Abstract
Background
The periosteum plays a crucial role in the development and injury healing process of bone. The purpose of this study was to construct a biomimetic periosteum with a double cell sheet for bone tissue regeneration.
Methods
In vitro, the human amniotic mesenchymal stem cells (hAMSCs) sheet was first fabricated by adding 50 μg/ml ascorbic acid to the cell sheet induction medium. Characterization of the hAMSCs sheet was tested by general observation, microscopic observation, live/dead staining, scanning electron microscopy (SEM) and hematoxylin and eosin (HE) staining. Afterwards, the osteogenic cell sheet and vascular cell sheet were constructed and evaluated by general observation, alkaline phosphatase (ALP) staining, Alizarin Red S staining, SEM, live/dead staining and CD31 immunofluorescent staining for characterization. Then, we prepared the double cell sheet. In vivo, rat calvarial defect model was introduced to verify the regeneration of bone defects treated by different methods. Calvarial defects (diameter: 4 mm) were created of Sprague–Dawley rats. The rats were randomly divided into 4 groups: the control group, the osteogenic cell sheet group, the vascular cell sheet group and the double cell sheet group. Macroscopic, micro-CT and histological evaluations of the regenerated bone were performed to assess the treatment results at 8 weeks and 12 weeks after surgery.
Results
In vitro, hAMSCs sheet was successfully prepared. The hAMSCs sheet consisted of a large number of live hAMSCs and abundant extracellular matrix (ECM) that secreted by hAMSCs, as evidenced by macroscopic/microscopic observation, live/dead staining, SEM and HE staining. Besides, the osteogenic cell sheet and the vascular cell sheet were successfully prepared, which were verified by general observation, ALP staining, Alizarin Red S staining, SEM and CD31 immunofluorescent staining. In vivo, the macroscopic observation and micro-CT results both demonstrated that the double cell sheet group had better effect on bone regeneration than other groups. In addition, histological assessments indicated that large amounts of new bone had formed in the calvarial defects and more mature collagen in the double cell sheet group.
Conclusion
The double cell sheet could promote to repair calvarial defects of rats and accelerate bone regeneration.
The translational potential of this article
We successfully constructed a biomimetic cell-sheet-engineered periosteum with a double cell sheet by a simple, low-cost and effective method. This biomimetic periosteum may be a promising therapeutic strategy for the treatment of bone defects, which may be used in clinic in the future.
Keywords: hAMSCs sheet, Osteogenic cell sheet, Vascular cell sheet, Double cell sheet, Biomimetic periosteum, Bone regeneration
Abbreviations: human amniotic mesenchymal stem cells, hAMSCs; scanning electron microscopy, SEM; hematoxylin and eosin, HE; alkaline phosphatase, ALP; extracellular matrix, ECM; polylactic-co-glycolic acid, PLGA; cell sheet technology, CST; human ethmoid sinus mucosa derived mesenchymal stem cells, hESMSCs; adiposetissue derivedstromalcells, ADSCs; bonemarrowmesenchymlstemcells, BMSCs; periodontal ligament-derived cells, PDLCs; cytokeratin 19, CK-19; bonevolume fraction, BV/TV; bone mineral density, BMD; Trabecular thickness, Tb.Th; Trabecular number, Tb.N
1. Introduction
Bone defects that occurred in the cases of trauma, tumor, and infection are common clinical diseases. Current clinical treatments of bone defects include autogenous, allogenic and artificial bone graft transplantation [1]. Although the optimized bone graft transplantation and the rapid development of surgical techniques, an approximate rate of 5–10% of patients will suffer from delayed fracture healing or even non-healing, which brings huge economic burden to patients and society [2]. With the rapid development of tissue engineering, tissue engineered bone has become a promising alternative strategy for bone regeneration and attracted many researchers’ attention. Nevertheless, most researchers focus on the bone defect itself, but neglect the importance of the periosteum. Periosteum is a thin and tough membrane that wraps around the outer surface of the bone tissue, which has an important role in the development and repair of bone tissue [3]. Periosteum is generally composed of two layers. The outer layer is fibrous layer, which contains elastic fibers, fibroblasts and microvessels. The inner layer is osteogenic layer that closely attaches on the surface of the bone and mainly contains progenitor cells that have the potential to develop into osteoblast or chondroblast after bone fracture and play an important role in the healing of bone fracture [3]. Studies have indicated that the periosteum is the main local source of skeletal progenitor/stem cells for bone healing and periosteum played a pivotal role in cytoskeletal reorganization [4,5]. Therefore, autologous periosteum is a promising therapy for bone repair. However, the limited availability of autologous periosteum hinder its widespread application in clinic. It is necessary and urgent to construct engineered biomimetic periosteum which can ideally simulate the structure and function of native periosteum to promote the healing and regeneration of bone tissue. To date, various biological and polymeric materials have been used to construct engineered biomimetic periosteum, including poly lactic-co-glycolic acid (PLGA) micropatterned nanosheet [6], cell composite hydrogel periosteum [7], acellular dermal periosteum [8]. Nevertheless, these tissue engineered periostea were mainly based on certain structural feature of the periosteum including macrostructural bionics and microstructural bionics. So far, tissue engineered periostea have also made great achievement in osteogenesis and angiogenesis of the regenerative periostea. Currenly, as a new therapy for tissue regeneration, cell sheet technology (CST) has promising application in bone regeneration due to the potential of realizing osteogenesis and angiogenesis.
CST, a new therapy for tissue regeneration, has been widely used in repairing cartilage, heart, cornea, and so on [[9], [10], [11]]. CST is capable of harvesting seed cells without utilizing enzymatic digestion, therefore it can preserve abundant extracellular matrix (ECM) and cell-to-cell junctions. It was reported that ECM had a crucial effect on recruiting plentiful endogenous stem cells or progenitor to injury sites and promote self-healing of lesions [12,13]. Additionally, the ECM plays a significant role in the proliferation and differentiation of the surrounding cells because of the dynamic interactions between the ECM and these surrounding cells [14]. For bone regeneration, CST has been used to serve as the convenient and effective measure to enhance allograft integration with improved outcomes [15]. What's more, CST can mimic periosteum, which provides nutrients to the bone defects and promotes the bone regeneration [15]. So far, many studies have used CST to promote the regeneration of bone. Xie et al. fabricated the human ethmoid sinus mucosa derived mesenchymal stem cells (hESMSCs) cell sheet and demonstrated it could promote the bone regeneration, which could serve as a promising method in the acceleration of bone regeneration [16]. Qi et al. prepared rat BMSCs sheet and combined it with CaP particles and PRP gel. After transplanting into bone defects of rat femurs, they found that incorporating PRP gel/CaP particles into BMSCs sheet could facilitate bone regeneration [17]. Currently, various types of cells have been applied to prepare cell sheet, including adipose tissue derived stromal cells (ADSCs) [18], bone marrow mesenchyml stem cells (BMSCs) [19], periodontal ligament-derived cells (PDLCs) [[20], [21], [22]], and so on. Compared with these cells, human amniotic mesenchymal stem cells (hAMSCs) have many special advantages. For instance, hAMSCs have the convenient and wide source, which come from the discarded placenta. Besides, the low immunogenicity of hAMSCs makes it is possible to widely used in vivo without immune rejection [[23], [24], [25], [26], [27], [28]]. A study by You et al. prepared have prepared hAMSCs sheet by using mechanical system and demonstrated that hAMSCs sheet could promote cartilage regeneration [29]. Therefore, it is promising to construct cell sheet by using hAMSCs as seed cells for bone regeneration. A variety of systems have been used to prepare cell sheet, including pH-responsive system [30,31], electro-responsive system [32,33], temperature-sensitive system [34,35], photo-responsive system [36,37], an so on. However, all of the above-mentioned methods have their disadvantages. For example, cells in the pH-responsive systems are easily damaged [31]; cells growth is suppressed by the coated materials in the electro-responsive systems [38]; cell aggregation and differentiation can be influenced by the temperature-sensitive system [34]; it is difficult to detach cell sheet in the photo-responsive system [39]. Compared with these methods, mechanical system may be easy to fabricate cell sheet without complex techniques or specific culture substrates. What's more, the viability of cells can not be influenced during the process of preparation cell sheet. Therefore, mechanical system has been widely used to prepare various cell sheets [[40], [41], [42], [43]].
Therefore, the purpose of this study was to construct a engineering biomimetic periosteum with a double cell sheet (the outer layer is the vascular cell sheet and the inner layer is the osteogenic cell sheet) and to explore the effect of the engineering biomimetic periosteum on bone regeneration. The schematic diagram of the experimental procedure is shown in Fig. 1.
Figure 1.
Schematic diagram of the experimental procedure. hAMSCs: human amniotic mesenchymal stem cells.
2. Materials and methods
2.1. Isolation and identification of hAMSCs
In this study, hAMSCs were isolated from the amnions of human placentas in accordance with a protocol from Zhang et al. and relevant informed consent was provided by each donor before the operation [[27], [28], [29]]. The morphology of the hAMSCs was observed under an inverted microscope after 3 days of cultivation. Third-generation (P3) hAMSCs were used in subsequent experiments. The stemness of the hAMSCs was tested by immunofluorescent staining with a stem cell marker (vimentin) and a human amniotic epithelial cell marker (cytokeratin 19: CK-19).
2.2. Construction and characterization of hAMSCs sheet
hAMSCs were seeded in six-well culture plates at a density of 1 × 106 cells/cm2. After cultivation in general medium for 2 days, the general medium was changed for cell sheet induction medium, which supplemented with 10% (v/v) FBS, 50 μg/ml ascorbic acid, 1% (v/v) P/S, 1% (v/v) glutamine, 1% (v/v) non-essential amino acids. The medium was refreshed every two days, and hAMSCs were continuous culture without passaging to obtain a dense hAMSCs sheet. After culturing for 7 days, the hAMSCs sheet was produced at the bottom of the six-well culture plates. Then, the hAMSCs sheet was harvested from the six-well culture plates with a cell scraper and the characterization of the hAMSCs sheet was assessed with the following experiments. First, the hAMSCs sheet was evaluated by general observation. Then, we oberved the cellular morphology of the hAMSCs sheet under the microscope. After culture for cell sheet, hAMSCs were stained with live/dead working solution to evaluate the viability of hAMSCs in the cell sheet according to the manufacturer's instructions. Then, the hAMSCs sheet was detected by scanning electron microscopy (SEM). For histological evaluation, the hAMSCs sheet were fixed in 4% buffered paraformaldehyde, dehydrated in graded alcohols, embedded in paraffin, and cut into sections. Sections were stained with hematoxylin-eosin (HE) to observe tissue structure.
2.3. Construction of osteogenic cell sheet
For construction of osteogenic cell sheet, we first construct hAMSCs sheet as mentioned above. After the formation of hAMSCs sheet, the cell sheet induction medium was changed for osteogenic cell sheet medium, which included 10% (v/v) FBS, 50 μg/ml ascorbic acid, 10 mM β-glycerophosphate, 10 nM dexamethasone, 1% (v/v) P/S. Then, we oberved the cellular morphology of the osteogenic cell sheet under the microscope and performed alkaline phosphatase (ALP) staining at day 3 day 5 and day 7. Besides, we performed Alizarin Red S staining according to the manufacturer's instructions at day 21. In addition, SEM was also used to detect the osteogenic cell sheet.
2.4. Construction of vascular cell sheet
To prepare a vascular cell sheet, we also first construct hAMSCs sheet as mentioned above. After the formation of hAMSCs sheet, we seeded a cell suspension of HUVECs onto the surface of the hAMSCs sheet at a cell density of 5 × 104/cm2. Subsequently, EBM-2 culture medium was used to the growing cells for continuous 7 days and the medium was refreshed every two days. The vascular cell sheet was tested by live/dead staining and CD31 immunofluorescent staining. The results of live/dead staining was taken by confocal microscopy.
2.5. Construction of the double cell sheet
To construct the double cell sheete, we first prepared the osteogenic and vascular cell sheet as mentioned above. Then, the osteogenic cell sheet was gently separated from the bottom of the six-well culture plate with a cell scraper and tweezers and then put into another six-well culture plate. Afterwards, we used the same method to separate the vascular cell sheet and slowly spread out onto the osteogenic cell sheet. Then, the double cell sheete was cultured for further 1 day and used in vivo experiment.
2.6. In vivo bone regeneration study
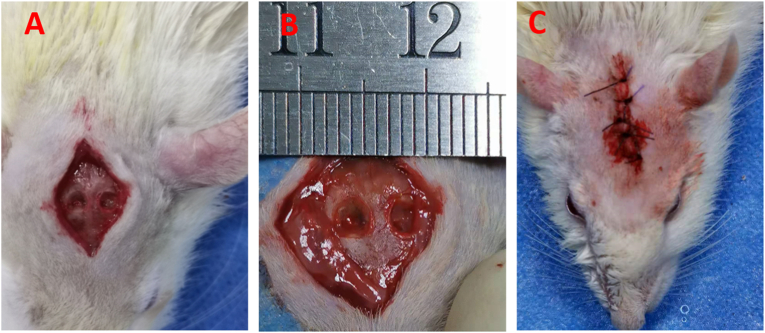
In this study, all animal studies adhered to the ARRIVE checklist. all Male Sprague–Dawley rats (SD, approximate weight of 300–350 g) were anesthetized by intraperitoneal injection of 2% sodium pentobarbital and a longitudinal incision was made in the middle of the surgical area after shaving and sterilizing. Then, the periosteum was stripped out. Afterwards, two bilateral bone defects with 4 mm in diameter were created on calvarium by dental trephine. The experiment was divided into 4 groups: group A, control group (defects were covered with nothing, n = 6); group B, osteogenic cell sheet group (defects were covered with osteogenic cell sheet, n = 6); group C, vascular cell sheet group (defects were covered with vascular cell sheet group, n = 6); group D, double cell sheet group (defects were covered with double cell sheetes, n = 6). After operation, penicillin was injected for three days to prevent infection. The rats were sacrificed at 8 weeks and 12 weeks, and the calvarial specimens were harvested for further assessments. Micro-CT was firstly used to assess the regenerative condition of the defect areas with the different treatments. The operative process of creation calvarial defects was shown in Fig. 2.
Figure 2.
The operative process of creation calvarial defects (A–B) Exposing calvarium and creating bilateral calvarial defects with 4 mm in diameter. (C) The calvarial defects were covered with different treatments and the wound was closed.
2.7. Histological analysis
After 8 weeks and 12 weeks of operation, the calvarial specimens were harvested and fixed in 4% paraformaldehyde for 24 h at room temperature, and decalcified for approximate 2 weeks. Then, they were embedded in paraffin for histological sectioning. The embedded specimens were cut into 5 μm-thick histological sections across the center of the defect area and stained with hematoxylin and eosin (HE) and Masson.
3. Statistical analysis
The data are expressed as the means ± standard deviations. Statistical analysis (GraphPad Prism 7.0 software, USA) was performed by one-way ANOVA followed by Tukey's multiple comparison test for further evaluation of the differences between the groups unless otherwise stated. P < 0.05 was considered to indicate statistical significance.
4. Results
4.1. Isolation and identification of hAMSCs
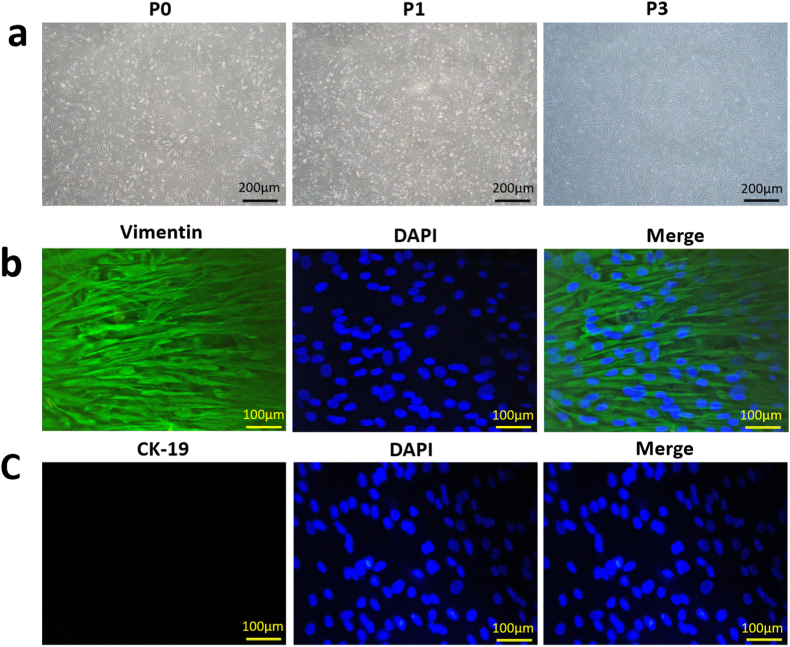
The morphology of different generations hAMSCs was observed under inverted phase contrast microscope. As shown in Fig. 3a, different generations hAMSCs all adhered to the culture bottle and exhibited the spindle-shaped exterior with radial-like growth. After several subcultures, highly pure hAMSCs were obtained and P3 hAMSCs showed a uniformly vortex-likeshape and used in the subsequent experiments. Afterwards, immunofluorescent staining was performed to test the stemness of the hAMSCs. As indicated in Fig. 3b and c, we could distinctly found that P3 hAMSCs highly express vimentin while did not express CK-19. Vimentin is the specific marker of hAMSCs, whereas CK-19 is the principal marker of human amniotic epithelial cells. Taken together, above results all indicated that we successfully isolated hAMSCs. Zhang et al. and You et al. have performed other detections to verify the stemness of the hAMSCs, including flow cytometry and multidirectional differentiation [28,29].
Figure 3.
Isolation and identification of hAMSCs. (a) Microscopic observations of passage-0 (P0), passage-1 (P1) and passage-3 (P3) hAMSCs. Scale bar: 200 μm. (b) The immunofluorescent staining of vimentin. Scale bar: 100 μm. (c) The immunofluorescent staining of CK-19. Scale bar: 100 μm.
4.2. Construction and characterization of hAMSCs sheet
After culturing for 7 days in cell sheet medium, high-density hAMSCs proliferated rapidly and hAMSCs sheet was formed in the six-well culture plate. As showed in Fig. 4a, hAMSCs sheet was white and transparent membrane and could be separated from the bottom of the six-well culture plate and folded easily into special shape. Plentiful long spindle-shaped cells were evenly distributed and secreted abundant ECM, which was observed under the inverted phase contrast microscope (Fig. 4b). Then, live/dead staining was introduced to examine the viability of hAMSCs embedded in hAMSCs sheet. The results demonstrated that most of the cells were live cells (green fluorescence) and only few dead cells (red fluorescence, Fig. 4c). The results suggested that after inducing for hAMSCs sheet, the hAMSCs still had the good viability. Then, SEM was also used to further detect the arrangement of hAMSCs embedded in hAMSCs sheet and the secretion of ECM. As shown in Fig. 4d and e, we could observed that vast ECM was formed around hAMSCs, and hAMSCs were evenly distributed and closely arranged. The result of HE staining further demonstrated that hAMSCs in cell sheet could secrete a large amount of ECM and hAMSCs sheet was composed of numerous cells (Fig. 4f).
Figure 4.
Characterization of hAMSCs sheet. (a) Gross observation of hAMSCs sheet. The hAMSCs sheet could be detached form the bottle of the six-well culture plate and folded into special shape. (b) hAMSCs sheet was observed under inverted phase contrast microscope. Many long spindle-shaped hAMSCs were closely arranged. (c) Live/dead staining of hAMSCs sheet. Green indicated live cells and red indicated dead cells. The red arrows indicated dead cells. (d) The surface of hAMSCs sheet was detected with SEM. hAMSCs were embedded in a large amount of ECM. (e) The cross section was observed with SEM. (f) HE staining of hAMSCs sheet. The hAMSCs sheet was composed of abundant ECM and plentiful cells.
4.3. Characterization of the osteogenic cell sheet
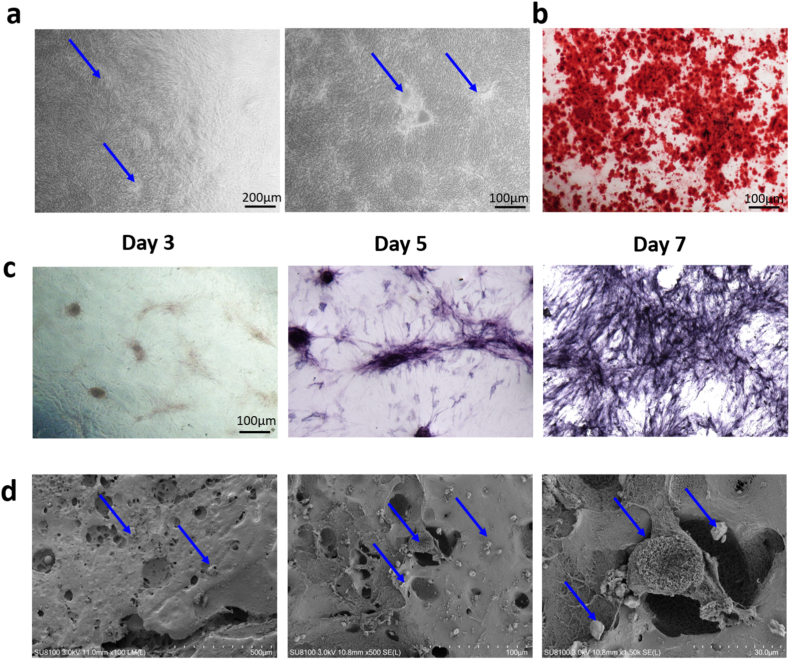
Under the inverted phase contrast microscope, we could observed many nodules located in the osteogenic cell sheet (Fig. 5a). ALP staining was used to determine the early osteogenic activity of osteogenic cell sheet. Besides, Alizarin Red S staining was introduced to evaluate the calcium deposition and further assessed the late osteogenic activity of osteogenic cell sheet. The ALP activities were significantly increased at day 7 compared with those at day 3 and day5 (Fig. 5c). The result of Alizarin Red S staining was positive and there was a large amount of calcium deposition in osteogenic cell sheet, indicating that mineralization occurred in osteogenic cell sheet (Fig. 5b). The result of SEM showed that many honeycomb-like mineralized nodules covered the surface of the osteogenic cell sheet (Fig. 5d). These above results suggested that the osteogenic cell sheet had a certain osteogenic potential.
Figure 5.
Characterization of the osteogenic cell sheet. (a) The osteogenic cell sheet was observed under the inverted phase contrast microscope. (b) The result of Alizarin Red S staining at 21 day. (c) ALP staining of the osteogenic cell sheet at day 3, day 5 and day 7. (d) SEM detection of the osteogenic cell sheet.
4.4. Characterization of the vascular cell sheet
To construct a vascular cell sheet, we first construct hAMSCs sheet. Then, the cell suspension of HUVECs was seeded onto the surface of the hAMSCs sheet to form vascular cell sheet. As shown in Fig. 6a–A, HUVECs adhered to the culture bottle and presented short fusiform. We could observe numerous HUVECs on hAMSCs sheet (Fig. 6a and B). After seeding HUVECs on hAMSCs sheet for 7days, HUVECs were embedded in hAMSCs sheet (Fig. 6a–C). Live/dead staining indicated HUVECs gradually increased both on six-well culture plate and hAMSCs sheet and HUVECs had good vitality (Fig. 6b). The number of live HUVECs on six-well culture plate and hAMSCs sheet was no statistic difference, indicating hAMSCs sheet possessed good cytocompatibility. CD31 immunofluorescent staining result indicated that the vascular cell sheet high expressed CD31, suggesting the vascular cell sheet had the potential capacity of angiogenesis (Fig. 6c).
Figure 6.
Characterization of the vascular cell sheet. (a) A) HUVECs adhered to the culture bottle and presented short fusiform. B) HUVECs were seeded on hAMSCs sheet. C) HUVECs were seeded on hAMSCs sheet for 7 days. (b) Live/dead staining of HUVECs cultured on six-well culture plate and hAMSCs sheet. (c) The result of CD31 immunofluorescent staining. (d) Quantitative analysis of the live/dead staining.
4.5. Evaluation of in vivo performance
To verify the regeneration of bone defect, rat calvarial bone defect model was introduced and the periosteum was stripped. Then, the biomimetic cell-sheet-engineered periosteum (the double cell sheet) was used to promote the regeneration of bone defect. After 8 weeks and 12 weeks of the surgery, we first took the general photos to assess the bone formation at the bone defect area. The double cell sheet group almost completely repaired the area of bone defect. Besides, we could observed a periosteum-like connective tissue was formed and coverd the bone defect area. However, a bare hole was distinct and a nonunion case occurred in the control group (Fig. 7).
Figure 7.
The general photos of calvarial specimens harvested at 8 weeks and 12 weeks after surgery. The double cell sheet group almost completely repaired the area of bone defect.
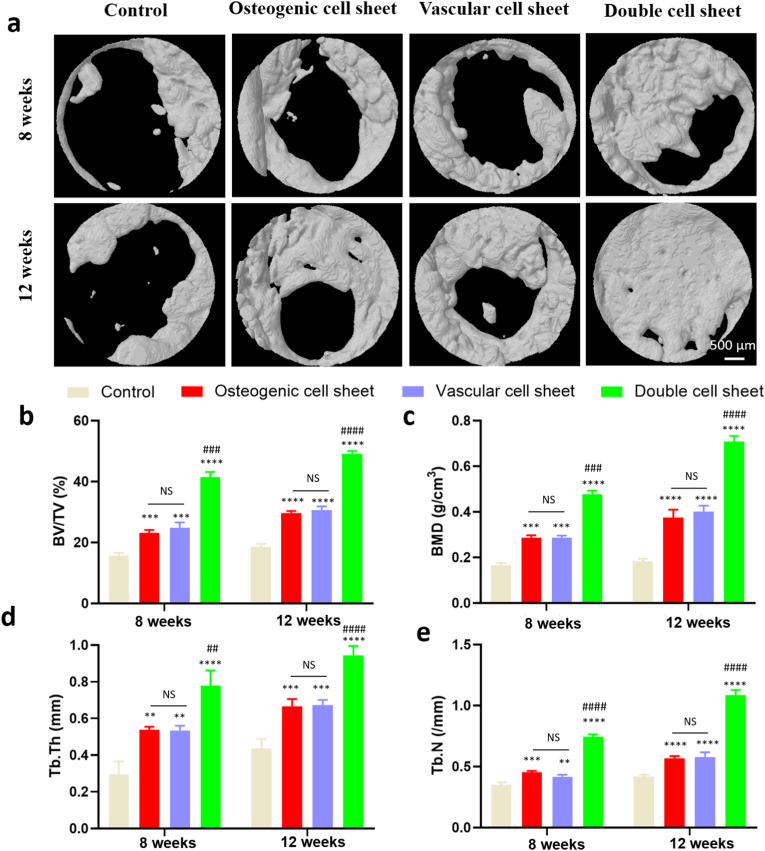
The micro-CT was also used to evaluate the bone forming process at 8 weeks and 12 weeks after the operation. The results showed that obvious differences between the double cell sheet group and other groups (Fig. 8a). The double cell sheet exhibited prominently better bone repairing effect, compared with the control group that only formed the indistinctive bone, which indicated that synergistic effect of the osteogenic cell sheet and vascular cell sheet on bone regeneration. The osteogenic cell sheet and vascular cell sheet groups showed better bone repairing effect than the control group, maybe due to the effect as a transient physical barrier to resist the invasion of the fibrous tissue (Fig. 8a). The further analysis of 3D reconstruction was performed and bone volume fraction (BV/TV), bone mineral density (BMD), Trabecular thickness (Tb.Th) and Trabecular number (Tb.N) were analysed to appraise the bone repairing effect. The results of BV/TV demonstrated the values of the double cell sheet group were higher than those of the other groups at 8 weeks and 12 weeks (Fig. 8b). Similarly, the results of BMD, Tb.Th and Tb.N also showed the values of the double cell sheet group were higher than other groups (Fig. 8c, d, 8e). These results of micro-CT were all consistent with the general assessment. The bone almost completely bridged the injury site for the double cell sheet group.
Figure 8.
Micro-CT analysis of in vivo performance at 8 weeks and 12 weeks. (a) 3D reconstruction images of bone defect areas; (b) Bone volume fraction (BV/TV); (c) Bone mineral density (BMD); (d) Trabecular thickness (Tb.Th); (e) Trabecular number (Tb.N). NS: no significant difference; ∗:Comparison between the control group and other groups; #: Comparison between the double cell sheet group and the vascular cell sheet group or the osteogenic cell sheet group. ∗∗: P < 0.01, ∗∗∗: P < 0.001, ∗∗∗∗: P < 0.0001, ##<0.01, ###: P < 0.001.
Histological detections were performed to further evaluate the bone repair from micro aspect at 8 weeks and 12 weeks after the operation. As showed in HE and Masson staining (Fig. 9a and b), compared with the other groups, the bone defect covered by double cell sheet formed more regularly-arranged bone, which were covered with cuboidal osteoblast-like cells. In comparison, the bone defect areas of the other groups were primarily filled with fibrous tissue and only partial regenerated bone, which indicate the inferior bone regenerative capacity. In the control groups, we could observe the distinct gap, indicating the extremely poor bone regenerative capacity. In the osteogenic cell sheet group and the vascular cell sheet group, a large amount of fibrous tissue and loose soft tissue grew into and covered the bone defect areas, and blocked the formation of new bone. The Masson staining also exhibited similar trend between the double cell sheet group and other groups at 8 weeks and 12 weeks after the operation. Under Masson staining, the mature collagen presented red color. Therefore, we could observed more mature collagen formed at the center of the bone defect in the double cell sheet group. In contrast, scattered newborn bone was found to fill the defect in other groups.
Figure 9.
Histological analysis of HE and Masson staining. (a–b) HE staining and Masson staining at 8 weeks. (c–d) HE staining and Masson staining at 12 weeks.
5. Discussion
In this study, we prepared a double cell sheet to construct the biomimetic cell-sheet-engineered periosteum and demonstrated that the double cell sheet promoted to repair calvarial defects of rats and accelerate bone regeneration. In vitro, we found that the double cell sheet had significant osteogenic potential and angiogenic potential. In vivo, the double cell sheet had better ability to promote bone regeneration compared with other groups. This result implied that the double cell sheet could simulate the structure of native periosteum and showed associated function of periosteum.
Our study used a simple method to construct the biomimetic cell-sheet-engineered periosteum to promote bone regeneration. Therefore, more in-depth researches are needed to explore the underlying mechanism. In the previous studies, Yang et al. elaborated the common strategies for constructing biomimicking artificial periosteum and introduced three novel versatile artificial periosteum, including healing phase-targeting biomimicking periosteum, heterogeneous structured biomimicking periosteum and physical-chemical combined artificial periosteum. These biomimicking periostea had unique advantages and had promising application prospects [44]. In addition, the other review summarized the importance of periosteum and summarized the advantages and disadvantages of different tissue engineering methods for construction of tissue-engineered periosteum [45].
Bone defects caused by various aetiologies are common clinical problems that impose huge burden on society and severely affect patients' quality of life [46]. Over the past two decades, the rapid development of tissue engineering has brought new direction for bone regeneration. However, the conventional bone tissue engineering mainly includes injection of cell suspension as well as the transplantation of scaffolds seeding with various cells, which has some problems need to be solved. For example, the injected cells are easily lost in vivo and the number of injected cells is considerably restricted with one injection. In addition, it is difficult to achieve the uniform distribution of cells after injection [47]. Currently, CST has gradually developed and attracted many researchers’ attention. CST is a new method to obtain seed cells without using enzymes, which effectively preserves ECM and cell-to-cell junctions. Therefore, it is easy to realize a uniform cell distribution and high cell density in vivo [48]. The periosteum is a thin and connective tissue covering the surface of bone except the joint, which plays a significant role in bone development and regeneration [49]. There are many studies using CST to construct engineering biomimetic periosteum for bone regeneration [[50], [51], [52]].
In this study, we used hAMSCs as seed cells to construct hAMSCs sheet in vitro by mechanical system. The prepared hAMSCs sheet could be folded into special shape with sharp pointed forceps. HE staining and SEM detection indicated that hAMSCs sheet had abundant ECM and intact proteins, which provided a favorable microenvironment for cell growth, migration and further vascularization [[53], [54], [55]]. Live/dead staining demonstrated that hAMSCs sheet had good viability and could be further used in vivo for bone regeneration. Then, we prepared osteogenic cell sheet and vascular cell sheet. The osteogenic cell sheet was positive for ALP and Alizarin red S staining on the tissue level. As we all know, ALP is the important marker of early osteogenesis, which is the enzyme protein that was secreted by osteoblasts [56]. The positive results of ALP indicated that osteogenic cell sheet had the osteogenic potential at the beginning of osteogenesis. Besides, the osteogenic cell sheet was positive for Alizarin red S staining at day 21, which demonstrated its capacity for late osteogenesis. The vascular cell sheet was positive for CD31, which is the important indicator of angiopoiesis. We used a simple detaching technique to detach cell sheet and overlaid the vascular cell sheet on the osteogenic cell sheet. The vascular cell sheet was used to mimic the outer fibrous layer of the native periosteum while the osteogenic cell sheet was used to mimic the inner cambium layer of the native periosteum. This tactics circumvented the drawbacks of using acellular human dermis or synthetic polymeric films for constructing the tissue engineered periosteum. Because, not only acellular human dermis but also synthetic polymeric films need revascularization [57,58].
In vivo, we further assessed the effect of the double cell sheet on bone regeneration. At 8 weeks and 12 weeks after using the double cell sheet to repair the calvarial defect of rat, micro-CT analysis, HE staining and Masson staining showed that the new bone formation was remarkably enhanced in the double sheet group in comparison with other groups. The possible reasons are as follows: the osteogenic cell sheet with good adhesive capacity could covered the areas of calvarial defects and provided temporary physical barrier, which provided a favorable microenvironment for bone regeneration. The vascular cell sheet could form an effective material and signal transmission pathway with the surrounding tissues. What's more, the vascular cell sheet could form blood vessel nets and provided necessary blood and oxygen supply for bone regeneration.
This study also had some limitations. First, we did not detect and test the double cell sheet in vitro. Further studies are needed to evaluate the characteristics of the double cell sheet in vitro. Second, we did not track the transplanted cell sheets (the osteogenic cell sheet, the vascular cell sheet and the double cell sheet) in vivo after surgery. Therefore, further studies are needed to explore the fate of cell sheets after transplantation into calvarial defects. Third, only one time point (8 week) was set to assess the bone regeneration. In the further study, multiple time pionts shoud be set to evaluate the bone regeneration because of the gradual process of bone healing. Last, the potential molecular mechanism involved in the better repair of calvarial defects via the double cell sheet was not explored in this study.
6. Conclusion
This study has demonstrated that the double cell sheet had the potential to repair rat calvarial defect. We successfully constructed hAMSCs sheet, osteogenic cell sheet, vascular cell sheet and the double cell sheet with a simple, low-cost and effective method. In addition, we demonstrated that the double cell sheet had significant osteogenic potential and angiogenic potential and could promote bone regeneration. This double cell sheet may be the promising method for the treatment of bone defects, which may be used in clinic in the future.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors'contributions
Author 6 conceived and designed the experiment. Author 1, 2 and 3 performed the experiment and collected the data. Author 4 and 5 analyzed the data. Author 6 contributed to the reagents, materials, and analysis tools. Author 1 wrote the manuscript. All authors read and approved the final manuscript.
Funding
The reported work was supported by the National Natural Science Foundation of China (NSFC) (nos. 8187090823). Ethics approval and consent to participate The study was reviewed and approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical University.
Consent for publication
Not applicable.
Declaration of competing interest
The all authors declare that they have no competing interests.
Acknowledgements
We are grateful for the Department of Orthopaedic Surgery, Affiliated Hospital of Zunyi Medical University and Professor Yi Liu.
Contributor Information
Jun Zhang, Email: 935876478@qq.com.
Xiaoji Luo, Email: cy2982@163.com.
References
- 1.Antalya H., Johanna B., Rustom L.E., et al. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells Current stage and future perspectives. Biomaterials. 2018;180:143–162. doi: 10.1016/j.biomaterials.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enrique G.B., Philippe R., Daniel L., et al. Bone fracture healing: cell therapy in delayed unions and nonunions. Bone. 2015;70:93–101. doi: 10.1016/j.bone.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Kang Y., Ren L., Yang Y. Engineering vascularized bone grafts by integrating a biomimetic periosteum and beta-TCP scaffold. Acs Appl Mater Inter. 2014;6(12):9622–9633. doi: 10.1021/am502056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichikawa Y., Watahiki J., Nampo T., et al. Differences in the developmental origins of the periosteum may influence bone healing. J Periodontal Res. 2015;50(4):468–478. doi: 10.1111/jre.12229. [DOI] [PubMed] [Google Scholar]
- 5.Dwek J.R. The periosteum: what is it, where is it, and what mimics it in its absence? Skeletal Radiol. 2010;39(4):319–323. doi: 10.1007/s00256-009-0849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi X., Fujie T., Saito A., et al. Periosteum-mimetic structures made from freestanding microgrooved nanosheets. Adv Mater. 2014;26(20):3290–3296. doi: 10.1002/adma.201305804. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman M.D., Xie C., Zhang X., et al. The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing. Biomaterials. 2013;34(35):8887–8898. doi: 10.1016/j.biomaterials.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schonmeyr B., Clavin N., Avraham T., et al. Synthesis of a tissue-engineered periosteum with acellular dermal matrix and cultured mesenchymal stem cells. Tissue Eng. 2009;15(7):1833–1841. doi: 10.1089/ten.tea.2008.0446. [DOI] [PubMed] [Google Scholar]
- 9.Matsuura K., Shimizu T., Okano T. Toward the development of bioengineered human three-dimensional vascularized cardiac tissue using cell sheet technology. Int Heart J. 2014;55(1):1–7. doi: 10.1536/ihj.13-337. [DOI] [PubMed] [Google Scholar]
- 10.Kobavashi T., Kan K., Nishida K., et al. Corneal regeneration by transplantation of corneal epithelial cell sheets fabricated with automated cell culture system in rabbit model. Biomaterials. 2013;34(36):9010–9017. doi: 10.1016/j.biomaterials.2013.07.065. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y.H., Zhang M., Liu N.X., et al. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. BIOMATERIALS. 2013;34(22):5506–5520. doi: 10.1016/j.biomaterials.2013.03.079. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal V., Johnson S.A., Reing J., et al. Badylak, Epimorphic regeneration approach to tissue replacement in adult mammals. Proc Natl Acad Sci USA. 2010;107(8):3351–3355. doi: 10.1073/pnas.0905851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vorotnikova E., Mcintosh D., Dewilde A., et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29(8):690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Benders K.E.M., Weeren P., Badylak S.F., et al. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013;31(3):169–176. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Ming L., Luo H., et al. Integration of a calcined bovine bone and BMSC-sheet 3D scaffold and the promotion of bone regeneration in large defects. Biomaterials. 2013;34(38):9998–10006. doi: 10.1016/j.biomaterials.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Xie Q., Wang Z., Huang Y., et al. Characterization of human ethmoid sinus mucosa derived mesenchymal stem cells (hESMSCs) and the application of hESMSCs cell sheets in bone regeneration. Biomaterials. 2015;66:67–82. doi: 10.1016/j.biomaterials.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Qi Y.Y., Niu L., Zhao T.F., et al. Combining mesenchymal stem cell sheets with platelet-rich plasma gel/calcium phosphate particles: a novel strategy to promote bone regeneration. Stem Cell Res Ther. 2015;6(1):1–16. doi: 10.1186/s13287-015-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tadashi, Sasagawa, Tatsuya, et al. Comparison of angiogenic potential between prevascular and non-prevascular layered adipose-derived stem cell-sheets in early post-transplanted period. J Biomed Mater Res A. 2014;102(2):358–365. doi: 10.1002/jbm.a.34707. [DOI] [PubMed] [Google Scholar]
- 19.Long T., Zhu Z., Awad H.A., et al. The effect of mesenchymal stem cell sheets on structural allograft healing of critical sized femoral defects in mice. Biomaterials. 2014;35(9):2752–2759. doi: 10.1016/j.biomaterials.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei F., Song T., Ding G., et al. Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem Cells Dev. 2013;22(12):1752–1762. doi: 10.1089/scd.2012.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei F., Qu C., Song T., et al. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J Cell Physiol. 2012;227(9):3216–3224. doi: 10.1002/jcp.24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsumanuma Y., Iwata T., Washio K., et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials. 2011;32(25):5819–5825. doi: 10.1016/j.biomaterials.2011.04.071. [DOI] [PubMed] [Google Scholar]
- 23.Naimisha, Beeravolu, Christina, et al. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J Vis Exp. 2017;3(122):1–13. doi: 10.3791/55224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silini A.R., Cancelli S., Signoroni P.B., et al. The dichotomy of placenta-derived cells in cancer growth. Placenta. 2017;59(2):154–162. doi: 10.1016/j.placenta.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Chen C.Y., Liu S.H., Chen C.Y., et al. Human placenta-derived multipotent mesenchymal stromal cells involved in placental angiogenesis via the PDGF-BB and STAT3 pathways. Biol Reprod. 2001;93(4):1–25. doi: 10.1095/biolreprod.115.131250. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder D.I., Blair J.D., Lott P., et al. The human placenta methylome. Proc Natl Acad Sci USA. 2013;110(15):6037–6042. doi: 10.1073/pnas.1215145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Liu Z., Li Y., et al. FGF-2-Induced human amniotic mesenchymal stem cells seeded on a human acellular amniotic membrane scaffold accelerated tendon-to-bone healing in a rabbit extra-articular model. Stem Cells Int. 2020;2020:1–14. doi: 10.1155/2020/4701476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J., Liu Z., Tang J., et al. Fibroblast growth factor 2-induced human amniotic mesenchymal stem cells combined with autologous platelet rich plasma augmented tendon-to-bone healing. J Orthop Transl. 2020;24:155–165. doi: 10.1016/j.jot.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You Q., Liu Z., Zhang J., et al. Human amniotic mesenchymal stem cell sheets encapsulating cartilage particles facilitate repair of rabbit osteochondral defects. Am J Sports Med. 2020;2020(6):1–13. doi: 10.1177/0363546519897912. [DOI] [PubMed] [Google Scholar]
- 30.Minxiong L.I., Jun M.A., Gao Y., et al. Cell sheet technology: a promising strategy in regenerative medicine. Cytotherapy. 2018;21(1):3–16. doi: 10.1016/j.jcyt.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Guillaume-Gentil O., Semenov O.V., Zisch A.H., et al. pH-controlled recovery of placenta-derived mesenchymal stem cell sheets. Biomaterials. 2011;32(19):4376–4384. doi: 10.1016/j.biomaterials.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 32.Mochizuki N., Kakegawa T., Osaki T., et al. Tissue engineering based on electrochemical desorption of an RGD-containing oligopeptide. J Tissue Eng Regen M. 2013;7(3):236–243. doi: 10.1002/term.519. [DOI] [PubMed] [Google Scholar]
- 33.Enomoto J., Mochizuki N., Ebisawa K., et al. Engineering thick cell sheets by electrochemical desorption of oligopeptides on membrane substrates. Regen Ther. 2016;3(C):24–31. doi: 10.1016/j.reth.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumashiro Y., Yamato M., Okano T. Cell attachment-detachment control on temperature-responsive thin surfaces for novel tissue engineering. Ann Biomed Eng. 2010;38(6):1977–1988. doi: 10.1007/s10439-010-0035-1. [DOI] [PubMed] [Google Scholar]
- 35.Zhonglan T., Teruo O. Recent development of temperature-responsive surfaces and their application for cell sheet engineering. Regen Biomater. 2014;2(1):91–102. doi: 10.1093/rb/rbu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y., Cheng Z., Weng W., et al. A facile synthesis of polydopamine/TiO2 composite films for cell sheet harvest application. Colloid Surface B. 2018;172:355–361. doi: 10.1016/j.colsurfb.2018.08.058. [DOI] [PubMed] [Google Scholar]
- 37.Liu C., Zhou Y., Sun M., et al. Light-induced cell alignment and harvest for anisotropic cell sheet technology. Acs Appl Mater Inter. 2017;9(42):36513–36524. doi: 10.1021/acsami.7b07202. [DOI] [PubMed] [Google Scholar]
- 38.Seto Y., Inaba R., Okuyama T., et al. Engineering of capillary-like structures in tissue constructs by electrochemical detachment of cells. Biomaterials. 2010;31(8):2209–2215. doi: 10.1016/j.biomaterials.2009.11.104. [DOI] [PubMed] [Google Scholar]
- 39.Shi D., Xu X., Ye Y., et al. Photo-cross-linked scaffold with kartogenin encapsulated nanoparticles for cartilage regeneration. ACS Nano. 2016;10(1):1292–1299. doi: 10.1021/acsnano.5b06663. [DOI] [PubMed] [Google Scholar]
- 40.Akahane M., Nakamura A., Ohgushi H., et al. Osteogenic matrix sheet-cell transplantation using osteoblastic cell sheet resulted in bone formation without scaffold at an ectopic site. Tissue Eng Regen Med. 2010;2(4):196–201. doi: 10.1002/term.81. [DOI] [PubMed] [Google Scholar]
- 41.Kang Y., Ren L., Yang Y. Engineering vascularized bone grafts by integrating a biomimetic periosteum and β-TCP scaffold. Acs Appl Mater Inter. 2014;6(12):9622–9633. doi: 10.1021/am502056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim A.Y., Kim Y., Lee S.H., et al. Effect of gelatin on osteogenic cell sheet formation using canine adipose-derived mesenchymal stem cells. Cell Transplant. 2016;26(1):115–123. doi: 10.3727/096368916X693338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F., Hu Y., He D., et al. Regeneration of subcutaneous tissue-engineered mandibular condyle in nude mice. J Cranio-Maxillo-Fac Surg. 2017;45(6):855–861. doi: 10.1016/j.jcms.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 44.YangYH, Rao J.D., Liu H.Q., et al. Biomimicking design of artificial periosteum for promoting bone healing. J Orthop Transl. 2022;36:18–32. doi: 10.1016/j.jot.2022.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W.T., Wang N.G., Yang M., et al. Periosteum and development of the tissue-engineered periosteum for guided bone regeneration. J Orthop Transl. 2022;33:41–54. doi: 10.1016/j.jot.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji C., Qiu M.L., Ruan H.T., et al. Transcriptome analysis revealed the symbiosis niche of 3D scaffolds to accelerate bone defect healing. Adv Sci. 2022;18 doi: 10.1002/advs.202105194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu Y., Zhang W., Wang J., et al. Recent advances in cell sheet technology for bone and cartilage regeneration: from preparation to application. Int J Oral Sci. 2019;11(2):1–13. doi: 10.1038/s41368-019-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cagdas Y.A., Esat K.A., Semih A., et al. A concise review on the use of mesenchymal stem cells in cell sheet-based tissue engineering with special emphasis on bone tissue regeneration. Stem Cells Int. 2017;2017:1–13. doi: 10.1155/2017/2374161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu L., Shang Y., Li C., et al. Hierarchical nanostructured electrospun membrane with periosteum-mimic microenvironment for enhanced bone regeneration. Adv Healthc Mater. 2021;10:1–14. doi: 10.1002/adhm.202101195. [DOI] [PubMed] [Google Scholar]
- 50.Hza B., Yza B., Wen Z.A., et al. Construction of vascularized tissue-engineered bone with a double-cell sheet complex-ScienceDirect. Acta Biomater. 2018;77:212–227. doi: 10.1016/j.actbio.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 51.Ren L.L., Kang Y.Q., Browne C., et al. Fabrication, vascularization and osteogenic properties of a novel synthetic biomimetic induced membrane for the treatment of large bone defects. Bone. 2014;64:173–182. doi: 10.1016/j.bone.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang D., Gao P., Li Q., et al. Engineering biomimetic periosteum with β-TCP scaffolds to promote bone formation in calvarial defects of rats. Stem Cell Res Ther. 2017;8(1):1–11. doi: 10.1186/s13287-017-0592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elin Y.M., Dixit V., Gitnick G. Extensive in vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artif Organs. 2001;25(7):558–565. doi: 10.1046/j.1525-1594.2001.025007558.x. [DOI] [PubMed] [Google Scholar]
- 54.Royce P.M., Kato T., Ohsaki K.I., et al. The enhancement of cellular infiltration and vascularisation of a collagenous dermal implant in the rat by platelet-derived growth factor BB. J Dermatol Sci. 1995;10(1):42–52. doi: 10.1016/0923-1811(95)93713-b. [DOI] [PubMed] [Google Scholar]
- 55.Chalupowicz D.G., Chowdhury Z.A., Bach T.L., et al. Fibrin II induces endothelial cell capillary TubeFormation. J Cell Biol. 1995;130:207–215. doi: 10.1083/jcb.130.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pirraco R.P., Marques A.P., Reis R.L. Cell interactions in bone tissue engineering. J Cell Mol Med. 2010;14(1-2):93–102. doi: 10.1111/j.1582-4934.2009.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bauer S.M., Bauer R.J., Liu Z.J., et al. Vascular endothelial growth factor-C promotes vasculogenesis, angiogenesis, and collagen constriction in three-dimensional collagen gels. J Vasc Surg. 2005;41(4):699–707. doi: 10.1016/j.jvs.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 58.Eppley B.L. Experimental assessment of the revascularization of acellular human dermis for soft-tissue augmentation. Plast Reconstr Surg. 2001;107(3):757–762. doi: 10.1097/00006534-200103000-00016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.