Abstract
Recurrent respiratory papillomatosis (RRP) is an insidious disease caused by human papillomavirus (HPV) infection. It is characterized by a variable clinical course that can include frequent disease recurrence, significant morbidity, and occasional mortality. The mechanisms responsible for the variability in the clinical course and the persistence of latent HPV infection remain unknown. Effective T-cell-mediated clearance of HPV-infected cells may be defective in patients with RRP, leading to recurrent disease and failure to suppress latent HPV reactivation. This study describes the down-regulation of the transporter associated with antigen presentation (TAP-1) and the major histocompatibility complex (MHC) class I protein expression in laryngeal papilloma tissue biopsies and cell culture of primary explants. There was a statistically significant correlation between reduction of TAP-1 expression in biopsy tissues and rapid recurrence of disease. Patients with RRP had less frequent recurrence if their papillomas expressed TAP-1 at levels close to that of normal tissue, compared with those with very low expression of TAP-1, who had frequent recurrence (32 versus 5 weeks to the next surgical intervention). These findings suggest that HPV may evade immune recognition by down-regulating class I MHC cell surface expression via decreased TAP-1 levels. Expression of TAP-1 could be used for prognostic evaluation of disease severity. Gamma interferon was able to restore class I MHC expression at the surfaces of laryngeal papilloma cells in culture. This up-regulation of class I MHC antigen at the cell surface potentially allows the infected cell to become a target for the immune system again. This finding provides some promise for nonsurgical treatment of laryngeal papillomas.
Recurrent respiratory papillomatosis (RRP) is a disease of viral origin caused by human papillomavirus type 6 or 11 (HPV-6 or -11) (10). The disease is characterized by periods of recurrent growth of benign warty lesions of the mucosal surfaces of the upper airway interspersed in some patients with variable periods of disease remission. The mainstay of treatment has been repeated surgical excision during periods of prolific growth. Latent HPV infection is widespread in the respiratory mucosa of patients with RRP (24), and complete eradication of HPV is rare, possibly because of a defect in the host cell-mediated immune response. We have detected low levels of HPV transcripts even during disease remission (19). In addition, we have previously shown that class I major histocompatibility complex (MHC-I) antigen can be variably down-regulated in RRP (1), which is consistent with reports of MHC-I antigen down-regulation in cervical cancers caused by associated HPV-16 and -18 (4). Therefore, one mechanism used by HPV to evade immune detection by HPV-specific cytotoxic T cells (CTC) is to down-regulate MHC-I expression on HPV-infected cells. Our hypothesis was that one or more factors were causing the down-regulation of MHC-I antigen. We proposed to determine whether TAP-1 expression is down-regulated in laryngeal papillomas, whether it was related to an MHC-I antigen down-regulation, and whether the down-regulation of TAP-1 was clinically significant.
For effective antigen presentation to occur in a virus-infected cell, a complex cascade of events must take place. The transporter associated with antigen presentation (TAP-1) is essential in assembling MHC-I proteins in the endoplasmic reticulum (12). TAP proteins facilitate the entry of viral peptides into the rough endoplasmic reticulum, making these peptides available to be complexed with MHC-I molecules (7). Binding of the viral peptide to the MHC-I molecule is then associated with binding and release of a series of calcium binding proteins, including calnexin, calreticulin, and tapasin (23). These proteins function as chaperones for the proper assembly and transport of MHC-I–peptide complex to the cell surface for CD8+-T-cell recognition and destruction.
Many viruses evade immune system recognition through interference with MHC assembly. Some adenoviruses produce a protein that directly binds MHC-I antigen, trapping it in the endoplasmic reticulum (2). Herpes virus produces a protein, ICP47, that blocks transport of viral peptides into the endoplasmic reticulum (9, 14). Cytomegalovirus also blocks peptide transport by producing a protein, US6, that blocks TAP-1 (13, 17). Cromme et al. (4) were the first to identify a similar mechanism for immune evasion in malignancies induced by HPV, with decreased TAP-1 and MHC-I protein in HPV-16- and HPV-18-infected carcinomas of the cervix. They further showed that regulation of MHC-I antigen was posttranslationally controlled in these tumor cells (5).
We have now observed a concomitant decrease in the expression of both TAP-1 and MHC-I antigen in benign papillomas infected with HPV-6 or -11 from patients with RRP. This decrease is apparent in both tissue biopsies and cultured cells from primary explants. More significantly, the amount of TAP-1 protein expression correlated inversely with the frequency of disease recurrence. These findings suggest that, in part, HPV-6 and -11 may evade T-cell recognition and killing of infected cells by decreasing the surface MHC-I complex through modulation of TAP-1.
MATERIALS AND METHODS
Patients.
Biopsy samples from the laryngeal mucosal surfaces of papillomas and from healthy patients who had undergone a single surgical laryngoscopy for benign, HPV-negative lesions such as vocal cord nodules, vocal cord paralysis, and subglottic stenosis were used in these studies. Biopsy samples from patients with adult- and juvenile-onset recurrent papillomatosis were used. There was no apparent correlation between age at disease onset and the data presented. The intervals described define times to the next surgery. The time of next surgery was determined by worsening patient symptoms (dysphonia or aphonia and respiratory distress) and airway obstruction as determined by office endoscopy.
Cell cultures.
For each subset of cell culture experiments, a minimum of three different papilloma and normal biopsy samples from six different patients were used. Biopsy samples from normal laryngeal mucosa and papillomas were minced and embedded in collagen gels containing type I collagen, Ham's F-12 medium, and 10% fetal bovine serum and cultured for 2 weeks as previously reported (24). The cells were then treated with collagenase, trypsinized, and replated on coverslips at 105/16-mm-diameter well in keratinocyte growth medium (KGM) (Clonetics, Walkersville, Md.) with 0.15 mM calcium as previously reported (27). When the cultured cells were near confluence, they were either shifted to high-calcium KGM (1.0 mM) or maintained in the same low-calcium KGM (0.15 mM) for an additional 72 h. The cells were routinely grown at 37°C. For MHC-I stability experiments, the cells were plated and grown at 26 and 37°C until confluence.
A subset of confluent cultured cells in either high- or low-calcium KGM were treated with 1,000 U of gamma interferon (Sigma, St. Louis, Mo.)/ml for 48 h. The cells were then immunostained.
Immunohistochemistry and immunofluorescence.
Snap-frozen laryngeal papilloma tissues and normal laryngeal tissue were sectioned, incubated with either polyclonal rabbit anti-human TAP-1 (a generous gift from H. L. Ploegh, Cambridge, Mass.) or monoclonal murine anti-human MHC-I (W6/32) antibody (Vector, Burlingame, Calif.) for 60 min at room temperature. Bound antibody was detected with the Vecastain horseradish peroxidase kit (Vector).
Sixteen paraffin-embedded archival specimens were deparaffinized by standard techniques and stained with either polyclonal anti-TAP-1 or monoclonal anti-CD3 antibodies. Immunohistochemical detection was done as for frozen sections, except that anti-MHC-I antibody could not be used on paraffin-embedded sections. The patterns of expression and distribution of TAP-1 were identical in paraffin embedded and frozen sections.
The cultured cells were permeabilized and fixed with a 30-s 1:1 acetone-methanol solution at −20°C, washed in phosphate-buffered saline, and stained with polyclonal immunoglobulin G (IgG) anti-TAP-1 antibodies, rabbit polyclonal IgG anti-calreticulin (Affinity Bioreagents, Golden, Colo.), or monoclonal IgG anti-MHC-I antibody clone W6/32 (Vector) for 1 h at room temperature. W6/32-bound antibody was detected with a fluorescein isothiocyanate-conjugated goat anti-mouse antibody. TAP-1- and calreticulin-bound antibodies were detected with a tetramethyl rhodamine isocyanate-conjugated anti-rabbit antibody.
Correlation between TAP-1 and clinical course.
Sixteen archival specimens were used to correlate the TAP-1 staining intensity with disease status. Ten biopsy samples of papilloma tissue were taken from patients with RRP who varied in the frequency of recurrent disease. Six biopsy samples of normal laryngeal tissue served as positive controls as defined above. The range of prior surgeries was from 2 to 63 in patients with papillomas at the time of biopsy analysis. Negative controls consisted of sections incubated with secondary antibody alone. A section of each tissue used in the experiment was routinely stained with hematoxylin and eosin to confirm the histology. The TAP-1 cellular staining intensity was scored blindly by two trained observers as normal/near normal, referred to as “high,” or minimal staining, called “low.” Patients whose TAP levels were in the high category had an average of 23 prior surgeries, whereas the low-TAP group had 7 surgeries prior to the time of the present biopsy. The average interval from previous surgery to the present surgery at which TAP levels were evaluated was 8 weeks in the low-TAP group and 38 weeks in the high-TAP group. The reviewers scored the slides independently and were in total (100%) agreement in assignment of categories. All normal tissue had high staining (n = 6), and some of the papillomas fit this category as well (n = 6). Following the scoring, the clinical charts of the patients with papillomas were studied to determine the elapsed time between the surgery at which the analyzed biopsy specimen was taken and the next required surgery to remove recurrent papillomas. The mean time to next surgery was determined for each staining category. Statistical significance was determined by the nonparametric Mann-Whitney two-tailed test. The results are expressed with their standard deviations. A similar analysis of MHC-I was not possible as the anti-MHC-I W6/32 clone could not be used for paraffin-embedded sections.
RESULTS
TAP-1 and MHC-I are co-down-regulated in laryngeal papilloma tissue.
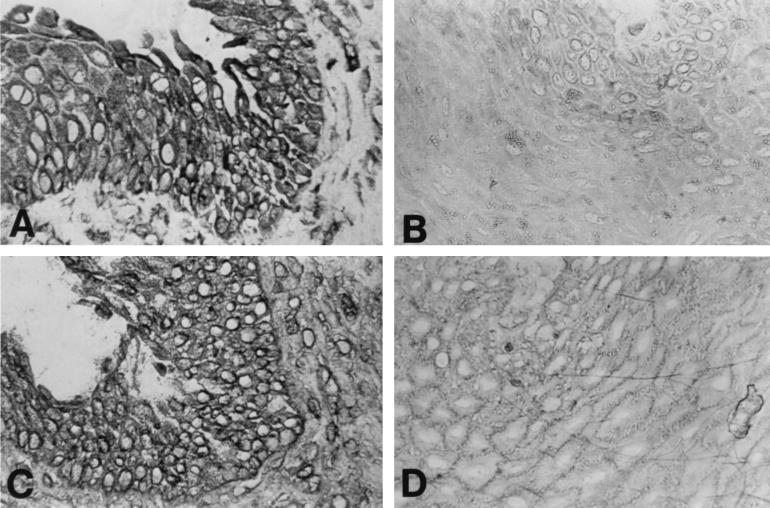
We first asked whether laryngeal papillomas and normal tissues expressed different levels of TAP-1 protein and whether down-regulation of TAP-1 correlated with reduced MHC-I. Figure 1 depicts the contrast in expression of TAP-1 and MHC-I between cryopreserved normal and papilloma tissues. The staining pattern of TAP-1 and MHC-I colocalized to the same cells, with TAP-1 perinuclear and MHC-I on the cell surface. However, expression in papilloma tissue was markedly less than in normal tissue. In different papilloma specimens, the staining intensities for both proteins varied somewhat, but they were always much less than in normal tissues. Staining of papillomas for TAP-1 was most detectable in the basal and suprabasal layers in both cryopreserved and paraffin-embedded tissues. HPV expression is very low in the basal layer and increases as the cells differentiate (15, 25). Thus, our staining was consistent with an inverse relationship to HPV expression. The observed, decreased TAP-1 expression is not a result of transcriptional regulation, as there was no difference between TAP-1 transcripts in normal and papilloma tissues or cultured cells by semiquantitative reverse transcription-PCR (data not shown).
FIG. 1.
Expression of TAP-1 and MHC-I in papilloma and normal laryngeal tissue biopsy samples. Serial frozen sections of papilloma tissue (B and D) and normal laryngeal mucosa (A and C) were immunostained for TAP-1 (A and B) and MHC-I (C and D) as described in Materials and Methods. Detection of bound antibody was done with the Vecastain horseradish peroxidase kit. Both TAP-1 and MHC-I were markedly reduced in papilloma tissue. Magnification, ×162.
TAP-1 expression by papillomas: correlation with relapse frequency.
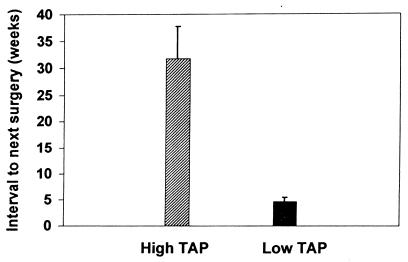
We then asked whether there was a correlation between the clinical course and the level of TAP-1 staining (Fig. 2). Ten papilloma specimens and six normal paraffin-embedded tissues were analyzed for TAP-1 staining intensity, and the results were compared to the intervals to the next required surgeries. Those patients with papillomas who had high TAP-1 expression (n = 6) had a significantly longer interval between surgical interventions (mean, 31.7 ± 6.0 weeks) than patients (n = 4) with low levels of TAP-1 (mean, 4.75 ± 0.6 weeks). This difference was significant (P = 0.0095). Therefore, increased TAP-1 expression by papillomas directly correlated with a longer disease-free interval in these patients, suggesting that TAP-1 expression is protective. The total T-cell presence was very low in all papilloma tissues, consistent with the studies of frozen sections, and did not correlate with the recurrence rate (data not shown).
FIG. 2.
Comparison of TAP-1 expression to clinical course. Archival tissue blocks of papillomas and normal laryngeal tissue were sectioned, deparaffinized, and immunostained with TAP-1 antibody. Two reviewers blinded to clinical data independently scored the staining intensity as low or high. There was complete agreement between the two reviewers. Low TAP-1 expression correlated with an aggressive clinical course. The error bars indicate standard deviations.
TAP-1 and MHC-I expression in cultured papilloma cells.
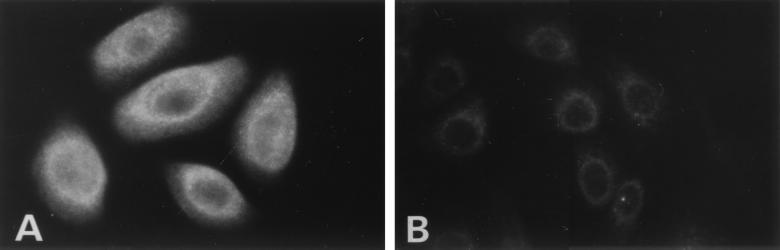
In order to study factors that could potentially up-regulate TAP-1 and MHC-I, we first needed confirmation that the culture conditions for papillomas and normal laryngeal keratinocytes produced results similar to those of tissue samples with respect to TAP-1 and MHC-I quantity and distribution. We used primary cells derived from both laryngeal papillomas and normal laryngeal epithelium to evaluate intracellular distribution. The cells were cultured in low-calcium serum-free medium, which maintains a basal cell phenotype for keratinocytes (20). We have previously shown that HPV is expressed in these cells (6). Immunostaining of cultured cells for TAP-1 (Fig. 3) was consistent with tissue results: down-regulation in papilloma cells compared with normal cells. Cellular distribution of TAP-1 between papilloma and normal cells was similar, demonstrating cytoplasmic staining in both.
FIG. 3.
Expression of TAP-1 in normal laryngeal and papilloma cultured cells. To determine whether findings with cultured basal cells replicated those with tissue, primary cells from surgical explants were used. Normal laryngeal mucosal cells cultured as described in Materials and Methods (A) and papilloma cells (B), grown in 0.15 mM calcium, were stained for TAP-1. A rhodamine-conjugated antibody was used for detection. Magnification, ×408.
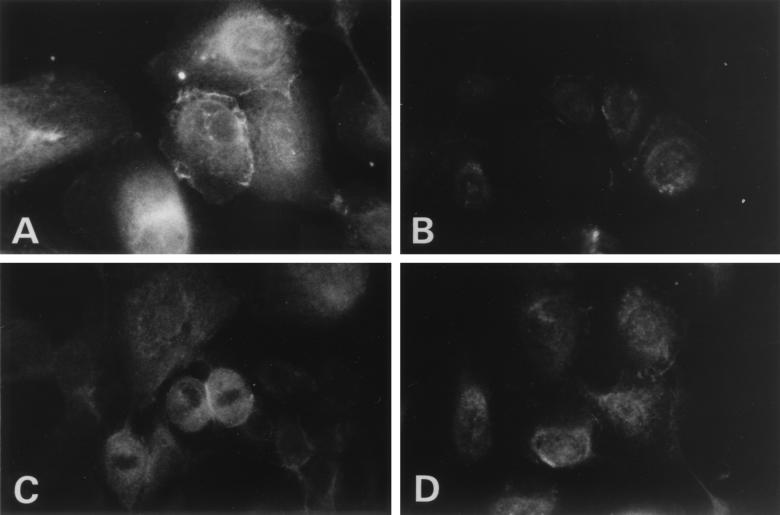
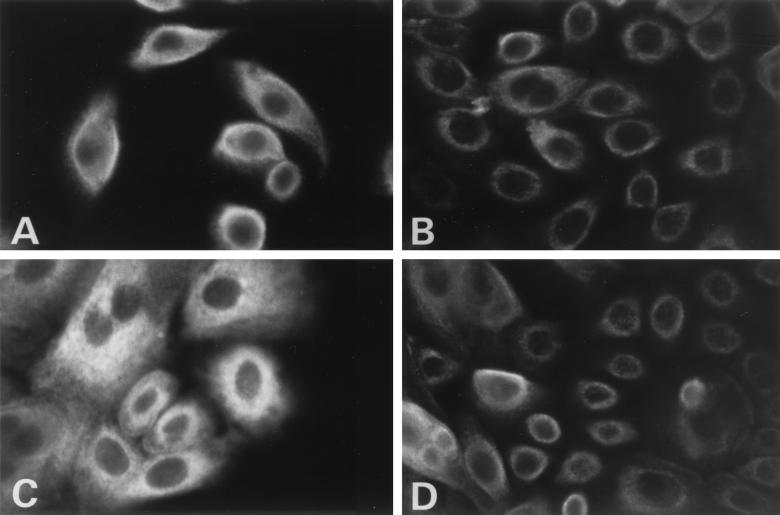
In contrast to the TAP-1 results, MHC-I expression in papillomas was not as expected (Fig. 4). Normal cells showed strong staining that was quite diffuse over the surfaces of the cells with some retention in the perinuclear region at low calcium levels (Fig. 4A). Papilloma cells also showed significant staining at 0.15 mM calcium, though less than in normal cells, primarily in a perinuclear distribution, suggesting that MHC-I was in the endoplasmic reticulum and not at the cell surface for antigen recognition (Fig. 4B). This did not entirely agree with the observed results in tissue showing that MHC-I was present at the cell surface in low abundance (Fig. 1). Papilloma cells were cultured at 26 and 37°C to assess MHC-I stability. There were no significant distribution changes at either temperature, suggesting the perinuclear clustering is not a result of MHC-I instability. To determine whether calcium-induced differentiation would give a class I staining pattern consistent with tissue biopsies, cultured cells were incubated in 1.0 mM calcium (Fig. 4C and D). Staining of the papilloma cells (Fig. 4D) was as previously observed, with faint staining compared to that of normal respiratory cells (Fig. 4C) and of MHC-I staining located at the cell membrane. Increasing the calcium concentration from 0.15 to 1.0 mM, which induces cellular differentiation, also resulted in a decrease in the amount of MHC-I in normal cells, thus more closely resembling the papilloma cells.
FIG. 4.
Expression of MHC-I in normal laryngeal and papilloma cells cultured in low- and high-calcium media. In order to determine if cellular differentiation has an effect on MHC-I expression, primary cells were cultured in either low- or high-calcium medium to maintain a basal phenotype or to induce cellular differentiation. Normal laryngeal cells (A and C) and papilloma cells (B and D), grown in 0.15 mM calcium (A and B) and 1 mM calcium (C and D), were stained for MHC-I. Detection was done with a fluorescein-conjugated second antibody. In papilloma cells at low calcium levels, only faint perinuclear staining was seen, with no cell surface staining. Magnification, ×408.
Calreticulin expression in cultured laryngeal papilloma cells.
Calreticulin, a calcium binding protein, is known to interact with TAP-1 and MHC-I in the binding of viral peptide in a complex series of events. Since we had seen a calcium-dependent change in MHC-I distribution, the expression and distribution of this molecular chaperone was analyzed in papilloma cells and normal laryngeal cells (Fig. 5). Expression of calreticulin was significantly more abundant in normal cells than in papilloma cells. Additionally, normal laryngeal or papilloma cells cultured in high-calcium medium had a modest increase in expression compared to cells cultured in low-calcium medium (Fig. 5C and D). There was no significant distribution change in papillomas compared with normal cells, as expression was predominantly perinuclear in all cells.
FIG. 5.
Expression of calreticulin in normal laryngeal and papilloma cells cultured in low- and high-calcium media. Assembly of MHC-I requires the interaction of several calcium binding proteins. Altering the calcium content did not significantly alter expression of calreticulin. Calreticulin expression was determined by immunohistochemistry in normal (A and C) and papilloma (B and D) cells grown in 0.15 mM (A and B) and 1 mM (C and D) calcium. Calreticulin was detected by a rhodamine-conjugated second antibody. Magnification, ×408.
Gamma interferon effect on cultured papilloma cells.
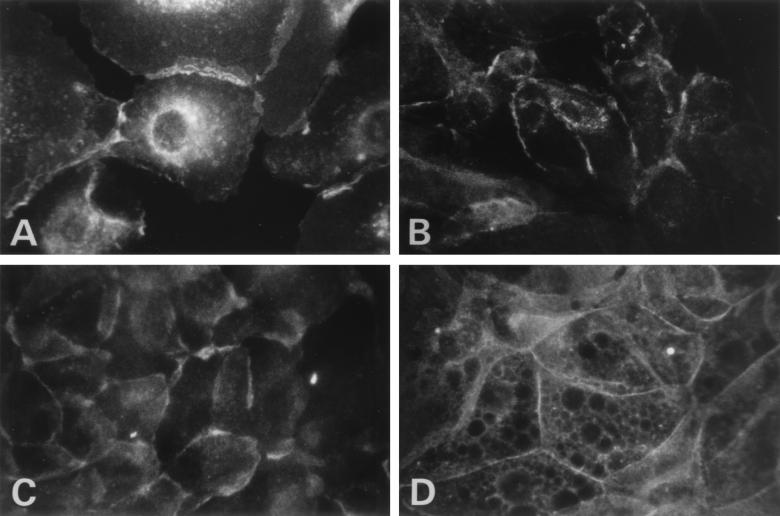
Because of the paucity of MHC-I in laryngeal papillomas, we asked if expression could be augmented. Treatment of papilloma and normal cells with gamma interferon increased MHC-I expression (Fig. 6). Additionally, cell surface expression in papillomas was restored with gamma interferon (Fig. 6B), while in its absence, MHC-I expression is predominantly perinuclear (Fig. 4B). Treatment of normal laryngeal keratinocytes also increased MHC-I cell surface expression. The observed up-regulation of TAP-1 and MHC-I in normal and papilloma cells with gamma interferon was transcriptional, as determined by semiquantitative reverse transcription-PCR (data not shown).
FIG. 6.
Expression of MHC-I in the presence of gamma interferon in normal laryngeal and papilloma cultured cells at high and low calcium levels. Gamma interferon is an inducer of both TAP-1 and MHC-I expression. MHC-I expression was determined in normal (A and C) and papilloma (B and D) cells grown in the presence of 1,000 U of gamma interferon/ml for 48 h prior to immunostaining. The cells were cultured in either 0.15 mM calcium (A and B) or 1.0 mM calcium (C and D) for at least 48 h prior to the introduction of gamma interferon. MHC-I was detected by a fluorescein-conjugated second antibody. Magnification, ×408.
DISCUSSION
Most of our present knowledge concerning immune regulation and HPV infection is based on studies of other HPV-associated diseases, namely cervical carcinomas and cutaneous warts. Unlike cervical carcinomas, which are associated with HPV-16 and -18, respiratory papillomatosis is most commonly associated with HPV-6 and -11, with low oncogenic potential. We previously reported that the global immune responsiveness in terms of T- and B-cell populations and subsets and response to other antigens of patients with RRP has been shown to be normal (1). However, we noted that MHC-I expression by papillomas is markedly decreased compared with that by normal respiratory epithelial tissue, although the down-regulation is variable within a given papilloma (1). Our observation was similar to that of others who showed a concomitant down-regulation of MHC-I and TAP-1 expression in cervical carcinomas (5).
Loading of MHC-I molecules with peptides (in this case, viral) involves a complex cascade of different protein interactions. Viral peptide is imported from the cytoplasm to the endoplasmic reticulum by TAP, an ATP-dependent transporter (21). Once in the endoplasmic reticulum, in the presence of TAP, the viral peptide associates with MHC-I and β2-microglobulin in the presence of several calcium binding proteins. Calnexin associates with the complex and drops off, followed by the association of calreticulin and, subsequently, tapasin (23). Once the peptide is properly loaded, MHC-I is transported to the cell surface in a stable configuration for antigen recognition. If MHC-I is not properly loaded with peptide, it is retained in the endoplasmic reticulum. Calreticulin, a calcium binding protein, is a chaperone and is one of the proteins responsible for the release of MHC-I molecules in the endoplasmic reticulum for transport to the cell surface (23). Unlike some of the other binding proteins, calreticulin maintains a stochiometric relationship with both TAP–MHC-I–tapasin complexes (11). The fact that both MHC-I and TAP-1 are reduced in laryngeal papillomas does not make the reduction in calreticulin surprising. Increasing the extracellular calcium concentration from 0.15 to 1 mM may modulate the release of MHC-I from its associated calcium binding protein and from the endoplasmic reticulum. However, even at this higher calcium concentration, expression of MHC-I is still reduced in papillomas compared with that in normal keratinocytes.
The present experiments show a concomitant decrease in the expression of TAP-1 and MHC-I in respiratory papillomas compared with normal respiratory epithelial tissue, most marked in the upper layers. The largest concentration of viral transcripts was identified in the suprabasal layers (6). This observation correlates well with the areas of the papilloma tissue in which we have found the most decreased TAP-1 expression. Taken together, these observations suggest that HPV may block TAP-1 expression and thereby decrease MHC-I assembly and expression by limiting peptide entry into the rough endoplasmic reticulum. It has yet to be determined which viral protein(s) is responsible for this interaction.
We have also noted that within respiratory papillomas no correlation could be made between the frequency of disease recurrence and increased or decreased concentrations of total T cells. The blood of patients with RRP did not show significant changes in the CD4+/CD8+-T-cell ratio (1), although a reduced CD4+/CD8+ ratio has been reported in HPV-infected patients with genital lesions (3).
Archival papillomas showed a correlation between the expression of TAP-1 and disease recurrence. Strong expression of TAP-1 could be associated with longer disease-free intervals; patients with near-normal tissue staining intensity for TAP-1 had a significantly longer interval between surgical procedures, although no quantitative value could be assigned. Although many have observed a down-regulation of MHC-I in viral disease (4), these results directly suggest a clinical significance of reduced TAP-1, with a resultant decrease in surface class I expression.
The decreased expression of TAP-1 is unlikely to be related to the frequency of surgical trauma. Normal cells when grown in culture develop a hyperproliferative phenotype, as in wound healing (24). We found significant differences between papillomas and normal laryngeal cells in culture, suggesting that the down-regulation of TAP-1 is not a wound-healing phenomenon. Additionally, the patients in the higher-TAP-expressing group had more prior surgeries on average than low TAP expressers.
Appropriate expression of MHC-I at the cell surface requires multiple factors to interact efficiently. At low calcium concentrations, MHC-I in cultured papilloma cells was identified by predominantly perinuclear staining, suggesting that molecules that react with W6/32 anti-MHC-I antibodies were still in the endoplasmic reticulum. W6/32 recognizes MHC-I molecules that are not complexed with TAP-1 proteins, suggesting that the clustering of perinuclear MHC molecules is not likely to be associated with peptides for presentation (22). A similar mechanism of viral evasion has been seen with herpes simplex. Herpes simplex virus creates the ICP47 protein that binds to TAP-1, inhibiting peptide loading onto MHC-I. MHC-I, in this disease, remains vacant and trapped in the endoplasmic reticulum (9). At higher calcium concentrations, there was expression as in normal cells, although greatly reduced in amount. This suggests that TAP-independent mechanisms for class I antigen expression may exist in these papillomas. Further studies are under way. Others have shown that certain cell lines are still able to express MHC-I in the absence of TAP-1 (28).
Gamma interferon is a known inducer of TAP-1 (17) and consequently causes up-regulation of MHC-I. The mechanism of gamma interferon induction is transcriptional (8). Treatment of cultured cells with gamma interferon produced an expected up-regulation of TAP-1. The cellular distribution remained unchanged (data not shown). In cultured papilloma cells and normal laryngeal cells, gamma interferon increased and altered the localization of MHC-I, inducing cell surface expression. The importance of this cannot be overemphasized: by allowing MHC-I to reach the cell surface, the virus-infected cell can once again become a target for attack by a functional immune system. Several clinical trials suggested that alpha interferon therapy was most effective initially or when used continuously. However, long-term improvement with alpha interferon was seen in 47 of 60 patients, with only 13 nonresponders in a 4-year study (18).
In summary, our results show a co-down-regulation of TAP-1 and MHC-I in respiratory papillomas compared with normal respiratory epithelial tissue. One would expect that patients with aggressive respiratory papilloma growth and frequent recurrence of disease, expressing the greatest amounts of viral peptides, should mount the strongest HPV-specific, CTC response. We have found that the patients with the most aggressive and rapidly progressive disease expressed the lowest levels of TAP-1. The resultant absence of surface MHC-I proteins would impede HPV-specific CTC recognition of HPV peptides at the cell membrane. Taken together with the reports that MHC-I and TAP-1 proteins are co-down-regulated in HPV-infected cervical carcinomas (4), our results provide evidence that HPVs in general may evade CTC effectors by blocking HPV peptide presentation through the inhibition of TAP-1 function. The mechanism(s) exploited by HPV that is responsible for down-regulation of TAP-1 function is yet to be defined. The clinical correlation of improved TAP expression with a more indolent course provides a possible target for immune modulation to temper disease recurrence. Gamma interferon has shown promise by up-regulating TAP-1 expression and by facilitating MHC-I reaching the cell surface for interaction with CD8 cells.
ACKNOWLEDGMENTS
We thank Hidde Ploegh for graciously providing the polyclonal TAP-1 antibody.
This work is funded by grants DC00203 (B.M.S.) and DC00155 (A.V.) from the National Institute on Deafness and Other Communication Disorders.
REFERENCES
- 1.Bonagura V B, Siegal F P, Abramson A L, Santiago-Schwartz F, O'Reilly M E, Shah K, Drake D, Steinberg B M. Enriched HLA-DQ3 phenotype and decreased class I major histocompatibility complex antigen expression in recurrent respiratory papillomatosis. Clin Diagn Lab Immunol. 1994;1:357–360. doi: 10.1128/cdli.1.3.357-360.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgert H G, Kvist S. The E3/19K protein of adenovirus type 2 binds to the domains of histocompatibility antigens required for CTL recognition. EMBO J. 1987;6:2019–2026. doi: 10.1002/j.1460-2075.1987.tb02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman N, Birley H D, Renton A M, Hanna N F, Ryait B K, Byrne M, Taylor-Robinson D, Stanley M A. Immunologic events in regressing genital warts. Am J Clin Pathol. 1994;102:768. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- 4.Cromme F V, Heemels M T, Ploegh H L, Keating P J, Stern P L, Meijer C J L M, Walboomers M M. Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J Exp Med. 1994;179:335–340. doi: 10.1084/jem.179.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cromme F V, Snijders P J F, van den Brule A J C, Meijer C J L M, Walboomers J M M. MHC class I expression in HPV 16 positive cervical carcinomas is post-translationally controlled and independent from c-myc overexpression. Oncogene. 1993;8:2969–2975. [PubMed] [Google Scholar]
- 6.DiLorenzo T P, Steinberg B M. Differential regulation of human papillomavirus type 6 and 11 early promoters in cultured cells derived from laryngeal promoters. J Virol. 1995;69:6865–6872. doi: 10.1128/jvi.69.11.6865-6872.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelhard V H. How cells process antigens. Sci Am. 1994;271:54–61. doi: 10.1038/scientificamerican0894-54. [DOI] [PubMed] [Google Scholar]
- 8.Epperson D E, Arnold D, Spies T, Cresswell P, Pober J S, Johnson D R. Cytokines increase transporter in antigen processing-1 expression more rapidly than HLA class I expression in endothelial cells. J Immunol. 1992;149:3297–3301. [PubMed] [Google Scholar]
- 9.Fruh K, Ahn K, Djaballah H, Sempe P, vanEndert P M, Tampe R, Peterson P A, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 10.Gissman L, deVilliers E M, zurHausen H. Molecular cloning and characterization of human papillomavirus DNA derived from a laryngeal papilloma. J Virol. 1982;44:393–400. doi: 10.1128/jvi.44.1.393-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris M R, Yu Y Y L, Kindle C S, Hansen T H, Solheim J C. Calreticulin and calnexin interact with different proteins and glycan determinants during the assembly of MHC class I. J Immunol. 1998;160:5404–5409. [PubMed] [Google Scholar]
- 12.Heemels M T, Ploegh H L. Generation, translocation, and presentation of MHC class I-restricted peptides. Annu Rev Biochem. 1995;64:463–491. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 13.Hengel H, Koopmann J O, Flohr T, Muranyi W, Goulmy E, Hammerling G J, Koszinowski U H, Momberg F. A viral resident glycoprotein inactivates the MHC encoded peptide transporter. Immunity. 1997;6:623–632. doi: 10.1016/s1074-7613(00)80350-7. [DOI] [PubMed] [Google Scholar]
- 14.Hill A, Jugovic P, York I, Russ G, Bennick J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 15.Iftner T, Oft M, Bohm S, Wilczynski S P, Pfister H. Transcription of the E6 and E7 genes of human papillomavirus type 6 in anogenital condylomata is restricted to undifferentiated cell layers of the epithelium. J Virol. 1992;66:4639–4646. doi: 10.1128/jvi.66.8.4639-4646.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lehner P J, Karttunen J T, Wilkinson G W G, Cresswell P. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc Natl Acad Sci USA. 1997;94:6904–6909. doi: 10.1073/pnas.94.13.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leventhal B G, Kashima H K, Mounts P, Thurmond L, Chapman S, Buckley S, Wold D. Long-term response of recurrent respiratory papillomatosis to treatment with lymphoblastoid interferon alfa-N1. Papilloma Study Group. N Engl J Med. 1991;325:613–617. doi: 10.1056/NEJM199108293250904. [DOI] [PubMed] [Google Scholar]
- 19.Maran A, Amella C A, DiLorenzo T P, Auborn K J, Taichman L B, Steinberg B M. Human papillomavirus type 11 transcripts are persistent at low abundance in latently infected respiratory tissues. Virology. 1995;212:285–294. doi: 10.1006/viro.1995.1486. [DOI] [PubMed] [Google Scholar]
- 20.Mendelsohn M G, Dilorenzo T P, Abramson A L, Steinberg B M. Retinoic acid regulates, in vitro, the two normal pathways of differentiation of human laryngeal keratinocytes. In Vitro Cell Dev Biol. 1991;27A:137–141. doi: 10.1007/BF02630999. [DOI] [PubMed] [Google Scholar]
- 21.Neefjes J J, Momburg F, Hammerling G J. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science. 1993;261:769–771. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- 22.Neisig A, Wubbolts R, Zang X, Melief C, Neefjes J. Allele-specific differences in the interaction of MHC class I molecules with the transporter associated with antigen processing. J Immunol. 1996;156:3196–3206. [PubMed] [Google Scholar]
- 23.Sadasivan B, Lehner P J, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg B M, Abramson A L, Meade R P. Culture of human laryngeal cells in vitro. Otolaryngol Head Neck Surg. 1982;90:728–735. doi: 10.1177/019459988209000610. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg B M, Topp W, Schneider P, Abramson A L. Laryngeal papillomavirus infection during clinical remission. N Engl J Med. 1983;308:1261. doi: 10.1056/NEJM198305263082104. [DOI] [PubMed] [Google Scholar]
- 26.Stoler M H, Wolinsky S M, Whitbeck A, Broker T R, Chow L T. Differentiation linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message specific RNA probes. Virology. 1989;172:331–340. doi: 10.1016/0042-6822(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 27.Vambutas A, DiLorenzo T P, Steinberg B M. Laryngeal papilloma cells have high levels of epidermal growth factor receptor and respond to epidermal growth factor by a decrease in epithelial differentiation. Cancer Res. 1993;53:910–914. [PubMed] [Google Scholar]
- 28.Young N T, Mulder A, Cerundolo V, Claas F H, Welsh K I. Expression of HLA class I antigens in transporter associated with antigen processing (TAP)-deficient mutant cell lines. Tissue Antigens. 1998;52:368–373. doi: 10.1111/j.1399-0039.1998.tb03057.x. [DOI] [PubMed] [Google Scholar]