This study evaluates whether use of postoperative surgical prophylaxis is correlated with surgical site infection rates in children undergoing nonemergent surgery.
Key Points
Question
In children undergoing nonemergent surgery, does continuation of antimicrobial prophylaxis following incision closure result in lower surgical site infection rates?
Findings
In this multicenter hospital-level cohort study of children undergoing nonemergent surgery, use of postoperative prophylaxis was not correlated with surgical site infection rates after adjusting for differences in procedure mix and patient characteristics among hospitals.
Meaning
These findings further support the recommendations of national and international organizations to discontinue postoperative prophylaxis after skin closure.
Abstract
Importance
Use of postoperative antimicrobial prophylaxis is common in pediatric surgery despite consensus guidelines recommending discontinuation following incision closure. The association between postoperative prophylaxis use and surgical site infection (SSI) in children undergoing surgical procedures remains poorly characterized.
Objective
To evaluate whether use of postoperative surgical prophylaxis is correlated with SSI rates in children undergoing nonemergent surgery.
Design, Setting, and Participants
This is a multicenter cohort study using 30-day postoperative SSI data from the American College of Surgeons’ Pediatric National Surgical Quality Improvement Program (ACS NSQIP-Pediatric) augmented with antibiotic-use data obtained through supplemental medical record review from June 2019 to June 2021. This study took place at 93 hospitals participating in the ACS NSQIP-Pediatric Surgical Antibiotic Prophylaxis Stewardship Collaborative. Participants were children (<18 years of age) undergoing nonemergent surgical procedures. Exclusion criteria included antibiotic allergies, conditions associated with impaired immune function, and preexisting infections requiring intravenous antibiotics at time of surgery.
Exposures
Continuation of antimicrobial prophylaxis beyond time of incision closure.
Main Outcomes and Measures
Thirty-day postoperative rate of incisional or organ space SSI. Hierarchical regression was used to estimate hospital-level odds ratios (ORs) for SSI rates and postoperative prophylaxis use. SSI measures were adjusted for differences in procedure mix, patient characteristics, and comorbidity profiles, while use measures were adjusted for clinically related procedure groups. Pearson correlations were used to examine the associations between hospital-level postoperative prophylaxis use and SSI measures.
Results
Forty thousand six hundred eleven patients (47.3% female; median age, 7 years) were included, of which 41.6% received postoperative prophylaxis (hospital range, 0%-71.2%). Odds ratios (ORs) for postoperative prophylaxis use ranged 190-fold across hospitals (OR, 0.10-19.30) and ORs for SSI rates ranged 4-fold (OR, 0.55-1.90). No correlation was found between use of postoperative prophylaxis and SSI rates overall (r = 0.13; P = .20), and when stratified by SSI type (incisional SSI, r = 0.08; P = .43 and organ space SSI, r = 0.13; P = .23), and surgical specialty (general surgery, r = 0.02; P = .83; urology, r = 0.05; P = .64; plastic surgery, r = 0.11; P = .35; otolaryngology, r = −0.13; P = .25; orthopedic surgery, r = 0.05; P = .61; and neurosurgery, r = 0.02; P = .85).
Conclusions and Relevance
Use of postoperative surgical antimicrobial prophylaxis was not correlated with SSI rates at the hospital level after adjusting for differences in procedure mix and patient characteristics.
Introduction
Surgical site infection (SSI) is the most common complication in pediatric surgery and is associated with prolonged hospitalization and excess hospital cost.1,2,3 Reported rates of SSI associated with elective pediatric surgery have ranged between 0% to 11.4%, depending on procedure and organ system involved.1,4,5,6,7,8,9 Appropriate use of intravenous antimicrobial prophylaxis is essential to mitigate SSI risk, however, overuse of prophylaxis has been associated with antimicrobial resistance and adverse events, including allergic reaction, acute kidney injury, and antibiotic-associated colitis.10,11
With the goal of optimizing antimicrobial stewardship and SSI prevention, duration-based guidelines for surgical prophylaxis were developed by the World Health Organization and US Centers for Disease Control Healthcare Infection Control Practices Advisory Committee, both recommending cessation of prophylaxis following incision closure for clean and clean-contaminated procedures.12,13 These recommendations were based on a growing body of evidence demonstrating a lack of benefit and an increased risk of adverse events associated with extended prophylaxis use.10,12,13,14,15,16 Despite available guidelines, continuation of intravenous prophylaxis following incision closure is common in pediatric surgery, with postoperative use reported in up to 86% of cases.10,17,18,19,20,21,22,23,24
The influence of postoperative prophylaxis on SSI in children remains poorly characterized and may underlie both practice variation and lack of compliance with existing guidelines. With these considerations, the goal of this study was to examine the association between postoperative prophylaxis use and SSI at the hospital level in the context of a national, multicenter pediatric antimicrobial stewardship collaborative. More specifically, we sought to determine whether more extensive use of postoperative prophylaxis was correlated with lower rates of SSI after adjusting for differences among hospitals in procedure mix, patient characteristics, and comorbidity profiles.
Methods
Study Design and Data Source
This was a retrospective multicenter cohort analysis of clinical outcomes and antibiotic use data from 93 hospitals participating in the American College of Surgeon’s Pediatric National Surgical Quality Improvement Program (ACS NSQIP-Pediatric) Surgical Antibiotic Prophylaxis Stewardship Collaborative. A broad array of clinical, laboratory, and outcomes data are collected by NSQIP-Pediatric for 6 pediatric surgical specialties (general surgery, otolaryngology, urology, neurosurgery, orthopedic surgery, and plastic surgery) to compare risk-adjusted adverse event data among its 151 participating hospitals.4 Data are abstracted at each hospital by dedicated, full-time NSQIP-Pediatric surgical clinical reviewers using standardized search criteria and a rigorous medical record review process to identify adverse events (including incisional and organ space SSIs) within the 30-day postoperative period.25 Consistency and accuracy of data are ensured by mandatory recertification of training for chart abstractors, periodic auditing of participating hospitals, and availability of an ACS NSQIP-Pediatric support team to address questions regarding data collection protocols and data definitions.
The NSQIP-Pediatric Surgical Antibiotic Prophylaxis Stewardship Collaborative was launched in 2018 as a partnership of 93 NSQIP-Pediatric hospitals with the goal of collecting an extended set of antimicrobial prophylaxis use measures to assess, compare, and benchmark compliance with consensus guidelines for appropriate use. Supplemental data were collected by NSQIP-Pediatric surgical clinical reviewers and included use and type of prophylactic agents, timing of administration, duration of use, medication allergies, and the presence of preexisting infections requiring antibiotic treatment. A manual of operations was distributed to all participating hospitals to ensure consistency and accuracy of data collection for the antibiotic prophylaxis variables and definitions were reviewed via webinar before the initiation of data collection (eMethods in the Supplement). A data-definitions support team for the collaborative was available to address questions regarding data collection protocol.
This study was approved by the Boston Children’s Hospital institutional review board. A waiver of informed consent was obtained from the institutional review board. This observational study followed Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study Cohort
Patients younger than 18 years who underwent nonemergent clean and clean-contaminated procedures between June 2019 and June 2021 at 93 hospitals participating in the ACS NSQIP-Pediatric Surgical Antibiotic Prophylaxis Stewardship Collaborative were considered for inclusion. A total of 417 nonemergent procedures, as defined by Current Procedure Terminology, were included in the analysis. Included procedures were then aggregated into 60 clinically related procedure groups based on organ system, operative indication, and recommended antimicrobial prophylaxis based on multidisciplinary consensus guidelines.14 Nonemergent procedures were defined as elective cases, as documented by the surgeon or anesthesiologist. Exclusion criteria included allergies to penicillin or cephalosporin antibiotics, conditions and medications associated with impaired immune function, infections or other conditions requiring intravenous antibiotics for treatment at time of surgery, and multiple procedures performed by different surgical specialties during the same operative encounter (eg, tracheostomy and gastrostomy).
Assessment of Postoperative Prophylaxis Use
Use of postoperative prophylaxis was defined as continuation after incision closure of 1 or more of the same intravenous antimicrobial agents used for prophylaxis for the procedure. The final dose of prophylactic antibiotics was considered the dose where no further administration of the same antimicrobial agent occurred. If multiple intravenous antimicrobial agents were continued past the time of incision closure, duration was determined based on the agent with the longest continuation following incision closure.
Assessment of SSIs
SSIs, including incisional SSI and organ space SSI, were identified by NSQIP-Pediatric surgical clinical reviewers through medical record review using standardized NSQIP-Pediatric definitions and medical record review methodology.25 For the purpose of this analysis, superficial and deep incisional SSIs were combined into a single incisional SSI category. The primary outcome for the correlation analysis included occurrence of any SSI (incisional or organ space SSI), and secondary outcomes included incisional and organ space infections that were analyzed separately.
Statistical Analysis
Hierarchical regression was used to estimate hospital-level odds ratios (ORs) for postoperative prophylaxis use and SSI measures. SSI ORs were adjusted for differences among hospitals in procedure mix, patient characteristics, and comorbidity profiles, while prophylaxis use ORs took into account differences in clinically related procedure groups. Following log-transformation of ORs (to meet assumptions of normality), Pearson correlation coefficients were used to explore associations between hospital-level ORs for postoperative prophylaxis use and SSI outcomes. Secondary models were created to examine correlations between postoperative prophylaxis use and SSI outcomes for extended postoperative use (longer than 24 hours) and for individual surgical specialties. A statistically significant result was defined as a P value less than .05. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
Forty thousand six hundred eleven patients were included in the analysis. Median hospital volume was 464 (interquartile range 238-616). In this study, 47.3% of patients were female, 66.7% of patients were White, and the median age was 7 years. Wound class categorizations identified 55.9% of cases as clean and 44.0% as clean contaminated.
Specialties with the largest contribution to the study cohort included general surgery (25.6% of all cases), followed by orthopedic surgery (22.4%), otolaryngology (11.6%), craniofacial surgery (multispecialty category including otolaryngology and plastic surgery [10.5%]), neurosurgery (8.9%), spine surgery (multispecialty category including neurosurgery and orthopedic surgery [8.2%]), and urology (7.6%) (Table 1).
Table 1. Postoperative Prophylaxis Use, Surgical Site Infection Rates, and Relative Contribution to the Study Cohort Associated With Nonemergent Pediatric Surgical Procedure Groups.
| Specialty | Procedure group | No. (%)a | Hospital median procedure volume (IQR) | Any SSI, % | Any postoperative prophylaxis, % | Postoperative prophylaxis >24 h, % |
|---|---|---|---|---|---|---|
| General surgery | Gastrostomy | 2475 (6.1) | 25 (10-41) | 3.8 | 10.3 | 2.0 |
| Pyloromyotomy | 1828 (4.5) | 19 (10-27) | 1.5 | 4.6 | 0.8 | |
| Cholecystectomy | 1319 (3.2) | 14 (7-19) | 0.9 | 5.5 | 1.7 | |
| Pectus | 764 (1.9) | 6 (3-14) | 1.8 | 62.4 | 21.6 | |
| Gastrostomy closure | 713 (1.8) | 7 (3-12) | 4.4 | 5.0 | 1.2 | |
| Gastroesophageal reflux | 527 (1.3) | 5 (3-8) | 3.2 | 14.0 | 3.8 | |
| Thoracic-lung resection | 463 (1.1) | 5 (3-8) | 1.9 | 17.9 | 8.1 | |
| Small bowel | 391 (1) | 4 (3-7) | 5.4 | 44.7 | 20.1 | |
| Colorectal-other | 383 (0.9) | 4 (2-6) | 6.5 | 39.5 | 13.9 | |
| Colorectal-colostomy | 276 (0.7) | 3 (1-5) | 6.9 | 44.7 | 15.3 | |
| Ovary-adnexa | 246 (0.6) | 3 (1-5) | 1.6 | 5.9 | 3.2 | |
| Colorectal-anorectal malformation | 244 (0.6) | 3 (1-5) | 1.2 | 66.1 | 36.0 | |
| Thyroid | 136 (0.3) | 3 (2-5) | 0.0 | 16.2 | 5.9 | |
| Colorectal-pullthrough | 133 (0.3) | 2 (1-3) | 5.3 | 79.2 | 33.2 | |
| Esophagus nonreflux | 124 (0.3) | 2 (1-3) | 11.3 | 56.0 | 20.9 | |
| Orthopedic surgery | Lower extremity | 3850 (9.5) | 38.5 (21-52) | 1.3 | 43.8 | 3.5 |
| Elbow | 2960 (7.3) | 22 (8-46) | 0.7 | 10.3 | 0.5 | |
| Hip | 1367 (3.4) | 14 (6-23) | 1.2 | 84.6 | 14.6 | |
| Foot | 554 (1.4) | 4 (2-9) | 0.7 | 45.8 | 3.0 | |
| Spine | 163 (0.4) | 3 (1-6) | 1.2 | 60.1 | 11.0 | |
| Hand | 160 (0.4) | 3 (1-4) | 3.8 | 2.9 | 0.7 | |
| ENT surgery | Endoscopic airway | 1332 (3.3) | 10 (3-21) | 0.5 | 23.1 | 8.7 |
| Tympanoplasty | 1112 (2.7) | 9 (3-27) | 0.9 | 2.1 | 0.9 | |
| Cochlear implant | 1052 (2.6) | 13 (5-21) | 1.4 | 14.6 | 1.1 | |
| Mastoid | 766 (1.9) | 8 (4-13) | 3.3 | 3.9 | 0.3 | |
| Tracheostomy | 207 (0.5) | 3 (1-5) | 17.9 | 8.9 | 0.8 | |
| ENT-salivary | 152 (0.4) | 2 (1-3) | 2.0 | 25.2 | 7.9 | |
| Multispecialty ENT and plastic surgery | Cleft palate | 1270 (3.1) | 12 (7-20) | 0.1 | 42.6 | 7.1 |
| Cleft lip | 1069 (2.6) | 12 (6-17) | 0.7 | 30.3 | 2.8 | |
| Rhinoplasty-nasal | 957 (2.4) | 10 (5-16) | 0.4 | 14.8 | 2.9 | |
| Cleft alveolar bone graft | 631 (1.6) | 8.5 (3.5-15.5) | 0.5 | 42.3 | 4.0 | |
| Pharyngeal | 237 (0.6) | 3 (1-5) | 0.8 | 31.1 | 7.1 | |
| Facial cartilage graft | 116 (0.3) | 2 (1-3) | 6.0 | 41.2 | 24.3 | |
| Neurosurgery | Neurosurgery (no foreign body) | 2003 (4.9) | 18.5 (9-30) | 2.2 | 75.3 | 12.4 |
| Neurosurgery (foreign body) | 1644 (4) | 18 (8-30) | 2.1 | 65.8 | 7.4 | |
| Multispecialty neurosurgery and orthopedics | Spine-idiopathic | 2318 (5.7) | 25 (14-40) | 0.7 | 93.4 | 32.1 |
| Spine-neuromuscular | 550 (1.4) | 5 (2-11) | 5.3 | 90.0 | 33.4 | |
| Spine-other | 469 (1.2) | 5 (2-9) | 2.1 | 92.9 | 35.0 | |
| Urology | Urinary reflux | 1122 (2.8) | 11 (7-17) | 0.4 | 70.9 | 15.1 |
| Ureteral reconstruction | 1051 (2.6) | 11 (6-18) | 0.8 | 71.2 | 10.8 | |
| Urology-other | 710 (1.7) | 7 (3-12) | 1.3 | 17.5 | 6.0 | |
| Urinary diversion | 164 (0.4) | 2 (2-4) | 4.3 | 37.9 | 5.8 | |
| Multispecialty general surgery and urology | Testicular | 817 (2) | 10 (4-14) | 1.4 | 1.3 | 1.3 |
| Nephrectomy | 218 (0.5) | 3 (2-5) | 0.9 | 62.2 | 11.7 | |
| Plastic surgery | Plastics-craniomaxillofacial | 332 (0.8) | 4 (2-6) | 2.7 | 71.4 | 31.6 |
| Plastics-breast | 217 (0.5) | 3 (1-6) | 2.8 | 15.1 | 1.4 |
Abbreviations: ENT, ear, nose, and throat; IQR, interquartile range.
Data are presented for procedure groups with more than 100 patients.
Postoperative Surgical Prophylaxis Use
The overall rate of postoperative prophylaxis use was 41.6%, and unadjusted rates ranged from 0% to 71.2% among hospitals. ORs for postoperative prophylaxis use varied 190-fold across hospitals (OR, 0.10-19.30). The overall rate of postoperative prophylaxis use extending more than 24 hours following incision closure was 9.2% with hospital unadjusted rates ranging from 0% to 35.1%. ORs for postoperative prophylaxis use extending more than 24 hours varied 100-fold among hospitals (OR, 0.10-9.65).
SSI Rates
The overall rate of any SSI in the study cohort was 1.8%, and unadjusted hospital rates ranged from 0% to 7.3%. Following adjustment for procedure mix, patient characteristics, and comorbidity profiles, ORs for any SSI varied 4-fold across hospitals (OR, 0.55-1.90). The overall rate of incisional SSI in the study cohort was 1.4%, and unadjusted hospital rates ranged from 0% to 6.5%. Risk-adjusted ORs for incisional SSI varied 4-fold among hospitals (OR, 0.48-2.22). The overall rate of organ space SSI in the study cohort was 0.43%, and unadjusted hospital rates ranged from 0% to 1.6% among hospitals. Following risk adjustment, ORs for organ space SSI varied 3-fold among hospitals (OR, 0.63-1.89).
Correlation Between Postoperative Prophylaxis Use and SSI Rates
Hospital-level correlation of log-transformed ORs revealed no association between postoperative prophylaxis use and SSI outcomes (r = 0.13; P = .20; Figure 1), nor between prophylaxis use extending more than 24 hours after incision closure and SSI outcomes (r = −0.02; P = .87; Figure 1). Correlations remained insignificant between log-transformed ORs for postoperative prophylaxis use and SSI outcomes when further stratified by incisional SSI (r = 0.08; P = .43; Figure 2) and organ space SSI (r = 0.13; P = .23; Figure 2), and when stratified by individual surgical specialty (general surgery, r = 0.02; P = .83; urology, r = 0.05, P = .64; plastic surgery, r = 0.11; P = .35; otolaryngology, r = −0.13; P = .25; orthopedic surgery, r = 0.05; P = .61; and neurosurgery, r = 0.02; P = .85) (Figure 3 and eFigure in the Supplement). Lack of correlation was also observed between log-transformed ORs for prolonged use greater than 24 hours and SSI outcomes at the level of each surgical specialty (Table 2).
Figure 1. Hospital-Level Correlation of Log-Transformed Odds Ratios (ORs) Between Any Surgical Site Infection (SSI).
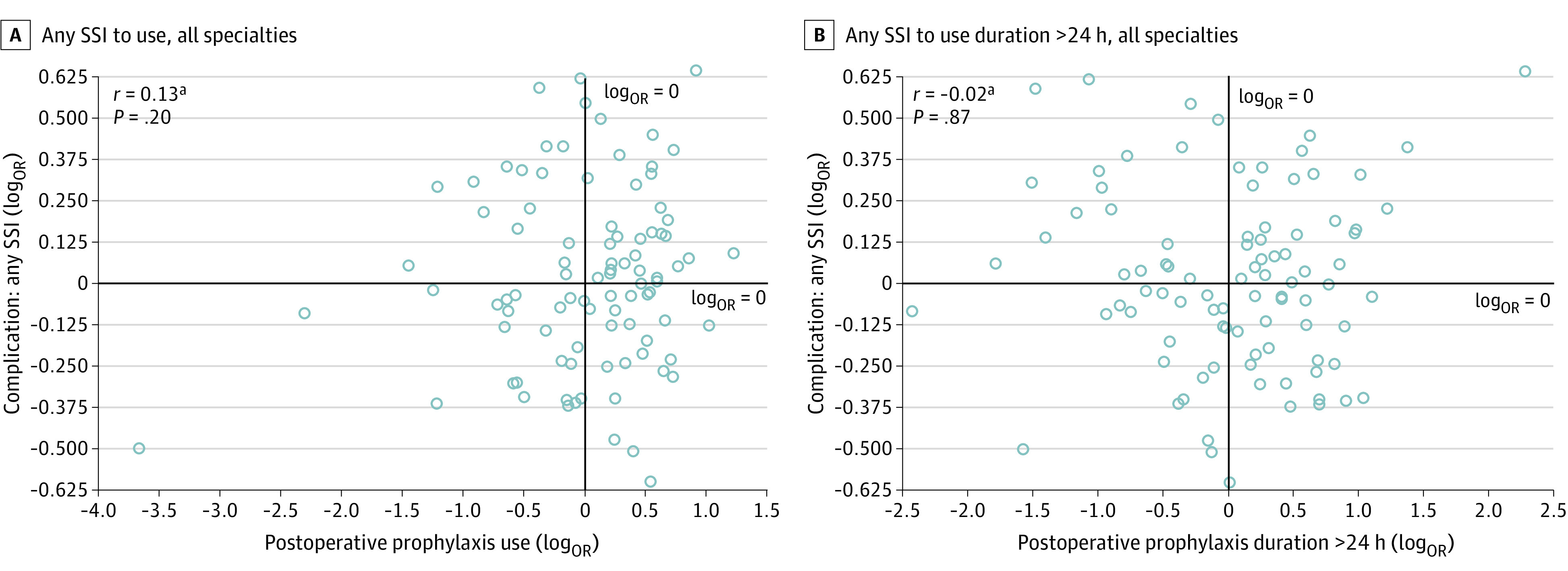
SSI ORs were adjusted for differences in procedure mix, patient characteristics, and comorbidity profiles, while prophylaxis-use ORs accounted for clinically related procedure groups.
aThe log transformation of ORs was used to meet assumptions of normality.
Figure 2. Hospital-Level Correlation of Log-Transformed Odds Ratios (ORs) Between Use of Any Postoperative Surgical Antimicrobial Prophylaxis and Surgical Site Infection (SSI).
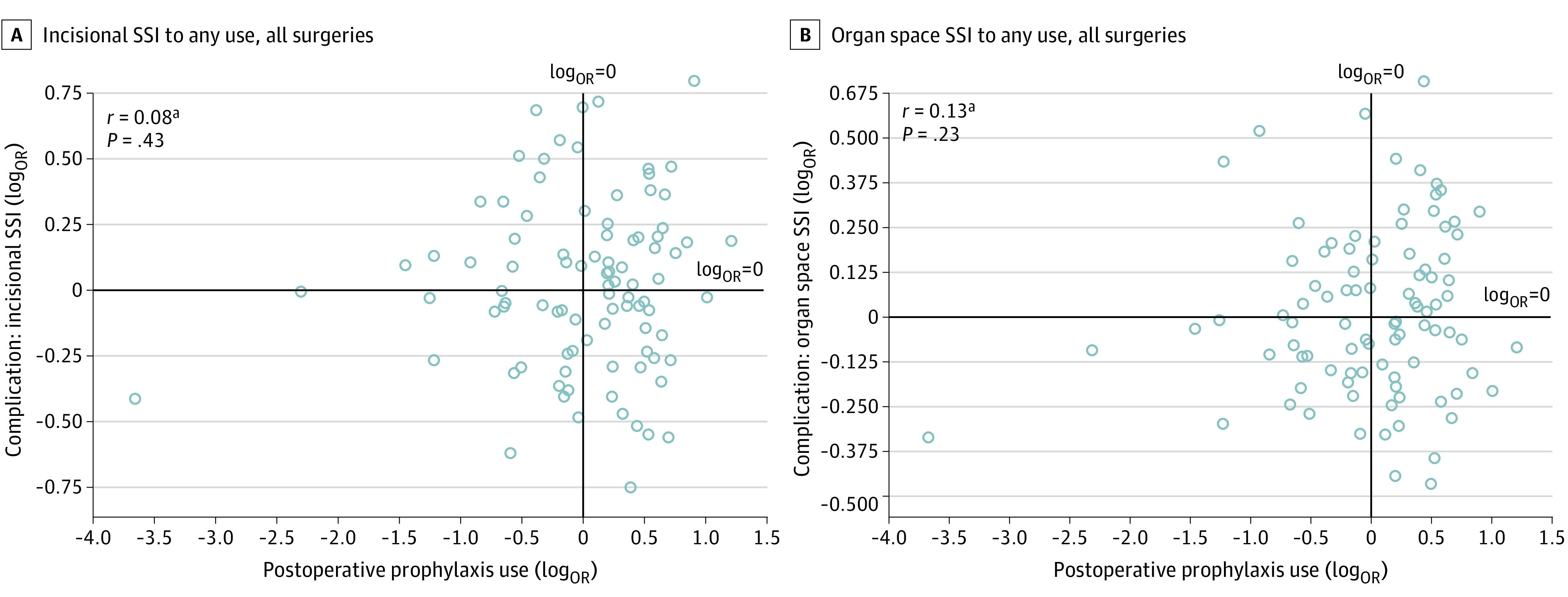
SSI ORs were adjusted for differences in procedure mix, patient characteristics, and comorbidity profiles, while prophylaxis-use ORs accounted for clinically related procedure groups.
aThe log transformation of ORs was used to meet assumptions of normality.
Figure 3. Hospital-Level Correlation of Log-Transformed Odds Ratios (ORs) Between Any Surgical Site Infection (SSI) and Use of Any Postoperative Surgical Antimicrobial Prophylaxis .
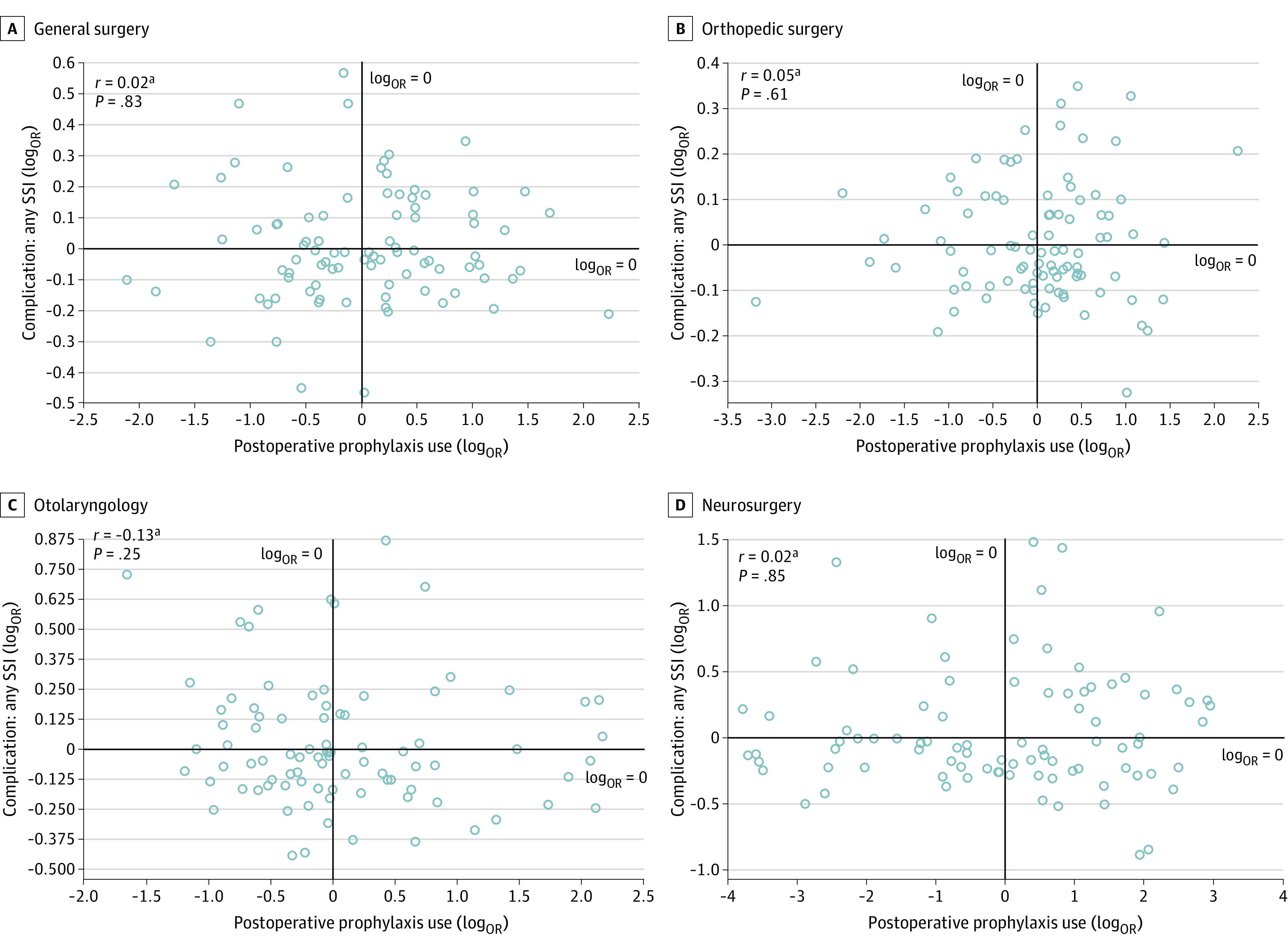
Analysis includes 93 hospitals, stratified by the 4 surgical specialties with the largest relative contribution to the study cohort. SSI ORs were adjusted for differences in procedure mix, patient characteristics, and comorbidity profiles, while prophylaxis-use ORs accounted for clinically related procedure groups.
aThe log transformation of ORs was used to meet assumptions of normality.
Table 2. Surgical Specialty-Level Correlation Between Surgical Site Infection (SSI) and Postoperative Antimicrobial Prophylaxis Use at 93 Hospitals, Stratified by SSI Type and Postoperative Duration.
| Specialty (N) | 30-d Postoperative SSI | Postoperative prophylaxis use | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Any | Extended (>24 h postincision closure) | ||||||||||
| SSI type | Specialty rate, % | Hospital range (adjusted OR, 95% CI)a | Specialty rate, % | Hospital range (adjusted OR, 95% CI) | Correlationb |
P value |
Specialty rate, % | Hospital range (adjusted OR, 95% CI) | Correlation | P value | |
| General surgery (10 398) | Any SSI | 3.00 | 0.63 (0.37-1.08) 1.76 (1.06-3.06) |
21.61 | 0.12 (0.04-0.33) 9.17 (6.18-13.62) |
0.02 | .83 | 6.67 | 0.24 (0.07-0.80) 7.06 (4.40-11.36) |
–0.09 | .40 |
| Incisional | 2.47 | 0.55 (0.29-1.08) 2.03 (1.17-3.57) |
–0.001 | .99 | –0.08 | .44 | |||||
| Organ space | 0.54 | 0.73 (0.27-1.85) 1.84 (0.55-6.16) |
0.07 | .80 | –0.03 | .80 | |||||
| Orthopedic surgery (9112) | Any SSI | 1.13 | 0.72 (0.31-1.69) 1.41 (0.56-3.58) |
40.33 | 0.04 (0.02-0.11) 9.68 (6.67-14.07) |
0.05 | .61 | 4.23 | 0.25 (0.06– to 0.97) 21.71 (14.15 to –33.32) |
–0.07 | .50 |
| Incisional | 1.06 | 0.70 (0.29-1.69) 1.50 (0.52-4.28) |
0.06 | .57 | –0.07 | .48 | |||||
| Organ space | 0.07 | NAc | NA | NA | NA | NA | |||||
| Otolaryngology (4712) | Any SSI | 2.25 | 0.64 (0.24-1.76) 2.38 (0.94-6.03) |
10.21 | 0.19 (0.05-0.78) 8.73 (4.21-18.10) |
–0.13 | .25 | 0.06 | 0.36 (0.06-2.17) 21.18 (5.62-79.91) |
0.08 | .45 |
| Incisional | 0.98 | 0.51 (0.07-3.49) 19.92 (2.40-34.87) |
–0.08 | .46 | 0.24 | .02 | |||||
| Organ space | 1.27 | 0.68 (0.22-2.05) 2.10 (0.44-9.91) |
–0.06 | .56 | –0.13 | .22 | |||||
| Multidisciplinary (ENT + plastic surgery) (4280) | Any SSI | 0.56 | 0.64 (0.11-3.77) 5.06 (0.75-34.19) |
33.76 | 0.06 (0.01-0.31) 13.58 (3.18-58.03) |
0.01 | .93 | 4.42 | 0.27 (0.05-1.40) 21.45 (7.61-60.46) |
0.09 | .39 |
| Incisional | 0.51 | 0.63 (0.11-3.76) 5.30 (0.68-41.42) |
0.03 | .78 | 0.14 | .20 | |||||
| organ space | 0.05 | NA | NA | NA | NA | NA | |||||
| Neurosurgery (3647) | Any SSI | 2.11 | 0.41 (0.12-1.44) 4.41 (1.34-14.53) |
71.02 | 0.02 (0.01-0.09) 19.29 (4.20-80.88) |
0.02 | .85 | 10.01 | 0.34 (0.09-1.32) 7.25 (3.82-13.78) |
0.03 | .77 |
| Incisional | 1.34 | 0.52 (0.14-1.86) 3.34 (0.64-17.56) |
–0.05 | .65 | –0.03 | .79 | |||||
| Organ space | 0.77 | NA | NA | NA | NA | NA | |||||
| Multidisciplinary (neurosurgery + orthopedics) (3337) | Any SSI | 1.65 | 0.86 (0.35-2.12) 1.29 (0.33-4.97) |
92.79 | 0.001 (0-0.05) 4.09 (0 to -50.67) |
0.01 | .90 | 32.51 | 0.03 (0.00-0.29) 125.44 (2.19-185.42) |
–0.01 | .95 |
| Incisional | 1.32 | 0.78 (0.28-2.15) 1.65 (0.37-7.30) |
0.03 | .76 | –0.04 | .75 | |||||
| Organ space | 0.36 | 0.68 (0.06-7.27) 17.21 (0.17-97.58) |
–0.11 | .33 | 0.01 | .91 | |||||
| Urology (3086) | Any SSI | 0.91 | 0.69 (0.20-2.44) 1.79 (0.29-9.30) |
57.00 | 0.03 (0.01-0.06) 5.97 (2.56-13.89) |
0.05 | .64 | 10.73 | 0.21 (0.06-0.76) 13.00 (4.49-37.67) |
0.18 | .10 |
| Incisional | 0.58 | 0.69 (0.13-3.35) 1.79 (0.19-5.35) |
–0.003 | .98 | 0.14 | .21 | |||||
| Organ space | 0.32 | 0.66 (0.08-5.43) 6.95 (0.14-39.15) |
0.07 | .52 | 0.08 | .44 | |||||
| Multidisciplinary (general surgery+ urology) (1049) | Any SSI | 1.24 | NA | 19.97 | 0.08 (0.01-0.60) 13.28 (0.72-140.21) |
–0.14 | .23 | 2.96 | 0.67 (0.15-3.03) 3.50 (0.51-24.16) |
0.13 | .28 |
| Incisional | 1.05 | NA | –0.11 | .31 | 0.13 | .29 | |||||
| Organ space | 0.19 | NA | –0.05 | .64 | 0.04 | .76 | |||||
| Plastic surgery (644) | Any SSI | 2.95 | 0.78 (0.18-3.49) 3.49 (0.13-91.92) |
46.70 | 0.10 (0.02-0.51) 14.29 (4.16-49.04) |
0.11 | .35 | 17.08 | 0.18 (0.03-1.02) 11.24 (1.66-75.94) |
–0.11 | .34 |
| Incisional | 2.95 | NA | NA | NA | NA | NA | |||||
| Organ space | 0 | NA | NA | NA | NA | NA | |||||
Abbreviations: ENT, ear, nose, and throat; NA, not applicable; OR, odds ratio.
SSI odds ratios were adjusted for differences in procedure mix, patient characteristics, and comorbidity profiles, while prophylaxis use odds ratios accounted for clinically related procedure groups.
Correlation coefficients were calculated on log-transformed ORs to meet assumptions of normality.
Due to low event rate, models for certain specialties did not converge (denoted with NA) and were thus left out of this analysis.
Discussion
In this analysis of 40 611 nonemergent pediatric surgical procedures from 93 hospitals, a high rate of noncompliance and practice variation was observed for consensus guidelines recommending against postoperative prophylaxis use. Hospitals with higher rates of postoperative use had similar SSI rates as compared with those with lower rates of postoperative antibiotic use, and findings were consistent across both incisional and organ space SSIs. Furthermore, high rates of noncompliance and negligible correlation between postoperative prophylaxis use and SSI measures were found within each individual surgical specialty.
The results of this study add to the growing body of evidence characterizing high rates of postoperative prophylaxis use in pediatric surgery.17,19,22,24 To date, published data examining the influence of postoperative prophylaxis use on SSI in pediatric surgery have been conflicting and largely limited to retrospective studies or small randomized clinical trials focusing on individual procedures.26,27,28,29,30 To our knowledge, the present study represents the most comprehensive and robust analysis to date examining the association between postoperative prophylaxis use and SSI rates in pediatric surgery. Furthermore, this is the first analysis to assess this association across multiple surgical procedures and pediatric surgical specialties using standardized definitions and a rigorous medical record review process for both exposures and outcomes.
Significant variability was observed in this analysis across hospitals for both SSI measures and postoperative prophylaxis use. However, it is noteworthy that the absolute magnitude of hospital-level variation was much greater for postoperative prophylaxis use than for SSI measures. Absolute differences in rates of incisional SSI among hospitals ranged from 0% to 6.5%, while use of postoperative prophylaxis ranged from 0% to 71.2%. This variation imbalance, along with the lack of correlation between postoperative use and SSI rates, provides important context for the prioritization of antimicrobial stewardship and SSI prevention efforts. Accordingly, opportunities to reduce postoperative prophylaxis use are likely to be far more impactful among most hospitals than improvement efforts aimed at reducing SSI rates. Also, the lack of correlation between postoperative prophylaxis use and SSI outcomes and similar variation imbalance was observed for each individual surgical specialty, suggesting that substantial opportunities to improve antibiotic stewardship may exist throughout all areas of pediatric surgery.
The reasons for poor compliance in pediatric surgery with guidelines recommending against the use of postoperative prophylaxis are likely multifactorial. Lack of knowledge regarding both evidence-based guidelines for antibiotic use and actual SSI risk associated with different procedures may drive postoperative continuation of antibiotics.31,32,33 Regional practice variation and prescriber risk aversion may also play a role.31 While the cost of antimicrobial resistance is difficult to quantify on a per-patient basis, the medical and financial consequences of a SSI are more easily quantified and may influence surgeons to prolong antibiotic duration.1,32,34 Furthermore, SSI events are often monitored by hospital and payor organizations, and pay-for-performance programs have been used by the latter to penalize hospitals with higher than average SSI rates.35 While antibiotic stewardship programs are required by the US Centers for Medicare and Medicaid Services, there are no direct financial consequences associated with poor stewardship, creating an environment in which antibiotic stewardship efforts may be undermined by pressure to reduce SSI rates.36 Additionally, there remains a paucity of pediatric-focused data in the published literature examining the association between postoperative prophylaxis use and SSI rates, and existing guidelines have largely been derived from adult literature.10,12,37 Pediatric surgeons may therefore not consider adult guidelines and the evidence to support them to be relevant to their patients.
The results of this analysis provide a call to arms for stewardship efforts to reduce postoperative prophylaxis use, particularly for hospitals that are relatively high users compared with their peers. Successful efforts to reduce prophylaxis use have included multidisciplinary peer-to-peer educational programs combined with electronic health record targeting prompts and routine feedback.32,38,39,40 Passive education alone, such as posting paper guidelines in areas of physician prescribing, has been less effective.32 Improvement in compliance with antibiotic duration guidelines has been demonstrated following implementation of bundled interventions, including local multidisciplinary guideline creation, antibiotic verification during the surgical timeout process, electronic order reminders discouraging routine antibiotic continuation, email notifications for inappropriate antibiotic use, and in-person educational sessions for nurses, attending surgeons, and residents.38,39,40 Importantly, successful implementation of comprehensive antibiotic stewardship initiatives has not been associated with increased SSI rates.38,40 Additionally, interhospital collaboration and peer benchmarking may have important roles in identifying opportunities for improved stewardship and for sharing best practices. The ACS NSQIP-Pediatric Surgical Antimicrobial Prophylaxis Stewardship Collaborative was developed with this goal in mind and has supported a multitude of best practice webinars to promote improved antimicrobial stewardship. Furthermore, the analysis reported in this study was also used to develop a benchmarking report card to provide comparative performance data for both postoperative use and SSI rates for participating collaborative hospitals.
Limitations
The results of this study should be considered in the context of its limitations. Although NSQIP-Pediatric uses a rigorous medical record review process and standardized definitions for both exposures and outcomes, data collected in NSQIP-Pediatric are retrospective and errors in misclassification, identification of outcomes, and antibiotic administration are possible. Intraoperative details, such as presence of gross contamination or use of surgical implants, were not available and may have influenced postoperative antibiotic use. Continuation of postoperative intravenous antibiotics may therefore have been appropriate for some cases as ongoing treatment rather than prolonged use of prophylaxis. However, the study cohort was limited to clean and clean-contaminated cases, which should limit the influence of this type of misclassification. Variation in the use of postoperative oral antibiotics for prophylactic indications other than for SSI prevention (eg, postoperative urinary prophylaxis) was not captured and may have influenced the findings of the study. The results of this analysis were based on outcomes and prophylaxis use profiles associated with NSQIP-Pediatric hospitals and may not be generalizable to other health care settings. Additionally, this analysis was performed at the hospital level and outcomes were based on a pooled analysis of many different surgical procedures. It is possible that some procedures may benefit from prolonged postoperative use, as has been reported for sternotomy-associated infections in cardiac surgery.18 Further efforts will need to examine practice variation and outcomes associated with postoperative use at a procedure level to identify conditions with the greatest opportunity for improved stewardship.
Conclusions
Despite the limitations above, the results of the study demonstrate that more extensive use of postoperative surgical prophylaxis was not associated with a correlation of lower rates of SSI at the hospital level after adjusting for differences in procedure mix and patient characteristics. These findings suggest that implementation of targeted antimicrobial stewardship efforts to reduce postoperative use is possible without an increase in SSI rate. Although this analysis focused on children undergoing nonemergent procedures, the approach may provide a framework to quantify and assess the effect of postoperative use for other areas of surgery where both high rates and practice variation exist around the use of postoperative prophylaxis.
eMethods. NSQIP-Pediatric Surgical Antibiotic Prophylaxis Variables
eFigure. Hospital-level correlation of log-transformed odds ratios between any surgical site infection (incisional or organ space) and use of any postoperative surgical antimicrobial prophylaxis at 93 hospitals, stratified by all surgical specialties.
References
- 1.de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387-397. doi: 10.1016/j.ajic.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 2.Kulaylat AN, Rocourt DV, Tsai AY, et al. Understanding readmissions in children undergoing surgery: a pediatric NSQIP analysis. J Pediatr Surg. 2018;53(7):1280-1287. doi: 10.1016/j.jpedsurg.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 3.Linnaus ME, Ostlie DJ. Complications in common general pediatric surgery procedures. Semin Pediatr Surg. 2016;25(6):404-411. doi: 10.1053/j.sempedsurg.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 4.Raval MV, Dillon PW, Bruny JL, et al. ; ACS NSQIP Pediatric Steering Committee . American College of Surgeons National Surgical Quality Improvement Program Pediatric: a phase 1 report. J Am Coll Surg. 2011;212(1):1-11. doi: 10.1016/j.jamcollsurg.2010.08.013 [DOI] [PubMed] [Google Scholar]
- 5.Horwitz JR, Chwals WJ, Doski JJ, Suescun EA, Cheu HW, Lally KP. Pediatric wound infections: a prospective multicenter study. Ann Surg. 1998;227(4):553-558. doi: 10.1097/00000658-199804000-00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng C, Sidhwa F, Cameron DB, Glass C, Rangel SJ. Rates and burden of surgical site infections associated with pediatric colorectal surgery: insight from the National Surgery Quality Improvement Program. J Pediatr Surg. 2016;51(6):970-974. doi: 10.1016/j.jpedsurg.2016.02.063 [DOI] [PubMed] [Google Scholar]
- 7.Alganabi M, Biouss G, Pierro A. Surgical site infection after open and laparoscopic surgery in children: a systematic review and meta-analysis. Pediatr Surg Int. 2021;37(8):973-981. doi: 10.1007/s00383-021-04911-4 [DOI] [PubMed] [Google Scholar]
- 8.Hollenbeak CS, Alfrey EJ, Sheridan K, Burger TL, Dillon PW. Surgical site infections following pediatric liver transplantation: risks and costs. Transpl Infect Dis. 2003;5(2):72-78. doi: 10.1034/j.1399-3062.2003.00013.x [DOI] [PubMed] [Google Scholar]
- 9.Rinke ML, Jan D, Nassim J, Choi J, Choi SJ. Surgical site infections following pediatric ambulatory surgery: an epidemiologic analysis. Infect Control Hosp Epidemiol. 2016;37(8):931-938. doi: 10.1017/ice.2016.98 [DOI] [PubMed] [Google Scholar]
- 10.Branch-Elliman W, O’Brien W, Strymish J, Itani K, Wyatt C, Gupta K. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019;154(7):590-598. doi: 10.1001/jamasurg.2019.0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anandalwar SP, Milliren C, Graham DA, et al. Trends in the use of surgical antibiotic prophylaxis in general pediatric surgery: are we missing the mark for both stewardship and infection prevention? J Pediatr Surg. 2020;55(1):75-79. doi: 10.1016/j.jpedsurg.2019.09.057 [DOI] [PubMed] [Google Scholar]
- 12.National Library of Medicine . Summary of a systematic review on surgical antibiotic. Accessed August 31, 2022. https://www.ncbi.nlm.nih.gov/books/NBK536429/
- 13.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. ; Healthcare Infection Control Practices Advisory Committee . Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784-791. doi: 10.1001/jamasurg.2017.0904 [DOI] [PubMed] [Google Scholar]
- 14.Bratzler DW, Dellinger EP, Olsen KM, et al. ; American Society of Health-System Pharmacists; Infectious Diseases Society of America; Surgical Infection Society; Society for Healthcare Epidemiology of America . Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14(1):73-156. doi: 10.1089/sur.2013.9999 [DOI] [PubMed] [Google Scholar]
- 15.Ban KA, Minei JP, Laronga C, et al. Executive Summary of the American College of Surgeons/Surgical Infection Society surgical site infection guidelines-2016 update. Surg Infect (Larchmt). 2017;18(4):379-382. doi: 10.1089/sur.2016.214 [DOI] [PubMed] [Google Scholar]
- 16.Balch A, Wendelboe AM, Vesely SK, Bratzler DW. Antibiotic prophylaxis for surgical site infections as a risk factor for infection with Clostridium difficile. PLoS One. 2017;12(6):e0179117. doi: 10.1371/journal.pone.0179117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciofi degli Atti M, Spila Alegiani S, Raschetti R, et al. ; APACHE Study Group . Surgical antibiotic prophylaxis in children: adherence to indication, choice of agent, timing, and duration. Eur J Clin Pharmacol. 2015;71(4):483-488. doi: 10.1007/s00228-015-1816-0 [DOI] [PubMed] [Google Scholar]
- 18.Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101(25):2916-2921. doi: 10.1161/01.CIR.101.25.2916 [DOI] [PubMed] [Google Scholar]
- 19.Giordano M, Squillace L, Pavia M. Appropriateness of surgical antibiotic prophylaxis in pediatric patients in Italy. Infect Control Hosp Epidemiol. 2017;38(7):823-831. doi: 10.1017/ice.2017.79 [DOI] [PubMed] [Google Scholar]
- 20.Gouvêa M, Novaes C de O, Pereira DMT, Iglesias AC. Adherence to guidelines for surgical antibiotic prophylaxis: a review. Braz J Infect Dis. 2015;19(5):517-524. doi: 10.1016/j.bjid.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinger G, Carmeli I, Feigin E, Freud E, Steinberg R, Levy I. Compliance with surgical antibiotic prophylaxis guidelines in pediatric surgery. Eur J Pediatr Surg. 2015;25(2):199-202. doi: 10.1055/s-0034-1368798 [DOI] [PubMed] [Google Scholar]
- 22.Kronman MP, Hersh AL, Gerber JS, et al. Identifying antimicrobial stewardship targets for pediatric surgical patients. J Pediatric Infect Dis Soc. 2015;4(4):e100-e108. doi: 10.1093/jpids/piv022 [DOI] [PubMed] [Google Scholar]
- 23.Gerber JS, Kronman MP, Ross RK, et al. Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol. 2013;34(12):1252-1258. doi: 10.1086/673982 [DOI] [PubMed] [Google Scholar]
- 24.Anandalwar SP, Milliren C, Graham DA, et al. Quantifying Procedure-level Prophylaxis Misutilization in Pediatric Surgery: Implications for the Prioritization of Antimicrobial Stewardship Efforts. Ann Surg. Published online July 7, 2022. doi: 10.1097/SLA.0000000000005480 [DOI] [PubMed] [Google Scholar]
- 25.American College of Surgeons . ACS NSQIP Pediatric Variables and Definitions. In: National Surgical Quality Improvement Project Pediatric Operations Manual 2019:1-189. [Google Scholar]
- 26.Caseris M, Ilharreborde B, Doit C, et al. Is Cutibacterium acnes early surgical site infection rate related to the duration of antibiotic prophylaxis in adolescent idiopathic scoliosis surgery? Eur Spine J. 2020;29(7):1499-1504. doi: 10.1007/s00586-020-06427-2 [DOI] [PubMed] [Google Scholar]
- 27.Weiss K, Simon A, Graf N, Schöpe J, Oertel J, Linsler S. Clinical practice audit concerning antimicrobial prophylaxis in paediatric neurosurgery: results from a German paediatric oncology unit. Childs Nerv Syst. 2017;33(1):159-169. doi: 10.1007/s00381-016-3279-8 [DOI] [PubMed] [Google Scholar]
- 28.Akgür FM, Cahit Tanyel F, Büyükpamukçu N, Hiçsönmez A. Prophylactic antibiotics for colostomy closure in children: short versus long course. Pediatr Surg Int. 1992;7(4):279-281. doi: 10.1007/BF00183980 [DOI] [Google Scholar]
- 29.Sayed-Hassan A, Hermann R, Chidiac F, et al. ; the Otolaryngology–Head and Neck Surgical Infection Survey Group (OSS Group) of Clermont-Ferrand . Association of the duration of antibiotic therapy with major surgical site infection in cochlear implantation. JAMA Otolaryngol Head Neck Surg. 2019;145(1):14-20. doi: 10.1001/jamaoto.2018.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holle J, Finger T, Lugonja J, et al. The influence of perioperative antibiotic prophylaxis on wound infection and on the colonization of wound drains in patients after correction of craniosynostosis. Front Pediatr. 2021;9:720074. doi: 10.3389/fped.2021.720074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cadieux G, Tamblyn R, Dauphinee D, Libman M. Predictors of inappropriate antibiotic prescribing among primary care physicians. CMAJ. 2007;177(8):877-883. doi: 10.1503/cmaj.070151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avorn J, Solomon DH. Cultural and economic factors that (mis)shape antibiotic use: the nonpharmacologic basis of therapeutics. Ann Intern Med. 2000;133(2):128-135. doi: 10.7326/0003-4819-133-2-200007180-00012 [DOI] [PubMed] [Google Scholar]
- 33.Malone SM, Seigel NS, Newland JG, Saito JM, McKay VR. Understanding antibiotic prophylaxis prescribing in pediatric surgical specialties. Infect Control Hosp Epidemiol. 2020;41(6):666-671. doi: 10.1017/ice.2020.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903-3910. doi: 10.2147/IDR.S234610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahn CN III, Ault T, Potetz L, Walke T, Chambers JH, Burch S. Assessing Medicare’s hospital pay-for-performance programs and whether they are achieving their goals. Health Aff (Millwood). 2015;34(8):1281-1288. doi: 10.1377/hlthaff.2015.0158 [DOI] [PubMed] [Google Scholar]
- 36.Department of Health and Human Services . Medicare and Medicaid Programs; regulatory provisions to promote program efficiency, transparency, and burden reduction; fire safety requirements for certain dialysis facilities; hospital and critical access hospital changes to promote innovation, flexibility, and improvement in patient care. 2019. https://s3.amazonaws.com/public-inspection.federalregister.gov/2019-20736.pdf
- 37.Vicentini C, Politano G, Corcione S, et al. Surgical antimicrobial prophylaxis prescribing practices and impact on infection risk: results from a multicenter surveillance study in Italy (2012-2017). Am J Infect Control. 2019;47(12):1426-1430. doi: 10.1016/j.ajic.2019.07.013 [DOI] [PubMed] [Google Scholar]
- 38.Dimopoulou A, Kourlaba G, Psarris A, Coffin S, Spoulou V, Zaoutis T. Perioperative antimicrobial prophylaxis in pediatric patients in Greece: compliance with guidelines and impact of an educational intervention. J Pediatr Surg. 2016;51(8):1307-1311. doi: 10.1016/j.jpedsurg.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 39.Donà D, Luise D, Barbieri E, et al. Effectiveness and sustainability of an antimicrobial stewardship program for perioperative prophylaxis in pediatric surgery. Pathogens. 2020;9(6):E490. doi: 10.3390/pathogens9060490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.So JP, Aleem IS, Tsang DS, Matlow AG, Wright JG; SickKids Surgical Site Infection Task Force . Increasing compliance with an antibiotic prophylaxis guideline to prevent pediatric surgical site infection: before and after study. Ann Surg. 2015;262(2):403-408. doi: 10.1097/SLA.0000000000000934 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. NSQIP-Pediatric Surgical Antibiotic Prophylaxis Variables
eFigure. Hospital-level correlation of log-transformed odds ratios between any surgical site infection (incisional or organ space) and use of any postoperative surgical antimicrobial prophylaxis at 93 hospitals, stratified by all surgical specialties.


