Abstract
COVID-19 was primarily considered a pulmonary disease with extrapulmonary manifestations. As the pandemic spread, there has been growing evidence that the disease affects various organs/systems, including the central and peripheral nervous systems. Accumulation of clinical data demonstrates that in a large population of survivors impairments in the function of one or more organs may persist for a long time, a phenomenon commonly known as post COVID or long COVID. Fatigue and cognitive dysfunction, such as concentration problems, short-term memory deficits, general memory loss, a specific decline in attention, language and praxis abilities, encoding and verbal fluency, impairment of executive functions, and psychomotor coordination, are amongst the most common and debilitating features of neuropsychatric symptoms of post COVID syndrome. Several patients also suffer from compromised sleep, depression, anxiety and post-traumatic stress disorder. Patients with long COVID may demonstrate brain hypometabolism, hypoperfusion of the cerebral cortex and changes in the brain structure and functional connectivity. Children and adolescents represent a minority of COVID-19 cases, so not surprisingly data on the long-term sequelae after SARS-CoV-2 infections in these age groups are scarce. Although the pathogenesis, clinical characteristics, epidemiology, and risk factors of the acute phase of COVID-19 have been largely explained, these areas are yet to be explored in long COVID. This review aims to provide an update on what is currently known about long COVID effects on mental health.
Keywords: Long COVID, Mental health, Depression, Anxiety, Post-traumatic stress disorder, Cognitive deficits
1. Introduction
In December 2019, the first cases of a novel infectious disease associated with pneumonia and acute respiratory distress were reported in the city of Wuhan, Hubei Province, China. A causative agent was quickly identified as a member of the Coronaviridae family and officially designed as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by the Coronavirus Study Group of the International Committee on Taxonomy of Viruses (Gorbalenya et al., 2020). The World Health Organization (WHO) termed the novel coronaviral pneumonia and CoV-associated diseases as “COVID-19”. The disease has quickly spread across the world, and on March 11, 2020 the pandemic outbreak was declared by the WHO. As of August 7, 2022, 581.8 million confirmed cases and 6.4 million deaths have been reported globally (World Health Organization, 2022). This devastating pandemic has badly disrupted normal social activities and economic growth worldwide.
In the beginning, COVID-19 was primarily considered as a pulmonary disease with extrapulmonary manifestations. As the pandemic spread, there has been growing evidence that COVID-19 affects various organs/systems. The clinical manifestations of the disease are broad, ranging from asymptomatic infection to life-threatening clinical conditions. Most patients demonstrate only mild (40%) or moderate (40%) symptoms; however, approximately 15% develop severe illness that requires oxygen support, and 5% have a critical illness with complications such as respiratory failure, acute respiratory distress syndrome (ARDS), sepsis and septic shock, thromboembolism, and multiorgan failure (Zawilska et al., 2021). Over time, a wide variety of central and/or peripheral nervous manifestations of COVID-19, such as headache, dizziness, sleep disorders, anosmia and dysgeusia, impaired consciousness, cerebrovascular disorders (including ischemic stroke and macro/microhemorrhages), encephalopathies, immune-mediated complications (Guillain-Barré syndrome and acute disseminated encephalomyelitis), seizures, psychosis, anxiety/depression, and psychological distress have been notified. It is estimated that during the acute phase of COVID-19 about one-third of patients develop neuropsychiatric symptoms (Helms et al., 2020; Hensley et al., 2022; Romero-Sánchez et al., 2020; Xu et al., 2021; Zawilska et al., 2021).
Since the pandemic has progressed, along with increasing numbers of infected patients, emerging data demonstrate that in a large population of survivors impairments in the function of one or more organs may persist for a long time, a phenomenon known as post COVID-19, long COVID, post-acute COVID syndrome (PACS), post-acute sequelae of COVID-19 (PASC), chronic COVID syndrome (CCS) or long haul COVID. Symptoms might be new onset after initial recovery from an acute COVID-19 episode or persist from the initial illness. Symptoms might also fluctuate or relapse over time (World Health Organization, 2021). Recently Fernández-de-las-Peñas et al. (2021b) defined four stages of the disease according to the period when a particular symptom appears in relation to the acute phase of SARS-CoV-2 infection: (1) potentially infection-related symptoms (up to 4–5 weeks after symptoms' onset), (2) acute post-COVID symptoms (from week 5 to week 12 after symptom's onset), (3) long post-COVID symptoms (from week 12 to week 24 after symptom's onset), and (4) persistent post-COVID symptoms (lasting more than 24 weeks after symptom's onset). Furthermore, based on a fluctuating or relapsing nature of post COVID symptoms, the group of Fernández-de-las-Peñas defined them as “exacerbated (when a patient suffered from a particular symptom before SARS-CoV-2 infection and this symptom worsens after), delayed-onset (a new symptom not experienced by a patient at the acute phase of the infection but appears after a latency period) or persistent (a new symptom experienced by a patient at the acute phase of the infection which persists without pain-free or remission periods)” (Fernández-de-Las-Peñas et al., 2021a). The number of subjects affected with the late sequelae after the acute COVID-19 episode remains unknown, but published reports indicate that approximately 10–20% of patients experience lingering symptoms for weeks to months following acute SARS-CoV-2 infection (World Health Organization, 2021).
Highlighted risk factors were disease severity, duration of symptoms, as well as female sex (Schou et al., 2021). The clinical manifestations of long COVID are highly variable in symptoms, intensity, and duration. People with the disease exhibit involvement and impairment in the structure and function of multiple systems, such as respiratory, cardiovascular, digestive, nervous (both central and peripheral), and hematological. These ongoing symptoms broadly fall into two basic categories: respiratory (e.g., cough, shortness of breath, chest tightness) and neuropsychiatric (e.g., post-exertional malaise or fatigue, insomnia and other sleep disturbances, cognitive dysfunction, headache, loss of smell or taste, depression and anxiety, post-traumatic stress disorder, psychosis), and generally have an impact on everyday functioning [for elegant reviews see (Lopez-Leon et al., 2021; Staffolani et al., 2022; Yan et al., 2021)]. A meta-analysis of prevalence of long COVID symptoms at different follow-up periods demonstrated that fatigue, dyspnoea, myalgia, and sleep disorders were most reported in the >12-month interval, while cough, headache, loss of taste, and loss of smell were most common at 6 to <9 months (Alkodaymi et al., 2022). Women report significantly higher number of symptoms than men: mean 2.25 versus 1.25 (Fernández-de-Las-Peñas et al., 2022), median 5 versus 3 (Staudt et al., 2022).
This review aims to provide an update on what is currently known about psychiatric and neurologic manifestations of long COVID.
2. Long COVID: neuropsychiatric manifestations
2.1. Adults
The symptoms observed in long COVID patients partly resemble chronic fatigue syndrome, which includes the presence of severe incapacitating fatigue, pain, neurocognitive disability, compromised sleep, symptoms suggestive of autonomic dysfunction. In a meta-analysis covering 25,268 long COVID patients, a pooled proportion of those experiencing fatigue 12 or more weeks after SARS-CoV-2 infection diagnosis was 0.32. A larger proportion of women reported fatigue as compared to men, but the inter-subgroup difference was not statistically significant. There was no statistically significant difference in the proportion of patients reporting fatigue between hospitalized and non-hospitalized respondents (Ceban et al., 2022). In a substantial population of COVID-19 survivors, cognitive deficits were reported. These include concentration problems, short-term memory deficits, general memory loss, a specific decline in attention, language and praxis abilities, encoding and verbal fluency, impairment of executive functions and psychomotor coordination, and an ICD-10 diagnosis of dementia (Boesl et al., 2021; Ceban et al., 2022; Fernández-de-Las-Peñas et al., 2022; Guo et al., 2022; Lauria et al., 2022; Miskowiak et al., 2021; Nalbandian et al., 2021; Sykes et al., 2021; Whiteside et al., 2021) (Table 1 ). Among 13,232 of long COVID patients, the pooled proportion of those experiencing cognitive impairment 12 or more weeks after SARS-CoV-2 infection diagnosis was 0.22. By analogy to fatigue, there was a non-significant trend towards a greater proportion of women than men who exhibited cognitive impairment. Additionally, the proportion of patients reporting cognitive impairment between hospitalized and non-hospitalized respondents did not differ significantly (Ceban et al., 2022). However, at an average four-months follow-up, neurological deficits and cognitive impairment were more frequently observed in severe COVID-19 patients admitted to intensive care unit (ICU) than in those suffering from a mild disease non-requiring oxygen support (Mattioli et al., 2021).
Table 1.
Neurological and psychiatric sequelae of long COVID.
| Study design | Time of assessment | Number of patients | Age (years) | Neurological and psychiatric complication | Location | Reference |
|---|---|---|---|---|---|---|
| CFQ-11 | Median interval between study assessment and discharge from hospital or a timepoint 14 days following diagnosis if managed as an outpatient: 72 days (IQR: 62–87) | 128; 53.9% women | Mean (SD): 49.5 ± 15 | Fatigue: <56 days (20.3%) 56–69 days (24.2%) 69–83 days (25.8%) >84 days (29.7%) |
St. James's Hospital, Dublin, Ireland | Townsend et al. (2020) |
| Ambidirectional cohort study Face-to-face interview with filling up questionaries: self-reported symptom questionnaire, mMRC dyspnea scale, EQ-5D-5L, EQ-VAS, ischemic stroke/cardiovascular event registration form |
Six months (Median = 186.0 days; IQR = 175.0–199.0) after acute infection (between symptom onset and hospital discharge) | 1,733, 48% women |
Median: 57.0; IQR: 47.0–65.0 |
Fatigue or muscle weakness (63%) Sleep difficulties (26%) Anxiety or depression (23%) Smell disorder (11%) Taste disorder (7%) Dizziness (6%) Myalgia (2%) Headache (2%) |
Jin Yin-tan Hospital and its outpatient clinic, Wuhan, China) | Huang et al. (2021) |
| Prospective cohort study BDI-13, IES-R, OCI, PCL-5, STAI-Y, WHIIRS, ZSDS |
One-three months (90.1 ± 13.4 days) after hospitalization | 226; 34% women | Mean (SD): 58.5 ± 12.8 Range: 26-87 |
Depression (persistent) PTSD, anxiety and insomnia (decreased during follow-up) |
IRCCS San Raffaele Hospital, Milan, Italy | Mazza et al. (2021) |
| GAD-7, IES-6, PHQ-9 | One month after hospitalization | 114; 46,5% women | Median: 40 IQR: 31.75–50.25 |
Provisional PTSD diagnosis (36%) | First Hospital of Changsha, Hunan, China | Ju et al. (2021) |
| CAPS-5 | 30–120 days after recovery from COVID-19 | 381; 43.6% women | Mean (SD): 55.26 ± 14.86 | PTSD (30.2%) | Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy | Janiri et al. (2021) |
| Online survey | Four-twelve weeks | 3,290; 78% women | Range: 45-54 | Fatigue (83.3%) Muscle weakness or joint stiffness (50.6%) Sleep disturbances (46.2%) Problems with mental abilities (45.9%) Changes in mood, anxiety and depression (43.1%) |
United Kingdom | Buttery et al. (2021) |
| Prospective observational study Questionnaire |
Patients were followed-up either at the institute's post COVID-19 clinic or by phone 91 (IQR: 45–181) days post discharged/post-end of isolation | 1,234; 30.6% women | Mean (SD): 41.4 ± 14.2 Range: 18-97 |
Myalgia (10.9%) Fatigue (5.5%) Insomnia (1.4%) Mood disturbances (0.48%) Anxiety (0.6%) |
Tertiary healthcare center, New Delhi, India | Naik et al. (2021) |
| Prospective observational study The primary outcome: mRS Secondary outcomes: ADL, Telephone MoCA, Neuro-QoL |
Six months (±1 month) from the onset of neurological symptoms among cases, or from the onset of COVID-19 symptoms among controls | 382; 65% women | Neurologic COVID-19 Median: 68 Range: 55-77 COVID-19 Control: Median = 69 Range: 57-78 |
Limited activities of daily living (56%) Abnormal cognition (50%) Worsen than average anxiety, depression, fatigue or sleep scores (62%) |
Four New York City area hospitals, USA | Frontera et al. (2021) |
| Prospective study Neurological examination, PROMIS. Evaluation of cognitive function was done with the National Institutes of Health (NIH) Toolbox (in-person visit) |
Mean (SD): 5.27 ± 1.83 months after infection | 100 [50 SARS-CoV-2 laboratory-positive; 50 laboratory-negative (suspected COVID-19)]; 70% women | Mean (SD): 43.2 ± 11.3 | Fatigue (85%) Non-specific cognitive complaints – “brain fog” (81%) Headache (68%) Numbness/tingling (60%) Dysgeusia (59%) Anosmia (55%) Myalgia (55%) Depression/Anxiety (47%) Dizziness (47%) Pain (43%) Insomnia (33%, Short-term memory deficit (32%) Blurred vision (30%) Tinnitus (29%) Attention deficit (27%) |
Neuro-Covid-19 Clinic of Northwestern Memorial Hospital, Chicago, USA | Graham et al. (2021) |
| Online survey | Seven months after COVID-19 onset | 3,762 (1020; antibody positive; 2,742 suspected – antibody negative or untested); 78.9% women |
≥18 |
Frequency of symptoms ≥ 60%: “Brain fog”, poor attention, difficulty thinking, anxiety, short-term memory loss, dizziness/vertigo, insomnia Frequency of symptoms ≥ 50%: Irritability, difficulty in executive functioning, difficulty in problem solving Frequency of symptoms ≥ 40%: Depression, mood liability, tearfulness, tingling/pins and needles, tremors, sleep problems, slowed thoughts, difficulty finding right words |
56 countries | Davis et al. (2021) |
| Neurological examination MoCA |
Six months after hospitalization | 165; 30.3% women | Mean (SD): 64.8 ± 12.6 | Fatigue (33.9%) Memory complains/attention deficits (31.5%) Insomnia (31.5%) Myalgia (30.3%) Depressive symptoms/anxiety (26.7%) Blurring/loss of vision (19.5%) Paresthesia (18.8%) Hyposmia/hypogeusia (16.4%) Confusion (13.3%) Headache (9.7%) |
COVID-19 Unit of the ASST Spedali Civili Hospital, Brescia, Italy | Pilotto et al. (2021) |
| Single-center study Interview, SF-12 MCS |
Five and twelve months after confirmed diagnosis of COVID-19 | 96; 55.2% women | Median: 57; IQR: 50–63 |
Five months: Fatigue (42%) Sleep problems (32%) Concentration problems (32%) Headache (20%) Twelve months: Fatigue (53%) Sleeping problems (25%) Concentration problems (39%) Headache (17%) |
University Hospital, Heidelberg, Germany | Seeβle et al. (2021) |
| Nationwide retrospective cohort study Data from the TriNetX electronic health records network |
Six months after confirmed diagnosis of COVID-19 | All patients: 236,379; 55.6% women | Mean (SD): 46.0 ± 19.7 |
Anxiety disorder (17.39%) Ischemic stroke (2.10%) Psychotic disorder (1.40%) Dementia (0.67%) Intracranial hemorrhage (0.56%) Parkinsonism (0.11%) Nerve, nerve root, or plexus disorders (2.85%) Guillain-Barré syndrome (0.08%) |
USA | Taquet et al. (2021) |
| Patients with ITU admission: 8,945; 46.7% women |
Mean (SD): 59.1 ± 17.3 | Anxiety disorder (19.15%) Ischemic stroke (6.92%) Psychotic disorder (2.77%) Dementia (1.74%), Intracranial hemorrhage (2.66%) Parkinsonism (0.26%) Nerve, nerve root, or plexus disorders (4.24%) Guillain-Barré syndrome (0.33%) |
||||
| Mental health examination QoL questionnaire EQ-5D-5L, PCFS, HADS-A, HADS-D |
Four-twelve weeks after mild/moderate COVID-19 infection | 64 inpatients; 51,56% women | Median: 46 | Depression (46.87%) Anxiety (34.37%) |
Romania | Giurgi-Oncu et al. (2021) |
| 79 outpatients; 56.96% women |
Median: 42 | Depression (27.84%) Anxiety (40.5%) |
||||
| Prospective online survey EQ5D index score |
Six and twelve months after recovery (Median: 454 days; IQR: 451–458) |
All: 1,141 12 months: 241; 68% women |
Median: 37; IQR: 26.0–51.0 |
Concentration difficulties (most frequent among 18–49 years old) Cognitive disfunction (most frequent among 50–59 years old) Amnesia, depression, fatigue (most frequent among ≥60 years old) Anxiety. |
Kyungpook National University Hospital, Korea | Kim et al. (2022) |
| Prospective monocentric cohort study SAS, SDS |
Six and twelve months after hospitalization | 64; 36% women | Median: 68; (1st-3rd quartiles: 56.5–75) |
Six months: Anxiety (48.5%), depression (56.4%), persistent fatigue (37.5%), memory and attention deficits (11%) Twelve months: Anxiety (50%), depression (61%), persistent fatigue (12.5%), memory and attention deficits (4.7%) |
Hospital of Fermo, Marche, Italy | Martino et al. (2022) |
| Prospective monocentric observational study MMSE, COWA S, COWA Ph, CVLT Immediate, CVLT Delayed, TOL, Rey figure copy, Rey figure recall |
IUC patients Mean (SD): 141 ± 4.22 after COVID-19 diagnosis |
52; 23% women |
Mean (SD): 60 ± 9.9 | Scores of tests, median (range) MMSE: 29 (26–30) COWA S: 48 (29–70) COWA Ph: 39 (15–59) CVLT Immediate: 55 (24–100) CVLT Delayed: 60 (20–100) TOL: 15 (0–22) Rey figure copy: Rey figure recall: 34 (18–36) |
University Hospital, Brescia, Italy | Mattioli et al., 2021 |
| Non-IUC patients Mean (SD): 121 ± 41.2 days after COVID-19 diagnosis |
163; 75% women |
Mean (SD): 49.6 ± 9.4 | Scores of tests - median (range) MMSE: 29 (27–30) COWA S: 46 (19–61) COWA Ph: 37 (3–58) CVLT Immediate: 70 (0–95) CVLT Delayed: 86 (0–107) TOL: 16 (1–22) Rey figure copy: 34 (18–36) Rey figure recall: 18 (2–31) |
|||
| Multicenter cohort study HADS-D, HADS-A, PSQI |
Mean (SD): 8.4 ± 1.5 months after onset of infection | 1,969; 46.4% women | Mean (SD): 61 ± 16 | Fatigue (61.3%) Pain, headache (45.1%) Poor sleep quality (34.2%) Depression (18.9%) Memory loss (17.3%) Anxiety (15.6%) Cognitive blurring “brain fog” (9.6%) Concentration loss (7.1%) Anosmia (4.05%) Ageusia (2.7%) |
Five public hospitals in Madrid, Spain | Fernández-de-Las-Peñas et al. (2022) |
| Retrospective observational study Surveys containing patient-reported outcomes: FSS, EQ-5D-5L, GAD-7, PHQ-2, Neuro-QoL test, VAFS |
Median: 351 (range 82–457) days after onset of COVID-19 | 156; 69% women | Median: 44; Range:13-79 |
Fatigue (82%) “Brain fog” (67%) Headache (60%) Sleep disturbances (59%) Dizziness (54%) At least mild cognitive impairment (63%) |
Mount Sinai's Post-acute COVID-19 Syndrome Clinic, New York, USA | Tabacof et al. (2022) |
| Prospective observational study Neuropsychological evaluation COMPASS-31, MoCA |
Four weeks – nine months (median: 59; range: 31–175 days) after onset of infection | 180; 70.6% women | Mean (SD): 51 ± 13 | “Brain fog”/cognitive deficit (42.3%) Hyposmia/hypogeusia (37.1%) Myalgia/asthenia (22.7%) Headache (13.4%) Sleep disturbances (10.3%) Dizziness (7.2%) |
University Hospital and Health Services, Trieste, Italy | Buoite Stella et al. (2022) |
| Multi-center observational study | Six months after acute neurological symptoms | 60 | NP | Impaired cognition (68.9%) Residual disability (51.7%) Smell/taste disorder (45%) Memory complaints (34%) Anxiety or depression (32%) |
NeuroCOVID hospital-based registry, France | Chaumont et al., 2022 |
| Follow-up cognitive assessment by phone MoCA-BLIND 7.1 |
Median: 270 days; range: 258–300 | 95; 42% women) | Median: 50 Range: 28-86 |
Easy fatigability (51.04%) Anxiety (38.54%) New-onset headache (38.54%) |
Large tertiary hospital, Tehran, Iran | Mirfazeli et al. (2022) |
| Prospective cohort study PHQ, EST-Q2 Depression subscale, EST-Q2 Anxiety subscale; The Primary Care PTSD Screen for DSM-5, the PTSD checklist for DSM-5, EST-Q2 Insomnia subscale, PSQI |
Mean (SD): 5.65 ± 4.26 months after diagnosis of COVID-19 | 247,249 COVID-19 diagnosis during the study period: 9,979 (67.9% women) No COVID-9 diagnosis: 237,270 (61.7% women) |
COVID-19 diagnosis - mean (SD): 46.6 ± 12.9; Non COVID-19 diagnosis – mean (SD): 48.9 ± 11.9 |
COVID-19 diagnosis vs. no Covid diagnosis, prevalence ratio: Depression – 1.18 Poorer sleep quality – 1.13 Anxiety – 0.97 COVID-19-related distress – 1.05 |
Denmark, Estonia, Iceland, Norway, Sweden, United Kingdom | Magnúsdóttir et al. (2022) |
| PHQ-9-D | Ten months after hospitalization | 101; 42% women | Median: 60.0 IQR: 50.8–66.0 Range: 28-69 |
Fatigue (49%) Cognitive impairment (39%) Signs of major depression (28%) |
RoMed hospital, Rosenheim, Germany | Staudt et al. (2022) |
| Observational study - online survey TSQ |
Three and six months after onset of COVID-19 symptoms | 239; 82.8% women | Median: 50 IQR: 39-56 |
Three months: PTSD (37.2%), anxiety (35.6%), depression (46.9%) Six months: PTSD (26.8%), anxiety (34.7%), depression (40.6%) |
The Netherland and Flanders | Houben-Wilke et al. (2022) |
| Structured questionnaire filled up during phone calls GAD-2 |
Five-eight months after confirmation of SARS-CoV-2 infection | 236; 61% women | Mean: 41.2 Range: 19-81 |
Sleep problems (45.8%) Feeling depressed (44.9%) Memory complaints (39.8%) Anxiety (36.9%) Fatigue (21.6%) Headache (19.1%) Myalgia (16.1%) |
Two public hospitals in the Federal District, Brazil | Titze-de-Almeida et al. (2022) |
| Self administered questionnaires: BDI-II, FACIT-F, IES-R, ISI, RS-14, SF-12, ZSAS | Mean (SD): 88.67 ± 12.62; range: 63–108 days after COVID-19 recovery | 21; 38,09% women | Mean (SD): 57.05 ± 11.02 Range: 39-83 | PTSD (28.6%) Moderate anxiety (14.3%) Moderate depressive symptoms (9.5%) Clinical insomnia (9.5%) |
Pulmonary Outpatient Clinic of Volterra Azienda USL, Pisa, Italy |
Vagheggini et al. (2022) |
| Single-center study Interview, questionaries/tests: DSB, DSF, FAB, HADS, K10, MFTC, MMSE, PSQI, RAVLT, TMT |
Days since first symptoms, mean (SD): 96.5 ± 45.3; since hospital discharge, 62.1 ± 39.7 |
100; 35% women | Mean (SD): 73.4 ± 6.1 | Fatigue (49%) Sleep problems (33%) Attention disorders (30%) Memory disorders (30%) Myalgias (17%) Anosmia (16%) Dysgeusia (12%) Language disorders (12%) Headache (5%) Vertigo (5%) |
Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy | Lauria et al. (2022) |
| Systematic review, meta-analysis of 15 publications Database search (LitCOVID and Embase) |
14–110 days post-viral infection | 47,910 | Range: 17-87 | Fatigue (58%) Headache (44%) Attention disorder (27%) Ageusia (23%) Anosmia (21%) Memory loss (16%) Anxiety (13%) Depression (12%) Sleep disorder (11%) Stroke (3%) PTSD (1%) |
UK/Europe (9), US (3), Australia (1), China (1), Egypt (1), Mexico (1) | Lopez-Leon et al. (2021) |
| Meta-analysis of 18 studies: 3 case-control, 6 cross-sectional, 8 prospective cohort, and 1 retrospective cohort study | Symptoms reported ≥ three months post onset of COVID-19 (mid-term: 3–6 months and long-term: > 6 months) | 10,530; 59% women | Mean (SD): 52 ± 10 | Fatigue (37%) “Brain fog” (32%) Sleep disturbances (31%) Memory problems (28%) Anxiety (23%) Attention disorder (22%) Depression (27%) Myalgia (17%) Headache (15%) Anosmia (12%) Dysgeusia (10%) |
Premraj et al. (2022) | |
| Systemic review and meta-analysis | 257,348 |
Three to < six months: Fatigue (32%), sleep disorder (24%), difficulty concentrating (22%) Six to < nine months: Fatigue (36%), sleep disorder (29%) Nine to 12< months Fatigue (37%) >12 months Fatigue (41%), sleep disorder (30%), myalgia (22%) |
North America (6), East Asia (12), Europe (37), North Africa, the Middle East or South Asia (8) |
Alkodaymi et al. (2022) |
Acronyms: ADL, Barthel Index for Activities of Daily Living; BDI-II, Beck Depression Inventory-II; BDI-13, 13-item Beck's Depression Inventory; CAPS-5, Clinician-Administered PTSD Scale for DSM-5; CFQ-11, Chalder Fatigue Scale; COMPASS-31, Composite Autonomic Symptom Scale 31 questionnaire; COWA, Controlled Oral Word Association (S - Semantic, Ph – phonemic); CVLT, California Verbal Learning Test; DSB, Digit Span Backward; DSF, Digit Span Forward; EQ-5D-5L, EuroQol five-dimension five-level questionnaire; EQ5D, EuroQol-5 dimension index score; EQ-VAS, EuroQol Visual Analog Scale; EST-Q2, Emotional State Questionnaire; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue subscale; FAB, Frontal Assessment Battery; FSS, Fatigue Severity Scale; GAD-2, Generalized Anxiety Disorder 2-item questionnaire; GAD-7, Generalized Anxiety Disorder 7-item questionnaire; HADS, Hospital Anxiety and Depression Scale or Hamilton Anxiety and Depression Scale; HADS-A, Hospital Anxiety and Depression Scale with subscale for anxiety symptoms; HADS-D, Hospital Anxiety and Depression Scale with subscale for depressive symptoms; IES-6, Impact of Event Scale-6; IES-R, Impact of Event Scale–Revised; IQR, Interquartile Range; ISI, Insomnia Severity Index; ITU, Intensive Therapy Unit; K10, Kessler Psychological Distress Scale; MCS, Mental Component Scale; MMSE, Mini Mental State Examination; MFTC, Multiple Features Target Cancellation Test; MoCA, Montreal Cognitive Assessment; mMRC, the modified Medical Research Council; mRS, modified Rankin Scale; Neuro-QoL, Quality of Life in Neurological Disorders; NP, Not provided; OCI, Obsessive-Compulsive Inventory; PCFS, Post-COVID-19 Functional Status Scale; PCL-5, PTSD Checklist for DSM-5; PHQ-2, Patient Health Questionnaire-2; PHQ-9-D (PHQ-9), Patient Health Questionnaire for Depression; PROMIS, Patient Reported Outcome Measurement Information System; PSQI, Pittsburgh Sleep Quality Index; PTSD, Post Traumatic Stress Disorder; RAVLT, Rey Auditory Verbal Learning Test; RS-14, 14-Item Resilience Scale; SAS, Self-Rating Anxiety Scale; SD, Standard Deviation; SF-12, 12-Item Short-Form Health Survey; STAI-Y, State-Trait Anxiety Inventory form Y; TMT, Trial Making Test; TOL, Tower of London; TSQ, Trauma Screening Questionnaire; VAFS, Visual Analog Fatigue Scale; WHIIRS, Women's Health Initiative Insomnia Rating Scale; ZSDS (SDS), Zung's Self-Rating Depression Scale.
An increasing number of studies indicate that psychiatric disturbances, such as anxiety and/or depression and PTSD, could persist after recovery from the primary infection [for elegant reviews see e.g. (Premraj et al., 2022; Renaud-Charest et al., 2021; Schou et al., 2021)]. The main factors associated with depression were female sex, previous psychiatric history, and psychopathology at one-month follow-up (e.g., Mazza et al., 2021; Renaud-Charest et al., 2021).
Unlike other symptoms analyzed, including but not limited to rhinitis, sore throut, skin rashes, tinnitus, chest tightness and palpitations/arrhythmias, psychiatric symptoms resolved relatively slow, and may last for more than 12 months (Kim et al., 2022; for reviews see Alkodaymi et al., 2022; Renaud-Charest et al., 2021) (Table 1). Moreover, reports from longitudinal studies demonstrated that patients may experience exacerbation of some neuropsychiatric symptoms (e.g., problems with mental abilities, anxiety, depression, insomnia, PTSD) after a longer interval (Buttery et al., 2021; Ceban et al., 2022; Giurgi-Oncu et al., 2021; Martino et al., 2022; Renaud-Charest et al., 2021) (Table 1). Alarmingly, several studies revealed that around 30% of patients who had recovered from COVID-19 met the criteria of PTSD (Houben-Wilke et al., 2022; Janiri et al., 2021; Ju et al., 2021; Vagheggini et al., 2022) (Table 1). However, the broad meta-analysis covering over 47,000 cases across the world indicates that PTSD was developed by 1% of patients (Lopez-Leon et al., 2021). This discrepancy may arise from different experiences during an active COVID-19 phase and background factors of the studied groups. According to Ju et al. (2021) female patients and patients with lower educational levels were more likely to develop PTSD symptoms during the rehabilitation stage. In addition, a higher anxiety level during hospitalization was a strong predictor of provisional PTSD in the post-discharge stage (Ju et al., 2021). Neurological abnormalities may be associated with older age, severity of acute COVID-19, longer hospitalization and pre-infection comorbidities (Pilotto et al., 2021). It is worth noting that patients with neurological complications during their COVID-19 admission had significantly worse functional outcomes, and were less likely to return to work, even after accounting for other premorbid factors (Frontera et al., 2021, Ziauddeen et al., 2022). Approximately one-third of subjects with cognitive disturbances (Nolen et al., 2022) and persistent fatigue (Townsend et al., 2020) following SARS-CoV-2 infection felt severely unable to function at work.
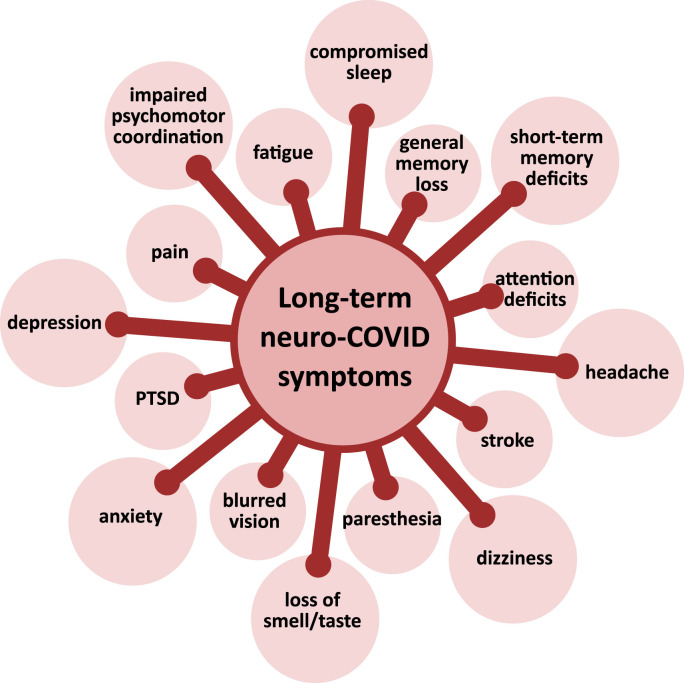
Common neuropsychiatric symptoms of long COVID are presented on Fig. 1 . Findings from diverse studies regarding prevalence of neuropsychiatric symptoms of long COVID in adult patients are summarized in Table 1.
Fig. 1.
Common neuropsychiatric symptoms of long COVID. PTSD – Post traumatic stress disorder.
2.2. Children and adolescents
Up to now, data on the long-term sequelae after SARS-CoV-2 infection in children and adolescents are scarce. The prevalence of long COVID in children varies remarkably depending on studies, ranging from 4% to 66% (Fainardi et al., 2022). Eighty nine children, mean age 12 ± 5 years, were assessed at a median of 112 days (range 33–410) after COVID-19 diagnosis. The two most common symptoms were fatigue and dyspnea, similar to what has been described in adults. The reported neurological symptoms included primarily sleep disturbances (33.3%), paresthesia (28.9%), headache (28.9%), anosmia-ageusia or parosmia/euosmia (25.6%), dizziness (18.9%), memory impairment (17.8%), tremor (13.3%) and difficulty in concentration (8.9%) (Ashkenazi-Hoffnung et al., 2021). In an Italian study, insomnia (18.6%) and concentration difficulties (10.1%) were the most frequently reported symptoms in a group of 109 children. These symptoms were particularly frequent in those assessed >60 days after the initial diagnosis (Buonsenso et al., 2021). Osmanov et al. (2022) conducted a study in a cohort of 518 subjects at the age of 3–15.2 years (median 10.4) previously hospitalized for COVID-19, assessing symptoms prevalence and duration of long COVID using the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) COVID-19 Pediatric follow-up questionnaire. After fatigue (10.6%), sleep disturbances were the second most frequent symptom (7.2%), followed by sensory problems (6.2%). Smell disturbances declined faster than headache and sleep problems. Multiple symptoms were experienced by 8.4% of participants. Risk factors for persistent symptoms were: older age (6–18 years) and a history of allergic diseases. Ashkenazi-Hoffnung et al. (2021) also demonstrated a positive correlation between increasing age and symptoms of long COVID. In a follow-up examination of 236 pediatric COVID-19 patients in Latvia, 44.5% of them had persistent symptoms at 73.5 days (IQR: 43–110) from the acute symptom onset, with irritability (27.6%), mood changes (26.7%), and fatigue (19.2%) being the most commonly reported ones (Roge et al., 2021). A Danish nationwide cohort study of 37,522 children aged 0–17 years with the verified SARS-CoV-2 infection revealed that 0.8% of them reported symptoms lasting longer than 4 weeks. The most common neurological symptoms were fatigue, loss of smell, loss of taste, and dizziness. The authors of the study noted that these symptoms can not be assigned to psychological sequelae of social restrictions (Borch et al., 2022). A case series of five Italian children (aged 2.75–15 years) demonstrating neuropsychiatric symptoms several weeks after diagnosis of SARS-CoV-2 infection was recently presented by Savino et al. (2022). Unusually, the most recurrent symptoms reported were abnormal movements (motor and vocal tics, ocular tics, repetitive and involuntary movements on the face and upper limbs); anxiety and emotional dysregulation were also described. One patient, a 15-years old boy, had prominent paranoid delirium, including feelings of concern and guilt, auditory and visual hallucinations, and showed poor insight and judgment regarding his condition. While positive symptoms disappeared completely after one year, negative ones persisted (Savino et al., 2022).
3. Alterations in anatomical and functional brain parameters in patients with long COVID
3.1. Hypometabolism
There are a few reports on changes in brain metabolism observed in patients with a confirmed diagnosis of SARS-CoV-2 infection and persistent functional complaints at least three weeks after the initial infection. In a retrospective analysis 18F-FDG brain PET scans of 35 patients with long COVID were compared with those of 44 healthy subjects controlled for age and sex. The median duration between this evaluation, including the PET scan, and the first symptoms of infection was 97 days. Among all patients, 80% complained of dyspnea, 66% of pain, 49% of memory/cognitive problems, 46% of insomnia, 29% of hyposmia/anosmia, and 26% of dysgeusia/ageusia. In comparison to healthy subjects, the patients with long COVID presented significant brain hypometabolism involving four clusters: (1) the bilateral rectal/orbital gyrus, including the olfactory gyrus; (2) the right temporal lobe, including the amygdala and the hippocampus, extending to the right thalamus; (3) the bilateral pons/medulla; (4) the bilateral cerebellum. Notably, pain was associated with lower metabolic values of all clusters, insomnia – with hypometabolism in the brainstem and cerebellum cluster, while hyposmia/anosmia and memory/cognitive complaints with decreased metabolism in the cerebellum cluster (Guedj et al., 2021). Brain metabolism of 13 adult COVID patients who complained for at least one persistent symptom for >30 days after infection recovery was compared with this of 26 melanoma patients, matched for sex/age, with negative PET/CT. Examination of 18F-FDG PET images revealed that COVID subjects with persistent anosmia/ageusia demonstrated a significant hypometabolism in parahippocampal gyri and orbitofrontal cortex, while those with persistent fatigue exhibited a relative hypometabolism in the right parahippocampal gyrus, the brainstem (substantia nigra), and the thalamus of both hemispheres (Sollini et al., 2021). A recent 18F-FDG PET study performed on 11 patients at a mean time of 38 days (range 14–91) after the first detection of SARS-CoV-2 infection, demonstrated multiple areas of hypometabolism in the parietal lobe (11 patients), temporal lobe (11 patients), frontal lobe (5 patients), occipital lobe (5 patients) and thalamus (1 patient) (Kiatkittikul et al., 2022). A case series of long COVID patients with cognitive decline, including “brain fog”, reported hypometabolic regions localized in the anterior and posterior cingulate cortex, precuneus and pons (Hugon et al., 2022a,b). Among 18F-FDG PET brain scans from 143 patients performed at 10.9 (±4.8) months from COVID-19 symptom onset, 53% were visually interpreted as normal, 21% as mildly to moderately or incompletely affected, and 26% as severely affected according to the COVID hypometabolic pattern. Major metabolic changes were found in the limbic/paralimbic regions, including fronto-orbital olfactory regions, pons and cerebellum (Verger et al., 2022). Dressing et al. (2022) examined a group of 31 outpatients with neurocognitive symptoms persisting for more than three months after confirmed COVID-19. All patients self-reported impaired attention, memory, and multitasking abilities, 27 of them word-finding difficulties, and 24 fatigue. Twelve patients could not return to the previous level of independence/employment. In contrast to the cited above data, no significant changes in regional cerebral glucose metabolism were found in the subgroup of 14 patients who underwent 18F-FDG PET examination (Dressing et al., 2022).
Metabolic brain findings in seven pediatric patients, mean age of 12 years, with persistent symptoms for more than four weeks following the diagnosis of COVID-19 without symptom-free interval, were confronted to those obtained in adult patients with post COVID-19. The most frequently reported symptoms in children were fatigue and cognitive impairment, such as memory and concentration difficulties. Despite a lower initial severity at the acute stage of the infection, pediatric patients demonstrated, on average five months later, a similar brain hypometabolic pattern as that found in adult long COVID patients, involving bilateral medial temporal lobes (amygdala, uncus, parahippocampal gyrus), pons and cerebellum (Morand et al., 2022).
2.2. Neuroimaging changes
There are a few neuroimaging studies on the brain structure and function in subjects suffering from long COVID. Detailed cross-sectional and longitudinal follow-up magnetic resonance imaging (MRI) analysis performed by Tian et al. (2022) characterized dynamic brain changes within 10 months after discharge from hospital in a group of 34 patients with COVID-19 (50-70-years old) without neurological manifestations, and compared them with alterations observed at 3 months follow-up. Cortical thickness differences changed dynamically. Ten months after discharge, gray matter atrophy in the frontal cortex, temporal-frontal cortex sensorimotor areas, temporal-parietal cortex, and left limbic areas were found; they were more pronounced compared to changes observed at 3 months. Regarding subcortical nuclei, in mild cases the volume of the right globus pallidus and left amygdala showed a downward trend along the time of recovery. On the other hand, in severe cases atrophy in the left putamen and thalamus observed at three months was still found at ten months. Additionally, newly emerging significant atrophy was observed in the right putamen and nucleus accumbens at ten months after discharge (Tian et al., 2022).
Douaud et al. (2022) investigated brain changes in participants of UK Biobank who were examined twice using MRI. Studies were done on two groups: 401 individuals (57.1% women) who had been tested positive for SARS-CoV-2 infections, and 384 (47.3% women) controls. Mean age at the scan one was 58.9 ± 7.0 years (range 46.9–80.2) for COVID-19 patients, and 60.2 ± 7.4 (47.1–79.8) for the controls. For the second scan the mean age was 62.1 ± 6.7 (51.3–81.4) for COVID-19 patients, and 63.3 ± 7.1 (51.3–81.3) for the controls. When compared to the controls, the SARS-CoV-2 subjects cases had: (1) a greater reduction in gray matter thickness and tissue contrast in the orbitofrontal cortex and parahippocampal gyrus; (2) greater changes in markers of tissue damage in regions that are functionally connected to the primary olfactory cortex; and (3) a greater reduction in global brain size. Furthermore, COVID-19 subjects showed on average a greater cognitive decline between these two time points.
Recently published data from a multimodal MRI study demonstrated that severity of depressive psychopathology is associated with decreasing gray matter volumes in the anterior cingulate, while post-traumatic symptoms are associated with decreasing gray matter volumes in the anterior cingulate and in the bilateral insular cortex. Both types of symptoms were associated with changes in the white matter microstructure and resting-state functional connectivity (Benedetti et al., 2021). Interestingly, overlapping associations between severity of inflammation during acute COVID-19, the brain structure and function, and severity of depression and PTSD in survivors were observed, suggesting that inflammation during acute COVID-19 predicted structural and functional changes in the brain and psychiatric complications in long haulers (Benedetti et al., 2021).
Smell disturbances, dysosmia and anosmia, were frequently reported in the acute phase of COVID-19, particularly during the first waves of the pandemic. They also occur in patients suffering from long COVID, albeit at a markedly lower frequency. It was suggested that viral-associated loss of smell could be linked to long-term changes in the brain (Boscolo-Rizzo et al., 2021). A pilot study done on six long COVID patients (median time between recovery and examination 20.6 weeks; range 16.6–27.6) and six healthy controls, reported lower olfactory function (measured using Sniffin’ Sticks 12-identification test) in COVID subjects. Surprisingly, signals from functional near-infrared spectroscopy recorded during olfactory stimuli did not differ between the two groups, suggesting that past COVID-19 infection may not affect frontotemporal cortex function. As emphasized by the authors, these preliminary results need to be verified in larger samples (Ho et al., 2021).
3. How much do we know about mechanisms underlying neuropsychiatric manifestation of long COVID?
At present little is known regarding mechanisms underlying the long-term distractive effects of COVID-19 on the nervous systems. It is believed that among various factors, neuronal damage triggered by reactive astrogliosis and microglial activation, microvascular thrombosis, damage and dysregulation of the blood-brain barrier that allows cytokines and leukocytes to infiltrate the brain parenchyma, neurotransmission alteration, oxidative stress, dysfunction of mitochondria, peripheral organ dysfunction (liver, kidney, lung), negative psychological and social factors associated with the COVID-19 pandemic all play important roles (Crook et al., 2021; Mohamed et al., 2022; Thye et al., 2022; Zhang et al., 2021).
The immunological processes behind the persistence of COVID-19 symptoms have been broadly investigated and discussed (Merad et al., 2022). Several lines of evidence indicate that neuropsychiatric disturbances in long COVID patients may be associated with hyperinflammatory state with elevated levels of pro-inflammatory cytokines, such as IL-6, IL-2, IL-17, granulocyte-colony stimulating factor, and TNF-α (Robinson-Agramonte et al., 2021). Moreover, a high level of antinuclear antibodies found in long COVID subjects suggests autoimmune etiology of neurocognitive symptoms (Seeβle et al., 2021).
One of the most frequently described symptoms in long COVID is fatigue. The most likely hypothesis of its pathomechanism involves the simultaneous presence of central, peripheral, and psychological factors. As previously mentioned, chronic neuroinflammation, along with neuromuscular mechanisms of injury (such as damages involving sarcolemma), may result in long-term fatigue, but non-well determined psychological and social factors might also play a part. Finally, long COVID symptoms in patients who received intensive care could result in post-intensive care syndrome, which is characterized by cognitive and psychiatric symptoms as well as immobility, microvascular and metabolic dysregulations (Nalbandian et al., 2021).
4. Conclusions
Since the outbreak of the COVID-19 pandemic, a major progress has been made in our understanding of the pathogenesis, clinical manifestations, and complications in the acute phase of the disease. However, knowledge about the long-term consequences of COVID-19 is still limited. Mounting clinical evidence indicates that some impairments may persist in COVID-19 survivors for over 24 weeks after the confirmed diagnosis of SARS-CoV-2 infection. The psychiatric and neurological symptoms seem to be characteristic of long COVID, pointing to an urgent need for on-going neurological and cognitive/affective monitoring of all cases of COVID-19 (Wijeratne and Crewther, 2020). The most frequently reported are chronic fatigue, cognitive deficits, depression, anxiety, sleep disturbances, and sensory issues. Nevertheless, it should be highlighted that the majority of the quoted data was gathered either by online surveys that were completed by patients themselves or patients' legal guardians, during telephone interviews, or by analyzing patients’ medical history. Thus, these results should be treated with some caution. Moreover, there is a limited number of reports regarding pediatric patients, and a true prevalence of long COVID within this group may be distinct from the generally recorded. It is unknown whether the long COVID impairs neurodevelopment in fetuses, children or adolescents, and whether the potential damage may persist into adulthood. There is also a need to identify if some patients are particularly prone to develop long COVID. Groups that may be at risk are patients with active neurological diseases or other comorbidities. However, it is crucial to distinguish symptoms of long COVID from those arising from the chronic disease. The possible connection between the different genotypic variants of SARS-CoV-2, the severity of the course of COVID-19, and the prevalence of long-lasting neurological symptoms need to be thoroughly explored. Finally, revealing the precise mechanism underlying long-term neuropsychiatric implications of long COVID19 could contribute to the establishment of successful treatment regimens that will bring great benefit to patients.
Declaration of competing interest
The Authors declare no conflict of interest.
Acknowledgments
This work was supported by Medical University of Lodz, Poland (grant 503/3-011-01/503-31-001-19-00).
References
- Alkodaymi M.S., Omrani O.A., Fawzy N.A., Shaar B.A., Almamlouk R., Riaz M., Obeidat M., Obeidat Y., Gerberi D., Taha R.M., Kashour Z., Kashour T., Berbari E.F., Alkattan K., Tleyjeh I.M. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2022;28(5):657–666. doi: 10.1016/j.cmi.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi-Hoffnung L., Shmueli E., Ehrlich S., Ziv A., Bar-On O., Birk E., Lowenthal A., Prais D. Long COVID in children: observations from a designated pediatric clinic. Pediatr. Infect. Dis. J. 2021;40(12):e509–e511. doi: 10.1097/INF.0000000000003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Palladini M., Paolini M., Melloni E., Vai B., De Lorenzo R., Furlan R., Rovere-Querini P., Falini A., Mazza M.G. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: a multimodal magnetic resonance imaging study. Brain Behav. Immun. Health. 2021;18 doi: 10.1016/j.bbih.2021.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesl F., Audebert H., Endres M., Prüss H., Franke C. A neurological outpatient clinic for patients with post-COVID-19 syndrome - a report on the clinical presentations of the first 100 patients. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.738405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borch L., Holm M., Knudsen M., Ellermann-Eriksen S., Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur. J. Pediatr. 2022;181(4):1597–1607. doi: 10.1007/s00431-021-04345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo-Rizzo P., Menegaldo A., Fabbris C., Spinato G., Borsetto D., Vaira L.A., Calvanese L., Pettorelli A., Sonego M., Frezza D., Bertolin A., Cestaro W., Rigoli R., D'Alessandro A., Tirelli G., Da Mosto M.C., Menini A., Polesel J., Hopkins C. Six-month psychophysical evaluation of olfactory dysfunction in patients with COVID-19. Chem. Senses. 2021;46:bjab006. doi: 10.1093/chemse/bjab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoite Stella A., Furlanis G., Frezza N.A., Valentinotti R., Ajcevic M., Manganotti P. Autonomic dysfunction in post-COVID patients with and without neurological symptoms: a prospective multidomain observational study. J. Neurol. 2022;269(2):587–596. doi: 10.1007/s00415-021-10735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonsenso D., Munblit D., De Rose C., Sinatti D., Ricchiuto A., Carfi A., Valentini P. Preliminary evidence on long COVID in children. Acta Paediatr. Oslo Nor. 2021;110(7):2208–2211. doi: 10.1111/apa.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttery S., Philip K.E.J., Williams P., Fallas A., West B., Cumella A., Cheung C., Walker S., Quint J.K., Polkey M.I., Hopkinson N.S. Patient symptoms and experience following COVID-19: results from a UK-wide survey. BMJ Open Respir. Res. 2021;8(1) doi: 10.1136/bmjresp-2021-001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceban F., Ling S., Lui L.M.W., Lee Y., Gill H., Teopiz K.M., Rodrigues N.B., Subramaniapillai M., Di Vincenzo J.D., Cao B., Lin K., Mansur R.B., Ho R.C., Rosenblat J.D., Miskowiak K.W., Vinberg M., Maletic V., McIntyre R.S. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont H., Meppiel E., Roze E., Tressières B., de Broucker T., Lannuzel A., Contributors to the French NeuroCOVID registry Long-term outcomes after NeuroCOVID: a 6-month follow-up study on 60 patients. Rev. Neurol. (Paris) 2022;178(1–2):137–143. doi: 10.1016/j.neurol.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook H., Raza S., Nowell J., Young M., Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re'em Y., Redfield S., Austin J.P., Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClin. Med. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L.R., Griffanti L., Duff E., Jbabdi S., Taschler B., Keating P., Winkler A.M., Collins R., Matthews P.M., Allen N., Miller K.L., Nichols T.E., Smith S.M. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressing A., Bormann T., Blazhenets G., Schroeter N., Walter L.I., Thurow J., August D., Hilger H., Stete K., Gerstacker K., Arndt S., Rau A., Urbach H., Rieg S., Wagner D., Weiller C., Meyer P.T., Hosp J.A. Neuropsychological profiles and cerebral glucose metabolism in neurocognitive Long COVID-syndrome. J. Nucl. Med. 2022;63(7):1058–1063. doi: 10.2967/jnumed.121.262677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainardi V., Meoli A., Chiopris G., Motta M., Skenderaj K., Grandinetti R., Bergomi A., Antodaro F., Zona S., Esposito S. Long COVID in children and adolescents. Life. 2022;12(2):285. doi: 10.3390/life12020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas C., Florencio L.L., Gómez-Mayordomo V., Cuadrado M.L., Palacios-Ceña D., Raveendran A.V. Proposed integrative model for post-COVID symptoms. Diabetes Metabol. Syndr. 2021;15 doi: 10.1016/j.dsx.2021.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas C., Martín-Guerrero J.D., Pellicer-Valero Ó.J., Navarro-Pardo E., Gómez-Mayordomo V., Cuadrado M.L., Arias-Navalón J.A., Cigarán-Méndez M., Hernández-Barrera V., Arendt-Nielsen L. Female sex is a risk factor Associated with long-term post-COVID related-symptoms but not with COVID-19 symptoms: the LONG-COVID-EXP-CM multicenter study. J. Clin. Med. 2022;11(2):413. doi: 10.3390/jcm11020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-de-las-Peñas C., Palacios-Ceña D., Gómez-Mayordomo V., Cuadrado M.L., Florencio L.L. Defining post-COVID symptoms (Post-acute COVID, long COVID, persistent Post-COVID): an integrative classification. Int. J. Environ. Res. Publ. Health. 2021;18(5):2621. doi: 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera J.A., Yang D., Lewis A., Patel P., Medicherla C., Arena V., Fang T., Andino A., Snyder T., Madhavan M., Gratch D., Fuchs B., Dessy A., Canizares M., Jauregui R., Thomas B., Bauman K., Olivera A., Bhagat D., Sonson M., Park G., Stainman R., Sunwoo B., Talmasov D., Tamimi M., Zhu Y., Rosenthal J., Dygert L., Ristic M., Ishii H., Valdes E., Omari M., Gurin L., Huang J., Czeisler B.M., Kahn D.E., Zhou T., Lin J., Lord A.S., Melmed K., Meropol S., Troxel A.B., Petkova E., Wisniewski T., Balcer L., Morrison C., Yaghi S., Galetta S. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J. Neurol. Sci. 2021;426 doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giurgi-Oncu C., Tudoran C., Pop G.N., Bredicean C., Pescariu S.A., Giurgiuca A., Tudoran M. Cardiovascular abnormalities and mental health difficulties result in a reduced quality of life in the post-acute COVID-19 syndrome. Brain Sci. 2021;11(11):1456. doi: 10.3390/brainsci11111456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham E.L., Clark J.R., Orban Z.S., Lim P.H., Szymanski A.L., Taylor C., DiBiase R.M., Jia D.T., Balabanov R., Ho S.U., Batra A., Liotta E.M., Koralnik I.J. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 "long haulers. Ann. Clin. Transl. Neurol. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj E., Campion J.Y., Dudouet P., Kaphan E., Bregeon F., Tissot-Dupont H., Guis S., Barthelemy F., Habert P., Ceccaldi M., Million M., Raoult D., Cammilleri S., Eldin C. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imag. 2021;48(9):2823–2833. doi: 10.1007/s00259-021-05215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P., Benito Ballesteros A., Yeung S.P., Liu R., Saha A., Curtis L., Kaser M., Haggard M.P., Cheke L.G. COVCOG 1: factors predicting physical, neurological and cognitive symptoms in long COVID in a community sample. A first publication from the COVID and cognition study. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.804922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley M.K., Markantone D., Prescott H.C. Neurologic manifestations and complications of COVID-19. Annu. Rev. Med. 2022;73:113–127. doi: 10.1146/annurev-med-042320-010427. [DOI] [PubMed] [Google Scholar]
- Ho R.C., Sharma V.K., Tan B.Y.Q., Ng A.Y.Y., Lui Y.S., Husain S.F., Ho C.S., Tran B.X., Pham Q.H., McIntyre R.S., Chan A.C.Y. Comparison of brain activation patterns during olfactory stimuli between recovered COVID-19 patients and healthy controls: a functional near-infrared spectroscopy (fNIRS) study. Brain Sci. 2021;11(8):968. doi: 10.3390/brainsci11080968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben-Wilke S., Goërtz Y.M.J., Delbressine J.M., Vaes A.W., Meys R., Machado F.V.C., van Herck M., Burtin C., Posthuma R., Franssen F.M.E., Vijlbrief H., Spies Y., van 't Hul A.J., Spruit M.A., Janssen D.J.A. The impact of long COVID-19 on mental health: observational 6-month follow-up study. JMIR Ment. Health. 2022;9(2) doi: 10.2196/33704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugon J., Msika E.F., Queneau M., Farid K., Paquet C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J. Neurol. 2022;269(1):44–46. doi: 10.1002/s00415-021-10655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugon J., Queneau M., Sanchez Ortiz M., Msika E.F., Farid K., Paquet C. Cognitive decline and brainstem hypometabolism in long COVID: a case series. Brain Behav. 2022 doi: 10.1002/brb3.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri D., Carfi A., Kotzalidis G.D., Bernabei R., Landi F., Sani G. Posttraumatic stress disorder in patients after severe COVID-19 infection. JAMA Psychiatr. 2021;78(5):567–569. doi: 10.1001/jamapsychiatry.2021.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y., Liu J., Ng R.M.K., Liu B., Wang M., Chen W., Huang M., Yang A., Shu K., Zhou Y., Zhang L., Liao M., Liu J., Zhang Y. Prevalence and predictors of post-traumatic stress disorder in patients with cured coronavirus disease 2019 (COVID-19) one month post-discharge. Eur. J. Psychotraumatol. 2021;12(1) doi: 10.1080/20008198.2021.1915576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiatkittikul P., Promteangtrong C., Kunawudhi A., Siripongsatian D., Siripongboonsitti T., Ruckpanich P., Thongdonpua S., Jantarato A., Piboonvorawong C., Fonghoi N., Chotipanich C. Abnormality pattern of F-18 FDG PET whole body with functional MRI brain in post-acute COVID-19. Nucl. Med. Mol. Imaging. 2022;56:29–41. doi: 10.1007/s13139-021-00730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Bitna Ha, Kim S.W., Chang H.H., Kwon K.T., Bae S., Hwang S. Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. BMC Infect. Dis. 2022;22(1):93. doi: 10.1186/s12879-022-07062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauria A., Carfì A., Benvenuto F., Bramato G., Ciciarello F., Rocchi S., Rota E., Salerno A., Stella L., Tritto M., Di Paola A., Pais C., Tosato M., Janiri D., Sani G., Pagano F.C., Fantoni M., Bernabei R., Landi F., Bizzarro A. Gemelli against COVID-19 post-acute care study group. Neuropsychological measures of long COVID-19 fog in older subjects. Geriatr. Med. 2022;38(3):593–603. doi: 10.1016/j.cger.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P.A., Cuapio A., Villapol S. More than 50 Long-term effects of COVID-19: a systematic review and meta-analysis. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnúsdóttir I., Lovik A., Unnarsdóttir A.B., McCartney D., Ask H., Kõiv K., Christoffersen L.A.N., Johnson S.U., Hauksdóttir A., Fawns-Ritchie C., Helenius D., González-Hijón J., Lu L., Ebrahimi O.V., Hoffart A., Porteous D.J., Fang F., Jakobsdóttir J., Lehto K., Andreassen O.A., Pedersen O.B.V., Aspelund T., Valdimarsdóttir U.A., COVIDMENT Collaboration Acute COVID-19 severity and mental health morbidity trajectories in patient populations of six nations: an observational study. Lancet Public Health. 2022;7(5):e406–e416. doi: 10.1016/S2468-2667(22)00042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino G.P., Benfaremo D., Bitti G., Valeri G., Postacchini L., Marchetti A., Angelici S., Moroncini G. 6 and 12 month outcomes in patients following COVID-19-related hospitalization: a prospective monocentric study. Intern. Emerg. Med. 2022 doi: 10.1007/s11739-022-02979-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli F., Stampatori C., Righetti F., Sala E., Tomasi C., De Palma G. Neurological and cognitive sequelae of Covid-19: a four month follow-up. J. Neurol. 2021;268(12):4422–4428. doi: 10.1007/s00415-021-10579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., COVID-19 BioB Outpatient Clinic Study group. Rovere-Querini P., Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Blish C.A., Sallusto F., Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375(6585):1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- Mirfazeli F.S., Sarabi-Jamab A., Pereira-Sanchez V., Kordi A., Shariati B., Shariat S.V., Bahrami S., Nohesara S., Almasi-Dooghaee M., Faiz S.H.R. Chronic fatigue syndrome and cognitive deficit are associated with acute-phase neuropsychiatric manifestations of COVID-19: a 9-month follow-up study. Neurol. Sci. 2022;43(4):2231–2239. doi: 10.1007/s10072-021-05786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K.W., Johnsen S., Sattler S.M., Nielsen S., Kunalan K., Rungby J., Lapperre T., Porsberg C.M. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M.S., Johansson A., Jonsson J., Schiöth H.B. Dissecting the molecular mechanisms surrounding post-COVID-19 syndrome and neurological features. Int. J. Mol. Sci. 2022;23(8):4275. doi: 10.3390/ijms23084275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand A., Campion J.Y., Lepine A., Bosdure E., Luciani L., Cammilleri S., Chabrol B., Guedj E. Similar patterns of [18F]-FDG brain PET hypometabolism in paediatric and adult patients with long COVID: a paediatric case series. Eur. J. Nucl. Med. Mol. 2022;49(3):913–920. doi: 10.1007/s00259-021-05528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik S., Haldar S.N., Soneja M., Mundadan N.G., Garg P., Mittal A., Desai D., Trilangi P.J., Chakraborty S., Begam N.N., Bhattacharya B., Maher G., Mahishi N., Rajanna C., Kumar S.S., Arunan B., Kirtana J., Gupta A., Patidar D., Kodan P., Sethi P., Ray A., Jorwal P., Kumar A., Nischal N., Sinha S., Biswas A., Wig N. Post COVID-19 sequelae: a prospective observational study from Northern India. Drug Discov. Ther. 2021;15(5):254–260. doi: 10.5582/ddt.2021.01093. [DOI] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen L.T., Mukerji S.S., Mejia N.I. Post-acute neurological consequences of COVID-19: an unequal burden. Nat. Med. 2022;28(1):20–23. doi: 10.1038/s41591-021-01647-5. [DOI] [PubMed] [Google Scholar]
- Osmanov I.M., Spiridonova E., Bobkova P., Gamirova A., Shikhaleva A., Andreeva M., Blyuss O., El-Taravi Y., DunnGalvin A., Comberiati P., Peroni D.M., Apfelbacher C., Genuneit J., Mazankova L., Miroshina A., Chistyakova E., Samitova E., Borzakova S., Bondarenko E., Korsunskiy A.A., Konova I., Wulf Hanson S., Carson G., Sigfrid L., Scott J.T., Greenhawt M., Whittaker E.A., Garralda E., Swann O.V., Buonsenso D., Nicholls D.E., Simpson F., Jones C., Semple M.G., Warner J.O., Vos T., Olliaro P., Munblit D., the Sechenov StopCOVID Research Team Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: a prospective cohort study. Eur. Respir. J. 2022;59(2) doi: 10.1183/13993003.01341-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Cristillo V., Cotti Piccinelli S., Zoppi N., Bonzi G., Sattin D., Schiavolin S., Raggi A., Canale A., Gipponi S., Libri I., Frigerio M., Bezzi M., Leonardi M., Padovani A. Long-term neurological manifestations of COVID-19: prevalence and predictive factors. Neurol. Sci. 2021;42(12):4903–4907. doi: 10.1007/s10072-021-05586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premraj L., Kannapadi N.V., Briggs J., Seal S.M., Battaglini D., Fanning J., Suen J., Robba C., Fraser J., Cho S.-M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J. Neurol. Sci. 2022;434 doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud-Charest O., Lui L.M.W., Eskander S., Ceban F., Ho R., Di Vincenzo J.D., Rosenblat J.D., Lee Y., Subramaniapillai M., McIntyre R.S. Onset and frequency of depression in post-COVID-19 syndrome: a systematic review. J. Psychiatr. Res. 2021;144:129–137. doi: 10.1016/j.jpsychires.2021.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Agramonte M.A., Gonçalves C.A., Noris-García E., Préndes Rivero N., Brigida A.L., Schultz S., Siniscalco D., García García R.J. Impact of SARS-CoV-2 on neuropsychiatric disorders. World J. Psychiatr. 2021;11(7):347–354. doi: 10.5498/wjp.v11.i7.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roge I., Smane L., Kivite-Urtane A., Pucuka Z., Racko I., Klavina L., Pavare J. Comparison of persistent symptoms after COVID-19 and other non-SARS-CoV-2 infections in children. Front. Pediatr. 2021;9 doi: 10.3389/fped.2021.752385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., González E., Redondo-Peñas I., Perona-Moratalla A.B., Del Valle-Pérez J.A., Gracia-Gil J., Rojas-Bartolomé L., Feria-Vilar I., Monteagudo M., Palao M., Palazón-García E., Alcahut-Rodríguez C., Sopelana-Garay D., Moreno Y., Ahmad J., Segura T. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino R., Polito A.N., Arcidiacono G., Poliseno M., Lo Caputo S. Neuropsychiatric disorders in pediatric long COVID-19: a case series. Brain Sci. 2022;12(5):514. doi: 10.3390/brainsci12050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou T.M., Joca S., Wegener G., Bay-Richter C. Psychiatric and neuropsychiatric sequelae of COVID-19 - a systematic review. Brain Behav. Immun. 2021;97:328–348. doi: 10.1016/j.bbi.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeßle J., Waterboer T., Hippchen T., Simon J., Kirchner M., Lim A., Müller B., Merle U. Persistent symptoms in adult patients 1 Year after COVID-19: a prospective cohort study. Clin. Infect. Dis. 2021;74(7):1191–1198. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollini M., Morbelli S., Ciccarelli M., Cecconi M., Aghemo A., Morelli P., Chiola S., Gelardi F., Chiti A. Long COVID hallmarks on [18F]FDG-PET/CT: a case-control study. Eur. J. Nucl. Med. Mol. Imag. 2021;48:3187–3197. doi: 10.1007/s00259-021-05294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffolani S., Iencinella V., Cimatti M., Tavio M. Long COVID-19 syndrome as a fourth phase of SARS-CoV-2 infection. Infez. Med. 2022;30(1):22–29. doi: 10.53854/liim-3001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt A., Jörres R.A., Hinterberger T., Lehnen N., Loew T., Budweiser S. Associations of Post-Acute COVID syndrome with physiological and clinical measures 10 months after hospitalization in patients of the first wave. Eur. J. Intern. Med. 2022;95:50–60. doi: 10.1016/j.ejim.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes D.L., Holdsworth L., Jawad N., Gunasekera P., Morice A.H., Crooks M.G. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. 2021;199(2):113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabacof L., Tosto-Mancuso J., Wood J., Cortes M., Kontorovich A., McCarthy D., Rizk D., Rozanski G., Breyman E., Nasr L., Kellner C., Herrera J.E., Putrino D. Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation. Am. J. Phys. Med. Rehabil. 2022;101(1):48–52. doi: 10.1097/PHM.0000000000001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatr. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thye A.Y.-K., Law J.W.-F., Tan L.T.-H., Pusparajah P., Ser H.-L., Thurairajasingam S., Letchumanan V., Lee L.-H. Psychological symptoms in COVID-19 patients: insights into pathophysiology and risk factors of long COVID-19. Biology. 2022;11(1):61. doi: 10.3390/biology11010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T., Wu J., Chen T., Li J., Yan S., Zhou Y., Peng X., Li Y., Zheng N., Cai A., Ning Q., Xiang H., Xu F., Qin Y., Zhu W., Wang J. Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight. 2022;7(4) doi: 10.1172/jci.insight.155827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze-de-Almeida R., da Cunha T.R., Dos Santos Silva L.D., Ferreira C.S., Silva C.P., Ribeiro A.P., de Castro Moreira Santos Júnior A., de Paula Brandão P.R., Silva A.P.B., da Rocha M.C.O., Xavier M.E., Titze-de-Almeida S.S., Shimizu H.E., Delgado-Rodrigues R.N. Persistent, new-onset symptoms and mental health complaints in Long COVID in a Brazilian cohort of non-hospitalized patients. BMC Infect. Dis. 2022;22(1):133. doi: 10.1186/s12879-022-07065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., O'Connor L., Leavy D., O'Brien K., Dowds D., Sugrue J.A., Hopkins D., Martin-Loeches I., Ni Cheallaigh C., Nadarajan P., McLaughlin A.M., Bourke N.M., Bergin C., O'Farrelly C., Bannan C., Conlon N. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagheggini G., Marzetti M., Miniati M., Bernardeschi L., Miccoli M., Boni Brivio G., Meini S., Panait E., Cini E., Gemignani A. Pulmonary function and psychological burden three months after COVID-19: proposal of a comprehensive multidimensional assessment protocol. Healtcare (Basel) 2022;10(4):612. doi: 10.3390/healthcare10040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger A., Kas A., Dudouet P., Goehringer F., Salmon-Ceron D., Guedj E. Visual interpretation of brain hypometabolism related to neurological long COVID: a French multicentric experience. Eur. J. Nucl. Med. Mol. Imag. 2022;49(9):3197–3202. doi: 10.1007/s00259-022-05753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside D.M., Oleynick V., Holker E., Waldron E.J., Porter J., Kasprzak M. Neurocognitive deficits in severe COVID-19 infection: case series and proposed model. Clin. Neuropsychol. 2021;35(4):799–818. doi: 10.1080/13854046.2021.1874056. [DOI] [PubMed] [Google Scholar]
- Wijeratne T., Crewther S. Post-COVID 19 Neurological Syndrome (PCNS); a novel syndrome with challenges for the global neurology community. J. Neurol. Sci. 2020;419 doi: 10.1016/j.jns.2020.117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization A clinical case definition of post COVID-19 condition by a Delphi consensus. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1
- World Health Organization Weekly epidemiological update on COVID-19 - 10 August 2022. 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-10-august-2022
- Xu Y., Zhuang Y., Kang L. A review of neurological involvement in patients with SARS-CoV-2 infection. Med. Sci. Monit. 2021;27 doi: 10.12659/MSM.932962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Yang M., Lai C.-L. Long COVID-19 syndrome: a comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines. 2021;9(8):966. doi: 10.3390/biomedicines9080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawilska J.B., Lagodzinski A., Berezinska M. COVID-19: from the structure and replication cycle of SARS-CoV-2 to its disease symptoms and treatment. J. Physiol. Pharmacol. 2021;72(4):479–501. doi: 10.26402/jpp.2021.4.01. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang F., Shen Y., Zhang X., Cen Y., Wang B., Zhao S., Zhou Y., Hu B., Wang M., Liu Y., Miao H., Jones P., Ma X., He Y., Cao G., Cheng L., Li L. Symptoms and health outcomes among survivors of COVID-19 infection 1 Year after discharge from hospitals in wuhan, China. JAMA Netw. Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen N., Gurdasani D., O'Hara M.E., Hastie C., Roderick P., Yao G., Alwan N.A. Characteristics and impact of long covid: findings from an online survey. PLoS One. 2022;17(3) doi: 10.1371/journal.pone.0264331. [DOI] [PMC free article] [PubMed] [Google Scholar]