Abstract
Occupational diseases are caused by zoonotic pathogens, which spread to humans through various types and intensities of human-livestock contact at work. In the present era, human brucellosis and tuberculosis remain the predominant occupational diseases throughout the world. However, the actual percentage of reported cases that are acquired from various livestock-related occupational groups is not well known. Therefore, we carried out a systematic review and meta-analysis of previous scatter studies mentioned the occurrence of human brucellosis and tuberculosis. From 2000 to 2021, a computer search of PubMed, Science Direct, Google Scholar, BioMed and Scopus was conducted and finally we found 71 studies (brucellosis = 54, tuberculosis = 17), which were included in this meta-analysis to calculate the aggregate prevalence using the random effects model. Moreover, I2 statistic, Cochran's Q statistic heterogeneity and subgroup analysis were also performed. The analysis of the data showed that among the various livestock-related occupational groups, the global pooled prevalence of tuberculosis was 19% (95% CI: 09–30), which was higher than brucellosis 14% (95% CI: 10–18). In addition, North America and Africa were reported as the continents of the maximum prevalence rate of 25% (95% CI: −08-58) and 16% (95% CI: 11–21) for tuberculosis and brucellosis than the other continents. Afterwards, the individual's occupation was broken down into the following four groups: farm worker, livestock owner, livestock connected person and abattoir worker. The significant association was found between slaughterhouse workers and brucellosis prevalence (20%; 95% CI: 13–27) as well as the livestock owners and tuberculosis prevalence (28%; 95% CI: 06–50). Likely, a maximum prevalence of tuberculosis was documented among workers ages 20 to 49 years, and of brucellosis among those between the ages of 20 and 25, which suggests that age also had a role. Therefore, it is concluded that the livestock-related occupational groups were found to be at an increased risk of adverse zoonotic disease outcomes. Future studies could be focused on specific occupational group that are in high risk of disease transmission to minimize the effect of these two hazardous pathogens.
Keywords: Tuberculosis, Brucellosis, Occupational groups, Livestock, Global, Meta-analysis
1. Introduction
Livestock supports the livelihoods of over 1.7 billion poor people throughout the world, and enormous demand for livestock product spurs the growth of ancillary job opportunities in ailed businesses such as animal husbandry, slaughterhouse, transportation and feed production [1]. However, a large number of livestock stakeholders are in danger due to transmission of zoonotic diseases, particularly bovine tuberculosis and brucellosis. Exposure to Mycobacterium tuberculosis (TB) among occupational groups is one of the most serious health risks- nowadays, one out of every four people is affected tuberculosis worldwide [2,3]. Throughout the year of 2016, globally, 147,000 confirmed cases and 12,500 fatalities of tuberculosis affected patients were reported [4]. In addition to TB, Mycobacterium bovis (bTB) has also the zoonotic importance due to its chronic nature and survival ability for months or even years within the human host without presenting clinical indications [5]. Therefore, the World Health Organization has categorized bTB as one of the eight dangerous zoonotic diseases with a high potential to infect the human population [6]. Human tuberculosis (HTB) of animal origin, specifically Mycobacterium bovis, is becoming more prevalent in both underdeveloped and developed countries because of sharing the same microenvironment with animal, living quarter and breathing during livestock handling [2]. According to reliable data from Tanzania, Nigeria and Uganda, >20% of the bacteria found in HTB infections were bTB [7]. Humans are accidental hosts of Mycobacterium bovis, with transmission occurring predominantly through the ingestion of contaminated cattle products: such as unpasteurized milk or raw meat products, close contact with infected cattle, aerosol inhalation of infective droplet or tissue, and in the presence of wounds from infected animals [8]. Moreover, human-animal contact at cattle markets and slaughterhouses as well as a lack of proper animal husbandry practices are also acted as the risk factor for tuberculosis transmission [9].
In contrast, brucellosis is one of the seven most neglected diseases in the world, with an estimated 5,000,000 to 12,500,000 cases annually [10,11]. In addition, it is suspected to be a re-emerging disease that affects 500,000 new cases of human infection each year [12]. Presently, human brucellosis is endemic in a number of developing nations, particularly in the Mediterranean, Central and South America, Asia, North and East Africa, and the Middle East: notably in Syria, Iraq, Egypt, Turkey and Iran [13]. In these regions, humans are typically exposed to Brucella abortus and Brucella melitensis through the consumption of raw milk and unpasteurized dairy products from the infected animal [14]. Consequently, transmission to humans is primarily accomplished through the close contact with contaminated placenta, urine, excrement, blood and aborted fetus [15]. As a result, brucellosis is spreading rapidly among shepherds, milkmen, butchers, knackers, veterinary assistants, and abattoir workers [16].
To summarize, despite taking eradicated or controlled programs, bovine tuberculosis and brucellosis remain endemic in many developing countries, particularly in African and Asian countries where the control measures are not existent in an adequate level [17]. Thus, it is essential to adopt risk management methods to limit human fatalities in order to reduce the danger of human exposure to lethal zoonotic diseases. Keeping this logic, in the present study, we focused on those who work as farmer, cow breeder, slaughterhouse staff and veterinarian since they regularly interact with animals, and are more prone to infection. Subsequently the global scenario of livestock-oriented tuberculosis and brucellosis is still in dearth, it is important to know the global prevalence with associated factors of tuberculosis and brucellosis from livestock-related occupational groups for taking any control measures in the near future. In this regard, meta-analysis is a highly valuable statistical tool whose goal is to synthesize, integrate and contrast the findings of numerous primary studies that investigate the same questions. Thus, we performed a meta-analysis study to calculate the pooled prevalence of these two diseases and provide policymakers with concise facts so they may decide whether to implement any control programs. Future studies could be focused on specific occupational groups which are high risk for the occurrence of tuberculosis and brucellosis and to define appropriate preventive measures.
2. Methods and materials
2.1. Study protocol
In this study, we used PRISMA standards for systematic review and meta-analysis to figure out the prevalence of tuberculosis and brucellosis among livestock-associated occupational groups [16].
2.2. Search strategy
From 2000 to 2021, a comprehensive literature search was undertaken in electronic databases such as Google Scholar, PubMed, Science Direct, BioMed and Scopus using the terms PICO (population, intervention, comparison and outcome)- veterinarians, laboratory staffs, abattoir workers and farmers: population, exposure to Brucella species and Mycobacterium species: intervention, occupational and job-related factors: comparison, and brucellosis or tuberculosis: outcome. Additionally, we performed manual searching of cross references or bibliography sections to identify the studies that matched the PICO criteria (supplementary file 1). However, the search criterion was restricted to English-language studies. Finally, the eligible studies were retrieved by two reviewers, and we employed a third reviewer to recheck the whole retrieval procedure to ensure that it was bias-free.
2.3. Inclusion criteria
The presented meta-analysis covered all original descriptive studies published in the English language that documented the prevalence of brucellosis and tuberculosis in humans who had come into contact with animals or related products. For further analysis, only studies with a clear contact history for tuberculosis (close contact with infected animal in farm or market or veterinary hospital or slaughterhouse; ingestion of unpasteurized milk or raw meat; and aerosol inhalation of infective droplets) and brucellosis (consumption of raw milk from affected animal and close contact with contaminated placenta, urine, excrement, blood and aborted fetus) were chosen. Then, Personnel from veterinary clinics, slaughterhouses, dairy cow owners, agricultural employees, and pastoralists with at least two years of work experience in their representative field were included.
2.4. Exclusion criteria
We excluded review articles, duplicate studies, qualitative studies, case reports and studies published in non-peer reviewed journals; studies concentrating on animal brucellosis or tuberculosis; studies focusing on immunology, microbiology, drug therapy; and studies focusing on genetics, immunology, microbiology, or drug therapy that did not include a diagnostic tool. Besides, intervention studies that lacked with baseline data on the association between animal exposure and disease were not considered for inclusion in the meta-analysis.
2.5. Quality assessment
For each original study, two authors independently assessed the risk of bias. Then, the study's quality was graded out of ten points using the Newcastle–Ottawa scale specially adapted for cross-sectional studies [18,19]. The tool is divided into three sections, the first of which is methodological quality and is rated with five stars: representativeness of the sample (1 star), sample size (1 star), non-respondents (1 star) and ascertainment of the exposure or risk factor (2 stars); the second section is study comparability: the subject in different outcome groups are comparable based on the study design or analysis (maximum 2 stars); and the third section is outcomes, which is related to statistical analysis: assessment of the outcome (maximum 2 stars), Statistical test (1 star). Following that, the mean score of two authors was applied to make the final decision, and studies with a score of six or higher were included in this systemic review and meta-analysis.
2.6. Screening and data extraction
Studies were included if they investigated occupational categories associated with domestic or household livestock as a risk factor for tuberculosis and brucellosis. Then, two authors independently reviewed and extracted the data before importing it into the pre-prepared form. Data from studies included the author's name, year of study, publication period, type of personnel, geographical area of the study, number of people examined and number of people who tested positive. In the event of a disagreement, the third author evaluated the article to determine its relevancy. A group conversation with the third author helped to establish a consensus.
2.7. Data analysis
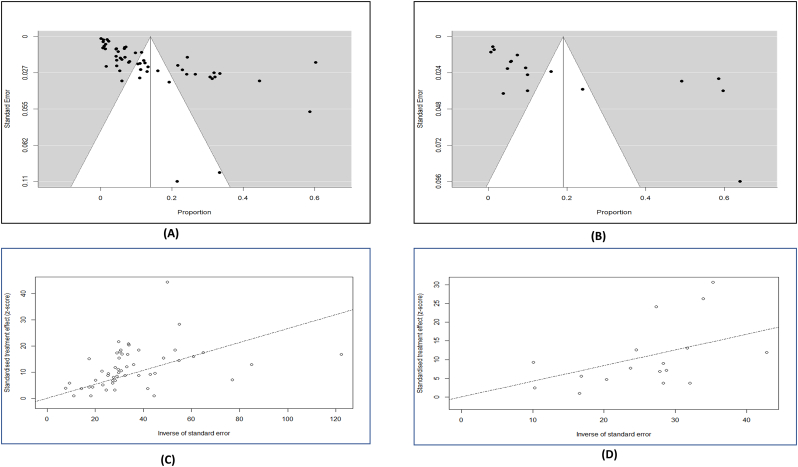
The random effect model was used to determine the pooled prevalence of tuberculosis and brucellosis infection in occupational categories using the meta-analysis approach with a 95% confidence interval (CI). Following that, various subgroup studies were undertaken separately based on the type of occupational categories linked with animal species exposures. Afterwards, the results of the meta-analysis were plotted in a forest plot, and the heterogenicity of study level was estimated by Galbraith plot (Fig. 4). Moreover, the Q and I2 tests were used to determine the studies heterogeneity (I2 > 50% indicates significant heterogeneity) [20,21]. Finally, funnel plot and egger's test were performed to confirm the study effect and publication bias. The statistical analysis was done applying the Jamovi software (keeping the significance level at <0.05), while the Galbraith plot and geographic distribution map were generated using RStudio. Additionally, sensitivity analysis was used to examine the robustness of the pooled estimate by removing studies with small sample sizes (n ≤ 200), studies with intermediate quality that had moderate risk of bias rating score between 6 and 7, and studies with outliers [22]. The presence of outliers was determined via a z-score approach- a score of > ± 1.96 was termed as potential outlier (Supplementary file 2, Fig. 1, Fig. 2) [23].
Fig. 4.
Visualization of funnel plot described studies heterogenicity: A) Brucellosis; B) Tuberculosis; Galbraith plot: C) Brucellosis; D) Tuberculosis.
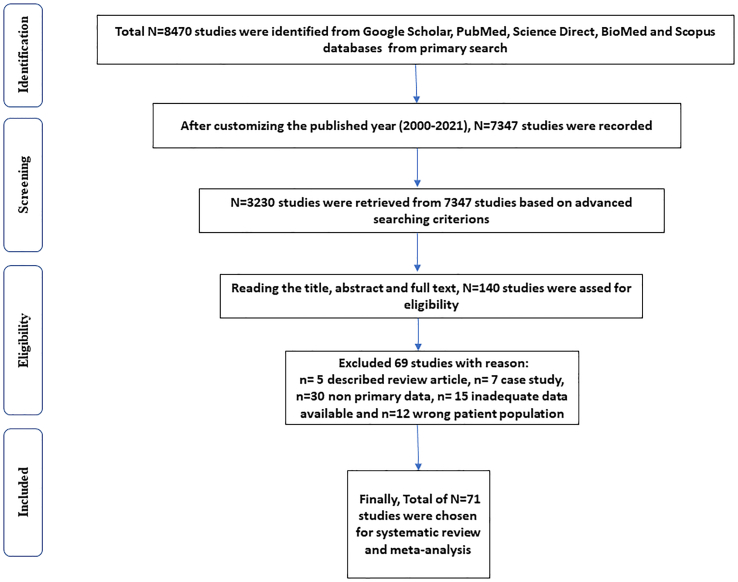
Fig. 1.
Flow chart denoting the selection procedures of eligible studies.
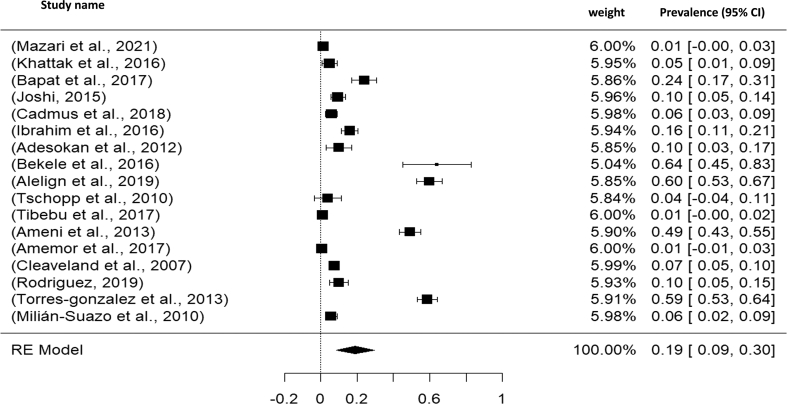
Fig. 2.
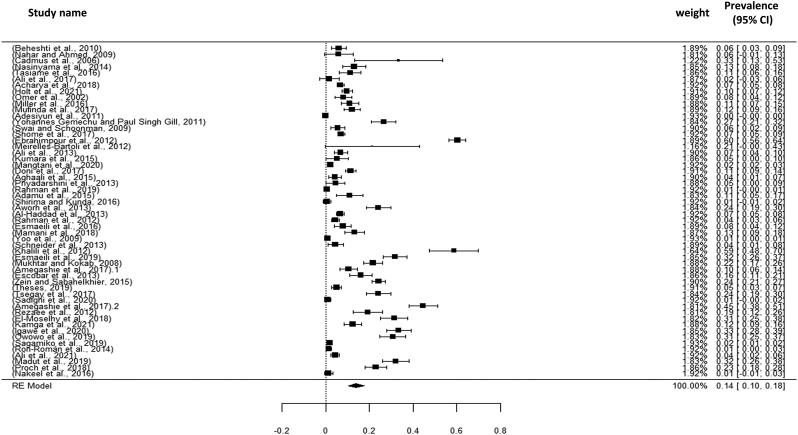
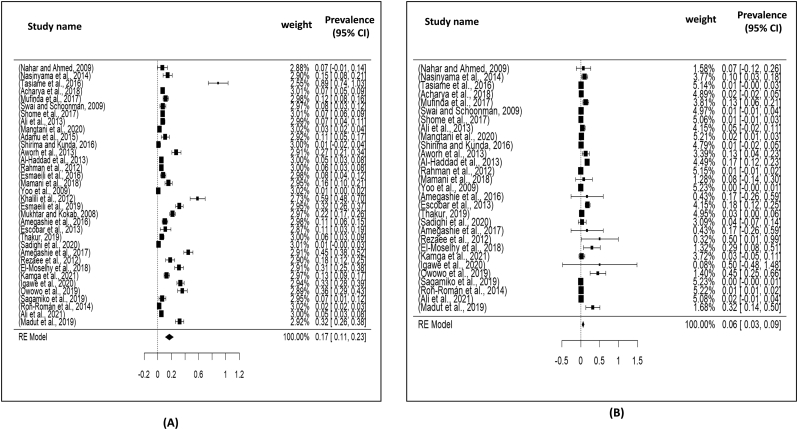
Visualizing forest plot described mean prevalence of zoonotic tuberculosis from different livestock related occupational groups.
3. Results
3.1. Characteristics of the articles included in the meta-analysis
The literature search identified a total of 7347 studies (brucellosis: 5204; tuberculosis: 2143) from the databases, and after assessing the inclusion and exclusion criteria, a total of 71 studies (Brucellosis: 54; Tuberculosis: 17) were included in this study (Fig. 1). For tuberculosis, Africa, Asia and America reported 10, 04 and 03 studies, respectively, while 29 studies from Asia, 21 studies from Africa and 04 studies from America (North and South) documented the prevalence of brucellosis (Table 1).
Table 1.
Characteristics of the included studies.
| Brucellosis | ||||||
|---|---|---|---|---|---|---|
| Reference | Author | Duration | Sample Name | Test Technique | Occupation Group (positive/Total) | Quality score |
| [33] | Beheshti et al. (2010) | N/A | Blood | Rose Bengal plate test (RBT), standard tube agglutination test (SAT) | Veterinary assistants (6/15), veterinarian students (0/42), butchers (2/52), slaughterhouse workers (2/17), and chefs (1/3) | 8 |
| [34] | Nahar and Ahmed (2009) | 2007 | Blood | Rose Bengal plate test (RBT), standard tube agglutination test (SAT) | Clinical attendant (1/13), animal owner (1/7), butchers (0/4), veterinary science Students (1/26) | 8 |
| [35] | Cadmus et al. (2006) | 2004 | Blood | Rose Bengal test (RBT) | butchers (7/11), herdsmen (0/11) | 7 |
| [36] | Nasinyama et al. (2014) | N/A | Blood | Standard Tube Agglutination Test and ELISA | cattle keepers (21/161) | 8 |
| [37] | Tasiame et al. (2016) | 2011 | Blood | Rose Bengal Plate Test and ELISA | Delivery assistants (10/160), cattle drovers (8/18) | 8 |
| [38] | Ali et al. (2017) | N/A | Blood | Rose Bengal Plate Test (RBT) | butchers (0/30) | 8 |
| [39] | Acharya et al. (2018) | 2012 | Blood | ELISA | N/A | 7 |
| [40] | Holt et al. (2021) | 2015–2017 | Blood | Rose Bengal Plate Test and ELISA | Dairy Farmers (57/585) | 8 |
| [41] | Omer et al. (2002) | 2000 | Blood | Rose Bengal Plate Test (RBT) | dairy farm workers (10/132), veterinary personnel (1/22), pastoral area (6/156) | 8 |
| [42] | Miller et al. (2016) | 2011 | Blood | ELISA | N/A | 6 |
| [43] | Mufinda et al. (2017) | 2012 | Blood | Rose Bengal Plate Test (RBT) | workers (7/131), cattle breeders (32/192) | 8 |
| [44] | Adesiyun et al. (2011) | 2006 | Blood | ELISA | livestock/farm workers (0/394), abattoir workers (0/99) | 7 |
| [45] | Yohannes Gemechu and Paul Singh Gill (2011) | 2008–2009 | Blood | Rose Bengal test and serum/ standard agglutination test | Veterinarians and pharmacists (43/126), para-veterinarians (3/16), animal attendants and dairy farmers (13/78), miscellaneous (5/21) | 8 |
| [46] | Swai and Schoonman (2009) | 2004 | Blood | Rose Bengal Plate Test (RBT) | Abattoir workers (8/41), livestock farmers (2/67), non-livestock keepers (0/38), veterinary/meat inspectors (0/11) | 8 |
| [47] | Shome et al. (2017) | N/A | Blood | Rose Bengal Plate Test and ELISA | Animal handlers (15/93), veterinarians (50/833), para-veterinarians (8/49), artificial inseminators (1/18), veterinary students (0/57) | 8 |
| [48] | Ebrahimpour et al. (2012) | 2010 | Blood | Wright Tube Agglutination test | Planter, farmer, housewives, worker, students | 6 |
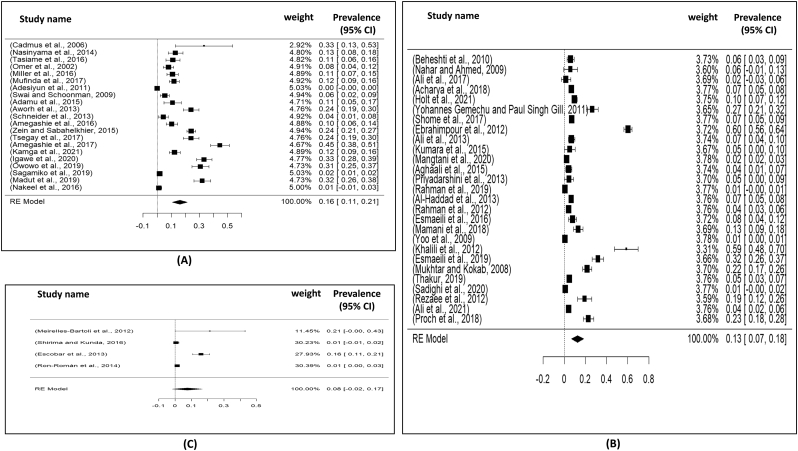
| [49] | Meirelles-Bartoli et al. (2012) | 2006 | Blood | Rose Bengal test and serum/ standard agglutination test | N/A | 6 |
| [14] | Ali et al. (2013) | 2011 | Blood | Rose Bengal test and serum/ standard agglutination test | Veterinary personnel (0/31), milker (1/53), abattoir worker (10/54), livestock farmer (7/107) | 8 |
| [50] | Kumara et al. (2015) | N/A | Blood | Rose Bengal test and serum/ standard agglutination test | Veterinarians (4/75) | 8 |
| [51] | Mangtani et al. (2020) | 2015–2017 | Blood | Rose Bengal Plate Test and ELISA | N/A | 7 |
| [52] | Doni et al. (2017) | 2013 | Blood | Rose Bengal test and serum/ standard agglutination test | Family farmers (42/461), seasonal migratory workers (39/246) | 8 |
| [53] | Aghaali et al. (2015) | 2013 | Blood | Standard tube agglutination test | N/A | 6 |
| [54] | Priyadarshini et al. (2013) | N/A | Blood | Rose Bengal Plate Test and ELISA | Veterinary officer (0/14), farmer (1/11), exposed animal handler (1/27), slaughter house worker (2/16) | 8 |
| [55] | Rahman et al. (2019) | N/A | Blood | Rose Bengal Plate Test (RBT) | Worker (3/437) | 7 |
| [56] | Adamu et al. (2015) | N/A | Blood | Rose Bengal Plate Test (RBT) | Animal handlers (5/40), livestock keepers (4/25), butchers (2/20), middle men (0/15) | 8 |
| [57] | Shirima and Kunda (2016) | 2005–2006 | Blood | Rose Bengal Plate Test (RBT) | N/A | 7 |
| [58] | Aworh et al. (2013) | 2010–2011 | Blood | Rose Bengal Plate Test and ELISA | Abattoir workers (54/224) | 7 |
| [59] | Al-Haddad et al. (2013) | 2009 | Blood | Standard tube agglutination test | Farmer (40/456), shepherd (3/47), butcher (0/5) | 8 |
| [60] | Rahman et al. (2012) | 2007–2008 | Blood | Rose Bengal Plate Test and ELISA | Livestock farmer (10/386), milker (10/55), butcher (1/40), veterinary practitioner (1/19) | 8 |
| [61] | Esmaeili et al. (2016) | 2011 | Blood | ELISA and standard tube agglutination test | Butchers and slaughterhouse workers (15/190) | 7 |
| [62] | Mamani et al. (2018) | 2014–2015 | Blood | Standard tube agglutination test | Butchers (19/93), slaughterhouse workers (7/79), veterinarians (3/17) | 8 |
| [63] | Yoo et al. (2009) | N/A | Blood | Standard tube agglutination test | Handlers of residual products (6/351), slaughterer (6/889), inspectors and their assistants (0/190), grading testers and their assistants (0/92) | 8 |
| [64] | Schneider et al. (2013) | N/A | Blood | Rose Bengal Plate Test (RBT) | Slaughterhouse workers (6/134) | 7 |
| [65] | Khalili et al. (2012) | 2011 | Blood | ELISA | Slaughterhouse workers (44/75) | 8 |
| [66] | Esmaeili et al. (2019) | 2017 | Blood | ELISA | Butchers and general population (92/289) | 7 |
| [67] | Mukhtar and Kokab (2008) | 2008 | Blood | ELISA | Animal keeper (15/40), loader (4/12), Vet/paravet (3/ 9), slaughterer (23/ 85), meat seller (30/16), cleaner (3/20), driver (0/28) | 8 |
| [68] | Amegashie et al. (2016) | 2014 | Blood | ELISA | Animal contact at work (5/54), meat processing (17/148) | 8 |
| [69] | Escobar et al. (2013) | 2005–2011 | Blood | Serum agglutination test (SAT) | Slaughterhouse workers (32/200) | 7 |
| [70] | Zein and Sabahelkhier (2015) | 2012 | Blood | Rose Bengal Plate Test (RBT) | Veterinarians (2/21), meat-inspectors (6/39), abattoir workers (99/407), animal handlers (76/286) | 8 |
| [71] | Theses (2019) | N/A | Blood | Rose Bengal Plate Test and ELISA | Hospital (6/127), veterinarians (4/23), students (0/41), pathological laboratory (8/171) | 8 |
| [27] | Tsegay et al. (2017) | 2013–2014 | Blood | Rose Bengal Plate Test (RBT) | Abattoir workers (54/224) | 8 |
| [72] | Sadighi et al. (2020) | N/A | Blood | ELISA | Dairy farm workers (2/196) | 7 |
| [73] | Amegashie et al. (2017) | 2013 | Blood | Rose Bengal tests, PCR | Slaughterhouse workers (98/220) | 7 |
| [74] | Rezaee et al. (2012) | 2010–2011 | Blood | Serum agglutination test (SAT) | Butcher (5/32), rancher (6/20), slaughter house worker (12/60), milk product seller (2/18) | 8 |
| [75] | El-Moselhy et al. (2018) | N/A | Blood | ELISA | Veterinary doctors (6/23), administrators (5/27), veterinary workers (17/46), peelers (38/115) | 8 |
| [76] | Kamga et al. (2021) | 2019 | Blood | Rose Bengal Plate Test and ELISA | Slaughterhouse workers (8/61), herdsmen (19/101), butchers (7/84), veterinarians (0/11), meat or milk sellers (0/16) | 8 |
| [77] | Igawe et al. (2020) | 2017 | Blood | Rose Bengal Plate Test and ELISA | Slaughterers (27/79), slaughterer/meat sellers (38/98), livestock seller/farmers (3/12), meat inspector/vets (8/20), meat sellers (20/66) | 8 |
| [78] | Owowo et al. (2019) | 2018 | Blood | ELISA | Herdsmen, abattoir, livestock worker | 7 |
| [79] | Sagamiko et al. (2019) | 2015–2016 | Blood | Rose Bengal Plate Test and ELISA | Shepherd (1/75), livestock officer (0/11), butcher men (3/57), abattoir worker (2/186), milking man (0/72), animal product (0/24) | 8 |
| [80] | Ron-Román et al. (2014) | 2006–2008 | Blood | ELISA | N/A | 6 |
| [81] | Ali et al. (2021) | N/A | Blood | Rose Bengal tests, PCR | Veterinary students (0/45), shepherds (0/44), veterinarians (1/44), dung cake makers (1/52), milkers (5/63), abattoir workers (13/211) | 8 |
| [82] | Madut et al. (2019) | 2015–2016 | Blood | Rose Bengal Plate Test and ELISA | Vet assistant (12/21), butcher (45/94), health worker (9/14), meat handler (25/57), administrator (2/4) | 8 |
| [83] | Proch et al. (2018) | 2015–2016 | Blood | Rose Bengal Plate Test and ELISA | Veterinarian (2/51), nurse (41/133), handler (21/95) | 8 |
| [84] | Nakeel et al. (2016) | N/A | Blood | Rose Bengal Plate Test and ELISA | N/A | 6 |
| Tuberculosis |
||||||
|---|---|---|---|---|---|---|
| Author, Year | Sample name | Test Technique | Occupation Group (positive/Total) | Quality score | ||
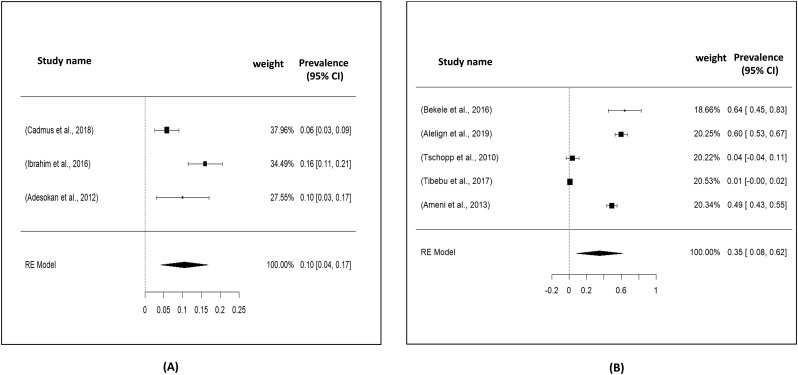
| [85] | Bekele et al. (2016) | 2012–2013 | Nasal swab | PCR | Dairy farm worker (16/25) | 7 |
| [7] | Alelign et al. (2019) | 2015–2018 | Sputum | PCR | Farmers (111/186) | 7 |
| [17] | Tschopp et al. (2010) | 2007–2009 | Sputum | Culture (Acid fast staining) | Livestock owner (4/26) | 7 |
| [87] | Tibebu et al. (2017) | 2010–2011 | Sputum | PCR | Dairy farm worker (3/256) | 7 |
| [88] | Ameni et al. (2013) | N/A | Sputum | M. bovis spoligotype | Cattle owner (141/287) | 7 |
| [89] | Amemor et al. (2017) | 2011–2012 | Sputum | Ziehl- Neelsen staining | Herdsman (0/68) | 6 |
| [90] | Bapat et al. (2017) | 2014–2015 | Blood | PCR | Farmers, dairy workers and livestock keepers (25/105), Zookeepers and animal handlers (11/45) | 8 |
| [91] | Torres-gonzalez et al. (2013) | 2009–2011 | Sputum | Tuberculin skin test (TST) and Interferon-gamma release assay (IGRA) | Dairy farm and abattoir workers (182/311) | 8 |
| [92] | Milián-Suazo et al. (2010) | 2006–2007 | Sputum | Mycobacterium bovis spoligotype | Farm worker (11/102), abattoir worker (0/91) | 8 |
| [93] | Joshi, (2015) | 2002–2003 | Sputum | Ziehl- Neelsen staining | Livestock owner and involve person (19/200) | 8 |
| [94] | Cadmus et al. (2018) | 2014–2015 | Sputum | PCR | Traders (7/136/206), butcher (5/70/206) | 8 |
| [95] | Ibrahim et al. (2016) | 2011–2013 | Sputum | Culture and biolin analysis | Livestock related person (40/250) | 7 |
| [28] | Adesokan et al. (2012) | N/A | Sputum | PCR | Livestock traders (63), worker in cattle market (7) | 8 |
| [96] | Mazari et al. (2021) | N/A | Nasal discharge | ELISA | Farm workers (3/200) | 7 |
| [97] | Khattak et al. (2016) | 2015 | Sputum | PCR | Abattoir workers (4/16), butchers (0/29), livestock farmers (1/50), Vet (0/3), Vet assistant (0/5) | 8 |
| [98] | Cleaveland et al. (2007) | N/A | Lymph node biopsies | PCR | TB infected cattle owner (65/457) | 8 |
| [99] | Rodriguez (2019) | 2016 | Blood | Tuberculosis assay | Dairy workers (14/140) | 7 |
3.2. Results of heterogeneity, publication bias and sensitivity analysis
In this meta-analysis, we evaluated the study's heterogeneity and publication bias. The analysis revealed significant heterogeneity among studies reporting brucellosis (H2 value = 342.99, I2 = 99.71%, p ≤0.001) and tuberculosis cases (H2 value =183.882, I2 = 99.46, p ≤0.001). Besides, the significant degree of heterogenicity between the studies was made apparent using the Galbraith plot test. Additionally, the Egger's test revealed no indication of bias in studies linked to tuberculosis (p-value = 0.7204); nevertheless, bias in papers connected to brucellosis was significant (p-value = 0.0023). As a result, we carried out sensitivity analysis for studies pertaining to brucellosis. Sensitivity analyses showed that the summary of the pooled estimates was unaffected significantly by the removal of outlier studies and moderate-quality studies (Supplementary file 2, Table 1). The prevalence rate stayed within the 95% CI for the relevant total prevalence [22]. Thus, we can conclude that the pooled estimated prevalence was validated by the reliability and rationality of our analyses. However, it is noted that we could not find any outlayer study for tuberculosis (Supplementary file, Fig. 2).
3.3. Meta-analysis result of tuberculosis
3.3.1. Meta-analysis of the prevalence of tuberculosis regarding continent and country
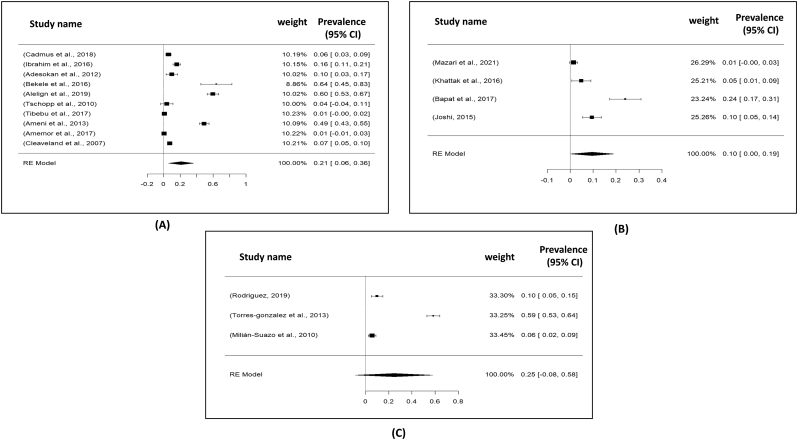
Total 17 studies obtained from Asia (04 studies), America (03 studies) and Africa (10 studies) were selected for the present meta-analysis. In this study, we analyzed a total of 3128 samples from the years 2000 to 2021, and found that the global prevalence of tuberculosis was 19% (95% CI: 09–30%), H2 value = 183.882, I2 = 99.46, p ≤0.001) (Fig. 2); besides funnel plot of all the studies were showed in Fig. 4. Then analyzing the continent-wise result (Fig. 5), the highest prevalence rate was found in America 25% (95% CI = −08–58%), followed by Africa 21% (95% CI = 06–36%) and Asia 10% (95% CI = 09–19%) (Table 2). After determining the continent-wise results, we analyzed the courtiers and found that Ethiopia, India and Mexico had the highest prevalence, while Ghana and Pakistan had the lowest (Fig. 7, Fig. 11).
Fig. 5.
Forest plot for representation of continent wise prevalence for tuberculosis: A) Africa; B) Asia and C) America (North and South).
Table 2.
Continent wise prevalence of brucellosis and tuberculosis.
| Disease | Continent | No. of Studies | Prevalence (%) (95% CI) | I2 (%) | H2 Value | Z-Test | Tau2 | Country Included |
|---|---|---|---|---|---|---|---|---|
| Brucellosis | Asia | 27 | 13 (07–18) | 99.69 | 319.310 | 4.27 | 0.022 | Pakistan, Bangladesh, India |
| Iran, South Korea | ||||||||
| Yemen | ||||||||
| Africa | 21 | 16 (11−21) | 99.6 | 263.08 | 5.94 | 0.014 | Angola, Cameroon, Ethiopia, Eritrea, Ghana, Kenya, Nigeria, Sudan, Tanzania | |
| Uganda | ||||||||
| America | 04 | 08 (−02–17) | 98.1 | 56.20 | 1.61 | 0.007 | Argentina, Ecuador, Brazil, Trinidad | |
| Tuberculosis | Africa | 10 | 21 (06–36) | 99.55 | 224.61 | 2.71 | 0.059 | Ethiopia, Ghana, Nigeria and Tanzania |
| Asia | 04 | 10 (0–19) | 96.14 | 25.89 | 2.00 | 0.086 | India, Nepal and Pakistan | |
| North America | 03 | 25 (−08–58) | 99.39 | 162.63 | 1.46 | 0.085 | Mexico and USA |
Fig. 7.
Forest plot showing the pooled prevalence of tuberculosis: A) Nigeria and B) Ethiopia.
Fig. 11.
Graphical representation of prevalence rate of tuberculosis from different countries.
3.3.2. Prevalence rate according to causal agent, diagnostic method and knowledge regarding tuberculosis
We found four serotypes of Mycobacterium bovis and Mycobacterium tuberculosis that are circulating rapidly throughout the world. The majority of studies reported Mycobacterium bovis infection, with a pooled prevalence of 15% (95% CI: 03–26), compared to a prevalence rate of 21% (95% CI: 03–38) for Mycobacterium tuberculosis (Table 3). Moreover, it is reported that 58% of tuberculosis pathogens were found from raw milk consumption, whereas only 36% of people have knowledge regarding zoonotic tuberculosis transmission.
Table 3.
Prevalence of tuberculosis according to different occupation groups and other risk factors.
| Variables | No. of Studies | Prevalence (%) (95% CI) | I2 (%) | H2 | Z-Test | Tau2 | P-value | Chi-square | |
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic method | PCR | 08 | 21 (04–38) | 99.47 | 188.41 | 2.46 | 0.058 | <0.001 | P = 0.808 |
| Other than PCR | 09 | 17 (03−31) | 99.35 | 153.33 | 2.41 | 0.045 | <0.001 | ||
| Age | (0–19) years | 04 | 06 (01−11) | 32.11 | 1.473 | 2.20 | 8e-04 | 0.353 | P = 0.926 |
| (20–49) years | 05 | 34 (02–66) | 99.19 | 122.7 | 2.06 | 0.133 | <0.001 | ||
| >50 years | 04 | 21 (06–37) | 87.23 | 7.832 | 2.80 | 0.018 | <0.001 | ||
| Occupational group | Farm's worker | 08 | 21 (03–38) | 99.67 | 301.23 | 2.32 | 0.061 | <0.001 | P = 0.563 |
| Livestock owner | 05 | 28 (06–50) | 98.87 | 88.88 | 2.51 | 0.061 | <0.001 | ||
| Livestock related person | 04 | 12 (05–19) | 87.10 | 7.75 | 3.45 | 0.004 | <0.001 | ||
| Abattoir workers | 04 | 03 (−01–08) | 64.46 | 2.81 | 1.54 | 0.001 | 0.025 | ||
| Causal agent | Mycobacterium bovis | 12 | 15 (03–26) | 99.49 | 197.84 | 2.49 | 0.040 | <0.001 | P = 0.251 |
| Mycobacterium tuberculosis | 07 | 21 (03–38) | 99.06 | 106.64 | 2.34 | 0.053 | <0.001 | ||
| Sex | Male | 07 | 20 (04–36) | 98.61 | 71.97 | 2.50 | 0.0447 | <0.001 | P = 1.00 |
| Female | 07 | 23 (−02–47) | 99.28 | 139.54 | 1.81 | 0.1081 | <0.001 | ||
3.3.3. Rate of prevalence according occupational group, age and sex
We categorized the individual's occupational groups as Farm's worker, livestock owner, livestock related person and abattoir worker. Among all of them, the maximum (28%; 95% CI: 06–50) prevalence rate of tuberculosis was found livestock owner, meanwhile, (21%; 95% CI: 03–38) prevalence rate was found from Farm's worker. However, the prevalence rate of tuberculosis was comparatively lower among the abattoir workers (03%; 95% CI: −01-08) (Table 3). Analyzing the age, the people from 20 to 49 years registered the maximum (34%; 95% CI: 02–66) prevalence rate; meanwhile, the lowest prevalence rate (34%; 06% CI: 01–11) was reported from the individuals between 0 and 19 years age. Moreover, in the term of sex category, Female had the maximum 23% (95% CI: −02-47) prevalence rate rather than male individuals 20% (95% CI: 04–36) (Table 3).
3.4. Meta-analysis result of brucellosis
3.4.1. Continent and country wise prevalence
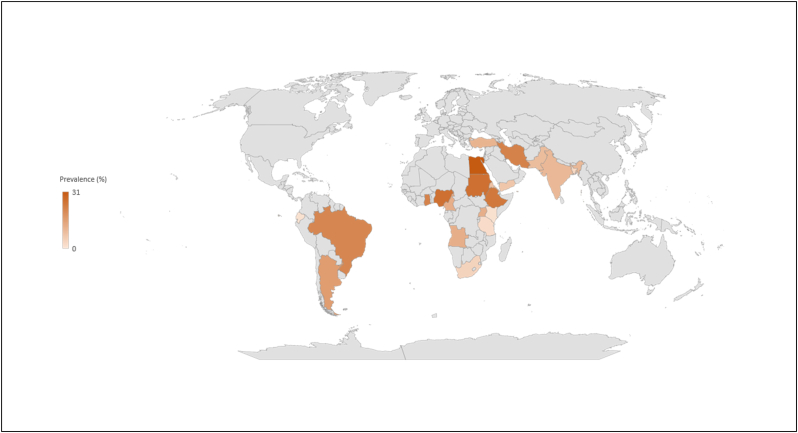
Total selected 54 studies were obtained from Asia (27 studies), Africa (21 studies), America (04 studies), single study from Egypt and Turkey. The current meta-analysis analyzed a total of 26,403 samples from the years 2000 to 2021, and revealed that the global prevalence of brucellosis was 14% (95% CI: 10–18%), H2 value = 342.99, I2 = 99.71%, p ≤0.001) (Fig. 3); besides funnel plot and radial plot of all the studies were showed in Fig. 4. Then analyzing the continent-wise result (Fig. 6), the highest prevalence rate was found in Africa 16% (95% CI = 11–21%), followed by Asia 13% (95% CI = 07–18%) and America 08% (95% CI = −02–17%), (Table 2). Likely, analyzed the courtiers, we found the maximum prevalence range (16–24) % in Egypt, Ethiopia, Ghana and Argentina; meanwhile, the lowest prevalence rate was registered from Kenya, Tanzania, Bangladesh and South Korea (Fig. 8, Fig. 10).
Fig. 3.
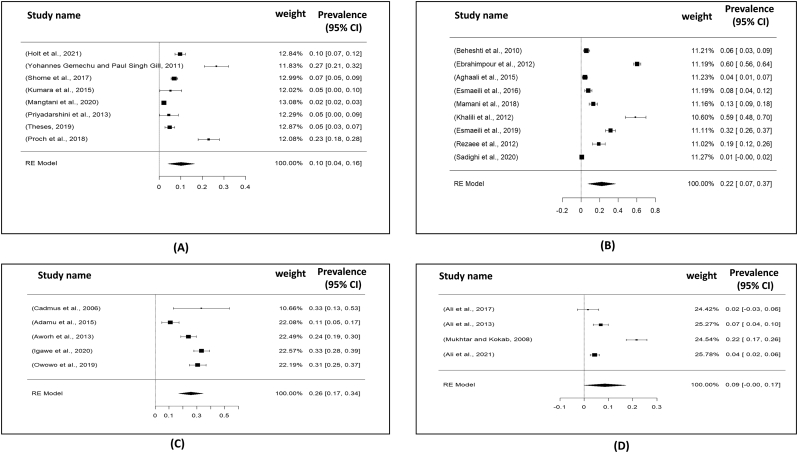
Visualizing forest plot described mean prevalence of zoonotic brucellosis from different livestock related occupational groups.
Fig. 6.
Forest plot for representation of continent wise prevalence for brucellosis: A) Africa; B) Asia and C) America (North and South).
Fig. 8.
Forest plot showing the pooled prevalence of brucellosis: A) India; B) Iran; C) Nigeria and D) Pakistan.
Fig. 10.
Visual representation of the prevalence rate of brucellosis from different countries.
3.4.2. Prevalence rate according diagnostic method
In this study, we categorized the test procedure into six distinct groups, including Rose Bengal Plate Test and enzyme-linked immunoassay (ELISA) (14 studies), Rose Bengal Plate Test (11 studies), ELISA (11 studies), Rose Bengal test/serum and standard agglutination test (07 studies), Standard tube agglutination test (04 studies) and Rose Bengal tests and PCR (03 studies). The maximum prevalence rate was found as 18% (95% CI: 08–29) from the ELISA test; in contrast, the lowest 06% (95% CI: 01–11) prevalence rate was documented in Standard tube agglutination test (Table 4).
Table 4.
Prevalence of brucellosis according to different occupation groups and other risk factors.
| Variable | Occupational Group | No. of Studies | Prevalence (%) (95% CI) | I2 (%) | H2 | Z-Test | Tau2 | P-value | Chi-square test |
|---|---|---|---|---|---|---|---|---|---|
| Occupational group | Slaughterhouse workers | 23 | 20 (13–27) | 99.08 | 108.50 | 5.79 | 0.026 | <0.001 | P = 0.002 |
| Butcher | 12 | 15 (05–24) | 94.11 | 16.98 | 3.07 | 0.023 | <0.001 | ||
| Farm workers | 12 | 10 (04–17) | 99.58 | 240.83 | 3.04 | 0.013 | <0.001 | ||
| Veterinary assistants | 12 | 19 (09–29) | 99.96 | 32.91 | 3.66 | 0.026 | <0.001 | ||
| Veterinarian | 11 | 13 (06–21) | 87.61 | 8.07 | 3.43 | 0.012 | <0.001 | ||
| Animal handlers | 10 | 16 (09–22) | 89.40 | 9.43 | 4.98 | 0.007 | <0.001 | ||
| Livestock farmers | 07 | 11 (04–18) | 73.35 | 3.75 | 3.28 | 0.004 | 0.001 | ||
| Shepherd | 06 | 10 (0–19) | 94.58 | 18.45 | 1.99 | 0.012 | <0.001 | ||
| Veterinarian students | 05 | 01 (0−03) | 0 | 1.00 | 1.58 | 2e-04 | 0.966 | ||
| Milker | 04 | 06 (−01−13) | 89.42 | 9.45 | 1.70 | 0.003 | 0.002 | ||
| Age | (20–45) years | 03 | 09 (06–12) | 0.0 | 1.00 | 6.46 | 7e-04 | 0.489 | P = 1.00 |
| 46 years-rest | 03 | 06 (04–07) | 1.34 | 1.01 | 6.39 | 3e-04 | 0.246 | ||
| Sex | Male | 32 | 17 (11–24) | 99.68 | 311.8 | 5.39 | 0.032 | <0.001 | P = 0.799 |
| Female | 30 | 06 (03–09) | 98.53 | 67.989 | 4.24 | 0.004 | <0.001 | ||
| Diagnostic test | Rose Bengal Plate Test and ELISA | 14 | 12 (06–18) | 99.48 | 190.81 | 4.10 | 0.011 | <0.001 | P = 0.036 |
| Rose Bengal Plate Test | 11 | 10 (05–16) | 97.87 | 47.02 | 3.53 | 0.008 | <0.001 | ||
| ELISA | 11 | 18 (08–29) | 99.91 | 1074.80 | 3.44 | 0.030 | <0.001 | ||
| Rose Bengal test and serum and standard agglutination test |
07 | 11 (5–17) | 92.74 | 13.77 | 3.49 | 0.005 | <0.001 | ||
| Standard tube agglutination test | 04 | 06 (01–11) | 96.43 | 27.98 | 2.36 | 0.002 | <0.001 | ||
| Rose Bengal tests and PCR | 03 | 18 (−07–44) | 99.72 | 355.43 | 1.43 | 0.049 | <0.001 |
3.4.3. Rate of prevalence according to occupational group, age and sex
We categorized the individual's occupation into ten groups: slaughterhouse workers, butchers, farm workers, veterinary assistants, veterinarian, animal handlers, livestock farmers, veterinary students and milker. Among all of them, the maximum (20%; 95% CI: 13–27) prevalence rate of brucellosis was found among slaughterhouse workers. Meanwhile, 19% (95% CI: 09–29) prevalence rate was recorded among the veterinary assistants. However, the prevalence rate of brucellosis was comparatively lower among veterinary students 01% (95% CI: 0–03) and milkers 06% (95% CI: -01-13). Analyzing the age, the people from 20 years to 25 years showed the maximum 09% (95% CI: 06–12) prevalence rate; meanwhile, the lowest prevalence rate 06% (95% CI: 04–07) was reported from the individuals 46 or above 46 years age. Moreover, in the term of sex category, male had 17% (95% CI: 11–24) prevalence rate rather than female individuals 06% (95% CI: 03–09) (Table 4) (Fig. 9).
Fig. 9.
Visualizing forest plot described mean prevalence of zoonotic brucellosis: A) Male and B) Female.
4. Discussion
The occurrence of zoonotic diseases like tuberculosis and brucellosis in the human population is associated with the presence of these diseases in the local livestock population. Therefore, understanding the different types of liability contact patterns between livestock and humans is extremely crucial in order to reduce disease transmission. As a result, the purpose of this systematic review was to describe the age, gender and diagnostic test variance in brucellosis and tuberculosis transmission patterns to livestock occupational groups. In the present study, a systematic procedure was applied to uncover current literature using preset criteria for two suspected zoonoses (brucellosis and tuberculosis) associated with livestock as well as try to distinguish among separate occupational groups preceding the zoonosis occurrence.
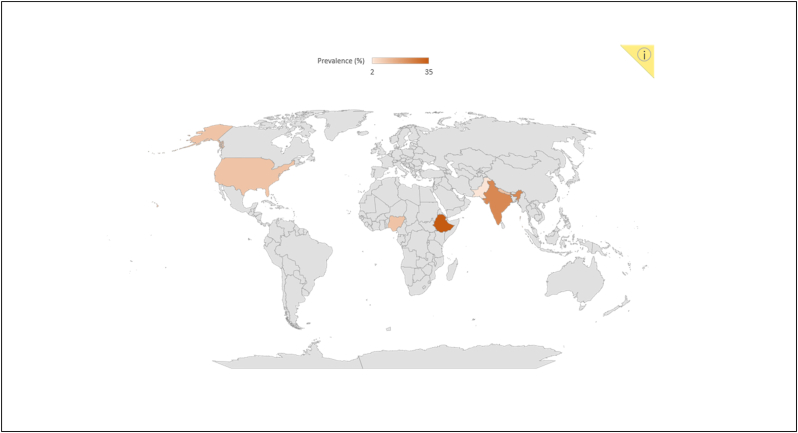
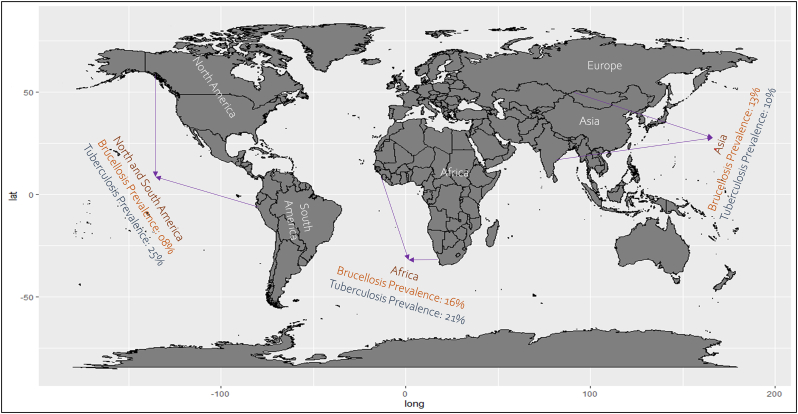
A total of 54 articles were identified for brucellosis as a solely livestock-associated pathogen with significant public health consequences for the livestock occupational groups (farmers, breeders, abattoir workers, veterinarians and veterinary technicians and hunters) as a result of constant aerosol exposure and contact of non-intact skin (wounds and abrasion) with infected materials (carcasses, viscera and live attenuated anti-brucellosis vaccines) and low adhesion to personal protective equipment in the work environment [24]. In contrast, 17 studies identified Mycobacterium tuberculosis as a zoonotic pathogen, which has gained considerable attention as a public health danger. Presently, Mycobacterium tuberculosis infection bears a significant risk in certain occupational groups that work closely with domestic and wild animals, such as livestock farmers, slaughterhouse employees, animal husbandry workers, veterinary staff, butchers, hunters, wildlife workers and live market workers [25]. Moreover, Numerous studies carried out in numerous nations have shown that animal handlers are susceptible to the zoonotic spread of Mycobacterium bovis if they have regular and uncontrolled interaction with livestock animals [8]. However, current data on the risk of brucellosis and tuberculosis infection in the workplace are sparse because the majority of studies are on a small magnitude in nature; thus, it is essential to identify the underlying risk factors and pathways associated with transmission of occupational related populations in order to facilitate and implementation of specific preventive measures and guidelines [25]. Therefore, it is crucial to understand the prevalence rate of these two zoonotic diseases among various occupational groups, as well as the association of multiple risk factors. In this regard, our study found a maximum 19% pooled prevalence rate for tuberculosis infection in different occupational categories than the prevalence rate of brucellosis (14%). When the pooled results were broken down by continent, brucellosis was more prevalent in Africa (16%) and Asia (13%); however, tuberculosis was more predominant in North America (25%) and Africa (21%) (Fig. 12). Immigrants, HIV-infected, and homeless people mostly live in urban areas in developed countries such as the United States of America, where they have a serious influence the predominant tuberculosis epidemiology [26].
Fig. 12.
Continent wise prevalence visualized in world map.
Furthermore, the current study documented that the low-income developing countries, such as Ethiopia, Ghana, Egypt and Mexico, are at risk for brucellosis and tuberculosis occurrence rates. Globally, the risks were enhanced in developing countries with a higher prevalence of the disease. A wide range of breeding techniques, in combination with a lack of veterinary health management to restrict infection in herds, could explain this elevated risk. Besides, proximity between human residences and animal shelters, shared material between farmers without disinfection precautions, consumption of unpasteurized dairy products and milk by farmers, regular physical contact with animals, lack of awareness concerning the disease [27], and inadequate hygienic practices are mainly responsible for rapid spared of these pathogens in the developing countries [28].
Analyzing the different occupational groups, slaughterhouse workers had the maximum prevalence rate of brucellosis (20%), whereas tuberculosis had the lowest prevalence rate (3%) among slaughterhouse workers. The majority of those people were from the United States, Africa, and Europe, with a small number from Asia. Previous research found that in Spain and Ethiopia, respectively, 12.26% and 48.72% of slaughterhouse workers reported cutting themselves with dirty sharp blades and coming into contact with animal fluids, aborted fetus, placenta, and viscera as the most common type of pathogen entry route [27].
Unlikely, in our study, the prevalence of tuberculosis among livestock owners was reported to be as high as 28%. In the same line, Adane et al. [29] found that the prevalence of tuberculosis appears to be higher in the livestock farmer community (59.7%) and this could be due to the lack of understanding about bovine tuberculosis, its transmission channels and prevention approach [30].
Comparing the risk factors including the age and sex, the maximum 34% and 23% prevalence of tuberculosis was reported from female individual and adult aged group (20–49 years). However, young aged group and male individuals have reported the maximum 9% and 17% prevalence rate for brucellosis. Other reports have shown a similar male predominance and this was probably related to the higher occupational exposure of these groups rather than greater ingestion of milk or dairy products. Likely, a previous study reported an increased tuberculosis rate among older adults aged 65 and above, which is in accordance with our study [31]. However, a prior study on the Western Cape's adolescent population's exposure to tuberculosis risk factors found that female adolescents had a 70% higher relative incidence rate of the disease than male adolescents. [32].
5. Conclusion
Infection with brucellosis and tuberculosis on a large scale among livestock-related personnel has become a major public health issue in both developing and developed countries. Working with animals carries a high risk, and yet frequent animal contact in general should also be taken into account for disease transmission. Going deeper, exposure to aborted fetuses, infectious after birth tissues, vaginal discharges, unpasteurized milk, sick animals handling and environmental factors are functioning as risk factors for disease transmission. In the present study we found, 19% and 14% pooled prevalence for tuberculosis and brucellosis among the different livestock related occupational groups. Additionally, brucellosis and tuberculosis were more prevalent in the African region; however, only tuberculosis was more widespread in the American region, and tuberculosis was expanding rapidly in Asian part. Furthermore, conferring to our findings, slaughterhouse workers and farm owners are at risk for getting brucellosis and tuberculosis, respectively. Thus, not only laboratory techniques for precise and rapid diagnosis, isolation and disposal of sick animals can aid in slowing the rapid transmission of these diseases, but also the implementation of proper preventive measures and specific guidelines among high-risk groups of people are also essential to curve the spread.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Credit author statement
Md. Mukthar Mia and Mahamudul Hasan: Conceptualization, Methodology and Analysis. Mahamudul Hasan and Faija Sadia Pory: Data collection. Md. Mukthar Mia, Mahamudul Hasan and Faija Sadia Pory: Reviewing and Editing.
Declaration of Competing Interest
Authors declare that they have no conflict of interests.
Acknowledgements
The authors would like to acknowledge the Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University for the technical support of the project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2022.100432.
Contributor Information
M. Mukthar Mia, Email: mukthar.psc@sau.ac.bd.
Mahamudul Hasan, Email: mhasan.student@sau.ac.bd.
Appendix A. Supplementary data
Keyword search strategy
Sensitivity analysis
References
- 1.F. and A.O. of the U.N. FAO Decent Rural Employment. 2022. https://www.fao.org/rural-employment/agricultural-sub-sectors/livestock/en/ (accessed April 25, 2022)
- 2.Bekele M., Mamo G., Mulat S., Ameni G., Beyene G., Tekeba E. Epidemiology of bovine tuberculosis and its public health significance in Debre-Zeit intensive dairy farms, Ethiopia. J Biomed Nurs. 2016;2:8–18. [Google Scholar]
- 3.Kelly R.F., Hamman S.M., Morgan K.L., Nkongho E.F., Ngwa V.N., Tanya V., Andu W.N., Sander M., Ndip L., Handel I.G. Others, knowledge of bovine tuberculosis, cattle husbandry and dairy practices amongst pastoralists and small-scale dairy farmers in Cameroon. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cousins S. The challenges of preventing bovine tuberculosis. Bull. World Health Organ. 2018;96:82–84. doi: 10.2471/BLT.18.020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ntivuguruzwa J.B., Michel A., Byaruhanga C., Gashururu R., Kolo F.B., et al. 2021. Awareness and Occupational Exposure to Brucellosis and Other Zoonotic Diseases Among Abattoir Workers in Rwanda; pp. 1–23. [DOI] [Google Scholar]
- 6.Malama S., Muma J.B., Godfroid J. A review of tuberculosis at the wildlife-livestock-human interface in Zambia. Infect. Dis. Poverty. 2013;2:1–5. doi: 10.1186/2049-9957-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alelign A., Zewude A., Petros B., Ameni G. Tuberculosis at farmer-cattle interface in the rural villages of South Gondar zone of Northwest Ethiopia. Tuberc. Res. Treat. 2019;2019 doi: 10.1155/2019/2106981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devi K.R., Lee L.J., Yan L.T., Syafinaz A.-N., Rosnah I., Chin V.K. Occupational exposure and challenges in tackling M. bovis at human--animal interface: a narrative review. Int. Arch. Occup. Environ. Health. 2021;94:1147–1171. doi: 10.1007/s00420-021-01677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins A.O., Cadmus S.I.B., Venter E.H., Pourcel C., Hauk Y., Vergnaud G., Godfroid J. Molecular epidemiology of human and animal tuberculosis in Ibadan, southwestern Nigeria. Vet. Microbiol. 2011;151:139–147. doi: 10.1016/j.vetmic.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 10.Tiwari H.K., Proch V., Singh B.B., Schemann K., Ward M., Singh J., Gill J.P.S., Dhand N.K. Brucellosis in India: comparing exposure amongst veterinarians, Para-veterinarians and animal handlers. One Heal. 2022;14 doi: 10.1016/j.onehlt.2021.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madut N.A., Ocan M., Muwonge A., Muma J.B., Nasinyama G.W., Godfroid J., Jubara A.S., Kankya C. Sero-prevalence of brucellosis among slaughterhouse workers in Bahr el Ghazal region. South Sudan. 2019:1–8. doi: 10.1186/s12879-019-4066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen K., Yu W.L. Serological diagnosis of brucellosis. Prilozi. 2010;31:65–89. [PubMed] [Google Scholar]
- 13.Sabzevari S., Shoraka H., Seyyedin M. Seroepidemiological survey of brucellosis and Q fever among high-risk occupations in northeast of Iran for first time. Iran. J. Microbiol. 2021;13:325. doi: 10.18502/ijm.v13i3.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali S., Ali Q., Neubauer H., Melzer F., Elschner M., Khan I., Abatih E.N., Ullah N., Irfan M., Akhter S. Seroprevalence and risk factors associated with brucellosis as a professional hazard in Pakistan. Foodborne Pathog. Dis. 2013;10:500–505. doi: 10.1089/fpd.2012.1360. [DOI] [PubMed] [Google Scholar]
- 15.Avila-Granados L.M., Garcia-Gonzalez D.G., Zambrano-Varon J.L., Arenas-Gamboa A.M. Brucellosis in Colombia: current status and challenges in the control of an endemic disease. Front. Vet. Sci. 2019:321. doi: 10.3389/fvets.2019.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerrier G., Daronat J.M., Morisse L., Yvon J.F., Pappas G. Epidemiological and clinical aspects of human Brucella suis infection in Polynesia. Epidemiol. Infect. 2011;139:1621–1625. doi: 10.1017/S0950268811001075. [DOI] [PubMed] [Google Scholar]
- 17.Tschopp R., Aseffa A., Schelling E., Berg S., Hailu E., Gadisa E., Habtamu M., Argaw K., Zinsstag J. Bovine tuberculosis at the wildlife-livestock-human interface in Hamer Woreda, South Omo, Southern Ethiopia. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newcastle-Ottawa O. Newcastle- Ottawa: Scale Customized for Cross-Sectional Studies. 2016. https://static-content.springer.com/esm/art%3A10.1186%2F1471-2458-13-154/MediaObjects/12889_2012_5111_MOESM3_ESM.doc
- 19.Rodriguez A.J., dos Santos Nunes V., Mastronardi C.A., Neeman T., Paz-Filho G.J. Association between circulating adipocytokine concentrations and microvascular complications in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of controlled cross-sectional studies. J. Diabetes Complicat. 2016;30:357–367. doi: 10.1016/j.jdiacomp.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Dalimi A., Tahvildar F. Others, molecular study on Cryptosporidium andersoni strains isolated from sheep based on 18S rRNA gene. Infect. Epidemiol. Microbiol. 2017;3:100–103. [Google Scholar]
- 21.Feller M., Huwiler K., Stephan R., Altpeter E., Shang A., Furrer H., Pfyffer G.E., Jemmi T., Baumgartner A., Egger M. Mycobacterium avium subspecies paratuberculosis and Crohn’s disease: a systematic review and meta-analysis. Lancet Infect. Dis. 2007;7:607–613. doi: 10.1016/S1473-3099(07)70211-6. [DOI] [PubMed] [Google Scholar]
- 22.Hajissa K., Islam M.A., Sanyang A.M., Mohamed Z. Prevalence of intestinal protozoan parasites among school children in africa: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0009971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howells K., Sivaratnam C., May T., Lindor E., McGillivray J., Rinehart N. Efficacy of group-based organised physical activity participation for social outcomes in children with autism spectrum disorder: a systematic review and meta-analysis. J. Autism Dev. Disord. 2019;49:3290–3308. doi: 10.1007/s10803-019-04050-9. [DOI] [PubMed] [Google Scholar]
- 24.Pereira C.R., de Almeida J.V.F., de Oliveira I.R., de Oliveira L., Pereira L.J., Zangeronimo M.G., Lage A.P., Dorneles E.M.S. Occupational exposure to Brucella spp.: a systematic review and meta-analysis, PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vayr F., Martin-Blondel G., Savall F., Soulat J.-M., Deffontaines G., Herin F. Occupational exposure to human Mycobacterium bovis infection: a systematic review. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caylà J.A., Orcau A. Control of tuberculosis in large cities in developed countries: an organizational problem. BMC Med. 2011;9:1–5. doi: 10.1186/1741-7015-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsegay A., Tuli G., Kassa T., Kebede N. Seroprevalence and risk factors of brucellosis in abattoir workers at Debre Zeit and Modjo export abattoir, Central Ethiopia. BMC Infect. Dis. 2017;17:1–8. doi: 10.1186/s12879-017-2208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adesokan H.K., Jenkins A.O., Van Soolingen D., Cadmus S.I.B. Mycobacterium bovis infection in livestock workers in Ibadan, Nigeria: evidence of occupational exposure. Int. J. Tuberc. Lung Dis. 2012;16:1388–1392. doi: 10.5588/ijtld.12.0109. [DOI] [PubMed] [Google Scholar]
- 29.Adane A., Damena M., Weldegebreal F., Mohammed H. Prevalence and associated factors of tuberculosis among adult household contacts of smear positive pulmonary tuberculosis patients treated in public health facilities of Haramaya district, Oromia region, eastern Ethiopia. Tuberc. Res. Treat. 2020;2020:1–7. doi: 10.1155/2020/6738532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khattak I., Mushtaq M.H., Ahmad M.U.D., Khan M.S., Haider J. Zoonotic tuberculosis in occupationally exposed groups in Pakistan. Occup. Med. (Chic. Ill). 2016;66:371–376. doi: 10.1093/occmed/kqw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C.-Y., Zhao F., Xia Y.-Y., Yu Y.-L., Shen X., Lu W., Wang X.-M., Xing J., Ye J.-J., Li J.-W. Others, prevalence and risk factors of active pulmonary tuberculosis among elderly people in China: a population based cross-sectional study. Infect. Dis. Poverty. 2019;8:26–35. doi: 10.1186/s40249-019-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahomed H., Ehrlich R., Hawkridge T., Hatherill M., Geiter L., Kafaar F., Abrahams D.A., Mulenga H., Tameris M., Geldenhuys H. Others, TB incidence in an adolescent cohort in South Africa. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beheshti S., Rezaian G.R., Azad F., Faghiri Z., Taheri F. Seroprevalence of brucellosis and risk factors related to high risk occupational groups in Kazeroon, south of Iran. Int. J. Occup. Environ. Med. 2010;1:62–68. [PubMed] [Google Scholar]
- 34.Nahar A., Ahmed M.U. Sero-prevalence study of brucellosis in cattle and contact human in Mymensingh district, Bangladesh. J. Vet. Med. 2009;7:269–274. [Google Scholar]
- 35.Cadmus S.I.B., Ijagbone I.F., Oputa H.E., Adesokan H.K., Stack J.A. Serological survey of brucellosis in livestock animals and workers in Ibadan, Nigeria, African. J. Biomed. Res. 2006;9:163–168. [Google Scholar]
- 36.Nasinyama G., Ssekawojwa E., Opuda J., Grimaud P., Etter E., Bellinguez A. Brucella sero-prevalence and modifiable risk factors among predisposed cattle keepers and consumers of un-pasteurized milk in Mbarara and Kampala districts. Uganda Abstract. 2014;14:2–5. doi: 10.4314/ahs.v14i4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tasiame W., Emikpe B.O., Folitse R.D., Fofie C.O., Burimuah V., Johnson S., Awuni J.A., Yebuah N., Wurapa F., West U., Hospital R. The prevalence of brucellosis in cattle and their handlers in North Tongu District of Volta Region. Ghana. 2016;10:111–117. doi: 10.21010/ajid.v10i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali S., Akbar A., Shafee M., Tahira B., Ullah N. Sero-epidemiological study of brucellosis in small ruminants and associated human beings in district Quetta. Balochistan. 2017;6:797–804. [Google Scholar]
- 39.Acharya D., Do Hwang S., Park J.-H. Seroreactivity and risk factors associated with human brucellosis among cattle slaughterhouse workers in South Korea. Int. J. Environ. Res. Public Health. 2018;15:2396. doi: 10.3390/ijerph15112396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holt H.R., Bedi J.S., Kaur P., Mangtani P., Sharma N.S., Gill J.P.S., Singh Y., Kumar R., Kaur M., McGiven J. Others, epidemiology of brucellosis in cattle and dairy farmers of rural Ludhiana, Punjab. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0009102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omer M.K., Assefaw T., Skjerve E., Tekleghiorghis T., Woldehiwet Z. Prevalence of antibodies to Brucella spp. and risk factors related to high-risk occupational groups in Eritrea. Epidemiol. Infect. 2002;129:85–91. doi: 10.1017/s0950268802007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller R., Nakavuma J.L., Ssajjakambwe P., Vudriko P., Musisi N., Kaneene J.B. The prevalence of brucellosis in cattle, goats and humans in rural Uganda: a comparative study. Transbound. Emerg. Dis. 2016;63:e197–e210. doi: 10.1111/tbed.12332. [DOI] [PubMed] [Google Scholar]
- 43.Mufinda F.C., Boinas F., Nunes C. Prevalence and factors associated with human brucellosis in livestock professionals. Rev. Saude Publica. 2017;51:51–57. doi: 10.1590/S1518-8787.2017051006051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adesiyun A., Campbell M., Rahaman S., Bissessar S., Stewart-Johnson A., Dookeran S., Gittens-St M., Hilaire Frequency of detection of immunoglobulins of Toxoplasma gondii, Leptospira spp., and Brucella abortus in livestock/farm and abattoir workers in Trinidad. J. Agromedicine. 2011;16:200–209. doi: 10.1080/1059924X.2011.581541. [DOI] [PubMed] [Google Scholar]
- 45.Yohannes Gemechu M., Gill J. Paul Singh. Seroepidemiological survey of human brucellosis in and around Ludhiana, India. Emerg. Health Threats J. 2011;4:7361. doi: 10.3402/ehtj.v4i0.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swai E.S., Schoonman L. Human brucellosis: seroprevalence and risk factors related to high risk occupational groups in Tanga Municipality, Tanzania. Zoonoses Public Health. 2009;56:183–187. doi: 10.1111/j.1863-2378.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 47.Shome R., Kalleshamurthy T., Padmashree B., Giribattanvar P., Chandrashekar N., Mohandoss N., Shome B.R., Kumar A., Sukhadeo B., Rahman H. Prevalence and risk factors of brucellosis among veterinary health care professionals, Pathog. Glob. Health. 2017;7724:1–6. doi: 10.1080/20477724.2017.1345366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebrahimpour S., Youssefi M.R., Karimi N., Kaighobadi M., Tabaripour R. The prevalence of human brucellosis in Mazandaran province, Iran, African. J. Microbiol. Res. 2012;6:4090–4094. [Google Scholar]
- 49.Meirelles-Bartoli R.B., Mathias L.A., Samartino L.E. Brucellosis due to Brucella suis in a swine herd associated with a human clinical case in the state of São Paulo, Brazil. Trop. Anim. Health Prod. 2012;44:1575–1579. doi: 10.1007/s11250-012-0108-2. [DOI] [PubMed] [Google Scholar]
- 50.Kumara M.S., Sindhib S.H., Dhanzea H., Mathapatib B.S. Sero-prevalence of brucellosis among veterinarians and livestock in Junagadh region of Gujarat state. J. Foodborne Zoonotic Dis. April. 2015;3:23–26. [Google Scholar]
- 51.Mangtani P., Berry I., Beauvais W., Holt H.R., Kulashri A., Bharti S., Sagar V., Nguipdop-Djomo P., Bedi J., Kaur M. Others, the prevalence and risk factors for human Brucella species infection in a cross-sectional survey of a rural population in Punjab, India. Trans. R. Soc. Trop. Med. Hyg. 2020;114:255–263. doi: 10.1093/trstmh/trz133. [DOI] [PubMed] [Google Scholar]
- 52.Doni N.Y., Gurses G., Simsek Z. Investigation of brucellosis in a female agricultural population in Turkey. Trop. Dr. 2017;47:132–136. doi: 10.1177/0049475516688148. [DOI] [PubMed] [Google Scholar]
- 53.Aghaali M., Mohebi S., Heydari H. Prevalence of asymptomatic brucellosis in children 7 to 12 years old. Interdiscip. Perspect. Infect. Dis. 2015;2015 doi: 10.1155/2015/187369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Priyadarshini A., Sarangi L.N., Palai T.K., Panda H.K., Mishra R., Behera P.C. Others, brucellosis in cattle and occupationally exposed human beings: a Serosurvey in Odisha, India. J Pure Appl Microbiol. 2013;7:3255–3260. [Google Scholar]
- 55.Rahman M.M., Rahman A., Hussain S.M.A., Al Maruf A., Hasan M.M., Rahman M.S. First report of brucellosis in dairy cattle and humans in military farms in Bangladesh. EC Vet. Sci. 2019;4:1–5. [Google Scholar]
- 56.Adamu N.B., Adeniyi S.O., Adamu S.G., Bale J.O.O., Okoh A.E.J., Umaru G.A., Umar Y.A. Seroprevalence of brucellosis among livestock workers at Maiduguri cattle market, Borno state, North eastern, Nigeria. J. Public Heal. Epidemiol. 2015;7:253–257. [Google Scholar]
- 57.Shirima G.M., Kunda J.S. Prevalence of brucellosis in the human, livestock and wildlife interface areas of Serengeti National Park, Tanzania: research communication. Onderstepoort J. Vet. Res. 2016;83:1–4. doi: 10.4102/ojvr.v83i1.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aworh M.K., Okolocha E., Kwaga J., Fasina F., Lazarus D., Suleman I., Poggensee G., Nguku P., Nsubuga P. Human brucellosis: seroprevalence and associated exposure factors among abattoir workers in Abuja, Nigeria-2011. Pan Afr. Med. J. 2013;16 doi: 10.11604/pamj.2013.16.103.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Haddad A.M., Al-Madhagi A.K., Talab A.A., Al-Shamahy H.A. The prevalence of human brucellosis in three selected areas in Al-Dala’a governorate, Yemen. Fac. Sci Bull. 2013;25:61–71. [Google Scholar]
- 60.Rahman A.K.M.A., Dirk B., Fretin D., Saegerman C., Ahmed M.U., Muhammad N., Hossain A., Abatih E. Seroprevalence and risk factors for brucellosis in a high-risk group of individuals in Bangladesh. Foodborne Pathog. Dis. 2012;9:190–197. doi: 10.1089/fpd.2011.1029. [DOI] [PubMed] [Google Scholar]
- 61.Esmaeili S., Naddaf S.R., Pourhossein B., Hashemi Shahraki A., Bagheri Amiri F., Gouya M.M., Mostafavi E. Seroprevalence of brucellosis, leptospirosis, and Q fever among butchers and slaughterhouse workers in South-Eastern Iran. PLoS One. 2016;11 doi: 10.1371/journal.pone.0144953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mamani M., Majzoobi M.M., Keramat F., Varmaghani N., Moghimbeigi A. Seroprevalence of brucellosis in butchers, veterinarians and slaughterhouse workers in Hamadan, western Iran. J. Res. Health Sci. 2018;18:406. [PubMed] [Google Scholar]
- 63.Yoo S.-J., Choi Y.-S., Lim H.-S., Lee K., Park M.-Y., Chu C.-S., Kang Y.-A. Seroprevalence and risk factors of brucellosis among slaughterhouse workers in Korea. J. Prev. Med. Public Heal. 2009;42:237–242. doi: 10.3961/jpmph.2009.42.4.237. [DOI] [PubMed] [Google Scholar]
- 64.Schneider R.C., Santos M.D., Lunardi M., Benetti A.H., Camargo L.M., Freitas S.H., Negreiro R.L., Costa D.S. Prevalence of brucellosis and risk factors associated with its transmission to slaughterhouse employees in the Cuiaba metropolitan area in the state of Mato Grosso. Semin. Ciências Agrárias. 2013;34:2367–2373. [Google Scholar]
- 65.Khalili M., Sami M., Aflatoonian M.R., Shahabi-Nejad N. Seroprevalence of brucellosis in slaughterhouse workers in Kerman city, Iran. Asian Pacific J. Trop. Dis. 2012;2:448–450. [Google Scholar]
- 66.Esmaeili S., Amiri F.B., Mokhayeri H., Kayedi M.H., Maurin M., Rohani M., Mostafavi E. Seroepidemiological study of Q fever, brucellosis and tularemia in butchers and slaughterhouses workers in Lorestan, western of Iran. Comp. Immunol. Microbiol. Infect. Dis. 2019;66 doi: 10.1016/j.cimid.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Mukhtar F., Kokab F. Brucella serology in abattoir workers. J Ayub Med Coll Abbottabad. 2008;20:57–61. [PubMed] [Google Scholar]
- 68.Amegashie E.A., Owusu-Dabo E., Salifu S.P., Awuah A.A.-A., Baffour-Awuah S., Addofoh N., Annan A., Winter C.H. Seroprevalence and occupational risk factors for Brucella infection among slaughterhouse workers and butchers in Kumasi, Ghana. J. Epidemiol. Res. 2016;3:17–22. doi: 10.5430/jer.v3n1p17. [DOI] [Google Scholar]
- 69.Escobar G.I., Jacob N.R., López G., Ayala S.M., Whatmore A.M., Lucero N.E. Human brucellosis at a pig slaughterhouse. Comp. Immunol. Microbiol. Infect. Dis. 2013;36:575–580. doi: 10.1016/j.cimid.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Zein A.M., Sabahelkhier M.K. Prevalence of brucellosis among high risk groups in northern state, Sudan. Nov J Med Biol Sci. 2015;4:1–4. [Google Scholar]
- 71.Luwumba Denice. Assessment of brucella infection status in abattoir workers and animals destined for slaughter at Dodoma modern abattoir. The Nelson Mandela African Institution of Science and Technology. 2019;2019:1–40. [Google Scholar]
- 72.Sadighi A., Samadi Kafil H., Maleki Chollou K., Maleki M., Babazadeh T., Ardeshiri Z., Bahadori A. Investigating the prevalence of brucellosis, as an occupational disease, in employees of traditional dairy workshops in Sarab. Infect. Epidemiol. Microbiol. 2020;6:11–19. [Google Scholar]
- 73.Amegashie E.A., Annan A.A., Awuah A.A.-A., Larbi R., Addofoh N., Feglo P.K., Owusu-Dabo E. Missed opportunities for the diagnosis of Brucella infection among slaughterhouse workers at the Kumasi abattoir, Ghana. J. Epidemiol. Res. 2017;32:58–64. doi: 10.5430/jer.v3n2p58. [DOI] [Google Scholar]
- 74.Rezaee M.A., Rashidi A., Motaharinia Y., Hossaini W., Rahmani R. Seroprevalence study of brucellosis among high-risk groups in comparison with other people of the population in Sanandaj (West of Iran), African. J. Microbiol. Res. 2012;6:1985–1989. doi: 10.5897/AJMR11.1095. [DOI] [Google Scholar]
- 75.El-Moselhy E.A., Zayet H., El-Khateeb A.S., Mohammed A.S., El-Tiby D.M., et al. Human Brucellosis: Seroprevalence, Risk Factors, and Barriers of Protection among Slaughterhouses’ Workers in El-Menia Governorate, Egypt. Arch Clin Pathol J. 2018;1(7):2. [Google Scholar]
- 76.Kamga R.M.N., Assongo B.S., Magang E.M.K., Fouapon A., Salihou M., Kuiate J.-R., Simo G. Seroprevalence of Brucella antibodies and risk factors associated with human brucellosis in high-risk occupational groups of the noun division in the West region of Cameroon. J. Biosci. Med. 2021;9:105–123. [Google Scholar]
- 77.Igawe P.B., Okolocha E., Kia G.S., Irmiya I.B., Balogun M.S., Nguku P. Seroprevalence of brucellosis and associated risk factors among abattoir workers in Bauchi state, Nigeria. Pan Afr. Med. J. 2020;35 doi: 10.11604/pamj.2020.35.33.18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Owowo E.E., Antia U.E., Christopher M.A., Okon I.E. Others, Sero-prevalence of brucellosis among nomadic herdsmen, abattoir and livestock Workers in Niger-Delta Region, Nigeria. J. Biosci. Med. 2019;7:32. [Google Scholar]
- 79.Sagamiko F.D., Muma J.B., Karimuribo E.D., Mwanza A.A., Mfune R.L., Sindato C., Kavunga H., Hang’ombe B.M. Seroprevalence of human brucellosis and associated risk factors among high risk occupations in Mbeya region of Tanzania. J. Epidemiol. Res. 2020;6:1–6. doi: 10.5430/jer.v6n1p1. 688705. [DOI] [Google Scholar]
- 80.Ron-Román J., Ron-Garrido L., Abatih E., Celi-Erazo M., Calva-Pacheco J., González-Andrade P., Berkvens D., Brandt J., et al. Human brucellosis in Northwest Ecuador: typifying Brucella spp., seroprevalence, and associated risk factors. Vector-Borne Zoonotic Dis. 2014;14:124–133. doi: 10.1089/vbz.2012.1191. [DOI] [PubMed] [Google Scholar]
- 81.Ali S., Saeed U., Rizwan M., Hassan L., Syed M.A., Melzer F., El-Adawy H., Neubauer H. Serosurvey and risk factors associated with Brucella infection in high risk occupations from district Lahore and Kasur of Punjab. Pakistan, Pathogens. 2021;10:620. doi: 10.3390/pathogens10050620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Madut N.A., Ocan M., Muwonge A., Muma J.B., Nasinyama G.W., Godfroid J., Jubara A.S., Kankya C. Sero-prevalence of brucellosis among slaughterhouse workers in Bahr el Ghazal region, South Sudan. BMC Infect. Dis. 2019;19:1–7. doi: 10.1186/s12879-019-4066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Proch V., Singh B.B., Schemann K., Gill J.P.S., Ward M.P., Dhand N.K. Risk factors for occupational Brucella infection in veterinary personnel in India. Transbound. Emerg. Dis. 2018;65:791–798. doi: 10.1111/tbed.12804. [DOI] [PubMed] [Google Scholar]
- 84.Nakeel M.J., Arimi S.M., Kitala P., Nduhiu G., Njenga J.M., Wabacha J.K. A sero-epidemiological survey of brucellosis, Q-fever and leptospirosis in livestock and humans and associated risk factors in kajiado county-Kenya. J Trop Dis. 2016;4:8. [Google Scholar]
- 85.Bekele M., Beyene G., Ameni G. Epidemiology of Bovine Tuberculosis and its public health significance in Debre- Zeit intensive dairy farms, Ethiopia. Biomed. Nurs. 2016;2:7–18. doi: 10.7537/marsbnj02021603. [DOI] [Google Scholar]
- 87.Tibebu M., Mekonnen W., Awoke T., Gebre-selassie S., Yamuah L., Berg S., Aseffa A. A high prevalence of tuberculosis among dairy farm Workers in Addis Ababa Mycobacterial Diseases a High Prevalence of tuberculosis among dairy farm Workers in Addis Ababa and its surroundings. Mycobact. Dis. 2017;4 doi: 10.4172/2161-1068.1000139. [DOI] [Google Scholar]
- 88.Ameni G., Tadesse K., Hailu E., Deresse Y., Medhin G., Aseffa A., Hewinson G., Vordermeier M., Berg S. Transmission of Mycobacterium tuberculosis between farmers and cattle in Central Ethiopia. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0076891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amemor E.A., Sackey S.O., Yebuah N., Folitse R.D., Benjamin O., Afari E., Wurapa F., Ohuabunwo C., Addo K., Mensah D., Gaglo E., Johnson S., Tasiame W., Amedzovor D., Nkunafa D., Bonsu F. THE PREVALENCE OF TUBERCULOSIS IN CATTLE AND THEIR HANDLERS IN NORTH TONGU, School of Veterinary Medicine, Kwame Nkrumah University of Science and Technology, Kumasi. Ghana Tuberculosis Control Programme, Ghana Health Service, 3 Veterinary Services. African J. Infect. Dis. 2017;11:12–17. doi: 10.21010/ajid.v11i1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bapat P.R., Dodkey R.S., Shekhawat S.D., Husain A.A., Nayak A.R., Kawle A.P., Daginawala H.F., Singh L.K., Kashyap R.S. Journal of epidemiology and Global Health prevalence of zoonotic tuberculosis and associated risk factors in central Indian populations. J. Epidemiol. Glob. Health. 2017;7:277–283. doi: 10.1016/j.jegh.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Torres-gonzalez P., Soberanis-ramos O., Martinez-gamboa A., Chavez-mazari B., Barrios-herrera M.T., Torres-rojas M., Cruz-hervert L.P., Garcia-garcia L., Singh M., Gonzalez-aguirre A., De Leon-gardun A.P., Bobadilla-del-valle M. Prevalence of latent and active tuberculosis among dairy farm workers exposed to cattle infected by Mycobacterium bovis. PLoS One. 2013;7:1–8. doi: 10.1371/journal.pntd.0002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milián-Suazo F., Pérez-Guerrero L. Molecular epidemiology of human cases of tuberculosis by Mycobacterium bovis in Mexico. Prev. Vet. Med. 2010;97:37–44. doi: 10.1016/j.prevetmed.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 93.Joshi Y.P. 2015. Prevalence of bovine tuberculosis among livestock and its relation with human tuberculosis in Kanchanpur district prevalence of bovine tuberculosis among livestock and its. [DOI] [Google Scholar]
- 94.Cadmus S., Akinseye V., Adegbulu A., Ovwighose N., Ayoola M., Ogugua J., Adesokan H., Cadmus E. Isolation of Mycobacterium tuberculosis from livestock workers and implications for zooanthroponotic transmission in Ibadan. J. Prev. Med. Hyg. 2018;59:212–218. doi: 10.15167/2421-4248/jpmh2018.59.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ibrahim S., Abubakar D., Usman A., Muhammad F.U., Musa G.A. Preliminary study on the prevalence of bovine tuberculosis and risk factors among pastoralists in Gombe state, North Eastern Nigeria. J Microbiol Exp. 2016;3:81. [Google Scholar]
- 96.Mazari M.Q., Kalhoro D.H., Baloch H., Kalhoro M.S., Abro S.H., Buriro R., Kaka A., Parveen F., Mangi M.H., Lochi G.M. Others, prevalence and risk factors of bovine tuberculosis in cattle and dairy farm Workers in Mirpurkhas and Badin Districts of Sindh. Pakistan. 2021 doi: 10.1186/2049-9957-2-13. [DOI] [Google Scholar]
- 97.Khattak I., Mushtaq M.H., Ayaz S., Ahmad M.U.D., Rahman S.U. Occurrence of zoonotic tuberculosis in occupationally exposed high-risk groups in Peshawar, Pakistan. Int. J. Mycobacteriology. 2016;5:S247. doi: 10.1016/j.ijmyco.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 98.Cleaveland S., Shaw D.J., Mfinanga S.G. Mycobacterium bovis in rural Tanzania : risk factors for infection in human and cattle populations. Tuberculosis. 2007;87:30–43. doi: 10.1016/j.tube.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 99.Rodriguez A. Tuberculosis among dairy workers in bailey county, texas. UT Sch. Public Heal. Diss. 2019;2019:24–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Keyword search strategy
Sensitivity analysis