Abstract
Different environmental and industrial technologies seek for fast and automatic ammonia detection systems, capable of the selective measurement of the concentration of its isotopes at sub-ppm levels, without any interference with the common contaminants. In this work, we report the quasi-simultaneous measurement of 14NH3 and 15NH3 concentrations based on a near-infrared diode laser-based photoacoustic system. Using a widely tunable external cavity diode laser, four nearby wavelengths within the range of 1531.3–1531.8 nm were optimal circumstances for sensitive detection, while avoiding interference with water vapor. Subsequently, a more robust distributed feedback diode laser was employed to tune the laser wavelength on the sub-second timescale by varying its driving current rather than using much slower temperature tuning. The detection limit of our system is 0.15 and 0.73 ppm for 14NH3 and 15NH3 (with an accuracy below 0.1%), respectively, and the response time is 3.5 s.
1. Introduction
Industrial ammonia (NH3) production through the Haber–Bosch process is a significant contributor to climate change, accounting for 1.2% of the global CO2 emissions. It has therefore become imperative in the scientific community to develop alternative methods for NH3 synthesis. Electrochemical nitrogen (N2) conversion to NH3 has become a popular research field during the past decade,1 as it holds the promise of substituting the energy-consuming and polluting Haber–Bosch process. Because of the infancy of this field, the NH3 generation rates are generally very small (often resulting in concentrations well below 1 ppm) to an extent that the detected amounts can easily originate from different contamination sources (air, human breath, N2 gas source, unstable N-containing compounds, etc.). In a recent article, rigorous isotopic labeling measurements were integrated in N2–to–NH3 conversion studies.2 Rather shockingly, it was found that the metallic catalysts, which are the most active in aqueous solutions, did not generate any NH3. This observation highlights that sensitive detection of NH3 is not enough, and one also needs to be able to detect 15NH3, in the presence of 14NH3 and N2 gases. Such a protocol can prevent false-positive results, while also providing information on the presence of contaminants. Furthermore, to perform mechanistic studies with appropriate time resolution, we need analytical methods, which can be connected in-line and can provide (quasi)-real-time information on the product formation (i.e., separation or derivative formation-based methods are excluded). In addition, isotopologues of NH3 (14NH3 and 15NH3) are commonly applied in physiology, for metabolic tracing studies where they help the identification of biosynthetic pathways used by cells.3 Another major application is in environmental monitoring (e.g., water and air quality and exhaust gas analysis) and in industrial process control (e.g., chemical, pharmaceutical, and propulsion).
Although there is a wealth of analytical techniques and methods for NH3 detection, their selectivity, sensitivity, and response time with an appropriate detection limit have been an important analytical problem for decades. Starting from wet chemical methods, through physical and chemical interaction-based ones, a wide sort of spectroscopic methods (spanning through the whole electromagnetic spectrum) have been employed (Table 1). As shown in Table 1, many of the physical and chemical methods are applicable for the bulk (nonselective) detection of 14NH3 and 15NH3 isotopologues.
Table 1. Most Commonly Used Methods for Detection of Ammoniaa.
| methods | principle of measurement | determination | adetection limit, brange of linearity, csensitivity | disadvantages, interference |
|---|---|---|---|---|
| physical methods for separated simultaneous measurement of 14NH3 and 15NH3 | ||||
| photoacoustic (PA) spectroscopy4 | PA effect | detection of sound waves following light absorption in a sample | a10 ppb | long path length |
| c10 ppb·m with 10 s averaging | ||||
| isotope ratio mass spectrometry5−9g,10g,11f,12f,13f,14e,15,16f,17f,18 | partition of the mass-to-charge ratio of ions by ionization | measurement of the mass-to-charge ratio of ions | a0.1 ppb in the gas phase | applicable mostly in the liquid phase with extensive sample preparation |
| b4 orders of magnitude | ||||
| nuclear magnetic resonance (NMR) method2h,19 | interaction between nuclei and external magnetic field | detection of NMR signals of target nuclei excited in magnetic field | a10 ppb | expensive |
| sensitive to contamination | ||||
| needs a large sensing volume | ||||
| Fourier transform infrared spectrometry20h,21h,22h,23 | multispectral absorption of materials | measurement of absorbance | a20–100 ppb | |
| c<10–15 W·Hz–1/2 | ||||
| physical methods | ||||
| PA spectroscopy4,24−28 | PA effect | detection of sound waves following light absorption in a sample | ≥a0.1 ppbd | |
| fluorometry29,30 | generation of fluorescence by reagents | detection of fluorescence signal | a0.02 ppb in liquid phase | complex instrument for automation |
| differential optical absorption spectroscopy31 | specific absorption of light by gas molecules | detection of extinction of light at a specific wavelength | <a1ppb | |
| chemical ionization mass spectrometry32−35 | partition of ions according to the mass/charge ratio after ionization | measurement of the mass spectrum | a0.01–0.3 ppb in gas phase | adsorption in tubing. Problem controlling background signal |
| 0.04 cHz/ppt with O2+ | ||||
| cavity ring-down spectrometry36−39 | absolute extinction of laser light in a detection cavity by scattering and absorption in a specific wavelength | measurement of the decay rate | a10 ppb | expensive limited availability of tunable laser light at the appropriate wavelength |
| chemical methodsb | ||||
| Nessler method40 | transformation into colored derivatives | extinction of the light beam in ≈400 nm | a0.02 ppm in the liquid phase | bias by amines, chloride, and alkaline earth metals |
| b0.02–2 ppm | ||||
| indophenol blue method41,42 | transformation into colored derivatives | extinction of the light beam in ≈670 nm | a0.04 ppm in the liquid phase | Fe(III) ion |
| b0.04–2 ppm | ||||
| ion chromatography42,43 | separation of ammonium ions in a column | conductivity measurement | a0.01 ppm in the liquid phase | influence of the sample matrix |
| b0.05–40 ppm | amines | |||
| metal cations | ||||
| annular denuderc,42,44,45 | absorption by acids in rotating annular denuder | conductivity measurement | a0.01 ppb in gas phase | high cost, continuous inspection during field measurements |
| ammonia selective electrode46−48 | pH change by ammonia diffusion through membrane | potentiometry | a0.01 ppb | ionic strength |
| b0.01–17,000 ppm in the liquid phase | precision decreases with low NH3 concentration | |||
| possible escape of gaseous ammonia | ||||
Note: Some of the methods based on transformation of ammonia into ammonium ions for determination in the liquid phase. Most of the concentration measurements involve special sampling procedures.
All of the methods involving chemical transformation (even NH3 ↔ NH4+) called chemical methods.
It can also be used for separation of ammonia in air sample by tubular or annular denuder for subsequent ion chromatographic or spectrophotometric determination.
Strongly depends on the light source, resonator, microphone used, and integration time.
Equipped with a GC–MS.
Equipped with an elemental analyzer.
Combined with an ion chromatograph.
Without isotope measurement.
The pool narrows drastically when an isotopic analysis is also required. Such methods are mostly based on mass spectrometry (MS), such as isotope ratio mass spectrometry for simultaneous detections of 14NH3 and 15NH3 isotopes. This method has a number of disadvantages; first of all, it involves complicated sample preparations (aqueous phase samples are analyzed), which is in many cases a source of artifact or other possible bias and error during measurements. Moreover, it is frequently combined by headspace, elemental analyzer, or ion chromatograph. The use of MS-based methods is challenging in general, because the mass difference between H2O and 15NH3 is very small (0.008 AMU); therefore, the suppression of the water signal becomes necessary to quantify the 15NH3 signal. This often yields to uncertainties in the detection – especially at low ammonia concentrations.49 Fourier transform infrared detection is based on the change in the vibrational frequencies because of the different molar masses. While this method is cheaper, easier to operate, and faster, compared to MS-based methods, it has disadvantages of requiring a considerably high amount of gas sample, low selectivity, and limited system stability.20−23 NMR spectroscopy is also an option, as both the 15NH3 signal and the splitting of the 1H resonance (because of the 1H and 15NH3/14NH3 interactions) can be traced. Unfortunately, both options are hindered at low concentrations and require relatively long measurement times. Overall, according to the best of our knowledge, there is no method that fulfills all the requirements listed in the first paragraph. Interestingly, most of the precedent literature specializes in the measurement of nitrogen isotope from either NH4+ ion or NH3 gas using various adaptations of the ammonia diffusion method.50 The reasons for this could be the low concentration of NH3 (in the sub-μg/L range), which presents a detection challenge because of the poor sensitivity of the currently used methods, mostly based on compound-specific isotope analysis (CSIA).51,52
Another possible measurement technique is the PA detection that in principle allows the in situ and nondestructive measurement of isotopes. PA spectroscopy is a powerful technique to measure concentrations at the low levels (from ppm to ppt ranges, depending on the available light source).4,24−28 In a PA detector, acoustic pressure waves recorded by a microphone are generated because of molecular absorption of modulated optical radiation in gases, liquids, and solids. In case of gases, the amplitude of the generated sound is directly proportional to the concentration of the absorbed gas component.
This work aims to develop a relatively simple, yet reliable, fully automatic, and robust system for the selective, rapid, and sensitive measurement of ammonia isotopes by using a near-infrared photoacoustic (NIR-PA) system. The PA method is efficient in determining NH3 concentration in general, and to the best of our knowledge, there is only one report on measuring 14NH3 and 15NH3 selectively.4 In this study, a procedure is used where the optical path length is of the order of 10 m through a generated plume making its application in laboratory impossible. Here, we describe a newly developed PA method for the simultaneous detection of 14NH3 and 15NH3 isotopologues, with a small and easy-to-use device.
2. Experimental Section
Spectral measurements, calibrations, cross-sensitivity determinations, and response time measurements were executed using the gas generation system shown in Figure S1. It has two main parts: the ammonia gas generation unit that generates various mixtures of 14NH3 and 15NH3 and the NIR-PA system, operated either by an external cavity diode laser (ECDL) or by a distributed feedback (DFB) diode laser. The gas generator unit was operated either in a mass-flow controller mixing mode or in a chemical reaction-based mode. In the first operation mode, the chemical reaction part of the system (marked by a dashed rectangle in Figure S1) is bypassed, and the calibrated mass-flow controllers are used to mix the gases from two cylinders. For the chemical reaction-based generation mode (for labeled NH3), a nitrogen cylinder was used to purge the gas mixture generated in the chemical reaction part of the system through the PA cell.
A longitudinal differential PA system was employed, similarly to our previous studies27,53−56 (see technical details in the SI). The PA spectra of the 14NH3 and 15NH3 isotopologues were recorded with gas samples either from the gas cylinders or from the chemical reaction (using pure 15NH4Cl), respectively. Measurement wavelength optimization started by recording the PA spectra of the two isotopologues and water vapor by an external cavity diode laser (ECDL, Sacher TEC 520), having an output light power of about 50 mW and its wavelength tunable between 1470 and 1590 nm. Two wavelength ranges were selected for further tests (see Discussion), from which the wavelength range of 1530.5–1533.5 nm has been chosen for further system optimization, using a telecommunication-type fiber-coupled DFB diode laser (type: FOL15DCWD-A82-19560-A, Furukawa Inc.) operating with an emitted light power of about 45 mW. This laser has been operated in a wavelength modulated mode with an unmodulated current set to be close to 300 mA, with a small amplitude sinusoidal modulation superimposed on it. Several PA spectra of the ammonia isotopes and water vapor were recorded with laser temperature tuning. For each temperature scan, the amplitude of the laser current modulation was kept fixed but changed from scan to scan. Based on the recorded spectra, an optimum laser modulation amplitude and a set of measurement wavelengths, that are least influenced by spectral interference, have been selected. Next, the measurements were accelerated by switching from temperature to current tuning. This latter operational mode was optimized in two steps: first, laser temperatures with which all the selected wavelengths are available with current tuning were screened, and then the laser temperature that provides highest possible PA signals yielding maximum sensitivity of the isotopologue concentration measurements was selected.
The concentration measurement subroutine of the operational software of the NIR-PA system was programmed in a way that after measuring at all the selected wavelengths, it converts the measured PA signals into two quantities: PA14 and PA15 (see below) characterized by high sensitivity for 14NH3 and 15NH3, respectively, while a minimal cross sensitivity against the other isotope as well as water vapor. The measurement sequence summarized in Table 2 was followed (see also a detailed description in the SI).
Table 2. Sequence of Procedures Applied during the Determination of the Sensitivity and Cross-Sensitivity Parameters.
| measured parameter | applied gas generator | supplementary data required | the procedure for generating the required supplementary data | |
|---|---|---|---|---|
| step 1 | 14NH3 sensitivity | mass-flow controller | none | none |
| step 2 | 15NH3 sensitivity | chemical reaction | actual value of c(15NH3) | c(15NH3) is determined in two steps: |
| (1) determination of c(14NH3) from the measured value of PA14 with the help of the 14NH3 sensitivity parameter determined in Step 1 | ||||
| (2) calculation of c(15NH3) from c(14NH3) by using the mixing ratio of the isotope labeled salts | ||||
| step 3 | 15NH3 cross sensitivity | mass-flow controller | actual value of c(14NH3) | c(14NH3) is calculated from the measured value of PA14 with the help of the 14NH3 sensitivity parameter determined in Step 1 |
| step 4 | 14NH3 cross sensitivity | chemical reaction | actual value of c(15NH3) | c(15NH3) is calculated from the measured value of PA15 with the help of the 15NH3 sensitivity parameter determined in Step 2 |
3. Results and Discussion
The PA spectra recorded using an ECDL are shown in Figure 1. To select the appropriate measurement wavelengths, the analysis of the amplitude modulation generated ECDL spectra was executed by searching for less than 1 nm wide wavelength ranges, in which strong absorption lines of both isotopes can be found, while the absorption lines of water vapor are as weak as possible. On the other hand, perfect spectral separation among the absorption lines was not a selection criterion in this phase of system optimization yet, because wavelength modulation modifies the spectra considerably. Wavelengths below 1500 nm had to be excluded because of the presence of strong water vapor absorption lines. On the other hand, both the 1520–1523 nm and the 1530.5–1533.5 nm wavelength ranges met the primary selection criteria; therefore, spectral measurements with wavelength modulated DFB lasers were executed in these wavelength ranges, by attempting the minimization of spectral cross sensitivities via the optimization of the laser operational parameters. No suitable cross interference-free wavelengths in the former range were found, that is why the latter one had been selected for further optimization.
Figure 1.
PA spectra of 15NH3 (blue line), 14NH3 (red line), and water vapor (black line) recorded by an ECDL. The two wavelength ranges marked with green rectangles were investigated in detail, by searching for optimal measurement lines.
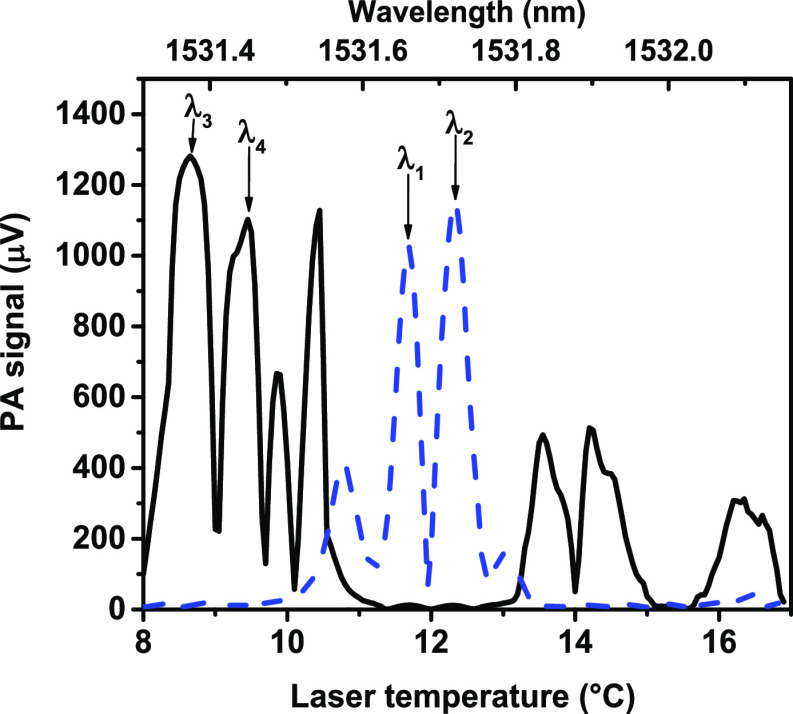
From the series of PA spectra of the isotopologues and water vapor, recorded by temperature tuning, the one using laser modulation amplitude of 12 mA was the least affected by spectral interferences. Indeed, these spectra contain well-separated absorption lines of both isotopically labeled compounds, while interference from water vapor absorption lines is negligible (Figure 2). The lower x-axis of Figure 2 corresponds to the temperature of one of the lasers, while the upper x-axis (i.e., laser emission wavelength) is approximated by comparing the PA spectrum of 14NH3 with data from the spectral database of PNNL.57,58 Based on this laser temperature to wavelength conversion, the selected measurement wavelengths are estimated to be the following: 1531.66, 1531.73, 1531.37, and 1531.45 nm, marked as λ1, λ2, λ3, and λ4, in Figure 2, respectively.
Figure 2.
PA spectra of the ammonia isotopologues as recorded by DFB diode laser temperature tuning. Solid-black and dashed-blue lines are the recorded spectrum of 15NH3 and 14NH3, respectively. The two wavelength pairs used for selective determination of the 14NH3 and 15NH3 isotopologue concentrations are indicated as λ1, λ2 and λ3, λ4 respectively.
We note that at the beginning of the system development the possibility of using the long-wavelength end of the near infrared (e.g., the wavelength range around 2000 nm), rather than the telecommunication window, was also considered. Because of the considerably stronger absorption lines in this long-wavelength region, this is indeed very attempting, but actually there are several counterarguments as well. First, one can compare the few mW light power of a typical diode laser operating in the longer wavelength range with the ≈50 mW light power of a telecommunication diode laser. Because the generated PA signal depends on the product of the optical absorption coefficient and the light power, this product is approximately equal for the two types of lasers; that is, the disadvantage of weaker absorption lines is compensated by the much higher light power of the telecommunication diode lasers. A considerable advantage of the telecommunication-type diode lasers is their availability in a fiber-coupled construction making them very robust and facilitating their fiber coupling for increased light power. Furthermore, they have long operational lifetime (exceeding 10 years), and they are much cheaper than their long-wavelength counterparts.
Because of the narrow width (≈0.5 nm) of the wavelength range that contains all the selected measurement wavelengths, the effort to switch from temperature to current tuning was successful. The laser temperature was optimized to achieve maximum system’s sensitivity. In this optimized operation mode, the software repeatedly sets the unmodulated part of the laser to 176.3, 185, 137.2, and 147.4 mA varying the measurement wavelengths among λ1, λ2, λ3, and λ4, respectively. PA signals measured at these four wavelengths by using a modulated current of 12 mA are marked in the following as PA(λ1), PA(λ2), PA(λ3), and PA(λ4), respectively. After the completion of a measurement cycle (having all four PA signals measured), the operational software of the PA system calculates the following quantities for the quantification of c(14NH3) and c(15NH3), respectively:
Increasing the sensitivity while simultaneously decreasing the cross sensitivity is always a primary goal of multiwavelength measurement system development. From this point of view, the definition of PA14 and PA15 as a measure of the concentration of 14NH3 and 15NH3 might be surprising at first, because Figure 2 suggests that for each isotope the subtracted signals are nearly equal, apparently resulting in reduced sensitivities. Actually this is not the case, as there is an approximately 180° degree phase difference between the subtracted PA signals; that is, PA(λ1) and PA(λ2) have opposite phases (as well as PA(λ3) and PA(λ4)), and consequently, the subtractions in the definition equations of PA14 and PA15 actually increase (almost double) these signals. This phase difference is the consequence of the applied wavelength modulation, and while it actually increases the sensitivity of the system, it is also an efficient tool for decreasing cross sensitivities. Indeed, whenever an interfering component generates roughly equal PA signals at the measurement wavelengths with the same phase, this subtraction diminishes its influence. Examples for efficiently suppressed spectral interferences include tails of absorption lines of small molecules, slowly varying absorption features generated by large molecules (or aerosol particles), and background PA signal generated by light absorption on the windows or walls of the PA cell.59
The result of the calibrations performed by mass-flow controllers and chemical reaction-based gas generation method is seen in Figure 3a,b. Rectangles in Figure 3a,b represent data points of PA14 vs c(14NH3) and PA15 vs c(15NH3), respectively, with the corresponding fitted calibration lines represented by solid lines. The slopes of the calibration lines give the sensitivity of 6.3 and 1.3 μV/ppm for c(14NH3) and c(15NH3) measurements, respectively. The noise of the measurements was 0.3 μV yielding 0.15 and 0.73 ppm as minimum detectable values of c(14NH3) and c(15NH3) respectively. Circles in Figure 3a,b represent the results of 14NH3 and 15NH3 cross-sensitivity determination, with the corresponding fitted calibration lines represented by dashed lines. The slopes of the fitted lines give the cross sensitivities of −1.3 × 10–4 and 7.4 × 10–3 ppm/ppm, for the measurements of c(14NH3) and c(15NH3), respectively.
Figure 3.
Results of the calibration and cross-sensitivity measurements of the NIR-PA system performed by the gas generation system operated either in the mass-flow controller mixing mode (a) or in the chemical reaction-based mode (b). PA14 and PA15 are the modified photoacoustic signals used to determine the concentration of 14NH3 and 15NH3, respectively. The error bars represent the standard deviation of three parallel measurements.
Finally, as shown in Figure 4, the developed NIR-PA system is capable of following sudden concentration variations with a response time of 3.5 s. Two unique properties of the system make its response time such remarkably low. First, as it is already discussed above, because of the close proximity of the selected measurement wavelengths, current tuning can be applied, and so one measurement cycle with measuring on the four selected wavelengths can be executed within less than a second. Second, the small volume (≈10 cm3) of the PA cell can be flushed through completely and rapidly, even at moderate gas flow rates. Roughly speaking, there is an inverse relationship between the gas flow rate and the response time, so whenever the gas production rate in the investigated process is slow, the flow rate can be reduced at the expense of the response time. At this point, it is worth comparing photoacoustics with one of their rivaling techniques: multipath optical absorption spectroscopy. It is true that the two methods have very similar analytical parameters and also that the same measurement wavelengths and current tuning method developed here can be applied with the latter technique, too. To achieve a similar response time, however, the large volume (≈1000 cm3) of the multipath cell has to be purged at much higher rates resulting in a considerable gas consumption. In the first approximation, the response time depends on the ratio of sample volume and the flow rate, respectively.60 At a high flow rate, the response time values of multipath (direct absorption) and PA methods will be comparable.61
Figure 4.
Response of the system to suddenly from 0 to 100 ppm 14NH3 concentration variation. Circles are measurement points; a line is drawn to guide the eye. The 10 and 90% of the concentration variation are indicated by horizontal dashed lines.
4. Conclusions
In this work, we have developed a DFB diode laser-based PA system for the sensitive, selective, and rapid detection of isotopically labeled NH3. Measurement wavelengths of the isotopologues, that are among the strongest in the targeted wavelength range and lay sufficiently close to each other to facilitate the application of current tuning of the diode lasers, were selected to program the operational software of the system in a way to complete a concentration measurement cycle in less than a second. This allows fully exploiting inherently fast response of the PA detection method even in such a demanding application. Altogether, because of its robustness, high sensitivity, low cross sensitivity, and short response time the presented system is expected to find numerous practical applications ranging from electrocatalytic N2 conversion to biological studies.
Acknowledgments
Emily Awuor wishes to acknowledge the Tempus Public Foundation for the award of the Stipendium Hungaricum Scholarship, which enabled and provided the platform to be able to carry out this research. This work was supported by the Hungarian Research and Technology Innovation Fund (OTKA), project no. K-138176. The authors also acknowledge the financial support of the 2018-2.1.3-EUREKA-2018-00026 project. Project no. TKP2021-NVA-19 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NVA funding scheme. The authors thank Dr. Balázs Endrődi (Univ. Szeged) for his support with the gas generation experiments.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.2c01191.
Additional experimental details of the gas generation, wavelength selection, and the operation of the system (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Qing G.; Ghazfar R.; Jackowski S. T.; Habibzadeh F.; Ashtiani M. M.; Chen C. P.; Smith M. R.; Hamann T. W. Recent Advances and Challenges of Electrocatalytic N2 Reduction to Ammonia. Chem. Rev. 2020, 120, 5437–5516. 10.1021/acs.chemrev.9b00659. [DOI] [PubMed] [Google Scholar]
- Andersen S. Z.; Čolić V.; Yang S.; Schwalbe J. A.; Nielander A. C.; McEnaney J. M.; Enemark-Rasmussen K.; Baker J. G.; Singh A. R.; Rohr B. A.; Statt M. J.; Blair S. J.; Mezzavilla S.; Kibsgaard J.; Vesborg P. C. K.; Cargnello M.; Bent S. F.; Jaramillo T. F.; Stephens I. E. L.; Nørskov J. K.; Chorkendorff I. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 2019, 570, 504–508. 10.1038/s41586-019-1260-x. [DOI] [PubMed] [Google Scholar]
- Spinelly J. B.; Kelley L. P.; Haigis M. C. An LC-MS Approach to quantitative measurement of ammonia isotopologues. Sci. Rep. 2017, 7, 10304. 10.1038/s41598-017-09993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. C.; Brumfield B. E.; Harilal S. S. Real-time standoff detection of nitrogen isotopes in ammonia plumes using a swept external cavity quantum cascade laser. Opt. Lett. 2018, 43, 4065–4068. 10.1364/ol.43.004065. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Wang Q.; Ching J. Y.; Wu J. C.; Zhang G.; Ren W. A portable low-power QEPAS-based CO2 isotope sensor using a fiber-coupled interband cascade laser. Sens. Actuators, B 2017, 246, 710–715. 10.1016/j.snb.2017.02.133. [DOI] [Google Scholar]
- Lehmann W. D. A Timeline of stable isotopes and mass spectrometry in the life sciences. Mass Spectrom. Rev. 2017, 36, 58–85. 10.1002/mas. [DOI] [PubMed] [Google Scholar]
- Simonova G.; Kalashnikova D. Isotope ratio mass spectrometry application for environmental investigations. E3S Web Conf. 2019, 98, 12020. 10.1051/e3sconf/20199812020. [DOI] [Google Scholar]
- Liu Y.; Asset T.; Chen Y.; Murphy E.; Potma E. O.; Matanovic I.; Fishman D. A.; Atanassov P. Facile all-optical method for in situ detection of low amounts of ammonia. iScience 2020, 23, 101757 10.1016/j.isci.2020.101757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.; Liu X.; Deng C.; Dore A. J.; Zhuang G. Source apportionment of atmospheric ammonia before, during, and after the 2014 APEC summit in Beijing using stable nitrogen isotope signatures. Atmos. Chem. Phys. 2016, 16, 11635–11647. 10.5194/acp-16-11635-2016. [DOI] [Google Scholar]
- Chang Y.; Zou Z.; Zhang Y.; Deng C.; Hu J.; Shi Z.; Dore A. J.; Collett J. L. Jr. Assessing contributions of agricultural and non-agricultural emissions to atmospheric ammonia in a Chinese megacity. Environ. Sci. Technol. 2019, 53, 1822–1833. 10.1021/acs.est.8b05984. [DOI] [PubMed] [Google Scholar]
- Felix J. D.; Elliot E. M.; Gay D. Spatial and temporal patterns of nitrogen isotopic composition of ammonia at U.S. ammonia monitoring network sites. Atmos. Environ. 2017, 150, 434–442. 10.1016/j.atmosenv.2016.11.039. [DOI] [Google Scholar]
- Lee C.; Hristov A. N.; Cassidy T.; Heyler K. Nitrogen isotope fractionation and origin of ammonia nitrogen volatilized from cattle manure in simulated storage. Atmosphere 2011, 2, 256–270. 10.3390/atmos2030256. [DOI] [Google Scholar]
- Lee C.; Feyereisen G. W.; Hristov A. N.; Dell C. J.; Kaye J.; Beegle D. Effects of dietary protein concentration on ammonia volatilization, nitrate leaching, and plant nitrogen uptake from dairy manure applied to lysimeters. J. Environ. Qual. 2014, 43, 398–408. 10.2134/jeq2013.03.0083. [DOI] [PubMed] [Google Scholar]
- Nômmik H. Assessment of volatilization loss of ammonia from surface-applied urea on forest soil by N15 recovery. Plant Soil 1973, 38, 589–603. 10.1007/BF00010699. [DOI] [Google Scholar]
- Taghizadeh-Toosi A.; Gough T. J.; Sherlock R. R.; Condron L. M. Biochar adsorbed ammonia is bioavailable. Plant Soil 2012, 350, 57–69. 10.1007/s11104-011-0870-3. [DOI] [Google Scholar]
- Tonn B.; Porath I.; Lattanzi F. A.; Isselstein J. Urine effects on grass and legume nitrogen isotopic composition: Pronounced short-term dynamics of δ15N. PLoS One 2019, 14, e0210623 10.1371/journal.pone.0210623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.-P.; Zhu H.; Liu Z.; Dai L.-H.; Zhang N.; Schwab J. J.; Yuan C.-S.; Yan J.-P. Nitrogen isotope composition of ammonium in PM2.5 in the Xiamen, China: impact of non-agricultural ammonia. Environ. Sci. Pollut. Res. 2019, 26, 25596–25608. 10.1007/s11356-019-05813-8. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Yan X.; Xie Y.; Wang S.; Xing G.; Zhu Z. Use of nitrogen isotope to determine fertilizer- and soil-derived ammonia volatilization in a rice/wheat rotation system. J. Agric. Food Chem. 2016, 64, 3017–3024. 10.1021/acs.jafc.5b05898. [DOI] [PubMed] [Google Scholar]
- Nielander A. C.; McEnaney J. M.; Schwalbe J. A.; Baker J. G.; Blair S. J.; Wang L.; Pelton J. G.; Andersen S. Z.; Enemark-Rasmussen K.; Čolić V.; Yang S.; Bent S. F.; Cargnello M.; Kibsgaard J.; Vesborg P. C. K.; Chorkendorff I.; Jaramillo T. F. A versatile method for ammonia detection in a range of relevant electrolytes via direct nuclear magnetic resonance techniques. ACS Catal. 2019, 9, 5797–5802. 10.1021/acscatal.9b00358. [DOI] [Google Scholar]
- McEnaney J. M.; Singh A. R.; Schwalbe J. A.; Kibsgaard J.; Lin J. C.; Cargnello M.; Jaramillo T. F.; Nørskov J. K. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy Environ. Sci. 2017, 10, 1621–1630. 10.1039/c7ee01126a. [DOI] [Google Scholar]
- Koletzko S.; Haisch M.; Seeboth I.; Braden B.; Hengels K.; Koletzko B.; Hering P. Isotope-selective non-dispersive infrared spectrometry for detection of Helicobacter pylori infection with 13C-urea breath test 13C-urea. Lancet 1995, 345, 961–962. 10.1016/s0140-6736(95)90704-1. [DOI] [PubMed] [Google Scholar]
- Murakami T.; Nohira T.; Goto T.; Ogata Y. H.; Ito Y. Electrolytic ammonia synthesis from water and nitrogen gas in molten salt under atmospheric pressure. Electrochim. Acta 2005, 50, 5423–5426. 10.1016/j.electacta.2005.03.023. [DOI] [Google Scholar]
- Griffiths P. R.; de Haseth J. A.. Fourier transform infrared spectrometry; John Wiley & Sons: Hoboken, New Jersey, 2007. [Google Scholar]
- Dang H.; Ma Y.; Liu F.; Lu J. Sensitive detection of ammonia based on quartz-enhanced photoacoustic spectroscopy. J. Russ. Laser Res. 2019, 40, 265–268. 10.1007/s10946-019-09800-9. [DOI] [Google Scholar]
- Schilt S.; Thévenaz L.; Niklès M.; Emmenegger L.; Hüglin C. Ammonia monitoring at trace level using photoacoustic spectroscopy in industrial and environmental applications. Spectrochim. Acta, Part A 2004, 60, 3259–3268. 10.1016/j.saa.2003.11.032. [DOI] [PubMed] [Google Scholar]
- von Bobrutzki K.; Braban C. F.; Famulari D.; Jones S. K.; Blackall T.; Smith T. E. L.; Blom M.; Coe H.; Gallagher M.; Ghalaieny M.; McGillen M. R.; Percival C. J.; Whitehead J. D.; Ellis R.; Murphy J.; Mohacsi A.; Pogany A.; Junninen H.; Rantanen S.; Sutton M. A.; Nemitz E. Field inter-comparison of eleven atmospheric ammonia measurement techniques. Atmos. Meas. Tech. 2010, 3, 91–112. 10.5194/amt-3-91-2010. [DOI] [Google Scholar]
- Huszár H.; Pogány A.; Bozóki Z.; Mohácsi Á.; Horváth L.; Szabó G. Ammonia monitoring at ppb level using photoacoustic spectroscopy for environmental application. Sens. Actuators, B 2008, 134, 1027–1033. 10.1016/j.snb.2008.05.013. [DOI] [Google Scholar]
- Pushkarsky M. B.; Webber M. E.; Baghdassarian O.; Narasimhan L. R.; Patel C. K. N. Laser-based photoacoustic ammonia sensors for industrial applications. Appl. Phys. B: Lasers Opt. 2002, 75, 391–396. 10.1007/s00340-002-0967-8. [DOI] [Google Scholar]
- Amornthammarong N.; Zhang J. Z.; Ortner P. B. An autonomous batch analyzer for the determination of trace ammonium in natural waters using fluorometric detection. Anal. Methods 2011, 3, 1501–1506. 10.1039/c1ay05095h. [DOI] [Google Scholar]
- Kéruel R.; Aminot A. Flourometric determination of ammonia in sea and estuarine waters by direct segmented flow analysis. Mar. Chem. 1997, 57, 265–275. 10.1016/S0304-4203(97)00040-6. [DOI] [Google Scholar]
- Gall R.; Perner D.; Ladstatter-Weissenmayer A. Simultaneous determination of NH3, SO2, NO and NO2 by direct UV absorption in ambient air, Fresen. J. Anal. Chem. 1991, 340, 646–649. 10.1007/BF00321528. [DOI] [Google Scholar]
- Nowak J. B.; Neuman J. A.; Kozai K.; Huey L. G.; Tanner D. J.; Holloway J. S.; Ryerson T. B.; Frost G. J.; McKeen S. A.; Fehsenfeld F. C. A chemical ionization mass spectrometry technique for airborne measurements of ammonia. J. Geophys. Res. Atmos. 2007, 112, D10S02. 10.1029/2006JD007589. [DOI] [Google Scholar]
- Norman M.; Spirig C.; Wolff V.; Trebs I.; Flechard C.; Wisthaler A.; Schnitzhofer R.; Hansel A.; Neftel A. Intercomparison of ammonia measurement techniques at an intensively managed grassland site (Oensingen, Switzerland) Atmos. Chem. Phys. 2009, 9, 2635–2645. 10.5194/acp-9-2635-2009. [DOI] [Google Scholar]
- Nowak J. B.; Huey L. G.; Russell A. G.; Tian D.; Neuman J. A.; Orsini D.; Sjostedt S. J.; Sullivan A. P.; Tanner D. J.; Weber R. J.; Nenes A.; Edgerton E.; Fehsenfeld F. C. Analysis of urban gas phase ammonia measurements from the 2002 Atlanta Aerosol Nucleation and Real-Time Characterization Experiment (ANARChE). J. Geophys. Res. 2006, 111, D17308. 10.1029/2006JD007113. [DOI] [Google Scholar]
- Norman M.; Hansel A.; Wisthaler A. O2+ as reagent ion in the PTR-MS instrument: detection of gas-phase ammonia. Int. J. Mass Spectrom. 2007, 265, 382–387. 10.1007/s00340-002-0967-8. [DOI] [Google Scholar]
- Vasileiou E.; Kyriakou V.; Garagounis I.; Vourros A.; Stoukides M. Ammonia synthesis at atmospheric pressure in a BaCe0.2Zr0.7Y0.1O2.9 solid electrolyte cell. Solid State Ionics 2015, 275, 110–116. 10.1016/j.ssi.2015.01.002. [DOI] [Google Scholar]
- Vasileiou E.; Kyriakou V.; Garagounis I.; Vourros A.; Manerbino A.; Coors W. G.; Stoukides M. Reaction rate enhancement during the electrocatalytic synthesis of ammonia in a BaZr0.7Ce0.2Y0.1O2.9 solid electrolyte cell. Top. Catal. 2015, 58, 1193–1201. 10.1007/s11244-015-0491-9. [DOI] [Google Scholar]
- Vasileiou E.; Kyriakou V.; Garagounis I.; Vourros A.; Manerbino A.; Coors W. G.; Stoukides M. Electrochemical enhancement of ammonia synthesis in a BaZr0.7Ce0.2Y0.1O2.9 solid electrolyte cell. Solid State Ionics 2016, 288, 357–362. 10.1016/j.ssi.2015.12.022. [DOI] [Google Scholar]
- Martin N. A.; Ferracci V.; Cassidy N.; Hoffnagle J. A. The application of a cavity ring-down spectrometer to measurements of ambient ammonia using traceable primary standard gas mixtures. Appl. Phys. B: Lasers Opt. 2016, 122, 219. 10.1007/s00340-016-6486-9. [DOI] [Google Scholar]
- Jeong H.; Park J.; Kim H. Determination of NH4+ in environmental water with interfering substances using the modified Nessler method. J. Chem. 2013, 2013, 359217 10.1155/2013/359217. [DOI] [Google Scholar]
- Koroleff F.Direct determination of ammonia in natural waters as indophenol blue. In Information on Techniques and Methods for Seawater Analysis; Charlottenlund, Internat. Counc. Exploration of the sea (Interlab. Rept. 3), 1970; pp 19 −22. [Google Scholar]
- EMEP/CCC-Report 1/95 Reference: O-7726 date: March 1996 Revision: November 2001 EMEP Co-operative Programme for Monitoring and Evaluation of the Long-range Transmission of Air Pollutants in Europe EMEP manual for sampling and chemical analysis (2001).
- Thomas D. H.; Rey M.; Jackson P. E. Determination of inorganic cations and ammonium in environmental waters by ion chromatography with a high-capacity cation-exchange column. J. Chromatogr. A. 2002, 956, 181–186. 10.1016/s0021-9673(02)00141-3. [DOI] [PubMed] [Google Scholar]
- Allegrini I.; de Santis F.; di Paolo V.; Febo A.; Perrino C.; Possanzini M. Annular denuder method for sampling reactive gases and aerosols in the atmosphere. Sci. Total Environ. 1987, 67, 1–16. 10.1016/0048-9697(87)90062-3. [DOI] [Google Scholar]
- Erisman J. W.; Otjes R.; Hensen A.; Jongejan P.; van den Bulk P.; Khlystov A.; Möls H.; Slanina S. Instrument development and application in studies and monitoring of ambient ammonia. Atmos. Environ. 2001, 35, 1913–1922. 10.1016/S1352-2310(00)00544-6. [DOI] [Google Scholar]
- Thomas R. F.; Booth R. L. Selective electrode measurement of ammonia in water and wastes. Environ. Sci. Technol. 1973, 7, 523–526. 10.1021/es60078a006. [DOI] [PubMed] [Google Scholar]
- ASTM . Manual of water and environmental technology, D1426-92 standard: test methods for ammonia nitrogen in water, 2015.
- Rice E.; Baird R.; Eaton A.; Clesceri L.. Standard methods for the examination of water and wastewater; American Public Health Association, American Water Works Association, Water Environment Federation, 2012. [Google Scholar]
- Song Y.; Johnson D.; Peng R.; Hensley D. K.; Bonnesen P. V.; Liang L.; Huang J.; Yang F.; Zhang F.; Qiao R.; Baddorf A. P.; Tschaplinski T. J.; Engle N. L.; Hatzell M. C.; Wu Z.; Cullen D. A.; Meyer H. M.; Sumpter B. G.; Rondinone A. J. A physical catalyst for the electrolysis of nitrogen to ammonia. Sci. Adv. 2018, 4, e1700336 10.1126/sciadv.1700336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger J.; Sigman D. M.; Prokopenko M. G.; Lehmann M. F.; Tortell P. D. A method for nitrite removal in nitrate N and O isotope analyses. Limnol. Oceanogr.: Methods 2006, 4, 205–212. 10.4319/lom.2006.4.205. [DOI] [Google Scholar]
- Schmidt T. C.; Zwank L.; Elsner M.; Berg M.; Meckenstock R. U.; Haderlein S. B. Compound-specific stable isotope analysis of organic contaminants in natural environments: A critical review of the state of the art, prospects, and compound-specific stable isotope analysis of organic contaminants in natural environments: a critical. Anal. Bioanal. Chem. 2004, 378, 283–300. 10.1007/s00216-003-2350-y. [DOI] [PubMed] [Google Scholar]
- Elsner M.; Imfeld G. Compound-specific isotope analysis (CSIA) of micropollutants in the environment – current developments and future challenges. Curr. Opin. Biotechnol. 2016, 41, 60–72. 10.1016/j.copbio.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Pogány A.; Weidinger T.; Bozóki Z.; Mohácsi Á.; Bieńkowski J.; Józefczyk D.; Eredics A.; Bordás Á.; Gyöngyösi A. Z.; Horváth L.; Szabó G. Application of a novel photoacoustic instrument for ammonia concentration and flux monitoring above agricultural landscape - results of a field measurement campaign in Choryń, Poland. Időjárás 2012, 116, 93–107. [Google Scholar]
- Tátrai D.; Bozóki Z.; Smit H.; Rolf C.; Spelten N.; Krämer M.; Filges A.; Gerbig C.; Gulyás G.; Szabó G. Dual-channel photoacoustic hygrometer for airborne measurements: Background, calibration, laboratory and in-flight intercomparison tests, Atmos. Meas. Tech. 2015, 8, 33–42. 10.5194/amt-8-33-2015. [DOI] [Google Scholar]
- Varga A.; Bozóki Z.; Szakáll M.; Szabó G. Photoacoustic system for on-line process monitoring of hydrogen sulfide (H2S) concentration in natural gas streams. Appl. Phys. B: Lasers Opt. 2006, 85, 315–321. 10.1007/s00340-006-2388-6. [DOI] [Google Scholar]
- Szabó A.; Mohácsi Á.; Gulyás G.; Bozóki Z.; Szabó G. In situ and wide range quantification of hydrogen sulfide in industrial gases by means of photoacoustic spectroscopy. Meas. Sci. Technol. 2013, 24, 065501 10.1088/0957-0233/24/6/065501. [DOI] [Google Scholar]
- Sharpe S. W.; Johnson T. J.; Sams R. L.; Chu P. M.; Rhoderick G. C.; Johnson P. A. Gas-phase databases for quantitative infrared spectroscopy. Appl. Spectrosc. 2004, 58, 14521461. 10.1366/0003702042641281. [DOI] [PubMed] [Google Scholar]
- Sharpe S. W.; Sams R. L.; Johnson T. J.; Chu P. M.; Rhoderick G. C.; Guenther F. R. Creation of 0.10-cm–1 resolution quantitative infrared spectral libraries for gas samples. In Proc. SPIE 4577, Vibrational Spectroscopy-based Sensor Systems (accessed February 22, 2002), 10.1117/12.455730. [DOI]
- Miklós A.; Bozóki Z.; Jiang Y.; Fehér M. Experimental and Theoretical Investigation of Photoacoustic-Signal Generation by Wavelength-Modulated Diode Lasers. Appl. Phys. B: Lasers Opt. 1994, 58, 483–492. 10.1007/BF01081079. [DOI] [Google Scholar]
- Schmohl A.; Miklós A.; Hess P. Detection of ammonia by photoacoustic spectroscopy with semiconductor lasers. Appl. Opt. 2002, 41, 1815–1823. 10.1364/AO.41.001815. [DOI] [PubMed] [Google Scholar]
- Bozóki Z.; Mohácsi Á.; Szabó G.; Bor Z.; Erdélyi M.; Chen W.; Tittel F. K. Near infrared diode laser based spectroscopic detection of ammonia: a comparative study of photoacoustic and direct optical absorption methods. Appl. Spectrosc. 2002, 56, 715–719. 10.1366/000370202760077658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.