Abstract
This is a case of congenital afibrinogenemia with multiple thrombotic and hemorrhagic events. His fibrinogen concentration was negatively correlated with thrombin time and prothrombin time and abnormally negatively correlated with plasma D‐dimer levels. The individualized standard for fibrinogen concentration may help to balance thrombotic and hemorrhagic events for this disease.
Keywords: congenital afibrinogenemia, fibrinogen infusion, hemorrhagic complication, individualized treatment, thrombotic complication
In congenital afibrinogenemia, a slightly elevated fibrinogen concentration caused significant improvements in thrombin time and prothrombin time, and fibrinogen level had a special negatively correlation with D‐dimer concentration. These coagulation disorders may contribute to the high risk of thrombotic and hemorrhagic events.

1. INTRODUCTION
Congenital afibrinogenemia (CA) is an autosomal recessive disease characterized by bleeding disorders induced by complete or extreme deficiency of circulating fibrinogen. 1 Bleeding is the main symptom, and the condition is diagnosed at birth with umbilical cord bleeding in up to 85% of patients. 2 Hemorrhages can be found in all tissues, including the skin, soft tissues, muscles, gastrointestinal tract, and urogenital and central nervous systems, but they are uncommon in joints. 3 , 4 Intracranial hemorrhage is the main cause of death. 4 Fibrinogen infusion therapy, including fibrinogen concentrates and antifibrinolytic agents, effectively replenishes fibrinogen levels and improves hemorrhagic diathesis in CA. 5
Paradoxically, fibrinogen infusion treatment is considered a possible risk factor and dramatically increases the risk of arterial and venous thromboembolism in CA patients, even when the concentration of fibrinogen is low. 5 The concentration of fibrinogen may play a key role in balancing thrombotic and hemorrhagic events. A fibrinogen level of 1 g/L (Clauss method) is the threshold to initiate clinical treatment for CA with prolonged thrombin time (TT) and prothrombin time (PT). 6 The ideal fibrinogen level for surgery is between 1.5 and 2 g/L. 6 , 7 In CA patients, a normal fibrinogen concentration (i.e., 2 g/L–4.5 g/L) has been reported to increase the risk of thromboembolic events, but the mechanism remains unclear. 7 , 8 Thus, the development of an individualized and more precise fibrinogen concentration standard may be more suitable for the management of CA patients.
Herein, we report a rare complicated case of a CA patient suffering thrombotic and hemorrhagic complications during fibrinogen infusion. Based on the results of 129 coagulation function tests and 82 assessments of plasma D‐dimers levels, we created a profile of thrombotic and hemorrhagic disorders in CA and describe a convenient method to explore individualized standards for CA treatment.
2. CASE PRESENTATION
A 44‐year‐old man complained of dizziness with nausea and vomiting for 2 days and went to the emergency department. Computed tomography (CT) scan revealed a hemorrhage in his left parietal and left occipital lobes surrounded with edema (Figure 1B). Magnetic resonance imaging (MRI) confirmed the hemorrhage and severe communicating hydrocephalus involving the lateral, third, and fourth ventricles (Figure 1C). His fibrinogen level was low at 0.82 g/L. He was diagnosed with CA, cerebral hemorrhage, and communicating hydrocephalus and the following accepted treatments: fresh frozen plasma (FFP), platelet suspensions, and continued fibrinogen concentrate (FC) infusion. The hemorrhage was not effectively controlled with FC infusion alone. His fibrinogen concentration fluctuated between <0.6 and 1.31 g/L during treatment. His prothrombin time (PT), activated partial thromboplastin time, and thrombin time (TT) ranged from 16.1 s to 46 s, 37.7 s to 61.7 s, and 25.3 s to 63.6 s, respectively. After FC infusion, his plasma D‐dimer concentration increased to 8045 ng/mL. Vascular ultrasonography revealed venous catheter‐related deep venous thrombosis in the right common femoral vein. Heparin sodium was administered to alleviate the hypercoagulable situation. When the fibrinogen concentration recovered to 2.0 g/L, he underwent ventriculoperitoneal (VP) shunt surgery to release the communicating hydrocephalus (Figure 1 D). After 2 weeks, most of his symptoms were resolved with only slight fatigue in both lower limbs.
FIGURE 1.

Transverse and sagittal MRI showed a long signal area around the bleeding site in the left parieto‐occipital lobe junction accompanied by enlargement of the bilateral lateral ventricle in T1‐weighted and T2‐weighted imaging in the patient at 41 year of age (A). Another MRI was performed at the age of 44 years (B), and CT confirmed a new increased signal at the old site of the left parieto‐occipital lobe junction and horn of the lateral cerebral ventricle (C). After an operation to insert a ventriculoperitoneal shunt, CT showed that the bleeding signal was no longer observed, and the enlarged bilateral lateral ventricle partly recovered (D)
Medical history: the patient was diagnosed with CA at birth based on umbilical cord bleeding for 7 days, and a fibrinogen level <0.01 g/L was detected using the Clauss method. He had a long‐term bleeding history and received irregular FC infusions as a child and younger adult. At 41 years of age, he experienced from his first severe hemorrhagic event in left parieto‐occipital lobe junction and communicating hydrocephalus (Figure 1A), right testicular hematoma and left testicular hemorrhage (Figure 2A,B). At the age of 43 years, he complained of left neck pain and limb fatigue for 10 days, and he was subsequently diagnosed with a hemorrhage in the spinal cord (Figure 2C,D). Two months ago, at the age of 44 years, he experienced ecchymosis of the right lower limb, dizziness, fatigue, and shock‐like symptoms after lifting heavy weights. Abdominal CT and ultrasonography revealed a massive retroperitoneal hemorrhage (Figure 2E,F). He accepted FFP, red blood cell suspension, and FC infusion, and his fibrinogen levels returned to 2.57 g/L. Then, he experienced chest pain, chest tightness, and shortness of breath. CT pulmonary arteriography (CTPA) revealed multiple severe pulmonary embolisms in both lungs, especially the pulmonary artery in the left upper pulmonary lingual segment, which was completely embolized (Figure 2G). Heparin sodium saline was administered and successfully relieved the symptoms.
FIGURE 2.

Sagittal and transverse MRI‐T2 showed increased signals in the right testicular hematoma (A,B) and unilateral spinal cord with infarction from C2 to C4 (C,D). Coronal and sagittal CT showed a retroperitoneal hematoma in the right abdomen that was 197 × 137 × 87 mm in size (E,F). Coronal CTPA showed a completely embolized pulmonary artery in the left upper pulmonary lingual segment and other small pulmonary embolisms (G)
During this 3‐year period, a total of 129 coagulation function tests and 82 assessments of plasma D‐dimer levels were performed. Correlation analysis was performed to analyze the data. The results showed that his fibrinogen concentration exhibited a negative correlation with TT and PT (Figure 3A,B). According to the fitted curves, the fibrinogen concentration must remain greater than 1.76 ng/nL and 1.98 ng/nL to maintain a TT and PT less than 14.5 s and 20 s, respectively. The plasma D‐dimer concentration exhibited an abnormal negative correlation with fibrinogen (Figure 3C). This correlation differed from the normal coagulation function in non‐CA individuals (Figure 3D).
FIGURE 3.
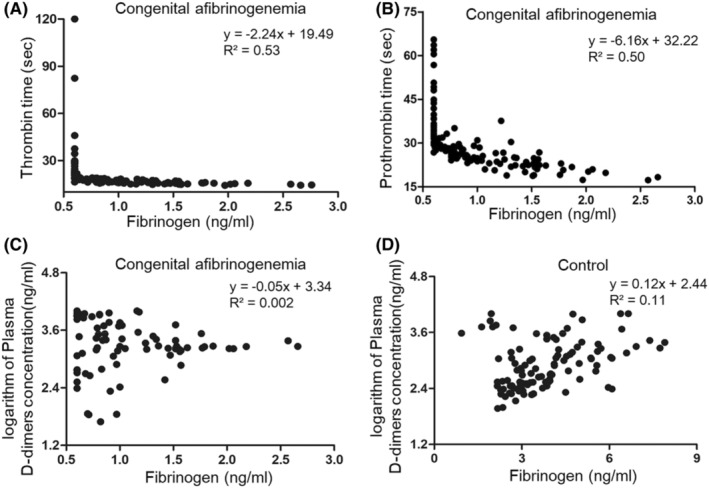
Correlation analysis between fibrinogen concentration and thrombin time (A), prothrombin time (B) and plasma D‐dimers (C) in this CA patient. The curve was fitted after excluding the points with fibrinogen <0.6 ng/mL outside of the detection range. A control group of 107 individuals without CA was established to evaluate the normal coagulation function parameters as a reference (D)
3. DISCUSSION
Here, we report a patient with CA combined with thrombotic and hemorrhagic complications with variation in spatial and temporal properties. During a three‐year period, he suffered a series of events, including scrotal hematoma, myelapoplexy, retroperitoneal hematoma, intraventricular hemorrhage, multiple pulmonary embolisms, and deep venous thrombosis. This combination of simultaneous complications has rarely been reported in one patient. Based on the results from 129 coagulation function tests, the dilemma regarding CA treatment was described in detail. These results suggest that a personalized fibrinogen concentration may serve as the key to balancing of thrombotic and hemorrhagic events in CA.
FC infusion represents a long‐term standard for maintaining constant fibrinogen concentrations in CA patients. 9 Patients with fibrinogen defects are associated with an increased risk of thrombotic events after fibrinogen infusion. 10 , 11 Thrombotic characteristics and mechanisms in CA are not clearly defined. In most cases, thrombosis occurred at a young age (median of 31.5 years) and mainly impaired large veins and arteries in CA patients. 9 Girolami et al. reported that 7/15 CA patients with thrombotic complications had a history of fibrinogen infusion. 12 Another review suggested that spontaneous bleeding and operations may increase the thrombotic risk of fibrinogen infusion. 13 , 14 In this case, we report a new observation in which anticoagulant treatment had a good therapeutic effect on thrombotic complications, including multiple pulmonary embolisms and deep venous thrombosis. 15 This finding may be based on the special features of thrombus formation in CA patients. Histological examination suggested that arterial thrombosis in CA is induced by a hematoma penetrating the vascular lumen and emphasized the notion that microhemorrhages in the vessel wall initiate the thrombosis event. 16 Regarding venous thrombosis, coagulation disorders may play an important role. 1 Although fibrinogen levels are insufficient in CA patients, the levels of other coagulation factors, such as von Wille brand factor, may increase in a compensatory manner to facilitate thrombus formation. 5 , 17 In this situation, plasmin generation may be inhibited. 18 These factors may strengthen the procoagulation effect of exogenous fibrinogen and increase the risk of thrombotic events. 18 In our case, we found that a slightly elevated fibrinogen concentration caused significant improvements in PT, TT, and plasma D‐dimer levels at some time points. We inferred that coagulation factor dysregulation may play a key role.
The fibrinogen concentration is important when evaluating the risk of thrombotic and hemorrhagic events in CA patients. 7 When fibrinogen levels are less than 1 g/L, blood coagulation indices, such as PT and TT, are abnormal. 19 In CA patients, 1 g/L is generally suggested as a threshold for FC infusion. 1 However, an individualized standard for CA treatment may be more suitable. Girolami et al. reported that 8/15 CA patients with different fibrinogen levels experienced thrombotic complications. 12 In our study, when the patient suffered severe hemorrhagic events, his fibrinogen levels were generally less than 1 g/L. When the patient's fibrinogen level increased to 1.5 g/L, he did not experience any hemorrhagic events. Based on the results from 129 coagulation function tests, we analyzed the relationship between fibrinogen concentration and PT, TT, and plasma D‐dimers. We suggest that 1.5 g/L–17.9 g/L may represent an ideal range to balance thrombotic and hemorrhagic events in this CA patient. More interestingly, the relationship between fibrinogen and D‐dimer concentration was negatively correlated in this case. The high D‐dimer levels are specifically observed during periods of low fibrinogen concentrations. We inferred that the significant levels of fibrinogen consumption, reduced fibrinogen production of and abnormal coagulation conditions may explain the complications noted in this patient. This negative correlation helped to profile the characteristics of CA in this patient and hinted that the low fibrinogen level was associated with a risk of both hemorrhage and thrombus formation with FC infusion. In other words, maintenance of fibrinogen concentrations at appropriate levels may help to control both thrombotic and hemorrhagic events.
In conclusion, due to the lack of fibrinogen and an activated compensatory mechanism, CA patients exhibit special profiles of thrombotic and hemorrhagic disorders during FC infusion. The establishment of an individualized fibrinogen concentration may help to balance the risk of thrombotic and hemorrhagic events in CA patients.
AUTHOR CONTRIBUTIONS
XPY and MMS analyzed the case and wrote the manuscript. TYT, WLJ, WZH, and MMS contributed to the diagnosis, treatment, and data collection. WZH and MMS analyzed the data and provided guidance to the rest of the authors. All authors read and approved the final manuscript.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (82171240), General Project of Basic and Applied Basic Research of Guangzhou Bureau of Science and Technology (2060206), Yang‐cheng Scholar Project of Guangzhou Municipal Bureau of Education (202032790), General Project of Natural Science Foundation of Guangdong Province (2021A1515011043), and Guangzhou Medical Key Discipline Project (2021–2023). The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
CONFLICT OF INTEREST
The authors declare no competing interests.
ETHICAL APPROVAL
It was approved to be reported by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University.
CONSENT
The patient gave written consent for their personal or clinical details along with any identifying images to be published in this study.
ACKNOWLEDGMENTS
We would like to thank the patient for his participation in this study.
Wei L, Tang Y, Wu Z, Xu P , Mo M. A case of congenital afibrinogenemia with multiple thrombotic and hemorrhagic disorders. Clin Case Rep. 2022;10:e06395. doi: 10.1002/ccr3.6395
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Stanciakova L, Kubisz P, Dobrotova M, Stasko J. Congenital afibrinogenemia: from etiopathogenesis to challenging clinical management. Expert Rev Hematol. 2016;9:639‐648. [DOI] [PubMed] [Google Scholar]
- 2. Kaur M, Kumar N, Bose SK, Rajendran A, Trehan A, Ahluwalia J. Congenital afibrinogenemia in a new born: a rare cause for bleeding. Blood Coagul Fibrinolysis. 2014;25:527‐529. [DOI] [PubMed] [Google Scholar]
- 3. Li Y, Ding B, Wang X, Ding Q. Congenital (hypo‐) dysfibrinogenemia and bleeding: a systematic literature review. Thromb Res. 2022;217:36‐47. [DOI] [PubMed] [Google Scholar]
- 4. Casini A, De Moerloose P, Neerman‐Arbez M. Clinical features and management of congenital fibrinogen deficiencies. Seminars in Thrombosis and Hemostasis. Thieme Medical Publishers; 2016:366‐374. [DOI] [PubMed] [Google Scholar]
- 5. Khayat C, Marchi R, Durual S, Lecompte T, Neerman‐Arbez M, Casini A. Impact of fibrinogen infusion on thrombin generation and fibrin clot structure in patients with inherited afibrinogenemia. Thromb Haemost. 2022;122:1461‐1468. [DOI] [PubMed] [Google Scholar]
- 6. Casini A, De Moerloose P. Fibrinogen concentrates in hereditary fibrinogen disorders: past, present and future. Haemophilia. 2020;26:25‐32. [DOI] [PubMed] [Google Scholar]
- 7. Nishihori M, Araki Y, Suzuki N, et al. Medical management of a mural thrombus inducing repeated ischemic strokes in a patient with congenital afibrinogenemia. J Stroke Cerebrovasc Dis. 2022;31:106526. [DOI] [PubMed] [Google Scholar]
- 8. Simurda T, Casini A, Stasko J, et al. Perioperative management of a severe congenital hypofibrinogenemia with thrombotic phenotype. Thromb Res. 2020;188:1‐4. [DOI] [PubMed] [Google Scholar]
- 9. Casini A, Neerman‐Arbez M, De Moerloose P. Heterogeneity of congenital afibrinogenemia, from epidemiology to clinical consequences and management. Blood Rev. 2021;48:100793. [DOI] [PubMed] [Google Scholar]
- 10. Nathoo N, Rydz N, Poon M‐C, Metz LM. Ischemic strokes in a man with congenital afibrinogenemia. Can J Neurol Sci. 2018;45:590‐592. [DOI] [PubMed] [Google Scholar]
- 11. Caimi G, Raso S, Napolitano M, Hopps E, Lo Presti R, Siragusa S. Haemorheological profile in congenital afibrinogenemia and in congenital dysfibrinogenemia: a clinical case report. Clin Hemorheol Microcirc. 2019;73:523‐530. [DOI] [PubMed] [Google Scholar]
- 12. Girolami A, Ruzzon E, Tezza F, Scandellari R, Vettore S, Girolami B. Arterial and venous thrombosis in rare congenital bleeding disorders: a critical review. Haemophilia. 2006;12:345‐351. [DOI] [PubMed] [Google Scholar]
- 13. Caimi G, Raso S, Napolitano M, Siragusa S, Presti RL. Plasma viscosity pattern and erythrocyte aggregation in two patients with congenital afibrinogenemia. Blood Coagul Fibrinolysis. 2020;31:330‐332. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Zuo X, Teng Y. Women with congenital hypofibrinogenemia/afibrinogenemia: from birth to death. Clin Appl Thromb Hemost. 2020;26:1076029620912819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Casini A. From routine to research laboratory: strategies for the diagnosis of congenital fibrinogen disorders. Hamostaseologie. 2020;40:460‐466. [DOI] [PubMed] [Google Scholar]
- 16. Teresa S, Marta M, Emiliano D, Mariangela F, Raffaele P, Ezio Z. Thrombosis of abdominal aorta in congenital afibrinogenemia: case report and review of literature. Haemophilia. 2015;21:88‐94. [DOI] [PubMed] [Google Scholar]
- 17. Dorgalaleh A, Rad F. Congenital bleeding disorders. Congenital Bleeding Disorders. Springer; 2018:27‐53. [Google Scholar]
- 18. Simurda T, Asselta R, Zolkova J, et al. Congenital afibrinogenemia and hypofibrinogenemia: laboratory and genetic testing in rare bleeding disorders with life‐threatening clinical manifestations and challenging management. Diagnostics. 2021;11:2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huissoud C, Carrabin N, Audibert F, et al. Bedside assessment of fibrinogen level in postpartum haemorrhage by thrombelastometry. BJOG. 2009;116:1097‐1102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


