This systematic review and meta-analysis evaluates data from 6 trials comparing ketamine and electroconvulsive therapy for the treatment of depression to assess their clinical efficacy and safety.
Key Points
Question
Is ketamine as effective as electroconvulsive therapy (ECT) in patients with major depressive episode?
Findings
This systematic review and meta-analysis of 6 trials with 340 patients suggests that ECT may be superior to ketamine in improving depression severity. Findings also suggest that ketamine and ECT each have unique adverse effect profiles.
Meaning
Although ECT may be more efficacious than ketamine in the acute phase, treatment options should be individualized and patient-centered, considering different adverse effect profiles and patient preferences.
Abstract
Importance
Whether ketamine is as effective as electroconvulsive therapy (ECT) among patients with major depressive episode remains unknown.
Objective
To systematically review and meta-analyze data about clinical efficacy and safety for ketamine and ECT in patients with major depressive episode.
Data Sources
PubMed, MEDLINE, Cochrane Library, and Embase were systematically searched using Medical Subject Headings (MeSH) terms and text keywords from database inception through April 19, 2022, with no language limits. Two authors also manually and independently searched all relevant studies in US and European clinical trial registries and Google Scholar.
Study Selection
Included were studies that involved (1) a diagnosis of depression using standardized diagnostic criteria, (2) intervention/comparator groups consisting of ECT and ketamine, and (3) depressive symptoms as an efficacy outcome using standardized measures.
Data Extraction and Synthesis
Data extraction was completed independently by 2 extractors and cross-checked for errors. Hedges g standardized mean differences (SMDs) were used for improvement in depressive symptoms. SMDs with corresponding 95% CIs were estimated using fixed- or random-effects models. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline was followed.
Main Outcomes and Measures
Efficacy outcomes included depression severity, cognition, and memory performance. Safety outcomes included serious adverse events (eg, suicide attempts and deaths) and other adverse events.
Results
Six clinical trials comprising 340 patients (n = 162 for ECT and n = 178 for ketamine) were included in the review. Six of 6 studies enrolled patients who were eligible to receive ECT, 6 studies were conducted in inpatient settings, and 5 studies were randomized clinical trials. The overall pooled SMD for depression symptoms for ECT when compared with ketamine was −0.69 (95% CI, −0.89 to −0.48; Cochran Q, P = .15; I2 = 39%), suggesting an efficacy advantage for ECT compared with ketamine for depression severity. Significant differences were not observed between groups for studies that assessed cognition/memory or serious adverse events. Both ketamine and ECT had unique adverse effect profiles (ie, ketamine: lower risks for headache and muscle pain; ECT: lower risks for blurred vision, vertigo, diplopia/nystagmus, and transient dissociative/depersonalization symptoms). Limitations included low to moderate methodological quality and underpowered study designs.
Conclusions and Relevance
Findings from this systematic review and meta-analysis suggest that ECT may be superior to ketamine for improving depression severity in the acute phase, but treatment options should be individualized and patient-centered.
Introduction
Major depressive disorder is one of the most common and disabling mental disorders, affecting 15.7 million adults 18 years and older in the United States,1,2 and is the subject of extensive prevention and treatment efforts.3,4 Unfortunately, more than 30% of individuals who experience major depressive episodes (MDEs) do not achieve remission after several trials of antidepressants.5,6 Such treatment-resistant depression (TRD) is associated with premature mortality, including suicide.7,8,9
Endorsed by multiple professional guidelines (eg, American Psychiatric Association),10,11,12 electroconvulsive therapy (ECT) is considered the gold standard treatment for TRD because of its proven high efficacy.13 However, ECT has been underused,14,15 due in part to health care professional barriers (eg, lack of well-trained ECT practitioners across the regions and lack of physical space) and patient barriers (eg, stigma or public attitude and transportation difficulties).16 Furthermore, this treatment has long been associated with adverse cognitive effects, although the risk for these adverse effects has reduced by recent procedural changes (eg, right unilateral with ultra-brief pulse width). Because of cognitive adverse effects and other issues (ie, difficulty of administration), physician-scientists have sought to identify alternative treatment modalities that approach ECT-equivalent efficacy with more tolerable adverse effect and acceptability profiles.
Since 2000, an increasing number of small- to medium-sized clinical trials have shown that low doses of (R,S)-ketamine delivered intravenously can have rapid and robust antidepressant effects in TRD.17,18,19,20,21 While conventional antidepressant medications and adjunctive second-generation antipsychotics target monoaminergic neurotransmission (ie, serotonin, norepinephrine, and dopamine), ketamine is a glutamate N-methyl-d-aspartate (NMDA) receptor antagonist.14 Ketamine has shown rapid and robust antidepressant effects in patients with MDE,22,23,24 and adverse effects are generally mild and self-limiting.25,26 However, unlike its S-enantiomer (esketamine), ketamine is not currently approved by regulatory agencies (eg, the US Food and Drug Administration) for the treatment of depression.27
Establishing the efficacy of ketamine as compared with ECT remains an important clinical question. Several review articles have highlighted the importance of resolving this issue,26,28,29 but no study has yet quantified the overall treatment effect sizes of efficacy and safety outcomes between ketamine and ECT. The aim of this study is to conduct a systematic review and meta-analysis of the clinical trials that compare the efficacy and safety of ketamine and ECT.
Methods
Search Strategy and Reporting Criteria
The protocol pertaining to this study was registered on PROSPERO (CRD42022338045). A systematic search was conducted from database inception to April 19, 2022. PubMed, MEDLINE, the Cochrane Library, and Embase were searched systematically using Medical Subject Headings (MeSH) terms and text keywords. We also manually searched all relevant studies in clinical trial registries funded by the National Institutes of Health, European Union clinical trial registries, and Google Scholar. No language restrictions were imposed. Sample search strategies are provided in eTable 1 in the Supplement.
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (eTable 2 in the Supplement).30 Our study used publicly available data and did not include human participant research. As per 45 CFR §46.102(f), this study was not submitted for institutional review board approval and did not require informed consent procedures.
Study Selection
Inclusion criteria were established prior to article reviews and were as follows: (1) patients with a diagnosis of depression using standardized diagnostic criteria (eg, DSM-5 or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10]); (2) intervention/comparator groups consisting of ECT and ketamine; and (3) severity of depressive symptoms as an efficacy outcome using standardized measures (eg, Montgomery-Åsberg Depression Rating Scale [MADRS] and Hamilton Depression Rating Scale [HDRS]); and (4) human-based clinical trials. We also considered suicidal ideation and cognition or memory measures as efficacy outcomes. Further, we considered safety-related events as secondary outcomes (eg, reports of adverse events: suicide attempts or deaths, headache, muscle pain, vertigo, diplopia/nystagmus, dissociative or depersonalization symptoms, and nausea). Exclusion criteria were (1) nonhuman studies and (2) no use of standardized measures for depression or the primary outcomes of interest.
Data Extraction
Titles and abstracts were independently screened by 2 reviewers (T.G.R. and S.R.S.), and articles identified as potentially relevant by at least 1 reviewer were retrieved and duplicates were removed. Full-text articles were independently screened by 2 reviewers (T.G.R. and S.R.S.); discrepancies were resolved through group discussions. Data from included articles were independently extracted by 2 reviewers (T.G.R. and S.R.S.) using a pilot-tested data extraction form and then corroborated, with discrepancies resolved through group discussions. Information to be extracted was established a priori and included the following: study characteristics, participant characteristics and subgroups, sample source and collection period, modes of ascertainment, methods of data analysis, selection of cases and controls, and quantitative data pertaining to any primary and secondary outcomes along with adjusted factors. To ensure the absence of overlapping data and to maintain the meta-integrity, data and references for each included study were carefully cross-checked.
Assessment of Bias and Methodological Quality
The risk of bias and methodological quality were evaluated using the Cochrane Collaboration Risk of Bias tool version 2.31,32 We assessed 5 parameters, including (1) randomization process, (2) deviations from the intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported result. Each domain was classified as having a high, low, or unclear risk of bias. We used Cochrane Library’s Review Manager software, RevMan version 5.4.1,33 when assessing biases and methodological qualities. We also assessed publication bias (or small-study effects) using a funnel plot.34 We used the Egger test (ie, linear regression test of funnel plot asymmetry) and Begg and Mazumdar test (ie, rank correlation test of funnel plot asymmetry) when assessing the publication bias.
Statistical Analysis
We used a Hedges g standardized mean difference (SMD) for improvement in severity of depressive symptoms because different studies used different standardized measures. The weight of each study was determined using an inverse-variance method.34 Relative risk (RR) was used for safety-related outcomes as they were all binary outcomes, and we used the Mantel-Haenszel method.34 We used both the Cochran Q statistic and I2 statistic to quantify the proportions of heterogeneity due to within- and between-study variations.34 To adequately estimate the overall effect sizes, SMDs or RRs with their corresponding 95% CIs were calculated using fixed- or random-effects models depending on the model assumptions.34 More specifically, a random-effects model was used when the I2 statistic was greater than 50%, and a fixed-effects model was used when I2 statistic was less than 50%. We reported both random- and fixed-effects models when the I2 statistic was 50%. Because some studies reported multiple effect sizes for severity of depressive symptoms, we used a 2-stage meta-analysis. In the first stage, we obtained the overall effect size estimate of multiple measures within the study using a 3-level meta-analysis. In the second stage, we pooled and obtained the overall effect size estimate using 1 effect size from each study. This approach avoids potential duplication of the samples included.
We also conducted moderator analyses using meta-regression analyses by study sample size, age, male sex (%), region, randomization status, presence of psychotic features, and treatment type (ie, right unilateral, bilateral, or mixed types for ECT). When identifying potential moderators, we used the variance of the true effects using a restricted maximum likelihood estimator.34 We used the statistical software R version 4.2.1 (R Foundation for Statistical Computing) for all analyses using the meta, rmeta, and metafor packages.35 A 2-sided P < .05 was considered statistically significant.
Results
Characteristics of the Studies Included
The literature search yielded 1248 articles, of which 60 were eligible after screening titles and abstracts and removing duplicates. Of these eligible studies, 56 were further excluded after full-text screening. Two independent investigators (T.G.R. and S.R.S.) discovered 2 additional studies by manually searching clinical trial databases and reference lists. Details of study selection are provided in eFigure 1 in the Supplement. Overall, 6 clinical trial studies36,37,38,39,40,41 with 340 patients (n = 162 for ECT and n = 178 for ketamine) were included in the review (Table 1). Five trials were conducted at single sites, and 1 study37 was a multicenter trial (6 clinics). From these studies, 6 effect sizes for depression severity were identified for meta-analysis. Two studies (33.3%) were conducted in Europe, and 4 other studies (66.7%) were conducted in Asia or the Middle East. Five studies followed randomized clinical trial designs, and 1 study,36 conducted in Germany, used a naturalistic, open-label clinical trial design.
Table 1. Characteristics of the Included Clinical Trials (n = 6).
| Source (country) | Study designa | Sample size, No. (% male sex) | Age, mean, y | Condition | Ketamine | ECT | Durationb | Key finding |
|---|---|---|---|---|---|---|---|---|
| Basso et al,36 2020 (Germany) | Open-label clinical trial | 50 (48) | 49.6 | TRD, no history of psychosis, ECT candidates | 0.5 mg/kg IV (n = 25) 3 times a week for 2 weeks; No. of infusions, mean (SD) [range]: 6.76 (1.23) [6-9] | UBP (0.3 ms) RUL ECT with spECTrum 5000 Q (n = 25) 3 times a week for 4 weeks; No. of sessions, mean (SD) [range]: 12.36 (1.75) [9-16] | 2-4 wk | ECT and ketamine administration were equally effective; however, the antidepressant effects of ketamine occurred faster. Ketamine improved neurocognitive functioning, especially attention and executive functions; ECT was related to a small overall decrease in cognitive performance. |
| Ekstrand et al,37 2022 (Sweden) | Open-label, multicenter RCT | 186 (36) | 52.5 | Unipolar depression, ECT candidates | 0.5 mg/kg IV (n = 95) 3 times a week for 2 weeks; No. of infusions, mean (SD) [range]: 6.8 (3.3) [5-9] | RUL ECT (n = 91) 3 times a week for 4 weeks; No. of sessions, mean (SD) [range]: 7.8 (2.4) [6-10] | 4 wk | Remission rates were statistically different (57/91 [62.6%] in ECT vs 44/95 [46.3%] in ketamine infusions; P = .03). Ketamine, despite being inferior to ECT, can be a safe and valuable tool in treating unipolar depression. |
| Ghasemi et al,38 2014 (Iran) | Blind RCT | 18 (44) | 37.6 | DSM-IV MDE, ECT candidates | 0.5 mg/kg IV (n = 9), 3 infusions every 48 h in a week | BP (1.0 ms) BL ECT with Thymatron DGx (n = 9); 3 sessions every 48 h in a week | 1 wk | Within 24 h, depressive symptoms significantly improved in patients receiving the first dose of ketamine compared with ECT. Compared with baseline, this improvement remained significant throughout the study. For depressive symptoms after the second dose, ketamine was lower than the second ECT. This study showed ketamine is as effective as ECT in improving depressive symptoms in MDE and has more rapid antidepressant effects compared with ECT. |
| Kheirabadi et al,39 2019 (Iran) | Blind RCT | 22 (59) | 38.8 | MDE, no history of psychosis, ECT candidates | 0.5 mg/kg IV over 40 min (n = 10) twice a week up to complete remission; range: 1-6 | BL ECT with Thymatron DGx (n = 12) twice a week weekly up to complete remission; range: 1-6 | 2-3 wk | Depressive symptoms in both groups improved with no statistically significant difference. Cognitive state was more favorable (but not significant) in the ketamine group. Treatment with IV ketamine in people with MDE has the same antidepressant effects as ECT treatment without any memory deficiency. |
| Kheirabadi et al,40 2020 (Iran) | Open-label RCT | 39 (33-53) | 39.1-41.6 | DSM-V MDE, ECT candidates | 0.5 mg/kg IM (n = 15), 6-9 injections in 3 weeks (with a 2- to 3-d interval); 1.0 mg/kg oral (n = 12), every 2-3 d up to 6-9 sessions in 3 weeks | BL ECT with Thymatron DGx (n = 12), 6-9 sessions in 3 weeks; range: 6-9 | 3 wk | Oral and IM ketamine probably have equal antidepressant in addition to more antisuicidal effects compared with ECT but had fewer cognitive adverse effects and higher preference by patients. Thereby, ketamine can be an alternative method in the treatment of patients with severe and/or suicidal MDE. |
| Sharma et al,41 2020 (India) | Blind, RCT | 25 (44) | 38.8 | ICD-10 criteria for severe depression (bipolar or unipolar), ECT candidates | 0.5 mg/kg IV (n = 12); 6 alternate-day sessions; range: 1-6 | BP (1.5 ms) bifrontal or RUL ECT with Niviqure (n = 13) for 6 alternate-day sessions; range: 1-6 | 2 wk | Compared with ketamine, ECT showed significantly greater reduction in HDRS and BDI scores. ECT patients had a higher response rate than ketamine patients. This study favored ECT over ketamine for a better efficacy over 6 treatment sessions in severe depression. |
Abbreviations: BDI, Beck Depression Inventory; BL, bilateral session; BP, brief pulse; ECT, electroconvulsive therapy; HDRS, Hamilton Depression Rating Scale; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; IM, intramuscular; IV, intravenous; MDE, major depressive episode; RCT, randomized clinical trial; RUL, right unilateral session; TRD, treatment-resistant depression; UBP, ultra-brief pulse stimuli.
Every study setting was inpatient.
Denotes a period for completing a series of treatment sessions.
Sample sizes ranged from 18 to 186, with mean age of the participants ranging from 37.5 to 52.5 years. A period for completing a series of treatment sessions for either ECT or ketamine was within a month. All studies recruited patients who were ECT candidates; 5 of 6 studies only enrolled patients with MDE, with 1 study41 recruiting patients with either unipolar or bipolar depression. Table 1 provides details (including the frequency and duration of treatment sessions) and summaries of applicable findings for all included studies.
Efficacy Outcome: Depressive Symptoms
The primary efficacy outcome of interest was the improvement of depressive symptoms (Figure 1). When stratified by individual measure, ECT was superior to ketamine across different depressive symptom measures (SMD, −0.59 [95% CI, −0.85 to −0.33] for MADRS; SMD, −0.83 [95% CI, −1.22 to −0.44] for HDRS; SMD, −0.86 [95% CI, −1.50 to −0.22] for Beck Depression Inventory) (Figure 1A). The overall pooled SMD for ECT, when compared with ketamine, was −0.69 (95% CI, −0.89 to −0.48; Cochran Q, P = .15; I2 = 39%), indicating that ECT was more efficacious than ketamine (Figure 1B).
Figure 1. Severity of Depressive Symptoms Between Electroconvulsive Therapy (ECT) and Ketamine in Patients With Major Depressive Episode.
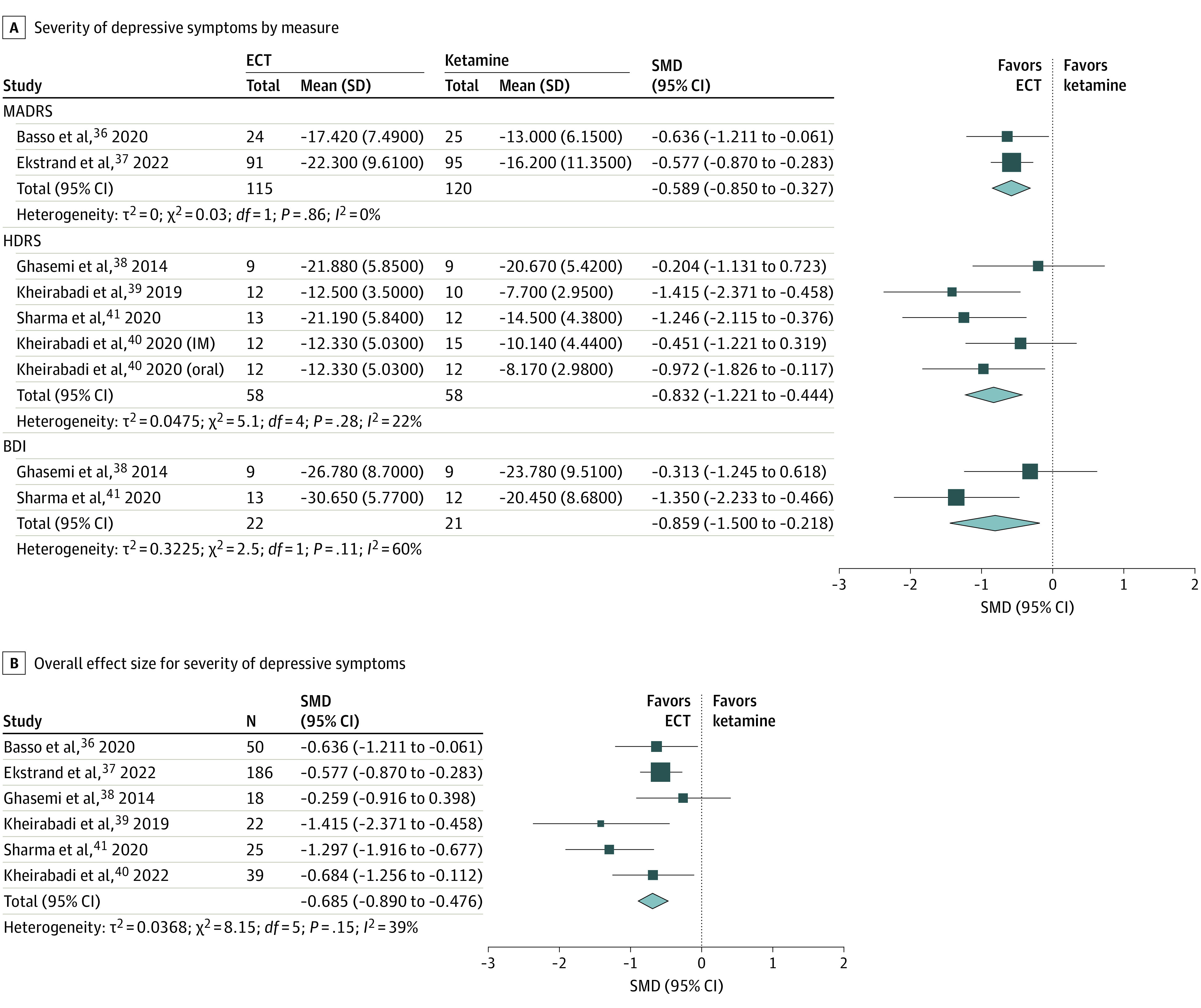
Analyses were done using a fixed-effects model and inverse variance. For the study by Kheirabadi et al (2020),40 we distinguish intramuscular ketamine and oral ketamine without duplications. A 2-stage model was used in B. In the first stage, we obtained the overall effect size estimate of multiple measures within the studies by Ghasemi et al,38 Sharma et al,41 and Kheirabadi et al (2020)40 using a 3-level meta-analysis. In the second stage, we pooled and obtained the overall effect size estimate using 1 effect size from each study. BDI indicates Beck Depression Inventory; HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; SMD, standardized mean difference.
Efficacy Outcome: Suicidal Ideation
Only 1 study40 investigated the trajectory of suicidal ideation. Using the Beck Scale for Suicidal Ideation, baseline scores were not statistically different across the 3 intervention groups (ie, ECT, oral ketamine, and intramuscular ketamine). While each group showed significant reductions in scores on Beck Scale for Suicidal Ideation at the end of the study, the group differences were not found at the 1-week and 1-month follow-up periods (P = .99 and P = .69, respectively).
Cognition and Memory
One study36 assessed neurocognitive performance as an outcome. The authors concluded that patients in the ketamine group performed better than those in the ECT group, with a small to moderate effect size (Cohen d, 0.40; P = .04). In individual domains, the ketamine group outperformed the ECT group in terms of attention, verbal memory, and executive functions with moderate to large effect sizes.36 There was no group difference for immediate memory or visual memory.36 The other study39 reported memory performance using a Wechsler Memory Scale and reported no group differences for memory performance.
Safety Outcomes
Only 4 of 6 studies (66.7%)37,38,39,40 explicitly reported adverse events. Of these, only 1 study37 reported serious adverse events, including suicide attempts and suicide deaths. In this study, the number of patients reporting any serious adverse events was not statistically significant between groups (23 of 90 in ECT vs 14 of 91 in ketamine; P = .09).37 In the same study,37 suicide attempts (6 of 90 in ECT vs 4 of 91 in ketamine) and suicide deaths (1 of 90 in ECT vs 0 of 91 in ketamine) were not different between groups.
Figure 2 presents individual types of adverse events. The ketamine group had lower risks than the ECT group for headache (RR, 0.37 [95% CI, 0.18-0.76]) and muscle pain (RR, 0.23 [95% CI, 0.13-0.38]) (Figure 2A). On the other hand, transient dissociative or depersonalization symptoms were more common in ketamine compared with ECT (RR, 5.04 [95% CI, 3.03-8.36]). Electroconvulsive therapy had lower risks than ketamine for blurred vision (RR, 26.47 [95% CI, 3.62-193.67]), vertigo (RR, 2.99 [95% CI, 2.03-4.40]), and diplopia/nystagmus (RR, 10.88 [95% CI, 3.52-33.63]) (Figure 2B).
Figure 2. Individual Safety Outcomes Between Electroconvulsive Therapy (ECT) and Ketamine in Patients With Major Depressive Episode.
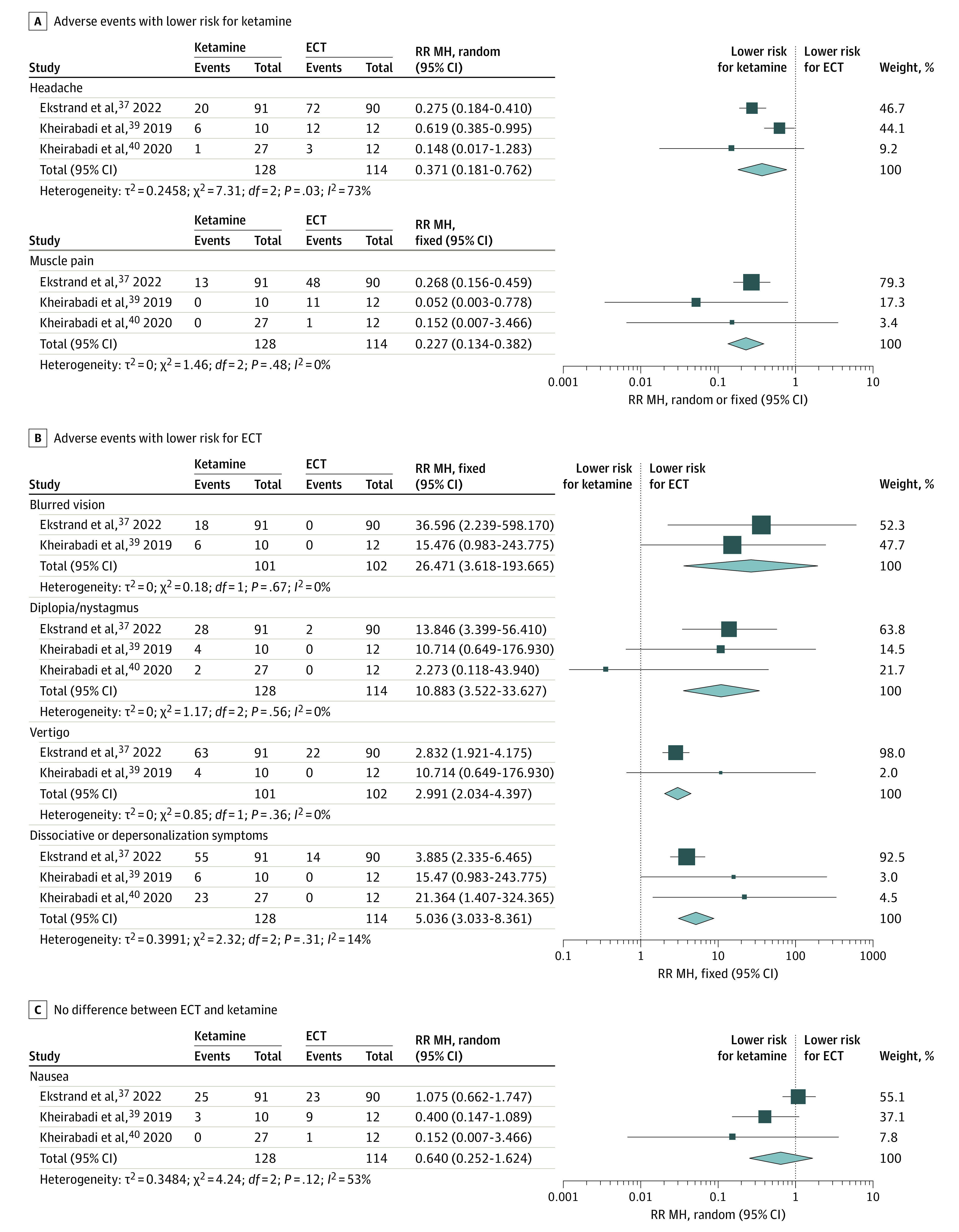
Fixed indicates fixed-effects model; MH, Mantel-Haenszel method; random, random-effects model; RR, relative risk.
Moderator Analysis
We also considered potential moderating roles of the following variables using meta-regression and meta–analysis of variance models: number of patients, age, male sex (%), geographic region, randomization status, treatment type, and presence of psychotic features (Table 2). We did not find any moderating effects of these factors on the main treatment effects.
Table 2. Moderator Analysis by Age, Sex, Sample Size, Region, Randomization, Type of ECT Treatment, and Presence of Psychotic Features Using Meta-Regression Analysesa.
| Moderator | k | Coefficient (95% CI) | SMD (95% CI) | P value |
|---|---|---|---|---|
| No. of total patients | 6 | 0.002 (−0.003 to 0.006) | .51b | |
| Age | 6 | 0.018 (−0.030 to 0.066) | .47b | |
| Male sex (%) | 6 | −4.490 (−4.687 to 1.708) | .36b | |
| Region | .25c | |||
| Asia/Middle East | 4 | NA | −0.839 (−1.171 to −0.507) | |
| Europe | 2 | NA | −0.589 (−0.850 to −0.327) | |
| Randomization | .86c | |||
| Yes | 5 | NA | −0.692 (−0.912 to −0.472) | |
| No | 1 | NA | −0.636 (−1.211 to −0.061) | |
| Type of ECT | .12c | |||
| Right unilateral | 2 | NA | −0.589 (−0.850 to −0.327) | |
| Bilateral | 3 | NA | −0.655 (−1.048 to −0.262) | |
| Mixed | 2 | NA | −1.297 (−1.916 to −0.677) | |
| Psychotic features | .78c | |||
| Yes | 2 | NA | −0.709 (−0.974 to −0.443) | |
| No | 4 | NA | −0.649 (−0.974 to −0.324) |
Abbreviations: ECT, electroconvulsive therapy; k, number of effect sizes; NA, not applicable; SMD, standardized mean difference (Hedges g).
Coefficient refers to the meta-regression coefficient. A restricted maximum likelihood was used.
Continuous moderators.
Categorical moderators.
Quality Assessment and Risk of Bias
Methodological quality of the included studies was low to moderate (Figure 3), and we provided our justification in eTable 3 in the Supplement. Except for 1 study,36 all studies used randomization, 4 of which had robust allocation concealment to reduce selection bias. Four of 6 studies (66.7%) had some concerns regarding deviations from intended intervention; this is partly due to difficulties in implementing blinding among participants and personnel. Further, 1 study37 explicitly reported potential missing outcome bias due to a higher dropout rate in the ketamine group than that of the ECT group. None of the included studies had selective reporting (ie, reporting bias) or other biases per study protocols, resulting in low risks for domains 4 and 5. In addition, we did not find any potential publication bias (eFigure 2 in the Supplement) using the Egger test (P = .32) and Begg and Mazumdar test (P = .57).
Figure 3. Risk-of-Bias Assessment for Individual Studies.
The risk-of-bias domains are described as follows: D1 indicates bias arising from the randomization process; D2, bias due to deviations from the intended intervention; D3, bias due to missing outcome data; D4, bias in measurement of the outcome; D5, bias in selection of the reported result. (See eTable 3 in the Supplement for details.)
Discussion
The present systematic review and meta-analysis includes 11 effect sizes for depression severity from 6 studies with 340 patients with a DSM or ICD-10 diagnosis of MDE. To our knowledge, this is the first meta-analysis to quantify the efficacy and safety of ketamine vs ECT in patients with MDE. At the end of completing a series of treatment sessions, we found that ECT was more efficacious than ketamine in reducing depression severity. While the meta-analysis suggests that ECT may be superior to ketamine in terms of efficacy, treatment options should still be individualized and patient-centered because ketamine’s faster antidepressant effects may still be desirable for certain patients with severe MDE who require quick recovery from the severity of depression. For instance, 3 studies36,38,39 qualitatively reported that ketamine had more rapid antidepressant effects than ECT during the initial course of treatment sessions, whereas 1 study41 found that patients receiving ECT recovered more quickly than those receiving ketamine.42,43 Additionally, ketamine and ECT have unique adverse effect profiles.
When reviewing articles systematically, we found that 2 studies37,39 had long-term follow-up (ie, 3 months or longer) after the trial completed. One study39 found no difference in depression severity during the 3-month follow-up between the ketamine and ECT groups. The other study37 reported that the remission rates were not different between groups by the 12-month follow-up period. It appears that the treatment effects may wane similarly in both groups over time. To our knowledge, a largely unexplored area is the evaluation of long-term strategies addressing maintenance, remission, and relapse issues between these 2 groups. For ECT, the best strategy for relapse prevention appears to be continuing ECT, continuing pharmacotherapy, or using some combination of both.44,45,46 Large and well-controlled studies examining relapse prevention approaches in ketamine are lacking. However, a large randomized withdrawal study of esketamine, a very similar therapy, suggests that continuing treatment at a less frequent interval is an effective relapse prevention approach.47 Future research is needed to further optimize long-term treatment outcomes for both ketamine and ECT to prevent relapse, which is of key importance for clinical practice.48,49
For cognition and memory performances, 1 study36 reported that the ketamine group outperformed the ECT group in cognition, but the effect size was small to moderate. Another study39 reporting memory performance found no difference between patients with ketamine and those with ECT, though this study was likely underpowered to detect such differences (total sample size of 32). Because of underpowered study designs, no firm conclusions regarding cognition and memory performance can be made in this meta-analysis. Future research should address this issue.
An important consideration for clinicians and patients with serious depression is the comparative tolerability and safety of ketamine vs ECT. The provision of ketamine only involves the administration of a low dose of anesthesia medicine, while the provision of ECT involves the administration of a full dose of anesthesia plus an electrical stimulus that induces a seizure. Hence, it is expected that ketamine would be better tolerated and safer than ECT. The studies included in this meta-analysis were limited in the exploration of this question. Only 1 of the studies reported formal serious adverse events. Additionally, both ketamine and ECT had unique adverse effect profiles (ie, ketamine had lower risks for headache and muscle pain whereas ECT had lower risks for blurred vision, vertigo, diplopia/nystagmus, and dissociative or depersonalization symptoms). No study assessed the relative tolerability or acceptability of these different adverse effect profiles. Future studies should consider patient tolerability and acceptability with respect to these 2 potential treatments. In addition, given the short-term nature of the studies included in this meta-analysis, all of the adverse events reported were acute. Thus, future research should assess long-term adverse events resulting from either ketamine or ECT and weigh the potential long-term benefits and risks of these treatment options.
Limitations
Several methodological limitations deserve comment. First, most of the studies had relatively small sample sizes and lacked long-term follow-up assessments, and thus, the study designs may be underpowered. There are 2 ongoing randomized clinical trials identified through the US clinical trial registry that may address this limitation in the near future. The Canadian Biomarker Integration Network in Depression (CAN-BIND) study50 plans to recruit 240 patients with unipolar or bipolar depression (accounting for a 20% dropout rate) to conduct a randomized, single-blinded crossover trial. In this trial, patients will be randomized to receive 0.5-mg/kg intravenous ketamine or ECT thrice weekly for 3 to 4 weeks. The other study, ECT Versus Ketamine in Patients With Treatment-Resistant Depression (ELEKT-D),51 aims to recruit 400 patients with unipolar TRD. This is an unblinded, open-label randomized trial to compare 0.5-mg/kg intravenous ketamine (2 times a week up to a total of 6 treatment sessions) and ECT (3 times a week up to a total of 9 treatment sessions) over 3 to 5 weeks. These studies are expected to be completed by March 2023 and December 2022, respectively. Additionally, no study has yet directly compared ECT with esketamine, which has substantially larger evidence from regulatory clinical trials and has received approval for treating TRD.
A second limitation is that the included studies were also slightly different in inclusion and exclusion criteria (eg, inclusion of bipolar depression and presence of psychotic features), which require careful interpretations of the findings. For example, while ECT is particularly effective in patients with psychotic depression,52 examining differences in the predictive value for response rates between ketamine and ECT by such patient characteristics may lead to more personalized medicine.26
A third limitation is that the included studies may have used different ketamine and/or ECT treatment protocols (eg, frequency and routes of administration), all of which could have influenced efficacy and safety outcomes. Although our moderator analyses accounted for some of these issues (eg, type of treatment modalities and geographic region), there may still be moderating or confounding factors (eg, frequency [eg, twice vs thrice weekly], stimulus width [eg, brief pulse vs ultra-brief pulse] for ECT, and medications used for anesthesia).
Conclusions
This meta-analysis suggests that ECT may be superior to ketamine for improving acute depression severity, but several major methodological limitations reported above should be considered. At least 2 large, ongoing comparative studies will lend further data on this important question that has significant relevance to patients, health care professionals, and policy makers.
eFigure 1. Study selection flowchart
eFigure 2. Funnel plot for publication bias (or small study effects) assessment
eTable 1. Search strategy
eTable 2. PRISMA 2020 checklist
eTable 3. Rationale for risk of bias for individual studies
References
- 1.World Health Organization . Depression. Accessed January 6, 2020. https://www.who.int/news-room/fact-sheets/detail/depression
- 2.National Institute of Mental Health . Major depression. Accessed May 13, 2020. https://www.nimh.nih.gov/health/statistics/major-depression.shtml
- 3.Rhee TG, Wilkinson ST, Steffens DC, Rosenheck RA, Olfson M. Prevalence of treatment for depression among US adults who screen positive for depression, 2007-2016. JAMA Psychiatry. 2020;77(11):1193-1195. doi: 10.1001/jamapsychiatry.2020.1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ormel J, Kessler RC, Schoevers R. Depression: more treatment but no drop in prevalence: how effective is treatment? and can we do better? Curr Opin Psychiatry. 2019;32(4):348-354. doi: 10.1097/YCO.0000000000000505 [DOI] [PubMed] [Google Scholar]
- 5.Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Spencer D, Fava M. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 2008;75(1):57-66. doi: 10.3949/ccjm.75.1.57 [DOI] [PubMed] [Google Scholar]
- 6.Rush AJ, Warden D, Wisniewski SR, et al. STAR*D: revising conventional wisdom. CNS Drugs. 2009;23(8):627-647. doi: 10.2165/00023210-200923080-00001 [DOI] [PubMed] [Google Scholar]
- 7.Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv. 2014;65(8):977-987. doi: 10.1176/appi.ps.201300059 [DOI] [PubMed] [Google Scholar]
- 8.Nemeroff CB. The burden of severe depression: a review of diagnostic challenges and treatment alternatives. J Psychiatr Res. 2007;41(3-4):189-206. doi: 10.1016/j.jpsychires.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson ST, Trujillo Diaz D, Rupp ZW, et al. Pharmacological and somatic treatment effects on suicide in adults: a systematic review and meta-analysis. Depress Anxiety. 2022;39(2):100-112. doi: 10.1002/da.23222 [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence . Guidance on the use of electroconvulsive therapy. Published April 26, 2003. https://www.nice.org.uk/guidance/ta59
- 11.American Psychiatric Association . The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association. American Psychiatric Publishing; 2008. [Google Scholar]
- 12.Weiss A, Hussain S, Ng B, et al. Royal Australian and New Zealand College of Psychiatrists professional practice guidelines for the administration of electroconvulsive therapy. Aust N Z J Psychiatry. 2019;53(7):609-623. doi: 10.1177/0004867419839139 [DOI] [PubMed] [Google Scholar]
- 13.Haq AU, Sitzmann AF, Goldman ML, Maixner DF, Mickey BJ. Response of depression to electroconvulsive therapy: a meta-analysis of clinical predictors. J Clin Psychiatry. 2015;76(10):1374-1384. doi: 10.4088/JCP.14r09528 [DOI] [PubMed] [Google Scholar]
- 14.Sackeim HA. Modern electroconvulsive therapy: vastly improved yet greatly underused. JAMA Psychiatry. 2017;74(8):779-780. doi: 10.1001/jamapsychiatry.2017.1670 [DOI] [PubMed] [Google Scholar]
- 15.Rhee TG, Olfson M, Sint K, Wilkinson ST. Characterization of the quality of electroconvulsive therapy among older Medicare beneficiaries. J Clin Psychiatry. 2020;81(4):19m13186. doi: 10.4088/JCP.19m13186 [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson ST, Kitay BM, Harper A, et al. Barriers to the implementation of electroconvulsive therapy (ECT): results from a nationwide survey of ECT practitioners. Psychiatr Serv. 2021;72(7):752-757. doi: 10.1176/appi.ps.202000387 [DOI] [PubMed] [Google Scholar]
- 17.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354. doi: 10.1016/S0006-3223(99)00230-9 [DOI] [PubMed] [Google Scholar]
- 18.Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142. doi: 10.1176/appi.ajp.2013.13030392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864. doi: 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
- 20.Sanacora G, Frye MA, McDonald W, et al. ; American Psychiatric Association (APA) Council of Research Task Force on Novel Biomarkers and Treatments . A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74(4):399-405. doi: 10.1001/jamapsychiatry.2017.0080 [DOI] [PubMed] [Google Scholar]
- 21.McIntyre RS, Rosenblat JD, Nemeroff CB, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;178(5):383-399. doi: 10.1176/appi.ajp.2020.20081251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y, Chen J, Zou D, et al. Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatr Dis Treat. 2016;12:2859-2867. doi: 10.2147/NDT.S117146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishimoto T, Chawla JM, Hagi K, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46(7):1459-1472. doi: 10.1017/S0033291716000064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruberto VL, Jha MK, Murrough JW. Pharmacological treatments for patients with treatment-resistant depression. Pharmaceuticals (Basel). 2020;13(6):E116. doi: 10.3390/ph13060116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5(1):65-78. doi: 10.1016/S2215-0366(17)30272-9 [DOI] [PubMed] [Google Scholar]
- 26.Veraart JKE, Smith-Apeldoorn SY, Spaans HP, Kamphuis J, Schoevers RA. Is ketamine an appropriate alternative to ECT for patients with treatment resistant depression? a systematic review. J Affect Disord. 2021;281:82-89. doi: 10.1016/j.jad.2020.11.123 [DOI] [PubMed] [Google Scholar]
- 27.Nikayin S, Rhee TG, Cunningham ME, et al. Evaluation of the trajectory of depression severity with ketamine and esketamine treatment in a clinical setting. JAMA Psychiatry. 2022;79(7):736-738. doi: 10.1001/jamapsychiatry.2022.1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrade C. Insights for the use of ketamine from randomized controlled trials that compared ketamine with electroconvulsive therapy in severe depression. J Clin Psychiatry. 2022;83(2):22f14451. doi: 10.4088/JCP.22f14451 [DOI] [PubMed] [Google Scholar]
- 29.Berman J, Ambrose AJ. Prioritizing patient preferences: a practical guide for tailoring treatment choices in interventional psychiatry. J Clin Psychiatry. 2022;83(3):22ac14436. doi: 10.4088/JCP.22ac14436 [DOI] [PubMed] [Google Scholar]
- 30.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-94. doi: 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 31.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 32.Cochrane Methods Bias . RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. Accessed June 6, 2022. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials
- 33.Cochrane Training . Cochrane Review Manager. Accessed June 6, 2022. https://training.cochrane.org/online-learning/core-software/revman
- 34.Cooper H, Hedges LV, Valentine JC, eds. The Handbook of Research Synthesis and Meta-analysis. Russell Sage Foundation; 2019. [Google Scholar]
- 35.Shim SR, Kim SJ. Intervention meta-analysis: application and practice using R software. Epidemiol Health. 2019;41:e2019008. doi: 10.4178/epih.e2019008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basso L, Bönke L, Aust S, et al. Antidepressant and neurocognitive effects of serial ketamine administration versus ECT in depressed patients. J Psychiatr Res. 2020;123:1-8. doi: 10.1016/j.jpsychires.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 37.Ekstrand J, Fattah C, Persson M, et al. Racemic ketamine as an alternative to electroconvulsive therapy for unipolar depression: a randomized, open-label, non-inferiority trial (KetECT). Int J Neuropsychopharmacol. 2022;25(5):339-349. doi: 10.1093/ijnp/pyab088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghasemi M, Kazemi MH, Yoosefi A, et al. Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res. 2014;215(2):355-361. doi: 10.1016/j.psychres.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 39.Kheirabadi G, Vafaie M, Kheirabadi D, Mirlouhi Z, Hajiannasab R. Comparative effect of intravenous ketamine and electroconvulsive therapy in major depression: a randomized controlled trial. Adv Biomed Res. 2019;8:25. doi: 10.4103/abr.abr_166_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kheirabadi D, Kheirabadi GR, Mirlohi Z, Tarrahi MJ, Norbaksh A. Comparison of rapid antidepressant and antisuicidal effects of intramuscular ketamine, oral ketamine, and electroconvulsive therapy in patients with major depressive disorder: a pilot study. J Clin Psychopharmacol. 2020;40(6):588-593. doi: 10.1097/JCP.0000000000001289 [DOI] [PubMed] [Google Scholar]
- 41.Sharma RK, Kulkarni G, Kumar CN, et al. Antidepressant effects of ketamine and ECT: a pilot comparison. J Affect Disord. 2020;276:260-266. doi: 10.1016/j.jad.2020.07.066 [DOI] [PubMed] [Google Scholar]
- 42.Kellner CH, Husain MM, Knapp RG, et al. ; CORE/PRIDE Work Group . Right unilateral ultrabrief pulse ECT in geriatric depression: phase 1 of the PRIDE study. Am J Psychiatry. 2016;173(11):1101-1109. doi: 10.1176/appi.ajp.2016.15081101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry. 2004;65(4):485-491. doi: 10.4088/JCP.v65n0406 [DOI] [PubMed] [Google Scholar]
- 44.Kellner CH, Husain MM, Knapp RG, et al. ; CORE/PRIDE Work Group . A novel strategy for continuation ECT in geriatric depression: phase 2 of the PRIDE study. Am J Psychiatry. 2016;173(11):1110-1118. doi: 10.1176/appi.ajp.2016.16010118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467-2474. doi: 10.1038/npp.2013.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sackeim HA, Haskett RF, Mulsant BH, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA. 2001;285(10):1299-1307. doi: 10.1001/jama.285.10.1299 [DOI] [PubMed] [Google Scholar]
- 47.Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903. doi: 10.1001/jamapsychiatry.2019.1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fourcade EW, Lapidus KAB. The basic and clinical pharmacology of ketamine. In: Mathew S, Zarate C Jr, eds. Ketamine for Treatment-Resistant Depression. Springer; 2016:13-29. doi: 10.1007/978-3-319-42925-0_2 [DOI] [Google Scholar]
- 49.Papakostas GI. Maintaining rapid antidepressant effects following ketamine infusion: a major unmet need. J Clin Psychiatry. 2020;81(2):19r12859. doi: 10.4088/JCP.19r12859 [DOI] [PubMed] [Google Scholar]
- 50.Phillips JL, Jaworska N, Kamler E, et al. ; CAN-BIND Investigator Team . A randomized, crossover comparison of ketamine and electroconvulsive therapy for treatment of major depressive episodes: a Canadian biomarker integration network in depression (CAN-BIND) study protocol. BMC Psychiatry. 2020;20(1):268. doi: 10.1186/s12888-020-02672-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathew SJ, Wilkinson ST, Altinay M, et al. Electroconvulsive therapy (ECT) vs. ketamine in patients with treatment-resistant depression: the ELEKT-D study protocol. Contemp Clin Trials. 2019;77:19-26. doi: 10.1016/j.cct.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 52.van Diermen L, van den Ameele S, Kamperman AM, et al. Prediction of electroconvulsive therapy response and remission in major depression: meta-analysis. Br J Psychiatry. 2018;212(2):71-80. doi: 10.1192/bjp.2017.28 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study selection flowchart
eFigure 2. Funnel plot for publication bias (or small study effects) assessment
eTable 1. Search strategy
eTable 2. PRISMA 2020 checklist
eTable 3. Rationale for risk of bias for individual studies



