]. Opening wedge HTO (OW-HTO) simply and easily corrects the tibial alignment in patients with minor deformities and is more commonly used. Although OW-HTO has lower risks of infection and aseptic loosening compared with total knee arthroplasty, a complication can occur with a nonunion at an opening gap created in the metaphysis of the tibia [6, 7]. The incidence of nonunion after OW-HTO has been reported to be 5–12% [8]. In particular, delayed union is more likely with an opening gap of more than 10 mm [9]. Several enhancements in bone filling have been used to fill this gap, including allograft, autograft, artificial bone, and recombinant human bone morphogenetic proteins [10–12]. Previous studies have evaluated bone filling using semi-quantitative scores on radiographs and computed tomography (CT) images. Reconstructed views can be created with CT and allow for a more accurate bone formation evaluation than the use of radiographs [13, 14]. However, routine CT scanning should be avoided due to the high cumulative radiation exposure.
The use of digital tomosynthesis (laminographs with a series of radiographic images) in clinical practice for the diagnosis of breast cancer or identification of lung nodules has recently increased [15, 16]. Recently, digital tomosynthesis has been used in many orthopedic clinics to evaluate bone formation [17, 18]. Compared with CT, digital tomosynthesis has a lower radiation exposure, requires fewer hospital resources, and can be performed quickly and in the presence of metal artifacts [19]. Furthermore, digital tomosynthesis has been reported to be equivalent to CT for the evaluation of bone integration after anterior cruciate ligament reconstruction [20]. Despite these advantages, digital tomosynthesis is not commonly used to evaluate the bone filling in the gap after OW-HTO, and few studies have quantitatively assessed bone filling.
The purpose of this study was to establish a novel quantitative evaluation method to assess bone formation using digital tomosynthesis and Fiji, an open-source image processing software [21]. As mentioned above, we compared the bone formation in the opening gap greater than and less than 10 mm. We hypothesized that tomosynthesis allows for an accurate and reproducible quantification of gap filling after OW-HTO.
Materials and Methods
Patients
From April 2014 to March 2019, 62 OW-HTO procedures were performed to treat medial unicompartmental knee OA at our institute. OW-HTO was contraindicated in patients with lateral knee OA with valgus deformity, severe patellofemoral joint OA, inflammation (including rheumatoid arthritis or psoriatic arthritis), or a deformity at the shaft of the tibia, femur, or other bone in the lower extremity. Since we aimed to detect the minute changes in bone formation using digital tomosynthesis, we assessed bone formation at specific timepoints (1, 2, 3, 6, 9, and 12 months postoperatively). Two patients (2 knees) who had a history of anterior cruciate ligament reconstruction and 35 patients (38 knees) who lacked tomosynthesis data (i.e., who stopped following up at our outpatient clinic) did not meet the inclusion criteria. The excluded patients showed no significant differences in demographic data. Finally, this study included 22 patients (8 men and 14 women) who underwent unilateral OW-HTO at our institute (Fig. 1). All patients were evaluated at 1, 2, 3, 6, 9, and 12 months postoperatively. The patients’ preoperative heights and weights were measured, and the body mass index was calculated for each patient. The ranges of knee extension and flexion were recorded preoperatively and at the final follow-up visit. The ethics committee of our institution approved the study design, and all patients provided informed consent. This study followed the principles of the Declaration of Helsinki.
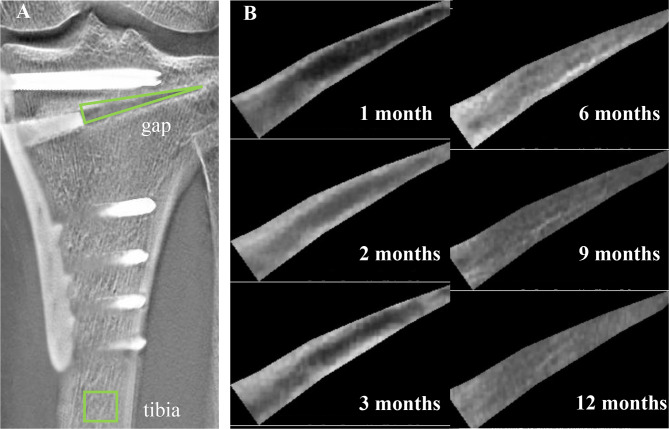
Fig. 1.
Quantitative evaluation of bone formation in the opening gap. A The intensity of the opening gap and tibia shaft are set using Fiji on each image. B A cropped image of the gap area at each time point is shown
Surgical Procedure
OW-HTO with a TriS medial HTO plate (Olympus Terumo Biomaterials, Japan) or a Puddu plate (Arthrex Inc., Naples, FL, USA) was performed at our institute. The correction angle was preoperatively calculated using radiographs of the entire lower extremity, with a target angle of 62.5% of the percent mechanical axis (%MA), which is the Fujisawa point [22]. First, the patient’s cartilage, meniscus, and ligamentous lesions were arthroscopically evaluated. In the present study, no patients needed any treatment for meniscal injury. The exposed subchondral bones of the medial femorotibial joint were added to the bone marrow stimulation. Osteotomy was performed after confirming that the lateral femorotibial joint and patellofemoral joint were intact. The medial aspect of the proximal tibia was approached, and a proximal osteotomy was performed 35 to 40 mm distal to the medial joint line of the proximal tibiofibular joint. The osteotomy site was opened using chisels, the specific type of which was determined during the preoperative planning. One or two wedged β-TCP blocks (SUPERPORE EX, HOYA Technosurgical, Co., Ltd., Japan) were inserted into the opening gap. Finally, the tibia was fixed using a TriS or Puddu plate.
Range of motion (ROM) and isometric muscle-strengthening exercises were initiated on postoperative day 1. Patients were permitted to begin partial weight-bearing 2 weeks after surgery, and full weight-bearing was permitted within 4 weeks postoperatively. After bone union was determined to be complete in the tibia, the plate and screws were removed. In this study, patients were divided into two groups based on the opening gap: the larger gap group (≥ 10 mm) and the smaller gap group (< 10 mm).
Imaging Evaluation
Digital tomosynthesis (Sonialvision Safire 17, Shimazhu, Kyoto, Japan) and radiography of the anterior–posterior (AP) view of the knee were performed. Tomosynthesis is more advantageous than plain radiography because of reduction of overlapping structures; moreover, it is routinely performed in our institution. The imaging conditions for digital tomosynthesis were as follows: X-ray tube voltage, 70 kV; current, 1.25 mAs; data acquisition time for 74 projections, 5.0 s; and field of view, 9 in.
The joint line conversion angle (JLCA) and medial proximal tibial angle (MPTA) were measured on pre- and postoperative weight-bearing, full-length, standing radiographs. All radiologic measurements were performed using Picture Archiving Communication System software (ShadeQuest/ViewR version 1.24.15; Fujifilm Medical Co. Ltd., Tokyo, Japan), which allows for a minimum measurable angle change of 0.01° and length of 0.01 mm. Gap filling was assessed semi-quantitatively using digital tomosynthesis and the modified van Hemert’s score at each time point (Table 1) [12, 20]. The modified van Hemert’s score was used as the comparator following quantitative evaluation of bone formation.
Table 1.
Modified van Hemert’s score
| Score | Explanation |
|---|---|
| 0 | The osteotomy line at the tibia is as clear as that immediately after surgery |
| 1 | The osteotomy line at the tibia appears unclear, with a distinct lucent line clearly visible at both the proximal and distal surfaces of the spacer |
| 2 | A blurred lucent line is visible in a limited part on both the proximal and distal surfaces of the spacer |
| 3 | A blurred lucent line is clearly visible on one surface of the spacer but is not visible on the other surface |
| 4 | A blurred lucent line is visible in a limited part on one surface of the spacer but is not visible on the other surface |
| 5 | No lucent line is visible on either surface of the spacer |
Quantitative Evaluation of Bone Formation Using Fiji
-
Preliminary investigations
The suitability of the bone formation evaluation was verified in all 22 patients prior to inclusion in the study. In this preliminary investigation, a slice of the tomosynthesis image with the largest gap area was used. Quantitative evaluations of bone formation were performed using Fiji, an image analysis software based on ImageJ (National Institutes of Health, Bethesda, MA). [21].
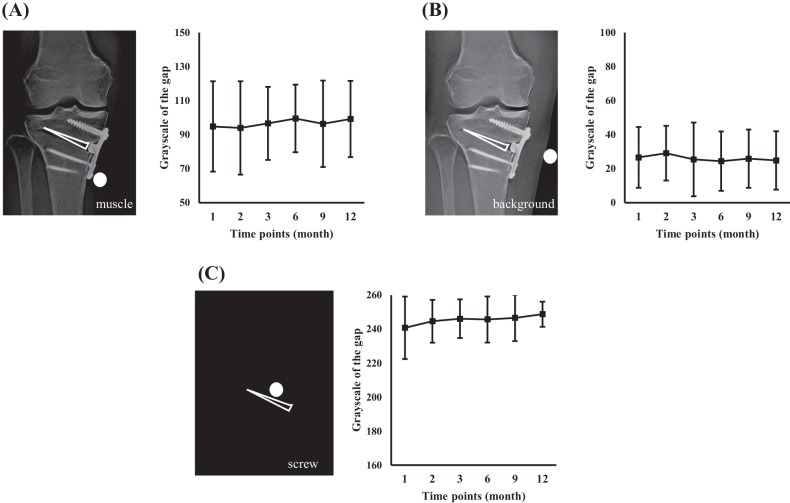
As an initial approach, we performed the calibration before measuring the grayscale intensity of the region of interest. First, we specified the baseline region in the different parts of the image, including the background, screw, and muscle areas. We measured the grayscale intensity of these areas. Then, we subtracted the grayscale of the overall image with each of the baseline grayscale using Process > Math > Subtract commands in Fiji. After calibration, the measured gap area was defined as a triangular area bound by the proximal and distal osteotomy lines and the lateral aspect of the apex of the β-TCP block (Fig. 1). The intensity (grayscale) of the gap area was recorded. The same gap area parameters (slice, area size, and position of the gap area) were used at all time points. However, we found that the trends of intensities did not reflect the time-dependent bone formation using these calibrated images (Fig. 2).
Stepwise Fiji protocol for measurement of gap filling value
Fig. 2.
Preliminary investigation for quantitative evaluation method of bone formation. The images are calibrated to standardize the intensity using the intensity of muscles (A), the background (B), and screws (C). Line graphs indicate the mean intensity at the opening gap
The intensity of the center of the shaft of the tibia was used to standardize the intensity at the opening gap (Fig. 1). The intensity was measured using the same method as in the preliminary investigations. The ratio of the intensity at the opening gap divided by the intensity of the tibial shaft was defined as the gap filling value (GFV). A high ratio indicates better bone formation at the opening gap. The following protocol was used to measure the GFV:
Import the image into Fiji with “drag-drop” option or through Open option under File drop-down menu.
Convert the image into an 8-bit black and white image using Image > Type > 8-bit.
Draw a square on the tibia area using the Rectangle selection, save the location as the ROI using Analyze > Tools > ROI manager, and then click Add [t] > More > Save. Measure the intensity of the ROI using Analyze > Measure (CTRL-M), and save it as a CSV file.
Draw a triangle on the gap area using the Polygon selection, save the location as ROI using Analyze > Tools > ROI manager, and then click Add [t] > More > Save.
Isolate the gap area using Edit > Clear Outside and Image > Crop. Measure the intensity in the triangle using Analyze > Measure (CTRL-M), and save it as a CSV file. The shape and size of the triangular ROI used to represent the gap area were the same for each time point and specific to each patient.
The above protocol was automated using ImageJ macro written in Python and applied to each patient. To evaluate the increase in the GFV at 2, 3, 6, 9, and 12 months postoperatively, Δ2M, Δ3M, Δ6M, Δ9M, and Δ12M were calculated as follows: (GFV at 2, 3, 6, 9, or 12 months postoperatively) – (the GFV at 1 month postoperatively). One observer re-evaluated the modified van Hemert’s score and GFV to examine the intraobserver reliability. In addition, two independent observers examined the interobserver reliability for the modified van Hemert’s score and the GFV using 20 randomly selected images.
Statistical Analysis
Demographic data and GFVs are shown as mean and standard deviation. Student’s t-test and Wilcoxon rank-sum test were performed to compare continuous variables between the larger and smaller gap groups, as appropriate depending on the distribution of the data. The modified van Hemert’s score and GFVs were compared using repeated measures analysis of variance (ANOVA) with Tukey’s post-hoc test. The gap filling evaluations were compared between the larger and smaller gap groups using the Wilcoxon rank-sum test. To evaluate whether GFV was acceptable for assessing bone formation in the gap after OW-HTO, the correlation between the semi-quantitative score and the quantitative value was assessed using Spearman’s rank correlation coefficient. In addition, the GFVs for each modified van Hemert’s score were compared using ANOVA and Tukey’s test. All statistical analyses were performed using JMP Pro software version 13 (SAS Institute Inc., Cary, NC, USA). A p-value < 0.05 was considered significant.
Results
This study included 22 patients (8 men and 14 women; mean age: 59.3 ± 6.3 years, range: 45–70 years). The larger gap group (n = 16) had a mean opening gap of 10.7 ± 1.1 mm (range: 5–9 mm), and the smaller gap group (n = 6) had a mean opening gap of 7.3 ± 1.3 mm (range: 10–12.5 mm) (p < 0.001). There were no significant differences in the patient demographic data or range of motion (pre- or postoperatively) between the two groups (Table 2). The mean preoperative MPTA was 83.9 ± 1.9° in the larger gap group and 86.0 ± 1.0° in the smaller gap group (p = 0.019). The mean postoperative MPTA was 90.2 ± 1.8° in the larger gap group and 89.9 ± 0.5° in the smaller gap groups (p = 0.734) (Table 2).
Table 2.
Patient characteristics
| Parameter | Larger gap group | Smaller gap group | p-value |
|---|---|---|---|
| Sex: female/male | 5/11 | 3/3 | |
| Age (years) | 57.8 ± 6.1 | 63.3 ± 5.6 | 0.058 |
| Height (m) | 1.60 ± 0.02 | 1.62 ± 0.03 | 0.465 |
| Weight (kg) | 64.2 ± 2.2 | 67.7 ± 3.6 | 0.421 |
| BMI (kg/m2) | 25.0 ± 0.7 | 25.5 ± 1.2 | 0.753 |
| Pre-flexion angle (°) | 136.7 ± 2.3 | 140.0 ± 3.5 | 0.436 |
| Pre-extension angle (°) | − 4.0 ± 1.8 | − 4.2 ± 1.2 | 0.941 |
| Pre-JLCA (°) | 3.6 ± 1.8 | 2.7 ± 2.4 | 0.379 |
| Pre-MPTA (°) | 83.9 ± 1.9 | 86.0 ± 1.0 | 0.019 |
| Opening gap (mm) | 10.7 ± 1.1 | 7.3 ± 1.3 | < 0.001 |
| Post-flexion angle (°) | 137.3 ± 2.7 | 141.3 ± 2.7 | 0.494 |
| Post-extension angle (°) | − 2.5 ± 5.0 | − 6.1 ± 7.9 | 0.331 |
| Post-JLCA (°) | 3.2 ± 2.3 | 1.2 ± 1.1 | 0.095 |
| Post-MPTA (°) | 90.2 ± 1.8 | 89.9 ± 0.5 | 0.734 |
Data are presented as mean ± SD. Student’s t-test and Wilcoxon rank sum test were used to compare the mean values between the larger and smaller gap groups
BMI body mass index, JLCA joint line convergence angle, MPTA medial proximal tibial angle
The interclass correlation coefficient (ICC) (1.2) for intraobserver reliability was 0.978 (95% confidence interval [CI]: 0.949–0.991; p < 0.001) for the modified van Hemert’s score and 0.958 (95% CI: 0.897–0.983; p < 0.001) for the GFV. The ICC (2.1) for the interobserver reliability was 0.950 (95% CI: 0.889–0.978; p < 0.001) for the modified van Hemert’s score and 0.975 (95% CI: 0.941–0.989; p < 0.001) for the GFV.
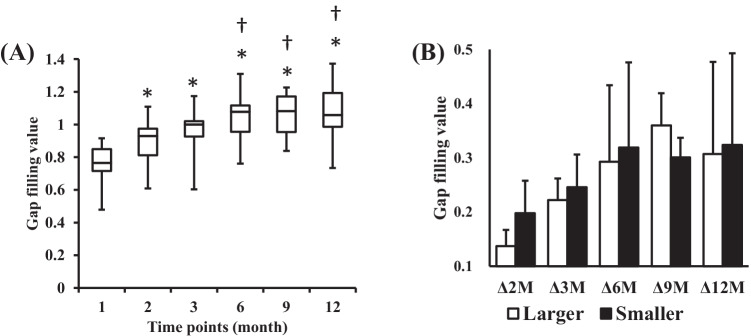
The mean modified van Hemert’s scores at 1, 2, 3, 6, 9, and 12 months postoperatively were 0.5 ± 0.5, 1.3 ± 0.9, 2.2 ± 1.0, 3.2 ± 0.9, 3.9 ± 0.8, and 4.7 ± 0.5, respectively. The mean GFVs at 1, 2, 3, 6, 9, and 12 months postoperatively were 0.75 ± 0.13, 0.89 ± 0.13, 0.97 ± 0.13, 1.04 ± 0.12, 1.06 ± 0.11, and 1.07 ± 0.15, respectively (Table 3). The serial modified van Hemert’s scores and GFVs of a representative case are shown in Fig. 3. The modified van Hemert’s score and the GFV increased at each time point (both p < 0.001). Although the GFV, Δ2M, Δ 3 M, Δ 6 M, Δ9M, and Δ12M were not significantly different between the two groups, the smaller gap group tended to have faster bone formation (Table 3) (Fig. 4). The mean GFVs that occurred with a modified van Hemert’s score of 0, 1, 2 3, 4, and 5 were 0.75 ± 0.13, 0.86 ± 0.13, 0.94 ± 0.15, 1.06 ± 0.12, 1.06 ± 0.13, and 1.02 ± 0.12, respectively. The GFV was positively correlated with the modified van Hemert’s score (r = 0.630, p < 0.001), and the GFV that occurred with each modified van Hemert’s score could be distinguished using Tukey’s test (Fig. 5). The GFV at 3 months postoperatively was significantly higher than that at 2 months postoperatively (p = 0.008).
Table 3.
Modified van Hemert’s score and gap filling value at each time point
| Modified van Hemert’s score | Gap filling value | |||||||
|---|---|---|---|---|---|---|---|---|
| Time points | Total | Larger gap group | Smaller gap group | p-value | Total | Larger gap group | Smaller gap group | p-value |
| 1 month | 0.5 ± 0.5 | 0.4 ± 0.5 | 0.4 ± 0.5 | 0.800 | 0.75 ± 0.13 | 0.74 ± 0.12 | 0.78 ± 0.11 | 0.485 |
| 2 months | 1.3 ± + 0.9 | 1.2 ± 0.7 | 1.5 ± 1.4 | 0.776 | 0.89 ± 0.13 | 0.88 ± 0.14 | 0.91 ± 0.11 | 0.768 |
| 3 months | 2.2 ± 1.0 | 2.1 ± 1.1 | 2.3 ± 1.0 | 0.699 | 0.97 ± 0.13 | 0.96 ± 0.14 | 0.97 ± 0.06 | 0.697 |
| 6 months | 3.2 ± 0.9 | 3.1 ± 0.9 | 3.7 ± 0.8 | 0.141 | 1.04 ± 0.12 | 1.04 ± 0.14 | 1.04 ± 0.06 | 0.740 |
| 9 months | 3.9 ± 0.8 | 3.8 ± 0.8 | 4.3 ± 0.8 | 0.147 | 1.06 ± 0.11 | 1.04 ± 0.12 | 1.08 ± 0.08 | 0.487 |
| 12 months | 4.7 ± 0.5 | 4.6 ± 0.5 | 4.8 ± 0.4 | 0.436 | 1.07 ± 0.15 | 1.03 ± 0.09 | 1.09 ± 0.17 | 0.458 |
Data are presented as mean ± SD. Gap filling value was defined as the intensity of the gap/the intensity of the tibial shaft. The Wilcoxon rank-sum test was used to compare the mean values between the larger and smaller gap groups
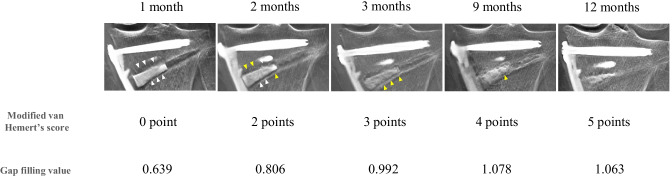
Fig. 3.
Time-dependent change of modified van Hemert’s score and gap filling values. The modified van Hemert’s scores and gap filling values at 1, 2, 3, 9, and 12 months postoperatively of a representative case are shown. White arrowheads indicate a lucent line. Yellow arrowheads indicate a blurred line
Fig. 4.
Change in the gap filling values after OW-HTO. (A) A box plot of the gap filling values (GFV) after surgery is shown. The center bar represents the median, the boundaries of the box represent the first and third quartiles, and the bars represent the maximum and minimum values of GFV at each time point. The GFV at 1, 2, 3, 9, and 12 months postoperatively are compared using analysis of variance and the Tukey test. p < 0.05 is considered significant in comparison to the scores at 1 month (*) or 2 months (†). (B) The change in GFV in the smaller gap group is greater than that in the larger gap group during the early postoperative phase. The change in GFV plateaus at approximately 6 months postoperatively. Abbreviations: GFV, gap filling value; OW-HTO, opening wedge high tibial osteotomy
Fig. 5.
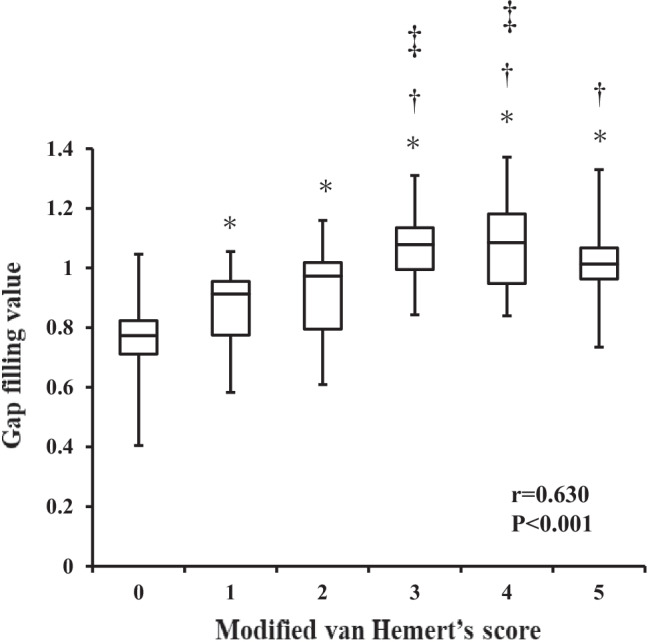
Correlation between quantitative and semi-quantitative evaluation of bone formation after OW-HTO. A strong correlation is observed between the GFV and modified van Hemert’s score (r = 0.630, p < 0.001). GFVs with modified van Hemert’s scores of 0, 1, 2, 3, 4, and 5 are compared using analysis of variance and Tukey test. p < 0.05 is considered significant in comparison with a score of 0 (*), 1 (†), or 2 (‡). Abbreviations: GFV, gap filling value; OW-HTO, opening wedge high tibial osteotomy
Discussion
The filling of the opening gap after OW-HTO can be quantitatively assessed using digital tomosynthesis, which reflects bone formation and its time-dependent changes. This quantification of the GFV was verified as having excellent reproducibility and validity with a semi-quantitative score and is a new technique that can be used for the quantitative assessment of bone formation. However, there was no significant difference in GFV between the larger and smaller groups. Image analysis in the field of orthopedics can benefit greatly from the use of specialized software, such as ImageJ. Our findings and methodology can help orthopedic surgeons to determine the phase of bone formation.
OW-HTO is a joint-preserving surgical procedure used to treat isolated medial or lateral compartment arthritis of the knee and corresponding varus or valgus malalignment of the lower extremity in young patients [2]. Accelerated bone formation in the gap plays an important role in the success of OW-HTO. A previous study reported the time to union in the gap after HTO using enhancements, such as allografts, artificial bones, and recombinant human bone morphogenetic proteins [8, 12, 13]. Koshino et al. reported that bone union was observed at 3–4 months when porous hydroxyapatite was used as an enhancement [23]. Brosset et al. reported that primary bone union occurred in 49 of 51 knees (96%) at an average time of 4.5 months [24]. These two previous studies evaluated bone formation using radiography. In contrast, Tanaka et al. evaluated the bone formation and resorption of β-TCP using CT-attenuation values [13]. A direct comparison of previous studies that evaluate bone formation is difficult due to variations in study design, follow-up duration, and imaging techniques. Although CT imaging has greater sensitivity in evaluating bone formation than radiography [13, 14], it is not appropriate for routine follow-up due to the higher radiation exposure. Tang et al. reported that the mean radiation exposure for tomosynthesis was similar to that for radiography, but was almost 6 times higher for CT [25, 26].
The diagnostic accuracy of digital tomosynthesis for assessing the osseointegration of cementless hip arthroplasty has been reported as much higher than that of radiography or CT imaging [26]. Compared with CT, digital tomosynthesis can equivalently assess pedicle width and pedicle screw breach with less metal artifacts and significantly less radiation exposure [27]. Furthermore, Alice et al. reported that in a series of 51 patients with wrist fractures treated with internal fixation, the rates of cortex obscuration by implant were 2% for CT, 8% for digital tomosynthesis, and 15% for radiography [18]. These results suggest that digital tomosynthesis may be an effective method to assess bone formation in patients undergoing OW-HTO. To our knowledge, this is the first study to quantitatively evaluate bone formation in the gap after OW-HTO using digital tomosynthesis.
This study is not without limitations. First, the region of the gap was determined by the observer. We excluded artificial bone from the region. Thus, human intervention is required to remove artifact from the ROI. Second, the influence of the patient’s position during digital tomosynthesis was unclear. However, the GFV had a modest upward trend with no outliers. Third, due to the restricted inclusion criteria, the sample size was relatively small. In addition, the mean age and MPTA showed significant differences between the two groups. Since the size of the opening gap was determined based on the preoperative, the smaller gap group tended to have a larger preoperative MPTA. Although the influence of degree of preoperative varus deformity on bone formation is not clearly known, age may affect bone formation [22]. Fourth, we used the modified van Hemert’s score, which is relatively little-known, as a control to validate the GFV. In addition, the modified van Hemert’s score mainly evaluated the surface of the spacer, which was not exactly the same as assessed by GFV. Although there are few validated assessment methods for bone formation, the modified van Hemert’s score is a simple method with high reliability [12]. Further studies are needed to validate GFV, either with a larger sample size, a different evaluation method of bone formation, or in conjunction with general imaging tools such as CT. Fifth, the intensity of the tibial shaft was used to standardize the intensity of the opening gap, but any changes in the intensity of the tibial shaft during the follow-up period were not accounted for. Sixth, the GFV between the two groups showed no significant difference. We hypothesized that bone formation in the larger gap group would be slower than in the smaller gap group. In fact, the smaller gap group tended to have faster bone formation. We should have determined the separation between the two groups larger than 10 mm. Despite these limitations, the results of this study suggest that GFV is an appropriate method for the evaluation of bone formation after OW-HTO.
Conclusion
Bone formation in the opening gap after OW-HTO can be quantified using digital tomosynthesis and ImageJ software, and GFV provides a good reference for the assessment of bone formation. Measurements of GFV are reproducible and correlate with the modified van Hemert’s score.
Author Contribution
K.I., E.S., E.W., and Y.I. contributed to the conception and design of the study. K.I., E.S., S.Y., S.S., and Y.K. contributed to the acquisition of the data. K.I., E.S., and E.W. contributed to the analysis and/or interpretation of the data. K.I., E.S., S.T., K.T., and Y.I. drafted the manuscript. Y.I. is the guarantor. The corresponding author attests that all the listed authors meet the authorship criteria and that no other authors meeting the criteria have been omitted.
Data Availability
The authors declare that they had full access to all of the data in this study, and the authors take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fürmetz J, Patzler S, Wolf F, Degen N, Prall WC, Soo C, Brocker W, Thaller PH. Tibial and femoral osteotomies in varus deformities - radiological and clinical outcome. BMC Musculoskelet Disord. 2020;21:201. doi: 10.1186/s12891-020-03232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pannell WC, Heidari KS, Mayer EN, Zimmerman K, Heckmann N, McKnight B, Hill JR, Vangsnes CT, Hatch GF, Weber AE. High tibial osteotomy survivorship: A population-based study. Orthop J Sports Med. 2019;7:2325967119890693. doi: 10.1177/2325967119890693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin QH, Lee W-G, Song E-K, Jin C, Seon J-K. Comparison of long-term survival analysis between open-wedge high tibial osteotomy and unicompartmental knee arthroplasty. J Arthroplasty. 10.1016/j.arth.2020.11.008, November 11, 2020 [DOI] [PubMed]
- 4.Sasaki E, Akimoto H, Iio K, Fujita Y, Saruga T, Kakizaki H, Ishibashi Y. Long-term survival rate of closing wedge high tibial osteotomy with high valgus correction: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2020 doi: 10.1007/s00167-020-06128-9,June29. [DOI] [PubMed] [Google Scholar]
- 5.Song SJ, Bae DK, Kim KI, Park CH. Long-term survival is similar between closed-wedge high tibial osteotomy and unicompartmental knee arthroplasty in patients with similar demographics. Knee Surg Sports Traumatol Arthrosc. 2019;27:1310–1319. doi: 10.1007/s00167-019-05390-w. [DOI] [PubMed] [Google Scholar]
- 6.Meehan JP, Danielsen B, Kim SH, Jamali AA, White RH. Younger age is associated with a higher risk of early periprosthetic joint infection and aseptic mechanical failure after total knee arthroplasty. J Bone Joint Surg Am. 2014;96:529–535. doi: 10.2106/JBJS.M.00545. [DOI] [PubMed] [Google Scholar]
- 7.Thiele K, Perka C, Matziolis G, Mayr HO, Sostheim M, Hube R. Current failure mechanisms after knee arthroplasty have changed: polyethylene wear is less common in revision surgery. J Bone Joint Surg Am. 2015;97:715–720. doi: 10.2106/JBJS.M.01534. [DOI] [PubMed] [Google Scholar]
- 8.auto vs allograft Bashar A, Bryant D, Kevin W, Robert GJ. Graft choice in medial opening wedge high tibial osteotomy. Orthopaedic Proceedings. 2011;93B:581–581. [Google Scholar]
- 9.Jung WH, Takeuchi R, Kim DH, Nag R. Faster union rate and better clinical outcomes using autologous bone graft after medial opening wedge high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2020;28(5):1380–1387. doi: 10.1007/s00167-019-05463-w. [DOI] [PubMed] [Google Scholar]
- 10.Chiari C, Grgurevic L, Bordukalo-Niksic T, Oppermann H, Valentinitsch A, Nemecek E, Staats K, Schreiner M, Trost C, Kolb A, Kainberger F, Pehar S, Milosevic M, Martinovic S, Peric M, Sampath TK, Vukicevic S, Windhager R. Recombinant human BMP6 applied within autologous blood coagulum accelerates bone healing: Randomized controlled trial in high tibial osteotomy patients. J Bone Miner Res. 2020;35:1893–1903. doi: 10.1002/jbmr.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawata M, Jo T, Taketomi S, Inui H, Yamagami R, Matsui H, Fushimi K, Yasunaga H, Tanaka S. Type of bone graft and primary diagnosis were associated with nosocomial surgical site infection after high tibial osteotomy: analysis of a national database. Knee Surg Sports Traumatol Arthrosc, 10.1007/s00167-020-05943-4April 1, 2020 [DOI] [PubMed]
- 12.Kim HJ, Seo I, Shin JY, Lee KS, Park KH, Kyuan HS. Comparison of bone healing in open-wedge high tibial osteomy between the use of allograft bone chips with autologous bone marrow and the use of allograft bone chips alone for gap filling. J Knee Surg. 2020;33:576–581. doi: 10.1055/s-0039-1681093. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Kumagae Y, Chazono M, Kitasato S, Kakuta A, Marumo K. A novel evaluation system to monitor bone formation and β-tricalcium phosphate resorption in opening wedge high tibial osteotomy. Knee Surg Sports Traumatol Arthrosc. 2015;23:2007–2011. doi: 10.1007/s00167-014-2870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fucentese SF, Tscholl PM, Sutter R, Brucker PU, Meyer DC, Koch PP. Bone autografting in medial open wedge high tibial osteotomy results in improved osseous gap healing on computed tomography, but no functional advantage: a prospective, randomised, controlled trial. Knee Surg Sports Traumatol Arthrosc, 27:2951–2957, 2019 [DOI] [PubMed]
- 15.Nelson G, Wu M, Hinkel C, Krishna G, Funk T, Rosenberg J. Improved targeting accuracy of lung tumor biopsies with scanning-beam digital x-ray tomosynthesis image guidance. Med Phys. 2016;43(12):6282. doi: 10.1118/1.4966025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tucker L, Gilbert FJ, Astley SM, Dibden A, Seth A, Morel J. Does reader performance with digital breast tomosynthesis vary according to experience with two-dimensional mammography? Radiology, 283:371—80, 2017 [DOI] [PubMed]
- 17.Blum A, Noël A, Regent D, Villani N, Gillet R, Gondim Teixeira P. Tomosynthesis in musculoskeletal pathology. Diagn Interv Imaging. 2018;99:423–441. doi: 10.1016/j.diii.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Ha AS, Lee AY, Hippe DS, Chou SHS, Chew FS. Digital tomosynthesis to evaluate fracture healing: Prospective comparison with radiography and CT. AJR Am J Roentgenol. 2015;205:136–141. doi: 10.2214/AJR.14.13833. [DOI] [PubMed] [Google Scholar]
- 19.Compton N, Murphy L, Lyons F, Jones J, MacMahon P, Cashman J. Tomosynthesis: A new radiologic technique for rapid diagnosis of scaphoid fractures. Surgeon. 2018;16:131–136. doi: 10.1016/j.surge.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Toyooka S, Masuda H, Nishihara N, Shimazaki N, Ando S, Kawano H, Nakagawa T. Tomosynthesis is equivalent to computed tomography for evaluating osseous integration after anterior cruciate ligament reconstruction. Anthrosc Sports Med Rehabil. 2020;2:e105–e112. doi: 10.1016/j.asmr.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longari M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji - an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujisawa Y, Masuhara K, Shiomi S. The effect of high tibial osteotomy on osteoarthritis of the knee. An arthroscopic study of 54 knee joints. Orthop Clin North Am, 10:585–608, 1979 [PubMed]
- 23.Koshino T, Murase T, Saito T. Medial opening-wedge high tibial osteotomy with use of porous hydroxyapatite to treat medial compartment osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85:78–85. doi: 10.2106/00004623-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Brosset T, Pasquier G, Migaud H, Gougeon F. Opening wedge high tibial osteotomy performed without filling the defect but with locking plate fixation (TomoFixTM) and early weight-bearing: Prospective evaluation of bone union, precision and maintenance of correction in 51 cases. Orthop Traumatol Surg Res. 2011;97:705–711. doi: 10.1016/j.otsr.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Xia W, Yin X-R, Wu J-T, Wu H-T. Comparative study of DTS and CT in the skeletal trauma imaging diagnosis evaluation and radiation dose. Eur J Radiol. 2013;82:e76–80. doi: 10.1016/j.ejrad.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Tang H, Yang D, Guo S, Tang J, Liu J, Wang D, Zhou Y. Digital tomosynthesis with metal artifact reduction for assessing cementless hip arthroplasty: a diagnostic cohort study of 48 patients. Skeltal Radiol. 2016;45:1523–1532. doi: 10.1007/s00256-016-2466-8. [DOI] [PubMed] [Google Scholar]
- 27.Witek L, Alifarag A-M, Tovar N, Lopec C-D, Gil L-F, Gorbonosov M, Hannan K, Neiva R, Coelho P-G. Osteogenic parameters surrounding trabecular tantalum metal implants in osteotomies prepared via osseodensification drilling. Med Oral Patol Oral Cir Bucal. 2019;24:e764–e769. doi: 10.4317/medoral.23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that they had full access to all of the data in this study, and the authors take complete responsibility for the integrity of the data and the accuracy of the data analysis.