Abstract
The recent emergence of monkeypox (MPX) has created a global threat. The number of infected and suspected cases of MPX is increasing in different parts of the world, especially in non-African countries. However, vaccines are available to fight against this disease. It has been observed that smallpox vaccines can be used to protect against MPX. The present article highlights the significant points and various issues for vaccines and vaccinations that should be considered related to MPX. This paper illustrates current vaccines for smallpox that can be utilized to protect against MPX infection. The article also describes the different significant research on MPXV, especially smallpox vaccines, and its outcome in MPX infection. We have also tried to depict the smallpox vaccination eradication model through the statistical interface using smallpox eradication data from Central and West Africa between 1967 and 1972. We suggest that these models might be helpful for the eradication of MPX in the middle to low-economic countries. Simultaneously, we have also discussed vaccination preparedness in different countries like the USA, UK, Canada, Denmark, Germany, etc. Our report might be helpful to scientists and policymakers in understanding the vaccines and vaccination against MPX and formulating effective strategies to fight against the disease.
Keywords: Vaccine: monkeypox, Vaccination program, Smallpox
1. Introduction
Recently monkeypox (MPX) has emerged when people worldwide have started recovering from the COVID-19 pandemic. The health communities are worried due to the re-emergence of this neglected disease. The recent MPX outbreak has created a unique challenge throughout the globe [1], [2]. The virus is very uncommon, and the disease is also life-threatening. The virus is called monkeypox virus (MPXV). Currently, MPX has sparse more than 96 non-African countries, and the outbreak has emerged in Australia, Canada, Belgium, Germany, France, Netherlands, Italy, the UK, Portugal, Sweden, Spain, and the USA. More than 61,753 suspected or confirmed cases have been recorded [3]. The virus has spread across multiple countries in several populations. Therefore, the viral disease appears as a global threat and has drawn people's attention worldwide [4].
This zoonotic virus was first identified in Denmark in 1958. The virus was noted in two monkey colonies which were preserved in a Danish research laboratory. Researchers found pox-like disease symptoms in affected animals. The researchers named the condition 'monkeypox' [5], [6]. However, it was noticed in animals, not in humans, during that period. The first case of MPX in a human was noted in 1970 in the Congo (Democratic Republic of Congo, DRC). The incidence was initially reported as smallpox [7], [8]. Later on, it was noted that MPX had re-emerged in Africa from time to time. Subsequently, researchers have found two distinct clades of the MPX. The two clades are the West African (WA) clade and Congo Basin (CB) clade [9], [10]. Among these two clades, the infected individuals with the CB clade show a higher case fatality rate (CFR) than those infected with WA clade. Usually, the CRF is recorded from 1% to 10%. The CFR was observed at approximately 10% for infected persons with CB clade. On the other hand, the CFR was noted to be about 1% for infected persons with WA clade [8], [10]. Recently, we have discussed the evolution and the Clade of the MPXV [11].
Several scientists have provided different hypotheses occasionally for the re-emergence of MPXV and other zoonotic diseases. Noteworthy hypotheses from the previously published work are rainforest exploitation, extremely mobile populations, climate change, and geopolitical-related conflicts in disease areas. However, the illegal animal trade is one of the significant causes of the jump of any infectious disease’s virus from animals to the human population, and it might be one of the reasons for starting the MPXV outbreak in non-epidemic countries. At the same time, some scientists have also provided immunological hypothesis, such as declining herd immunity [8], [12].
No traditional vaccine using the MPXV is available. At the same time, no next-generation MPX vaccine is expected shortly. However, the vaccinia virus vaccine is in use for immunization against smallpox. The vaccine might prevent the onset of clinical disease if the vaccine is administered within four days of smallpox infection [8]. The vaccine was successful in eradicating smallpox. It has been observed that, after the eradication of smallpox, the routine vaccination of smallpox was paused in several places. However, MPX emerged in the unvaccinated human populations with augmented frequency during this phase. Currently, smallpox vaccines are being used to protect against MPX.
At the moment, there is an urgent need to vaccinate people to protect against MPX. Therefore, proper strategies are required for vaccinating people. The present article highlights major areas for vaccines and vaccination strategies related to MPX. In this direction, the paper illustrates currently available vaccines for smallpox that are being used to protect against MPX infection and significant research to fight against MPX using smallpox vaccines. Moreover, we have tried to depict the smallpox vaccination eradication model in Central and West Africa between 1967 and 1972. We suggested that these models might be helpful for the eradication of MPX in the middle to low-economic countries. Recently, we also urged to adopt the quick contact tracing and ring vaccination strategy to control the MPX, which is presently used by several countries.
2. Available smallpox vaccines that are being used to protect against the infection of MPX
Currently, two smallpox vaccines are available in the USA: one is JYNNEOSTM, and another is ACAM200. These vaccines are administered to people having an exposure chance to any Orthopoxviruses [13]. This condition is entitled PrEP (pre-exposure prophylaxis). The people who might have a chance to get PrEP include research laboratory workers, clinical laboratory people, public health workers, and healthcare professionals.
2.1. JYNNEOSTM vaccine
The vaccine is given to individuals as a non-replicating, live virus [13]. It is a modified vaccinia virus Ankara (MVA) vaccine. The USFDA first approved the vaccine in 2019 for adults in the USA [14]. Different studies have shown that the vaccine is about 85% effective against MPX. The vaccine is administered in two doses (0.5 mL each dose), and these two dosages are administered after four weeks. The vaccine is given as subcutaneous injections. Presently, the vaccine is made by Bavarian Nordic in Denmark. The company has also collaborated with the USA to develop a freeze-dried vaccine form (MVA-BN® freeze-dried). The company has completed the phase 3 clinical trial for MVA-BN®. The vaccine is available under different names in different countries. For instance, in the European Union, the vaccine is known as Imvanex, and in Canada, it is Imvamune.
2.2. ACAM2000 vaccine
This smallpox vaccine is prepared using the live vaccinia virus, and the vaccinia strain was derived from the New York City Board of Health, the USA. The live virus preparation is administrated to the individuals for vaccination. The Emergent BioSolutions developed the vaccine with an agreement with USCDC. The vaccination procedure is different from the usual injecting form. The vaccination technique is called the multiple puncture technique. The vaccine is administered using a bifurcated stainless steel needle. During vaccination, the needle is dipped into the solution (vaccine solution), and the individual's skin is pierced in the arm with the needle a few times during the administration. It has been noted that 100 doses are available in each vaccine vial containing approximately 0.0025 mL of live vaccinia virus. It has also been observed that the amount contains 2.5–12.5 ×105 plaque-forming units. The preparation of purified live viruses includes some non-active recipients: human serum albumin, HEPES, mannitol sodium chloride, etc. [15]. The renowned vaccine development company Sanofi Pasteur is manufacturing this vaccine. The USFDA approved the vaccine in August 2007.
2.3. Dryvax vaccine
The Dryvax vaccine has been recorded as the oldest smallpox vaccine in the world. American home product was created during the late 19th century. It was synthesized by Wyeth's forebear, a well-known pharmaceutical company in the USA. The vaccine contains a live vaccinia virus (VACV) in freeze-dried form. The vaccine is prepared from calf lymph. USFDA licensed it in 1931. It was used to protect against smallpox; however, the vaccine can be used to protect against MPX. It has been noted that the vaccine can provide immunogenic protection for about 95% of vaccinated individuals. The vaccine has some side effects in about 1% to 2% of cases [16]. However, the Dryvax was replaced by ACAM2000 in 2008, the national strategic stockpile [17]. The vaccine is no longer manufactured.
Several smallpox vaccines have been used from time to time for MPX vaccines by different countries. We have provided a list of the smallpox vaccines that might help eradicate the MPXV (Table 1 ).
Table 1.
Different smallpox vaccines are used for the MPXV infection from time to time by several countries.
| Sl. No. | Vaccine category | Vaccine name | Vaccine type | Present status |
|---|---|---|---|---|
|
Old smallpox vaccines |
Western Reserve | Replicative, vacinia strain | Model virus for best studies on features of poxvirus biology | |
| VACV Lister strain (VACV-LST) | Replicative, attenuated vaccinia virus | Extensively used as smallpox vaccine | ||
| Modified vaccinia virus Ankara (MVA) | Non-replicative, highly attenuated and modified vaccinia virus Ankara (MVA) strain | Approved as third-generation vaccine against the smallpox | ||
| New York City Board of Health (NYCBOH)(Dryvax) | Replicative, multi clonal vaccinia strain | Stopped in the year 1982 | ||
| Aventis Pasteur Smallpox Vaccine (APSV) | Replication-competent of vaccinia virus | Under investigational new drug application or emergency use authorization for smallpox emergency |
||
|
Recent-advanced smallpox vaccines |
ACAM2000® | Live vaccinia virus (replication competent) |
Approved against smallpox and human MPXV |
|
| JYNNEOS™ (Imvamune or Imvanex or MVA-BN) | Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) strain, attenu- ated Vaccinia virus |
3. Major researches to fight against MPX using smallpox vaccines: From molecular biology to the mathematical model
It has been noted that different researchers have used smallpox vaccines as protection against MPXV. Some researchers have also tried to develop next-generation vaccines to protect against the virus. Kennedy et al. performed a study to understand the humoral immune responses of the recipients who have received the smallpox vaccine. In this study, a poxvirus proteome array was used to evaluate the response of the antibody. The array analyzed the protein from a cohort who received the smallpox vaccine (n=1,037). The antibody responses were measured against the viral proteins using Dryvax and ACAM2000 [18]. A routine smallpox vaccine Dryvax was observed to protect nonhuman primates from MPX. Another study was performed by Hooper et al. using the Dryvax vaccine on rhesus macaques. They have noted that vaccinated monkeys with Dryvax have no clinical symptoms of the disease. In this study, the researchers have used a DNA vaccine that consists of four genes from the vaccinia virus (B5R, A33R, A27L, and L1R), demonstrating a protective effect against MPX [19]. This study might have used the first subunit vaccine for the immunization against MPX or smallpox. Similarly, Edghill-Smith et al. have reported that the vaccination with live vaccinia virus, the active agent of the smallpox vaccine, provides adequate safety against the MPXV. It can stimulate protective antibody responses, which are long-lasting against the MPXV [20]. At the same time, Earl et al. used two vaccines Dryvax and attenuated MVA strain, in a monkey model. They analyzed T-cell responses, neutralizing titers, and antibody binding after one dose of MVA with subsequent Dryvax or two MVA doses. They found that vaccinated animals were healthier than infected animals, a challenge with the MPXV [21]. Another study developed a multivalent smallpox DNA vaccine. It was administered to nonhuman primates using intradermal electroporation to induce immunity against the lethal MPXV. It shows a high antibody -titer and might offer animals safety from the pathogenic MPX challenge [22]. Likewise, Marriott et al. developed clonal vaccinia virus isolated from Dryvax, manufactured through the cell culture. The protective activity of the clonal vaccinia virus was tested in a nonhuman primate model. It showed protection in monkeys challenged with a lethal dose of MPXV [23].
At the same time, it was observed that some researchers had developed mathematical or statistical models to evaluate the vaccination program. In this direction, researchers developed a mathematical model to understand the transmission dynamics of the virus, which might help formulate vaccination strategies [24]. Bankuru et al. developed a game-theoretic model that might help evaluate different vaccination approaches [25]. However, more research is needed to fight against the neglected disease, mostly to develop the next-generation MPX vaccine and understand the immunological aspect of vaccination.
4. Can we use the smallpox or COVID-19 vaccination eradication model to eradicate MPX?
4.1. Continuous immunization programs in developing and underdeveloped countries for smallpox eradication
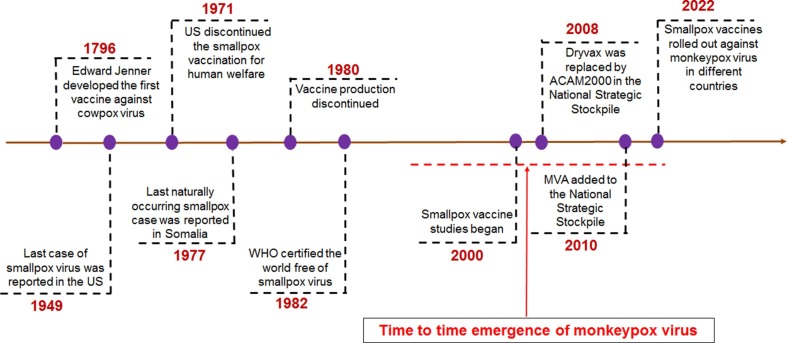
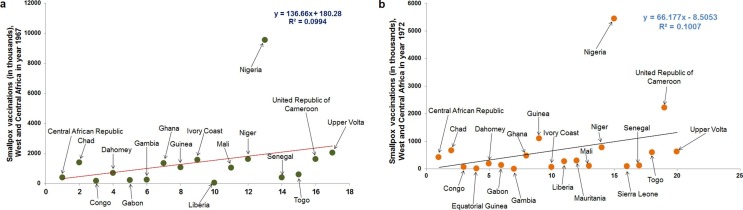
The first origin of smallpox is not known. In 1949, the last smallpox case was reported in the USA. However, continuous immunization programs against smallpox in developing and underdeveloped countries continued to eradicate smallpox. The last case of smallpox was noted in Bangladesh in 1975, which might be the latest case of smallpox worldwide. Afterward, WHO declared the smallpox-free world in May 1980 (Fig. 1 ). However, if we look back, the smallpox global eradication program was started in 1959. After the continuous effort of 21 years of this eradication program, the WHO declared in the thirty-third World Health Assembly that the ‘universal disease’ has been eradicated globally [26]. In 1960, it might be observed that different smallpox vaccination programs were conducted aggressively to eliminate smallpox, especially in Central and West Africa. One report illustrated that aggressive smallpox vaccination programs between 1967 and 1969 vaccinated 100 million people and 28 million additional children during 1972 [27]. We have tried to depict smallpox vaccination programs from the previously published data through two statistical models conducted between 1967 and 1972 in Central and West Africa (Fig. 2 a and Fig. 2b). The first model illustrates the smallpox vaccination programs in 1967 in Central and West Africa (Fig. 2a). The second model demonstrates the smallpox vaccination programs in 1972 in Central and West Africa (Fig. 2b). The first model illustrated that Nigeria is the most vaccinated country, and the Congo, Gabon, Liberia, and the Gambia were less vaccinated. Similarly, the second model presented that Nigeria again was the highest vaccinated country. Several countries were less vaccinated, such as Congo, Equatorial Guinea, Gabon, Gambia, Liberia, etc. The second model illustrated that the program might eradicate the maximum number of smallpox in more countries where fewer vaccination programs occurred. It also illustrated that the vaccination program might be successful in those countries before 1972. However, these vaccination models can be adopted for MPX eradication if the viruses spread in developing or underdeveloped countries or middle to low-economic countries.
Fig. 1.
The timeline shows the different milestones of smallpox, such as the vaccination and eradication of smallpox. The timeline also describes the emergence period of MPX, and currently, the smallpox vaccine has been rolled out to fight against the MPX in different countries.
Fig. 2.
Vaccination for the smallpox eradication program in Central and West Africa from 1967 to 1972. (a) A statistical regression plot describes the number of smallpox vaccinations during the smallpox elimination program in different countries in Central and West Africa in 1967 (b). A statistical regression plot represents the number of smallpox vaccinations during the smallpox eradication program in Central and West Africa in various countries in 1972.
4.2. Rapid contact tracing and ring vaccination
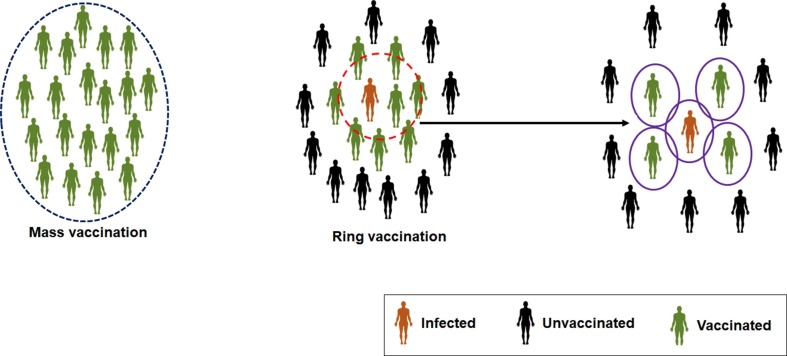
Ring vaccination is a strategy to control a disease spreading by vaccinating individuals who are most likely to be infected. Contact tracing and ring vaccination are essential strategies developed to eradicate smallpox. The method was noted successful compared to mass vaccination (Fig. 3 ). Several researchers have developed statistical or mathematical-based contact tracing and ring vaccination models from time to time. Porco et al. have developed a stochastic network model for contact tracing and ring vaccination [28]. At the same time, Kretzschmar et al. have illustrated a stochastic model to constrain the spread of smallpox. The researchers have considered the basic reproduction number (R0) during development and analysis. They have concluded that the ring vaccination could be a successful strategy if infectious cases are quickly diagnosed [29]. Several researchers have recently tried to enlist some ring vaccination strategies to eradicate the MPXV [30], [31]. However, it is necessary to consider the previously developed ring vaccination models when researchers depict the present strategies of ring vaccination.
Fig. 3.
A schematic diagram illustrates the mass vaccination and ring vaccination strategies. It is important to consider three components during ring vaccination which are infected individuals, unvaccinated individuals and the vaccinated individuals.
4.3. Epidemiological pattern to develop the vaccination strategy of MPX
It has been noted that epidemiological pattern is one of the essential criteria for developing the vaccination strategy. Therefore, epidemiological models are an indispensable tool for controlling any disease. Several mathematical models have been used during the COVID-19 pandemic to understand the epidemiological pattern of the disease. Several researchers have intimated that epidemiological models are effective tools to intervene in the COVID-19 pandemic [32], [33], [34]. However, these models might not fit to control the current MPX infection because of the specific age group of current MPX infection. Several recent studies have illustrated that the age group pattern of present MPX infection differs from the previous MPX infection. We have also provided an epidemiological model through a recent work [11]. We also noted from the extensive literature survey that the infected individuals in the UK are 31–43 age group and the 28–61 age group for the USA. However, we found from the USCDC data that the USA is vaccinating those groups of individuals more compared to the 0–17 age group. It might be vital information to develop vaccination strategies. However, more studies are needed to illustrate the epidemiological pattern to establish the vaccination strategy of MPX.
5. Present vaccination statistics and preparedness against the current emergence of the MPXV
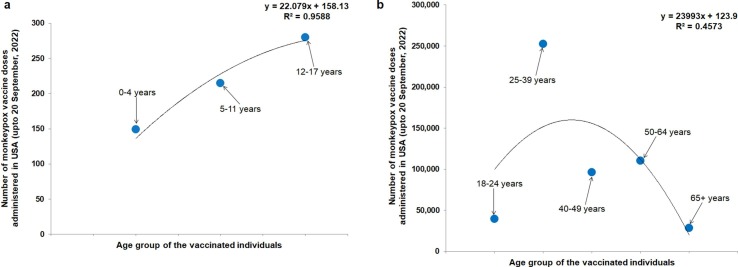
The recent outbreak of the MPX has created an alert in different countries. Although, the emergence of the virus occurred during the last phase of the COVID-19 pandemic. Some countries have started vaccination drives to fight against MPX. However, if we analyze, we can observe two significant developments. First is the rollout of the MPX vaccine in some countries where the suspected individuals or high-risk groups have been vaccinated. Secondly, some countries have acted quickly and firmly to fight against diseases. In the USA, MPX vaccines have been rolled out. It was noted that about 684,980 doses of vaccines were administrated as of September 20, 2022 to suspected individuals. An age-wise MPX vaccination information has been collected from USCDC and plotted (Fig. 4 ). We found that very high vaccine doses were administered in the 18–65+ age group compared to the 0–17 age group. Among the 18–65+ age group, more vaccines were administered among 25–39 old individuals (Fig. 4a and 4b). At the same time, several countries in Europe are preparing to fight against the disease. The countries have ordered several vaccine dosages as preparedness to fight against MPX. Contract manufacturing is one vaccine manufacturing strategy. Several countries are using contract manufacturing for MPXV manufacturing. The countries from European Union and others are using the contract manufacturing order to Bavarian Nordic. The Bavarian Nordic, an MPXV/smallpox manufacturing company, can develop JYNNEOS /IMVAMUNE / IMVANEX. According to a report, the company produced several MPXV vaccine doses for different countries which are as follows 750,000 doses for Canada, 450,000 doses for Australia, 250,000 doses for France, 240,000 doses for Germany, 154,000doses the UK, and 10,000 doses for Israel [35]. At the same time, we have also found from the clinical trial database (clinicaltrial.gov) that several clinical trials have been initiated using smallpox vaccines to understand the safety and efficiency against the MPXV (Table 2 ).
Fig. 4.
The statistical regression plot shows the present vaccine doses administered, by age of MPX vaccine (JYNNEOS Vaccine) in the USA. In the USA, MPX vaccines have been rolled out, and the number of rolled-out vaccines (age-wise) has been plotted. (a) The figure shows the vaccine doses administered in the 0–17 age group (b) The figure shows the vaccine doses administered in the 18–65+ age group. The data is represented up to September 20, 2022, and the information is adapted from CDC, USA. The plots have been depicted using the first vaccine administrated dosage.
Table 2.
List of clinical trials initiated using smallpox vaccines to understand the safety and efficiency against the MPXV. The data has been collected from clinicaltrial.gov.
| Sl. No. | Vaccine name | Clinical trials no. | Status | Sponsoring agency (Company/Institute name) | Description of clinical trials | Remarks |
|---|---|---|---|---|---|---|
|
IMVAMUNE |
NCT02977715 | Phase 3 | CDC, USA Ministry of Public Health, Democratic Republic of the Congo, Bavarian Nordic, Denmark |
It was aninterventional, open label trial of 1600 participants (healthcare personnel) | Two doses of attenuated live virus vaccine received by adult individuals at risk for MPX in the Democratic Republic of the Congo | |
| IMVAMUNE | NCT03745131 | Completed | Public Health England, Bavarian Nordic, Denmark |
It was an observational study of 120 adult, older adult participants (healthcare personnel) | It examine the Ab responses in studied groups associated to control groups for specific MXPV involved in the UK outbreak | |
| Treatment by scabs, blood and pus samples | NCT05058898 | _ | Institut Pasteur, France | It was an observational, case-Control study of 280 participants | Treatment by scabs, blood and pus samples of MXPV confirmed individuals for biological diagnosis at Republic of the Congo | |
| Live Modified Vaccinia Virus Ankara | NCT05522296 | Recruiting | Foundation Fight against AIDS and Infectious Diseases, Spain | Observational, cohort study of 4638 participants for target trial emulation | Evaluate the protection of single dose (0.1 mL) smallpox pre-exposure vaccination against the infection with risk factors for MPX. | |
| JYNNEOS | NCT05512949 | Phase 2 | National Institute of Allergy and Infectious Diseases (NIAID), United States | The interventional, randomized, open-label, non-placebo controlled clinical trial of 210 participants | Access the reactogenicity of modified vaccinia ankara-bavariannordic (MVA-BN) vaccine followed the dose reduction strategies |
6. Conclusion
After 42 years of eradication of smallpox, the fight has again begun for the eradication of the MPX. The recent spread of MPXV informs us that this disease is no longer rare. Now rigorous studies are needed to focus on epidemiology and transmission patterns. At the same time, more studies are needed to unfold the zoonotic hosts of this virus [36]. It has been noted that the infection of the CB clade has increased from 2010 to 2019 [37]. We need to understand which clade is infecting more during the current spread of infections. At the same time, it is also necessary to understand the epidemiological pattern of the virus. Several researchers have tried to analyze the case studies of a recent outbreak pattern in Italy [38], Australia [39], the UK [40], and Portugal [41]. However, we need a clear understanding of the epidemiological pattern of this virus. We have noted that the epidemiological pattern, especially the virulence pattern of human MPX, has variable data in various available reports [3], [11].
We need to develop quick vaccination strategies to fight against the virus. In this direction, our report might be helpful to scientists, ordinary people, and policymakers to understand the vaccine and vaccination status against MPX. However, it is necessary to vaccinate people quickly to fight against the disease. We need to identify high-risk individuals before they are vaccinated. Therefore, pregnant women, children, and elderly adults should be considered before vaccination. However, scientists have suggested quick contact tracing, and ring vaccination to stop the virus's spread [42], [43], [44], [45]. We urge scientists and policymakers to assemble together to form the proper strategies to contain the recent spread of the virus.
Funding
There is not any financial support for this study.
CRediT authorship contribution statement
Chiranjib Chakraborty: Conceptualization, Data curation, Writing – original draft, Supervision. Manojit Bhattacharya: Validation, Data curation. Ashish Ranjan Sharma: Validation. Kuldeep Dhama: Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.Velavan T.P., Meyer C.G. Monkeypox 2022 outbreak: an update. Trop. Med. Int. Health. 2022;27(7):604–605. doi: 10.1111/tmi.13785. [DOI] [PubMed] [Google Scholar]
- 2.Zumla A., Valdoleiros S.R., Haider N., Asogun D., Ntoumi F., Petersen E., Kock R. Monkeypox outbreaks outside endemic regions: scientific and social priorities. Lancet. Infect. Dis. 2022;22(7):929–931. doi: 10.1016/S1473-3099(22)00354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO, Multi-country outbreak of monkeypox, External situation report #6 - 21 September 2022 (2022). https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox--external-situation-report--6---21-september-2022 (Accessed on September 24, 2022).
- 4.Yang Z. Monkeypox: a potential global threat? J. Med. Virol. 2022;94(4):4034–4036. doi: 10.1002/jmv.27884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shchelkunov S., Totmenin A., Safronov P., Mikheev M., Gutorov V., Ryazankina O., Petrov N., Babkin I., Uvarova E., Sandakhchiev L. Analysis of the monkeypox virus genome. Virology. 2002;297(2):172–194. doi: 10.1006/viro.2002.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnus P.V., Andersen E.K., Petersen K.B., Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Pathologica Microbiologica Scandinavica. 1959;46(2):156–176. [Google Scholar]
- 7.Center For Disease. Control, Prevention, Human monkeypox--Kasai Oriental, Zaire, 1996-1997, MMWR. Morbidity and mortality weekly report 46(14) (1997) 304-307. [PubMed]
- 8.Simpson K., Heymann D., Brown C.S., Edmunds W.J., Elsgaard J., Fine P., Hochrein H., Hoff N.A., Green A., Ihekweazu C. Human monkeypox–After 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38(33):5077–5081. doi: 10.1016/j.vaccine.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., Davidson W., Galloway R., Khristova M.L., Reynolds M.G. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86(10):2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 10.Beer E.M., Rao V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl.Trop. Dis. 2019;13(10):e0007791. doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty C., Bhattacharya M., Sharma A.R., Dhama K. Evolution, epidemiology, geographical distribution, and mutational landscape of newly emerging monkeypox virus. GeroScience. 2022;12:1–7. doi: 10.1007/s11357-022-00659-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds M.G., Doty J.B., McCollum A.M., Olson V.A., Nakazawa Y. Monkeypox re-emergence in Africa: a call to expand the concept and practice of One Health. Expert review of anti-infective therapy. 2019;17(2):129–139. doi: 10.1080/14787210.2019.1567330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao A.K. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal. Wkly Rep. 2022;71(22):734–742. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.USFDA, FDA approves first live, non-replicating vaccine to prevent smallpox and monkeypox, FDA Bethesda, MD, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-live-non-replicating-vaccine-prevent-smallpox-and-monkeypox (Acessed on September 13, 2022).
- 15.Petersen B.W., Damon I.K., Pertowski C.A., Meaney-Delman D., Guarnizo J.T., Beigi R.H., Edwards K.M., Fisher M.C., Frey S.E., Lynfield R. Clinical guidance for smallpox vaccine use in a postevent vaccination program. Morbidity and Mortality Weekly Report: Recommendations and Reports. 2015;64(2):1–26. [PubMed] [Google Scholar]
- 16.Metzger W., Mordmueller B.G. Vaccines for preventing smallpox. Cochrane Database of Systematic Reviews (3) 2007:CD004913. doi: 10.1002/14651858.CD004913.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frey S.E. New smallpox vaccines for an ancient scourge. Mo. Med. 2014;111(4):332–336. [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy R.B., Ovsyannikova I.G., Haralambieva I.H., Grill D.E., Poland G.A. Proteomic assessment of humoral immune responses in smallpox vaccine recipients. Vaccine. 2022;40(5):789–797. doi: 10.1016/j.vaccine.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooper J., Thompson E., Wilhelmsen C., Zimmerman M., Ichou M.A., Steffen S., Schmaljohn C., Schmaljohn A., Jahrling P. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 2004;78(9):4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., Nalca A., Hooper J.W., Whitehouse C.A., Schmitz J.E. Smallpox vaccine–induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 21.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., Eisenberg R.J., Hartmann C.J., Jackson D.L., Kulesh D.A. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428(6979):182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 22.Hirao L.A., Draghia-Akli R., Prigge J.T., Yang M., Satishchandran A., Wu L., Hammarlund E., Khan A.S., Babas T., Rhodes L. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J. Infect. Dis. 2011;203(1):95–102. doi: 10.1093/infdis/jiq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marriott K.A., Parkinson C.V., Morefield S.I., Davenport R., Nichols R., Monath T.P. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine. 2008;26(4):581–588. doi: 10.1016/j.vaccine.2007.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usman S., Adamu I.I. Modeling the transmission dynamics of the monkeypox virus infection with treatment and vaccination interventions. J. Appl. Math. Phys. 2017;5(12):2335–2353. [Google Scholar]
- 25.Bankuru S.V., Kossol S., Hou W., Mahmoudi P., Rychtář J., Taylor D. A game-theoretic model of Monkeypox to assess vaccination strategies. PeerJ. 2020;8:e9272. doi: 10.7717/peerj.9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foege W.H. Commentary: smallpox eradication in west and central Africa revisited. Bull. World Health Organ. 1998;76(3):233–235. [PMC free article] [PubMed] [Google Scholar]
- 27.Foege W.H., Millar J.D., Henderson D.A. Smallpox eradication in West and Central Africa. Bull. World Health Organ. 1975;52(2):209–222. [PMC free article] [PubMed] [Google Scholar]
- 28.Porco T.C., Holbrook K.A., Fernyak S.E., Portnoy D.L., Reiter R., Aragón T.J. Logistics of community smallpox control through contact tracing and ring vaccination: a stochastic network model. BMC Public Health. 2004;4(1):1–20. doi: 10.1186/1471-2458-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kretzschmar M., Van den Hof S., Wallinga J., Van Wijngaarden J. Ring vaccination and smallpox control. Emerg. Infect. Dis. 2004;10(5):832–841. doi: 10.3201/eid1005.030419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R.Sah, A. Abdelaal, A. Asija, S. Basnyat, Y.R. Sedhai, S. Ghimire, S. Sah, D.K. Bonilla-Aldana, A.J. Rodriguez-Morales. Monkeypox virus containment: the application of ring vaccination and possible challenges. J. Travel Med. 29(6) (2022) :taac085. [DOI] [PubMed]
- 31.O. Choudhary, M. Fahrni, A. Saied, H. Chopra. Ring vaccination for monkeypox containment: Strategic implementation and challenges–Correspondence. International journal of surgery (London, England) 105:(106873) (2022) doi: 10.1016/j.ijsu.2022.106873. [DOI] [PMC free article] [PubMed]
- 32.Thompson R.N. Epidemiological models are important tools for guiding COVID-19 interventions. BMC Med. 2020;18(1):1–4. doi: 10.1186/s12916-020-01628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry K., Willenbring K., Crossley K. Human immunodeficiency virus antibody testing: a description of practices and policies at US infectious disease—teaching hospitals and Minnesota hospitals. JAMA. 1988;259(12):1819–1822. doi: 10.1001/jama.259.12.1819. [DOI] [PubMed] [Google Scholar]
- 34.C. Chakraborty, A.R. Sharma, M. Bhattacharya, B Mallik, S. S. Nandi, S-S Lee. Comparative genomics, evolutionary epidemiology, and RBD-hACE2 receptor binding pattern in B. 1.1. 7 (Alpha) and B. 1.617. 2 (Delta) related to their pandemic response in UK and India. Infection, Genetics and Evolution 101 (2022):105282. [DOI] [PMC free article] [PubMed]
- 35.NPR. (2022) Is there enough monkeypox vaccine to go around? Maybe yes, more likely no https://www.npr.org/sections/goatsandsoda/2022/08/16/1117658734/the-who-wants-to-help-low-income-nations-combat-the-monkeypox-outbreak#:∼:text=For%20instance%20Canada%20has%20contracted,Kingdom%20154%2C000%3B%20Israel%2010%2C000%20doses. (Accessed on September 24, 2022).
- 36.Chakraborty C., Bhattacharya M., Nandi S.S., Mohapatra R.K., Dhama K., Agoramoorthy G. Appearance and re-appearance of zoonotic disease during the pandemic period: Long-term monitoring and analysis of zoonosis is crucial to confirm the animal origin of SARS-CoV-2 and monkeypox virus. Veterinary Quarterly. 2022;42(2):119–124. doi: 10.1080/01652176.2022.2086718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen J. Global outbreak puts spotlight on neglected virus. Science (New York, NY) 2022;376(6597):1032–1033. doi: 10.1126/science.add2701. [DOI] [PubMed] [Google Scholar]
- 38.Jin X., Lian J.-S., Hu J.-H., Gao J., Zheng L., Zhang Y.-M., Hao S.-R., Jia H.-Y., Cai H., Zhang X.-L. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammerschlag Y., MacLeod G., Papadakis G., Sanchez A.A., Druce J., Taiaroa G., Savic I., Mumford J., Roberts J., Caly L. Monkeypox infection presenting as genital rash, Australia, May 2022. Eurosurveillance. 2022;27(22):2200411. doi: 10.2807/1560-7917.ES.2022.27.22.2200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vivancos R., Anderson C., Blomquist P., Balasegaram S., Bell A., Bishop L., Brown C.S., Chow Y., Edeghere O., Florence I. Community transmission of monkeypox in the United Kingdom, April to May 2022. Eurosurveillance. 2022;27(22):2200422. doi: 10.2807/1560-7917.ES.2022.27.22.2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duque M.P., Ribeiro S., Martins J.V., Casaca P., Leite P.P., Tavares M., Mansinho K., Duque L.M., Fernandes C., Cordeiro R. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance. 2022;27(22):2200424. doi: 10.2807/1560-7917.ES.2022.27.22.2200424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho C.T., Wenner H.A. Monkeypox virus. Bacteriol. Rev. 1973;37(1):1–18. doi: 10.1128/br.37.1.1-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakraborty S., Mohapatra R.K., Chandran D., Alagawany M., Sv P., Islam M.A., Chakraborty C., Dhama K. Monkeypox vaccines and vaccination strategies: Current knowledge and advances. An update – correspondence. Int. J. Surg. 2022;05(106869) doi: 10.1016/j.ijsu.2022.106869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattacharya M., Dhama K., Chakraborty C. Recently spreading human monkeypox virus infection and its transmission during COVID-19 pandemic period: A travelers' prospective. Travel Med. Infect. Dis. 2022;49(102398) doi: 10.1016/j.tmaid.2022.102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohapatra R.K., Tuli H.S., Sarangi A.K., Chakraborty S., Chandran D., Chakraborty C., Dhama K. Unexpected sudden rise of human monkeypox cases in multiple non-endemic countries amid COVID-19 pandemic and salient counteracting strategies: another potential global threat? Int. J. Surg. 2022;103(106705) doi: 10.1016/j.ijsu.2022.106705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.