Abstract
Earlier studies have supported a significant role for cocaine in the susceptibility to and the progression of human immunodeficiency virus type 1 (HIV-1) infection. Recently, several unique HIV-1 entry coreceptors (e.g., CCR5 and CCR3) and a trio of HIV-1-specific suppressor chemokines, namely, RANTES (regulated-upon-activation T expressed and secreted), macrophage inflammatory protein 1α (MIP-1α) and MIP-1β, were identified. Although cocaine has been linked to the immunopathogenesis of HIV-1 infection, the corresponding cellular and molecular mechanism(s) have not been well defined. We hypothesize that cocaine mediates these pathologic effects through the downregulation of HIV-1-suppressing chemokines and/or upregulating HIV-1 entry coreceptors in HIV-1-infected subjects, resulting in disease progression to AIDS. Our results show that cocaine selectively downregulates endogenous MIP-1β secretion by normal peripheral blood mononuclear cells (PBMC), while cocaine did not affect the MIP-1β production by PBMC from AIDS patients. Cocaine also selectively suppresses lipopolysaccharide-induced MIP-1β production by PBMC from HIV-infected patients. Further, cocaine significantly downregulates endogenous MIP-1β gene expression, while it upregulates HIV-1 entry coreceptor CCR5 by normal PBMC. These studies suggests a role for cocaine as a cofactor in the pathogenesis of HIV infection and support the premise that cocaine increases susceptibility to and progression of HIV-1 infection by inhibiting the synthesis of HIV-1 protective chemokines and/or upregulating the HIV-1 entry coreceptor, CCR5.
Human immunodeficiency virus type 1 (HIV-1) mainly infects CD4+ T (T-helper) lymphocytes and macrophages. Although the CD4 molecule is the primary receptor for virus entry, several studies have shown that, in addition to CD4, other coreceptors may be required for efficient viral entry. Recently, a major HIV-1 coreceptor and β-chemokine receptor, CCR5, was identified for macrophage (M)-tropic HIV-1 strains (2, 10).
Chemokines are chemoattractant cytokines that have gained major attention because of their specific inhibitory effects on HIV-1 infection (6). Members of the β-chemokine family, RANTES, macrophage inflammatory protein 1α (MIP-1α), and MIP-1β were shown to inhibit infection of target cells by primary M-tropic virus strains, but not T-cell (T)-tropic virus strains (6, 10). Recent studies showed that these chemokines specifically block the CCR5 coreceptor and thereby inhibit the entry of HIV-1 into target cells (6, 10, 11; for a review, see reference 14). These studies clearly demonstrate that the β-chemokines and the HIV-1 entry coreceptor, CCR5, are important host factors that can influence susceptibility to M-tropic HIV-1 infection.
Cocaine is one of the most widely abused drugs in the United States. In 1994, according to the National Household Survey on Drug Abuse, an estimated 1.4 million Americans were current cocaine users (NIDA Notes, September/October, 1995). The last decade has witnessed a great, entangled epidemic of cocaine abuse and HIV-1 infections. In vivo and in vitro studies indicate that cocaine alters cytokine production and cell trafficking, stimulates susceptibility of peripheral blood leukocytes to HIV-1 infection, and increases viral titers in the brain, thus facilitating disease progression (6, 8, 13; for a review, see reference 25). However, the molecular mechanisms underlying these effects of cocaine on HIV-1 infections remain poorly understood. Given the substantial literature describing the association of cocaine use with the susceptibility to and the progression of HIV-1 infections and the exciting recent studies demonstrating the potential role of chemokines, it is reasonable to hypothesize that cocaine can modulate the HIV-suppressing chemokines and their receptors. The present study examines the effect of cocaine on MIP-1β synthesis by lymphocytes from normal and HIV-infected subjects and MIP-1β gene expression by normal peripheral blood mononuclear cells (PBMC).
MATERIALS AND METHODS
Blood donors were apprised of this study, and consents were obtained in accordance with the policies of the appropriate institutions and the National Institutes of Health. Peripheral blood samples from healthy, HIV-negative individuals and also from HIV-1-infected patients were drawn into a syringe containing heparin (20 U/ml). The HIV-infected subjects were recruited from the Immunodeficiency Services Clinic of the Erie County Medical Center and were at different stages of the disease. The mean CD4 and CD8 numbers of HIV-1-infected patients were 273.1 ± 227.2 (standard deviation [SD]) and 958.1 ± 554.4 (SD)/mm3, respectively. Monocyte numbers in HIV-1-infected subjects varied from 296 to 1,000/mm3 with a mean of 499.4 ± 252.1 (SD). HIV-1-infected subjects were not using cocaine at the time of the study, and their age varied from 25 to 40 years. Mononuclear cells from uninfected and HIV-infected subjects were isolated from heparinized venous blood by using a modified method of Boyum (5). Blood was diluted with an equal volume of normal saline and was centrifuged at 400 × g for 30 min at 18°C over a cushion of Ficoll-Hypaque. The PBMC band was harvested, washed three times with saline, and resuspended in RPMI 1640 medium containing 25 mM HEPES buffer supplemented with 5% heat-inactivated fetal bovine serum (Gibco/BRL, Gaitherburg, Md.), 80 mg of gentamicin per ml (Schering, Kenilworth, N.J.), and 300 μg of fresh glutamine per ml. Tissue culture-treated, polystyrene, sterile, nonpyrogenic 12-well cell culture plates (Costar, Corning, N.Y.) were used to culture cells in 1-ml quantities. Triplicate cultures containing 3 × 106 cells/ml received different concentrations of cocaine. Cocaine hydrochloride was provided by the National Institute on Drug Abuse (Rockville, Md.) and used at 10−6, 10−9, and 10−12 M final concentrations. Cocaine was originally dissolved in sterile distilled water and was subsequently diluted in Hanks' balanced salt solution to the required concentrations. Control and treated cultures were incubated at 37°C for 24 h in 5% CO2 and 95% air. The cultures were centrifuged at 900 × g for 10 min, and the culture supernatants were examined for MIP-1β by enzyme-linked immunosorbent assay (ELISA).
MIP-β levels in culture supernatants were measured by a quantitative sandwich ELISA technique by using the Quantikine Kit (R&D Systems, Minneapolis, Minn.) as described by the manufacturer. Briefly, 200 μl of diluted culture fluid or control standard sample was added to each well containing 50 μl of assay diluent and then incubated for 1.5 h at room temperature. Plates were vigorously washed three times with wash buffer, and then 200 μl of MIP-1β conjugate was added and incubated for 1.5 h at room temperature. The plates were washed again, and 200 μl of substrate solution was added and incubated at room temperature for 20 min. The reaction was terminated by adding 50 μl of stop solution, and the plates were measured for absorbance at 450 nm by using an ELISA plate reader (Flow Laboratories). The concentration of MIP-1β in the culture supernatants was determined by comparing the absorbancy of the samples with absorbancy curves obtained with standard samples run simultaneously with test samples and expressed as picograms per milliliter.
RNA isolation.
Cell cultures treated with various concentrations of cocaine were harvested at 8 h, and cytoplasmic RNA was extracted by an acid guanidinium thiocyanate-phenol-chloroform method as described elsewhere (9). The rational for selecting an 8-h time period for in vitro treatment of PBMC with cocaine was based on previous experiments which produced maximum modulatory effects on MIP-1β and CCR5 gene expression. Briefly, cultured cells were pelleted by centrifugation and resuspended in a 4 M solution of guanidinium thiocyanate. Cells were pipetted 7 to 10 times to lyse them and then were phenol-chloroform extracted in the presence of sodium acetate. After centrifugation, the RNA was precipitated from the aqueous layer by isopropanol. An equal volume of isopropanol was added, and the mixture was kept at −20°C for 1 h and then centrifuged to pellet the RNA. The pellet was resuspended in the guanidinium solution, and an equal volume of isopropanol was added. The solution was kept at −20°C for 1 h and then centrifuged again to pellet the RNA. The pellet was washed once with 75% ethanol to remove any remaining guanidinium. The final pellet was dried and resuspended in diethyl pyrocarbonate-treated water, the amount of RNA was determined, and the mixture was stored at −70°C until used in further experiments.
RT-PCR.
After extraction, RNA was reverse transcribed to make a DNA copy for use in PCR. The reverse transcriptase PCR (RT-PCR) analyses were performed by using a Perkin-Elmer kit (catalog number N808-0143) according to the directions of the manufacturer. Briefly, 1 μg of RNA was added to a tube containing 5 mM Mg Cl2, a 1 mM concentration of each deoxynucleoside triphosphate (A, C, G, and T), 50 mM KCl, 10 mM Tris (pH 8.3), 2.5 μM oligo(dT), 20 U of RNasin, and 50 U of murine leukemia virus RT. The mixture was incubated at room temperature for 10 min, and the temperature was raised to 42°C for 35 min, then heated to 99°C for 5 min, and placed on ice until used in the PCR reaction. For the PCR, the newly synthesized cDNA was tested with housekeeping β-actin control primers separately or together with the second set of specific primers. To each tube was added a 10-μl sample of the RT product in a final mixture of 2 mM MgCl2, 10 mM Tris (pH 8.3), and 50 mM KCl, plus 0.02 mM concentrations of both the 5′ and 3′ primers and 2.5 U of Taq polymerase. The mixture was overlaid with mineral oil and placed in a thermocycler for 30 cycles of 95°C for 30 s, 60°C for 30 s, and 74°C for 1 min. A 60°C incubation was done for 5 min prior to the start of the 30 cycles. A 10-μl sample of each of the PCR reactions was analyzed on 1.2% agarose gels containing ethidium bromide with a phi X174 DNA/HaeIII-digested molecular weight standard to determine the fragment sizes. Bands were visualized under UV light and photographed by using Polaroid film. Densitometric scans of the experimental signals in each lane were normalized to the corresponding housekeeping β-actin gene signals to correct for gel loading error, if any (experimental optical density [OD]/β-actin OD × 100), and the corrected OD values from the untreated control lane were compared to the corrected OD values of the treated lanes. Decreases or increases from control values were calculated as follows: % change = (corrected experimental OD/corrected control OD) × 100. The following primer sequences were used in the experiments: β-actin, 5′-GTGGGGCGCCCCAGGCACCA-3′ (upstream) and 5′-CTCCTTAATGTCACGCACGATTTC-3′ (downstream) (548 bp); MIP-1β, 5′-CCAAACCAAAAGAAGCAAGC-3′ (upstream) and 5′-AGAAACAGTGACAGTGGACC-3′ (downstream) (320 bp); and CCR5, 5′-CTCGGATCCGGTGGAACAAGATGGATTAT-3′ (upstream) and 5′-CTCGTCGACATGTGCACAACTCTGACTG-3′ (downstream) (1,117 bp).
RESULTS
Cocaine selectively suppresses endogenous MIP-1β production by normal lymphocytes.
The data presented in Table 1 demonstrate that cocaine selectively inhibited the endogenous production of MIP-1β by lymphocytes from normal subjects and not from HIV-infected patients in a dose-dependent manner. PBMC from normal subjects cultured in medium alone produced 153 pg of MIP-1β per ml. PBMC cultured with cocaine at 10−6 M concentration produced significantly lower level of MIP-1β (76 pg/ml; 50% inhibition, P < 0.03) compared to control culture (153 pg/ml). Cocaine at 10−9 and 10−12 M concentrations also produced lower levels of MIP-1β (87 pg/ml, 43% inhibition, P < 0.06, and 99 pg/ml, 34% inhibition, P < 0.13, respectively) compared to control culture (153 pg/ml), although these inhibitions were statistically not significant. PBMC from HIV-1-infected patients cultured in medium alone produced 210 pg/ml of MIP-1β. PBMC from HIV-1-infected subjects cultured with cocaine at similar concentrations of 10−6, 10−9, and 10−12 M produced 210, 223, and 195 pg of MIP-1β per ml, respectively. The mean percent inhibitions compared to the untreated controls were 0 (P < 0.9), 6 (P < 0.9), and 7% (P < 0.8), respectively, for the 10−6, 10−9, and 10−12 M cocaine concentrations. These results demonstrate that cocaine selectively downregulates endogenous production of HIV protective chemokines by normal PBMC, while cocaine at a similar concentration did not affect MIP-1β production by PBMC from HIV-1-infected subjects.
TABLE 1.
Effect of cocaine on MIP-1β productiona
| Group (concn [M]) | Uninfected subjects
|
HIV-1-infected subjects
|
||
|---|---|---|---|---|
| MIP-1β (pg/ml) | % Inhibition (P) | MIP-1β (pg/ml) | % Inhibition (P) | |
| Cell control | 153.5 ± 35.4 | 210.0 ± 140.4 | ||
| Cocaine (10−12) | 99.8 ± 34.7 | 34.9 (<0.13) | 195.5 ± 113.0 | 7.1 (<0.8) |
| Cocaine (10−9) | 87.1 ± 29.2 | 43.2 (<0.06) | 223.3 ± 131.4 | −6.1 (<0.9) |
| Cocaine (10−6) | 76.2 ± 26.5 | 50.1 (<0.03) | 210.7 ± 143.1 | 0 (<0.9) |
PBMC (3 × 106/ml) from normal subjects and HIV-1-infected patients were cultured alone or with different concentrations of cocaine for 24 h, and culture supernatants were quantitated for MIP-1β by ELISA. The results represent the mean ± the SD of three independent experiments performed with PBMC from three separate patients and three different healthy individuals. The statistical significance of difference was calculated by the t test. Although the mean control level of MIP-1β in HIV-1-infected subjects was slightly higher (210 pg/ml) than in uninfected subjects (153 pg/ml), the difference was statistically not significant (P < 0.53).
Cocaine selectively suppresses LPS-induced β-chemokine production by lymphocytes from HIV-infected patients.
The data presented in Table 2 show the effect of cocaine on lipopolysaccharide (LPS)-induced MIP-1β production by lymphocytes from normal donors and HIV-infected patients. Lymphocytes from uninfected and HIV-1-infected patients cultured in medium alone produced similar levels of MIP-1β (155 and 142 pg/ml, respectively; P < 0.16). Addition of LPS (10 μg/ml) to cultures of lymphocytes from uninfected donors significantly enhanced MIP-1β production to 1,529 pg/ml (P < 0.0001) compared to an untreated culture (155 pg/ml). Lymphocytes (from HIV-1-infected patients) cultured with similar concentrations of LPS also produced enhanced levels of MIP-1β (1,490 pg/ml, P < 0.0001) compared to untreated control culture (142 pg/ml). Cocaine did not affect LPS-induced MIP-1β production by normal lymphocytes. Normal PBMC cultured with LPS plus 10−6, 10−9, and 10−12 M cocaine produced 1,479, 1,500, and 1,471 pg/ml, respectively, compared to 1,529 pg/ml produced by culture treated with LPS alone. In contrast, cocaine significantly suppressed LPS-induced MIP-1β production by PBMC from HIV-1-infected patients in a dose-dependent manner; the levels of MIP-1β produced were 930 (P < 0.007), 1,153 (P < 0.04), and 1,184 (P < 0.04) pg/ml, respectively, for 10−6, 10−9, and 10−12 M cocaine compared to 1,490 pg/ml produced by PBMC from HIV-infected patients treated only with LPS. These results demonstrate that cocaine selectively suppresses LPS-induced MIP-1β production by PBMC from HIV-infected patients, while leaving cells from normal donors unaffected.
TABLE 2.
Effect of cocaine on LPS-induced MIP-1β production by PBMC from normal donors and HIV-infected patientsa
| Compound added
|
MIP-1β titer (pg/ml)
|
||
|---|---|---|---|
| LPS (10 μg/ml) | Cocaine (M) | Uninfected subjects | HIV-infected subjects |
| 0 | 0 | 155.0 ± 15.2 | 142.2 ± 10.8 |
| LPS | 0 | 1,529.9 ± 80.4 | 1,490.0 ± 78.0 |
| LPS | 10−12 | 1,471.0 ± 33.0 (NS) | 1,184.6 ± 28.1 (P < 0.04) |
| LPS | 10−9 | 1,500.4 ± 70.1 (NS) | 1,153.5 ± 76.8 (P < 0.04) |
| LPS | 10−6 | 1,479.5 ± 66.2 (NS) | 930.6 ± 64.4 (P < 0.007) |
PBMC (3 × 106/ml) from normal and HIV-infected patients were cultured alone or with LPS plus different concentrations of cocaine for 24 h, and culture supernatants were quantitated for MIP-1β by ELISA. The data are the mean ± the SD values of five independent experiments performed with PBMC from five different healthy subjects and five different HIV-infected patients. The control levels of MIP-1β produced by PBMC from uninfected subjects and HIV-1-infected subjects were similar (P < 0.1). Statistical significance of difference was calculated by the t test.
Cocaine modulates the expression of the chemokine MIP-1β and its receptor gene, CCR5, by PBMC from normal donors.
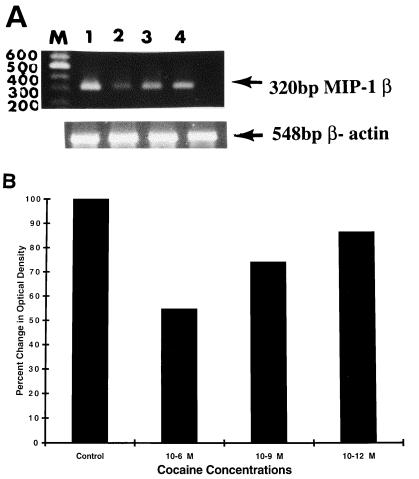
Several chemokine receptors also serve as entry coreceptors for HIV, and this function can be blocked by their natural chemokine ligands. The chemokine, MIP-1β, can suppress HIV-1 infection of permissive target cells. Conversely, drugs of abuse such as cocaine are associated with increased replication of HIV-1. Since cocaine significantly downregulated MIP-1β production by normal PBMC, we undertook the following experiments to determine whether cocaine can enhance susceptibility to HIV infection by upregulating HIV coreceptor gene expression and/or inhibiting HIV-suppressing chemokine gene expression. PBMC were cultured with or without 10−6 to 10−9 M cocaine for 8 h. Total RNA was extracted and subjected to RT-PCR assay by using specific primers for MIP-1β and the housekeeping gene, β-actin. Figure 1A demonstrates that treatment of PBMC with cocaine suppressed transcription of the MIP-1β gene. cDNA from amplified PCR products by using β-actin primers migrated to the expected region of 548 bp. MIP-1β-specific PCR products banded at the expected region of 320 bp. Lane M reflects molecular size markers. Cocaine at 10−6 M (lane 2, OD = 17.3), 10−9 M (lane 3, OD = 23.0), and 10−12 M concentration (lane 4, OD = 27.4) downregulated MIP-1β gene expression in a dose-dependent manner compared to the control culture (lane 1, OD = 31.6). Figure 1B shows the percent change of OD values from Fig. 1A. Cocaine at 10−6, 10−9, and 10−12 M suppressed MIP-1β gene expression in a dose-dependent manner; the percent suppression levels were 45.2, 25.9, and 18.0%, respectively, for 10−6 M (lane 2), 10−9 M (lane 3), and 10−12 M (lane 4) compared to the untreated control culture (lane 1).
FIG. 1.
(A) Cocaine suppresses MIP-1β gene expression by lymphocytes as measured by RT-PCR. PBMC (3 × 106/ml) from normal donors were treated with 10−6 and 10−9 M cocaine for 8 h, mRNA extracted, reverse transcribed, amplified with MIP-1β and the housekeeping gene β-actin primers, and electrophoresed. cDNA from amplified PCR products of MIP-1β and β-actin banded at 320 and 548 bp, respectively. Lanes: M, molecular weight markers; 1, untreated control; 2, cocaine at 10−6 M; 3, cocaine at 10−9 M; 4, cocaine at 10−12 M. These data represent results from a single experiment. The experiment was repeated independently four times with PBMC from four different subjects with similar results. (B) Quantitation of changes in MIP-1β gene expression. The percent changes in the laser densitometry readings of the photographic negatives of experimental values after normalization with respective β-actin values were determined and then compared with control values from Fig. 1A. Lanes: 1, untreated control; 2, cocaine at 10−6 M; 3, cocaine at 10−9 M; 4, cocaine at 10−12 M.
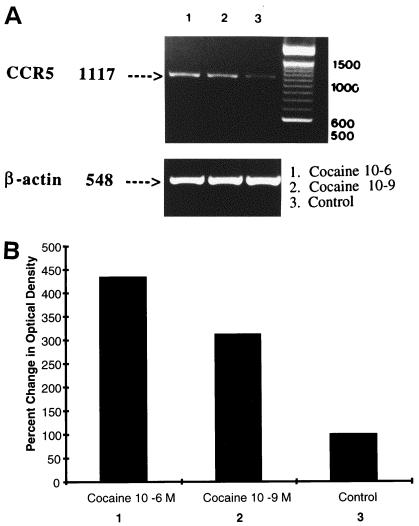
Figure 2A shows the effects of cocaine on gene expression of the CCR5 chemokine receptor and HIV coreceptor. Normal PBMC were cultured with 10−6 or 10−9 M cocaine for 8 h. RNA was extracted and reverse transcribed, and cDNA was amplified by PCR by using primers specific for the housekeeping gene β-actin and CCR5. Lanes 1 and 2 reflect cultures treated with cocaine at 10−6 and 10−9 M, respectively, while lane 3 represents the untreated control culture. Housekeeping β-actin cDNA (lower panel) migrated, as expected, to the 548-bp region. Treatment of cultures with cocaine did not affect the constitutively expressed β-actin gene (lanes 1 and 2) and was comparable to the control (lane 3). The CCR5 PCR product banded at the expected region of 1,117 bp. Control PBMC (lane 3) incubated in medium alone for 8 h demonstrated detectable CCR5 gene expression (lane 3, OD = 11.5). Cultures treated with cocaine at 10−6 (lane 2, OD = 50) and 10−9 M (lane 1, OD = 36) upregulated CCR5 gene expression compared to the control culture (lane 3, OD = 11.5). Data presented in Fig. 2B show the percent change of the OD values from those in Fig. 2A. Cocaine significantly upregulated CCR5 gene expression in a dose-dependent manner compared to the control culture; the levels of percent upregulation were 334 and 213%, respectively, for 10−6 M (lane 2) and 10−9 M (lane 1) compared to the untreated control culture (lane 3).
FIG. 2.
(A) Cocaine upregulates CCR5 gene expression by lymphocytes as measured by semiquantitative RT-PCR. RNA from PBMC (3 × 106/ml) cultures either untreated or treated with cocaine for 8 h were reverse transcribed and amplified with CCR5 primers and electrophoresed. Lanes: 1, cocaine at 10−6 M; 2, cocaine at 10−9 M; 3, untreated control. These data represent results from a single experiment. The experiment was repeated independently four times with PBMC from four different subjects with similar results. (B) Quantitation of changes in CCR5 gene expression. The percent changes in the densitometry reading of the photographic negatives after normalization with respective β-actin values were determined and then compared with the control values from Fig. 2A. Lanes: 1, cocaine at 10−6 M; 2, cocaine at 10−9 M; 3, untreated control. Cocaine significantly upregulated CCR5 gene expression in dose-dependent manner (lanes 1 and 2) compared to the control (lane 3).
DISCUSSION
Several human and animal studies have demonstrated that various recreational drugs, including cocaine, alcohol, and opiates, exert immunomodulating activities and therefore could serve as cofactors in susceptibility to HIV-1 infections (for a review, see references 18 and 19). After sexual transmission, intravenous substance abuse is the second greatest risk factor for HIV-1 infection (12, 15). The advent of the cocaine abuse epidemic during the last decade has coincided with and also has had profound effects on the AIDS epidemic (19). Various investigators have shown that crack (cocaine in smokeable form) use is more closely linked to HIV-1 infection than heroin injection in the United States (7, 12, 17), and crack cocaine is independently associated with clinical progression to AIDS (25). Previous studies showed that cocaine enhanced the replication of HIV-1 in a cell culture system and suggest a link between cocaine use and HIV-1 disease progression (3, 22, 26). Thus, this “co-factor hypothesis” is supported by a number of clinical studies, animal models, and in vitro investigations (3, 19, 25, 26).
Paxton and colleagues (20) showed that CD4+ T lymphocytes from subjects who remained uninfected despite repeated high-risk sexual exposures were resistant to in vitro infection by M-tropic HIV-1 isolates; this result was associated with the secretion of high levels of CC chemokines. Using experimental vaccines in monkey models, several investigators demonstrated the correlation of increased chemokine levels with protection from lentivirus infections (1, 16, 23). RANTES, MIP-1α, and MIP-1β were also shown to selectively inhibit cell fusion mediated by HIV-1 envelope glycoproteins (2). These studies clearly demonstrate that β-chemokines play a significant role in resistance to HIV-1 infection and its clinical progression to AIDS. From these observations showing that cocaine is a major risk factor for exposure and increased susceptibility to HIV-1 infection and that β-chemokines manifest significant anti-HIV-1 effects, we hypothesize that cocaine downregulates β-chemokine gene expression and production by lymphocytes from HIV-1-infected subjects, thereby fostering progression of the disease. Our results demonstrate that cocaine selectively suppresses LPS-induced MIP-1β production by PBMC from HIV-infected patients, while leaving cells from normal donors unaffected. These studies suggest that, although lymphocytes from HIV-infected patients who are not using cocaine can produce normal levels of MIP-1β in response to external stimuli, lymphocytes from HIV-infected patients who use cocaine may produce lower levels of MIP-1β in response to external stimuli such as infections, thereby increasing the risk of acquisition or progression of HIV-1 infection. Cocaine did not significantly inhibit the endogenous production of MIP-1β by PBMC from HIV-infected patients. In our studies, despite a varying number of monocytes present in HIV-1-infected subjects, the control levels of MIP-1β produced by PBMC from these patients were similar to those in uninfected subjects, suggesting that although monocytes are robust producers of MIP-1β, other cells also produce MIP-1β and may be targets for the cocaine's effect. An interesting paradox exists regarding the selective effects of cocaine on PBMC from HIV-infected patients and normal donors. While cocaine did not affect constitutive production of MIP-1β by cells from HIV-infected patients, it suppressed constitutive MIP-1β production by normal PBMC. By contrast, however, cocaine suppressed LPS-induced MIP-1β production by PBMC from HIV-infected patients, leaving LPS-induced MIP-1β production by normal cells unaffected. Nevertheless, our data support a model wherein cocaine may be a cofactor in increasing the susceptibility of cocaine users to infection with HIV-1 and, once infected, hastening the progression of the disease (25). Although PBMC from HIV-infected patients produce levels of MIP-1β comparable to normal cells in response to external stimuli such as other concomitant infections, they are selectively sensitive to the immunosuppressive effects of cocaine. Our results suggest that one mechanism of cocaine-associated immunosuppression in HIV-infected subjects may be cocaine-mediated inhibition of the production HIV protective chemokine(s) by PBMC.
Recently Biti and coworkers (4) observed that in patients with active HIV infections, the virus uses an expanded range of coreceptors, including the chemokine receptors CCR3, CCR2b, CCR5, and CXCR4; this adaptation was associated with progression to AIDS. The emergence of HIV variants with these coreceptors was correlated with a switch from a non-syncytium-inducing to a syncytium-inducing phenotype and resistance to virus inhibition by the chemokine ligands of these receptors. A recent study also shows an increased frequency of CCR5-positive perivascular mononuclear cells and macrophages in the brains of HIV-1-infected subjects with encephalitis (24). In our investigations we demonstrate that cocaine can upregulate CCR5 gene expression by PBMC in a dose-dependent manner. These studies support our hypothesis that cocaine is a cofactor in susceptibility to HIV infection and disease progression. Cocaine may mediate these effects by two previously unrecognized, interrelated and self-reinforcing mechanisms: upregulation of HIV entry coreceptors and inhibition of host-protective chemokines.
In summary, our studies demonstrate that cocaine can inhibit the production of the HIV-suppressing chemokine, MIP-1β, and HIV-1 entry coreceptor, CCR5, by normal PBMC. Cocaine also selectively inhibits LPS-induced MIP-1β production by PBMC from AIDS patients. Our studies on the modulation of β-chemokines by cocaine in HIV-1-infected subjects may yield new information on the role of drugs of abuse in the natural history of HIV-1 infections. These findings open up an entirely new area for potential therapeutic strategies using (i) inexpensive synthetic blockers of the HIV-1 entry coreceptor and chemokine receptors (CCR5), (ii) custom-designed vaccines to increase the levels of HIV-1-suppressing chemokines, and/or (iii) specific cocaine receptor antagonists in drug addicts with HIV-1 infection. These studies are currently under way in our laboratory. Further, studies on the modulation of chemokines by cocaine and the reversal or blocking of these effects by receptor-specific antagonist may open up new perspectives for the development of unique therapeutic approaches to patients with HIV-1 infections.
ACKNOWLEDGMENTS
Supported by National Institutes of Health grants RO3 NIDA 1119-01, RO1 NIMH 47225,1 and RO1 DA 010632-01A1 and a grant from the Margaret Duffy and Robert Cameron Troup Memorial Fund of the Buffalo General Hospital.
REFERENCES
- 1.Abimiku A G, Franchini G, Tartaglia J, Aldrich K, Myagkiikh M, Markham P D, Chong P, Klein M, Kieny M P, Paoletti E. HIV-1 recombinanat poxvirus vaccine induces cross-protection against HIV-2 challenge in rheses macaques. Nat Med. 1995;1:321–329. doi: 10.1038/nm0495-321. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Bergert E A. CC CKR5: a RANTES, MIP-1a, MIP-1b receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Bagasra O, Pomerantz R J. Human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells in the presence of cocaine. J Infect Dis. 1993;168:1157–1164. doi: 10.1093/infdis/168.5.1157. [DOI] [PubMed] [Google Scholar]
- 4.Biti R, Ffrench R, Young J. HIV-1 infection in an individual homozygous for the CCR5 deletion allele. Nat Med. 1997;3:252. doi: 10.1038/nm0397-252. [DOI] [PubMed] [Google Scholar]
- 5.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Investig. 1968;21:77–89. [PubMed] [Google Scholar]
- 6.Broder C C, Collman R G. Chemokine receptors and HIV. J Leukoc Biol. 1997;62:20–29. doi: 10.1002/jlb.62.1.20. [DOI] [PubMed] [Google Scholar]
- 7.Chaisson R E, Bacchetti P, Osmond D, Brodie B, Sandie M A, Moss A R. Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA. 1989;261:561–566. [PubMed] [Google Scholar]
- 8.Chiappelli F, Kung M A, Nguyen P, Villanueva P, Shapshak P, McCoy C, Weatherby N, Page B, Stitt F, Mash D, Shah S, Netsch L, Beall G, Kruger S, Purser J, Sanwo M, Saitoh A. Progression to AIDS among HIV-seropositive cocaine alcohol drug abusers. San Juan, Puerto Rico: 4th Annual Symposium on AIDS; 1996. p. 23. [Google Scholar]
- 9.Chomczynski P, Saachi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1a MIP-1b as the major HIV suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 11.Combadiere C, Ahuja S K, Tiffany H L, Murphy P M. Cloning and funtional expression of a CCR-5, a human monocyte CC chemokine receptor selective for MIP-1α and MIP-1β and RANTES. J Leukoc Biol. 1996;60:147. doi: 10.1002/jlb.60.1.147. [DOI] [PubMed] [Google Scholar]
- 12.Des Jarlais D C, Friedman S R, Stoneburner R L. Development of AIDS, HIV seroconversion and potential cofactors for T4 cell loss in a cohort of intravenous drug users. Rev Infect Dis. 1988;10:151–158. [Google Scholar]
- 13.Donahoe R M. Drug abuse and AIDS: causes for the connection. NIDA Res Monogr. 1990;96:181–191. [PubMed] [Google Scholar]
- 14.Garzino-Demo A, Devico A L, Cocchi F, Gallo R C. Beta chemokines and protection from HIV. AIDS Res Hum Retrovir. 1998;14:S177–S184. [PubMed] [Google Scholar]
- 15.Hahn R A, Onarato I M, Jones T S, Dougherty J. Opiates, opioids, and addiction. JAMA. 1989;261:2677–2684. [PubMed] [Google Scholar]
- 16.Heeney J, Jonker R, Koorstra W, Dubbes R, Niphuis H, DiRienzo A M, Gougeon M L, Montagnier L. The resistance of HIV-infected chimpanzees to pregression to AIDS correlates with absence of HIV-related T cell dysfunction. J Med Primatol. 1993;22:194–200. [PubMed] [Google Scholar]
- 17.Hubbard R L, Marden M E, Cavanaugh E, Rachal J V, et al. Role of drug-abuse treatment in limiting the spread of AIDS. Rev Infect Dis. 1988;10:377–384. doi: 10.1093/clinids/10.2.377. [DOI] [PubMed] [Google Scholar]
- 18.Kreek M. Opiates, opioids, and addiction. Mol Psychiatry. 1996;1:232–254. [PubMed] [Google Scholar]
- 19.Larrat E P, Zierler S. Entangled epidemics: cocaine use and HIV disease. J Psychoactive Drugs. 1993;25:207. doi: 10.1080/02791072.1993.10472272. [DOI] [PubMed] [Google Scholar]
- 20.Paxton W A, Martin S R, Tse D, O'Brien T R, Skurnick J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, Koup R A. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposures. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrino T, Bayer B M. In vivo effects of cocaine on immune cell function. J Neuroimmunol. 1998;83:139–147. doi: 10.1016/s0165-5728(97)00230-0. [DOI] [PubMed] [Google Scholar]
- 22.Peterson P K, Gekker G, Chao C C, Schut R, Verhoef J, Edelman C K, Erice A, Balfour H H., Jr Cocaine amplifies HIV-1 replication in cytomegalovirus-stimulated peripheral blood mononuclear cell cocultures. J Immunol. 1992;149:676–680. [PubMed] [Google Scholar]
- 23.Putkonen P, Nilsson C, Walther L, Ghavamzadeh L, Hild K, Broliden K, Biberfeld G, Thorstensson R. Efficacy of inactivated whole HIV-2 vaccines with various adjuvants in cyno molgens monkeys. J Med Primatol. 1994;23:89–94. doi: 10.1111/j.1600-0684.1994.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 24.Vallat A, DeGirolami U, He J, Mhashilkar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith T, Gabuzda D. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152:167–178. [PMC free article] [PubMed] [Google Scholar]
- 25.Webber M P, Schoenbaum E E, Gourevitch M N, Buono D, Klein R S. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;4:257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Looney D, Taub D, Chang S L, Way D, Witte M H, Graves M C, Fiala M. Cocaine opens the blood-brain barrier to HIV-1 invasion. J Neurobiol. 1998;4:619–626. doi: 10.3109/13550289809114228. [DOI] [PubMed] [Google Scholar]