Abstract
Purpose
This study aimed to investigate whether MOS methylation can be useful for the prediction of metachronous recurrence after endoscopic resection of gastric neoplasms.
Materials and Methods
From 2012 to 2017, 294 patients were prospectively enrolled after endoscopic resection of gastric dysplasia (n=171) or early gastric cancer (n=123). When Helicobacter pylori was positive, eradication therapy was performed. Among them, 124 patients completed the study protocol (follow-up duration > 3 years or development of metachronous recurrence during the follow-up). Methylation levels of MOS were measured at baseline using quantitative MethyLight assay from the antrum.
Results
Median follow-up duration was 49.9 months. MOS methylation levels at baseline were not different by age, sex, and current H. pylori infection, but they showed a weak correlation with operative link on gastritis assessment (OLGA) or operative link on gastric intestinal metaplasia assessment (OLGIM) stages (Spearman’s ρ=0.240 and 0.174, respectively; p < 0.05). During the follow-up, a total of 20 metachronous gastric neoplasms (13 adenomas and 7 adenocarcinomas) were developed. Either OLGA or OLGIM stage was not useful in predicting the risk for metachronous recurrence. In contrast, MOS methylation high group (≥ 34.82%) had a significantly increased risk for metachronous recurrence compared to MOS methylation low group (adjusted hazard ratio, 4.76; 95% confidence interval, 1.54 to 14.79; p=0.007).
Conclusion
MOS methylation can be a promising marker for predicting metachronous recurrence after endoscopic resection of gastric neoplasms. To confirm the usefulness of MOS methylation, validation studies are warranted in the future (ClinicalTrials No. NCT04830618).
Keywords: Methylation, Stomach neoplasms, Second primary neoplasms, Recurrence
Introduction
Lung cancer is one of the most prevalent malignant neoplGastric cancer (GC) is the sixth most diagnosed cancer and the third leading cause of cancer mortality with 1,090,103 incident cases, and more than 768,793 deaths in 2020 [1]. Helicobacter pylori infection is associated with peptic ulcer disease, mucosa-associated lymphoid tissue lymphoma, and GC.
H. pylori infection induces chronic inflammation, increased secretion of inflammatory cytokines, and aberrant DNA methylation including promoter CpG island hypermethylation and global DNA hypomethylation [2,3]. In result, prolonged H. pylori infection results in epigenetic field defect [4,5], suggesting that methylation could be a surrogate marker for GC [6,7]. Previously, we performed a genome-wide DNA methylation chip study in H. pylori–induced gastric carcinogenesis and identified several methylation markers [8]. Then we validated these methylation markers in a case-control study, and among the candidate genes, methylation of MOS, a, proto-oncogene, was associated with the duration of H. pylori exposure and the risk of GC [9]. Interestingly, MOS methylation decreased after H. pylori eradication in controls, but it remained significantly increased in patients with gastric dysplasia or GC even after H. pylori eradication [10].
In Korea, biannual upper gastrointestinal endoscopy is covered by national insurance for adults over 40 years of age to detect the early gastric cancer (EGC) before progression to advanced GC. This has led to an increase both in diagnosis and endoscopic resection (ER) of EGC [11]. H. pylori eradication after ER of EGC reduced the risk for metachronous recurrence [12]. However, many patients still develop metachronous gastric cancers or gastric dysplasia even after H. pylori eradication treatment [13,14]. Thus, there is a need for a surrogate marker that can predict the risk of GC after H. pylori eradication [15].
From this background, we performed a prospective cohort study to investigate whether MOS methylation can be useful for the prediction of metachronous recurrence after ER of gastric neoplasms.
Materials and Methods
1. Study subjects
The study was designed as a prospective cohort study. From 2012 to 2017, 294 patients were prospectively enrolled after ER of gastric dysplasia (n=171) or EGC (n=123). All lesions were assessed by endoscopy with biopsy before ER. Endoscopic mucosal resection or endoscopic submucosal dissection (ESD) was performed for gastric dysplasia and early gastric cancers which met the absolute indication (differentiated adenocarcinoma, intramucosal cancer, lesions < 20 mm, and no endoscopic evidence of ulceration). All lesions were curatively resected; if non-curatively resected, then the patients were not enrolled in the study. All subjects, who provided informed consent at the time of initial endoscopic treatment, were asked to complete a questionnaire under the supervision of a well-trained interviewer. The questionnaire included questions regarding demographic data (age, sex), socioeconomic data (smoking, alcohol, and education), their family history of GC in first-degree relatives, and history of H. pylori eradication therapy.
Among the 294 subjects, MOS methylation level at baseline could be determined in 261 patients from noncancerous gastric mucosae at antrum. When H. pylori was positive by CLOtest or histology at baseline or during the follow-up, eradication therapy was done. To evaluate whether H. pylori was eradicated, 13C-urea breath testing was performed at least 4 weeks after completion of eradication therapy. The definition of the completion of the study protocol was (1) endoscopic and/or radiologic follow-up for more than 3 years, or (2) development of metachronous gastric neoplasm (gastric dysplasia or cancer) during the follow-up. Metachronous recurrence was defined as secondary dysplasia or cancers detected > 1 year after initial diagnosis. Finally, 124 of 261 subjects completed the study protocol and were included for the survival analysis.
2. Follow-up after endoscopic resection
All study subjects were closely followed up since recurrent tumors at previous ER sites can be easily detected on endoscopy with biopsy and treated during follow-up. Patients with local recurrence underwent further treatments, including repeated ESD, argon plasma coagulation, and gastrectomy based on pathology, and patients who refused treatment received supportive care.
All patients underwent endoscopy with biopsy within 6 months, then at 12 months after ESD to check for metachronous lesions or local recurrences. After 12 months, endoscopy with biopsy was performed annually. In case of EGCs, abdominal computed tomography scan was performed in the first year and biennially thereafter to detect lymph node or distant metastases.
3. H. pylori testing and histologic assessment
At each endoscopy, 12 biopsy specimens were obtained for histological analysis, Campylobacter-like organism test, to determine the presence of a current H. pylori infection. This methodology has been presented previously [10,16]. In brief, two biopsy specimens from the antrum and two from the corpus (1 from the lesser curvature, 1 from the greater curvature) were fixed in formalin to assess the presence of H. pylori by modified Giemsa staining and the degree of inflammatory cell infiltration, atrophy and intestinal metaplasia (all by hematoxylin and eosin staining). These histologic features of the gastric mucosa were recorded using the updated Sydney scoring system (0, none; 1, mild; 2, moderate; and 3, marked) [17]. One specimen from each of the lesser curvature of the antrum and the body was used for rapid urease testing (CLOtest, Delta West, Bentley, Australia). The remaining six noncancerous mucosal biopsy specimens (3 antrum and 3 body each) were immediately frozen at −70°C until DNA extraction.
4. Operative link on gastritis assessment and operative link on gastric intestinal metaplasia assessment staging
Operative link on gastritis assessment (OLGA) or operative link on gastric intestinal metaplasia assessment (OLGIM) stages were made by histological examination of gastric biopsy samples (antrum and corpus) following the updated Sydney System [18]. Two independent gastrointestinal pathologists, who were blinded to clinical information, assessed the biopsies. if there was a disagreement, the biopsies were assessed by a third pathologist again.
5. DNA extraction, bisulfite modification, and MethyLight assay
Genomic DNA was extracted directly from noncancerous antral biopsy specimens using sodium bisulfite. The methodology was reported previously [19]. Briefly, specimens were homogenized in proteinase K solution (20 mmol/L Tris–HCl [pH 8.0], 10 mmol/L ethylenediaminetetraacetic acid, 0.5% sodium dodecyl sulfate, and 10 mg/mL proteinase K) using a sterile micropestle, followed by incubation for 3 hours at 52°C. DNA was isolated from homogenates using phenol/chloroform extraction and ethanol precipitation. Genomic DNA (1 μg) was bisulfite modified using the EZ DNA Methylation Kit (Zymo Research, Irvine, CA) by following the manufacturer’s instructions. The methylation status of MOS from bisulfite-modified DNA samples was quantified using real-time polymerase chain reaction–based MethyLight technology. MethyLight, as a sensitive, high-throughput methylation assay, allows the highly specific detection of methylation using probes that cover methylation sites, as well as methylation-specific primers [20]. The primer and probe sequences used in the reaction are as follows: forward primer sequence, TTCACTCCAACGACCCTAATATCC; backward primer sequence, GGGAAAATTCGTTTCGGAGGTAG; pro-be oligo sequence, 6FAM-AATACGATACCCTCGCCCCTA-ACCCTACG-BHQ-1 [19]. The quantified level of MOS was reported as a percentage of methylated reference, which is the relative methylation ratio of the target gene to the ALU gene of a sample, divided by the ratio of the target gene to the Alu gene of sodium bisulfite and CpG methyltransferase (M-SssI)–treated sperm DNA, multiplied by 100.
6. Statistical analysis
For sample size calculation, the expected incidence of metachronous recurrence in low-risk group (low methylation group) is presumed to be 0.01 per year, and that in high-risk group increases by 4-fold. Assuming that the ratio of the number of the low-risk and high-risk individuals is 1:1, the number of patients in each group was calculated as 131 at a statistical power of 0.80 with a two-sided significance level of 0.05. Considering a dropout rate of ~10%, the sample size was determined as 290 (145 in each group).
Continuous variables were presented as mean±standard deviation. Categorical variables were presented as numbers with proportions. To compare continuous variables, Student t test was used. For categorical variables, chi-square test was used for analysis. For determining the optimal diagnostic cutoff value on predicting metachronous recurrence, receiver operating characteristic curve was used. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. For survival analysis, Kaplan-Meier curves for cumulative incidences were used with log-rank test. Cox proportional hazard model was adopted under adjustment with clinically important variables. All the statistical analyses were performed using R ver. 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org). All tests were two-sided and p < 0.05 were considered statistically significant.
Results
1. Optimal cutoff value of MOS methylation level to predict metachronous recurrence
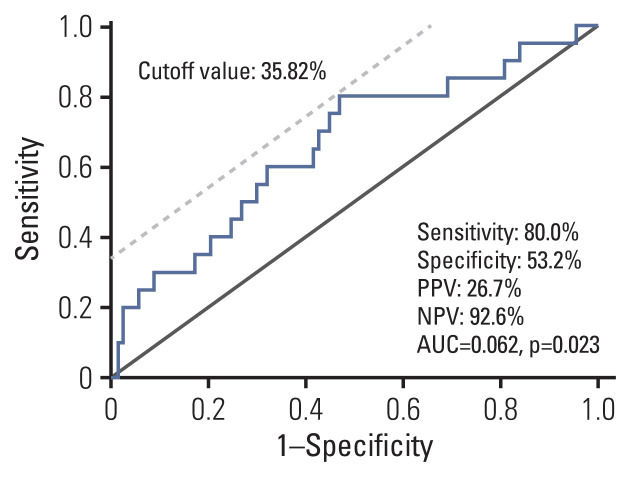
Among the study subjects who completed the study protocol (n=124), 20 metachronous gastric lesions (13 adenomas and 7 adenocarcinomas) were developed during the follow-up (median of the follow-up duration: 49.9 months [range, 13.1 to 96.2 months], median follow-up visits: 4.9 times). To determine the optimal cutoff value of MOS methylation level to predict metachronous recurrence, receiver operating characteristics curve analysis was performed (Fig. 1), and the optimal cutoff value was 35.82% (sensitivity, specificity, PPV, and NPV: 80.0%, 53.2%, 26.7%, and 92.6%, respectively.). In MOS methylation low group (n=74), eight metachronous recurrences (4 adenomas and 4 adenocarcinomas) were developed; in MOS methylation high group (n=50), 12 metachronous lesions (9 adenomas and 3 adenocarcinomas) were developed during the follow-up.
Fig. 1.
Receiver operating characteristic curve analysis to determine a cutoff value of MOS methylation level to predict the risk for metachronous recurrence (n=124). Optimal cutoff value was 35.82% and sensitivity, specificity, PPV and NPV were 80.0%, 53.2%, 26.7%, and 92.6%, respectively. AUC, area under curve; NPV, negative predictive value; PPV, positive predictive value.
2. Characteristics of the study subjects at baseline
The clinical and pathological characteristics of the study subjects at baseline were summarized in Table 1. There was no significant difference between the methylation high group (MOS methylation level ≥ 35.82%) and the methylation low group (methylation level < 35.82%) except for follow-up duration and follow-up visits (p < 0.001), which was attributed to a higher metachronous recurrence in the methylation high group.
Table 1.
Characteristics of the study subjects at baseline
| Variable | Total (n=294) | MOS methylation level (n=261) | p-value | |
|---|---|---|---|---|
| Low (n=99) | High (n=162) | |||
| Age (yr) | 63.2±8.7 | 62.4±9.1 | 64.1±8.6 | 0.132 |
| Male sex | 200 (68.0) | 68 (68.7) | 108 (66.7) | 0.735 |
| Follow-up duration (day) | 998.1±670.1 | 1,250.5±712.4 | 837.4±589.8 | < 0.001 |
| No. of endoscopic follow-up | 3.6±2.4 | 4.4±2.7 | 3.1±2.1 | < 0.001 |
| Helicobacter pylori positive | 110 (37.4) | 33 (33.3) | 63 (38.9) | 0.366 |
| Current or ex-smoker | 127 (43.2) | 57 (47.9) | 62 (52.1) | 0.572 |
| Current or ex-drinker | 158 (53.7) | 70 (48.3) | 75 (51.7) | 0.787 |
| Family history of GC in 1° relatives | 49 (17.4) | 20 (20.6) | 25 (16.2) | 0.378 |
| Body mass index (kg/m 2 ) | 24.4±3.2 | 24.3±3.5 | 24.4±3.0 | 0.678 |
| Education | ||||
| Elementary-Middle-High | 149 (65.6) | 62 (72.1) | 79 (64.2) | 0.232 |
| University | 78 (34.4) | 24 (27.9) | 55 (35.8) | |
| Pathology | ||||
| Low-grade dysplasia | 147 (50.0) | 44(44.9) | 77 (48.7) | 0.662 |
| High-grade dysplasia | 24 (8.2) | 7 (7.1) | 14 (8.9) | |
| Adenocarcinoma | 123 (41.8) | 47 (48.0) | 67 (42.4) | |
| OLGA stage | ||||
| Stage 0 | 26 (22.4) | 11 (28.2) | 13 (18.3) | 0.760 |
| Stage 1 | 28 (24.1) | 8 (20.5) | 18 (25.4) | |
| Stage 2 | 28 (24.1) | 8 (20.5) | 19 (26.8) | |
| Stage 3 | 22 (19.0) | 8 (20.5) | 13 (18.3) | |
| Stage 4 | 12 (10.3) | 4 (10.3) | 8 (11.8) | |
| OLGIM stage | ||||
| Stage 0 | 51 (17.3) | 21 (21.6) | 25 (16.0) | 0.430 |
| Stage 1 | 59 (20.1) | 16 (16.5) | 35 (22.4) | |
| Stage 2 | 87 (29.6) | 34 (35.1) | 44 (28.2) | |
| Stage 3 | 52 (17.7) | 15 (15.5) | 30 (19.2) | |
| Stage 4 | 35 (11.9) | 11 (11.3) | 22 (14.1) | |
| Synchronous EGCs/dysplasia a) | 25 (10.2) | 8 (8.7) | 16 (10.9) | |
Values are presented as mean±SD or number (%). p-values were calculated using chi-square test or Student’s t test. The cutoff value (35.82%) of high or low MOS methylation levels was determined by receiver operating curve analysis. Statistically significant at p < 0.001. EGC, early gastric cancer; GC, gastric cancer; OLGA, operative link on gastritis assessment; OLGIM, operative link on gastric intestinal metaplasia assessment; SD, standard deviation.
Synchronous lesions were defined as secondary dysplasia or cancers detected within 1 year after initial diagnosis.
Also, the clinicopathological characteristics of the 124 patients completed the study protocol according to meta-chronous recurrence were presented in S1 Table. In patients with metachronous recurrence, initial pathology was low- or high-grade dysplasia rather than adenocarcinoma (p < 0.001), and synchronous lesions (dysplasia or EGCs) were more prevalent (p=0.053). OLGA and OLGIM stages were not different between the two groups (p > 0.05), but MOS methylation level was higher in patients with metachronous recurrence (p=0.009).
3. Association between MOS methylation level and clinical and histologic variables
Next, we evaluated whether MOS methylation levels were different by age, family history of GC, synchronous gastric lesions, current H. pylori infection, and OLGA and OLGIM stages. There was no correlation between age and MOS methylation level (Pearson’s correlation coefficient=0.063, p=0.312) (Fig. 2A). Family history of GC in 1° relatives, synchronous gastric neoplasms, current H. pylori infection did not affect MOS methylation levels (p > 0.05) (Fig. 2B-D). In contrast, MOS methylation levels correlated with OLGA or OLGIM stages (Spearman’s ρ=0.240 and 0.174, respectively, both p < 0.05) (Fig. 2E and F).
Fig. 2.
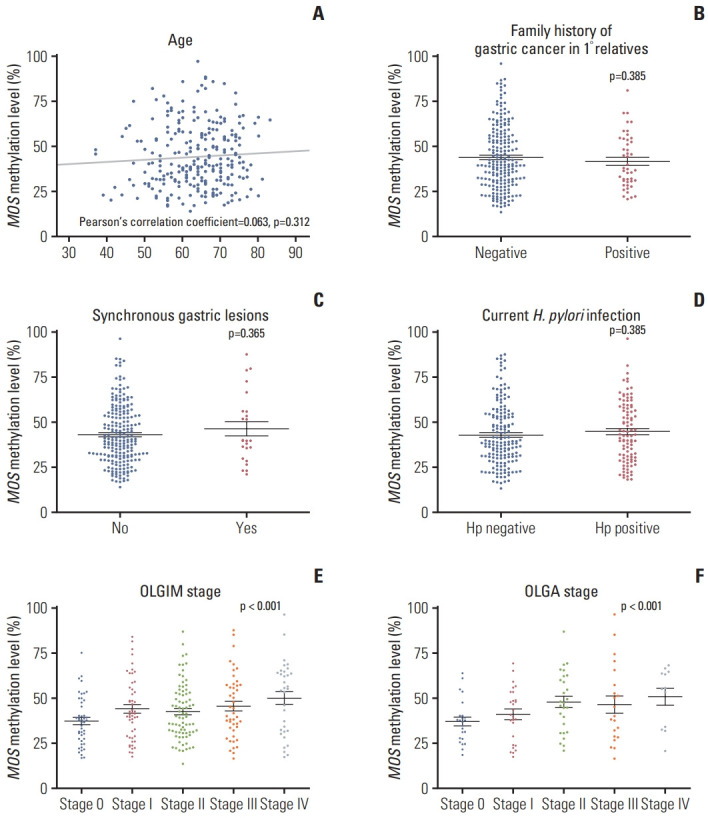
MOS methylation levels according to age (A), family history of gastric cancer (B), synchronous gastric lesions (C), current Helicobacter pylori infection (D), and OLGA (E) and OLGIM (F) stages (n=261). OLGA, operative link on gastritis assessment; OLGIM, operative link on gastric intestinal metaplasia assessment.
4. Clinical implication of mucosal atrophy, intestinal metaplasia, and MOS methylation in the prediction of meta-chronous gastric recurrence after endoscopic resection
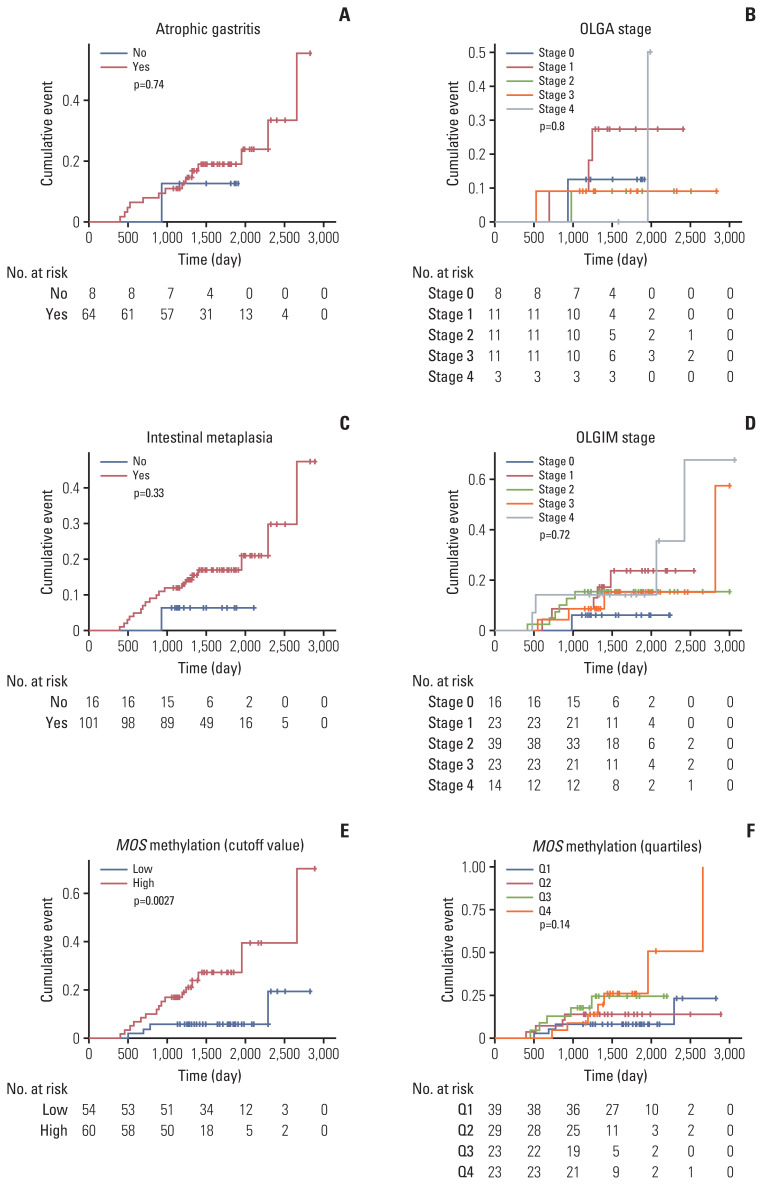
Then, we evaluated whether atrophic gastritis, intestinal metaplasia, or MOS methylation level could predict the metachronous recurrence after ER of gastric neoplasms (Table 2, Fig. 3). Kaplan-Meier curves for cumulative incidences of metachronous recurrence showed that presence or absence of atrophic gastritis and intestinal metaplasia did not predict the risk for metachronous recurrence in this high-risk population (Fig. 3A and C). Also, OLGA and OLGIM stages were not useful in predicting the risk (Fig. 3B and D); if the analysis was performed comparing low-risk (grade 0 to 2) and high-risk (grade 3 and 4) groups, it was not statistically significant (S2 Fig.).
Table 2.
Univariate and multivariate Cox proportional regression analyses of the metachronous recurrence (n=124)
| Crude HR (95% CI) | p-value | p for trend | Adjusted HRa) (95% CI) | p-valuea) | p for trend | |
|---|---|---|---|---|---|---|
| MOS high b) | 4.73 (1.56–14.40) | 0.006c) | 4.76 (1.54–14.79) | 0.007c) | ||
| Atrophic gastritis | 1.40 (0.18–10.88) | 0.746 | 1.31 (0.16–10.75) | 0.802 | ||
| Intestinal metaplasia | 2.62 (0.35–19.74) | 0.349 | 2.32 (0.30–18.29) | 0.423 | ||
| MOS quartile | ||||||
| Q1 | 1 (reference) | 1 (reference) | ||||
| Q2 | 1.64 (0.41–6.61) | 0.485 | 1.42 (0.35–5.87) | 0.624 | ||
| Q3 | 3.36 (0.87–13.03) | 0.080 | 3.11 (0.78–12.46) | 0.109 | ||
| Q4 | 3.53 (1.02–12.22) | 0.047 | 0.027c) | 3.29 (0.94–11.53) | 0.062 | 0.034c) |
| OLGA stage | ||||||
| 0 | 1 (reference) | 1 (reference) | ||||
| 1 | 1.79 (0.18–17.56) | 0.615 | 1.65 (0.13–20.16) | 0.697 | ||
| 2 | 0.61 (0.04–9.90) | 0.728 | 0.57 (0.03–10.28) | 0.705 | ||
| 3 | 0.60 (0.04–27.31) | 0.719 | 0.51 (0.02–10.59) | 0.665 | ||
| 4 | 1.56 (0.09–27.31) | 0.761 | 0.689 | 1.63 (0.09–30.55) | 0.745 | 0.677 |
| OLGIM stage | ||||||
| 0 | 1 (reference) | 1 (reference) | ||||
| 1 | 3.26 (0.38–27.93) | 0.282 | 2.95 (0.33–26.56) | 0.334 | ||
| 2 | 2.22 (0.27–18.54) | 0.461 | 2.10 (0.24–18.20) | 0.503 | ||
| 3 | 2.21 (0.24–20.15) | 0.481 | 1.75 (0.18–16.83) | 0.627 | ||
| 4 | 3.78 (0.42–34.44) | 0.238 | 0.452 | 3.38 (0.34–33.17) | 0.297 | 0.617 |
CI, confidence interval; HR, hazard ratio; OLGA, operative link on gastritis assessment; OLGIM, operative link on gastric intestinal metaplasia assessment.
Adjusted for age, sex, Helicobacter pylori infection status, and smoking,
The cutoff value (35.82%) of high or low MOS methylation levels was determined by receiver operating curve analysis. Atrophic gastritis and intestinal metaplasia were defined as the presence of histologic atrophy (score 1–3) and intestinal metaplasia (score 1–3), respectively, at either antrum or corpus by the updated Sydney scoring system,
Statistically significant.
Fig. 3.
Kaplan-Meier curves for cumulative incidences of metachronous recurrence according to atrophic gastritis (A), OLGA stage (B), intestinal metaplasia (C), OLGIM stage (D), MOS methylation status (E, F, n=124). Atrophic gastritis and intestinal metaplasia were defined as the presence of histologic atrophy (score 1–3) and intestinal metaplasia (score 1–3), respectively, at either antrum or corpus by the updated Sydney scoring system. The cutoff value (35.82%) of high or low level of MOS methylation was determined by receiver operating characteristic curve analysis. OLGA, operative link on gastritis assessment; OLGIM, operative link on gastric intestinal metaplasia assessment.
In contrast, MOS methylation could be useful to determine the high-risk group in metachronous recurrence. That is, MOS methylation high group (≥ 34.82%) had a significantly increased risk for metachronous recurrence compared to MOS methylation low group (adjusted hazard ratio [HR], 4.76; 95% confidence interval [CI], 1.54 to 14.79; p=0.007) (Table 2). In adjusted Cox proportional regression model, the risk of metachronous recurrence significantly increased in the highest quartile level (Q4) compared with the lowest quartile level (Q1) (HR, 3.53; 95% CI, 1.02 to 12.22; p=0.047). However, this was not statistically significant after adjusting for age, sex, H. pylori infection, and smoking (p=0.062) (Table 2). Nevertheless, a significant increasing linear trend was observed between MOS methylation and the risk of meta-chronous recurrence (adjusted p for trend=0.034).
When the same analyses were performed in the entire cohort (n=261), the results were not different (S3 Table, S4 Fig.).
Discussion
This study showed that MOS methylation could be useful in predicting metachronous recurrence after H. pylori eradication in the high-risk patients who had undergone ER of gastric neoplasm. The patients who underwent ER of EGC or gastric dysplasia are regarded as a high-risk population of metachronous gastric neoplasms [15]. In the previous studies, the incidence of metachronous GC was reported to be 1.9%–25.3% when observed up to 4–7 years [21], and H. pylori eradication reduced the incidence of metachronous GC by ~50% [12]. However, metachronous recurrence still develops even after H. pylori eradication; thus, we need a surrogate marker for the risk of metachronous GC after H. pylori eradication [15].
Differentiated GCs are frequently found after H. pylori eradication, showing characteristic endoscopic features such as reddish depression; benign reddish depression is difficult to be distinguished from GC because of the histological alterations in the surface structures (non-neoplastic epithelium or epithelium with low-grade atypia) as well as multiple appearances of benign reddish depression [22]. Furthermore, submucosal invasive cancers were not infrequently found after H. pylori eradication despite of the annual endoscopic surveillance [22]. In this study, all cases of metachronous recurrence (n=20) were either gastric dysplasia or EGC; six of seven metachronous gastric cancers (85.7%) were differentiated gastric cancers, but three cases (42.9%) invaded submucosa.
There have been several studies that aberrant DNA methylation could be a surrogate marker for the risk of metachronous GC [6,23]. Previously, a Japanese group published the impact of aberrant DNA methylation accumulation on metachronous GC in a 5-year follow-up of a multicenter prospective cohort study [24,25]. They showed that the higher quartiles of methylation levels in miR-124a-3, EMX1, and MKX6-1 showed an increased risk for metachronous GCs. Another study has shown that aberrant methylation of microRNA-34b/c is a predictive marker of metachronous GC risk [23].
In the present study, the rationale for choosing MOS methylation as a marker is based on the results of previous studies. Previously, we evaluated the usefulness of several candidate methylation markers to define a high-risk group for GC [8]. Among them, methylation of MOS was associated with the duration of H. pylori exposure. MOS methylation was also increased in remote past infection in which H. pylori disappeared in gastric mucosa, and it was significantly increased in patients with GC regardless of H. pylori infection [9]. Interestingly, MOS methylation decreased after H. pylori eradication in controls, but it remained significantly increased in patients with gastric dysplasia or GC even after H. pylori eradication [10]. In a retrospective study, we have shown that MOS methylation levels at baseline were significantly higher among patients with metachronous gastric neoplasms [26].
We paid attention to the results of previous studies in that there are two types of methylation occurring in the gastric mucosa. One is temporary components of methylation (induced in progenitor or differentiated cells) and the other is permanent components (induced in stem cells) [2,4]. During active H. pylori infection, both temporary and permanent components of methylation increase as the duration of infection increases. When H. pylori infection discontinues, the temporary component will disappear, leaving only the permanent component. The remaining permanent components correlate with the risk of developing gastric cancers.
From this point of view, MOS methylation could be an ideal marker for predicting the risk of GC. The MOS methylation we analyzed in this study does not originate from the promoter region (promoter CpG island), but the exon region [8]. Although methylation of some marker genes is not directly involved in carcinogenesis, their methylation levels correlate with those of tumor-suppressor genes and thus GC risks. Methylation of a marker gene is not requisite for gastric carcinogenesis [4]. Methylation levels of MOS in GC tissues did not correlate with those in their background gastric mucosa. Rather, we found that hypomethylation of MOS in GC tissues was associated with tumor invasion, nodal metastasis, and undifferentiated histology, suggesting that MOS methylation occurs in a complex manner depending on the stages of gastric carcinogenesis [9].
In the present study, MOS methylation was not affected by age (Table 1, Fig. 2). Therefore, MOS methylation might not be an aging process. There was no significant difference in MOS methylation level between H. pylori–positive and –negative patients. This is because most of the subjects were high-risk patients in this study. Even if some of them had no evidence of active H. pylori infection at present, most of them might be in remote past H. pylori infection [27]. Likely, MOS methylation levels did not differ according to the presence or absence of synchronous gastric neoplasm.
In contrast, MOS methylation level positively correlated with OLGA and OLGIM staging (Fig. 2). Atrophic gastritis and intestinal metaplasia are not only important precancerous lesions of GC but have been reported to be significantly associated with the occurrence of metachronous GC [13,28]. In this study, however, OLGA and OLGIM stages failed to show the relations to metachronous recurrence. This might be attributed to the fact that the frequencies of patients with high OLGA and OLGIM stages (stage 3–4) at baseline were much lower than those reported in GC patients (Table 1). In contrast, we found that MOS methylation may predict the risk of metachronous gastric neoplasms better than atrophy or metaplasia (Table 2, Fig. 3). Unlikely with the previous studies, the reason of insignificant results in atrophic gastritis and intestinal metaplasia might be attributed to the relatively small sample size; if the sample size is sufficiently large, significant results could be shown for atrophic gastritis and metaplasia as well. However, the fact that MOS methylation was found to be significantly related to the risk for metachronous recurrence despite the relatively small sample size in this study indicates that MOS methylation can be a more powerful marker to predict the recurrence of metachronous gastric neoplasms after endoscopic resection. Recently, we found that metachronous GC occurred in the 35 patients among 3,044 patients (1.1%) in the remaining stomach after curative gastric partial resection with GC [29]. In this population, the metachronous GC was only related to older age and surgical methods used. Thus, it might be valuable to perform further study whether the MOS methylation can be beneficial in predicting the metachronous recurrence after gastrectomy.
Our study has several limitations as the following. First, the sample size was relatively small. In addition, the dropout rate (follow-up loss within 3 years after initial endoscopic treatment) was much higher than expected (137/261, 52.5%). In South Korea, it is recommended that the patients be returned to the local clinic for screening endoscopy if there are no problems after endoscopic treatment. As a result, many subjects were dropped out, and only 124 subjects were followed up for more than 3 years. Thus, this study might be underpowered. Nevertheless, MOS methylation showed statistically significant results. In addition, the results were not different when the survival analyses were performed in the entire cohort (n=261) (S3 Table, S4 Fig.). However, the results of our study should be verified through a large prospective study. Second, serum gastrin-17, anti–H. pylori IgG antibody, and pepsinogen I/II levels were not measured in this study. They have been shown to be a surrogate marker of metachronous recurrence after ER of EGC [30,31]. Third, H. pylori–positive rate was relatively low (~37%) for the study population, which was EGC or dysplasia patients. It might be because most of the patients who were H. pylori–negative in this study were patients with a remote past infection. However, since OLGA and OLGIM stages were not high at baseline, there is a possibility that H. pylori infection rate was actually low. Fourth, the interpretation of OLGA and OLGIM staging should be cautious because gastric mucosae were not obtained at gastric angle. Furthermore, OLGA staging was possible in 110 of 261 (42.1%) patients only, because in many cases either antrum or corpus biopsy specimen was inappropriate to assess the degree of atrophy. Despite these limitations, the results of this study show the possibility of MOS methylation as a surrogate marker for metachronous gastric neoplasms, and also prove the importance of aberrant DNA methylation in gastric carcinogenesis.
In conclusion, MOS methylation can be a promising marker for predicting metachronous gastric neoplasms after ER of gastric neoplasms. To confirm the usefulness of MOS methylation, large prospective studies (validation studies) are warranted in the future.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant for the Global Core Research Center (GCRC) funded by the Korea government (MSIP) (No. 2011-0030001).
Footnotes
Ethical Statement
The study protocol was approved by the Ethical Committee at Seoul National University Bundang Hospital (IRB No. B1204/152-005). All study participants signed a consent form before enrolling in the study.
Author Contributions
Conceived and designed the analysis: Shin CM, Kim N, Lee DH.
Collected the data: Shin CM, Yoon H, Choi YJ, Park YS.
Contributed data or analysis tools: Kim N, Park JH, Lee DH.
Performed the analysis: Shin CM, Park JH.
Wrote the paper: Shin CM.
Revised the manuscript: Kim N, Yoon H, Choi YJ, Park YS, Lee DH.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima T, Yamashita S, Maekita T, Niwa T, Nakazawa K, Ushijima T. The presence of a methylation fingerprint of Helicobacter pylori infection in human gastric mucosae. Int J Cancer. 2009;124:905–10. doi: 10.1002/ijc.24018. [DOI] [PubMed] [Google Scholar]
- 3.Shin CM, Kim N, Jung Y, Park JH, Kang GH, Kim JS, et al. Role of Helicobacter pylori infection in aberrant DNA methylation along multistep gastric carcinogenesis. Cancer Sci. 2010;101:1337–46. doi: 10.1111/j.1349-7006.2010.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ushijima T. Epigenetic field for cancerization. J Biochem Mol Biol. 2007;40:142–50. doi: 10.5483/bmbrep.2007.40.2.142. [DOI] [PubMed] [Google Scholar]
- 5.Padmanabhan N, Ushijima T, Tan P. How to stomach an epigenetic insult: the gastric cancer epigenome. Nat Rev Gastroenterol Hepatol. 2017;14:467–78. doi: 10.1038/nrgastro.2017.53. [DOI] [PubMed] [Google Scholar]
- 6.Grady WM, Yu M, Markowitz SD. Epigenetic alterations in the gastrointestinal tract: current and emerging use for biomarkers of cancer. Gastroenterology. 2021;160:690–709. doi: 10.1053/j.gastro.2020.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leja M, Line A. Early detection of gastric cancer beyond endoscopy: new methods. Best Pract Res Clin Gastroenterol. 2021;50–51:101731. doi: 10.1016/j.bpg.2021.101731. [DOI] [PubMed] [Google Scholar]
- 8.Shin CM, Kim N, Jung Y, Park JH, Kang GH, Park WY, et al. Genome-wide DNA methylation profiles in noncancerous gastric mucosae with regard to Helicobacter pylori infection and the presence of gastric cancer. Helicobacter. 2011;16:179–88. doi: 10.1111/j.1523-5378.2011.00838.x. [DOI] [PubMed] [Google Scholar]
- 9.Shin CM, Kim N, Park JH, Kang GH, Kim JS, Jung HC, et al. Prediction of the risk for gastric cancer using candidate methylation markers in the non-neoplastic gastric mucosae. J Pathol. 2012;226:654–65. doi: 10.1002/path.2990. [DOI] [PubMed] [Google Scholar]
- 10.Shin CM, Kim N, Lee HS, Park JH, Ahn S, Kang GH, et al. Changes in aberrant DNA methylation after Helicobacter pylori eradication: a long-term follow-up study. Int J Cancer. 2013;133:2034–42. doi: 10.1002/ijc.28219. [DOI] [PubMed] [Google Scholar]
- 11.Kim SG, Lyu DH, Park CM, Lee NR, Kim J, Cha Y, et al. Current status of endoscopic submucosal dissection for early gastric cancer in Korea: role and benefits. Korean J Intern Med. 2019;34:785–93. doi: 10.3904/kjim.2017.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018;378:1085–95. doi: 10.1056/NEJMoa1708423. [DOI] [PubMed] [Google Scholar]
- 13.Take S, Mizuno M, Ishiki K, Kusumoto C, Imada T, Hamada F, et al. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J Gastroenterol. 2020;55:281–8. doi: 10.1007/s00535-019-01639-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan MC, Graham DY. Gastric cancer risk stratification and surveillance after Helicobacter pylori eradication: 2020. Gastrointest Endosc. 2019;90:457–60. doi: 10.1016/j.gie.2019.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim N. Chemoprevention of gastric cancer by Helicobacter pylori eradication and its underlying mechanism. J Gastroenterol Hepatol. 2019;34:1287–95. doi: 10.1111/jgh.14646. [DOI] [PubMed] [Google Scholar]
- 16.Hwang YJ, Choi Y, Kim N, Lee HS, Yoon H, Shin CM, et al. The difference of endoscopic and histologic improvements of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication. Dig Dis Sci. 2022;67:3055–66. doi: 10.1007/s10620-021-07146-4. [DOI] [PubMed] [Google Scholar]
- 17.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Yun CY, Kim N, Lee J, Lee JY, Hwang YJ, Lee HS, et al. Usefulness of OLGA and OLGIM system not only for intestinal type but also for diffuse type of gastric cancer, and no interaction among the gastric cancer risk factors. Helicobacter. 2018;23:e12542. doi: 10.1111/hel.12542. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Kim N, Kim HW, Park JH, Shin CM, Lee DH. Promising aberrant DNA methylation marker to predict gastric cancer development in individuals with family history and long-term effects of H. pylori eradication on DNA methylation. Gastric Cancer. 2021;24:302–13. doi: 10.1007/s10120-020-01117-w. [DOI] [PubMed] [Google Scholar]
- 20.Trinh BN, Long TI, Laird PW. DNA methylation analysis by MethyLight technology. Methods. 2001;25:456–62. doi: 10.1006/meth.2001.1268. [DOI] [PubMed] [Google Scholar]
- 21.Nishizawa T, Yahagi N. Long-term outcomes of using endoscopic submucosal dissection to treat early gastric cancer. Gut Liver. 2018;12:119–24. doi: 10.5009/gnl17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito M, Tanaka S, Chayama K. Characteristics and Early Dia-gnosis of gastric cancer discovered after Helicobacter pylori eradication. Gut Liver. 2021;15:338–45. doi: 10.5009/gnl19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki R, Yamamoto E, Nojima M, Maruyama R, Yamano HO, Yoshikawa K, et al. Aberrant methylation of microRNA-34b/c is a predictive marker of metachronous gastric cancer risk. J Gastroenterol. 2014;49:1135–44. doi: 10.1007/s00535-013-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asada K, Nakajima T, Shimazu T, Yamamichi N, Maekita T, Yokoi C, et al. Demonstration of the usefulness of epigenetic cancer risk prediction by a multicentre prospective cohort study. Gut. 2015;64:388–96. doi: 10.1136/gutjnl-2014-307094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda M, Nakajima T, Oda I, Shimazu T, Yamamichi N, Maekita T, et al. High impact of methylation accumulation on metachronous gastric cancer: 5-year follow-up of a multicentre prospective cohort study. Gut. 2017;66:1721–3. doi: 10.1136/gutjnl-2016-313387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon H, Kim N, Shin CM, Lee HS, Kim BK, Kang GH, et al. Risk factors for metachronous gastric neoplasms in patients who underwent endoscopic resection of a gastric neoplasm. Gut Liver. 2016;10:228–36. doi: 10.5009/gnl14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang HY, Kim N, Park YS, Hwang JH, Kim JW, Jeong SH, et al. Progression of atrophic gastritis and intestinal metaplasia drives Helicobacter pylori out of the gastric mucosa. Dig Dis Sci. 2006;51:2310–5. doi: 10.1007/s10620-006-9276-0. [DOI] [PubMed] [Google Scholar]
- 28.Maeda M, Moro H, Ushijima T. Mechanisms for the induction of gastric cancer by Helicobacter pylori infection: aberrant DNA methylation pathway. Gastric Cancer. 2017;20:8–15. doi: 10.1007/s10120-016-0650-0. [DOI] [PubMed] [Google Scholar]
- 29.Choi Y, Kim N, Yoon H, Shin CM, Park YS, Lee DH, et al. The incidence and risk factors for metachronous gastric cancer in the remnant stomach after gastric cancer surgery. Gut Liver. 2022;16:366–74. doi: 10.5009/gnl210202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764–8. doi: 10.1136/gut.2004.055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terasawa T, Nishida H, Kato K, Miyashiro I, Yoshikawa T, Takaku R, et al. Prediction of gastric cancer development by serum pepsinogen test and Helicobacter pylori seropositivity in Eastern Asians: a systematic review and meta-analysis. PLoS One. 2014;9:e109783. doi: 10.1371/journal.pone.0109783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.