Abstract
Background
Osteonecrosis of the femoral head (ONFH) is a refractory disease due to its unclear pathomechanism. Therapies during the early stage of ONFH have not achieved satisfactory results. Therefore, this study aims to explore the available evidence for the therapeutic effect of human umbilical cord mesenchymal stem cells (HUCMSCs) on early-stage traumatic ONFH.
Methods
Early-stage traumatic ONFH was established. The femoral heads of rats were then locally administered HUCMSCs. Four weeks and eight weeks after surgery, bone repair of the necrotic area in the femoral head was analyzed to evaluate the therapeutic effect of HUCMSCs using micro-CT, histopathological staining, immunofluorescence staining, Luminex.
Results
HUCMSCs were still present in the femoral head four weeks later, and the morphological, micro-CT and histopathological outcomes in the 4-week HUCMSC-treated group were better than those in the model, NS and 8-week HUCMSC-treated groups. Local transplantation of HUCMSCs promoted bone repair and prevented bone loss in the necrotic area of the femoral head.
Conclusions
HUCMSCs can survive and positively affect the femoral head through local transplantation in early-stage traumatic ONFH. The conclusions of this study can provide a treatment option for patients who have ONFH and can serve as basic research on the advanced development of this disease.
The Translational potential of this article
The study indicated that the positive effect of exogenous HUCMSCs in the treatment of early-stage traumatic ONFH provides the solid basis and guidance for the clinical application of HUCMSCs.
Keywords: Osteonecrosis of the femoral head, HUCMSCs, Therapeutic effect
1. Introduction
Osteonecrosis of the femoral head (ONFH) is a refractory disease that is characterized by subchondral microfractures, impaired microcirculation and interrupted bone remodeling processes [1,2]. ONFH is a global problem. In the United States [3], an estimated 20,000 to 30,000 patients are diagnosed with osteonecrosis each year. In addition, 8.2 million patients over the age of 15 years have nontraumatic ONFH each year in China [4]. As ONFH mostly occurs in young and active middle-aged patients, the long-term outcomes after total hip arthroplasty (THA) are not satisfactory due to the shortcomings of a high revision rate and short prosthesis life. Therefore, to protect joint function, early intervention for ONFH is particularly important. At present, there are many clinical methods of joint preservation, including medication, physical therapy and surgery, such as core decompression (CD) and bone grafting with and without vascularization [5]. However, studies of these methods have not achieved consistent results, and their efficacy varies widely. Ongoing efforts are needed to develop more effective treatments for ONFH.
As a promising therapy, many studies have concentrated on mesenchymal stem cell (MSC) treatment [6]. MSCs have been verified to play an essential role in bone healing. A series of clinical studies have reported on MSC transplantation, which has gradually become a prospective treatment for ONFH [[7], [8], [9], [10]].
Bone marrow mesenchymal stem cells (BMMSCs) are widely used in a variety of diseases. However, they have a low yield. The outcomes were closely related to the number, biological activity, and proliferation potential of the transplanted MSCs. Therefore, to obtain stable efficacy of cellular therapy, it is necessary to determine and establish an exhaustless and standardized source of MSCs. Among the various types of stem cells, human umbilical cord mesenchymal stem cells (HUCMSCs) have obvious advantages, such as easy access, low immunogenicity, high yield and stable amplification [11], and are used as a treatment in many diseases with no immunological rejection or toxic reaction [12]. Currently, there are few basic research studies on the treatment of femoral head necrosis with HUCMSCs, and most of them focus on glucocorticoid-induced ONFH; thus, adequate research is needed. Hence, in this study, we explored the precise effect of HUCMSCs on traumatic ONFH.
2. Materials and methods
2.1. Materials
HUCMSCs were purchased from Beijing Tuohua Biotechnology Co., Ltd.Co., Ltd.
2.2. Animals and experimental grouping
Thirty-six adult male SPF Sprague–Dawley (SD) rats (purchased from the Animal Center of the General Hospital of the PLA, weighing 350 ± 50 g) were randomly divided into 7 groups: a 4-week model group (n = 6), 8-week model group (n = 6), 4-week normal saline group (NS group) (n = 6), 8-week normal saline group (NS group) (n = 6), 4-week HUCMSC-treated group (n = 6), 8-week HUCMSC-treated group (n = 6) and normal group, which consisted of the 6 normal hips from the model group. All rats were housed under the same standard laboratory conditions (the same temperature and humidity) with unlimited access to water and a standard diet. All the samples were harvested in the fourth week and eighth week after surgery.
All experiments were approved by the Animal Care and Use Committee of the General Hospital of the PLA, according to the National Institutes of Health guidelines for the use of experimental animals.
2.3. Establishment of the traumatic ONFH model groups, NS groups and HUCMSC-treated groups
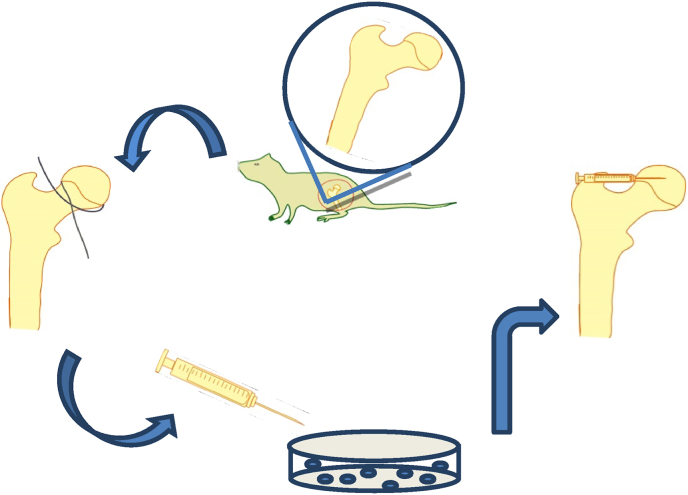
We adopted a model of traumatic ONFH [13]. All thirty-six rats were anesthetized by injecting 2% pentobarbital (0.3 ml/100 g) into the abdominal cavity; then, the rats were placed on the operating table. Once the rats were deeply anesthetized, the fur was removed from the operative area; the skin was cleansed, sterilized, and covered with a sterile sheet; and a right hip lateral incision of approximately 2 cm was made from the center of the trochanter. The muscle was separated and pulled along the direction of the muscle fibers, the hip joint capsule was cut to expose the femoral head, and the hip joint was dislocated. The round ligament was tightly wrapped with a 3-0 suture around the base of the femoral head, sliding it side to side 5 times to disrupt the blood supply nourishing the femoral head. According to the results of a preliminary experiment, the volume of the rat femoral head was approximately 14 μl. To ensure that enough HUCMSCs were retained in the femoral head in the HUCMSC-treated group, approximately 100 μl (1 × 107/ml) of HUCMSC suspension was injected into the channel, which was approximately 0.2 cm long, with the needle of a 2 ml syringe. In the NS group, 100 μl of normal saline was injected using the same methods. During the operation, the sciatic nerve was bypassed to avoid injury, the joint capsule was enhanced, and the integrity of the surrounding muscle of the hip joint was maintained to avoid dislocation of the hip joint. To prevent infection, the wound was rinsed with normal saline, and the incision was sutured in layers. After awakening, all rats were active and fed without any abnormalities (Fig. 1).
Figure 1.
The process of establishing a rat model of traumatic osteonecrosis of the femoral head and local injection of HUCMSCs in the femoral head.
2.4. Preparation of tissue samples
The animals were sacrificed at 4 and 8 weeks after the operation or treatment for the model groups, NS groups and HUCMSC-treated groups. Blood samples were obtained from the retro-orbital sinuses of rats at the time of sacrifice and centrifuged immediately. Then, the supernatant was collected and stored at −80 °C until analysis. Whole femurs were harvested from each rat, and all samples were fixed in 10% neutral calcium formaldehyde for 1 week and then decalcified in EDTA for 4 weeks. Next, half of the specimens were embedded in paraffin and cut into 5 μm sections for histopathology, and the other half were soaked in 30% sucrose solution overnight for dehydration. Then, the specimens were embedded in OCT gel and sectioned by a frozen microtome at a thickness of 8 μm for immunofluorescence staining analysis.
2.5. Assessment of the traumatic ONFH model
Four weeks after surgery, the femurs of rats in the 4-week model group were removed from the body and soaked in 10% neutral calcium formaldehyde. ONFH was evaluated by using micro-CT and H&E staining analyses.
2.6. Micro-CT evaluation
All the animals in each group were euthanized, the right and left femoral heads were dissected, and the soft tissue on the femoral head was carefully removed. All the samples were scanned and reconstructed with a micro-CT scanner (GE eXplore Locus, USA) to assess the relevant bone parameters, such as the bone mineral density (BMD), bone volume (BV), BV/tissue volume (TV), bone surface (BS)/BV, trabecular space (Tb. Sp) and trabecular thickness (Tb. Th). The region of interest (ROI) size was X: 2.8207; Y: 2.8413; Z: 2.2854 (millimeters), the position of ROI was above and below the epiphyseal plate in the middle of the femoral head. the scanning resolution was 27 μm, the scanning voltage was 74.01 kV, the current was 133.0 μA, and the threshold was 75–255. All the data were analyzed by GE Micro View software.
2.7. Histopathological staining
The specimens were fixed in 10% neutral calcium formaldehyde for 7 days and then decalcified with 10% EDTA solution for 28 days. Some specimens in each group were embedded in paraffin, sliced into 5 μm sections in the coronary position, dewaxed in xylene, and rehydrated in graded ethanol, and residual water was removed from the slide using distilled water for hematoxylin and eosin (H&E) staining. Saffron O solid green staining was performed according to the respective specifications for histological observation.
2.8. Immunofluorescence staining
The other half of the specimens, which were prepared for immunofluorescence staining, were incubated with normal goat serum for 2 h to block nonspecific antibody interference and then incubated overnight at 4 °C with anti-mitochondria (1:400; Abcam), anti-SOX9 (1:200; Abcam), and anti-SP7/Osterix (1:200; Abcam). The slices were incubated in secondary antibodies—goat anti-mouse fluorescein-conjugated antibodies (1:100, Zhong Shan-Golden Bridge) or goat anti-rabbit fluorescein-conjugated antibodies (1:100, Abcam)—for 1 h at 37 °C. Next, these slices were counterstained with the nuclear marker 4,6-diamino-2-phenylindole (DAPI). All images were observed using a fluorescence microscope (Olympus).
2.9. Luminex analysis
All the rats in each group were anesthetized at different time points after treatment. The serum from each rat was isolated and centrifuged immediately, the supernatant was collected, and the production of specific cytokines in the serum was evaluated according to the instructions of Bio-Rad (USA).
2.10. Statistical analyses
All statistical analyses were performed by IBM SPSS Statistics 20 software. Values are presented as the mean ± standard deviation, and differences with a P value < 0.05 were considered statistically significant. All data satisfying the normal distribution and the test of homogeneity of variance were analyzed using ANOVA for multiple comparisons, and the data that did not satisfy the normal distribution and the test of homogeneity of variance were analyzed using nonparametric tests.
3. Results
3.1. Assessment of the traumatic ONFH model
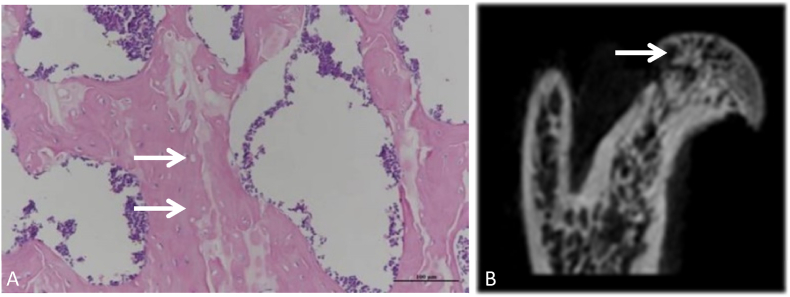
Micro-CT and H&E staining were used to evaluate the effect of the traumatic ONFH model. All the right femurs from the 4-week model group were analyzed by these two means. In the micro-CT images, the trabecular continuity of the subchondral bone tissue was interrupted and disordered, and the local trabeculae were sparse and thin. Furthermore, corresponding with the results of bone mass formation from micro-CT, histological outcomes based on H&E staining also showed conspicuous osteonecrosis, in which many empty lacunae appeared. Sparse and thin trabeculae were observed in the femoral head, bone mineral loss was obvious in the subchondral area of the femoral head, and abnormal cystic changes were observed in many femoral heads in the 4-week model group (Fig. 2).
Figure 2.
H&E staining and micro-CT assessment of the model group (A) Image in the coronal plane of the femoral head stained by H&E at 20 × magnification. The white arrow indicates empty lacunae, the trabeculae were disordered, and the local trabeculae were sparse and thin. (B) Image in the coronal plane of the femoral head scanned by micro-CT. The white arrow indicates where the bone became less dense and more porous and that the trabecular continuity was interrupted, disordered and thin (scale bars: 20 × = 100 μm).
3.2. Quantitative analysis of the femoral head by micro-CT
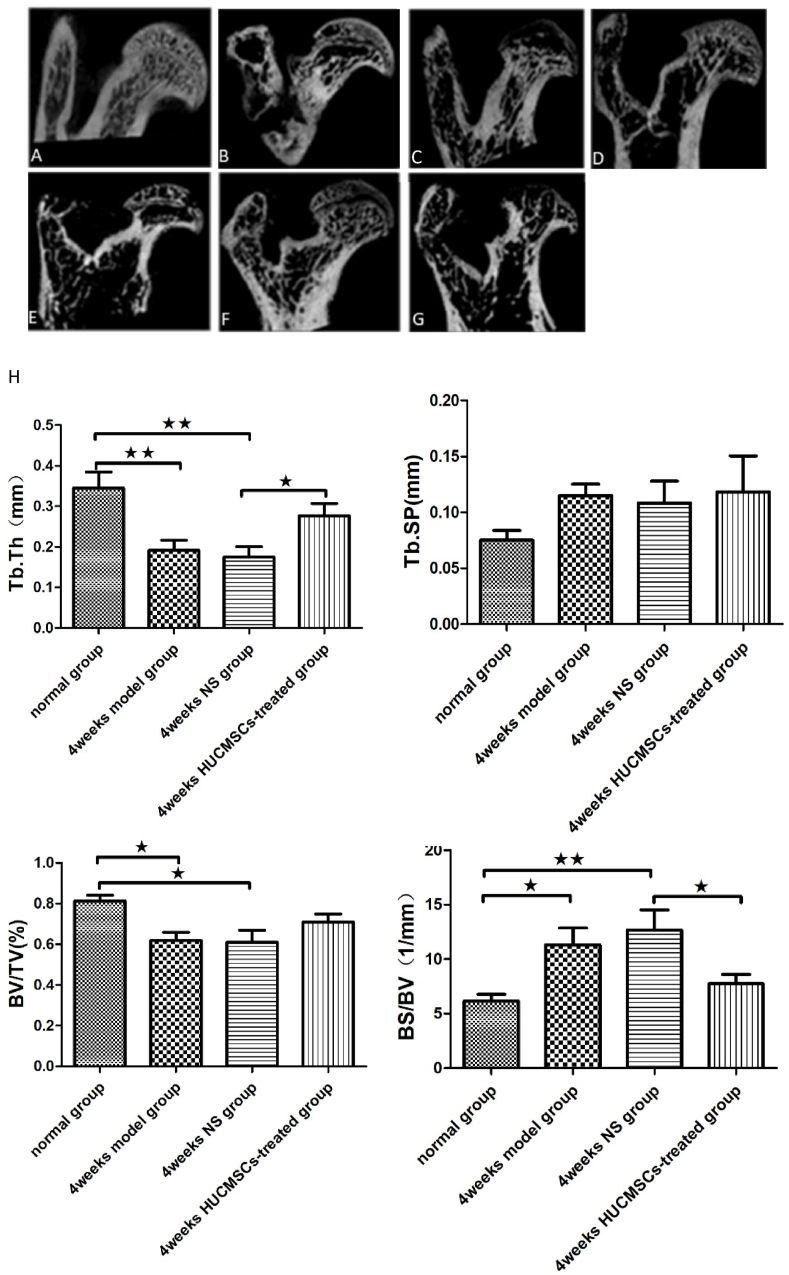
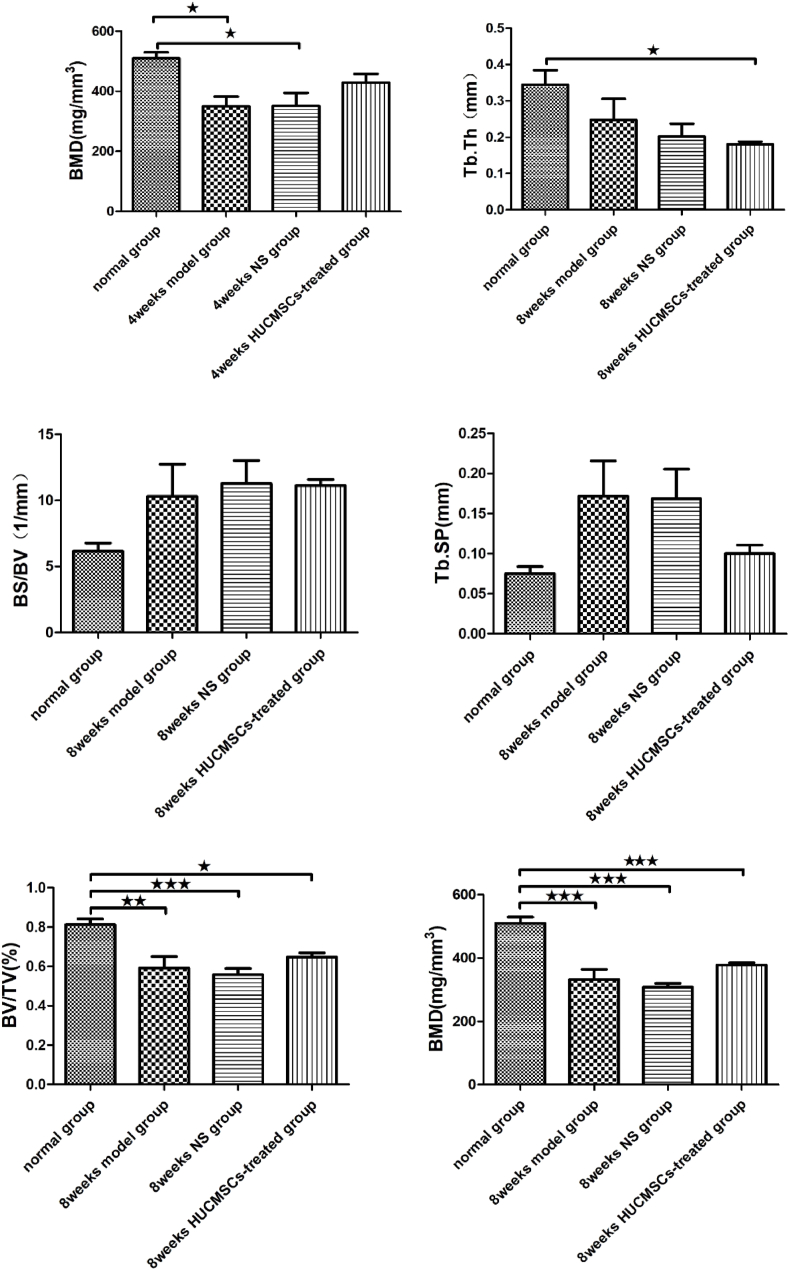
To explore the therapeutic effect of HUCMSCs on early traumatic ONFH, rat models were established by impairing the blood supply to the femoral head with 3–0 sutures, followed by local administration of HUCMSCs and normal saline to the femoral head in the HUCMSC-treated groups and NS groups, respectively. The femoral heads of the traumatic ONFH group showed significant changes compared to those of the normal group, including a decrease in BMD in the subchondral area of the femoral head and the appearance of cystic changes.
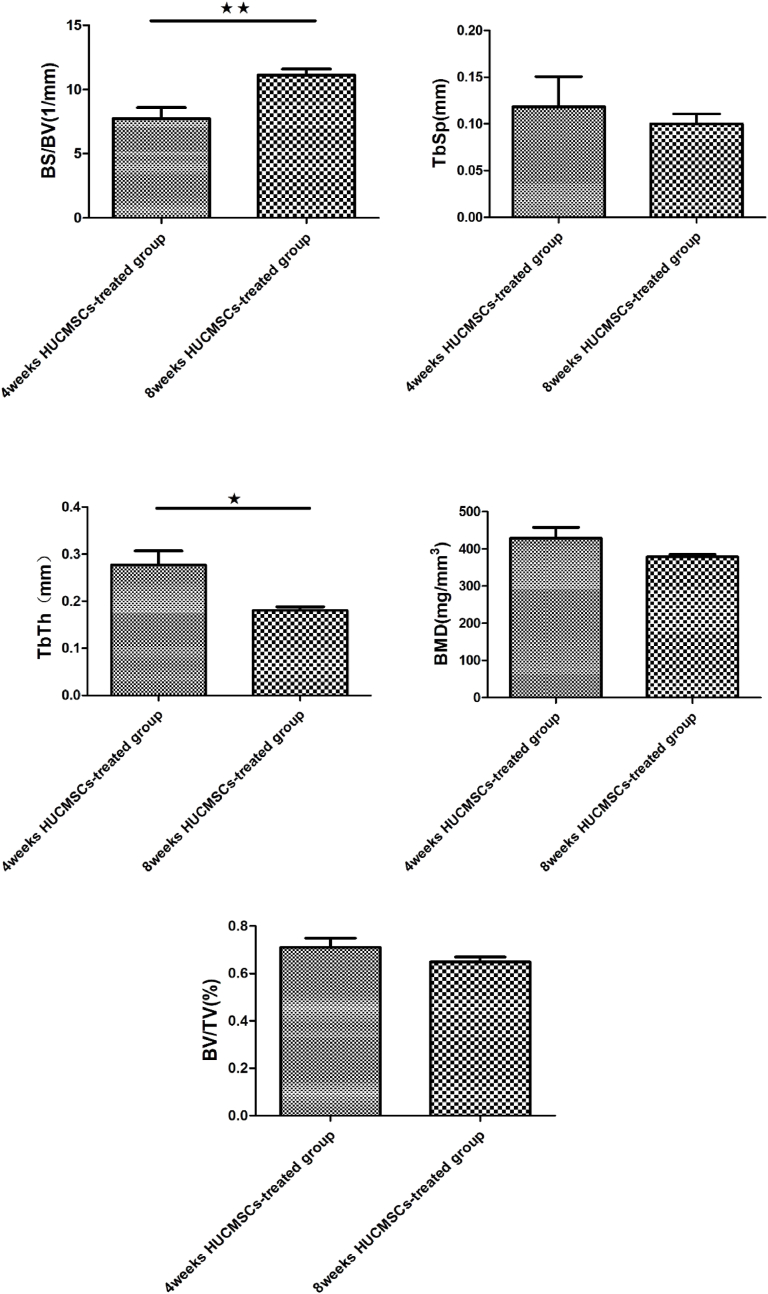
The femoral heads were scanned and analyzed by Micro CT. Quantitative analysis revealed that the internal structure and morphology of the femoral head were significantly different among these groups. After treatment with HUCMSCs, the bone trabeculae in the femoral head, especially around the subchondral area, showed notable improvement compared to those the NS groups and model groups. Therefore, a trend of recovery in the femoral head was observed after treatment with HUCMSCs. The results of micro-CT parameters proved that treatment with HUCMSCs had an obvious therapeutic effect in early traumatic ONFH. However, the femoral heads in the 8-week HUCMSC-treated group did not exhibit better outcomes than those in the 8-week NS group or the 8-week model group (Fig. 3).
Figure 3.
Morphometric evaluation of micro-CT images among the seven groups (n = 42): A: normal group; B: 4-week model group; C: 4-week normal saline group; D: 8-week model group; E: 8-week normal saline group; F: 4-week HUCMSC-treated group; G: 8-week HUCMSC-treated group; H: comparison of the bone metrology parameters of the seven groups. (★p < 0.05,★★p < 0.01,★★★p < 0.001).
3.3. Histopathological findings in all groups
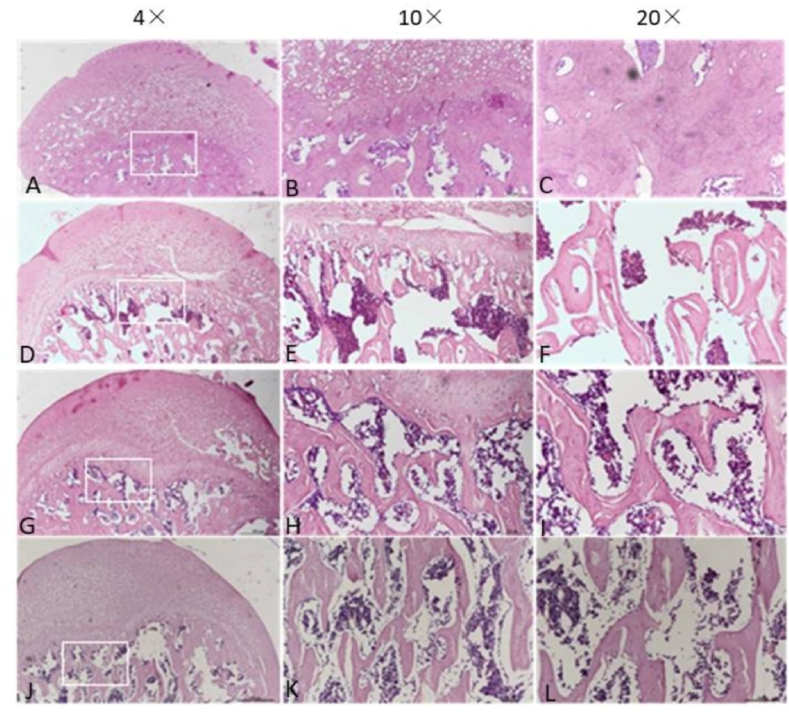
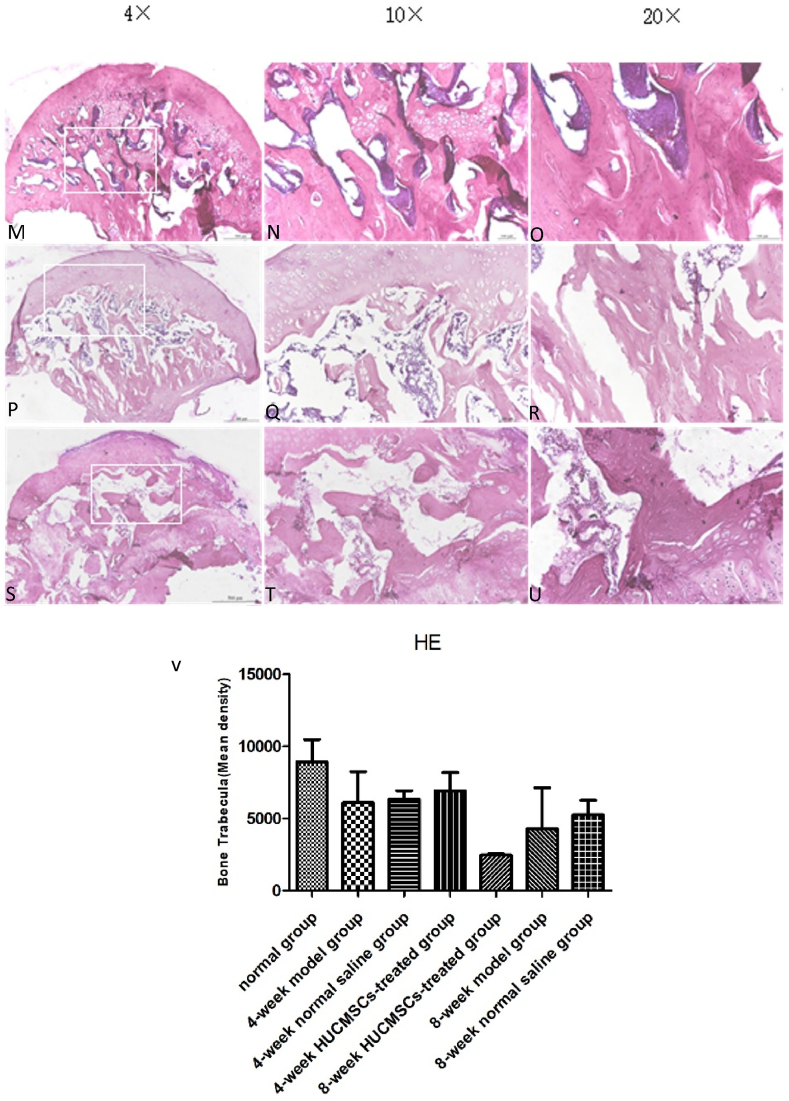
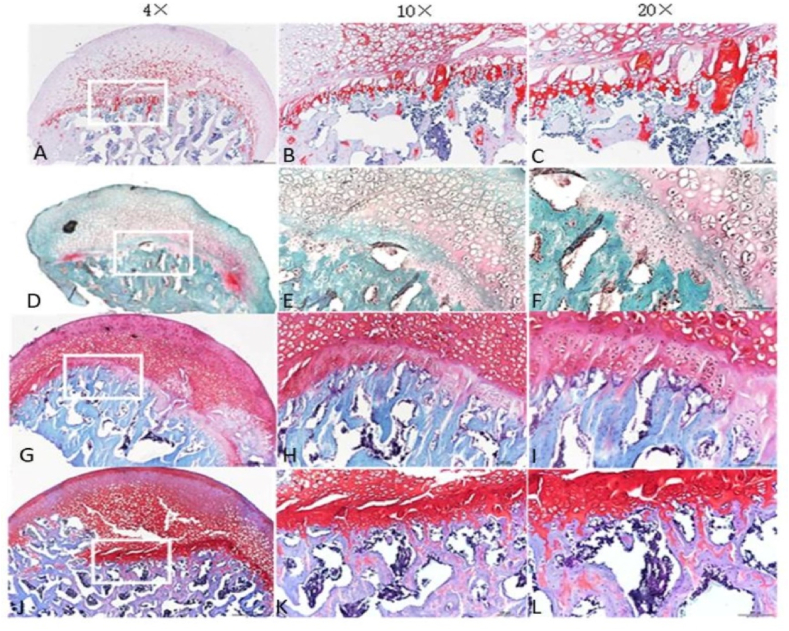
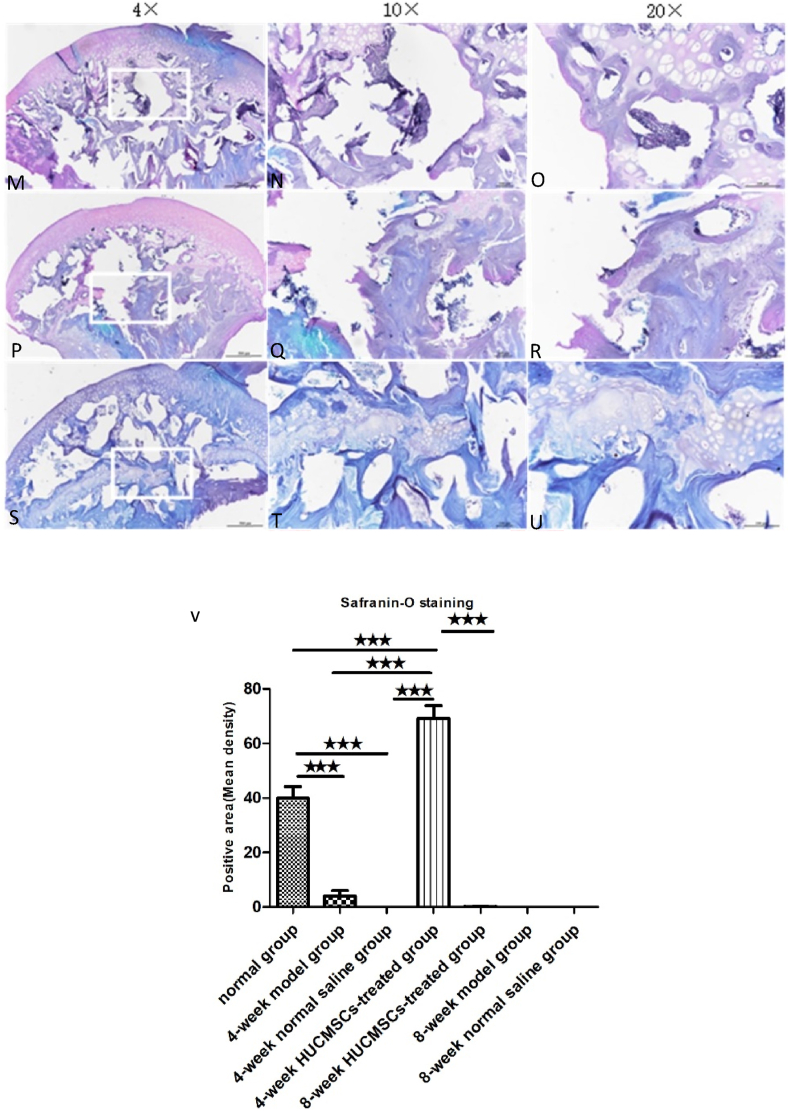
The femoral heads of each group were collected 4 and 8 weeks after surgery. The tissue was dehydrated and embedded in paraffin. The thickness of each section was 5 μm, and H&E staining was performed. Bone trabecular sparseness, fracture, continuity interruption and extensive apoptosis of osteocytes were observed in the 4-week model group, 4-week NS group, 8-week model group, 8-week NS group and 8-week HUCMSC-treated group. In the normal group and 4-week HUCMSC-treated group, bone trabeculae were dense and arranged in an orderly manner, fewer empty bone lacunae were observed in the trabeculae, and the thickness of trabeculae was improved. Safranin-o staining showed that the cartilage of the 4-week HUCMSC-treated group was normal and had little necrosis. The 8-week HUCMSC-treated group, NS groups and model groups demonstrated obvious cartilage degeneration with almost no staining (Figure 4, Figure 5).
Figure 4.
H&E staining analysis of coronal plane sections of the femoral head in the seven groups with different magnifications: A–C: 4-week model group; D–F: 4-week normal saline group; G–I: 4-week HUCMSC-treated group; J–L: normal group; M–O: 8-week model group; P–R: 8-week normal saline group; S–U: 8-week HUCMSC-treated group; V: Bone Trabecula in each group (scale bars: 4 × = 500 μm, 10 × = 100 μm, 20 × = 100 μm).
Figure 5.
Safranin-O staining analysis of coronal plane sections of the femoral head in the seven groups with different magnifications: A–C: 4-week model group; D–F: 4-week normal saline group; G–I: 4-week HUCMSC-treated group; J–L: normal group; M–O: 8-week model group; P–R: 8-week normal saline group; S–U: 8-week HUCMSC-treated group; V: cartilage area in each group (scale bars: 4 × = 500 μm, 10 × = 100 μm, 20 × = 100 μm,★★★p < 0.001).
3.4. Cell tracking in the femoral head of the HUCMSC-treated groups
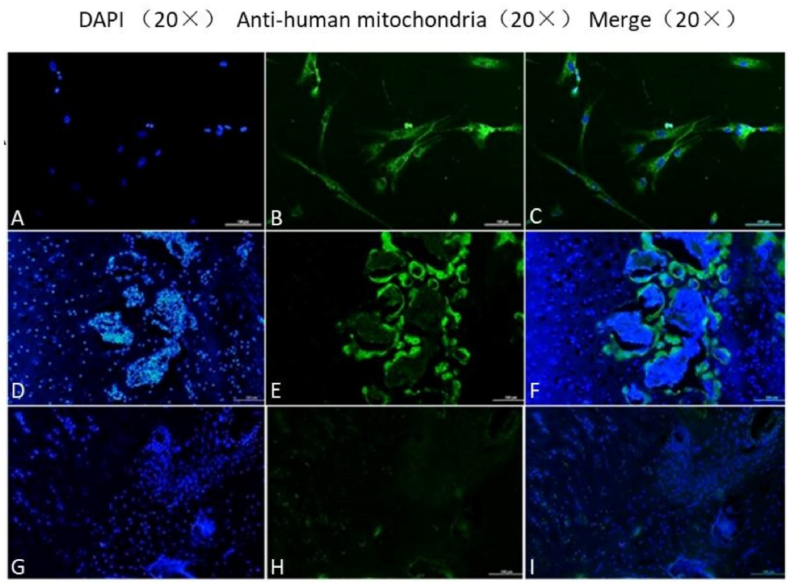
Anti-human mitochondrial antibody reacts only with antigen derived from humans. This property can be used to identify and track HUCMSCs injected into the femoral head. Many HUCMSCs (green staining) were detected in the femoral head in the 4-week HUCMSC-treated group, but no HUCMSCs were found in that of the 8-week HUCMSC-treated group (Fig. 6).
Figure 6.
A–C: HUCMSCs; D–F: HUCMSCs in the femoral head of the 4-week HUCMSC-treated group; G–I: HUCMSCs in the femoral head of the 8-week HUCMSC-treated group (scale bars: 20 × = 100 μm).
3.5. HUCMSCs enhanced osteogenesis and the chondrogenic ability in the femoral head in a traumatic ONFH rat model
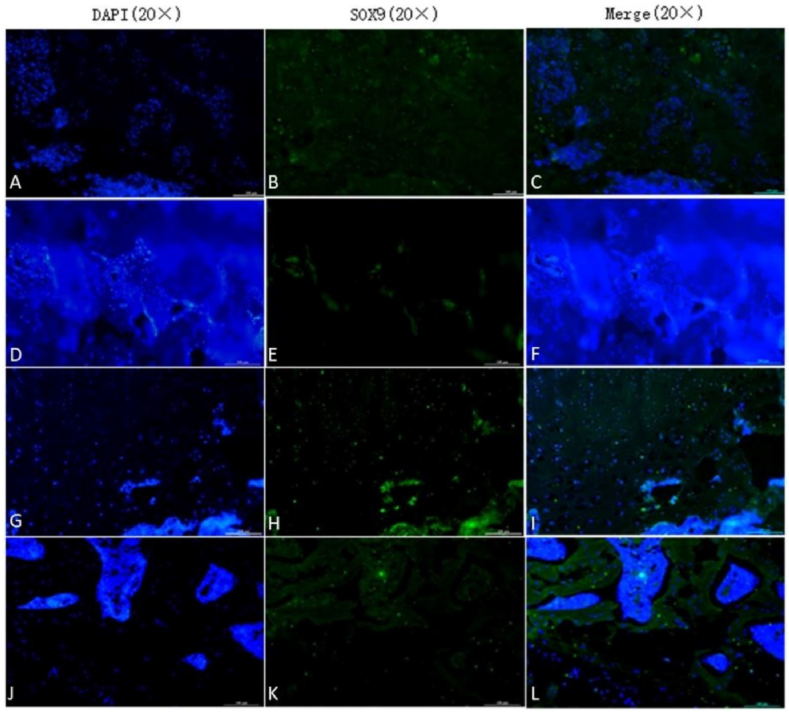
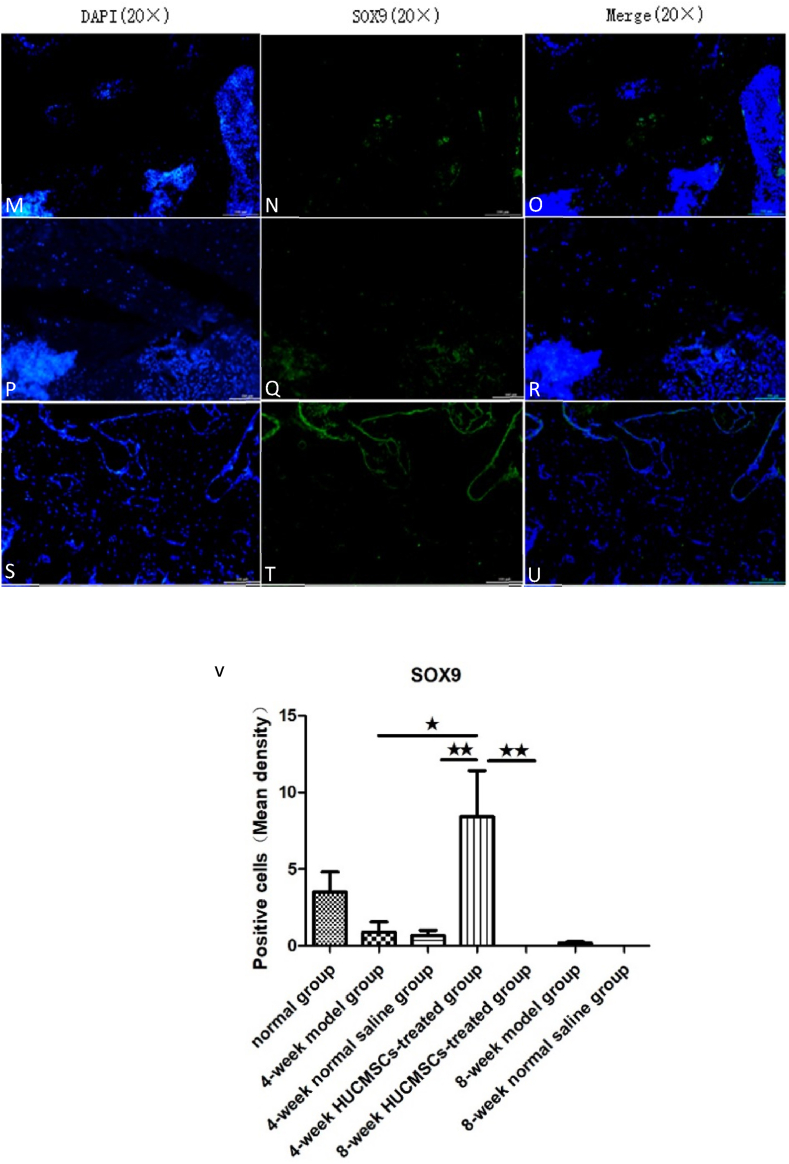
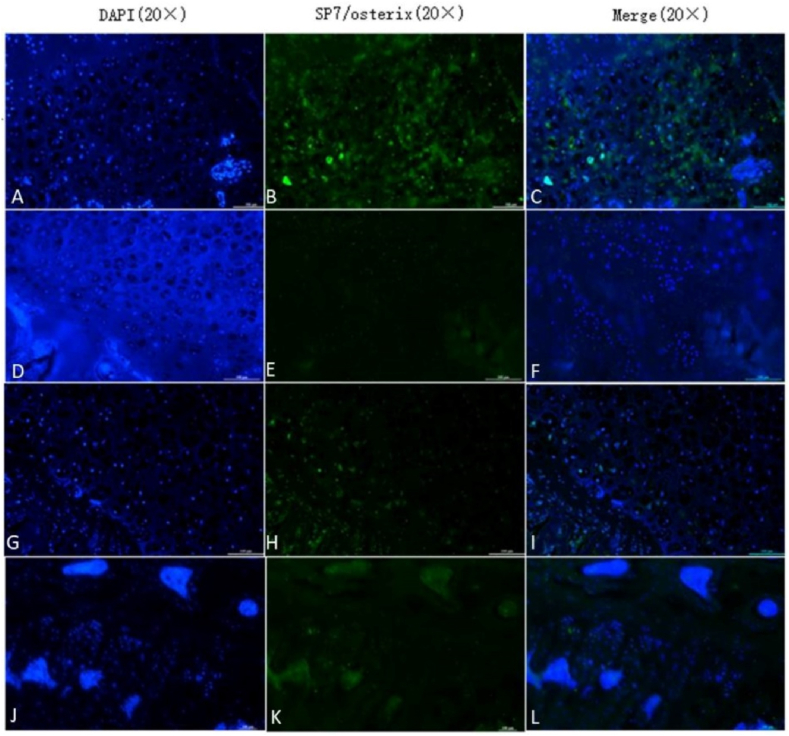
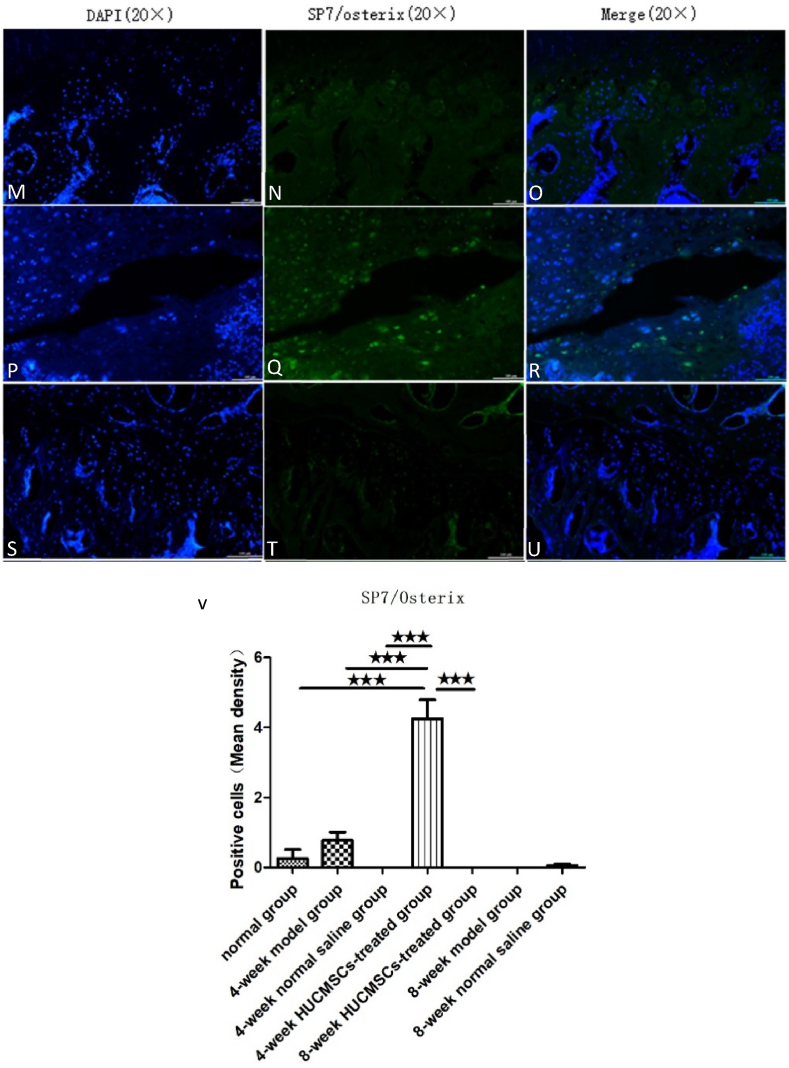
SOX9 and SP7/Osterix are important markers of cartilage growth and bone tissue growth, respectively. We used an immunofluorescence technique to identify chondrogenic and osteogenic capabilities in the rat femoral head using an anti-SOX9 antibody and anti-SP7/Osterix. Immunofluorescence staining showed that the expression of SOX9 was higher in the 4-week HUCMSC-treated group than in the NS groups, model groups and 8-week HUCMSC-treated group, and the expression of SP7/Osterix was the same as that of SOX9. The results of the experiment verified that HUCMSCs injected into the femoral head promoted chondrogenesis and osteogenesis in a 4-week period. However, over time, the positive effect of HUCMSCs waned, as observed in the 8-week HUCMSC-treated group. This indicated that HUCMSCs may no longer home to the lesion site at 8 weeks (Figure 7, Figure 8).
Figure 7.
The expression of SOX9 in the femoral heads of the seven groups: A–C: 4-week model group; D–F: 4-week normal saline group; G–I: 4-week HUCMSC-treated group; J–L: normal group; M–O: 8-week model group; P–R: 8-week normal saline group; S–U: 8-week HUCMSC-treated group; V: SOX9 expression in each group (scale bars: 20 × = 100 μm, ★p < 0.05,★★p < 0.01).
Figure 8.
The expression of SP7/Osterix in the femoral heads of the seven groups: A–C: 4-week model group; D–F: 4-week normal saline group; G–I: 4-week HUCMSC-treated group; J–L: normal group; M–O: 8-week model group; P–R: 8-week normal saline group; S–U: 8-week HUCMSC-treated group; V: SP7/Osterix expression in each group (scale bars: 20 × = 100 μm,★★★p < 0.001).
3.6. Cytokine levels in the serum of rats
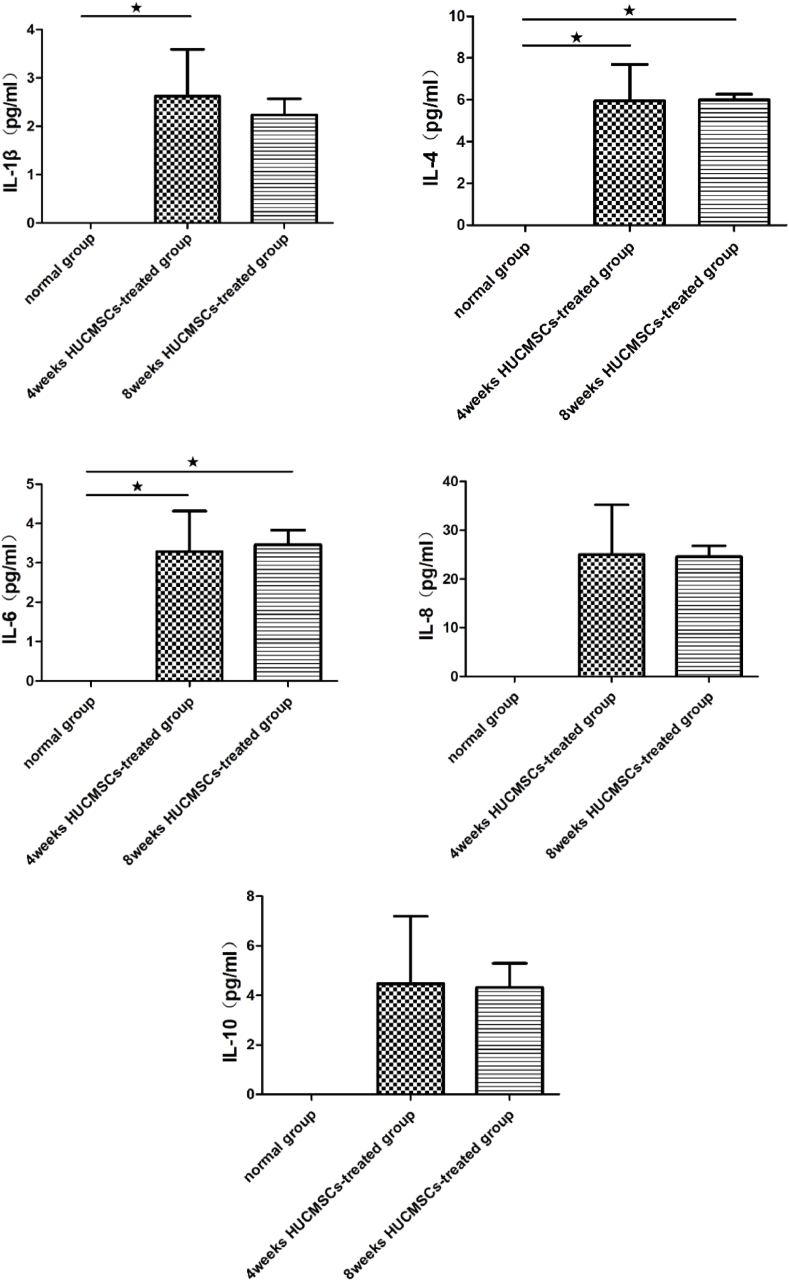
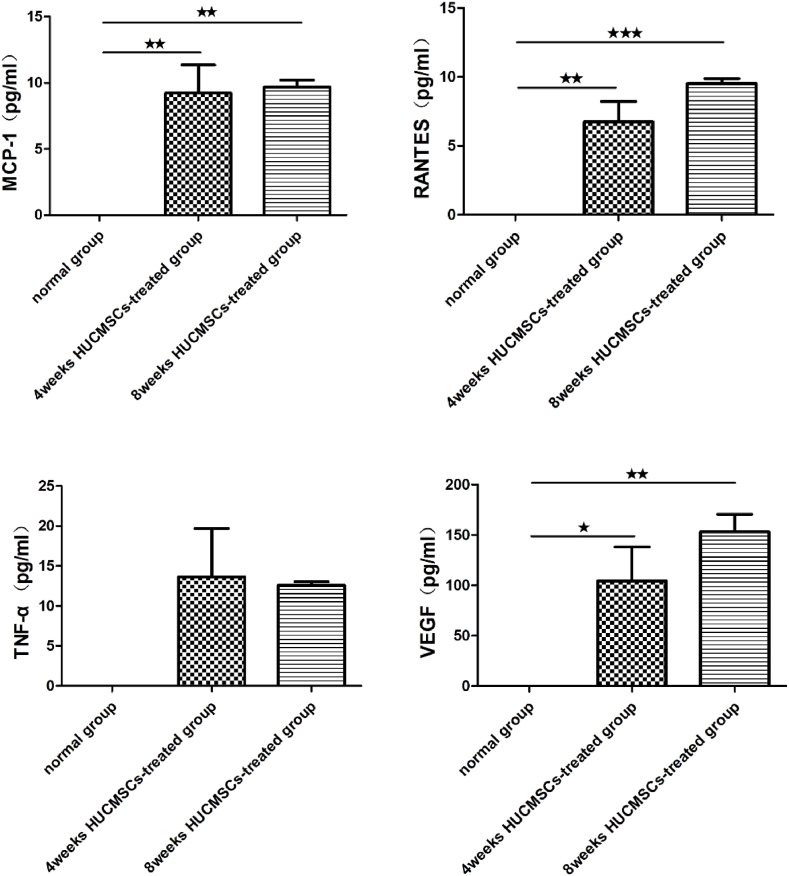
The therapeutic effect of MSCs was generated mainly through paracrine action, promoting secretion of a large number of cytokines. The levels of cytokines in the serum of rats at 4 and 8 weeks after treatment with HUCMSCs was measured by Luminex, along with the expression of VEGF, MCP-1, IL-1β, IL-4, IL-6, IL-8, IL-10, TNF-α, and RANTES. As shown in Fig. 9, the expression levels of cytokines in the serum of rats were measured in both the 4-week HUCMSC-treated group and the 8-week HUCMSC-treated group, and there was no significant difference between the two groups. The results of Luminex indicated that local administration of HUCMSCs in the femoral head was successful and that HUCMSCs could survive in the femoral head and exert their function. However, HUCMSCs were not detected in the femoral head in the 8-week HUCMSC-treated group.
Figure 9.
Comparison of the levels of cytokines in the serum of rats between the 4-week HUCMSC-treated group and the 8-week HUCMSC-treated group. (★p < 0.05,★★p < 0.01,★★★p < 0.001).
4. Discussion
The pathologic process of ONFH is characterized by ischemic death of bone marrow and bone cells with a limited self-repairing capability. Previous studies have shown that the osteogenic and proliferative abilities of MSCs are significantly reduced in patients with ONFH caused by alcohol and steroid use [[14], [15], [16], [17]]. After ONFH, the balance of osteogenesis and osteolysis in the femoral head is impaired [18].
During the process of osteonecrosis, the number of osteoclasts increased, and the process of bone absorption was active because of the dead bone. Moreover, osteogenic ability was weakened, resulting in changes in bone structure in the femoral head. Ultimately, bone trabecular strength decreased, and the femoral head collapsed at the end.
Exogenous MSCs can be added to compensate for a reduction in cell number and proliferation ability. The progression of the disease is delayed, and even collapse can be avoided by improving and enhancing the strength of bone trabeculae [8]. Allogeneic MSCs from healthy humans are one treatment for ONFH. There is much evidence that MSCs exhibit low immunogenicity [19], which allows them to be transplanted into other individuals without immune rejection. Currently, MSCs have been reported to be a promising method for regenerating various tissues. MSCs that were first identified in human bone marrow are called BMMSCs. MSCs can be isolated from many sources other than bone marrow, including adipose tissue, the synovium and the umbilical cord [20,21].
Among MSCs from various tissues, BMMSCs are the most used and studied. Rastogi et al. [22] compared the therapeutic effects of isolated monocytes and untreated bone marrow. Considerable improvement in hip function was observed. However, the limited number of BMMSCs, which is affected by patient age, trauma and pain, and their proliferative capacity restrict their application in ONFH [23,24]. Thus, HUCMSCs may be a better choice. Umbilical cord collection is simple, and no ethical arguments around it. HUCMSCs have a high yield and low immunogenicity [25,26]. Therefore, we chose HUCMSCs in view of their advantages, such as easy access, no ethical controversy, high yield, strong proliferative ability, and commercialization.
But the safety of stem cells is an unavoidable issue. Stem cells have some features of cancer cells including relative apoptosis resistance, long lifespan, and ability to replicate for extended periods of time. In addition, similar growth regulators and control mechanisms are involved in both cancer and stem cell maintenance. Therefore, stem cells may undergo malignant transformation which is often seen as a key obstacle to the safe use of stem-cell-based medicinal products [27]. After reviewing many studies that used stem cells to treat ONFH, we found that most of the current studies reported that no severe complications were observed. Only a few studies reported that patients had some complications, such as mild headache, flushing, and fever [[28], [29], [30]]. Cai et al. [31] analyzed the therapeutic effect of the transplantation of autologous BMMSCs and allogeneic UCMSCs for treating ONFH and no severe adverse effects were observed at 12 months after transplantation. Chenet al. [32] evaluated the clinical effects of transplantingallogeneic HUCMSCs to treat ONFH and obtained clear results in which no obvious side-effects were found after a three-year follow-up. Compared to other therapies, transplantation of the HUCMSCs could secret neurotrophic factors and anti-inflammatory factors to improve the microenvironment in the injured environment and has low immunogenicity and no side effects were detected [[33], [34], [35]]. Zhang et al. demonstrated that HUCMSCs transplantation improves the disorder of sexual cycle, modulates the serum hormone expression to a better state and restores ovarian function in a rat model of premature ovarian failure with no severe adverse effects [36]. To verify the immunogenicity of allogeneic MSCs, we injected HUCMSCs isolated from human umbilical cord into the right gastrocnemius muscle of nude mice and KM mice. Then, H&E staining and immunohistochemical staining were performed on the muscle sections from the injection site on days 2, 3, 7 and 14. No obvious infiltration of inflammatory cells, such as neutrophils and monocytes, was found. On day 7, immunohistochemistry showed that many HUCMSCs were still present in the gastrocnemius of KM mice without being rejected by their immune systems. No abnormal cells, such as tumor cells, were found, and the safety was verified. In terms of effectiveness, intravenous stem cell therapy for early ONFH has some efficacy, but the results varied considering the limited number of stem cells that reached the injured area after intravenous injection [37].
To retain sufficient stem cells locally in the femoral head, we must first maximize the stem cell concentration. Many studies have shown that positive treatment results are closely related to high cell counts [38,39]. However, the ideal concentration of HUCMSCs has not been determined. Preliminary experiments indicated that the most appropriate concentration of HUCMSCs without blocking the needle was 1 × 106/100 μl. Second, sufficient HUCMSCs were injected into the femoral head through local injection. Since the volume of the rat femoral head is approximately 14 μl after calculation [13], we injected approximately 100 μl into each femoral head, so 14 μl of HUCMSC suspension could be retained locally. The survival of HUCMSCs was observed at 4 and 8 weeks after the operation to evaluate the therapeutic effect. At 4 weeks after surgery, many HUCMSCs were detected locally in the femoral head by immunofluorescence and anti-human mitochondrial antibody staining, but a large number of HUCMSCs could not be observed at 8 weeks after surgery. It was reported that transplanted stem cells could proliferate and divide in the early time period after translation, However, over time, most of the cells showed either die or migrate elsewhere [40]. Therefore, We speculated that HUCMSCs migrated out of the femoral head 8 weeks after transplantation, so few HUCMSCs were detected in the femoral head. H&E staining and Saffron O solid green staining showed that the outcome of the HUCMSC-treated group at 4 weeks was better than that of the ONFH model groups, normal saline groups and 8-week HUCMSC-treated group. The results of bone parameters from micro-CT were consistent with histological staining.
The expression of SOX9 and SP7/Osterix in the 4-week HUCMSC-treated group was higher than that in the ONFH model groups, normal saline groups and 8-week HUCMSC-treated group.
Luminex showed that there were human-derived cytokines, such as IL-1β, IL-8, IL-4, IL-6, IL-10, McP-1, TNF-α, RANTES and VEGF, in the serum of rats in the 4-week and 8-week HUCMSC-treated groups. Much recent evidence suggests that mesenchymal stem cell regeneration may be mainly mediated by the secretion of some bioactive factors, such as cytokines, exosomes, growth factors, and chemokines [41,42]. VEGF is an essential angiogenic peptide that is predominantly secreted by osteoblasts in the bone environment. It affects angiogenesis by promoting endothelial cell proliferation and migration and promotes bone development and osteogenesis [43]. HUCMSCs inhibit the proliferation of immune cells and reduce inflammation by secreting IL-4 (interleukin-4) and IL-10 (interleukin-10). These changes in the immune response promote tissue repair [44]. HUCMSCs suppress cell apoptosis by inhibiting expression of the inflammatory factors TNF-α (tumor necrosis factor-α), IL-8 (interleukin-8), and IL-1β (interleukin-1β) [45,46].
These results indicated that HUCMSCs could survive in the hypoxic and ischemic microenvironment of the femoral head and still have secretory function, which has a positive impact on the pathological process of ONFH and bone tissue metabolism and was consistent with many research results.
The guidelines also indicated that for patients with Association Research Circulation Osseous (ARCO) stages 1 and 2, core decompression (CD) in combination with autologous BMMSCs or stem cell transplantation was recommended [47]. Our research was more in depth, providing a reliable reference for the treatment of ONFH in the early stage.
Some researchers implanted BMMSCs containing 2% and 20% oxygen through CD in rabbits, and the results showed that the apoptosis of tissue treated with BMMSCs containing 2% oxygen was significantly reduced and that the angiogenesis ability was significantly increased. BMMSCs pretreated with hypoxia could reverse BMMSCs in the damaged site and enhance the therapeutic effect [48]. Other studies have similarly concluded that osteogenesis of MSCs was enhanced if they were exposed to low oxygen levels in the early stage of differentiation [[49], [50], [51]]. However, with time, we found that the number of local HUCMSCs decreased and that the bone structure in the femoral head was also changed, which fully indicated that HUCMSCs play an important role in the bone metabolism of ONFH; however, sufficient and intermittent infusions of HUCMSCs were needed to maintain the therapeutic effect on ONFH. In subsequent experiments, it is necessary to increase the sample size to further evaluate the efficacy in the 8-week HUCMSC-treated group. Cai et al. [31,32]. Evaluated the cotransplantation of autologous BMMSCs and allogeneic UCMSCs for ONFH and observed a therapeutic effect 12 months after transplantation, and no serious adverse reactions were found. Chen et al. analyzed the clinical effect of HUCMSC transplantation in the treatment of ONFH. After 3 years of follow-up, the effect was clear without obvious side effects. However, only 30 patients and 9 patients were involved in the two studies [32]. To further evaluate the efficacy and safety of HUCMSCs in ONFH, more studies with many patients and longer follow-up times are needed.
After analyzing several clinical studies, Wang et al. [52] found that CD combined with BMMSCs had a better therapeutic effect on ONFH than CD alone, and the combined therapy significantly reduced symptoms and had a positive effect on preventing collapse. However, the author did not specify the degree of necrosis in the ONFH patients, and there are still many problems to be further studied, such as tumorigenicity, performance of transplanted cells in vivo, and immune rejection.
There are still some further studies to be done in our research. First, few molecular mechanisms of HUCMSCs were involved in the treatment of early femoral head osteonecrosis in rats. Second, the molecular pathways involved in the effect of HUCMSCs on the pathological change process of ONFH are still unclear. Regarding the result of HUCMSC implantation at 8 weeks after surgery, we believe that with the extension of time after surgery, HUCMSCs cannot remain in the femoral head for a long time, but they can still survive and enter the blood circulation and other parts of the body, which can be seen from the Luminex assay of serum from the rats. Therefore, intermittent implantation of HUCMSCs during treatment will be required to maintain their efficacy. At the same time, we believe that it is still necessary to increase the sample size to further evaluate the efficacy of HUCMSCs at 8 weeks after surgery, and further studies will be carried out in future experiments.
5. Conclusions
As a refractory orthopedic disease, ONFH needs to be further studied. Stem cell therapy is a promising treatment, and local transplantation of HUCMSCs in the femoral head can promote the stability of bone structure and avoid further deterioration due to ONFH.
Ethic approval
All experiments were approved by the Animal Care and Use Committee of the General Hospital of the PLA, according to the National Institutes of Health guidelines for the use of experimental animals.
Declaration of competing interest
Author Jun Zhao, Author Haoye Meng, Author Sida Liao, Author Yaoyu Su, Author Li Guo, Author Aiyuan Wang, Author Wenjing Xu, Author Hao Zhou and Author Jiang Peng declare that they have no conflicts of interest.
Acknowledgment
This research was funded by the National Natural Science Foundation of China (81972047).
References
- 1.Moya-Angeler J., Gianakos A.L., Villa J.C., Ni A., Lane J.M. Current concepts on osteonecrosis of the femoral head. World J Orthoped. 2015;6(8):590–601. doi: 10.5312/wjo.v6.i8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah K.N., Racine J., Jones L.C., Aaron R.K. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med. 2015;8(3):201–209. doi: 10.1007/s12178-015-9277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mankin H.J. Nontraumatic necrosis of bone (osteonecrosis) N Engl J Med. 1992;326(22):1473–1479. doi: 10.1056/NEJM199205283262206. [DOI] [PubMed] [Google Scholar]
- 4.Zhao D.W., Yu M., Hu K., Wang W., Yang L., Wang B.J., et al. Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: results from a nationally representative survey. Chin Med J. 2015;128(21):2843–2850. doi: 10.4103/0366-6999.168017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mont M.A., Salem H.S., Piuzzi N.S., Goodman S.B., Jones L.C. Nontraumatic osteonecrosis of the femoral head: where do we stand today?: a 5-year update. J Bone Joint Surg Am. 2020;102(12):1084–1099. doi: 10.2106/JBJS.19.01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rackwitz L., Eden L., Reppenhagen S., Reichert J.C., Jakob F., Walles H., et al. Stem cell- and growth factor-based regenerative therapies for avascular necrosis of the femoral head. Stem Cell Res Ther. 2012;3(1):7. doi: 10.1186/scrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernigou P., Poignard A., Zilber S., Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43(1):40–45. doi: 10.4103/0019-5413.45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao D., Cui D., Wang B., Tian F., Guo L., Yang L., et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–330. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Mao Q., Jin H., Liao F., Xiao L., Chen D., Tong P. The efficacy of targeted intraarterial delivery of concentrated autologous bone marrow containing mononuclear cells in the treatment of osteonecrosis of the femoral head: a five year follow-up study. Bone. 2013;57(2):509–516. doi: 10.1016/j.bone.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu P., Sun D.L., Shu Z.X., Tian S., Pan Q., Wen C.J., et al. Therapeutic applications of genes and gene-engineered mesenchymal stem cells for femoral head necrosis. Hum Gene Ther. 2020;31(5–6):286–296. doi: 10.1089/hum.2019.306. [DOI] [PubMed] [Google Scholar]
- 11.Wang H.S., Hung S.C., Peng S.T., Huang C.C., Wei H.M., Guo Y.J., et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cell. 2004;22(7):1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 12.Fu Y.S., Cheng Y.C., Lin M.Y., Cheng H., Chu P.M., Chou S.C., et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cell. 2006;24(1):115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J., Yue T., Lu S., Meng H., Lin Q., Ma H., et al. Local administration of zoledronic acid prevents traumatic osteonecrosis of the femoral head in rat model. J Orthop Translat. 2021;27:132–138. doi: 10.1016/j.jot.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J.S., Lee J.S., Roh H.L., Kim C.H., Jung J.S., Suh K.T. Alterations in the differentiation ability of mesenchymal stem cells in patients with nontraumatic osteonecrosis of the femoral head: comparative analysis according to the risk factor. J Orthop Res : official publication of the Orthopaedic Research Society. 2006;24(4):604–609. doi: 10.1002/jor.20078. [DOI] [PubMed] [Google Scholar]
- 15.Suh K.T., Kim S.W., Roh H.L., Youn M.S., Jung J.S. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2005;(431):220–225. doi: 10.1097/01.blo.0000150568.16133.3c. [DOI] [PubMed] [Google Scholar]
- 16.Houdek M.T., Wyles C.C., Packard B.D., Terzic A., Behfar A., Sierra R.J. Decreased osteogenic activity of mesenchymal stem cells in patients with corticosteroid-induced osteonecrosis of the femoral head. J Arthroplasty. 2016;31(4):893–898. doi: 10.1016/j.arth.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Wang B.L., Sun W., Shi Z.C., Lou J.N., Zhang N.F., Shi S.H., et al. Decreased proliferation of mesenchymal stem cells in corticosteroid-induced osteonecrosis of femoral head. Orthopedics. 2008;31(5):444. doi: 10.3928/01477447-20080501-33. [DOI] [PubMed] [Google Scholar]
- 18.Gangji V., Hauzeur J.P. Cellular-based therapy for osteonecrosis. Orthop Clin N Am. 2009;40(2):213–221. doi: 10.1016/j.ocl.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Lee H.J., Kang K.S., Kang S.Y., Kim H.S., Park S.J., Lee S.Y., et al. Immunologic properties of differentiated and undifferentiated mesenchymal stem cells derived from umbilical cord blood. J Vet Sci. 2016;17(3):289–297. doi: 10.4142/jvs.2016.17.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedenstein A.J., Deriglasova U.F., Kulagina N.N., Panasuk A.F., Rudakowa S.F., Luria E.A., et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2(2):83–92. [PubMed] [Google Scholar]
- 21.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 22.Rastogi S., Sankineani S.R., Nag H.L., Mohanty S., Shivanand G., Marimuthu K., et al. Intralesional autologous mesenchymal stem cells in management of osteonecrosis of femur: a preliminary study. Musculoskelet Surg. 2013;97(3):223–228. doi: 10.1007/s12306-013-0273-0. [DOI] [PubMed] [Google Scholar]
- 23.Fellows C.R., Matta C., Zakany R., Khan I.M., Mobasheri A. Adipose, bone marrow and synovial joint-derived mesenchymal stem cells for cartilage repair. Front Genet. 2016;7:213. doi: 10.3389/fgene.2016.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang J.S., Suh Y.J., Moon K.H., Park J.S., Roh T.H., Park M.H., et al. Clinical efficiency of bone marrow mesenchymal stem cell implantation for osteonecrosis of the femoral head: a matched pair control study with simple core decompression. Stem Cell Res Ther. 2018;9(1):274. doi: 10.1186/s13287-018-1030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding D.C., Chang Y.H., Shyu W.C., Lin S.Z. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24(3):339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 26.Bongso A., Fong C.Y. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton's jelly of the human umbilical cord. Stem Cell Rev Rep. 2013;9(2):226–240. doi: 10.1007/s12015-012-9418-z. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Han Z.B., Song Y.P., Han Z.C. Safety of mesenchymal stem cells for clinical application. Stem Cell Int. 2012;2012 doi: 10.1155/2012/652034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aoyama T., Goto K., Kakinoki R., Ikeguchi R., Ueda M., Kasai Y., et al. An exploratory clinical trial for idiopathic osteonecrosis of femoral head by cultured autologous multipotent mesenchymal stromal cells augmented with vascularized bone grafts. Tissue Eng B Rev. 2014;20(4):233–242. doi: 10.1089/ten.teb.2014.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilge H., Bittersohl B., Schneppendahl J., Hesper T., Zilkens C., Ruppert M., et al. Bone marrow aspirate concentrate in combination with intravenous iloprost increases bone healing in patients with avascular necrosis of the femoral head: a matched pair analysis. Orthop Rev. 2016;8(4):6902. doi: 10.4081/or.2016.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R., Lin Q.X., Liang X.Z., Liu G.B., Tang H., Wang Y., et al. Stem cell therapy for treating osteonecrosis of the femoral head: from clinical applications to related basic research. Stem Cell Res Ther. 2018;9(1):291. doi: 10.1186/s13287-018-1018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai J., Wu Z., Huang L., Chen J., Wu C., Wang S., et al. Cotransplantation of bone marrow mononuclear cells and umbilical cord mesenchymal stem cells in avascular necrosis of the femoral head. Transplant Proc. 2014;46(1):151–155. doi: 10.1016/j.transproceed.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Chen C., Qu Z., Yin X., Shang C., Ao Q., Gu Y., et al. Efficacy of umbilical cord-derived mesenchymal stem cell-based therapy for osteonecrosis of the femoral head: a three-year follow-up study. Mol Med Rep. 2016;14(5):4209–4215. doi: 10.3892/mmr.2016.5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima A., Tator C.H. Intrathecal administration of epidermal growth factor and fibroblast growth factor 2 promotes ependymal proliferation and functional recovery after spinal cord injury in adult rats. J Neurotrauma. 2002;19(2):223–238. doi: 10.1089/08977150252806974. [DOI] [PubMed] [Google Scholar]
- 34.Liu A.-M., Chen B.-L., Yu L.-T., Liu T., Shi L.-L., Yu P.-P., et al. Human adipose tissue- and umbilical cord-derived stem cells: which is a better alternative to treat spinal cord injury? 2020;15(12):2306–2317. doi: 10.4103/1673-5374.284997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martens D.J., Seaberg R.M., van der Kooy D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur J Neurosci. 2002;16(6):1045–1057. doi: 10.1046/j.1460-9568.2002.02181.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang X., Zhang L., Li Y., Yin Z., Feng Y., Ji Y. Human umbilical cord mesenchymal stem cells (hUCMSCs) promotes the recovery of ovarian function in a rat model of premature ovarian failure (POF) Gynecol Endocrinol : Off J Int Soc Gynecol Endocrinol. 2021;37(4):353–357. doi: 10.1080/09513590.2021.1878133. [DOI] [PubMed] [Google Scholar]
- 37.Mao Q., Wang W., Xu T., Zhang S., Xiao L., Chen D., et al. Combination treatment of biomechanical support and targeted intra-arterial infusion of peripheral blood stem cells mobilized by granulocyte-colony stimulating factor for the osteonecrosis of the femoral head: a randomized controlled clinical trial. J Bone Miner Res. 2015;30(4):647–656. doi: 10.1002/jbmr.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim Y.W., Kim Y.S., Lee J.W., Kwon S.Y. Stem cell implantation for osteonecrosis of the femoral head. Exp Mol Med. 2013;45:e61. doi: 10.1038/emm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Houdek M.T., Wyles C.C., Collins M.S., Howe B.M., Terzic A., Behfar A., et al. Stem cells combined with platelet-rich plasma effectively treat corticosteroid-induced osteonecrosis of the hip: a prospective study. Clin Orthop Relat Res. 2018;476(2):388–397. doi: 10.1007/s11999.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balsam L.B., Wagers A.J., Christensen J.L., Kofidis T., Weissman I.L., Robbins R.C. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 41.Liang X., Ding Y., Zhang Y., Tse H.F., Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 42.Hyun I., Hochedlinger K., Jaenisch R., Yamanaka S. New advances in iPS cell research do not obviate the need for human embryonic stem cells. Cell Stem Cell. 2007;1(4):367–368. doi: 10.1016/j.stem.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Hu K., Olsen B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–38. doi: 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dabrowski F.A., Burdzinska A., Kulesza A., Sladowska A., Zolocinska A., Gala K., et al. Comparison of the paracrine activity of mesenchymal stem cells derived from human umbilical cord, amniotic membrane and adipose tissue. J Obstet Gynaecol Res. 2017;43(11):1758–1768. doi: 10.1111/jog.13432. [DOI] [PubMed] [Google Scholar]
- 45.Sun X., Hao H., Han Q., Song X., Liu J., Dong L., et al. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther. 2017;8(1):241. doi: 10.1186/s13287-017-0668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao J., Zheng J., Cai J., Zeng K., Zhou C., Zhang J., et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. Faseb J : Off Publ Fed Am Soc Exp Biol. 2019;33(2):1695–1710. doi: 10.1096/fj.201800131RR. [DOI] [PubMed] [Google Scholar]
- 47.Zhao D., Zhang F., Wang B., Liu B., Li L., Kim S.Y., et al. Guidelines for clinical diagnosis and treatment of osteonecrosis of the femoral head in adults (2019 version) J Orthop Translat. 2020;21:100–110. doi: 10.1016/j.jot.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan L., Zhang C., Yu Z., Shi Z., Dang X., Wang K. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and osteogenesis in rabbit femoral head osteonecrosis. Bone. 2015;81:544–553. doi: 10.1016/j.bone.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Hung S.P., Ho J.H., Shih Y.R., Lo T., Lee O.K. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J Orthop Res Off Publ Orthop Res Soc. 2012;30(2):260–266. doi: 10.1002/jor.21517. [DOI] [PubMed] [Google Scholar]
- 50.Ding H., Chen S., Yin J.H., Xie X.T., Zhu Z.H., Gao Y.S., et al. Continuous hypoxia regulates the osteogenic potential of mesenchymal stem cells in a time-dependent manner. Mol Med Rep. 2014;10(4):2184–2190. doi: 10.3892/mmr.2014.2451. [DOI] [PubMed] [Google Scholar]
- 51.Fotia C., Massa A., Boriani F., Baldini N., Granchi D. Prolonged exposure to hypoxic milieu improves the osteogenic potential of adipose derived stem cells. J Cell Biochem. 2015;116(7):1442–1453. doi: 10.1002/jcb.25106. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z., Sun Q.M., Zhang F.Q., Zhang Q.L., Wang L.G., Wang W.J. Core decompression combined with autologous bone marrow stem cells versus core decompression alone for patients with osteonecrosis of the femoral head: a meta-analysis. Int J Surg. 2019;69:23–31. doi: 10.1016/j.ijsu.2019.06.016. [DOI] [PubMed] [Google Scholar]