Abstract
The incidence of the avian influenza virus in late 2016, different genotypes of highly pathogenic avian influenza (HPAI) H5N8 clade 2.3.4.4b have been reported among different domestic and wild bird species. The virus became endemic in the poultry population, causing a considerable economic loss for the poultry industry. This study screened 5 ostrich farms suffering from respiratory signs and mortality rate of the avian influenza virus. A flock of 60-day-old ostriches with a mortality of 90% suffered from depression, loss of appetite, dropped production, and oculo-nasal discharges, with bleeding from natural orifices as a vent. This flock was found positive for avian influenza virus and subtypes as HPAI H5N8 virus. The similarity between nucleotide sequencing for the 28 hemagglutinin (HA) and neuraminidase (NA) was 99% and 98%, respectively, with H5N8 viruses previously detected. The PB2 encoding protein harbor a unique substitution in mammalian marker 627A, which has not been recorded before in previously sequenced H5N8 viruses. Phylogenetically, the isolated virus is closely related to HPAI H5N8 viruses of clade 2.3.4.4b. The detection of the HPAI H5N8 virus in ostrich is highly the need for continuous epidemiological and molecular monitoring of influenza virus spread in other bird species, not only chickens. Ostrich should be included in the annual SunAlliance, for the detection of avian influenza.
Key words: H5N8, hemagglutinin, neuraminidase, PB2, ostrich
Abbreviations: HPAI, highly pathogenic avian influenza; AIV, avian influenza virus; HA, hemagglutinin; NA, neuraminidase; LPAIVs, low pathogenic avian influenza viruses; SPF, specific pathogen-free; ECE, embryonated chicken eggs; NDV, newcastle disease virus; IBV, infectious bronchitis virus; PCR, polymerase chain reaction; NCBI, National Center for Biotechnology Information
Introduction
Highly pathogenic avian influenza (HPAI) circulates in several bird species worldwide viruses, causing a substantial negative impact on the poultry industry and threatening public health via zoonotic influenza subtypes (Dhingra et al., 2018; Abd El-Hack et al., 2022). In the last decade, HPAI of H5 subtypes (e.g., H5N1 and H5N8) have been the most circulating HPAI viruses in wild birds and domestic poultry (Naguib et al., 2019).
There are numerous genetically different clades (0–9) and subclades circulating. The HP H5 clade 2.3.4 was discovered in China in 2008 (Gu et al., 2011) and has since evolved into several clades, including 2.3.4.4, in China in 2013 (Zhou et al., 2016). By reassortment with other Low Pathogenic Avian Influenza Viruses (LPAIVs) circulating in wild birds, HPAI viruses with a HA of clade 2.3.4.4 have acquired many neuraminidases (NAs), including N1, N2, N5, N6, and N8. They have further diversified into 8 genetically different groups (a–h) (Kandeil et al., 2022).
Since the emergence of HPAI H5N8 virus of clade 2.3.4.4 in the live bird markets in China (Lee et al., 2014), subsequent spread into several countries was reported (Kanehira et al., 2015; Marchenko et al., 2015). The virus was disseminated to Europe and North America by migratory birds by the end of 2014 (Bouwstra et al., 2015; Hanna et al., 2015; Pasick et al., 2015).
Following their spread via active reassortment, the 2.3.4.4b H5N8 viruses evolved further (Chang et al., 2022). Among the bird species, ostrich has shown susceptibility to the avian influenza virus (AIV) and is associated with mortality up to 60% (Verwoerd, 2000).
Many avian influenza outbreaks were recorded in ostriches globally, including H7N1, H5N9, H9N2, H6N8, H5N3, H5N1, H5N2, and H5N8 (Yang et al., 2010; Abolnik et al., 2012; Hagag et al., 2014; Hemida et al., 2019). However, the HPAI H5 has spread significantly in ostriches, with zoonotic potential and socioeconomic consequences (OIE, 2009). In January 2014, HPAI H5N8 (A/ostrich/Korea/H829/2014) was detected in ostriches in South Korea belonged to clade 2.3.4.4, which is closely related to three South Korean H5N8 viruses isolated from ducks and wild birds (Kim et al., 2016), Later in 2017, outbreaks with HPAI H5N8 viruses were reported in commercial ostriches in South Africa (Valley‐Omar et al., 2020).
Although the ostrich industry has been viewed recently as an economic and a viable agricultural investment (Hagag et al., 2014), there is not enough data on the endemicity of AIV concerning them. Since the introduction of HPAI H5N1 viruses in 2006, only 2 cases have been reported in ostrich (Hagag et al., 2014).
The emergence of the virus of clade 2.3.4.4 HPAI H5N8 in November 2016 and large spreading even in wild birds and chickens (Naguib et al., 2019; Tarek et al., 2021), there was no detection for this virus in ostrich due to the exclusion of this species from the surveillance.
During our narrow passive surveillance in this study, we isolated the HPAI H5N8 for the first time in ostrich. So, we have tried in this study to clarify the identity of this isolated virus genetically and pathologically.
MATERIALS AND METHODS
Sampling and Pathological Examination
In 2021, 5 ostrich flocks were tested for avian influenza viruses. Geographically, 3 flocks have located in Qaliobia, one from Cairo and one from Menofia. All the flocks suffered from clinical signs and pathognomonic lesions characteristic of avian influenza suspicion, including loss of appetite, drop in production, oculo-nasal discharges, depression, and diarrhea, with different degrees of high mortalities. The age of birds ranged from young chicks at 2 wk old to breeders at 3 to 5 yr old. The examined birds died recently and were transported to the laboratory on ice; the biopsy, real-time RT-qPCR, virus isolation and sequencing were done in Reference Laboratory for Veterinary Quality Control on Poultry Production, Animal Health Research Institute.
Virus Detection and Isolation
Real-time RT-qPCR was used to detect and genotype viruses. Viral RNA was extracted from samples according to the instructions in the QIAamp viral RNA mini kit (Qiagen, Germany, GmbH). QuantiTect RT Mix was used for real-time RT-PCR amplification in a final volume of 25 uL, following the kit's instructions. The primers and probes that have been used for H5N8 real-time RT-qPCR subtyping (Löndt et al., 2008; Hoffmann et al., 2016), were provided by Metabion (Germany). Virus was isolated on specific pathogen-free (SPF) embryonated chicken eggs (ECE) at 9 to 11 d old embryos, following the OIE manual (OIE, 2009), then retested for H5N8 subtype and other viral pathogens including H9N2, NDV, and IBV (Spackman et al., 2002; Wise et al., 2004; Meir et al., 2010; Shabat et al., 2010).
Genetic and Phylogenetic Characterization
The isolated strain's HA, NA, and PB2 genes have been amplified by Easyscript one-step RT-PCR kit (Trans, China), using specific primers for each gene (Yehia et al., 2018). The reactions have been applied following the kit's instructions. Then, the final PCR products were separated by gel electrophoresis and a gel documentation system determined the results.
The positive PCR reactions were purified by the Qiaquick gel extraction kit (Qiagen, USA), following the kit's instructions, then the sequence was applied to the purified products following the instructions of the Bigdye terminator sequencing kit (Applied Biosystems, USA); the sequencing products have been purified to remove the excess dye by DyeEx pure kit (Qiagen, USA), following the kit instruction. Finally, the purified sequence reactions have been loaded in a plate for reading in genetic sequencer 3500 series (Applied Biosystem, Waltham, MA).
The nucleotide sequences of complete HA and NA genes and partial sequence of PB2 gene have been uploaded to Genbank (NCBI) by BLAST to identify the sequenced genes. Bioedit software has been used to create alignments for each gene containing representative sequences for the different related clades. MEGA 11 software has created the phylogenetic trees for the targeted genes to find out the genetic evolution and relationship with other strains. The phylogenetic trees have been constructed by neighbor joining logarithm with maximum composite likelihood substation model (Gamma+1) and bootstrapping 1000 iterations. N-Glycosylation sites have been detected using by N-glyc server https://openebench.bsc.es/tool/nglyc.
RESULTS
Pathological Analysis
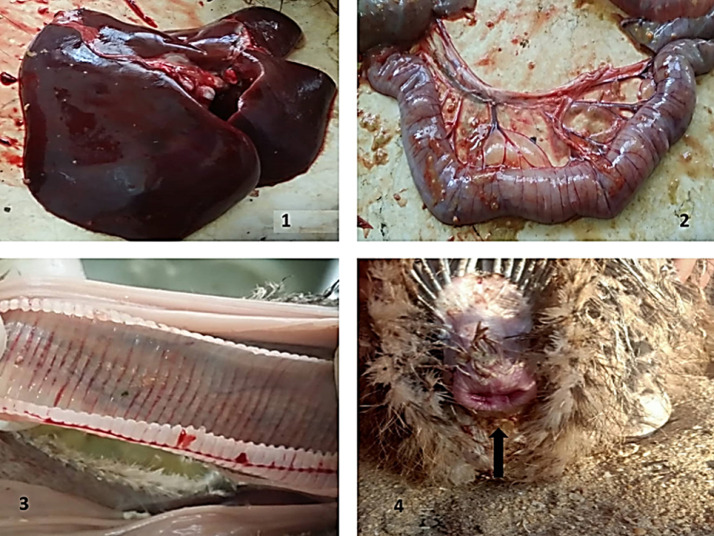
The characteristic pathological lesions for avian influenza have been observed in the internal organs of the dead birds. Hemorrhage in intestine and trachea with congested mucosa, bleeding from natural orifices as vent, congested liver, as elaborated in Figure 1
Figure 1.
Pathological findings in the suspected case. 1) congested liver, 2) congested intestine with hemorrhage, 3) hemorrhage in tracheal mucosa, and 4) bleeding from the vent (elaborated with black arrow).
Genetic Characterization of HA, NA, and PB2 Genes
The generated sequences in this study were uploaded to the GenBank (NCBI) under accession numbers: HA: ON430504, NA gene: ON430505, and PB2 gene: ON430506. Ostrich originated isolate showed a similarity of 99% with the most recent circulating strains from 2019. However, it possessed less similarity to the ostrich originated viruses that have been circulated in Korea, Saudi Arabia, and South Africa, which are H5N8 of clade 2.3.4.4; as it has identity percent = 96% with the South African and Saudi Arabian viruses, which are genetically related to clade 2.3.4.4b, while the identity percent was 94% with the Korean viruses that related to clade 2.3.4.4a.
A substitution of R169Q on HA molecule is similar to the viruses of ostrich origin that have been isolated from Korea and KSA. A substitution of N236D was specific for the Egy/Ostrich/2021and the strains related to the clade 2.3.4.4b of European II-2020, this site is located adjacent to the left edge of the receptor binding sites. Table 1 compares the clades 2.3.4.4a, 2.3.4.4b and recently circulating viruses in receptor binding sites, cleavage sites, and antigenic sites.
Table 1.
Comparison between the amino acids of Hemagglutinin (HA) of the Egypt-ostrich-2021 and subclades of 2.3.4.4 H5N8.
| Antigenic sites |
Cleavage sites | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor binding sites |
Antigenic site A |
Antigenic site B |
Antigenic site E |
|||||||||||||
| 103 | 129 | 186 | 221 | 222 | 224 | 133 | 140 | 141 | 154 | 156 | 184 | 71 | 83 | 86 | ||
| 2.3.4.4a | H | L | E | G | Q | G | A | A | S | N | A | A | I | A | A | RERRRKRGLF |
| 2.3.4.4b | H | L | E | G | Q | G | A | T | P | N | A | A | I | A/T | A/V | REKRRKRGLF |
| EuroII2020 | H | L/S | E | G | Q | G | A | A | P | N | A | A | I | A | A | REKRRKRGLF |
| Egy-ost-2021 | H | L | E | G | Q | G | A | A | P | N | A | A | I | A | A | REKRRKRGLF |
The nucleotide sequence of the NA gene of ostrich/2021 was similar to the most recent circulating H5N8 with 98% identity and the European viruses of subclade II of clade 2.3.4.4b in 2020 and Chinese strains that circulating in 2020. However, it was highly similar to the ostrich originated viruses in South Africa and KSA (clade 2.3.4.4b) with identity% = 95% to 92%, with lower identity with Korean Ostrich originated viruses (clade 2.3.4.4a) with % identity = 90%. ostrich/2021 lost the glycosylation site 293NWTG and the viruses of the EuroII-2020 subclade, as shown in Table 2.
Table 2.
Comparison between the amino acids of Neuraminidase (NA) of the Egypt-ostrich-2021 and subclades of 2.3.4.4 H5N8.
| Mutation along neuraminidase |
Glycosylation sites |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 27 | 79 | 88 | 89 | 106 | 201 | 245 | 265 | 295 | 359 | 46 | 54 | 67 | 84 | 144 | 293 | 398 | |
| 2.3.4.4a | V | E | P | I | V | V | A | T | T | V | NGTV | NETV | NTSV | NNTE | NGTV | NWTG | NWSG |
| 2.3.4.4b | V | D/E | P | I/L/V | V | V | A | T | T | V | —– | NETV | NTSV | NNTE | NGTV | NWTG | NWSG |
| EuroII2020 | L | E | P | I | I | I | S | A | M | M | —— | NETV | NTSV | NNTE | NGTV | —- | — |
| Egy-ost-2021 | L | E | P | I | I | I | S | A | M | M | —— | NETV | NTSV | NNTE | NGTV | —- | NWSG |
Also, there is no change in the amino acid sequences of the 2 regions responsible for the NA hemadsorption activity (361RTISRTSRSGFE372 and 390RQVVVDNLNWSGYSGS404). Additionally, there is no deletion in the stalk region. The PB2 similarity between the circulating H5N8 viruses during 2018–2021 was 98.8%, and the recently Chines and Nigerian strains that have been isolated in 2020–2021. The amino acid sequence of partial PB2 of Egy/ostrich/2021 harbored novel mutations as A627, R660, and M697.
Phylogenetic Analysis of the Ostrich H5N8
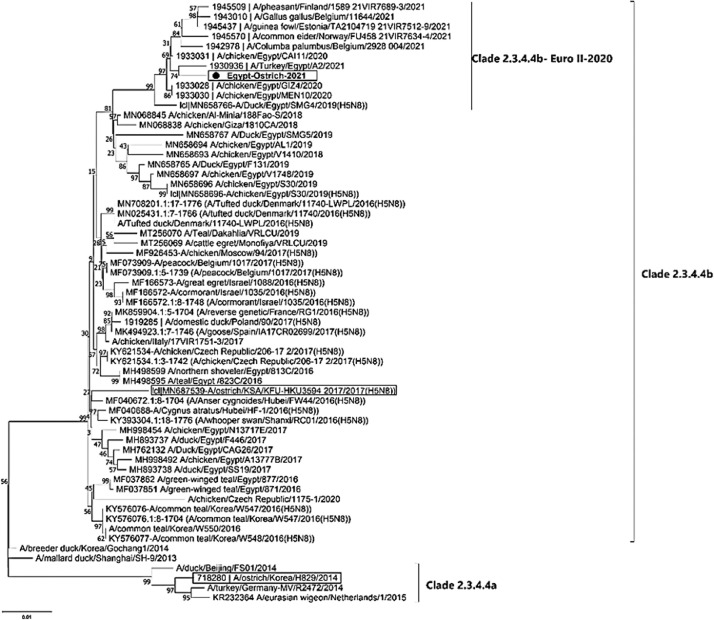
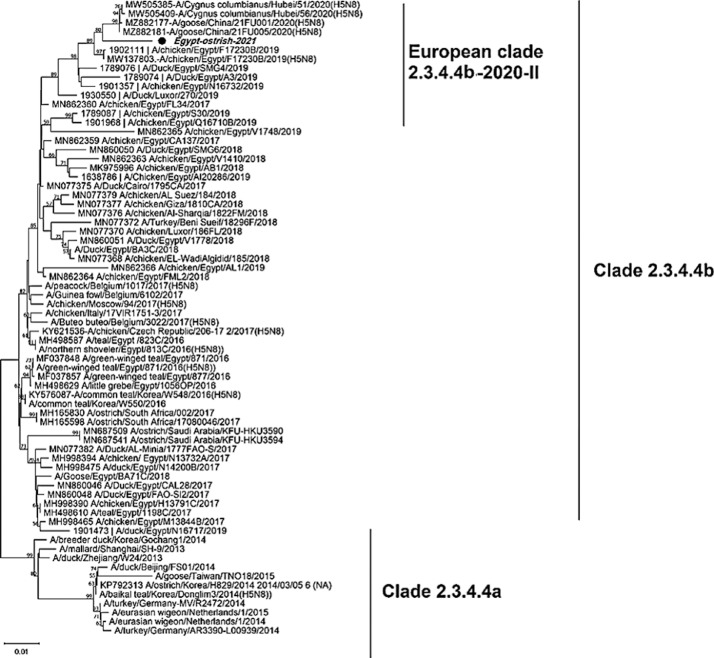
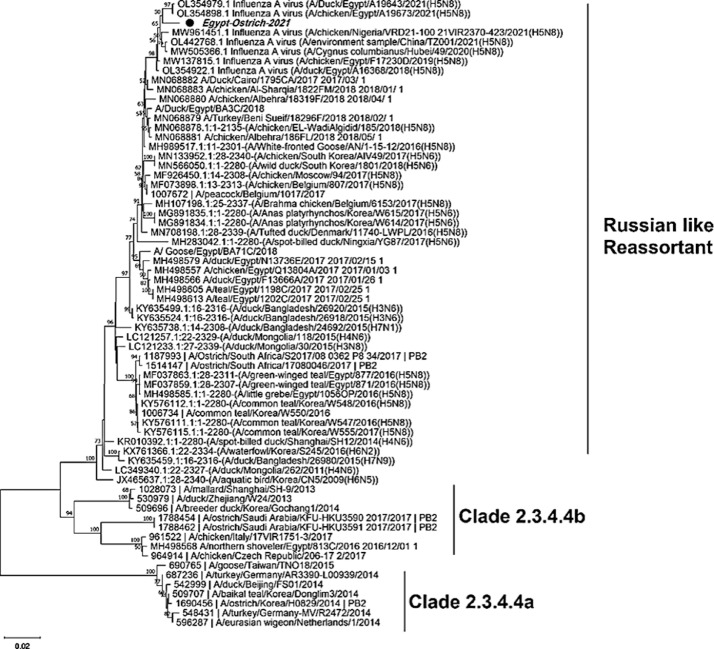
The phylogenetic tree of HA gene revealed the ostrich virus related to the European sub-clade II- 2.3.4.4b of 2020 and Nigerian viruses that have been identified in 2021, that also enclose the recent circulating viruses (Figure 2). As well as, the phylogenetic analysis of NA gene elaborated that the ostrich-2021 belongs to the same European subclade II-2020 of 2.3.4.4b (Figure 3). While, the phylogenetic tree of the partial PB2 gene showed that the virus was closely related to the clade 2.3.4.4b of the Russian reassortant H5N8 viruses, which included viruses from Egypt, India, and China since 2016 (Figure 4).
Figure 2.
Phylogenetic tree of HA gene of 80 nucleotide sequence for H5N8 viruses represent the clade 2.3.4.4a and 2.3.4.4b. The Ostrich originated strain (Egy/ostrich/2021) in bold italic font labeled with a black circle; it has related to the clade 2.3.4.4b of European strains II-2020. All the Ostrich originated viruses were enclosed in a square.
Figure 3.
Phylogenetic tree of NA gene of 70 nucleotide sequence for H5N8 viruses represent the clade 2.3.4.4a and 2.3.4.4b. The Ostrich originated strain (Egy/ostrich/2021) in bold italic font labeled with a black circle; it has related to the clade 2.3.4.4b of European strains II-2020.
Figure 4.
Phylogenetic tree of HA gene of 65 nucleotide sequence for H5N8 viruses represent the clade 2.3.4.4a, 2.3.4.4b, European reassortants and Russian reassortants. The Ostrich originated strain (Egy/ostrich/2021) in bold italic font labeled with a black circle; it has related to the Russian reassortants and most circulating Egyptian viruses in 2020.
DISCUSSION
Respiratory affections contribute a serious hazard causing severe economic losses to the poultry industry (Setta et al., 2018; Marouf et al., 2020,2022). There was no record of avian influenza infection in ostrich since the isolation of classic HPAI H5N1 2.2.1 in 2010 (Hagag et al., 2014; Abolnik et al., 2019, 2021). However, the new avian influenza virus isolated from ostrich belongs genetically to the HPAI H5N8 clade 2.3.4.4b viruses that have been circulated since 2016–2017 (Kandeil et al., 2017; Selim et al., 2017 ). First, H5N8 clade 2.3.4.4b have been spilled over from migratory wild birds and then transmitted to different domestic birds like chicken, duck, and goose in the commercial sector and backyard rearing, with multiple introductions of genetic reassortant H5N8 viruses (Yehia et al., 2018; Shriner & Root, 2020).
In Menofia governorate, a herd of 60-day-old ostriches kept next to backyard chickens had clinical indications of avian influenza. Because backyard flocks are known to be major connectors for virus spillover (Naguib et al., 2019), it was assumed that the isolated virus in ostrich had been spread across from nearby infected backyard birds (Abolnik et al., 2016).
Here we have studied the genetic variations in the HA, NA, and PB2 genes of the focal isolate of ostrich. The HA gene has been closely related to the most recent circulating H5N8 viruses since 2019, which were found to belong to the European subclade-II of 2.3.4.4b that have been isolated since 2020 (Tarek et al., 2021).The 6 possible glycosylation sites at locations 10NNST13, 23NVTV26, 165NNTN168, 286NSSM289, 483NGTY486, and 542NGSL545 have been distinctive of H5N8 viruses since their first introduction (Kandeil et al., 2017), have remained unchanged. The receptor-binding site (RBS) promotes the development of human viruses from avian origins and is important for receptor specificity (Eggink et al., 2020). Within the RBS, all H5N8 isolates showed Q222 and G224 (H5 numbering), indicating that avian cell-surface receptors preferentially bind to sialic acid coupled to galactose by 2,3-a-linkages (Sia 2,3-a-Gal) (Kandeil et al., 2017; Yehia et al., 2018).
In comparison to Egyptian strains, there is no difference in oseltamivir resistance markers in the NA gene. The major marker at amino acid residue I314/312V (N2/N8 numbering) substitution (Orozovic et al., 2011; Kandeil et al., 2017), as well as the stability of additional oseltamivir resistant molecular markers (I117V, E119V, D198N, H274Y, R292K, and N294S). Furthermore, zanamivir resistance markers indicated no substitutions among Egyptian H5N8 viruses, including V116A, R118K, E119G/A/D, Q136K, D151E, R152K, R224K, E276D, R292K, and R371K by N2 numbering (Orozovic et al., 2011). 54NETV57, 67NTSV70, 84NNTE87, 144NGTV147, 293NWTG296, 398NWSG401 are N-linked glycosylation sites in clade 2.3.4.4b. Ostrich-2021, like the viruses of the EuroII-2020 subclade to which it belonged, lacked the glycosylation site 293NWTG296.
The PB2 protein did not show E627K or D701N substitution mutations, which are associated with mammalian host adaptation and virulence (Gabriel et al., 2013; Czudai-Matwich et al., 2014). Egypt-ostrich-2021 possesses a unique substitution in amino acid residue E627A, which has not been recorded before, contrary to the other H5N8 viruses with amino acid D701. Regarding the importance of this residue in mammalian transmission, it is strongly recommended to be studied in a further pathogenic study.
CONCLUSIONS
The predominant avian influenza subtype is now HPAI H5N8. Ostrich were not included in the annual SunAlliance, hence there was a shortage in detecting avian influenza in these birds. It is advised to regularly check flocks of ostriches for avian influenza in light of this study. Essentially, it has emerged as a significant meat supplier.
Acknowledgments
The authors would like to thank Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R318), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, and also, the authors would like to thank Dr. Mahmoud Naguib, Zoonosis Science Centre, Dep. of Medical Biochemistry and Microbiology, Uppsala University, Sweden, for reviewing the manuscript and his valuable remarks.
Disclosures
The authors disclose any financial and personal relationships with other people or organizations that might inappropriately influence or bias this work.
References
- Abd El-Hack M.E., El-Saadony M.T., Alqhtani A.H., Swelum A.A., Salem H.M., Elbestawy A.R., El-Tarabily K.A. The relationship among avian influenza, gut microbiota and chicken immunity: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abolnik C., Olivier A.J., Grewar J., Gers S., Romito M. Molecular analysis of the 2011 HPAI H5N2 outbreak in ostriches, South Africa. Avian Dis. 2012;56:865–879. doi: 10.1637/10171-041012-Reg.1. [DOI] [PubMed] [Google Scholar]
- Abolnik C., Olivier A., Reynolds C., Henry D., Cumming G., Rauff D., Romito M., Petty D., Falch C. Susceptibility and status of avian influenza in ostriches. Avian Dis. 2016;60:286–295. doi: 10.1637/11110-042815-Reg. [DOI] [PubMed] [Google Scholar]
- Abolnik C., Strydom C., Landman D., Pieterse R. Identification of bacteria in the tracheal swabs of farmed ostriches and their effect on the viability of influenza A virus. J. Vet. Diagn. Invest. 2021;33:1089–1095. doi: 10.1177/10406387211034483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abolnik C., Pieterse R., Peyrot B.M., Choma P., Phiri T.P., Ebersohn K., van Heerden C.J., Vorster A.A., van der Zel G., Geertsma P.J., Laleye A.T., Govindasamy K., Rauff D.L. The incursion and spread of highly pathogenic avian influenza H5N8 clade 2.3. 4.4 within South Africa. Avian Dis. 2019;63:149–156. doi: 10.1637/11869-042518-Reg.1. [DOI] [PubMed] [Google Scholar]
- Bouwstra R., Heutink R., Bossers A., Harders F., Koch G., Elbers A. Full-genome sequence of influenza A (H5N8) virus in poultry linked to sequences of strains from Asia, the Netherlands, 2014. Emerg. Infect. Dis. 2015;21:872. doi: 10.3201/eid2105.141839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N., Zhang C., Mei X., Du F., Li J., Zhang L., Du H., Yun F., Aji D., Shi W. Novel reassortment 2.3. 4.4 b H5N8 highly pathogenic avian influenza viruses circulating in Xinjiang, China. Prev. Vet. Med. 2022;199 doi: 10.1016/j.prevetmed.2021.105564. [DOI] [PubMed] [Google Scholar]
- Czudai-Matwich V., Otte A., Matrosovich M., Gabriel G., Klenk H.-D. PB2 mutations D701N and S714R promote adaptation of an influenza H5N1 virus to a mammalian host. J. Virol. 2014;88:8735–8742. doi: 10.1128/JVI.00422-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra M.S., Artois J., Dellicour S., Lemey P., Dauphin G., Von Dobschuetz S., Van Boeckel T.P., Castellan D.M., Morzaria S., Gilbert M. Geographical and historical patterns in the emergences of novel highly pathogenic avian influenza (HPAI) H5 and H7 viruses in poultry. Front. Vet. Sci. 2018;5:84. doi: 10.3389/fvets.2018.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggink D., Spronken M., van der Woude R., Buzink J., Broszeit F., McBride R., Pawestri H.A., Setiawaty V., Paulson J.C., Boons G.-J. Phenotypic effects of substitutions within the receptor binding site of highly pathogenic avian influenza H5N1 virus observed during human infection. J. Virol. 2020;94 doi: 10.1128/JVI.00195-20. e00195-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel G., Czudai-Matwich V., Klenk H.-D. Adaptive mutations in the H5N1 polymerase complex. Virus Res. 2013;178:53–62. doi: 10.1016/j.virusres.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Gu M., Liu W., Cao Y., Peng D., Wang X., Wan H., Zhao G., Xu Q., Zhang W., Song Q. Novel reassortant highly pathogenic avian influenza (H5N5) viruses in domestic ducks, China. Emerg. Infect. Dis. 2011;17:1060. doi: 10.3201/eid1706.101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagag N.M., Arafa A., El-Hussieny M.H., Aly M.M., El-Sanousi A.A., Shalaby M.A. Molecular characterization of highly pathogenic Avian influenza H5N1 in Ostrich. Int. J. Virol. 2014;10:103–111. [Google Scholar]
- Hanna A., Banks J., Marston D.A., Ellis R.J., Brookes S.M., Brown I.H. Genetic characterization of highly pathogenic avian influenza (H5N8) virus from domestic ducks, England, November 2014. Emerg. Infect. Dis. 2015;21:879. doi: 10.3201/eid2105.141954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida M.G., Chu D., Abdelaziz A., Alnaeem A., Chan S.M.S., Peiris M. Molecular characterisation of an avian influenza (H5N8) outbreak in backyard flocks in Al Ahsa, Eastern Saudi Arabia, 2017–2018. Vet. Rec. Open. 2019;6 doi: 10.1136/vetreco-2019-000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B., Hoffmann D., Henritzi D., Beer M., Harder T.C. Riems influenza a typing array (RITA): an RT-qPCR-based low density array for subtyping avian and mammalian influenza a viruses. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep27211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeil A., Kayed A., Moatasim Y., Webby R.J., McKenzie P.P., Kayali G., Ali M.A. Genetic characterization of highly pathogenic avian influenza A H5N8 viruses isolated from wild birds in Egypt. J. Gen. Virol. 2017;98:1573. doi: 10.1099/jgv.0.000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeil A., Moatasim Y., El Taweel A., El Sayes M., Rubrum A., Jeevan T., McKenzie P.P., Webby R.J., Ali M.A., Kayali G. Genetic and antigenic characteristics of highly pathogenic avian influenza A (H5N8) viruses circulating in domestic poultry in Egypt, 2017–2021. Microorganisms. 2022;10:595. doi: 10.3390/microorganisms10030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehira K., Uchida Y., Takemae N., Hikono H., Tsunekuni R., Saito T. Characterization of an H5N8 influenza A virus isolated from chickens during an outbreak of severe avian influenza in Japan in April 2014. Arch. Virol. 2015;160:1629–1643. doi: 10.1007/s00705-015-2428-9. [DOI] [PubMed] [Google Scholar]
- Kim H.-R., Kwon Y.-K., Lee Y.-J., Kang H.-M., Lee E.-K., Song B.-M., Jung S.-C., Lee K.-H., Lee H.-K., Baek K.-H. Ostrich (Struthio camelus) infected with H5N8 highly pathogenic avian influenza virus in South Korea in 2014. Avian Dis. 2016;60:535–539. doi: 10.1637/11357-122315-CaseR. [DOI] [PubMed] [Google Scholar]
- Lee Y.-J., Kang H.-M., Lee E.-K., Song B.-M., Jeong J., Kwon Y.-K., Kim H.-R., Lee K.-J., Hong M.-S., Jang I. Novel reassortant influenza A (H5N8) viruses, South Korea, 2014. Emerg. Infect. Dis. 2014;20:1087. doi: 10.3201/eid2006.140233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löndt B.Z., Nunez A., Banks J., Nili H., Johnson L.K., Alexander D.J. Pathogenesis of highly pathogenic avian influenza A/turkey/Turkey/1/2005 H5N1 in Pekin ducks (Anas platyrhynchos) infected experimentally. Avian Pathol. 2008;37:619–627. doi: 10.1080/03079450802499126. [DOI] [PubMed] [Google Scholar]
- Marchenko V.Y., Susloparov I.M., Kolosova N.P., Goncharova N.I., Shipovalov A.V., Durymanov A.G., Ilyicheva T.N., Budatsirenova L.V., Ivanova V.K., Ignatyev G.A. Influenza A (H5N8) virus isolation in Russia, 2014. Arch. Virol. 2015;160:2857–2860. doi: 10.1007/s00705-015-2570-4. [DOI] [PubMed] [Google Scholar]
- Marouf S., Moussa I.M., Salem H., Sedeik M., Elbestawy A., Hemeg H.A., Dawoud T.M., Mubarakb A.S., Mahmouda H., Alsubki R.A., Bahkali Ali H. A picture of Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Egypt: phenotypic and genotypic characterization. J. King Saud Univ. Sci. 2020;32:2263–2268. [Google Scholar]
- Marouf S., Khalf M.A., Alorabi M., El-Shehawi A.M., El-Tahan A.M., Abd El-Hack M.E., El-Saadony M.T., Salem H.M. Mycoplasma gallisepticum: a devastating organism for the poultry industry in Egypt. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir R., Maharat O., Farnushi Y., Simanov L. Development of a real-time TaqMan® RT-PCR assay for the detection of infectious bronchitis virus in chickens, and comparison of RT-PCR and virus isolation. J. Virol. Methods. 2010;163:190–194. doi: 10.1016/j.jviromet.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib M.M., Verhagen J.H., Samy A., Eriksson P., Fife M., Lundkvist Å., Ellström P., Järhult J.D. Avian influenza viruses at the wild-domestic bird interface in Egypt. Infect. Ecol. Epidemiol. 2019;9 doi: 10.1080/20008686.2019.1575687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE. 2009. [OIE] World Organisation for Animal Health. (2009). Chapter 2.3. 4. Avian influenza. Manual of diagnostic tests and vaccines for terrestrial animals [Internet].
- Orozovic G., Orozovic K., Lennerstrand J., Olsen B. Detection of resistance mutations to antivirals oseltamivir and zanamivir in avian influenza A viruses isolated from wild birds. PLoS One. 2011;6:e16028. doi: 10.1371/journal.pone.0016028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasick J., Berhane Y., Joseph T., Bowes V., Hisanaga T., Handel K., Alexandersen S. Reassortant highly pathogenic influenza A H5N2 virus containing gene segments related to Eurasian H5N8 in British Columbia, Canada, 2014. Sci. Rep. 2015;5:1–4. doi: 10.1038/srep09484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim A.A., Erfan A.M., Hagag N., Zanaty A., Samir A.-H., Samy M., Abdelhalim A., Arafa A.-S.A., Soliman M.A., Shaheen M. Highly pathogenic avian influenza virus (H5N8) clade 2.3. 4.4 infection in migratory birds, Egypt. Emerg. Infect. Dis. 2017;23:1048. doi: 10.3201/eid2306.162056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setta A., Salem H.M., Elhady M., El-Hussieny A., Arafa A.A. Molecular and genetic characterization of infectious bronchitis viruses isolated from commercial chicken flocks in Egypt between 2014 and 2016. J. World Poult. Res. 2018;8:01–08. [Google Scholar]
- Shabat M.Ben, Meir R., Haddas R., Lapin E., Shkoda I., Raibstein I., Perk S., Davidson I. Development of a real-time TaqMan RT-PCR assay for the detection of H9N2 avian influenza viruses. J. Virol. Methods. 2010;168:72–77. doi: 10.1016/j.jviromet.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Shriner S.A., Root J.J. A review of avian influenza A virus associations in synanthropic birds. Viruses. 2020;12:1209. doi: 10.3390/v12111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E., Senne D.A., Myers T.J., Bulaga L.L., Garber L.P., Perdue M.L., Lohman K., Daum L.T., Suarez D.L. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarek M., Naguib M.M., Arafa A.-S., Tantawy L.A., Selim K.M., Talaat S., Sultan H.A. Epidemiology, genetic characterization, and pathogenesis of Avian Influenza H5N8 viruses circulating in Northern and Southern parts of Egypt, 2017–2019. Animals. 2021;11:2208. doi: 10.3390/ani11082208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valley-Omar Z., Cloete A., Pieterse R., Walaza S., Salie-Bassier Y., Smith M., Govender N., Seleka M., Hellferscee O., Mtshali P.S. Human surveillance and phylogeny of highly pathogenic avian influenza A (H5N8) during an outbreak in poultry in South Africa, 2017. Influenza Other Respi. Viruses. 2020;14:266–273. doi: 10.1111/irv.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd D.J. Ostrich diseases. Rev. Sci. Tech. Int. des Epizoot. 2000;19:638–652. doi: 10.20506/rst.19.2.1235. [DOI] [PubMed] [Google Scholar]
- Wise M.G., Suarez D.L., Seal B.S., Pedersen J.C., Senne D.A., King D.J., Kapczynski D.R., Spackman E. Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. J. Clin. Microbiol. 2004;42:329–338. doi: 10.1128/JCM.42.1.329-338.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Wang C., Tang C., Xing L., Luo D., Zhan Z., Duan Y., Jia W., Peng D., Liu X. Characterization of a highly pathogenic avian influenza H5N1 virus isolated from an ostrich. Biochem. Biophys. Res. Commun. 2010;396:973–977. doi: 10.1016/j.bbrc.2010.05.035. [DOI] [PubMed] [Google Scholar]
- Yehia N., Naguib M.M., Li R., Hagag N., El-Husseiny M., Mosaad Z., Nour A., Rabea N., Hasan W.M., Hassan M.K. Multiple introductions of reassorted highly pathogenic avian influenza viruses (H5N8) clade 2.3. 4.4 b causing outbreaks in wild birds and poultry in Egypt. Infect. Genet. Evol. 2018;58:56–65. doi: 10.1016/j.meegid.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Zhou L.-C., Liu J., Pei E.-L., Xue W.-J., Lyu J.-M., Cai Y.-T., Wu D., Wu W., Liu Y.-Y., Jin H.-Y. Novel avian influenza A (H5N8) viruses in migratory birds, China, 2013–2014. Emerg. Infect. Dis. 2016;22:1121. doi: 10.3201/eid2206.151754. [DOI] [PMC free article] [PubMed] [Google Scholar]