Key Points
Question
Can proton magnetic resonance spectroscopy (1H-MRS) at 3 T detect metabolic changes in individuals with major depressive disorder (MDD), and are these changes associated with acute depressive episodes?
Findings
In this cross-sectional study including 396 mostly medication-free young adults, past MDD was associated with low prefrontal γ-aminobutyric acid (GABA) and glutamate levels and high glutamine levels.
Meaning
In this study, GABA and glutamate metabolic disturbances were detected by 3-T 1H-MRS; these disturbances were associated with MDD diagnosis rather than to acute symptoms.
This cross-sectional study investigates changes in γ-aminobutyric acid, glutamate, and glutamine levels in a voxel in the left dorsolateral prefrontal cortex of participants with no, past, and current major depressive disorder using proton magnetic resonance spectroscopy.
Abstract
Importance
Major depressive disorder (MDD) is one of the most prevalent illnesses worldwide. Perturbations of the major inhibitory and excitatory neurotransmitters, γ-aminobutyric acid (GABA) and glutamate (Glu), respectively, as well as Glx (Glu or glutamine [Gln]) have been extensively reported in a multitude of brain areas of individuals with depression, but few studies have examined changes in Gln, the metabolic counterpart of synaptic Glu.
Objective
To investigate changes in GABA, Glx, Glu, and Gln levels in a voxel in the left dorsolateral prefrontal cortex of participants with no, past, and current MDD using proton magnetic resonance spectroscopy (1H-MRS).
Design, Setting, and Participants
This community-based study used a cross-sectional design using 3-T 1H-MRS in participants not taking MDD medication recruited from the community. The sample consisted of 251 healthy controls, 98 participants with a history of past MDD, and 47 participants who met the diagnostic criteria for current MDD. Diagnostic groups were comparable regarding age, education, income, and diet. Data were collected from March 2014 to October 2021, and data were analyzed from October 2021 to June 2022.
Main Outcomes and Measures
GABA, Glx, Glu, and Gln concentrations in the left dorsolateral prefrontal cortex.
Results
Of 396 included participants, 258 (65.2%) were female, and the mean (SD) age was 25.0 (4.7) years. Compared with healthy controls, those with past MDD and current MDD had lower GABA concentrations (mean [SEM] concentration: healthy controls, 2.70 [0.03] mmol/L; past MDD, 2.49 [0.05] mmol/L; current MDD, 2.54 [0.07] mmol/L; 92 with past MDD vs 236 healthy controls: r = 0.18; P = .002; 44 with current MDD vs 236 healthy controls: r = 0.13; P = .04). Compared with healthy controls, those with past MDD also had lower Glu concentrations (mean [SEM] concentration: healthy controls, 7.52 [0.06] mmol/L; past MDD, 7.23 [0.11] mmol/L; 93 with past MDD vs 234 healthy controls: r = 0.16; P = .01) and higher Gln concentrations (mean [SEM] concentration: healthy controls, 1.63 [0.04] mmol/L; past MDD, 1.84 [0.07] mmol/L; 66 with past MDD 153 healthy controls: r = 0.17; P = .04).
Conclusions and Relevance
In a large, mostly medication-free community sample, reduced prefrontal GABA concentrations were associated with past MDD, consistent with histopathologic studies reporting reduced glial cell and GABA cell density in the prefrontal cortex in individuals with depression. Patients with MDD also demonstrated increased Gln levels, indicative of increased synaptic Glu release, adding to previous evidence for the Glu hypothesis of MDD.
Introduction
There is substantial evidence for a GABAergic deficiency in individuals with major depressive disorder (MDD) from various types of studies, including pharmacological, postmortem, and genetic studies.1,2 A 2016 meta-analysis3 including 230 patients with MDD and 230 healthy controls found reduced brain γ-aminobutyric acid (GABA) concentrations in patients with active depression but not in those with depression in remission in various prefrontal and occipital brain regions. These findings have been confirmed by more recent studies.2,4,5 However, at least 1 recent study found conflicting results,6 reporting increased prefrontal GABA concentrations in 31 patients with first-episode depression relative to 64 healthy controls. Previous authors have explained heterogeneity across studies by substantial variation of clinical characteristics, including medication, clinical states, and diagnostic methods.2,7
There is also growing evidence for glutamatergic abnormalities in individuals with MDD. A 2019 meta-analysis8 of magnetic resonance spectroscopy (MRS) studies including 1180 patients and 1066 healthy controls found significant decreases in Glx (glutamate [Glu] or glutamine [Gln]) in the medial prefrontal cortex in patients with depression. However, subanalyses on Glu and Gln measured separately remained inconclusive, and the authors underline the methodological difficulties, such as referencing methods, that might have led to inconsistent results among MRS studies. In addition, results were more prominent in patients taking medications, suggesting that antidepressant medications rather than MDD pathogenesis might explain some of the observed changes in Glu. One 2019 study9 found increased Gln levels in medication-free patients with MDD in the pregenual anterior cingulate cortex, while another study found normal Gln levels in the anterior cingulate cortex but increased Gln levels in the putamen in medication-free patients with MDD.10 Another MRS study in 34 medication-free, symptomatic, chronically ill patients with MDD and 32 healthy volunteers demonstrated increased Glx levels in patients,4 adding to the disagreement in the literature and emphasizing the need to measure Glu and Gln levels separately in larger medication-free MDD samples.
The goal of our study was to clarify some of the inconsistent results and substantial heterogeneity in the MRS literature relating to changes in GABA, Glx, Glu, and Gln levels in individuals with MDD. In most previous studies, patients were recruited from a clinical setting, such as from a dose-finding trial of ketamine,4 from an outpatient service,6 or an inpatient department of a psychiatric hospital.11 As a result, recruitment methods for patients and controls differed considerably, which is a well-known and important confounding factor in psychiatric research because of important differences in educational level, activity level, and living conditions between diagnostic groups caused by the recruitment strategy. To address this problem, we recruited all participants from the community, resulting in a sample with almost no sociodemographic differences between diagnostic groups. In previous studies, including our own,12,13 there were differences in the recruitment for participants with remitted and current MDD. In contrast, in the current study, we used the same community-based methods to make these MDD groups as comparable as possible. Because medication has been a major confounding factor in previous MRS research, we studied a largely medication-free and never-medicated sample in this study. Since psychiatric conditions may have an impact on brain creatinine levels,8 we applied water referencing with correction for tissue composition. To clarify inconsistent Glx results in participants with MDD, we selected a voxel region in the dorsolateral prefrontal cortex (DLPFC), where high spectral quality can be achieved because of the high field homogeneity, thereby increasing the chances for reliable separation of Glu from Gln.14,15,16 A standardized set of measurements were used for voxel positioning to ensure a consistent voxel position between participants.
Based on the findings from previous MRS, histopathologic, and neurochemical studies, we hypothesize that GABA and Glu levels, reflecting GABA cell and glia cell integrity, respectively, would be decreased in the left DLPFC of participants with MDD. We hypothesized that such decreases would be more prominent in acute MDD compared with remitted or partially remitted MDD. In addition, based on our previous observations of an association between Gln levels and neuroticism,16 we hypothesized that Gln levels, which potentially reflects Glu neurotransmission, would be increased in participants with MDD. Finally, we examined the associations between GABA, Glx, Glu, and Gln levels with depression severity and with specific depressive symptoms in explanatory analyses.
Methods
Recruitment
Participants were admitted to the prescreening process of the study after a full explanation of the risks and goals and after giving verbal consent. Written informed consent was obtained from all participants after confirming the following criteria during the prescreening process: age between 18 and 40 years, generally no intake of psychoactive medication or drugs for 3 months prior to the study assessment (we made exceptions in 10 participants with irregular or low-dose intake), and abstinence from caffeine, nicotine, and alcohol the day before and on the day of testing. We excluded participants with a history or family history of severe psychiatric disorders, such as psychosis, substance use disorder, and acute eating disorders, as well as those with a history of neurological or severe physical disorders (eg, heart disease). Participants were recruited by advertisements in local newspapers or by blackboard webpages of the University of Zurich for this cohort study. We oversampled the study population for current and past MDD by adding the information on some ads that we were interested in participants with depression. Psychiatric diagnoses were made based on the German version of the Structured Clinical Interview for DSM-IV.17 Depression severity was assessed using the 21-item Hamilton Depression Rating Scale (HAM-D),18 the Montgomery-Åsberg Depression Rating Scale (MADRS),19 and the 21-item Beck Depression Inventory (BDI).20 The study was approved by the local ethics committee (Kantonale Ethikkommission Zürich). The study was carried out in accordance with the Declaration of Helsinki for experiments involving humans. Study procedures are described in eMethods 1 in the Supplement.
We initially contacted 578 potential participants, 160 of whom had to be excluded due to meeting exclusion criteria or not meeting inclusion criteria, eg, because they were taking medication or they had a piercing or a tattoo that was not compatible with our magnetic resonance imaging (MRI) safety guidelines. Of the 418 remaining individuals, 19 canceled before attending the study, and 3 canceled while participating for personal reasons (eg, time conflicts, resumption of medication intake, loss of interest). The final sample included 396 young adults from the general population, of which 251 were considered healthy (mean [SD] HAM-D score, 1.0 [2.0]), 98 had a history of past MDD (mean [SD] HAM-D score, 6.2 [5.0]), and 47 met the diagnostic criteria for current MDD (mean [SD] HAM-D score, 12.8 [8.3]). Of the 396 participants included in this study, 83 had a current disorder other than depression (mainly anxiety disorders), while 80 were diagnosed with a past disorder (mainly anxiety disorders but also substance use disorder, posttraumatic stress disorder, and eating disorders). Ten participants took psychoactive medication in the 3 months before the study, 21 took such substances in the past with a 3-month or longer drug-free interval prior to the study, and 365 were medication naive. Results are shown in eResults 1 in the Supplement.
MRI Data Acquisition and Analyses
MRI data were obtained on a 3-T GE Discovery MR750 whole-body MRI scanner (GE Healthcare), equipped with an 8-channel receive-only head coil and a body transmit coil. We acquired MR spectra from a 25 × 40 × 30-mm voxel in the left frontal lobe, centered in the DLPFC (Figure 1) using the MEGA-PRESS method (echo time, 69 milliseconds; repetition time, 2000 milliseconds; 320 averages; and an 8-step phase cycle). More details on MRS data acquisition and analyses can be found in eMethods 2 in the Supplement. DLPFC has been implicated in depression by various types of evidence,21 including evidence of reduced glia cell counts in the left dorsal anterolateral PFC,22 which is included in our voxel. This is relevant because glia cell pathology is generally supposed to underlie Glu and Gln disturbances in individuals with MDD.9 In addition, the left side was chosen because of the direction of the chemical shift effect on our scanner, where by default water is shifted to the right and fat is shifted to the left. Because of the relatively large voxel size, some subcortical gray matter was also included in the most inferior part of the voxel, although the center of the voxel was positioned in the DLPFC. Spectra were analyzed with LCModel (eMethods 2 in the Supplement), and the quality of the spectra was evaluated by the Cramer Rao lower bounds (CRLB) of the LCModel fit. CRLB cutoffs of 20% for GABA, Glu, and Glx and 30% for Gln were used, resulting in the exclusion of 24 GABA values, 23 Glu values, and 149 Gln values from the statistical analyses.
Figure 1. Magnetic Resonance Spectroscopy Voxel Position and Representative Spectrum.
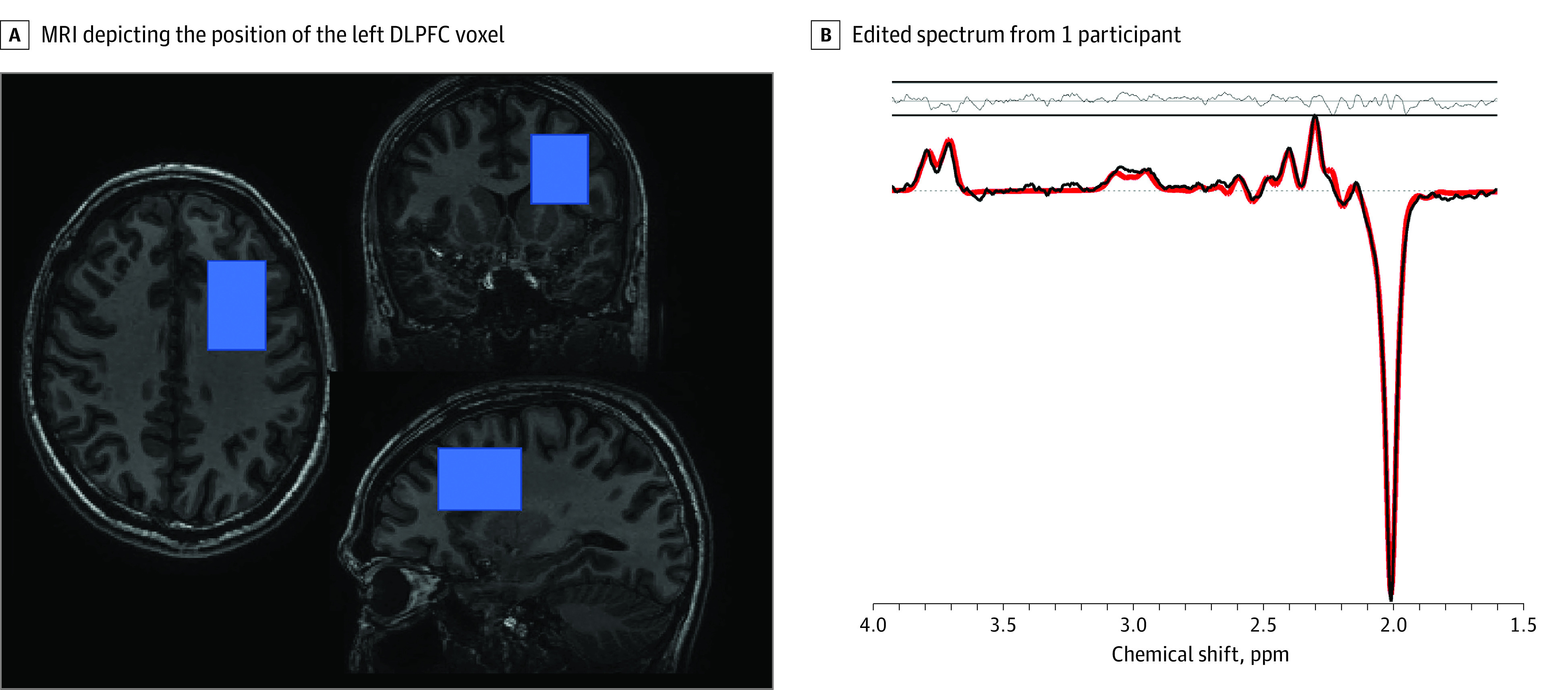
A, Triplanar 3-dimensional T1-weighted magnetic resonance imaging (MRI) showing the position of the dorsolateral prefrontal cortex (DLPFC) magnetic resonance spectroscopy voxel (overlaid in blue). B, The reconstructed spectrum is depicted in black, with the mean LCModel fit overlaid in red. The residuals between the data and the fit are depicted above the spectrum.
Statistical Analysis
Statistical analyses were performed with Jamovi version 1.6.23.0 and R version 4.0.3 (The R Foundation). As the distribution of most of the variables, apart from Glu, differed significantly from normality in a Shapiro-Wilk test, nonparametric tests were used for the analyses. Calculations of groupwise differences were carried out using pairwise Wilcoxon rank sum tests (healthy controls vs participants with past MDD vs participants with current MDD) with R. r Values in these analyses were calculated as the absolute (positive) standardized z statistic, divided by the square root of the sample size using the wilcox_effsize function in the rstatix package; see Rosenthal23 for detailed information. All statistical significance values were adjusted for false discovery rate (FDR).
Between-group analyses were FDR-corrected for the metabolites GABA, Glx, Glu, and Gln and the diagnostic groups (past MDD, current MDD, and controls) in the whole-group and sex-split subgroup analyses. All partial Spearman analyses included the voxel gray matter fraction as a covariate and incorporated the following variables: GABA, Glx, Glu, and Gln concentrations as well as HAM-D, MADRS, and BDI scores.
There is a considerable sexual dimorphism in MDD, with up to 60% of pathogenetic paths differing between sexes.24 In particular, sex hormones may substantially influence GABA and Glu neurotransmission in the brain.25 In addition, we observed different sex distributions in our samples (Table). As a result, we reported differences in GABA, Glu, and Gln concentrations between the sexes,26 and we also calculated post hoc pairwise Wilcoxon rank sum comparisons and Spearman correlation analyses in subgroups split by sex. Post hoc partial Spearman correlation analyses were calculated using the partial.r function of the psych package in R. Significance was set at P < .05, and all P values were 2-tailed.
Table. Characteristics of the Diagnostic Groups.
| Characteristic | Mean (SD) | Test | ||
|---|---|---|---|---|
| Healthy controls | Participants with past MDD | Participants with current MDD | ||
| Sex, No. (%) | ||||
| Female | 153 (61) | 75 (77) | 30 (64) | χ22 = 7.57a |
| Male | 98 (39) | 23 (23) | 17 (36) | |
| Age, y | 24.92 (4.47) | 24.43 (4.78) | 25.74 (5.46) | F2,389 = 1.26 |
| Education, y | 14.13 (2.82) | 13.75 (2.94) | 13.60 (3.05 | F2,369 = 0.95 |
| Incomeb | 2.06 (1.5) | 1.77 (1.03) | 2.00 (1.49) | F2,390 = 1.52 |
| MEDAS total score | 6.71 (1.99) | 7.25 (2.05) | 7.00 (2.58) | F2,148 = 0.98 |
Abbreviations: MDD, major depressive disorder; MEDAS, Mediterranean Diet Adherence Screener.
P < .05.
Income was recorded on an ordinal scale from 1 to 9 (1, CHF <$10 000 [US <$10 367]; 2, CHF $10 000 to $30 000 [US $10 367 to $31 100]; 3, CHF $30 000 to $50 000 [US $31 100 to $51 834]; 4, CHF $50 000 to $70 000 [US $51 834 to $72 568]; 5, CHF $70 000 to $100 000 [US $72 568 to $103 669]; 6, CHF $100 000 to $130 000 [US $103 669 to $134 769]; 7, CHF $130 000 to $160 000 [US $134 769 to $165 870]; 8, CHF $160 000 to $200 000 [US $165 870 to $207 338]; 9 = CHF >$200 000 [US >$207 338]).
Results
Main Analyses
Of 396 included participants, 258 (65.2%) were female, and the mean (SD) age was 25.0 (4.7) years. Diagnostic groups did not differ significantly concerning gray matter ratio (χ22 = 2.52; P = .28), age, education, income, or Mediterranean diet (Table) but showed significantly different sex distributions (eResults 2 in the Supplement). Therefore, between-group analyses of neurometabolite concentrations were not corrected for any of these variables, but we conducted sex subgroup analysis to account for the significant distribution of sex between the groups. The mean (SD) water linewidth of the included spectra was 5.5 (1.02) Hz or 0.043 (0.008) ppm, and Gln was reliably separated from Glu in 248 participants (62.6%).
Compared with healthy controls, those with past MDD and current MDD had lower left DLPFC GABA concentrations (mean [SEM] concentration: healthy controls, 2.70 [0.03] mmol/L; past MDD, 2.49 [0.05] mmol/L; current MDD, 2.54 [0.07] mmol/L; 92 with past MDD vs 236 healthy controls: r = 0.18; FDR-corrected P = .002; 44 with current MDD vs 236 healthy controls: r = 0.13; FDR-corrected P = .04), but no significant differences between the past and current MDD groups (r = 0.01; FDR-corrected P = .90). In general, results were more prominent in the female than in the male subgroup. Sex-specific analyses are reported in eResults 3 in the Supplement.
We found no significant differences in left DLPFC Glx concentration between diagnostic groups. When calculating pairwise Wilcoxon rank sum comparisons, we found significantly lower left DLPFC Glu concentrations in participants with a history of MDD than in healthy controls (mean [SEM] concentration: healthy controls, 7.52 [0.06] mmol/L; past MDD, 7.23 [0.11] mmol/L; current MDD, 7.44 [0.13] mmol/L; 93 with past MDD vs 234 healthy controls: r = 0.16; FDR-corrected P = .01), but there were no significant differences between controls and participants with current MDD (44 with current MDD vs 234 healthy controls: r = 0.02; FDR-corrected P = .78) or between the MDD groups (93 with past MDD vs 44 with current MDD: r = 0.16; FDR-corrected P = .10) (Figure 2).
Figure 2. Boxplots of Left Dorsolateral Prefrontal Cortex (DLPFC) Neurotransmitter Concentrations by Major Depressive Disorder (MDD) Diagnosis.
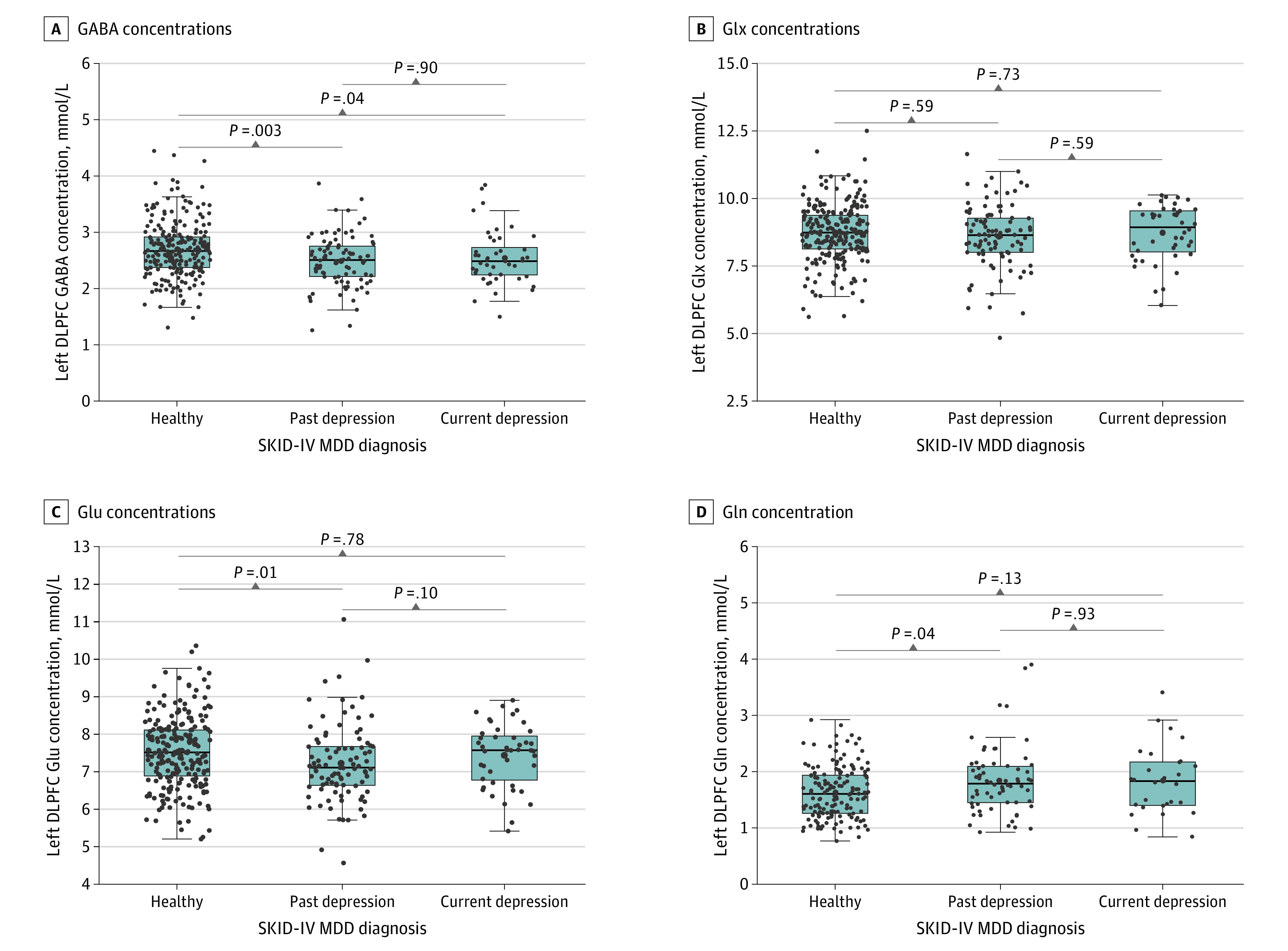
A, Kruskal-Wallis χ22 = 13.597; P = .001. B, Kruskal-Wallis χ22 = 1.214; P = .55. C, Kruskal-Wallis χ22 = 8.940; P = .01. D, Kruskal-Wallis χ22 = 7.480; P = .02. All measures were corrected for atrophy and water scaling. Boxes indicate the IQRs; midlines, the median; and error bars, 95% CIs. GABA indicates γ-aminobutyric acid; Gln, glutamine; Glu, glutamate; Glx, glutamate or glutamine; SKID-IV, German version of the Structured Clinical Interview for DSM-IV.
Left DLPFC Gln concentration was significantly higher in the past MDD group compared with healthy controls (mean [SEM] concentration: healthy controls, 1.63 [0.04] mmol/L; past MDD, 1.84 [0.07] mmol/L; current MDD, 1.84 [0.11] mmol/L; 66 with past MDD vs 153 healthy controls: r = 0.17; FDR-corrected P = .04), but there were no differences between the current MDD group and healthy controls (28 with current MDD vs 153 healthy controls: r = 0.13; FDR-corrected P = .13) or between the MDD groups (66 with past MDD vs 28 with current MDD: r = 0.01; FDR-corrected P = .93).
Results of GABA, Glu, and Gln differences between depression groups survived the introduction of comorbid psychiatric disorders as covariates in an analysis of covariance, although the Gln results diminished to nonsignificant levels when anxiety disorders were included as a covariate. Excluding the 10 participants who took psychoactive therapeutic drugs at the time of the examinations did not change the results of this study (eResults 1 in the Supplement). Including gray matter fraction as a covariate did not change the results of this study, except for the GABA difference between the current MDD group and the control group, which no longer reached statistical significance.
Post Hoc Analyses
In the female subgroup, we found significantly lower Glu concentrations in participants with past MDD compared with healthy controls (mean [SEM] concentration: healthy controls, 7.52 [0.08] mmol/L; past MDD, 7.16 [0.12] mmol/L; current MDD, 7.58 [0.13] mmol/L; 75 with past MDD vs 153 healthy controls: r = 0.19; FDR-corrected P = .02) and significantly higher Glu concentrations in participants with current MDD compared with participants with past MDD (30 with past MDD vs 153 healthy controls: r = 0.25; FDR-corrected P = .01). See eFigures 1 and 2 in the Supplement for boxplots of the female and male subgroups.
Generally, we did not find statistically significant correlations between MRS measures and depression severity in the current and past MDD groups, with a single exception: Glx level was significantly associated with BDI total score (ρ = 0.251; P = .02; n = 87) in the past MDD group. We found no significant associations with severity in the current MDD group, but there were several nonsignificant correlations with comparable effect sizes to those of the correlation between Glx and BDI (eg, Gln and HAM-D total score: ρ = −0.302; P = .13; n = 27; GABA and HAM-D total score: ρ = −0.191; P = .25; n = 39). For an exploratory factor analysis of BDI items and a Spearman correlation analysis of neurometabolites and the identified factors, see eResults 4 in the Supplement.
Discussion
The major findings of this study include reduced GABA and Glu levels and increased Gln levels in the left DLPFC of individuals with past MDD, ie, remitted and partially remitted MDD. Glx concentration was not associated with diagnostic groups, possibly because of the inverse direction of changes observed for Glu and Gln, but it is important to note the difference in statistical power for the different metabolite analyses because of the difference in data points available, particularly for Gln.
In this study, we attempted to address a number of limitations inherent in previous studies, particularly with regard to the participant recruitment and medication status, resulting in diagnostic groups comparable in age, education, income, and diet and including a largely never-medicated subgroup with MDD. In addition, the MRS protocol was selected to maximize spectral quality (examining a large brain region in which the field homogeneity is typically high, enabling spectra to be acquired with high signal-to-noise ratio and a narrow spectral linewidth), and reliability (using standardized measurements for voxel localization to account for differences in head position between participants). The lack of a reliable separation of Gln from Glu in 38% of participants underscores the challenges of robust Gln measurement at 3 T, even with a protocol selected for high spectral quality. However, because of the large group size, the remaining set of Gln values passing the quality criteria (n = 248, including 94 with current or past MDD) comprises a larger data set than has been available to date, particularly at 3 T.
In a large mostly medication-free sample recruited from the community, we observed decreased GABA and Glu levels and elevated Gln levels in in the left DLPFC of participants with remitted or partially remitted MDD as well as in those with a current MDD diagnosis in the case of GABA, while we found no differences for Glx levels. To our knowledge, this is the first 3-T MRS study concerning GABA metabolism and MDD to calculate GABA levels in the DLPFC referenced to water rather than creatine or N-acetyl aspartate (NAA). In addition, in two-thirds of the spectra, we were able to separate Glu from Gln, elucidating some of the Glx changes reported previously and providing important insight into glutamatergic changes in individuals with depression. Although a meta-analysis suggests that NAA and creatine levels do not differ in individuals with depression,27 another study has found referencing to creatine to yield questionable results.8 Our findings can confirm that changes reported in studies referencing GABA to NAA or creatine are indeed specific to GABA and Gln (rather than the reference metabolite), and our results are also consistent with those from 7-T studies, also investigating water-scaled GABA concentrations in individuals with depression.28
In general, more significant groupwise differences were observed for the subgroup of participants with past depression (compared with controls) than those for the subgroup with current depression, possibly attributable to lack of power because of the smaller number of participants who reached the diagnosis criteria for current MDD (n = 44) compared with the number of participants who reached the diagnostic criteria for a past MDD phase (n = 92). In fact, effect sizes were similar for GABA (past MDD vs healthy controls: r = 0.18; current MDD vs healthy controls: r = 0.13), Gln (past MDD vs healthy controls: r = 0.17; current MDD vs healthy controls: r = 0.12), and Glx (past MDD vs healthy controls: r = 0.05; current MDD vs healthy controls: r = 0.02) but different for Glu (past MDD vs healthy controls: r = 0.16; current MDD vs healthy controls: r = 0.02). This lack of power in the current MDD group may particularly affect the Gln analysis where the group numbers were even smaller (66 with past MDD and 28 with current MDD), although these group numbers are still comparable with the sample sizes for many previous MRS studies in individuals with MDD.8,27
Lower GABA levels seen in participants with depression are consistent with reports from previous studies29 describing reduced GABA concentrations in a multitude of cortical regions, such as the PFC,6 the anterior cingulate cortex,11,30,31 and occipital cortex.28,32,33 Our findings are also consistent with the GABAergic deficit hypothesis of MDD1,34 that emphasizes the role of cortical GABAergic interneurons in the PFC in MDD where most of the prefrontal GABA pool exists.
The significant between-group alterations observed in GABA, Glu, and Gln concentrations, the latter of which is thought to represent a marker for synaptic Glu release,35,36 provide further evidence for the prefrontal excitation-inhibition imbalance hypothesis of MDD.34 Increased Gln levels in individuals with MDD is consistent with preclinical studies showing that stress increases extracellular Glu levels in the prefrontal cortex, potentially leading to glia cell pathology, reflected by reduced glia cell counts, density and gene products, and impaired Glu-Gln cycling,37 which is in agreement with our finding of reduced total Glu concentration in individuals with MDD.
The lack of a significant group effect for Glx is likely to arise from the inverse direction of effects found in the Glu and Gln concentrations from which Glx was calculated, indicating that Glx concentration might be a suboptimal measure of Glu pathology in individuals with MDD if Glu and Gln can be separated reliably.
Within the past MDD and current MDD groups, we did not find correlations between GABA, Glu, and Gln concentrations with depression severity. In addition, decreases in GABA concentration were more consistent in those with past MDD than in those with current MDD. As a result, we conclude that the association between GABA concentration in the left DLPFC and the severity of acute depressive symptoms was inconsistent. An exploratory analysis revealed that reduced GABA concentrations was associated with somatic symptoms, such as weight loss and loss of energy, while increased Gln concentration was associated with a negative self-schema. This is in line with our preliminary report that glutamatergic therapeutics, such as ketamine, have the power to improve symptoms related to a negative self-schema.38
With regards to the sexual dimorphism of MDD,39,40,41 we conducted post hoc analyses in sex subgroups to account for the influence of sex on the above analyses. Comparisons in the female subgroup echoed those of the whole sample.
Limitations
The following limitations merit comment. We used a 3-T scanner; higher field strengths may have yielded better peak separation, particularly for Gln measurement. Our voxel contained tissues outside the DLPFC, including anterior insula, dorsal caudate, and white matter and therefore cannot be interpreted as purely a DLPFC voxel, although it was centered on the DLPFC. However, our voxel position offered high spectral quality and reliability, which is a prerequisite for reliable separation of Gln from Glu at 3 T. Because of the low cerebral concentration of Gln, we used a more liberal cutoff (30%), as applied previously for Gln,14,15,16 since the CRLB percentage correlate inversely with the metabolite concentration. Since the controls demonstrated lower Gln levels, the more liberal cutoff resulted in the inclusion of more controls with CRLB between 20% and 30% (n = 82) than patients with past MDD (n = 33) or current MDD (n = 11). We used a measure of GABA+ rather than pure GABA to minimize the effects of field offsets and other sources of experimental instability, which could potentially degrade the spectral quality.42 The cross-sectional design of our study did not allow for inferring causality because a temporal sequence could not be established. Although our total sample was relatively large for a MRS study, the number of participants with current MDD was rather small, especially in the male subgroup, and the depression severity rather low. The preponderance of young and highly educated participants in our sample limited the generalizability of the results.
Conclusions
In conclusion, these findings converge with the results of previous research studies using MRS and other approaches to investigate GABAergic and glutamatergic alterations in the pathogenesis of MDD. In addition, the results demonstrate that dysfunctions of these neurotransmitter systems are not limited to acute phases of the illness but are also present in states of remission. The observation of persistent GABAergic and glutamatergic alterations in individuals with remitted MDD encourages the use of longitudinal MRS to study GABA and Glu abnormalities related to MDD over the course of the disease and underlines the importance of novel antidepressant treatment options, such as ketamine and esketamine, that modulate GABAergic and glutamatergic function.
eMethods 1. Study Procedures
eMethods 2. MRI Data Acquisition and Analyses
eResults 1. Comorbidity Analyses
eResults 2. ANCOVA Correcting for Gray Matter Ratio, Age, and Sex
eResults 3. Analyses in Female and Male Groups
eResults 4. Exploratory Factor Analysis of BDI Items
eFigure 1. Female Subgroup Boxplots
eFigure 2. Male Subgroup Boxplots
eReferences.
References
- 1.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16(4):383-406. doi: 10.1038/mp.2010.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romeo B, Choucha W, Fossati P, Rotge JY. Meta-analysis of central and peripheral γ-aminobutyric acid levels in patients with unipolar and bipolar depression. J Psychiatry Neurosci. 2018;43(1):58-66. doi: 10.1503/jpn.160228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schür RR, Draisma LWR, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37(9):3337-3352. doi: 10.1002/hbm.23244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantrowitz JT, Dong Z, Milak MS, et al. Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder. Transl Psychiatry. 2021;11(1):419. doi: 10.1038/s41398-021-01541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Wang X, Luo M-T, Wang H, Li Y-H. Gamma-aminobutyric acid levels in the anterior cingulate cortex of perimenopausal women with depression: a magnetic resonance spectroscopy study. Front Neurosci. 2019;13:785. doi: 10.3389/fnins.2019.00785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Draganov M, Vives-Gilabert Y, de Diego-Adeliño J, Vicent-Gil M, Puigdemont D, Portella MJ. Glutamatergic and GABA-ergic abnormalities in first-episode depression. a 1-year follow-up 1H-MR spectroscopic study. J Affect Disord. 2020;266:572-577. doi: 10.1016/j.jad.2020.01.138 [DOI] [PubMed] [Google Scholar]
- 7.Luykx JJ, Laban KG, van den Heuvel MP, et al. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36(1):198-205. doi: 10.1016/j.neubiorev.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 8.Moriguchi S, Takamiya A, Noda Y, et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. 2019;24(7):952-964. doi: 10.1038/s41380-018-0252-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colic L, von Düring F, Denzel D, et al. Rostral anterior cingulate glutamine/glutamate disbalance in major depressive disorder depends on symptom severity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(12):1049-1058. doi: 10.1016/j.bpsc.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 10.Godlewska BR, Masaki C, Sharpley AL, Cowen PJ, Emir UE. Brain glutamate in medication-free depressed patients: a proton MRS study at 7 Tesla. Psychol Med. 2018;48(10):1731-1737. doi: 10.1017/S0033291717003373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter M, Henning A, Grimm S, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry. 2009;66(5):478-486. doi: 10.1001/archgenpsychiatry.2009.39 [DOI] [PubMed] [Google Scholar]
- 12.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64(2):193-200. doi: 10.1001/archpsyc.64.2.193 [DOI] [PubMed] [Google Scholar]
- 13.Hasler G, Neumeister A, van der Veen JW, et al. Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;58(12):969-973. doi: 10.1016/j.biopsych.2005.05.017 [DOI] [PubMed] [Google Scholar]
- 14.Bollmann S, Ghisleni C, Poil SS, et al. Developmental changes in gamma-aminobutyric acid levels in attention-deficit/hyperactivity disorder. Transl Psychiatry. 2015;5:e589. doi: 10.1038/tp.2015.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhury FA, O’Gorman RL, Nashef L, et al. Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J Magn Reson Imaging. 2015;41(3):694-699. doi: 10.1002/jmri.24611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasler G, Buchmann A, Haynes M, et al. Association between prefrontal glutamine levels and neuroticism determined using proton magnetic resonance spectroscopy. Transl Psychiatry. 2019;9(1):170. doi: 10.1038/s41398-019-0500-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wittchen H-U, Zaudig M, Fydrich T. Strukturiertes Klinisches Interview für DSM-IV. Hogrefe; 1997. [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. doi: 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 20.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 21.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16(1):61-71. doi: 10.1016/j.tics.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 22.Rajkowska G, Miguel-Hidalgo JJ, Wei J, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45(9):1085-1098. doi: 10.1016/S0006-3223(99)00041-4 [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal R. Parametric measures of effect size. In: Cooper H, Hedges LV, eds. The Handbook of Research Synthesis. Russell Sage Foundation; 1994:231-244. [Google Scholar]
- 24.Kendler KS, Gardner CO. Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. Am J Psychiatry. 2014;171(4):426-435. doi: 10.1176/appi.ajp.2013.13101375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology. 2019;44(1):111-128. doi: 10.1038/s41386-018-0148-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flint J, Kendler KS. The genetics of major depression. Neuron. 2014;81(3):484-503. doi: 10.1016/j.neuron.2014.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147(1):1-25. doi: 10.1016/j.pscychresns.2005.12.004 [DOI] [PubMed] [Google Scholar]
- 28.Song XM, Hu X-W, Li Z, et al. Reduction of higher-order occipital GABA and impaired visual perception in acute major depressive disorder. Mol Psychiatry. 2021;26(11):6747-6755. doi: 10.1038/s41380-021-01090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarawagi A, Soni ND, Patel AB. Glutamate and GABA homeostasis and neurometabolism in major depressive disorder. Front Psychiatry. 2021;12:637863. doi: 10.3389/fpsyt.2021.637863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabbay V, Bradley KA, Mao X, Ostrover R, Kang G, Shungu DC. Anterior cingulate cortex γ-aminobutyric acid deficits in youth with depression. Transl Psychiatry. 2017;7(8):e1216-e1216. doi: 10.1038/tp.2017.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price RB, Shungu DC, Mao X, et al. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65(9):792-800. doi: 10.1016/j.biopsych.2008.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanacora G, Mason GF, Rothman DL, et al. Reduced cortical γ-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56(11):1043-1047. doi: 10.1001/archpsyc.56.11.1043 [DOI] [PubMed] [Google Scholar]
- 33.Bhagwagar Z, Wylezinska M, Jezzard P, et al. Reduction in occipital cortex γ-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol Psychiatry. 2007;61(6):806-812. doi: 10.1016/j.biopsych.2006.08.048 [DOI] [PubMed] [Google Scholar]
- 34.Fogaça MV, Duman RS. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci. 2019;13(87):87. doi: 10.3389/fncel.2019.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Gorman Tuura R, Warnock G, Ametamey S, et al. Imaging glutamate redistribution after acute N-acetylcysteine administration: a simultaneous PET/MR study. Neuroimage. 2019;184:826-833. doi: 10.1016/j.neuroimage.2018.10.017 [DOI] [PubMed] [Google Scholar]
- 36.Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68(9):785-794. doi: 10.1016/j.biopsych.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duman RS, Sanacora G, Krystal JH. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102(1):75-90. doi: 10.1016/j.neuron.2019.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasler G, Suker S, Schoretsanitis G, Mihov Y. Sustained improvement of negative self-schema after a single ketamine infusion: an open-label study. Front Neurosci. 2020;14:687. doi: 10.3389/fnins.2020.00687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritter C, Buchmann A, Müller ST, et al. Cerebral perfusion in depression: relationship to sex, dehydroepiandrosterone sulfate and depression severity. Neuroimage Clin. 2021;32:102840. doi: 10.1016/j.nicl.2021.102840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynch CJ, Gunning FM, Liston C. Causes and consequences of diagnostic heterogeneity in depression: paths to discovering novel biological depression subtypes. Biol Psychiatry. 2020;88(1):83-94. doi: 10.1016/j.biopsych.2020.01.012 [DOI] [PubMed] [Google Scholar]
- 41.Labonté B, Engmann O, Purushothaman I, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23(9):1102-1111. doi: 10.1038/nm.4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edden RA, Oeltzschner G, Harris AD, et al. Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. J Magn Reson Imaging. 2016;44(6):1474-1482. doi: 10.1002/jmri.25304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Study Procedures
eMethods 2. MRI Data Acquisition and Analyses
eResults 1. Comorbidity Analyses
eResults 2. ANCOVA Correcting for Gray Matter Ratio, Age, and Sex
eResults 3. Analyses in Female and Male Groups
eResults 4. Exploratory Factor Analysis of BDI Items
eFigure 1. Female Subgroup Boxplots
eFigure 2. Male Subgroup Boxplots
eReferences.


