Abstract
Background
Tourniquet-induced ischemia and reperfusion (I/R) has been related to postoperative muscle atrophy through mechanisms involving protein synthesis/breakdown, cellular metabolism, mitochondrial dysfunction, and apoptosis. Ischemic preconditioning (IPC) could protect skeletal muscle against I/R injury. This study aims to determine the underlying mechanisms of IPC and its effect on muscle strength after total knee arthroplasty (TKA).
Methods
Twenty-four TKA patients were randomized to receive either sham IPC or IPC (3 cycles of 5-min ischemia followed by 5-min reperfusion). Vastus medialis muscle biopsies were collected at 30 min after tourniquet (TQ) inflation and the onset of reperfusion. Western blot analysis was performed in muscle protein for 4-HNE, SOD2, TNF-ɑ, IL-6, p-Drp1ser616, Drp1, Mfn1, Mfn2, Opa1, PGC-1ɑ, ETC complex I-V, cytochrome c, cleaved caspase-3, and caspase-3. Clinical outcomes including isokinetic muscle strength and quality of life were evaluated pre- and postoperatively.
Results
IPC significantly increased Mfn2 (2.0 ± 0.2 vs 1.2 ± 0.1, p = 0.001) and Opa1 (2.9 ± 0.3 vs 1.9 ± 0.2, p = 0.005) proteins expression at the onset of reperfusion, compared to the ischemic phase. There were no differences in 4-HNE, SOD2, TNF-ɑ, IL-6, p-Drp1ser616/Drp1, Mfn1, PGC-1ɑ, ETC complex I–V, cytochrome c, and cleaved caspase-3/caspase-3 expression between the ischemic and reperfusion periods, or between the groups. Clinically, postoperative peak torque for knee extension significantly reduced in the sham IPC group (−16.6 [-29.5, −3.6] N.m, p = 0.020), while that in the IPC group was preserved (−4.7 [-25.3, 16.0] N.m, p = 0.617).
Conclusion
In TKA with TQ application, IPC preserved postoperative quadriceps strength and prevented TQ-induced I/R injury partly by enhancing mitochondrial fusion proteins in the skeletal muscle.
The translational potential of this article
Mitochondrial fusion is a potential underlying mechanism of IPC in preventing skeletal muscle I/R injury. IPC applied before TQ-induced I/R preserved postoperative quadriceps muscle strength after TKA.
Keywords: Ischemia reperfusion injury, Ischemic preconditioning, Knee arthroplasty, Mitochondrial dynamics, Tourniquet
Abbreviations: 4-HNE, 4-hydroxy-2-nonenal; ADP, Adenosine diphosphate; ASA, American Society of Anesthesiologists; ATP, Adenosine triphosphate; BSA, Bovine serum albumin; CAT, Catalase; CHOP, C/EBP homologous protein; Drp1, Dynamin-related protein-1; ER, Endoplasmic reticulum; ETC, Electron transport chain; Fis1, Fission protein-1; FGF21, Fibroblast growth factor 21; GPx, Glutathione peroxidase; IL-6, Interleukin-6; IPACK, Interspace between the popliteal artery and capsule of the posterior knee; IPC, Ischemic preconditioning; I/R, Ischemia and reperfusion; MDA, Malondialdehyde; Mfn, Mitofusin; MnSOD, Manganese superoxide dismutase; mPTP, Mitochondrial permeability transition pore; NF-κB, Nuclear factor kappa B; OXPHOS, Oxidative phosphorylation; PGC-1ɑ, Peroxisome proliferator-activated receptor-gamma coactivator-1ɑ; RIPC, Remote ischemic preconditioning; ROS, Reactive oxygen species; SBP, Systolic blood pressure; SOD, Superoxide dismutase; TKA, Total knee arthroplasty; TNF, Tumor necrosis factor; TQ, Tourniquet; UPR, Unfolded protein response
Clinical Trial Number and Registry URL: clinicaltrials.in.th; TCTR20190128001.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Data availability statement: The authors confirm that the data supporting the findings of this study are available within the article.
1. Introduction
Skeletal muscle ischemia and reperfusion (I/R) is routinely induced by a tourniquet (TQ) application during extremity surgery. Even though its use in total knee arthroplasty (TKA) has shown benefits by shortening surgical time, improving quality of cementation, reducing intraoperative blood loss, and restricting blood transfusion, TQ negatively affects quadriceps and hamstring muscles volume and strength which are consequently related to the impaired or delayed return of functional activities [[1], [2], [3]]. Limitation of the TQ inflation time to 90 min for a lower extremity is recommended in clinical practice to avoid ischemic injury to the human skeletal muscle [4]. However, changes in genomic responses in the insulted skeletal muscle have been reported at 45–60 min of ischemia in TKA patients [3,[5], [6], [7], [8]].
Muscle atrophy following TKA with TQ has been linked to alterations of multiple signaling pathways in the skeletal muscle cells. These cascades of cellular events induce a reduction in protein synthesis [3], increase in protein degradation [5], upregulation of the genes in cell stress pathways [7], and induction of endoplasmic reticulum (ER) stress [6]. As a part of mitochondrial quality control, mitochondrial dynamics is also a key process in modulating skeletal muscle health [9]. Alterations of mitochondrial dynamics and morphology have been determined to play a causal role in aging sarcopenia and other muscle-wasting conditions [[9], [10], [11], [12]]. In aging rat skeletal muscles, the levels of dynamin-related protein-1 (Drp1), mitochondrial fission protein-1 (Fis1), and mitofusin-2 (Mfn2) proteins are upregulated, and these are linked to a decline in muscle mass and strength [10]. Morphologically, aging increases fragmented subsarcolemmal and intermyofibrillar mitochondria in oxidative muscle fibers, while showing enlarged subsarcolemmal mitochondria and branched intermyofibrillar mitochondria in glycolytic fibers. On the other hand, deletion of Drp1 decreases adenosine diphosphate (ADP)-stimulated respiration, increases oxidative stress, alters calcium homeostasis, induces ER stress, reduces protein synthesis, inhibits autophagy, and consequently produces muscle wasting [11,12]. Accordingly, changes in mitochondrial fission and fusion proteins, causing mitochondrial dynamics imbalance, seem to be distinctive among various settings. The role of mitochondrial dynamics in skeletal muscle weakness following TQ-induced I/R has not been elucidated.
Ischemic preconditioning (IPC), which occurs when tissues are exposed to a short period of ischemia and then followed by a restoration of blood flow, prevents the I/R injury by rendering the preconditioned tissues resistant to subsequent I/R. IPC has shown its protective effects against skeletal muscle I/R injury in preclinical and clinical studies [2,[13], [14], [15], [16], [17]]. In rat skeletal muscle, IPC enhanced maximal oxidative capacities [13], downregulated Bax and Bax/Bcl2 genes [15], upregulated superoxide dismutase (SOD), glutathione peroxidase (GPx) as well as catalase (CAT) genes [17], and increased ER stress-related C/EBP homologous protein (CHOP) [17]. These findings indicated that IPC restored mitochondrial respiratory complex activities, normalized pro-apoptotic genes, induced enzymatic antioxidant defense, and enhanced elimination of damaged cells by activation of an ER stress response [13,15,17]. In human myocytes, the genomic response to IPC has been identified in TKA patients [14,16]. The results demonstrated that IPC upregulated the mitochondrial biogenesis and antioxidant genes, while downregulated the pro-apoptotic genes [14]. No previous study has investigated the mechanisms related to mitochondrial dynamics of IPC in preventing I/R injury.
Thus, this study aims to determine the effects of IPC on the expression of skeletal muscle proteins involving mitochondrial biogenesis, mitochondrial dynamics, apoptosis, oxidative stress, and inflammation at 30 min after TQ inflation and the onset of reperfusion. Moreover, the secondary objective was to investigate the effects of IPC on postoperative functional outcomes including thigh muscle strength and quality of life.
2. Materials and methods
2.1. Patient recruitment and randomization
This randomized, placebo-controlled trial was approved by the Ethics Committee of Faculty of Medicine, Chiang Mai University (30/01/2019) and registered at clinicaltrials.in.th (24/01/2019, TCTR20190128001) before patient enrollment. Primary knee osteoarthritis patients with ages ranging from 60 to 80 years who were scheduled for unilateral TKA were screened for eligibility of recruitment. Patients with any of these conditions: body mass index >35 kg/m2, current use of antioxidants or steroids, atherosclerotic diseases, neurodegenerative diseases, malignancy diseases, chronic renal disease, and active heavy smoking, were excluded. Informed consent was obtained from all individual participants included in the study. Patients who signed the consent were randomly allocated to receive either the sham IPC or IPC. An opaque sealed envelope containing a computer-generated random number was disclosed 1 h before the operation.
2.2. Study intervention
Before spinal anesthesia, the sham IPC or IPC was performed in the anesthesia induction room. A research investigator who did not involve in the anesthesia care and outcome assessment applied the TQ cuff on the patient's operated thigh. For patients in the IPC group, the cuff was inflated to 100 mmHg above systolic blood pressure (SBP) for 5 min and deflated for 5 min. This intermittent inflation and deflation were repeated for three cycles. For patients in the sham IPC group, the unpressurized cuff was applied for 30 min.
2.3. Anesthesia and surgical protocol
Standard American Society of Anesthesiologists (ASA) monitors were applied throughout the operation. All patients received spinal anesthesia (0.5% isobaric bupivacaine; 10–15 mg), interspace between the popliteal artery and capsule of the posterior knee (IPACK) block (0.25% bupivacaine with 1:200,000 epinephrine; 20 mL), and femoral triangle block (0.25% bupivacaine with 1:200,000 epinephrine; 20 mL) preoperatively. Midazolam and oxygen supplements were administered intraoperatively if required. Intraoperative thigh TQ was inflated to 100 mmHg above SBP before the anterior longitudinal midline incision. The medial parapatellar approach of TKA was performed by two orthopedic surgeons (NS, KK). The first muscle biopsy was taken from the vastus medialis muscle at exactly 30 min after ischemia. The second muscle biopsy was dissected at the onset of TQ release, which was within 5 min after the closure of parapatellar arthrotomy.
2.4. Biochemical outcomes
The western blot analysis was performed in muscle protein. A 600 μg/mL of protein was mixed with loading buffer, loaded onto 10% SDS-Acrylamide gels, and transferred to nitrocellulose membranes in a glycine/methanol-transfer buffer using a Wet/Tank blotting system (Bio-Rad Laboratories, CA, USA). Membranes were blocked in 5% Bovine Serum Albumin (BSA) in Tris-Buffered saline and tween buffer. To detect the level of protein expression, the membranes were incubated with antibodies used for immunoblotting: 4-HNE, SOD2, TNF-ɑ, IL-6, p-Drp1ser616, Drp1, Mfn1, Mfn2, Opa1, PGC-1ɑ, ETC complex I–V, cytochrome c, cleaved caspase-3 and caspase-3 (1:1000 dilution), and actin (1:2000 dilution, Santa Cruz Biotechnology) was used as an internal control. Bound antibodies were detected using horseradish peroxidase-conjugated with either anti-rabbit IgG (1:2000 dilution). The membranes were exposed to an ECL Western blotting substrate (Bio-Rad Laboratories, CA, USA), and the densitometric analysis was performed using a ChemiDoc Touch Imaging System (Bio-Rad Laboratories, CA, USA).
2.5. Functional outcomes
Muscle strength was assessed using an isokinetic dynamometer (Con-Trex MJ; CMV AG, Zurich, Switzerland). Torque measurements were performed twice at two weeks before and four weeks after surgery. Average peak torque for concentric isokinetic knee extension (quadriceps) and flexion (hamstring) at an angular velocity of 60°/s (Newton-meter) were measured.
Health-related quality of life was evaluated using the Thai version of the EQ-5D instrument [18]. The 5-level EQ-5D version (EQ-5D-5L) consists of two pages: the EQ-5D descriptive system and the EQ visual analog scale (EQ-VAS). After receiving permission from the instrument developer, the EQ-5D-5L test was performed twice at two weeks before and four weeks after surgery.
2.6. Statistical analysis
Data were analyzed using STATA 16 software (StataCorp LLC, TX, USA). Continuous variables were examined for normality using the Shapiro–Wilk normality test. Two-sample t-test or Wilcoxon rank-sum test was performed to assess differences between two groups. Differences between preoperative and postoperative data were analyzed using the dependent-sample t-test. Fisher's exact test was used to assessed differences of categorical data. Differences associated with p-value < 0.05 were considered significant.
3. Results
Twenty-four patients were recruited from February 2019 to August 2020. No patients were excluded from the analysis (Fig. 1). Demographic and intraoperative data including TQ pressure and duration were comparable between the sham IPC and IPC groups as shown in Table 1.
Figure 1.
CONSORT Flow for screening, exclusion, allocation, and lost to follow-up of the study participants
Abbreviations: IPC, ischemic preconditioning.
Table 1.
Demographic data of knee osteoarthritis patients receiving sham IPC vs IPC.
| Variables | Sham IPC (n = 10) | IPC (n = 14) | Mean difference (95% CI) | p-value |
|---|---|---|---|---|
| Age (y), mean ± SD | 66.1 ± 5.5 | 66.8 ± 6.3 | −0.7 (−5.8, 4.4) | 0.784 |
| BMI (kg/m2), mean ± SD | 27.1 ± 3.7 | 25.3 ± 3.5 | 1.9 (−1.2, 4.9) | 0.220 |
| Female, n (%) | 10 (100%) | 10 (71%) | 0.114 | |
| Current medication, n (%) | ||||
| Metformin | 1 (10%) | 1 (7%) | 1.000 | |
| Statin | 5 (50%) | 4 (29%) | 0.403 | |
| Right TKA, n (%) | 5 (50%) | 8 (57%) | 1.000 | |
| Intraoperative oxygen use, n (%) | 3 (30%) | 4 (29%) | 1.000 | |
| Intraoperative midazolam, n (%) | 6 (60%) | 11 (79%) | 0.393 | |
| TQ pressure (mmHg), mean ± SD | 255.0 ± 28.8 | 251.4 ± 14.6 | 3.6 (−14.9, 22.1) | 0.661 |
| TQ duration (min), mean ± SD | 86.5 ± 18.1 | 88.4 ± 16.8 | −1.9 (−16.8, 13.0) | 0.791 |
| Operation time (min), mean ± SD | 112.0 ± 24.2 | 117.9 ± 33.2 | −5.9 (−31.5, 19.7) | 0.724 |
Abbreviations: BMI, body mass index; IPC, ischemic preconditioning; TKA, total knee arthroplasty; TQ, tourniquet.
3.1. Quadriceps and hamstring muscles strength
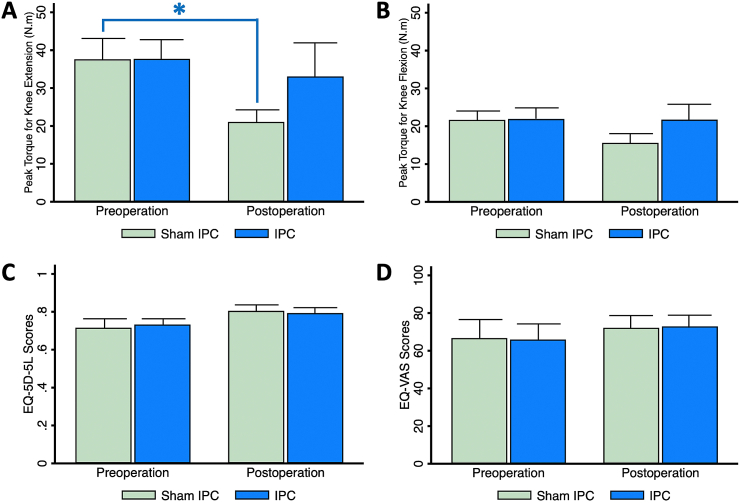
Quadriceps weakness after TKA occurs and persists during the first three months [1]. Recovery of the quadriceps and hamstring muscles strength is the main determinant of return to physical activities. This study showed that postoperative peak torque for knee extension significantly reduced in the sham IPC group (Mean difference; −16.6 [−29.5, −3.6] N.m, p = 0.020), while that in the IPC group was marginally less than the preoperative value (Mean difference; −4.7 [−25.3, 16.0] N.m, p = 0.617) (Fig. 2A). There was no significant difference between the two groups in the hamstring muscle strength (Fig. 2B).
Figure 2.
Functional outcomes at two weeks before and four weeks after TKA (A) Extension peak torque or quadriceps muscle strength, (B) Flexion peak torque or hamstring muscle strength (C) Quality of life scores assessed by 5-dimension questionnaire (EQ-5D-5L), and (D) Overall quality of life scores assessed by visual analog scale (VAS). ∗represents p < 0.05 in comparison with preoperative data.
Abbreviations: IPC, ischemic preconditioning; TKA, total knee arthroplasty.
3.2. Quality of life
The EQ-5D-5L utility scores include a detailed assessment of five health dimensions and the EQ-VAS directly evaluates a patient's feeling of his/her overall health status. This study showed no difference in both scores between preoperative and postoperative assessment, or between the groups (Fig. 2C and D).
3.3. Oxidative stress and antioxidative defense
A key molecular mechanism underlying skeletal I/R injury is reactive oxygen species (ROS) production exceeding antioxidant defenses capacity [19]. Superoxide anion has been reported as one of the most abundant ROS produced in skeletal muscle. It induces the generation of further ROS resulting in lipid peroxidation, protein carboxylation, and DNA damage. IPC has demonstrated its protection against skeletal muscle I/R injury by reducing tissue malondialdehyde (MDA) and increasing catalase levels [20]. However, this study showed no differences in 4-hydroxynonenal (4-HNE) and SOD2 proteins (Fig. 3A and B, Fig. 4).
Figure 3.
Muscle protein expression at 30-min ischemia and the onset of reperfusion in TKA patients receiving preoperative sham IPC (n = 10) or IPC (n = 14) (A) 4-HNE, (B) SOD2 (C) TNF-ɑ, and (D) IL-6.
Abbreviations:4-HNE, 4-hydroxynonenal; IL-6, interleukin-6; IPC, ischemic preconditioning; SOD, superoxide dismutase; TNF- ɑ, tumor necrosis factor-ɑ.
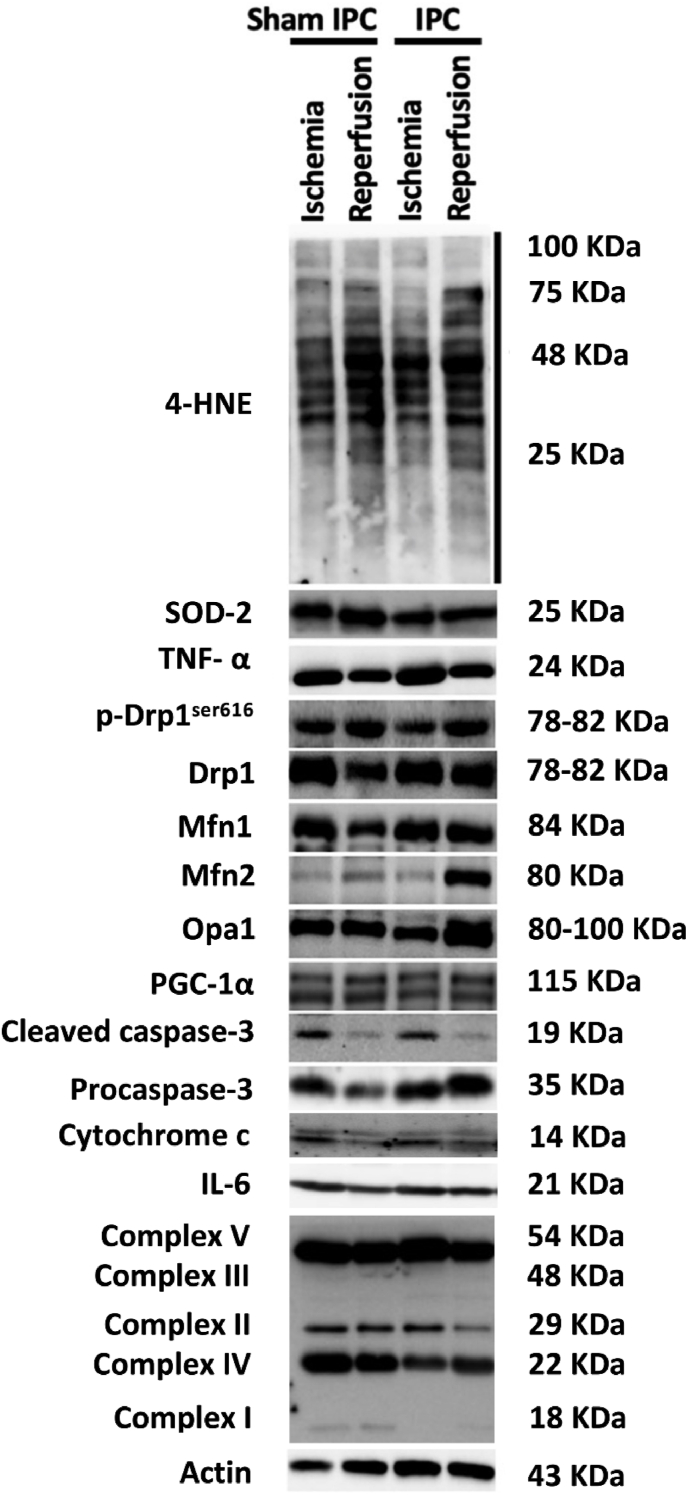
Figure 4.
Representative western blot images of all protein bands.
3.4. Inflammation
Acute local inflammatory reaction following skeletal muscle I/R is induced by excessive cellular ROS production and these inflammatory processes possibly cause remote organ injuries [19]. Activated endothelial cells stimulate adhesion molecules, facilitate leukocyte adhesion as well as transmigration, and produce proinflammatory cytokines. In TKA model, interleukin-6 (IL-6) levels in systemic circulation increased during the first 4 h after surgery, while the level of IL-6 in the ischemic limb and those of tumor necrosis factor-ɑ (TNF-ɑ) in both ischemic and non-ischemic limbs were not influenced by surgery with skeletal muscle I/R [21]. This study showed that TNF-ɑ and IL-6 expressions in the skeletal muscle were not different between the ischemic and reperfusion periods, or between the groups (Fig. 3C and D, Fig. 4).
3.5. Mitochondrial dynamics
Mitochondrial fission is mediated mainly by Drp1. Phosphorylation of Drp1 at Serine616 activates morphological fission and facilitates multiple mitochondrial metabolic functions involving mitochondrial transportation, mitophagy, and apoptosis [22]. This study showed no difference in p-Drp1ser616/Drp1 expression between the ischemic and reperfusion periods, or between the groups (Fig. 5A, Fig. 4).
Figure 5.
Mitochondrial dynamics protein expression at 30-min ischemia and onset of reperfusion in TKA patients receiving preoperative sham IPC (n = 10) or IPC (n = 14) (A) p-Drp1ser616/Drp1, (B) Mfn1 (C) Mfn2, and (D) Opa1. ∗represents p < 0.05 in comparison with ischemic data.
Abbreviations: Drp1, dynamin-related protein-1; p-Drp1ser616, phosphorylated Drp1 at Serine616; IPC, ischemic preconditioning; Mfn, mitofusin; Opa1, optic atrophy-1.
Mitochondrial fusion is a two-step process. The outer membrane fusion requires Mfn1 and Mfn2 proteins, whereas the inner membrane fusion is mediated by optic atrophy-1 (Opa1). Mitochondrial fusion plays a role in the metabolism of skeletal muscle cells, especially the oxidative muscle fibers which rely mainly on oxidative phosphorylation (OXPHOS) capacity [23]. During ischemia, oxygen deprivation inhibits mitochondrial OXPHOS and electron transport chain (ETC) leading to adenosine triphosphate (ATP) depletion and ROS overproduction [24]. A previous study has reported that stress can trigger Mfn1-and Opa1-dependent mitochondrial fusion and restore ATP through OXPHOS [25]. This study showed that IPC significantly increased Mfn2 (2.0 ± 0.2 vs 1.2 ± 0.1, p = 0.001) and Opa1 (2.9 ± 0.3 vs 1.9 ± 0.2, p = 0.005) proteins expression at the onset of reperfusion, compared to the ischemic phase (Fig. 5C and D, Fig. 4). The Mfn2 expression at the onset of reperfusion in the IPC group was also moderately higher than that in the sham IPC group (2.0 ± 0.2 vs 1.4 ± 0.2, p = 0.062) (Figs. 5C and 4). These findings indicate that IPC promoted mitochondrial fusion in both outer and inner mitochondrial fusion processes.
3.6. Mitochondrial biogenesis
Peroxisome proliferator-activated receptor-gamma coactivator-1ɑ (PGC-1ɑ) is a transcriptional coactivator involved in the regulation of genes related to mitochondrial biogenesis, respiration, and dynamics [26]. Overexpression of PGC-1ɑ regulates mitochondrial fusion proteins and prevents muscle disuse atrophy [26]. However, this study showed no difference in skeletal muscle PGC-1ɑ between the ischemic and reperfusion periods, or between the groups (Fig. 6A, Fig. 4).
Figure 6.
Muscle protein expression at 30-min ischemia and onset of reperfusion in TKA patients receiving preoperative sham IPC (n = 10) or IPC (n = 14) (A) PGC-1ɑ, (B) ETC complex I (C) ETC complex II, (D) ETC complex III (E) ETC complex IV, (F) ETC complex V (G) cytochrome c, and (H) cleaved caspase-3/caspase-3.
Abbreviations:IPC, ischemic preconditioning; ETC, electron transport chain; OXPHOS, oxidative phosphorylation; PGC-1ɑ, peroxisome proliferator-activated receptor-gamma coactivator-1ɑ.
3.7. Mitochondrial oxidative phosphorylation
During ischemic phase of the skeletal muscle I/R, mitochondrial OXPHOS is inhibited, which results in a reduction of mitochondrial membrane potential and ATP synthesis [24]. In rat skeletal muscle with 3- to 5-h ischemia, ETC complexes I and II were impaired and IPC showed protection against ischemic-induced mitochondrial dysfunction [13,15]. However, this study showed no differences in skeletal muscle ETC complex I–V between the ischemic and reperfusion periods, or between the groups (Fig. 6B–F, Fig. 4).
3.8. Apoptosis
Skeletal muscle I/R injury induces mitochondrial permeability transition pore (mPTP) opening leading to mitochondrial OXPHOS uncoupling, mitochondrial membrane potential depolarization, mitochondrial swelling, and rupture [24]. Release of pro-apoptotic factors including cytochrome c and apoptosis-inducing factors from the mitochondrial intermembrane activates the intrinsic apoptotic pathway. IPC has been reported to induce reperfusion tolerance by modulating the caspase-dependent apoptotic pathway [20]. However, this study showed that cytochrome c and cleaved caspase-3/caspase-3 expressions were not different between the ischemic and reperfusion periods, or between the groups (Fig. 6G and H, Fig. 4).
4. Discussion
This clinical study demonstrated the beneficial effect of IPC on the preservation of postoperative quadriceps muscle strength following TKA with TQ use. We observed that the molecular mechanism associated with this protection was an enhancement of the mitochondrial fusion proteins, Mfn2 and Opa1. This introduces the role of mitochondrial fusion as one of the various mechanisms underlying the IPC in preventing skeletal muscle I/R injury.
In accordance with our results regarding quadriceps muscle strength, quadriceps muscle atrophy following TKA with TQ has been reported within the first two to six weeks after surgery in elderly patients [27]. The underlying mechanisms of skeletal muscle atrophy from the TQ use during TKA have been serially investigated and comprehensively reviewed by Dreyer H.C. and colleagues [3,[5], [6], [7]]. Besides, other molecular mechanisms of TQ-induced I/R in TKA have been reported by Jawhar A. and colleagues [8,28,29]. In this study, we provided additional cellular and subcellular evidence to support these previous findings by focusing on mitochondrial biogenesis, mitochondrial dynamics, caspase-dependent apoptosis, oxidative stress, and inflammation.
Mitochondrial biogenesis and dynamics are key processes of mitochondrial quality control, which is necessary for maintaining mitochondrial integrity and function in skeletal muscle cells [9]. Mitochondrial ROS overgeneration, increased mitochondrial fragmentation, and mPTP opening activate the FoxO3 transcriptional factors, and subsequently upregulate atrophy-related genes, including Atorgin-1 and MuRF-1, resulting in loss of muscle mass. Previous studies reported that overexpression of PGC-1ɑ, which is a co-transcriptional regulatory factor of mitochondrial biogenesis, protected muscle from proteolytic [30]. In this study, we failed to find a significant role of the PGC-1ɑ in preserving postoperative muscle strength. An explanation might be because the subjects in our study are in the aging population and senescent muscle cells possibly have a blunt or delayed response to cellular stress. A previous investigation demonstrated a decline in PGC-1ɑ mRNA and nuclear PGC-1ɑ protein content in aged skeletal muscle [31]. Besides, modulation of PGC-1ɑ following oxidative stress is tissue-specific and time-dependent [32]. In skeletal muscle, short-term exposure of lipopolysaccharide induces a transient rise followed by a gradual drop in PGC-1ɑ expression within 24 h.
Regarding the role of mitochondrial dynamics in skeletal muscle atrophy, elevated mitochondrial fission and reduced mitochondrial fusion are linked to increased cellular apoptosis and muscle fiber atrophy in disuse muscle and sarcopenia [9]. As for mitochondrial fission, inhibition of Fis1 reduced atrophy-related ubiquitin ligases expression and Drp1 inhibition blocked FoxO3-mediated autophagosome formation [33]. These two mechanisms of mitochondrial fission suppression inhibit proteolytic pathways and therefore are potential targets of muscle loss prevention. Concerning mitochondrial fusion, mitofusin-depleted muscle showed ultrastructure signs of dysfunction of both subsarcolemmal and intermyofibrillar mitochondria and a shift in the composition of skeletal muscle fiber types, which resulted in muscle atrophy [34]. Also, both Mfn1 and Mfn2 are required for mitochondrial DNA stability. In Mfn2 deficient mice, there was inhibition of mitophagy and accumulation of damaged mitochondria, which contributed to metabolic alterations inducing sarcopenia [35]. In Opa1 knockout mice, muscle loss and weakness occurred as results of mitochondrial oxidative stress, ER stress, activation of unfolded protein response (UPR), reduction in Akt and AMPK phosphorylation, upregulation of fibroblast growth factor 21 (FGF21) and nuclear factor kappa B (NF-κB), induction of atrophy-related genes, and autophagy [36]. These findings emphasize the importance of mitochondrial dynamics balance in the maintenance of skeletal muscle function. In this study, we observed an increase in Mfn2 and Opa1 proteins expression at the onset of reperfusion in skeletal muscle pretreated with IPC and speculated that the protective effect of IPC in preserving quadriceps muscle strength was partly mediated by enhancing mitochondrial fusion.
Oxidative stress and inflammation are also involved in TQ-induced skeletal muscle I/R during TKA [2]. In humans, increased glutathione oxidation, lipid peroxidation, and pro-inflammatory cytokines in local and systemic circulation have been reported after 75–125 min of TQ time [21]. In this study, we found that muscle TNF-ɑ, IL-6, 4-HNE, and SOD2 proteins at the onset of reperfusion was comparable to that at 30-min ischemia. Both unaltered inflammatory response and antioxidant system demonstrated in this study might be because we extracted proteins from the vastus medialis muscle which composes mainly of the slow oxidative fibers [37]. This muscle fiber type has a high basal mitochondrial respiratory rate as well as antioxidant pools and therefore is more tolerable to I/R-induced injury than the glycolytic muscle fibers such as the gastrocnemius muscle [38].
Apoptosis is a late process following skeletal muscle I/R injury [24]. As a result of mitochondrial calcium, ROS, and H+ accumulation during ischemia, mPTP is formed. Thereafter, at the beginning of reperfusion, a surge of ROS production triggers mPTP opening and pro-apoptotic factors release. Besides, at high levels of cellular stress, mitochondrial fission also facilitates apoptosis to remove damaged mitochondria [19]. In this study, cytochrome c and caspase-3 activation were not observed at the onset of reperfusion. We postulate that the limited TQ time in our study has not reached the critical time point for apoptotic cell death of skeletal muscle. A previous in vitro model reported that human skeletal muscle cell injury occurred after three to 4 h of hypoxia followed by 2 h of reoxygenation [39].
IPC has been investigated as a protective strategy against TQ-induced I/R injury in TKA [2]. It triggers protective genomic responses by upregulation of immediate early response genes, oxidative stress defense genes, and prosurvival genes [14,16]. As for the effects on mitochondrial function of skeletal muscle, IPC restores ETC complex I and II activities resulting in enhanced maximal oxidative capacities [13,15]. The mechanism of IPC related to mitochondrial dynamics in the skeletal muscle has not been reported. However, an imbalance of mitochondrial fission and fusion plays a significant role in myocardial I/R injury. Inhibition of Drp1 by Mdivi-1 and activation of mitochondrial fusion proteins inhibit ROS-induced mPTP opening, preserve mitochondrial morphology, reduce cell death, and maintain cardiac function. Only one previous study examined the effect of remote IPC (RIPC) on mitochondrial dynamics in rat cardiomyocytes and demonstrated that RIPC increased Opa1 and decreased Drp1 protein expression, leading to a reduction in infarct size [40].
Our study contains some limitations. First, the sample size was relatively small and not priori estimated. Second, other higher sensitivity analyses, such as ELISA or metabolomics, possibly detect subtle alterations in cellular and subcellular pathways and provide more insight into the mechanisms underlying TQ-induced I/R and the protective effects of IPC. Third, aging process is a significant factor that impairs the mitochondrial quality and quantity in skeletal muscle and causes sarcopenia [9]. In our study, all recruited patients in both groups were possible sarcopenia because of their age and low physical ability due to knee OA. Further studies excluding this aging factor by recruiting younger subjects as non-sarcopenia groups would suggest for investigating the mechanisms of IPC protection. In addition, chronic smoking or alcohol exposure could lead to mitochondrial damage. However, smoking or drinking status were not taken into consideration in our study because only one patient in the IPC group was a current smoker (0–1 cigarette per day) and none of patients was an active drinker. Last, different IPC protocols, such as different numbers of cycles or duration of ischemic and reperfusion periods, might exert superior or inferior effects. Moreover, the timing of IPC application might affect the outcomes. Our study conducted IPC before spinal anesthesia because we purposed to preserve all possible protective mechanisms, including neuronal mechanisms of remote IPC, to protect distant organs from TQ-induced I/R injury as well [2,40]. Studies with this or modified IPC protocols are recommended to further demonstrate the mechanisms underlying the preconditioning to protect the skeletal muscle against I/R injury and the effects of IPC on functional outcomes.
5. Conclusion
IPC application (3 cycles of 5-min ischemia followed by 5-min reperfusion) before TQ inflation for TKA preserved postoperative quadriceps muscle strength. The potential mechanism related to this protection was Mfn2 and Opa1 proteins upregulation. This is the first study introducing the role of mitochondrial fusion as one mechanism underlying the IPC in preventing skeletal muscle I/R injury. Further animal and clinical investigations are encouraged to support this proposed mechanism.
Funding statement
This work was supported by the National Research Council of Thailand [contact grant number: Senior Research Scholar Grant (SCC)]; the National Science and Technology Development Agency of Thailand [contact grant number: NSTDA Research Chair Grant (NC)]; Chiang Mai University [contact grant number: Center of CMU Excellence Award (NC)]; and the Faculty of Medicine Research Fund of Chiang Mai University [grant number 014/2562 (PL)].
Authorship statement
Conception and design of study: P. Leurcharusmee, P. Sawaddiruk, Y. Punjasawadwong, N Sugundhavesa, K. Klunklin, S. Tongprasert, P. Sirilertpisan, N. Chattipakorn, S.C. Chattipakorn; acquisition of data: P. Leurcharusmee, N. Apaijai; analysis and/or interpretation of data: P. Leurcharusmee, N. Apaijai, S.C. Chattipakorn. Drafting the manuscript: P. Leurcharusmee; revising the manuscript critically for important intellectual content: P. Leurcharusmee, P. Sawaddiruk, Y. Punjasawadwong, N Sugundhavesa, K. Klunklin, S. Tongprasert, P. Sirilertpisan, N. Apaijai, N. Chattipakorn, S.C. Chattipakorn. Approval of the version of the manuscript to be published (the names of all authors must be listed): P. Leurcharusmee, P. Sawaddiruk, Y. Punjasawadwong, N Sugundhavesa, K. Klunklin, S. Tongprasert, P. Sirilertpisan, N. Apaijai, N. Chattipakorn, S.C. Chattipakorn.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:P. Leurcharusmee receives a grant from the Faculty of Medicine Research Fund of Chiang Mai University [grant number 014/2562].
N. Chattipakorn receives grants from the National Science and Technology Development Agency of Thailand [contact grant number: NSTDA Research Chair Grant (NC)] and Chiang Mai University [contact grant number: Center of CMU Excellence Award (NC)].
S.C. Chattipakorn receives a grant from the National Research Council of Thailand [contact grant number: Senior Research Scholar Grant (SCC)].
Acknowledgements
None.
References
- 1.Moon Y.W., Kim H.J., Ahn H.S., Lee D.H. Serial changes of quadriceps and hamstring muscle strength following total knee arthroplasty: a meta-analysis. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leurcharusmee P., Sawaddiruk P., Punjasawadwong Y., Chattipakorn N., Chattipakorn S.C. The possible pathophysiological outcomes and mechanisms of tourniquet-induced ischemia-reperfusion injury during total knee arthroplasty. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/8087598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratchford S.M., Bailey A.N., Senesac H.A., Hocker A.D., Smolkowski K., Lantz B.A., et al. Proteins regulating cap-dependent translation are downregulated during total knee arthroplasty. Am J Physiol Regul Integr Comp Physiol. 2012;302(6):R702–R711. doi: 10.1152/ajpregu.00601.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostman B., Michaelsson K., Rahme H., Hillered L. Tourniquet-induced ischemia and reperfusion in human skeletal muscle. Clin Orthop Relat Res. 2004;418:260–265. doi: 10.1097/00003086-200401000-00045. [DOI] [PubMed] [Google Scholar]
- 5.Bailey A.N., Hocker A.D., Vermillion B.R., Smolkowski K., Shah S.N., Jewett B.A., et al. MAFbx, MuRF1, and the stress-activated protein kinases are upregulated in muscle cells during total knee arthroplasty. Am J Physiol Regul Integr Comp Physiol. 2012;303(4):R376–R386. doi: 10.1152/ajpregu.00146.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hocker A.D., Boileau R.M., Lantz B.A., Jewett B.A., Gilbert J.S., Dreyer H.C. Endoplasmic reticulum stress activation during total knee arthroplasty. Physiological reports. 2013;1(3) doi: 10.1002/phy2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muyskens J.B., Hocker A.D., Turnbull D.W., Shah S.N., Lantz B.A., Jewett B.A., et al. Transcriptional profiling and muscle cross-section analysis reveal signs of ischemia reperfusion injury following total knee arthroplasty with tourniquet. Physiological reports. 2016;4(1) doi: 10.14814/phy2.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jawhar A., Brenner D., De La Torre C., Sticht C., Obertacke U., Ponelies N. Gene expression analysis of vastus medialis cells after tourniquet-induced ischemia during total knee arthroplasty: a randomized clinical trial. Eur J Trauma Emerg Surg. 2021;47(1):233–240. doi: 10.1007/s00068-019-01196-0. [DOI] [PubMed] [Google Scholar]
- 9.Leduc-Gaudet J.P., Hussain S.N.A., Barreiro E., Gouspillou G. Mitochondrial dynamics and mitophagy in skeletal muscle health and aging. Int J Mol Sci. 2021;22(15) doi: 10.3390/ijms22158179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faitg J., Leduc-Gaudet J.P., Reynaud O., Ferland G., Gaudreau P., Gouspillou G. Effects of aging and caloric restriction on fiber type composition, mitochondrial morphology and dynamics in rat oxidative and glycolytic muscles. Front Physiol. 2019;10:420. doi: 10.3389/fphys.2019.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favaro G., Romanello V., Varanita T., Andrea Desbats M., Morbidoni V., Tezze C., et al. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nat Commun. 2019;10(1):2576. doi: 10.1038/s41467-019-10226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dulac M., Leduc-Gaudet J.P., Reynaud O., Ayoub M.B., Guerin A., Finkelchtein M., et al. Drp1 knockdown induces severe muscle atrophy and remodelling, mitochondrial dysfunction, autophagy impairment and denervation. J Physiol. 2020;598(17):3691–3710. doi: 10.1113/JP279802. [DOI] [PubMed] [Google Scholar]
- 13.Thaveau F., Zoll J., Rouyer O., Chafke N., Kretz J.G., Piquard F., et al. Ischemic preconditioning specifically restores complexes I and II activities of the mitochondrial respiratory chain in ischemic skeletal muscle. J Vasc Surg. 2007;46(3):541–547. doi: 10.1016/j.jvs.2007.04.075. ; discussion 47. [DOI] [PubMed] [Google Scholar]
- 14.Murphy T., Walsh P.M., Doran P.P., Mulhall K.J. Transcriptional responses in the adaptation to ischaemia-reperfusion injury: a study of the effect of ischaemic preconditioning in total knee arthroplasty patients. J Transl Med. 2010;8:46. doi: 10.1186/1479-5876-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansour Z., Bouitbir J., Charles A.L., Talha S., Kindo M., Pottecher J., et al. Remote and local ischemic preconditioning equivalently protects rat skeletal muscle mitochondrial function during experimental aortic cross-clamping. J Vasc Surg. 2012;55(2):497–505 e1. doi: 10.1016/j.jvs.2011.07.084. [DOI] [PubMed] [Google Scholar]
- 16.Sha Y., Xu Y.Q., Zhao W.Q., Tang H., Li F.B., Li X., et al. Protective effect of ischaemic preconditioning in total knee arthroplasty. Eur Rev Med Pharmacol Sci. 2014;18(10):1559–1566. [PubMed] [Google Scholar]
- 17.Park U.J., Kim H.T., Cho W.H., Park J.H., Jung H.R., Kim M.Y. Remote ischemic preconditioning enhances the expression of genes encoding antioxidant enzymes and endoplasmic reticulum stress-related proteins in rat skeletal muscle. Vasc Specialist Int. 2016;32(4):141–149. doi: 10.5758/vsi.2016.32.4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattanaphesaj J., Thavorncharoensap M. Measurement properties of the EQ-5D-5L compared to EQ-5D-3L in the Thai diabetes patients. Health Qual Life Outcome. 2015;13:14. doi: 10.1186/s12955-014-0203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lejay A., Meyer A., Schlagowski A.I., Charles A.L., Singh F., Bouitbir J., et al. Mitochondria: mitochondrial participation in ischemia-reperfusion injury in skeletal muscle. Int J Biochem Cell Biol. 2014;50:101–105. doi: 10.1016/j.biocel.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Kocman E.A., Ozatik O., Sahin A., Guney T., Kose A.A., Dag I., et al. Effects of ischemic preconditioning protocols on skeletal muscle ischemia-reperfusion injury. J Surg Res. 2015;193(2):942–952. doi: 10.1016/j.jss.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Clementsen T., Reikeras O. Cytokine patterns after tourniquet-induced skeletal muscle ischaemia reperfusion in total knee replacement. Scand J Clin Lab Investig. 2008;68(2):154–159. doi: 10.1080/00365510701528587. [DOI] [PubMed] [Google Scholar]
- 22.Mishra P., Chan D.C. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212(4):379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra P., Varuzhanyan G., Pham A.H., Chan D.C. Mitochondrial dynamics is a distinguishing feature of skeletal muscle fiber types and regulates organellar compartmentalization. Cell Metabol. 2015;22(6):1033–1044. doi: 10.1016/j.cmet.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paradis S., Charles A.L., Meyer A., Lejay A., Scholey J.W., Chakfe N., et al. Chronology of mitochondrial and cellular events during skeletal muscle ischemia-reperfusion. Am J Physiol Cell Physiol. 2016;310(11):C968–C982. doi: 10.1152/ajpcell.00356.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tondera D., Grandemange S., Jourdain A., Karbowski M., Mattenberger Y., Herzig S., et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28(11):1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannavino J., Brocca L., Sandri M., Grassi B., Bottinelli R., Pellegrino M.A. The role of alterations in mitochondrial dynamics and PGC-1alpha over-expression in fast muscle atrophy following hindlimb unloading. J Physiol. 2015;593(8):1981–1995. doi: 10.1113/jphysiol.2014.286740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreyer H.C., Strycker L.A., Senesac H.A., Hocker A.D., Smolkowski K., Shah S.N., et al. Essential amino acid supplementation in patients following total knee arthroplasty. J Clin Invest. 2013;123(11):4654–4666. doi: 10.1172/JCI70160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jawhar A., Hermanns S., Ponelies N., Obertacke U., Roehl H. Tourniquet-induced ischaemia during total knee arthroplasty results in higher proteolytic activities within vastus medialis cells: a randomized clinical trial. Knee Surg Sports Traumatol Arthrosc : official journal of the ESSKA. 2016;24(10):3313–3321. doi: 10.1007/s00167-015-3859-2. [DOI] [PubMed] [Google Scholar]
- 29.Jawhar A., Ponelies N., Schild L. Effect of limited ischemia time on the amount and function of mitochondria within human skeletal muscle cells. Eur J Trauma Emerg Surg. 2016;42(6):767–773. doi: 10.1007/s00068-015-0600-2. [DOI] [PubMed] [Google Scholar]
- 30.Cannavino J., Brocca L., Sandri M., Bottinelli R., Pellegrino M.A. PGC1-alpha over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J Physiol. 2014;592(20):4575–4589. doi: 10.1113/jphysiol.2014.275545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang C., Chung E., Diffee G., Ji L.L. Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1alpha. Exp Gerontol. 2013;48(11):1343–1350. doi: 10.1016/j.exger.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Yu X.X., Barger J.L., Boyer B.B., Brand M.D., Pan G., Adams S.H. Impact of endotoxin on UCP homolog mRNA abundance, thermoregulation, and mitochondrial proton leak kinetics. Am J Physiol Endocrinol Metab. 2000;279(2):E433–E446. doi: 10.1152/ajpendo.2000.279.2.E433. [DOI] [PubMed] [Google Scholar]
- 33.Romanello V., Guadagnin E., Gomes L., Roder I., Sandri C., Petersen Y., et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010;29(10):1774–1785. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H., Vermulst M., Wang Y.E., Chomyn A., Prolla T.A., McCaffery J.M., et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141(2):280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sebastian D., Sorianello E., Segales J., Irazoki A., Ruiz-Bonilla V., Sala D., et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016;35(15):1677–1693. doi: 10.15252/embj.201593084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tezze C., Romanello V., Desbats M.A., Fadini G.P., Albiero M., Favaro G., et al. Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metabol. 2017;25(6):1374–13789 e6. doi: 10.1016/j.cmet.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Travnik L., Pernus F., Erzen I. Histochemical and morphometric characteristics of the normal human vastus medialis longus and vastus medialis obliquus muscles. J Anat. 1995;187(Pt 2):403–411. [PMC free article] [PubMed] [Google Scholar]
- 38.Charles A.L., Guilbert A.S., Guillot M., Talha S., Lejay A., Meyer A., et al. Muscles susceptibility to ischemia-reperfusion injuries depends on fiber type specific antioxidant level. Front Physiol. 2017;8:52. doi: 10.3389/fphys.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martou G., O'Blenes C.A., Huang N., McAllister S.E., Neligan P.C., Ashrafpour H., et al. Development of an in vitro model for study of the efficacy of ischemic preconditioning in human skeletal muscle against ischemia-reperfusion injury. J Appl Physiol. 2006;101(5):1335–1342. doi: 10.1152/japplphysiol.00278.2006. 1985. [DOI] [PubMed] [Google Scholar]
- 40.Cellier L., Tamareille S., Kalakech H., Guillou S., Lenaers G., Prunier F., et al. Remote ischemic conditioning influences mitochondrial dynamics. Shock. 2016;45(2):192–197. doi: 10.1097/SHK.0000000000000500. [DOI] [PubMed] [Google Scholar]