Abstract
This study examines adverse events and durability of response of SER-109, an investigational microbiome therapeutic comprised of purified Firmicutes spores, compared with placebo for Clostridioides difficile infection.
Antibiotics are necessary but often insufficient to treat recurrent Clostridioides difficile infection (rCDI) due to persistent spores that germinate into toxin-producing bacteria within a disrupted gut microbiome.1,2 In a phase 3 trial (ECOSPOR III), SER-109, an investigational microbiome therapeutic composed of purified Firmicutes spores, was superior to placebo in reducing the rate of rCDI by week 8 (12% vs 40%; relative risk [RR], 0.32 [95% CI, 0.18-0.58]).3 We assessed the prespecified secondary end points of adverse events and durability of response through 24 weeks and time to recurrence.
Methods
ECOSPOR III was a double-blind, randomized, multicenter trial conducted from July 2017 to September 2020. The trial was approved by investigational review boards and participants provided written informed consent. Adults with rCDI (≥3 CDI episodes within 12 months, inclusive of the qualifying episode) were randomized to receive SER-109 or matching placebo administered as 4 capsules daily for 3 days. All patients were required to have a positive C difficile test result for toxin production and symptom resolution after standard-of-care antibiotics.3
Secondary end points included rCDI rates at 4, 12, and 24 weeks and time to recurrence, with confirmed CDI recurrence defined as at least 3 unformed stools daily over 2 days continuing until antibiotic initiation, positive C difficile test result for toxin production, and investigator decision to treat.
Treatment-emergent adverse events (AEs) were collected through week 8; serious AEs and AEs of special interest (eg, bacteremia, abscess, meningitis) were collected through week 24.
Efficacy analyses were performed using SAS, version 9.4 (SAS Institute), and included all randomized participants analyzed according to randomized treatment assignment. The RR (ie, recurrence rate with SER-109 divided by recurrence rate with placebo), adjusted for randomization strata, was tested (1-sided test [α = .025] of the null hypotheses that RR ≥1) using Cochran-Mantel-Haenszel methods. Risk difference (ie, SER-109 recurrence rate minus placebo recurrence rate) was tested using a 2-sided χ2 test (α = .05). Time to CDI recurrence was analyzed using Kaplan-Meier methods and a log-rank test. As previously specified, patients who left the study early, were lost to follow-up, or died were imputed as recurrence. Patients who were missing components for rCDI criteria were also imputed if the documented components were consistent with recurrence (eg, positive toxin test result). These criteria were also applied for the Kaplan-Meier analysis of time to recurrence over 24 weeks.
Results
There were 182 patients randomized (mean age, 65.5 years; 59.9% women). Comorbidities were common (mean Charlson Comorbidity Index score of 4.1 in the SER-109 group and 4.2 in the placebo group).4 Sixty-three of 182 patients had rCDI through 24 weeks (19 [21.3%] in the SER-109 group vs 44 [47.3%] in the placebo group). A significantly lower proportion of patients in the SER-109 group vs the placebo group had rCDI at weeks 4, 8, 12, and 24 (Table).
Table. Cumulative Recurrent Clostridioides difficile Infection Rates, Rate Differences, and Relative Risks at Weeks 4, 8, 12, and 24a.
| Time pointb | No. (%) | Rate difference (95% CI)c | P value | Relative risk (95% CI)d | P value | |
|---|---|---|---|---|---|---|
| SER-109 (n = 89) | Placebo (n = 93) | |||||
| 4 wk | 10 (11.2) | 31 (33.3) | −22.1 (−33.4 to −10.1) | <.001 | 0.35 (0.19 to 0.67) | <.001 |
| 8 wk | 11 (12.4) | 37 (39.8) | −27.4 (−38.9 to −14.8) | <.001 | 0.32 (0.18 to 0.58) | <.001 |
| 12 wk | 16 (18.0) | 43 (46.2) | −28.3 (−40.3 to −14.8) | <.001 | 0.40 (0.24 to 0.65) | <.001 |
| 24 wk | 19 (21.3) | 44 (47.3) | −26.0 (−38.4 to −12.2) | <.001 | 0.46 (0.30 to 0.73) | <.001 |
Patients with recurrence (confirmed and imputed) shown for all time points. Confirmed recurrence was defined as ≥3 unformed stools per day over 2 consecutive days, a positive C difficile stool toxin assay (enzyme immunoassay or cell cytotoxicity neutralization assay), a decision by the investigator to treat, and the requirement that diarrhea continued until antibiotics were initiated. Patients who were lost to follow-up, left the study prematurely, or died were imputed as a recurrence. If a patient had 1 missing criterion (eg, toxin test) but other criteria were documented (eg, diarrhea, decision to treat), recurrence was imputed.
Week 8 was the study primary efficacy end point. Weeks 4, 12, and 24 are secondary efficacy end points defined in the statistical analysis plan.
SER-109 minus placebo recurrence rate. P value is from a χ2 test for differences between treatment groups. 95% CIs are based on the Newcombe-Wilson method. Negative values favor SER-109.
SER-109 divided by placebo recurrence rate adjusted for stratification based on Cochran-Mantel-Haenszel method. 95% CI based on Greenland-Robins method. Values less than 1 favor SER-109.
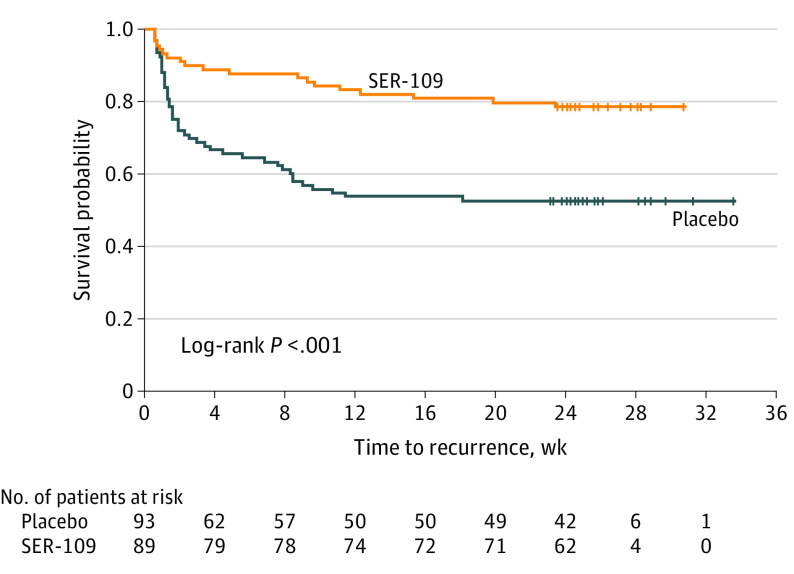
Among patients who experienced recurrence, 65.1% had a recurrence by week 4; only 6.3% of patients had a recurrence between week 12 and week 24 (Table). There was a significant difference between treatment groups for Kaplan-Meier estimates of time to recurrence (log-rank P < .001). Benefit from SER-109 was evident at week 2 and was durable through 24 weeks (Figure). The median (range) time of rCDI was 3.3 (0.6-23.4) weeks for SER-109 and 1.6 (0.6-18.1) weeks for placebo.
Figure. Survival Function for Time to Clostridioides difficile Infection Recurrence.
Patients' duration of participation may be longer than the planned study duration. The last contact date was used as the censoring date.
Treatment-emergent AEs that occurred in at least 5% of patients, and more frequently in the SER-109 vs placebo group, included abdominal distension, constipation, diarrhea, and urinary tract infection. Serious AEs occurred in 15 patients in the SER-109 group and 19 in the placebo group; none were considered drug-related. Serious urinary tract infections occurred in 3 SER-109 recipients and in no placebo recipients and AEs of special interest were reported in 7 patients (4 in the SER-109 group and 3 in the placebo group; all resolved). No isolated pathogens were SER-109 species. Three patients (1 in the SER-109 group and 2 in the placebo group) discontinued the study secondary to worsening of preexisting conditions. One patient in each group withdrew due to serious treatment-emergent AEs. Three deaths occurred in the SER-109 group 15, 60, and 164 days after receiving the intervention; none were deemed drug-related.3
Discussion
SER-109 durably reduced rCDI rates and was well-tolerated through 24 weeks in patients with prevalent comorbidities. The benefit of SER-109 was evident as early as week 2, highlighting the need for rapid microbiome repair after completing standard-of-care antibiotics. One study limitation was the exclusion of patients with first recurrence, although this subgroup is similarly characterized by microbiome disruption.5 These data support a potential role for this investigational oral microbiome therapeutic in the treatment of patients with this debilitating infection.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Senior Editor.
References
- 1.Seekatz AM, Young VB. Clostridium difficile and the microbiota. J Clin Invest. 2014;124(10):4182-4189. doi: 10.1172/JCI72336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theriot CM, Young VB. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu Rev Microbiol. 2015;69(1):445-461. doi: 10.1146/annurev-micro-091014-104115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med. 2022;386(3):220-229. doi: 10.1056/NEJMoa2106516 [DOI] [PubMed] [Google Scholar]
- 4.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 5.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435-438. doi: 10.1086/525047 [DOI] [PubMed] [Google Scholar]