Abstract
Type I Chiari malformation (CMI) is a neurological condition in which the cerebellar tonsils descend into the cervical spinal subarachnoid space resulting in cervico-medullary compression. Early case-control investigations have indicated cognitive deficits in the areas of attention, memory, processing speed, and visuospatial function. The present study further examined cognitive and emotional processing deficits associated with CMI using a dual-task paradigm. Nineteen CMI patients were recruited during pre-surgical consultation and 19 matched control participants identified emotional expressions in separate single and asynchronous dual-task designs. To extend earlier behavioral studies of cognitive effects in CMI, we recorded event-related potentials (ERPs) in the dual-task design. Though response times were slower for CMI patients across the two tasks, behavioral and ERP analyses indicated that patients did not differ from matched controls in the ability to allocate attentional resources between the two tasks. P1 ERP component analyses provided no indication of an emotional arousal deficit in our CMI sample while P3 ERP component analyses suggested a CMI-related deficit in emotional regulation. P3 analysis also yielded evidence for a frontalization of neurophysiological activity in CMI patients. Pain and related depression and anxiety factors accounted for CMI deficits in single-task, but not dual-task, response times. Results are consistent with a dysfunctional fronto-parietal attentional network resulting from either the indirect effects of chronic pain or the direct effects of CMI pathophysiology stemming from cervico-medullary compression.
Keywords: Chiari malformation, Divided attention, Emotion processing, Affective arousal, Emotion regulation, Chronic pain, Cerebellar cognitive affective syndrome
Introduction
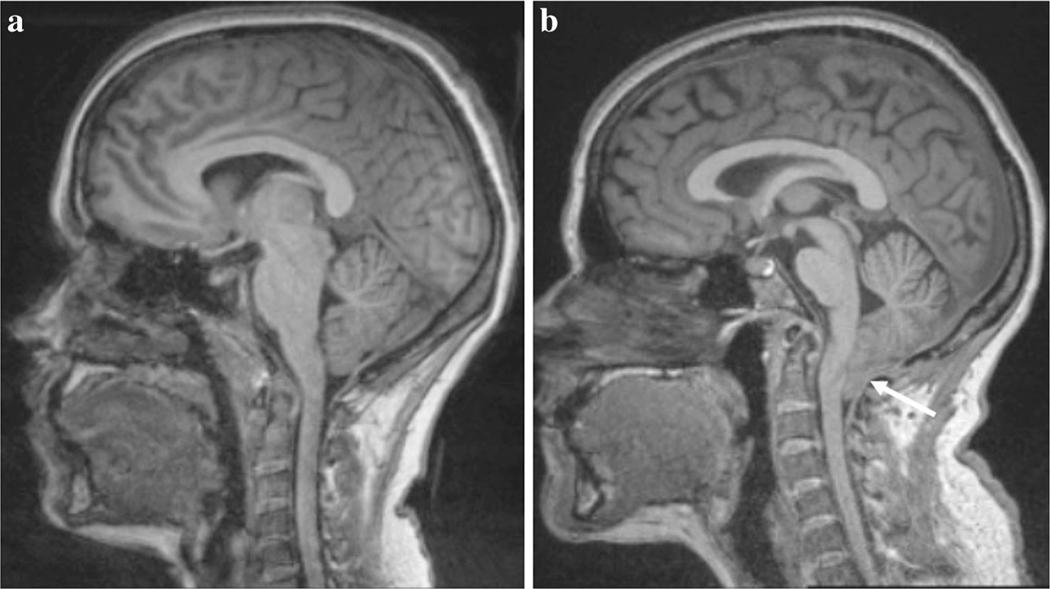
Chiari Malformation Type I (CMI) is a brain dysmorphism in which the cerebellar tonsils are displaced 3 to 5 mm, or greater, below the foramen magnum (see Fig. 1; [18, 37]). Prevalence of symptomatic CMI is approximately 0.1%, although estimates vary in part due to incidental radiographic identification [15, 19, 35, 61]. However, radiographic evidence of tonsillar ectopia in CMI has not been found to strongly correlate with symptomatology [59]. One possibility is that tonsillar ectopia is a “necessary but not sufficient” variable in determining whether an individual will show symptoms in CMI.
Fig. 1.
T1-weighted midsagittal presentation of a a healthy participant and b a CMI patient. The arrow indicates the location of the tonsillar descent through the foramen magnum
The Cognitive Profile of CMI
Many CMI patients anecdotally report experiencing “brain fog” and disruptions in concentration. Though these descriptions do not well inform the underlying processes behind patients’ cognitive disruptions, they have provided a starting point for empirical investigations. For example, previous efforts have established cognitive and motor symptoms in CMI [1, 2, 21, 28]. Herweh et al. [21] was early to empirically identify cognitive deficits in a CMI sample using a neuropsychological assessment. Relative to standardized norms, four of five patients exhibited low threshold or subnormal IQ. Deficits in visuospatial memory and verbal memory were also apparent in their sample. Kumar et al. [28] examined the integrity of white matter pathways in a group of ten CMI patients and ten matched controls. In addition to a DTI scan, participants also completed a neuropsychological assessment consisting of motor coordination, focused and sustained attention, processing speed, and memory. Results showed that CMI patients exhibited deficits in verbal and visuospatial reasoning and processing speed relative to controls, with no apparent differences in visuomotor coordination or divided attention. However, the sample size of Kumar et al. [28] was small and the tasks used to assess cognitive function (e.g., the trail making test for divided attention) were brief clinical assessments that may lack the precision of more elaborate laboratory testing.
In contrast to Kumar et al. [28], the results from Allen et al. [2] yielded evidence for a cognitive deficit profile in CMI that included attention processes. Using 24 post-surgery CMI patients and age and education matched controls, Allen et al. identified deficits in processing speed, response inhibition, and working memory in CMI patients using digit symbol coding, Stroop, and operation span tasks, respectively. Moreover, after covarying for depression and anxiety, deficits in processing speed and working memory dissipated, yet group differences in response inhibition remained. This response inhibition deficit was further determined to be unique from the slower processing speed in the CMI group. Supporting a multiple source model of cognitive decline, the authors argued that CMI is indirectly associated with cognitive decline due to comorbid anxiety and depression and directly associated with decline due to CMI pathophysiological processes. Expanding upon this work, Allen et al. [1] assessed a sample of 638 CMI patients (341 decompressed and 297 non-decompressed) and 102 healthy controls on an immediate-recall task (Rey Auditory Verbal Learning Test; [58]). While Allen et al. [2] using a much smaller sample found a trend toward CMI patients showing poorer immediate recall, Allen et al. [1] found conclusive evidence of a deficit in immediate recall in CMI patients relative to controls.
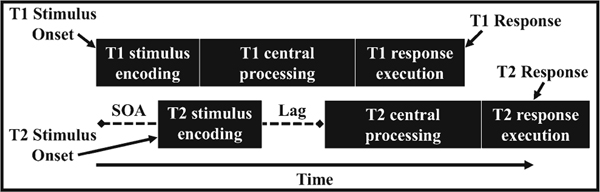
The present study advances on the work of these previous investigations along several fronts. First, building on Kumar et al. [28], the present study examines divided attention processes in CMI using a more elaborate task and a larger sample size in which patients and controls are matched for gender, age, and educational achievement. We chose to assess divided attention through a commonly used psychological refractory period (PRP) paradigm [41, 42]. In PRP tasks, participants must perform two tasks (Task 1 and Task 2) on two asynchronously presented stimuli. The critical manipulation is the temporal overlap between the processing of these two tasks, which was manipulated by varying the time between the two stimuli onsets (the stimulus onset asynchrony [SOA]). The typical finding is that response times for Task 2 are longer at short SOAs than at long SOAs, known as the PRP effect, or slack effect. The PRP effect is commonly attributed to a central processing bottleneck in which the attention resources, occupied by Task 1, are not available for Task 2 at short SOAs. Importantly, the PRP effect is not believed to reflect peripheral processes such as perceptual encoding, which are thought to function at least partially in parallel (see Fig. 2). Processes identified as being sensitive to the central processing bottleneck have been identified in attention [30, 45], working memory [11, 26, 52], and lexical processing [31]. Therefore, if CMI patients and control participants show similar magnitudes of slack effects in the PRP paradigm, this provides further evidence of the sparing of divided attention in CMI.
Fig. 2.
Visual representation of a trial from the psychological refractory period (PRP) model. T1—Task 1; T2—Task 2; SOA—stimulus onset asynchrony
In addition to the focus on divided attention, the present study has been designed to replicate processing speed deficits in CMI [2, 28]. In addition to the dual-task PRP task, participants also completed a single task which only included Task 2. As a result, we will be able to use the response times from Task 1 and Task 2 in the PRP design and response times from the single task to provide further evidence regarding potential processing speed deficits in CMI patients.
Linking CMI and Emotion Processing
In addition to examining CMI-related deficits in divided attention and processing speed, the present study also aims to explore whether CMI is associated with emotion processing deficits. There is precedence for such an investigation given the chronic pain consistently reported in CMI patients. A growing body of literature is beginning to provide evidence that long-term chronic pain influences not only cognitive function [1, 4, 8, 9, 38, 39, 63, 69], but also modulates the processing of information with an emotional context [9, 22, 39, 62, 63]. For example, Apkarian et al. [3] used the Iowa gambling task [6] and identified impaired emotional decision-making in participants with chronic back pain. Results such as these suggest that investigation into CMI-modulated emotion processing is warranted in part to better understand the impact of the chronic pain experienced by CMI patients.
In addition to the impact of chronic pain on cognition and emotion processing, the structural cerebellar abnormalities that are present in CMI may be uniquely associated with a cognitive and affective (i.e., emotion processing) deficit profile [53–57]. While classic investigations have explored in depth the role of the cerebellum in coordinating movement (see [34], for a review), historical clinical records have also provided early suggestion that cerebellar abnormalities were associated with more than motor control, noting mood fluctuations, dementia-like conditions, psychosis, and limited cognitive functioning in cerebellar disease and psychiatric patient records [55]. There is also reported evidence of clinically relevant cognitive deficits in patients with cerebellar lesions. For example, Schmahmann and Sherman [57] identified deficits in executive function, visuospatial function, working memory, attentional control, and verbal fluency. These results have led to the proposal of the cerebellar cognitive affective syndrome (CCAS), or Schmahmann’s syndrome [33, 55–57]. The CCAS is proposed to be characterized by (1) disturbances in executive function, working memory, and verbal fluency, (2) impaired visuospatial function, (3) personality change including flat affect and disinhibition, and (4) linguistic deficits.
Based upon the established links between chronic pain, the CCAS, and affect processing, we hypothesize that CMI patients will exhibit an emotion processing deficit characterized by the lack of different response times to emotional (angry, happy) facial expressions relative to neutral expressions. In an effort to parse the driving factor behind the effects of chronic pain and CCAS, follow-up analyses were also performed in which measures of chronic pain and the related factors of depression and anxiety were treated as control variables. The rationale behind these supplementary analyses is that if response time effects are eliminated after controlling for the additional variables, then this provides evidence that any CMI-related deficits are likely due to the indirect effects of chronic pain rather than the direct result of CMI pathophysiology.
Divided Attention, Emotion Processing, and Event-Related Potentials
While Kumar et al. [28] associated behavioral performance with white matter integrity using DTI, participants in the present study completed the divided attention task while having their neural activity recorded using electroencephalography (EEG). Though lacking in the spatial resolution of fMRI methods, EEG has the millisecond temporal precision to identify component neurophysiological processes using non-invasive, scalp-based electrodes. When time-locked to external events such as stimulus presentations, summed EEG activities are referred to as event-related potentials (ERPs). The physiological processes that establish ERPs vary based upon the temporal and spatial characteristics. For example, early ERPs (relative to stimulus onset) such as a P1 waveform are recorded over occipital channels and generally represent integrative visual processing and threat signaling that are viewed as preconscious in nature [13, 16]. By comparison, later ERPs such as the P3 waveform can manifest at frontal and parietal recording locations and reflect effortful cognitive control and conscious motivational processes [25, 27, 48, 49, 64].
No specific hypotheses are made regarding the P1 (early, preconscious processing) and P3 (later, conscious processing) ERP waveforms. However, in accordance with the ERP methodology, we expect that they will supplement the behavioral data and provide information regarding the locus of behavioral effects. P1 waveforms are expected to coincide with differences in PRP effects between CMI patients and control participants and supplement behavioral findings related to the divided attention capacities of CMI patients. Related to emotion processing, there are two possibilities as to how the hypothesized CMI-related deficits may manifest in the ERP data. First, CMI patients may exhibit a P1 waveform that does not differ across the type of emotional expression, indicative of a breakdown in arousal processes and early threat signaling. The second possibility is that we will identify the lack of a distinct P3 waveforms across emotional expressions. This would be suggestive of a breakdown in conscious emotion processing or emotion regulation in CMI.
Methods
Institutional Review Approval
This study was submitted and approved by the local institutional review board of the University of Akron and the Cleveland Clinic.
Participants
Data from a new series of 20 CMI patients and 20 healthy control participants participated in the study. CMI patients were surgical candidates recruited during pre-surgical consultation and were diagnosed with CMI by a neurosurgeon specializing in decompression surgery (either M.G.L. or S.V.). No CMI patients were prescribed opioid use for pain relief at the time of assessment. Control participants were selected to best match CMI patients based upon age, gender, and years of education. Our final sample consisted of 36 females and 4 males. However, one CMI male could not complete the data collection session. As a result, his matched control participant was also removed from the analysis, resulting in a final dataset of 38 participants, with 18 females and 1 male in each group. Of the 19 CMI patients in the final dataset, 17 had not undergone decompression surgery. Due to scheduling conflict, the remaining two CMI patients could not complete the data collection session prior to undergoing surgery. For these two participants, data was collected approximately 3 months post-surgery. This delay was purposeful to avoid post-surgery complications and surgery-related pain. Upon review of the data, the inclusion of these post-surgery participants did not change the outcome of analyses from the 17 pre-surgery participants to their matched controls. As a result, we included post-surgery participants for all analyses.
Apparatus, Stimuli, and Procedure
Thirty facial images, taken from the NimStim database, were used (see Fig. 3 for example male and female images). They comprised three emotional expressions (happy, angry, and neutral) from ten different actors (five male, five female). The actors in these images were a mixture of African-, Asian-, European-, and Latin-American descent. The emotional expressions in the NimStim database were standardized so that angry and happy faces were extreme versions of these emotional expressions.
Fig. 3.
Example images of angry, neutral, and happy expressions from actors 1 and 43 from the NimStim dataset
Prior to engaging in the emotion perception task, self-reported age, gender, education, and symptom information was collected. No participant reported problems with their vision or audition. Participants also completed the digit symbol coding task and forward digit-span task from the RBANS-revised Book A (Pearson Education, Upper Saddle River, NJ). The digit symbol coding served as a measure of gross processing speed and the digit span served as a baseline measure of attentional capacity. The short form Depression, Anxiety, and Stress Scale (DASS 21) was used as a self-reported measure of depression and anxiety [20], and the self-reported chronic pain was assessed using the short-form McGill Pain Questionnaire [36].
Face stimuli were presented centrally on a 23-in. Dell LCD monitor appending approximately 9.53° (height) by 6.75° (width). For the single emotion perception task, each trial consisted of a single stimulus presentation consisting of a color image of a face presented against a black background. Each trial started with the presentation of a white fixation cross on a black background that persisted for 800 ms. After a 100, 300, or 900 ms onset delay, randomized within blocks, the stimuli appeared and remained until a response was collected. Participants were asked to determine the emotional expression of the target face by pressing the keys “V,” “B,” and “N” for angry, happy, and neutral emotions, respectively, with their right hand. The participants performed one practice block of 36 trials, followed by four experimental blocks of 27 faces each. The three expressions for each of the ten actors were presented at least three times across experimental blocks.
The dual-task emotion perception task used the same actors and face stimuli as the single task for Task 2 with an auditory Task 1. Each trial began with an 800 ms fixation. A pure tone or white noise (22 kHz, 8 bits) stimulus was then randomly presented over two speakers adjacent to the presentation monitor for 100 ms. Participants made responses to the auditory presentations (Task 1) using the “Z” and “X” keys for pure tone and white noise responses, respectively, with their left hand. An SOA of 100, 300, or 900 ms separated the auditory stimulus presentation and the emotional face presentation (Task 2), which was presented on the monitor until responses to the auditory and visual stimuli were recorded. The same response values of “V,” “B,” and “N” were used to identify the emotional expressions as in the single-task design. The participants performed one practice block of 36 trials, followed by 16 blocks of 72 experimental trials each. The three expressions across the ten actors were presented at least 38 times each, and each of the two auditory stimuli was presented approximately half of the trials.
Across tasks, participants were asked to respond as quickly as possible while maintaining accuracy. Mean response time and accuracy feedback were provided after each block and participants were encouraged to take breaks after completing individual blocks. Following standard PRP instructions, participants were instructed to respond to Task 1 before Task 2 and to avoid grouping their responses.
EEG Recording and Analyses
Electrophysiological data were recorded using a 32 channel Neuroscan EEG amplifier using Quik-caps with silver chloride (AgCl) electrodes. Channel values were measured in reference to the average of the left and right mastoid. A horizontal electrooculogram (HEOG) was recorded from the outer canthi of both eyes and a vertical electrooculogram (VEOG) was recorded above and below the midpoint of the left eye. Impedance was kept below 5 kΩ. The EEG, HEOG, and VEOG were amplified using a Synamps1 amplifier (Compumedics, Victoria, Australia) and digitized at 500 Hz.
Waveforms were analyzed using the ERPLab 4.0 and EEGLabv.13 toolboxes in Matlab2016b [14, 32].A bandpass filter consisting of half-amplitude high pass filter of 0.1 Hz and low pass filter of 30 Hz with a 12 dB/octave roll-off was applied to the data prior to artifact detection. Independent components were calculated from the continuous data using the runica algorithm in EEGLab for artifact detection. Components identified as artifacts in the continuous data were rejected based upon spatial and spectral waveform characteristics with the assistance of the MARA toolbox plugin [67]. After component rejection, 1000 ms epochs were established and time-locked to 200 ms pre-Task 2 stimulus onset for trials in which correct responses were identified between 200 and 3000 ms. A 200-ms pre-stimulus onset was used as a baseline for artifact rejections in EEGLab. This rejection process resulted in the loss of 12.2% of experimental trials for the PRP task ERP analysis. Greater trial loss was apparent for CMI patients compared to control participants (MCMI = 14.1%, MCON = 10.2%).
Event-Related Potentials
The P1 waveform was operationalized as the average positive amplitude in microvolts (μV) at O1, O2, and Pz in the window of 70–170 ms after face stimulus onset relative to the baseline. The P1 ERP component is believed to reflect integrative visual processing and physiological arousal [13, 16]. Using a similar PRP task as the present study, Pollock et al. [45] identified P1 ERP differences across all SOA levels with the greatest amplitudes for the 100 ms, followed by the 300 and 900 ms SOAs. Greater amplitudes have also been identified in response to threatening relative to neutral stimuli, including faces depicting fearful and angry expressions (e.g., [5, 17, 45, 46], though see [25]).
The late P3, or P3b, ERP measurement was established by taking the average amplitude (μV) at Fz, Cz, and Pz in the window of 400 to 600 ms after the face stimulus onset relative to the − 200 ms stimulus onset baseline [29]. The P3 has been associated with effortful cognitive control and motivational processes [25, 27, 48, 49, 64]. Related to the processing of emotional expressions, the P3 window reflects conscious, goal-driven processes and has been suggested to measure conscious appraisal of emotional information or emotion regulation [25]. These two ERP waveforms, the P1 and late P3, have been studied together in the work of Rellecke and colleagues [48]. They identified emotional modulations of the ERP waveform from 50 to 100 ms post-stimulus at parieto-occipital channel locations (i.e., P1) and between 350 and 450 ms at temporo-occipital and parieto-occipital channel locations (i.e., P3), where both waveforms were larger in amplitude for emotional expressions compared to neutral expressions.
Results
All analyses were performed using R v3.4.3 (R Core Team) and Microsoft Excel. Group differences were assessed using Student’s t tests or Wilcoxon rank-sum tests, dependent upon the normality of group distributions. For all analyses of variance (ANOVA), Greenhouse-Geisser corrections for repeated measures effects are reported to correct for departures from compound symmetry. To control for Type I error, a false discovery rate correction, as described by Benjamin and Yekutieli [7], was used to identify all significant simple effects.
Background Sample Characteristics
Demographic and baseline task characteristics of the CMI patients and control participants are presented in Table 1. The groups did not reliably differ in age, t(36) = − 0.66, p = 0.510, d = 0.22, or years of education age, t(36) = − 0.42, p = 0.676, d = 0.14. Control participants showed significantly faster processing speed compared to CMI patients, t(36) = − 2.46, p = 0.019, d = 0.80. Control participants also exhibited greater span capacities compared to CMI patients t(36) = − 2.17, p = 0.036, d = 0.71. As control participants reported near floor levels of depression, anxiety, and pain, non-parametric Wilcoxon rank-sum comparisons, rather than t tests, were performed to examine differences. CMI patients reported higher levels of anxiety, W = 288.0, p = 0.002, d = 1.07, and pain, W = 309.5, p < 0.001, d = 1.34, compared to control participants. However, there was only a non-significant trend for CMI patients to report higher levels of depression compared to control participants, W = 242.0, p = 0.069, d = 0.57.
Table 1.
Sample background characteristic by CMI status
| Measure | CMI status |
|
|---|---|---|
| CMI patients | Control participants | |
|
| ||
| Sample size | 19 | 19 |
| Age | 35.4 (11.7) | 38.2 (14.5) |
| Years of education | 14.0 (2.5) | 14.3 (2.1) |
| RBANS Coding* | 50.5 (14.6) | 61.9 (13.9) |
| RBANS Digit Span* | 10.2 (2.4) | 11.9 (2.5) |
| DASS depression | 6.1 (6.9) | 2.8 (4.4) |
| DASS anxiety* | 7.0 (5.6) | 2.1 (3.0) |
| SFMPQ total pain* | 29.8 (22.4) | 6.0 (11.4) |
Values from RBANS, DASS, and SFMPQ assessments reflect scale scores
RBANS Repeatable Battery for the Assessment of Neurological Status, DASS 21-item Depression, Anxiety, and Stress Scale, SFMPQ short-form McGill Pain Questionnaire
Denotes a significant difference between groups at p < .05
Single-Task Emotion Perception Behavioral Performance
Trials with response times less than 200 ms and greater than 3000 ms were excluded from analyses. This resulted in greater trial loss for CMI patients relative to control participants, W = 117.5, p = 0.018, d = 0.75 (MCMI = 1.6%, MCON = 0.1%). Accuracy and response time for the single-task emotion perception was assessed using a two-factor ANOVA with CMI status (patient vs. control) as a between-groups factor and facial expression (angry vs. happy vs. neutral) as a within-subjects factor. Table 2a provides the means of accuracy and response time for each facial expression in each group. For brevity, tables for main effects and interactions for all behavioral performance ANOVAs are presented in Table 3a.
Table 2.
Behavioral emotion perception performance values by CMI status
| a | Single-task emotion perception | T2 expression | SOA | CMI patients | Control participants |
| Accuracy | Angry | – | 97.1% (3.9%) | 97.1% (3.4%) | |
| Happy | – | 94.5% (7.4%) | 95.9% (4.6%) | ||
| Neutral | – | 98.4% (2.3%) | 98.2% (2.3%) | ||
| Response time | Angry | – | 1066 (369) | 824 (138) | |
| Happy | – | 1037 (397) | 765 (125) | ||
| Neutral | – | 985 (364) | 809 (120) | ||
| b | Dual-task emotion perception | T2 expression | SOA | CMI patients | Control participants |
| T2 accuracy | Angry | 100 | 93.3% (7.8%) | 95.6% (3.3%) | |
| Angry | 300 | 94.1% (7.0%) | 95.2% (4.6%) | ||
| Angry | 900 | 94.3% (5.9%) | 94.9% (4.3%) | ||
| Happy | 100 | 95.5% (4.2%) | 95.5% (4.1%) | ||
| Happy | 300 | 95.0% (4.1%) | 95.3% (3.9%) | ||
| Happy | 900 | 95.3% (3.1%) | 95.5% (4.5%) | ||
| Neutral | 100 | 95.8% (5.3%) | 96.9% (5.1%) | ||
| Neutral | 300 | 94.9% (5.1%) | 96.8% (4.0%) | ||
| Neutral | 900 | 94.6% (5.8%) | 96.9% (4.2%) | ||
| T2 response time | Angry | 100 | 1722 (372) | 1376 (216) | |
| Angry | 300 | 1574 (393) | 1183 (243) | ||
| Angry | 900 | 1282 (372) | 936 (216) | ||
| Happy | 100 | 1636 (352) | 1316 (214) | ||
| Happy | 300 | 1493 (376) | 1135 (226) | ||
| Happy | 900 | 1169 (348) | 855 (184) | ||
| Neutral | 100 | 1665 (339) | 1334 (181) | ||
| Neutral | 300 | 1525 (358) | 1167 (212) | ||
| Neutral | 900 | 1203 (324) | 899 (187) |
Accuracy values presented in percentage correct. Response time values presented in milliseconds (ms) T2 Task 2, SOA stimulus onset asynchrony
Table 3.
Behavioral emotion perception performance ANOVAs tables
| a | Single-task emotion perception—omnibus accuracy | df(num) | df(den) | F | p | η g 2 |
| Group | 1 | 36 | 0.17 | 0.681 | 0.002 | |
| Emotion* | 2 | 72 | 6.36 | 0.007 | 0.084 | |
| Group x emotion* | 2 | 72 | 0.48 | 0.567 | 0.007 | |
| Single-task emotion perception—omnibus response time | df(num) | df(den) | F | p | η g 2 | |
| Group | 1 | 36 | 6.75 | 0.014 | 0.015 | |
| Emotion | 2 | 72 | 3.82 | 0.027 | 0.006 | |
| Group x emotion | 2 | 72 | 3.22 | 0.046 | 0.005 | |
| Single-task emotion perception—Chiari response time | df(num) | df(den) | F | p | η g 2 | |
| Emotion | 2 | 36 | 2.99 | 0.063 | 0.008 | |
| Single-task emotion perception-control response time | df(num) | df(den) | F | p | η g 2 | |
| Emotion | 2 | 36 | 5.18 | 0.011 | 0.038 | |
| b | Dual-task emotion perception—omnibus accuracy | df(num) | df(den) | F | p | η g 2 |
| Group | 1 | 36 | 0.64 | 0.429 | 0.013 | |
| Emotion | 2 | 36 | 3.05 | 0.053 | 0.015 | |
| SOA* | 2 | 72 | 0.28 | 0.709 | 0.000 | |
| Group x emotion | 2 | 72 | 1.03 | 0.362 | 0.005 | |
| Group x SOA* | 2 | 72 | 0.02 | 0.968 | 0.000 | |
| Emotion x SOA | 4 | 144 | 0.55 | 0.699 | 0.001 | |
| Group x emotion x SOA | 4 | 144 | 1.43 | 0.226 | 0.003 | |
| Dual-task emotion perception—omnibus response time | df(num) | df(den) | F | p | η g 2 | |
| Group | 1 | 36 | 13.56 | 0.001 | 0.261 | |
| Emotion* | 2 | 36 | 16.63 | < 0.001 | 0.012 | |
| SOA* | 2 | 72 | 647.28 | < 0.001 | 0.297 | |
| Group x emotion* | 2 | 72 | 0.82 | 0.429 | 0.001 | |
| Group x SOA* | 2 | 72 | 1.96 | 0.159 | 0.001 | |
| Emotion x SOA | 4 | 144 | 1.74 | 0.145 | 0.000 | |
| Group x emotion x SOA | 4 | 144 | 0.27 | 0.895 | 0.000 |
Italic values represent significant effects and interactions
ηg2 generalized eta squared effect size, see [40]
Indicates that probability values were corrected for departures from compound symmetry using the Greenhouse-Geisser correction
There was no difference in accuracy across groups (p = 0.681). However, there was an effect of emotion (p = 0.007) in which happy expressions were identified less accurately compared to angry and neutral expressions, which did not differ. There was no group by emotion interaction (p = 0.567). For response time, CMI participants were slower to respond than CMI patients (p = 0.014). There was also a significant CMI status by emotion expression interaction (p = 0.046). Control participants exhibited differences in response time by emotion (p = 0.011) with happy expressions being reliably faster compared to angry expressions and neutral expressions with no differences between the latter two expressions. By comparison, the effect of emotional expression was trending (p = 0.063), but not significant for CMI patients.
To examine whether the group differences in response time were independent of chronic pain and associated depression and anxiety, a logistic regression model was performed in which it was determined whether group status could be predicted by response time after controlling for DASS depression, DASS anxiety, and McGill total pain scores (see Table 4a for odds ratios and regression coefficients). In this model, pain (p < 0.001), depression (p = 0.010), and anxiety (p = 0.004) were significant predictors of group membership. However, RT was no longer a predictor of group status (p = 0.503), suggesting that the CMI-related deficit in identifying the emotional faces likely stemmed from CMI-related symptoms.
Table 4.
Logistic regression models predicting CMI status from response time and pain-related factors
| a | Single-task emotion perception | B | Wald Z | p | OR | 95% CI |
| SFMPQ pain | 0.070 | 3.75 | < 0.001 | 1.077 | [1.168 2.139] | |
| DASS depression | − 0.312 | − 2.59 | 0.010 | 0.732 | [0.004 0.723] | |
| DASS anxiety | 0.435 | 2.85 | 0.004 | 1.545 | [0.565 0.910] | |
| Response time | 0.001 | 0.67 | 0.503 | 1.001 | [0.998 1.005] | |
| b | Dual-task emotion perception | B | Wald Z | p | OR | 95% CI |
| SFMPQ Pain | 0.069 | 6.14 | < 0.001 | 1.072 | [1.050 1.097] | |
| DASS Depression | − 0.293 | − 4.29 | < 0.001 | 0.746 | [0.647 0.847] | |
| DASS Anxiety | 0.428 | 5.14 | < 0.001 | 1.533 | [1.312 1.819] | |
| Response Time | 0.001 | 2.35 | 0.019 | 1.001 | [1.000* 1.002] |
B parameters reflect the log odds of being a CMI patient for a one unit increase in the predictor variable. For future comparison, the Akaike Information Criterion (AIC) for the two models were as followed – single task = 103.45; dual-task = 286.11
DASS 21-item Depression, Anxiety, and Stress Scale, SFMPQ short-form McGill Pain Questionnaire
Lower bound = 1.0002
Dual-Task Emotion Perception Behavioral Performance
Trials with response times for auditory tones or facial expressions less than 200 ms and greater than 3000 ms were excluded from analyses. This resulted in a loss of 16.0% of the trials for the dual-task condition. Trial loss was greater for CMI patients relative to control participants, W = 79, p = 0.002, d = 0.86 (MCMI = 21.8%, MCON = 10.1%). As Task 1 serves as a control task in which we made no specific hypotheses, we report analyses only for Task 2 (T2). T2 response times were yolked with Task 1 so that T2 response times were calculated only for trials in which Task 1 and T2 responses were correct. T2 analyses were performed using a three-factor ANOVA with CMI status (patient vs. control) as a between-groups factor and emotion expression (angry vs. happy vs. neutral) and SOA (100 vs. 300 vs. 900 ms) as within-subjects factors. Table 2b shows the mean response time and accuracy for T2.
The ANOVA for T2 accuracy yielded no significant main effects or interactions (all p’s > 0.053; Table 3b). However, for response time, there was a significant effect of emotion (p < 0.001) in which happy faces were identified the fastest, followed by neutral faces, followed by angry faces. There were differences in response time across all SOA conditions (p < 0.001) and an overall PRP effect of 451 ms (100 ms SOA–900 ms SOA). CMI patients were also slower to identify the facial expressions compared to the control participants (p = 0.001). There were no reliable interactions (all p’s > 0.144).
To further explore the group effect on response time, depression, anxiety, and pain scores were again entered into a logistic regression model to predict group membership. Even after controlling for the three symptom variables of pain (p < 0.001), depression (p < 0.001), and anxiety (p < 0.001), response time remained a significant predictor of group status (p = 0.019; Table 4b). This suggests that the differences in dual-task response time cannot be entirely accounted for by CMI-related symptoms and were rather the direct result of CMI pathophysiology.
Dual-Task Emotion Perception Event-Related Potentials
P1 (70 to 170 ms post-stimulus) mean amplitudes were anchored to T2 onset and analyzed using a four-factor ANOVA with CMI status (patient vs. control) as a between-groups factor and location (O1 vs. O2 vs. Pz), emotion (angry vs. happy vs. neutral), and SOA (100 vs. 300 vs. 900 ms) as within-subjects factors. The P3 (400 to 600 ms post-stimulus) was identical in design to the P1 analysis, though the location factor was characterized by midline locations (Fz vs. Cz vs. Pz). Mean amplitudes by location, emotion, and SOA across groups are provided by Table 5.
Table 5.
Event-related potential mean amplitude values by CMI status
| a | P1 mean amplitude | CMI patients | Control participants | |||||
| T2 expression | SOA | O1 | O2 | Pz | O1 | O2 | Pz | |
| Angry | 100 | 2.90 (2.73) | 4.15 (2.26) | − 0.02 (1.76) | 2.21 (2.40) | 3.46 (2.22) | 0.40 (2.87) | |
| Angry | 300 | 3.39 (3.13) | 3.23 (3.27) | 1.95 (2.18) | 2.21 (3.05) | 1.71 (2.85) | 1.54 (2.96) | |
| Angry | 900 | 1.32 (2.12) | 2.66 (2.60) | − 0.07 (1.63) | 0.84 (2.22) | 1.86 (2.24) | − 0.19 (1.44) | |
| Happy | 100 | 2.82 (2.74) | 3.93 (2.23) | 0.04 (1.59) | 2.73 (2.23) | 3.68 (2.20) | 0.59 (2.93) | |
| Happy | 300 | 3.30 (3.09) | 3.10 (3.08) | 1.99 (2.31) | 1.89 (3.04) | 1.72 (2.74) | 1.30 (2.98) | |
| Happy | 900 | 1.30 (2.17) | 2.71 (2.26) | 0.11 (1.81) | 0.63 (2.24) | 1.86 (2.16) | − 0.49 (1.74) | |
| Neutral | 100 | 2.78 (2.83) | 4.05 (2.37) | 0.10 (1.49) | 2.59 (2.55) | 3.64 (2.15) | 0.21 (2.84) | |
| Neutral | 300 | 3.30 (2.92) | 3.10 (3.04) | 1.86 (2.16) | 2.12 (2.69) | 1.85 (2.50) | 1.37 (3.12) | |
| Neutral | 900 | 0.93 (1.96) | 2.27 (2.62) | − 0.38 (1.74) | 1.02 (2.27) | 2.01 (2.19) | − 0.28 (1.60) | |
| b | P3 mean amplitude | CMI patients | Control participants | |||||
| T2 expression | SOA | Fz | Cz | Pz | Fz | Cz | Pz | |
| Angry | 100 | 3.87 (5.43) | − 1.46 (3.83) | 2.09 (3.42) | 3.51 (6.24) | − 0.16 (4.32) | 3.18 (3.99) | |
| Angry | 300 | 4.27 (5.89) | − 0.71 (3.21) | 2.13 (3.40) | 4.49 (6.30) | 1.63 (4.41) | 3.33 (3.08) | |
| Angry | 900 | 4.69 (3.65) | 3.76 (3.23) | 2.98 (1.96) | 4.59 (5.73) | 5.19 (2.92) | 3.04 (3.77) | |
| Happy | 100 | 3.66 (5.70) | − 2.06 (3.32) | 2.03 (3.29) | 3.61 (5.79) | − 0.27 (4.47) | 3.10 (3.90) | |
| Happy | 300 | 4.48 (5.16) | − 0.80 (3.12) | 1.98 (3.18) | 3.80 (5.91) | 0.89 (3.76) | 2.70 (2.97) | |
| Happy | 900 | 4.95 (3.96) | 3.74 (3.31) | 2.73 (1.96) | 4.06 (5.32) | 4.52 (3.12) | 2.38 (3.58) | |
| Neutral | 100 | 3.46 (5.81) | − 1.95 (3.41) | 1.87 (3.53) | 3.39 (5.88) | − 1.22 (4.53) | 2.27 (3.66) | |
| Neutral | 300 | 4.19 (5.34) | − 1.23 (2.95) | 1.85 (3.39) | 4.05 (5.87) | 0.44 (4.56) | 2.39 (3.29) | |
| Neutral | 900 | 4.56 (3.53) | 3.32 (2.92) | 2.68 (2.31) | 4.83 (5.13) | 4.44 (2.76) | 2.29 (2.88) | |
All values presented in microvolts (μV)
T2 Task 2, SOA stimulus onset asynchrony
P1 Waveform
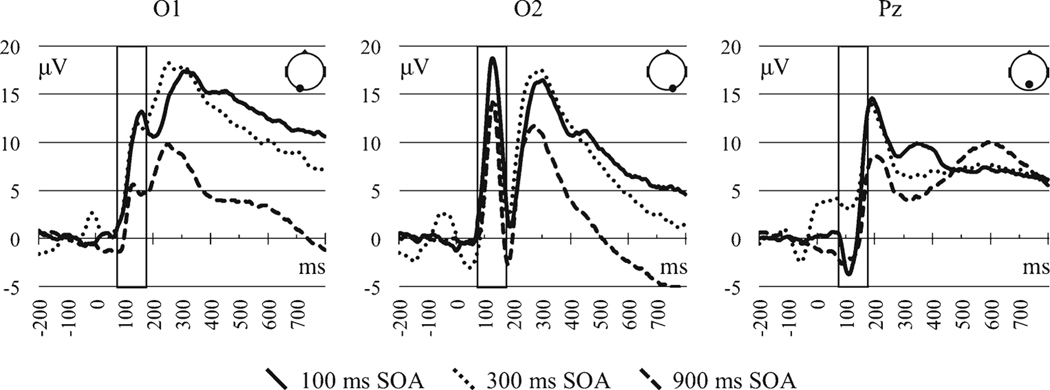
The P1 mean amplitude did not differ by group status (p = 0.289) or emotional expression (p = 0.702; see Table 6a for effect tables). A significant effect of SOA (p < 0.001) was the result of 100 and 300 ms SOAs eliciting greater amplitudes relative to the 900 ms, with no differences between the former two SOAs. There was also an effect of channel (p < 0.001) in which the greatest amplitudes were identified at the right occipital channel, followed by the left occipital channel, followed by the midline parietal. There was also a single SOA by channel interaction (p < 0.001). The SOA pattern at Pz did not resemble the decreasing P1 amplitude with greater SOA at the occipital channels. See Fig. 4 for waveforms by SOA and channel location collapsed across CMI status.
Table 6.
Event-related potential mean amplitude ANOVA tables
| a | Dual-task emotion perception—P1 | df(num) | df(den) | F | p | η g 2 |
| Group | 1 | 36 | 1.16 | 0.289 | 0.012 | |
| Emotion | 2 | 72 | 0.36 | 0.702 | 0.000 | |
| SOA* | 2 | 72 | 11.32 | < 0.001 | 0.057 | |
| Channel* | 2 | 72 | 19.36 | < 0.001 | 0.136 | |
| Group x emotion | 2 | 72 | 1.66 | 0.198 | 0.000 | |
| Group x SOA* | 2 | 72 | 1.22 | 0.298 | 0.006 | |
| Group x channel* | 2 | 72 | 0.47 | 0.586 | 0.004 | |
| Emotion x SOA | 4 | 144 | 0.68 | 0.609 | 0.000 | |
| Emotion x channel* | 4 | 144 | 0.65 | 0.570 | 0.000 | |
| SOA x channel* | 4 | 144 | 17.04 | < 0.001 | 0.040 | |
| Group x emotion x SOA | 4 | 144 | 2.13 | 0.080 | 0.001 | |
| Group x emotion x channel* | 4 | 144 | 2.16 | 0.104 | 0.000 | |
| Group x SOA x channel* | 4 | 144 | 0.22 | 0.844 | 0.001 | |
| Emotion x SOA x channel | 8 | 288 | 0.55 | 0.817 | 0.000 | |
| Group x emotion x SOA x channel | 8 | 288 | 0.55 | 0.820 | 0.000 | |
| Dual-task emotion perception—P1 at O1 | df(num) | df(den) | F | p | η g 2 | |
| SOA* | 2 | 72 | 14.17 | < 0.001 | 0.089 | |
| Dual-task emotion perception—P1 at O2 | df(num) | df(den) | F | p | η g 2 | |
| SOA | 2 | 72 | 13.27 | < 0.001 | 0.076 | |
| Dual-task emotion perception—P1 at Pz | df(num) | df(den) | F | p | η g 2 | |
| SOA* | 2 | 72 | 12.18 | < 0.001 | 0.119 | |
| b | Dual-task emotion perception—P3 | df(num) | df(den) | F | p | η g 2 |
| Group | 1 | 36 | 0.54 | 0.466 | 0.005 | |
| Emotion | 2 | 72 | 8.52 | < 0.001 | 0.002 | |
| SOA* | 2 | 72 | 19.98 | < 0.001 | 0.049 | |
| Channel* | 2 | 72 | 9.36 | 0.002 | 0.089 | |
| Group x emotion | 2 | 72 | 1.37 | 0.261 | 0.000 | |
| Group x SOA* | 2 | 72 | 0.40 | 0.568 | 0.001 | |
| Group x channel* | 2 | 72 | 0.64 | 0.470 | 0.007 | |
| Emotion x SOA | 4 | 144 | 0.63 | 0.644 | 0.000 | |
| Emotion x channel* | 4 | 144 | 3.97 | 0.008 | 0.001 | |
| SOA x channel* | 4 | 144 | 11.63 | 0.001 | 0.053 | |
| Group x emotion x SOA | 4 | 144 | 1.90 | 0.113 | 0.001 | |
| Group x emotion x channel* | 4 | 144 | 2.78 | 0.041 | 0.000 | |
| Group x SOA x channel* | 4 | 144 | 0.17 | 0.749 | 0.001 | |
| Emotion x SOA x channel* | 8 | 288 | 0.37 | 0.879 | 0.000 | |
| Group x emotion x SOA x channel* | 8 | 288 | 0.57 | 0.734 | 0.000 | |
| Dual-task emotion perception—Chiari P3 | df(num) | df(den) | F | p | η g 2 | |
| Emotion | 2 | 36 | 1.6 | 0.216 | 0.001 | |
| Channel* | 2 | 36 | 8.28 | 0.006 | 0.155 | |
| Emotion x channel* | 4 | 72 | 1.23 | 0.309 | 0.000 | |
| Dual-task emotion perception—control P3 | df(num) | df(den) | F | p | η g 2 | |
| Emotion | 2 | 36 | 9.903 | < 0.001 | 0.004 | |
| Channel* | 2 | 36 | 2.333 | 0.133 | 0.044 | |
| Emotion x channel* | 4 | 72 | 4.497 | 0.006 | 0.002 | |
| Dual-task emotion perception—control P3 at Fz | df(num) | df(den) | F | p | η g 2 | |
| Emotion | 2 | 36 | 1.174 | 0.321 | 0.001 | |
| Dual-task emotion perception—control P3 at Cz | df(num) | df(den) | F | p | η g 2 | |
| Emotion | 2 | 36 | 14.47 | < 0.001 | 0.011 | |
| Dual-task emotion perception—control P3 at Pz | df(num) | df(den) | F | p | η g 2 | |
| Emotion | 2 | 36 | 11.79 | < 0.001 | 0.011 |
Italic values represent significant effects and interactions
ηg2 generalized eta squared effect size, see [40]
Indicates that probability values were corrected for departures from compound symmetry using the Greenhouse-Geisser correction
Fig. 4.
P1 event-related potentials by stimulus onset asynchrony (SOA) across channel locations. Rectangular boxes indicate the time window used to assess P1
P3 Waveform
CMI patients and control participants did not differ in overall P3 amplitudes (p = 0.466; Table 6b). However, P3 mean amplitudes differed by emotional expression (p < 0.001). Angry expressions elicited greater P3 amplitudes compared to happy and neutral expressions. Participants also exhibited greater P3 amplitudes in response to happy expressions relative to neutral expressions. P3 amplitudes also differed by SOA (p < 0.001). The greatest amplitudes were observed at the 900 ms SOA, followed by the 300 ms SOA, followed by the 100 ms SOA. There was also an overall effect of channel location (p = 0.002). Differences were observed between all three channel locations in the order of the frontal, parietal, and central from greatest to smallest amplitudes.
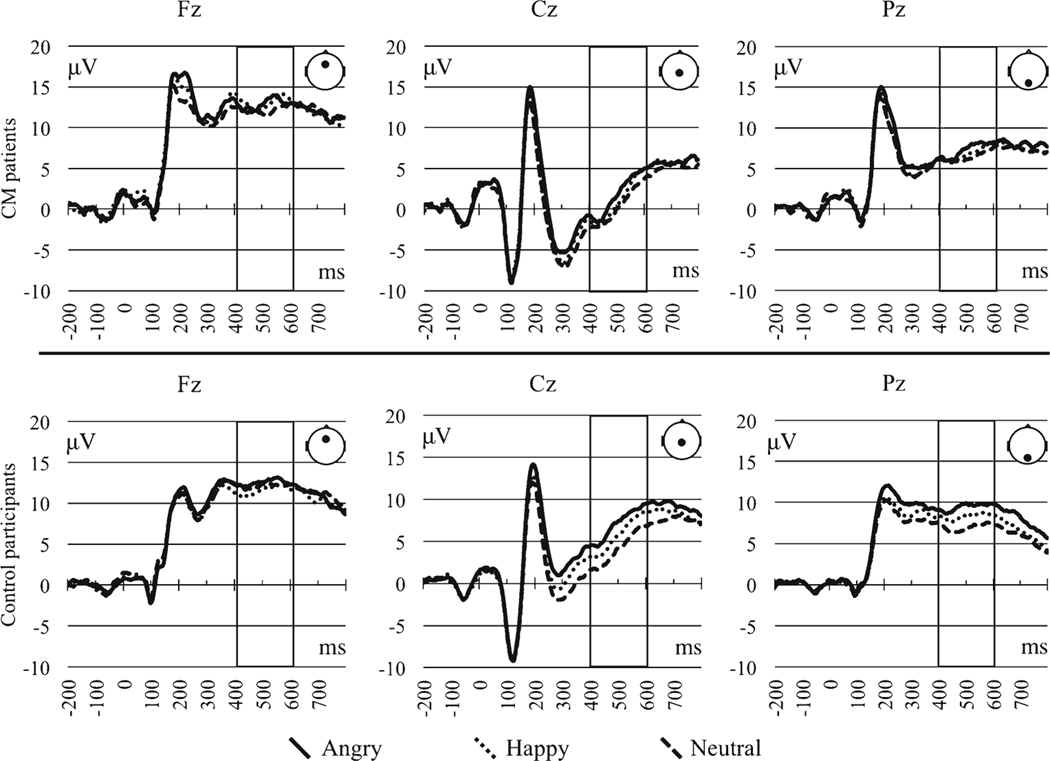
There were also several significant interactions. Emotion interacted with location (p = 0.008) and SOA interacted with location (p = 0.001). Moreover, there was a reliable three-way interaction of emotion by location by CMI status (p = 0.041; see Fig. 5 for the ERP waveform visual of the three-way interaction). As only the three-way interaction involved CMI status, we report only our interpretation of this interaction. To break down the three-way emotion by location by CMI status interaction, we examined the emotion by location simple interactions separately by CMI status.
Fig. 5.
P3 event-related potentials by emotion across channel locations for the CMI and control participants. Rectangular boxes indicate the time window used to assess P3
For CMI patients, there was no effect of emotion (p = 0.216). However, CMI patients exhibited different P3 amplitudes across channel locations (p = 0.006) with the greatest mean amplitudes identified at Fz and the lowest amplitudes identified at Cz. Moreover, there was no emotion by location interaction (p = 0.309).
In contrast, the P3 of control participants differed across emotional expression (p < 0.001). Angry expressions elicited greater P3 amplitudes compared to happy and neutral expressions, with no differences between the latter expressions. P3 amplitudes did not differ across channel locations (p = 0.133). There was also a reliable emotion by location interaction (p = 0.006), which was broken down by examining emotional effects separately by channel location. At both the central (p < 0.001) and parietal channel locations (p < 0.001), the greatest P3 amplitudes were identified for angry expression, followed by happy expressions, followed by neutral expressions. By comparison, there were no differences in P3 mean amplitudes by emotional expression at the frontal channel location (p = 0.321).
In summary, the three-way interaction of emotion, location, and CMI status was driven by control participants demonstrating P3 modulations by emotional expression, which were in turn driven by activations at the midline central and parietal channel locations. CMI patients, on the other hand, exhibited no P3 modulation by emotional expression. Unlike control participants, CMI patients also exhibited a frontalization of their maximal P3 waveform.
Discussion
We hypothesized that CMI patients would exhibit cognitive deficits due to either the indirect effect of chronic pain or the direct effect of damage to cognitive processing centers associated with brain areas affected by cervico-medullary compression. Results indicated a sparing of attentional control in CMI despite the finding of slower response times across two emotional expression identification tasks in CMI patients. Nevertheless, CMI patients did exhibit differences in neurophysiological activity believed to represent conscious, controlled processing of emotional information (i.e., emotion regulation).
We view several of our findings as being theoretically valuable to understand the cognitive deficits manifested in CMI. However, given that minimal group differences were observed for accuracy and that accuracy results for both tasks were high (all above 93%), we will focus the discussion section on response time and ERP results.
Maintained Cognitive Control in CMI
Through the use of the PRP method and emotional face stimuli, we aimed to better understand cognitive and affective processing associated with CMI. We found that CMI patients exhibited slower response times relative to age and education-matched control participants. Despite the slower processing speeds in the PRP task, there were no behavioral group differences in attentional control. That is, CMI patients and control participants showed an analogous PRP effect in which shorter SOAs (i.e., higher temporal overlap between the two tasks) were associated with longer response times. These results converge with the null divided attention finding in Kumar et al. [28], though using a larger, matched sample of CMI patients and healthy controls. These results also suggest that the previously identified cognitive deficits for CMI individuals are likely due to either pre- or post-central processing (e.g., perceptual or response initiation).
The P1 ERP waveform, a marker for integrative visual processing that derives from secondary visual areas, showed no differences in our CMI patients relative to matched control participants. In both groups, P1 ERPs were greater for the 100 and 300 ms SOAs than the 900 ms SOA. This pattern of effects was previously observed in Pollock et al. [45] in a sample of younger and older adults using the same task design as the present study. Based on the reasoning that ERP modulation by SOA indicates that the component in question involves central processing resources (i.e., N2pc in [60]; P1 in [45]), our P1 results converge with our behavioral data and suggest that a divided attention deficit is not a component of the cognitive profile of CMI.
Like the earlier P1, our results suggest that the P3 amplitude is maintained—consistent with the idea that divided attention is maintained in CMI. Both CMI patients and control participants exhibited P3 amplitudes that were inversely associated with SOA. While the P1 reflects early visual processes that are generally perceived to be preconscious in nature [51], the P3 is known to measure effortful and motivational (conscious) cognitive processes [27, 44, 64]. This suggests that CMI patients could act upon their motivation to identify the faces in a way that did not vary from control participants.
The observed group difference in the spatial distribution of the ERP amplitudes provides evidence of yet another factor to consider for the cognitive profile of CMI. Independent of attention effects, CMI patients exhibited a frontalization of ERP amplitudes that were not apparent in matched controls. That is, ERP amplitudes were greatest at frontal electrode locations positioned over the frontal association areas. This pattern of effects is frequently identified in normal aging and believed to be indicative of compensatory recruitment [10, 25, 50]. Though more evidence is needed to substantiate this initial finding, it is possible that compensatory mechanisms may also account for the lack of a behavioral group difference in the PRP effect in the present study.
Emotion Processing and Type I Chiari Malformation
In addition to the cognitive deficits of CMI, two findings suggest that CMI patients also experience difficulty in processing emotional content. First, response times for control participants varied dependent upon emotional expression in the single-task emotion perception, with faster responses to happy expressions than angry or neutral faces. This pattern of effects was previously observed in Houston et al. [25] in a sample of healthy younger and older adults. However, the response times of CMI patients did not vary as a function of emotional expression. It is also of interest that in the dual-task expression identification task, both control participants and CMI patients showed response times that were sensitive to emotional expression. This effect was very similar across single and dual tasks for the control participants with the fastest responses occurring in response to happy expressions. Yet, some aspect of the single-task design resulted in a non-significant emotion effect in the CMI patients. As there was no interaction between emotional expression and SOA, it is unlikely that the different results across tasks stemmed from an attention deficit. Additional investigation is needed to more fully understand this emotion and resource load relationship in CMI.
In addition to the behavioral results, CMI patients did not exhibit P3 modulation by emotion, whereas control participants exhibited greater P3 amplitudes for angry and happy expressions relative to neutral expressions in the dual task, indicating a negativity bias (see also [60]). Related to emotion processing functions, the P3 is likely associated with controlled regulation processes [25, 68]. This evidence further depicts CMI-related emotional deficits as originating from conscious, controlled processes (i.e., rather than from preconscious object perception).
Cerebellar Structure and Pain Accounts of Cognitive and Emotional Deficits
We proposed two accounts to understand cognitive and emotional deficits in our study. The first account, chronic pain, was partially supported by our data. After controlling for chronic pain and the related factors of depression and anxiety, the CMI status could be no longer predicted by response time in the single task. This suggests that the processing speed deficits in the single task could be accounted for by the indirect effects of chronic pain and related depression and anxiety. However, dual-task response times remained a significant predictor of CMI status after controlling for the three status variables, suggesting that the group difference in response time was due to unique influences beyond chronic pain and related variables. Therefore, the inconsistent relationship between the group differences in processing speed and the chronic pain factors suggests that, while chronic pain likely plays a factor in the cognitive deficits that are present in CMI, there are also likely cognitive deficits stemming directly from CMI pathophysiology.
As an alternative to the chronic pain account, the cerebellar dysfunction account described by the CCAS, or Schmahmann’s syndrome, appears to best explain the emotional processing deficit manifested in CMI patients [33, 56]. Stemming primarily from clinical observation, the CCAS suggests that widespread, clinically relevant cognitive deficits stem from structural abnormalities in the cerebellum and associated subcortical and cerebral pathways [55]. The characterization of the CCAS includes deficits in executive function, working memory, verbal skills, and visuospatial function as well as personality change including flattening of affect and disinhibition. While the lack of a PRP group difference observed in the present study suggests that divided attention is maintained in CMI—the lack of an emotion P3 effect in CMI patients coincides with the flat affect component of the CCAS. Notably, previous empirical investigations have found support for the other cognitive components of the CCAS in CMI [2, 21, 28]. In particular, Allen et al. [2] identified Stroop response disinhibition in CMI patients relative to matched control participants, a finding that remained after controlling for chronic pain. The response disinhibition finding of Allen et al., along with the divided attention finding of the present study, suggests that not all aspects of cognitive function are dysfunctional in CMI. These findings also suggest that other conditions characterized by structural cerebellar abnormalities may also exhibit specific, rather than general, attentional control deficits.
Implications and Substrates of Chiari Malformation-Related Deficits
We hypothesize that tonsillar ectopia can be a “necessary but not sufficient” factor in determining whether an individual may experience CMI-related symptoms (though not necessarily symptoms stemming from other types of Chiari malformations). In addition to tonsillar ectopia, we propose that CMI-related cognitive deficits are the result of a combination of a direct biological response to compression dynamics at the cervico-medullary junction that may not necessarily be related to tonsillar ectopia and an indirect result of long-term chronic pain experienced by CMI patients. While there is a considerable body of evidence supporting the latter’s association with the deterioration of cognitive function [9, 39], less is understood about malignant biological responses in CMI patients.
Though early, we are beginning to understand the implications of cervico-medullary compression and related factors on the neural networks underlying the cognitive deficits of CMI, a brain malformation that is traditionally considered to be confined to the posterior cranial fossa compartment (though see [24]). Allen et al. [2] suggested that CMI-related deficits in Stroop inhibitory control could likely be traced to dysfunction in the fronto-parietal attentional pathway (FPAP; [12, 23, 43]). The present finding of no CMI-related differences in the PRP effect magnitude (i.e., divided attention) is inconsistent with this proposition. However, emotional influences on human attention have been localized to pre-frontal and parietal areas that are known to overlap with the FPAP [47, 65, 66]. Thus, it is possible that the emotional processing differences observed in this study could be traced to activity in the FPAP despite evidence for spared cognitive processes thought to operate along overlapping neural pathways.
There are two additional sources of evidence to suggest that there is merit to the FPAP hypothesis. First, the effects of chronic pain are known to influence cognitive and emotional processing through areas involved in the FPAP. Bushnell et al. [9] described pain modulation as operating from the complex interaction of networks involving the brainstem, midbrain periaqueductal gray, anterior cingulate, frontal association areas, and the FPAP as described by Corbetta and Shulman [12]. Second, research on cerebrocerebellar circuitry describes several feedforward (i.e., corticopontine and pontocerebellar) and feedback (i.e., cerebellothalamic and thalamocortical) projections between the cerebellum and components of the pain-modulating centers and FPAP (see [57], for a review). Though speculative, Schmahmann and other researchers have suggested that these pathways are the likely substrates of the cerebellar cognitive affective syndrome [33, 57]. Future efforts in functional neuroimaging, particularly fMRI, diffusion tensor imaging, and resting state fMRI, are needed to better understand the impact of CMI on these interconnected networks.
Conclusion
Cervico-medullary compression in CMI has been suggested to have malignant effects on cognitive function [2]. The present findings suggest that CMI, a structural cerebellar abnormality characterized by cervico-medullary compression, manifests with specific, rather than general, attentional control deficits. While CMI patients did not experience deficits in cognitive control, response times and emotion regulation appear to be affected by CMI. Moreover, while single-task response time was no longer a predictor of CMI status after controlling for chronic pain and related factors, dual-task response time remained a significant predictor of CMI status, suggesting that the CMI-related deficits at least partially stem from CMI pathophysiology rather than chronic pain and related factors.
More work is needed on several fronts to better understand the constraints of cognitive deficits in CMI. Functional neuroimaging studies are needed to better understand the cognitive and emotional networks that are affected by CMI and whether CMI patients exhibit compensatory neural activity. Apart from these issues, it is also unknown as to how CMI is affected by demographic characteristics such as sex and age. Sex, in particular, is a characteristic with clinical implications that poses a challenge due to the difficulty in recruiting male CMI patients for laboratory study. Continued recruitment efforts are vital to establish representative samples of greater size than have been traditional to the CMI research field. Lastly, it is critical to continue taking steps to develop cognitive tasks that are clinically relevant and viable as diagnostic tools to complement traditional approaches to CMI diagnosis.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no competing interest.
References
- 1.Allen PA, Delahanty D, Kaut KP, Li X, Garcia M, Houston JR, et al. Chiari 1000 Registry Project: assessment of surgical outcome on self-focused attention, pain and delayed recall. Psychol Med. 2017. 10.1017/S0033291717003117. [DOI] [PubMed] [Google Scholar]
- 2.Allen PA, Houston JR, Pollock JW, Buzzelli C, Li X, Harrington AK, … Luciano MG (2014) Task-specific and general cognitive effects in Chiari malformation type I. PLoS One, 9(4), e94844, DOI: 10.1371/journal.pone.0094844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apkarian AV, et al. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108(1–2):129–36. 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Attridge N, Noonan D, Eccleston C, Keogh E. The disruptive effects of pain on n-back task performance in a large general population sample. Pain. 2015;156(10):1885–91. 10.1097/j.pain.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batty M, Taylor MJ. Early processing of the six basic facial emotional expressions. Cogn Brain Res. 2003;17(3):613–20. 10.1016/S0926-6410(03)00174-5. [DOI] [PubMed] [Google Scholar]
- 6.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1):7–15. 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165–88. [Google Scholar]
- 8.Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, Moseley GL. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev. 2014;34(7): 563–79. 10.1016/j.cpr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Bushnell MC, Čeko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502–11. 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46(4):462–73. 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrier LM, Pashler H. Attentional limits in memory retrieval. J Exp Psychol: Learn Mem Cognit. 1995;21(5):1339–48. [DOI] [PubMed] [Google Scholar]
- 12.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–15. 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 13.Dehaene S, Changeux J-P. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70(2):200–27. 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Method. 2004;134(1):9–21. 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Doberstein CA, Torabi R, Klinge PM. Current concepts in the pathogenesis, diagnosis, and management of Type I Chiari malformations. Rhode Island Med J: Recent Adv Neurosurg. 2017;100:47–9. [PubMed] [Google Scholar]
- 16.Eason RG, Harter R. Effects of attention and arousal on visually evoked cortical potentials and reaction time in man. Physiol Behav. 1969;4(3):283–9. 10.1016/0031-9384(69)90176-0. [DOI] [Google Scholar]
- 17.Eimer M, Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45(1):15–31. 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischbein R, Saling JR, Marty P, Kropp D, Meeker J, Amerine J, et al. Patient-reported Chiari malformation type I symptoms and diagnostic experiences: a report from the national Conquer Chiari Patient Registry database. Neurol Sci. 2015;36(9):1617–24. 10.1007/s10072-015-2219-9. [DOI] [PubMed] [Google Scholar]
- 19.Furuya K, Sano K, Segawa H, Ide K, Yoneyama H. Symptomatic tonsillar ectopia. J Neurol Neurosurg Psychiatry. 1998;64(2):221–6. 10.1136/jnnp.64.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44(2): 227–39. 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 21.Herweh C, Akbar M, Wengenroth M, Blatow M, Mair-Walther J, Rehbein N, et al. DTI of commissural fibers in patients with Chiari II-malformation. NeuroImage. 2009;44(2):306–11. 10.1016/j.neuroimage.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Hess LE, Haimovici A, Munoz MA, Montoya P. Beyond pain: modeling decision-making deficits in chronic pain. Front Behav Neurosci. 2014;8:263–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hesselmann G, Flandin G, Dehaene S. Probing the cortical network underlying the psychological refractory period: a combined EEG-fMRI study. Neuro Image. 2011;56(3):1608–21. 10.1016/j.neuroimage.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Houston JR, Eppelheimer MS, Pahlavian SH, Biswas D, Urbizu A, Martin BA, et al. A morphometric assessment of type I Chiari malformation above the McRae line: a retrospective case-control study in 302 adult female subjects. J Neuroradiol. 2017. 10.1016/j.neurad.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Houston JR, Pollock JW, Lien M-C, & Allen PA (in press-b [B]). Emotional arousal deficit or emotional regulation bias? An electrophysiological study of age-related differences in emotion perception. Experimental Aging Research. [DOI] [PubMed] [Google Scholar]
- 26.Jolicoeur P.Modulation of the attentional blink by on-line response selection: evidence from speeded and unspeeded task1 decisions. Mem Cogn. 1998;26(5):1014–32. 10.3758/BF03201180. [DOI] [PubMed] [Google Scholar]
- 27.Krolak-Salmon P, Fischer C, Vighetto A, Mauguière F. Processing of facial emotional expression: spatio-temporal data as assessed by scalp event-related potentials. Eur J Neurosci. 2001;13(5):987–94. 10.1046/j.0953-816x.2001.01454.x. [DOI] [PubMed] [Google Scholar]
- 28.Kumar M, Rathore RK, Srivastava A, Yadav SK, Behari S, Gupta RK. Correlation of diffusion tensor imaging metrics with neurocognitive function in Chiari I malformation. World Neurosurg. 2011;76(1–2):189–94. 10.1016/j.wneu.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Lien M-C, Allen PA, Crawford C. Electrophysiological evidence of different loci for case-mixing and word frequency effects in visual word recognition. Psychon Bull Rev. 2012;19(4):677–84. 10.3758/s13423-012-0251-9. [DOI] [PubMed] [Google Scholar]
- 30.Lien M-C, Proctor RW, Allen PA. Ideomotor compatibility in the psychological refractory period effect: 29 years of oversimplification. J Exp Psychol Human Percept Perform. 2002;28(2):396–409. 10.1037/0096-1523.28.2.396. [DOI] [PubMed] [Google Scholar]
- 31.Lien M-C, Ruthruff E, Cornett L, Goodin Z, Allen PA. On the nonautomaticity of visual word processing: electrophysiological evidence that word processing requires central attention. J Exp Psychol Hum Percept Perform. 2008;34(3):751–73. 10.1037/0096-1523.34.3.751. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. 2014;8 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manto M, Mariën P. Schmahmann’s syndrome—identification of the third cornerstone of clinical ataxiology. Cerebellum Ataxias. 2015;2(2):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manto M, Bower JM, Conforto AB, Delgado-Garcia JM, da Guarda SN, Gerwig M, et al. Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11(2):457–87. 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari type I malformations identified on magnetic resonance imaging. J Neurosurg. 2000;92(6):920–6. 10.3171/jns.2000.92.6.0920. [DOI] [PubMed] [Google Scholar]
- 36.Melzack R.The short-form McGill Pain Questionnaire. Pain. 1987;30(2):191–7. 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 37.Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44(5):1005–17. 10.1097/00006123-199905000-00042. [DOI] [PubMed] [Google Scholar]
- 38.Moriarty O, Finn DP. Cognitionand pain. Curr Opin Support Palliat Care. 2014;8(2):130–6. 10.1097/SPC.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 39.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93(3):385–404. 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Olejnik S, Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol Methods. 2003;8(4):434–47. 10.1037/1082-989X.8.4.434. [DOI] [PubMed] [Google Scholar]
- 41.Pashler H.Processing stages in overlapping tasks: evidence for a central bottleneck. J Exp Psychol. 1984;10(3):358–77. [DOI] [PubMed] [Google Scholar]
- 42.Pashler H.Dual-task interference in simple tasks: data and theory. Psychol Bull. 1994;116(2):220–44. 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- 43.Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23(10):3990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polich J.Meta-analysis of P300 normative aging studies. Psychophysiology. 1996;33(4):334–53. 10.1111/j.1469-8986.1996.tb01058.x. [DOI] [PubMed] [Google Scholar]
- 45.Pollock JW, Khoja N, Kaut KP, Lien M-C, Allen PA. Electrophysiological evidence for adult age-related sparing and decrements in emotion perception and attention. Front Integr Neurosci. 2012;6 10.3389/fnint.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pourtois G, Thut G, Grave de Peralta R, Michel C, Vuilleumier P. Two electrophysiological stages of spatial orienting towards fearful faces: early temporo-parietal activation preceding gain control in extrastriate visual cortex. Neuro Image. 2005;26(1):149–63. 10.1016/j.neuroimage.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Pourtois G, Schettino A, Vuilleumier P. Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biol Psychol. 2013;92(3):492–512. 10.1016/j.biopsycho.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Rellecke J, Palazova M, Sommer W, Schacht A. On the automaticity of emotion processing in words and faces: event-related brain potentials evidence from a superficial task. Brain Cogn. 2011;77(1): 23–32. 10.1016/j.bandc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Rellecke J, Sommer W, Schacht A. Does processing of emotional facial expressions depend on intention? Time-resolved evidence from event-related brain potential. Biol Psychol. 2012;90(1):23–32. 10.1016/j.biopsycho.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17(3):177–82. 10.1111/j.1467-8721.2008.00570.x. [DOI] [Google Scholar]
- 51.Rotshtein P, Richardson MP, Winston JS, Kiebel SJ, Vuilleumier P, Eimer M, et al. Amygdala damage affects event-related potentials for fearful faces at specific time windows. Hum Brain Mapp. 2010;31(7):1089–105. 10.1002/hbm.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruthruff E, Miller J, Lachmann T. Does mental rotation require central mechanisms? J Exp Psychol Hum Percept Perform. 1995;21(3):552–70. 10.1037/0096-1523.21.3.552. [DOI] [PubMed] [Google Scholar]
- 53.Schmahmann JD. An emerging concept the cerebellar contribution to higher function. Arch Neurol. 1991;48(11):1178–87. 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- 54.Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4(3):174–98. . [DOI] [PubMed] [Google Scholar]
- 55.Schmahmann JD. The cerebellar cognitive affective syndrome: clinical correlations of the dysmetria of thought hypothesis. Int Rev Psychiatry. 2001;13(4):313–22. 10.1080/09540260120082164. [DOI] [Google Scholar]
- 56.Schmahmann JD. The cerebellar cognitive affective syndrome and the neuropsychiatry of the cerebellum. Essentials of Cerebellum and Cerebellar Disorders: A Primer for Graduate Students. 2016: 499–511. 10.1007/978-3-319-24551-5_68. [DOI] [Google Scholar]
- 57.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(4):561–79. 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt M.Rey auditory verbal learning test: A handbook (p. 1996). Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- 59.Sekula RFJ, Jannetta PJ, Casey KF, Marchan EM, Sekula LK, McCrady CS. Dimensions of the posterior fossa in patients symptomatic for Chiari I malformation but without cerebellar tonsillar descent. Cerebrospinal Fluid Res. 2005;2(1):11. 10.1186/1743-8454-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaw K, Lien M-C, Ruthruff E, Allen PA. Electrophysiological evidence of emotion perception without central attention. J Cogn Psychol. 2011;23(6):695–708. 10.1080/20445911.2011.586624. [DOI] [Google Scholar]
- 61.Smith BW, Strahle J, Bapuraj JR, Muraszko KM,Garton HJ, Maher CO. Distribution of cerebellar tonsil position: implications for understanding Chiari malformation. J Neurosurg. 2013;119(3):812–9. 10.3171/2013.5.JNS121825. [DOI] [PubMed] [Google Scholar]
- 62.Tamburin S, Maier A, Schiff S, Lauriola MF, Di Rosa E, Zanette G, et al. Cognition and emotional decision-making inchronic low back pain: an ERPs study during Iowa gambling task. Front Psychol. 2014;5:1350–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Leeuw G, Eggermont LH, Shi L, Milberg WP, Gross AL, Hausdorff JM, … Leveille SG (2016). Pain and cognitive function among older adults living in the community. J Gerontol A Biol Sci Med Sci, 71(3), 398–405, DOI: 10.1093/gerona/glv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogel EK, Luck SJ, Shapiro KL. Electrophysiological evidence for a postperceptual locus of suppression during the attentional blink. J Exp Psychol Hum Percept Perform. 1998;24(6):1656–6. 10.1037/0096-1523.24.6.1656. [DOI] [PubMed] [Google Scholar]
- 65.Vuilleumier P.How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9(12):585–94. 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 66.Vuilleumier P, Huang Y-M. Emotional attention uncovering the mechanisms of affective biases in perception. Curr Dir Psychol Sci. 2009;18(3):148–52. 10.1111/j.1467-8721.2009.01626.x. [DOI] [Google Scholar]
- 67.Winkler I, Haufe S, Tangermann M. Automatic classification of artifactual ICA-components for artifact removal in EEG signals. Behav Brain Funct. 2011;7(1):30. 10.1186/1744-9081-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wood S, Kisley MA. The negativity bias is eliminated in older adults: age-related reduction in event-related brain potentials associated with evaluative categorization. Psychol Aging. 2006;21(4): 815–20. 10.1037/0882-7974.21.4.815. [DOI] [PubMed] [Google Scholar]
- 69.Zhou S, Despres O, Pebayle T, Dufour A. Age-related decline in cognitive pain modulation induced by distraction: evidence from event-related potentials. J Pain. 2015;16(9):862–72. 10.1016/j.jpain.2015.05.012. [DOI] [PubMed] [Google Scholar]