Abstract

During the past years, the synthesis of polymer prodrug structures, based on natural phytochemical compounds with a great range of valuable biological properties, has become a promising solution in cancer prevention, imaging, and detection. Curcumin (Curc) remains one of the most studied natural products, due to the impressive palette of biological properties and the possibility to be easily loaded in various micro- and nanostructures and chemically modified. In this study, pegylated curcumin derivatives were prepared by a direct esterification reaction between poly(ethylene glycol)diacid (PEG of 600 g/mol molar mass, PEG600) and Curc in the presence of N,N′-dicyclohexylcarbodiimide (PEG600-Curc). The successful reaction resulted in a water-soluble stable product that was characterized by infrared spectroscopy (Fourier transform infrared (FT-IR)) and proton (1H) and carbon (13C) NMR. The effect of the pH values of buffer solutions on PEG600-Curc spectral properties (absorption and photoluminescence) was investigated by UV–vis and fluorescence spectrophotometry. Based on the biological tests, it was confirmed that PEG600-Curc exhibits cytotoxic activity against Graffi cell lines, as a function of the Curc concentration in the conjugate and the incubation time. PEG600-Curc antibacterial activity was validated in microbiological tests against pathogenic microorganisms such as Staphylococcus aureus. Most importantly, despite the covalent attachment of Curc to PEG and the slight reduction in the therapeutic index of the conjugate, both the anticancer and antimicrobial activities remain the highest reported, thus opening the gate for further, more clinically oriented studies.
Introduction
Cancer is one of the major health problems worldwide, with an annual increase in morbidity and mortality.1 Despite the undoubted recent progress in tumor prevention and early detection and the developed cancer treatments, two main issues still remain unsolved dealing with the formed multiple drug resistance and the undesired side effects (life-threatening or dose-limiting).2 In this regard, there are still insufficiently effective and safe means that use mechanisms with a direct impact on carcinogenesis.3 Promising results have been shown by some natural phytochemicals—taxol analogues, vincristine, vinblastine, and podophyllotoxin analogues. Their specific mechanism of action includes increasing antioxidant status, carcinogen inactivation, proliferation inhibition, cell cycle arrest, and apoptosis induction, thus regulating the immune system.4 Today, curcumin (Curc), also known as the “yellow gold”, is a polyphenol herbal product of great research and clinician interest due to the plethora of valuable therapeutical properties.
Curc or [1,7-bis(4-hydroxy-3-methoxy)-1,6-heptadien-3,5-dion] is extracted from the rhizomes of the turmeric Curcuma longa plant and possesses valuable biological activities: antibacterial,5 anticoagulant,6 antitumor,7,8 antioxidant,9 and anti-inflammatory.10 Therapeutically, Curc is safe (8–12 g/day, even for extensive consumption for 3 months) and brings added value to a series of commercial products (capsules, tablets, ointments, energy drinks, soaps, and cosmetic formulations).11 Despite the therapeutical advantages, Curc has poor water solubility (<1 μg/mL) and it exhibits thermal- and photosensitivity, easy oxidation under alkaline conditions, and sulfation and glucuronidation in various biological tissues, leading to its poor absorption, rapid metabolism, and elimination in the living organisms.12,13 Therefore, it is of common clinical concern to improve Curc bioavailability.
In the literature, different approaches can be found aiming at an increase in Curc bioavailability, mainly by developing prodrug structures with suitable biopharmaceutic and pharmacokinetic properties.14 Curc chemical modification with biocompatible oligomers and polymers is a great strategy for the design of polymeric drug formulations (nanoparticles, liposomes, micelles, phospholipid complexes) for a prolongated half-life, improved membrane permeability, and increased metabolic stability of the bioactive molecule. Pegylation of Curc was reported as one of the most promising approaches, to improve the polyphenol water solubility with the possibility to adjust the Curc:PEG molar ratio for the desired final product properties.15−18 The obtained formulations present limited drug release and enhanced robustness in the physiological gastrointestinal tract pH and accelerated release in the colon in response to the bacterial reduction. Low cytotoxicity and increased transmembrane permeability were evidenced by in vivo pharmacokinetic assays with rats.16,17 Cytotoxicity assays show that the ester-linked conjugate was inactive and the urethane-linked conjugate demonstrated anticancer activity against a series of cells (human prostate, colon, and pancreatic carcinoma cell lines).15 The synthesis of stimuli-responsive (redox and pH) conjugates was explored in the design of polymer–drug conjugate micelles for efficient drug delivery, extended systemic circulation, and tumor imaging modalities.17−25
Overall, Curc chemical modification by pegylation or glycosylation brings beneficial pharmacological advantages to the natural molecule, by improving its water solubility and bioavailability for reduced dose administration.26−29 In addition, the obtained materials presented an improved circulation period and additional protection and stability against enzymatic degradation accompanied with low immunogenicity and antigenicity effects.29,30 However, the available processes showed a decrease in the therapeutic index of the conjugates, which impedes their commercial applicability, drives the research back to physical incorporation of Curc in nanosized objects (which may bring post-administration concerns, such as burst effect, low drug circulation time, etc.), and opens the gate for further investigations.
Based on the gathered knowledge, the present study applied direct esterification between Curc and a relatively low-molar mass PEG-diacid (600 g/mol, and PEG600-diacid/Curc molar ratio of 1:1 in the starting mixture) in the presence of N,N′-dicyclohexylcarbodiimide (DCC) to prepare a PEG-Curc conjugate with a specially chosen hydrophilic–hydrophobic balance (curcumin saturation index of about 131,32), which was expected to ensure (1) higher Curc water solubility, (2) improved stability, and (3) preserved therapeutic index. The presence of Curc in the final product was demonstrated by Fourier transform infrared (FT-IR) spectroscopy analysis. Information on the obtained product’s chemical structure is obtained from 1H NMR and 13C NMR analysis, and the thermal characteristics of PEG600-Curc were characterized by differential scanning calorimetry (DSC). Special attention was paid to the material water solubility and pH stability and spectral properties (absorption and photoluminescence). The therapeutic index of the conjugate was evaluated by modeling its anticancer activity viain vitro tests against the Graffi tumor cell line and against the pathogenic microorganism Staphylococcus aureus as a conventional model.
Experimental Part
Materials
Poly(ethylene glycol)diacid (PEG 600 g/mol; PEG600), curcumin (368.38 g/mol, Curc), 4-(N,N-dimethylamino)pyridine (DMAP), and N,N′-dicyclohexylcarbodiimide (DCC) were supplied by Sigma-Aldrich. Toluene (VWR) was dried using a MBraun solvent purification system under nitrogen. Methylene chloride (DCM, VWR) is freshly distilled under nitrogen. All other solvents and salts for the preparation of buffer solutions were supplied by VWR and were qualified as pure for analyses.
Synthesis of PEG600-Curc
To synthesize the PEG600-Curc conjugate, 10.1 g (0.017 mol) of PEG600 and 6.15 g (0.017 mol) of Curc were dried in a 250 mL reaction flask by azeotropic distillation with anhydrous toluene (3 × 50 mL). DMAP (0.047 g) and 50 mL of freshly distilled DCM were then added. DCC (3.45 g, 0.017 mol) was finally added to the resulting yellow suspension followed by the addition of another 50 mL of DCM. The reaction was carried out at room temperature under stirring for 48 h. The reaction mixture was concentrated to a final volume of 50 mL and precipitated in cold diethyl ether (DCM/diethyl ether = 1/7 v/v). The precipitate was isolated, and a minimum amount of DCM was added to dissolve and thaw it in cold diethyl ether. The second precipitate was filtered and centrifuged for complete isolation (10 000 rpm, 10 min, acceleration 9, at 10 °C), with a final yield value of 81%.
Methods
Chemical Structure
Information about the chemical structure of the conjugate was obtained by FT-IR, 1H NMR, and 13C NMR analyses.
FT-IR
FT-IR spectra of PEG, curcumin, and the conjugate were obtained with an IRAffinity-1 spectrophotometer (Shimadzu Co., Japan) equipped with a MIRacle ATR attachment (diamond crystal; IR penetration depth in the sample of about 2 μm) (PIKE Technologies) in the range of 600–4000 cm–1 with a resolution of 4 cm–1 using a thermally controlled DLATGS detector. All spectra were adjusted for H2O and CO2 using a software program, IRsolution.
1H and 13C NMR
The structures of Curc, PEG600, and the resulting product PEG600-Curc were further confirmed by 1H NMR and 13C NMR analyzes. The 1H NMR spectra were recorded in deuterated acetone on a Bruker Avance II+500 spectrometer. The 13C NMR spectra were acquired in deuterated chloroform (CDCI3) on a Bruvance A spectrometer II+600.
Thermal Properties
The thermal behavior was analyzed by differential scanning calorimetry, DSC model TA Q2000. Experiments were performed under a nitrogen atmosphere (50 mL/min), using around 10 mg of each sample. The experimental procedure is set at the temperature range from −80 to 200 °C: first heating to 200 °C, followed by cooling to −80 °C, and finally second heating to 200 °C; all three of them were at a rate of 10 °C/min. The glass transition (Tg), crystallization (Tc), and melting temperatures (Tm) of the conjugate and the reactants were obtained from the second heating curve to erase the previous thermal history.
Solubility
The solubility assessment for PEG600-Curc was performed in water, acetone, ethanol, methanol, chloroform, dichloromethane, dimethyl sulfoxide, tetrahydrofuran, diethyl ether, heptane, and toluene. For this purpose, a predetermined amount of the conjugate (5 mg) was placed in a test tube and 2 mL of solvent was added. The experiments were performed at room temperature for a period of 10 min.
UV–Vis Absorption and Photoluminescence
UV–Vis Absorption
Buffer solutions with pH 2 (aqueous solution of hydrochloric acid), 4 (acetate buffer), 8 (phosphate buffer), and 9 (carbonate buffer) were prepared to monitor the effect of pH on the absorption and photoluminescence of water-soluble PEG600-Curc. Then, 3 mg of PEG600-Curc was dissolved in 20 mL of buffer solution, in which dilution was performed to obtain a solution with a concentration of 8.1 × 10–5 mol/L. The absorption of Curc in each solution was measured using a DU 800 UV spectrophotometer (Beckman Coulter) at wavelengths of 280 and 404 nm for pH = 2; 383 nm for pH = 4; 385 nm for pH = 8; and 404 nm for pH = 9.
Photoluminescence
Photoluminescent spectra of aqueous solutions of PEG600-Curc with pH = 4; 8; and 9 and at a concentration of 8.1 × 10–5 mol/L were recorded on a Varian Cary Eclipse fluorescence spectrophotometer at room temperature at an excitation length of 420 nm.
Anticancer Activity
For in vitro experiments, 6.3 mg of PEG600-Curc was dissolved in 2 mL of distilled water to obtain a stock concentrated solution, from which working solutions of concentrations of 11 and 22 μg/mL were prepared.
Graffi Tumor Cells
Graffi tumor cell culture was maintained in Roswell Park Memorial Institute (RPMI)-1640 cell culture medium enriched with 10% fetal calf serum, with added 100 U/mL penicillin and 0.1 mg/mL streptomycin in 25 cm3 plastic tissue culture vessels, under standard conditions in a CO2 incubator (37 °C, 90% humidity, and 5% CO2).
Quantitative Cytotoxicity Assay
The effect of the synthesized product PEG600-Curc (concentrations of 11 and 22 μg/mL) on the viability of the tested cells was measured by a quantitative cytotoxicity test according to the method described by Mosmann et al. (1983).33 The test assesses the metabolic activity of the cells using tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (MTT). After entering the cell, the yellow tetrazolium salt is converted by a reduction reaction into insoluble formazan crystals. The formation of formazan crystals is proportional to the activity of mitochondrial enzymes and, accordingly, to the viability of the cells. Each concentration was tested in six replicates.
Seeding of Graffi Tumor Cells in a 96-Well Plate
To perform the experiments, Graffi cells were trypsinized with trypsin–ethylenediaminetetraacetic acid (EDTA), counted with trypan blue, diluted to the desired concentration, seeded in a 96-well plate (2 × 104 cells/well, 0.1 mL volume), cultivated for 24 h in a thermostat to obtain a monolayer, and then treated with PEG600-Curc according to a preapproved scheme.
The cells were treated by adding PEG600-Curc solution with Curc concentrations of 11 and 22 μg/mL (six wells per concentration) and incubated for another 24 h in a thermostat (5% CO2, 37 °C, 95% humidity). Graffi cells cultured in RPMI medium with 10% fetal bovine serum (FBS) were used as controls. The effect of PEG600-Curc at 24, 48, and 72 h was then reported by the MTT test. After culturing Graffi tumor cells with PEG600-Curc at the indicated concentrations and times, the cells were washed twice with PBS (pH 7.4), and 100 μL/well MTT working solution (Sigma Chemical Co.) was added to each well, and the cells were incubated again at 37 °C for 3 h; the supernatants were removed, and 100 μL of lysis solution (DMSO/ethanol = 1:1, v/v) was added to each well to dissolve the formazan crystals in the cells. The results of the MTT test were reported using an enzyme-linked immunosorbent assay (ELISA) plate reader (TECAN, Sunrise, Grödig/Salzburg, Austria). The absorption of the dissolved formazan was measured spectrophotometrically at a wavelength of 540 nm, reference 620 nm. Cell viability was calculated by the following formula
Statistical Analysis
One-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test was performed to determine the statistical significance of the data, and values of *p < 0.05, **p < 0.01, and ***p < 0.001 were considered significant.
Antimicrobial Activity
A stock solution of PEG600-Curc (0.003 g in 10 mL of distilled water) was prepared for microbiological tests. The appropriate amount of PEG600-Curc solution was then added to 1 mL of bacterial suspension to give mixtures with final concentrations of 5, 21, 42, and 74 μg/mL. The concentration of cells in the bacterial suspensions was 106 cells/mL.
The development of the pathogen was assessed after illumination for 1 h 15 min at λ = 420 nm and without illumination. The distance between the lamp (10 W) and the surface of the Petri dish was 10 cm. Aliquots were then taken from each suspension in contact with the PEG600-Curc solution. From the aliquots, a series of dilutions were performed by application on solid agar. The plates were incubated at 37 °C for 24 h. The number of surviving cells was determined relative to the colony-forming cells (CFUs).
Results and Discussion
Prodrugs are considered as pharmacologically inactive derivatives of bioactive molecules (drugs or natural products), biotransformed chemically or enzymatically to an active drug with improved biopharmaceutic or pharmacokinetic properties. In the case of Curc, such a chemical modification (attachment of promoieties to the phenolic hydroxyl groups via a biocompatible and biodegradable linkage) is a commonly applied approach. Here, synthesis of a water-soluble PEG-Curc conjugate by direct esterification between PEG diacid and Curc in the presence of N,N′-dicyclohexylcarbodiimide was proposed, as shown in Scheme 1. For this purpose, PEG diacid with a molar mass of 600 g/mol (PEG600-Curc) was used, and the molar ratio between PEG diacid and Curc was 1:1. As a result, two phenolic hydroxyl groups of Curc were engaged in covalent ester bonds with PEG600, resulting in a stable water-soluble Curc derivate. Pegylation involving the phenol groups is reported to enhance Curc chemical stability under hydrolytic conditions, due to the slow release of Curc from the conjugates.16 Indeed, free Curc phenol groups are easily ionized and oxidized.33
Scheme 1. Synthetic Pathway to PEG600-Curc (Yield = 81%).
The obtained product was first characterized by FT-IR analysis, and the spectra of PEG600 and PEG600-Curc are presented in Figure 1. From the literature, it is known that the Curc characteristic bands are observed at 1622 and 1506 cm–1, valence oscillations for vC=O and vC=C bonds, respectively, at 1425 cm–1, corresponding to deformation oscillations for C-bonds H (δCH) and C=C (δCC), at 1275 cm–1, for the C–O bonds involved in the construction of the enol bond in the Curc structure, and at 854 and 808 cm–1, characteristic bands for out-of-plane deformation oscillations of the aromatic nucleus (γAr–H).34−36 In the spectrum of PEG600, several characteristic bands were observed: at 3447 cm–1 and 1738 cm–1 for the valence oscillations of −OH and C=O groups, respectively. A signal was also observed at 1092 cm–1, characteristic for the ether bond, and at 2872 cm–1, for the valence oscillations of CH2. In the case of PEG600-Curc, band shifts from 1092 to 1101 cm–1 for the ether bond and from 1738 to 1778 cm–1 for the −C=O valence vibrations of the PEG600 carboxyl group were noticed. This last shift shows that PEG and Curc in the resulting product are most probably linked by ester bonds.
Figure 1.

FT-IR spectra of PEG600 and the PEG600-Curc conjugate.
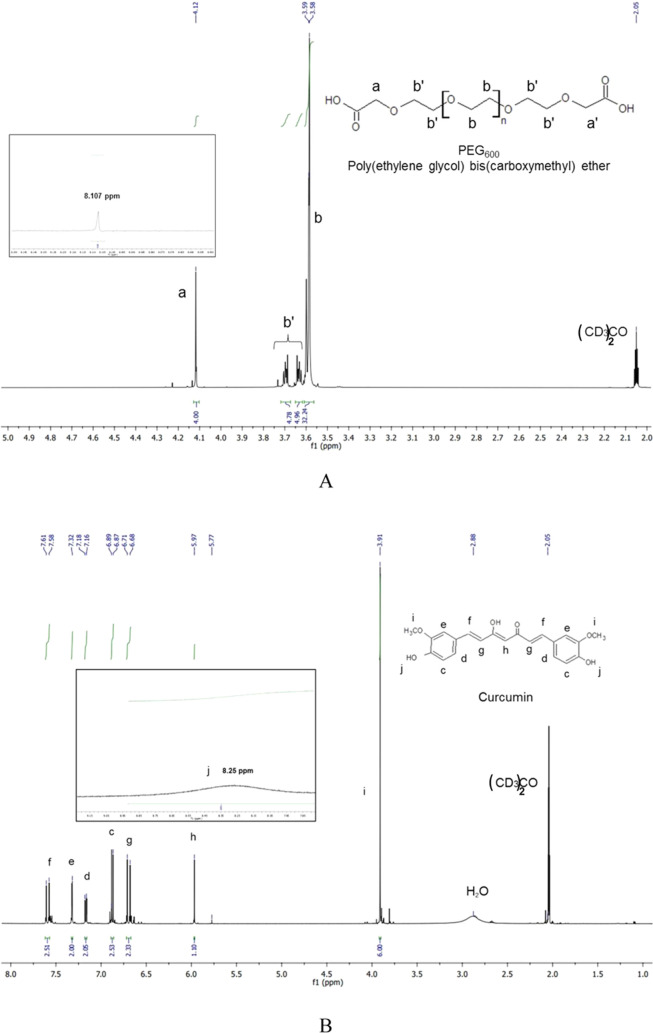
The obtained PEG600-Curc conjugate was further analyzed by 1H NMR ((CD3)2CO) as presented in Figure 2. Based on the obtained results, it might be concluded that the final product is a mixture of mono- and disubstituted PEG600, 25% monosubstituted PEG600-Cur and 75% disubstituted Curc-PEG600-Cur, as calculated from the ratio of the integral intensities of −O–CH2–C(O)O– versus the sum of the integral intensities of −O–CH2–C(O)O– and CH2COOH (Figure 2B).
Figure 2.
1H NMR spectra of PEG600 diacid (A), Curc (B), and PEG600-Curc conjugate (C).
These conclusions were confirmed by 13C NMR and distortionless enhancement by polarization transfer (DEPT)-135 spectra of PEG600-Curc (Figure 3). As can be seen, in the spectrum of PEG600-Curc, chemical shifts were observed, characteristic for (−(C=O)– of Curc) at 183.6 ppm (5); (−COOH of PEG600) at 172.4 ppm (3); (−(C=O)–O–CH−) at 168.7 (14); (−OCH3) at 148.4 ppm (7); (Ar–CH=CH– in Curc) at 140.2 ppm (8); (−CH=C–C– for Cuc) at 123.2 ppm (10); (OH–C=CH–CH=O) at 101.5 ppm (4); (−CH–C=CH from the aromatic core of Curc) at 127.8 ppm (9); (−C=CH–CH of the Curc aromatic nucleus) at 121.3 ppm (11), (−C=CH–OH of the Curc aromatic nucleus) at 115.4 ppm (12); (−C=COCH3 of the Curc aromatic nucleus) at 111.7 ppm (13); (−CH2–CH2–O) at 70.4 ppm (2); (−O–CH2–COOH) at 68.9 ppm (1); and (CH3O−) at 56.2 ppm (7).
Figure 3.
13C NMR spectra of PEG600-Curc in the range from 10 to 80 ppm (A) and in the range from 80 to 200 ppm (B) and DEPT 135 spectra (C).
All along this contribution, the PEG600-Curc conjugate thus will refer to the mixture of mono- and disubstituted PEG: 25% monosubstituted and 75% disubstituted PEG600-Curc.
As mentioned in the Introduction section, the poor water solubility of Curc reduces its bioavailability. Based on the previously published data, pure Curc is described as highly unstable in alkaline aqueous solutions (pH ≥ 7.0), accompanied with fast chemical degradation. On the other hand, the polyphenol compound tends to crystallize out of acidic aqueous solutions (pH < 7).37 To increase Curc water solubility and to preserve the natural substance valuable biological activity (antibacterial, anti-inflammatory, anticancer), conjugation with PEG might be useful. Therefore, the PEG600-Curc water solubility was studied. It was also found that the resulting product was soluble in a wide range of solvents such as water, acetone, ethanol, methanol, chloroform, dichloromethane, dimethyl sulfoxide, and tetrahydrofuran, confirming that the product can be easily handled. However, PEG600-Curc was found to be insoluble in diethyl ether, heptane, and toluene, indicating that the solubility of the natural polyphenolic compound was successfully modulated by covalently binding it to PEG600. The difference in Curc and the synthesized PEG600-Curc conjugate water solubilities is illustrated in Figure 4. This allowed the next steps in the material characterization relative to the pH stability using UV–vis and photoluminescence.
Figure 4.

Solubility in water of Curc (A) and PEG600-Curc (B).
UV–vis spectra of PEG600-Curc aqueous solutions at different aqueous solution pH values (pH = 2, 4, 8, and 9) are presented in Figure 5. It was noticed that PEG600-Curc was slightly soluble in strongly acidic medium (pH = 2), as evidenced by the low absorption and low color of the solution (Figure 5A). However, the increase in the pH of the medium led to an increase in the solubility of PEG600-Curc, a change in the color of the solutions, and a shift in the wavelength (λmax), 383, 385, and 404 nm, respectively, for pH values of 4, 8, and 9 (Figure 5B–D). The observed data are in accordance with the literature, where a color change of Curc solutions, from yellow to brownish orange, is observed at pH values between 2 and 13.38 This is explained with the keto–enol tautomerism: in acidic and neutral media, Curc is pronominally in the keto form (yellowish color), while in alkaline, it has an enol confirmation (color degradation—brownish orange).39 Additionally, Curc degradation under alkaline pH might occur, leading to derivative products such as feruloyl methane,40 rapidly forming a brown condensation product,41 causing a bathochromic shift from 420 nm (pH 2–10) to 460 nm (pH 12–13).42 Based on the results obtained here, no condensation product is observed in the PEG600-Curc conjugate solutions in the entire pH range, revealing good solubility in aqueous solutions and improved pH stability.
Figure 5.
UV–vis spectra and digital photographs of PEG600-Curc aqueous solutions at pH = 2 (A); 4 (B); 8 (C); and 9 (D).
As UV–vis in aqueous solutions was not comparable to Curc, solutions of Curc and PEG600-Curc in acetone (a good solvent for both forms) at a concentration of 0.1 mg/mL were also characterized (Figure 6). The obtained spectra showed that the maximum wavelength (λmax) for Curc is at 420 nm but presents a shoulder at 445 nm (black line). The obtained product shows a hypsochromic shift with about 20 nm; λmax is at 400 nm and the absorption of the arm is at 425 nm, suggesting the presence of disrupted conjugated structures.43
Figure 6.
UV–vis spectra of Curc and PEG600-Curc in acetone.
The results were confirmed by the photoluminescence emission spectra of PEG600-Curc solutions at pH 4, 8, and 9 (Figure 7). Curc spectral properties (photoluminescence), also varying with pH, allow traceability in cancer prevention and in situ cell imaging. It was found that the increase of the pH of the medium again led to a shift of λmax = 521, 524, and 543 nm, with the highest intensity obtained for the most alkaline solution. This pH-induced bathochromic effect can be explained by a change in the keto–enol form of curcumin associated with pKa1.22 It is interesting to note the absence of isosbestic points and additional bands characteristic of the protonation of phenolic groups at both opposite ends of the molecule. This phenomenon can be explained by the inclusion of terminal phenolic groups in ester bonds with PEG600–COOH and confirms the expected structure of the conjugate. The obtained results are in accordance with the data of other author groups on amphiphilic conjugate solutions.44 Moreover, the greater intensity in the case of the most alkaline solution (pH 9) confirmed the improved Curc solubility and stability in such solutions as discussed previously.37
Figure 7.
Photoluminescence spectra of PEG600-Curc aqueous solutions at pH = 4 (A), pH = 8 (B), and pH = 9 (C) and (D) overlay of the photoluminescence spectra taken at pH values of 4, 8, and 9.
After confirming the chemical structure of the synthetic products, the thermal properties of the conjugate were evaluated by DSC (Figure 8), as they are of considerable importance in biomedical applications. Based on the obtained thermograms, PEG600 diacid presented a glass transition temperature (Tg) of −54 °C, cold crystallization temperature (Tcc) of about −25 °C (enthalpy of cold crystallization (ΔHcc) of 67.3 J/g), and melting temperature (Tm) of about 12 °C (ΔHm of 79.7 J/g). The DSC thermogram of PEG600-Curc showed a significant shift of Tg to a higher temperature (−14 °C), again attesting the efficiency of the esterification reaction. Additional evidence for the successful synthesis might be found in the absence of cold crystallization and the significant decrease in crystallinity (ΔHm = 0.37 J/g).
Figure 8.
DSC thermograms of PEG600 diacid and the PEG600-Curc conjugate.
In addition to the enhanced water solubility of the obtained PEG600-Curc of great importance in the present work was to preserve the natural product biological properties. Previously published data, on more than 700 Curc and Curc analogues’ pharmacological properties, revealed a direct relationship between the Curc chemical structure and its specific therapeutic properties. In fact, the anticancer properties of curcuminoids were linked to the presence of hydroxyl groups in the phenolic ring, acting as an electron donor to free radicals. On the other hand, the methoxy group was responsible for the increased antioxidant properties of the natural product; substitutions in positions 2 and 2′ were responsible for the enhancement in the properties of analogues in comparison to the unsubstituted one. In general, cyclization in the central part of the molecule and introduction of heteroatoms, such as oxygen and nitrogen, led to the generation of compounds with pronounced antitumor and antiangiogenic activity, and the attachment of solubilizing groups to the hydroxyl group enhanced the curcuminoid cytotoxicity characteristics. Elimination of one methoxy group is related to tuberculosis activity, and conversion of methoxy groups to hydroxyl is linked to anti-HIV activity.45 Having in mind this information, PEG600-Curc was subjected to cytotoxic activity tests and the antimicrobial activity was evaluated.
In Vitro Cytotoxicity Studies
As a next step, the synthesized conjugate was tested for cytotoxicity properties against the Graffi tumor cell line, as presented in Figure 9. The MTT test was monitored at 24, 48, and 72 h, and it revealed the concentration and time dependency response of the cells. After the first 24 h of incubation in the presence of PEG600-Curc, the cell viability decreased to 62.8 and 39% for the Curc content in PEG600-Curc 11 and 22 μg/mL, respectively (Figure 9A). After 48 and 72 h of incubation, a greater cytotoxic effect was achieved in the case of PEG600-Curc with 22 μg/mL with only 4.4 and 2.3% cell growth. At the end of the test, PEG600-Curc with a concentration of 11 μg/mL did not present a significant change in the cell viability, remaining close to 68%. In this study, Curc was used as a positive control. During the first 24 h of cell incubation, the cell viability was reduced to 15.4 and 3.2% for Curc concentrations of 11 and 22 μg/mL, respectively. This concentration dependency was not observed after 48 and 72 h, where the cell viability was 2.96 and 0.65% for Curc concentrations of 11 and 22 μg/mL, respectively, after 48 h of incubation and 2.5 and 0.4% after 72 h (Figure 9B).
Figure 9.
Viability of Graffi tumor cells after 24 (A), 48 (B), and 72 h (C) contact with a control; 1—PEG600; 2—PEG600-Curc (22 μg/mL); 3—PEG600-Curc (11 μg/mL); 4—Curc (22 μg/mL); and 5—Curc (11 μg/mL).
The obtained results demonstrated the good antitumor activity of PEG600-Curc at a relatively low active concentration, compared to other formulations in the literature, where Cur-S and Cur-S-mPEG are used at much higher concentrations (between 50 and 200 μg/mL) against Hep G2 cancer cells46 or against HeLa cells, the concentration effect starting from 40 μg/mL, when acid-responsive polymeric micelles based on Curc-mPEG-PLA conjugates are used.19 Although the mechanism of action was not studied here, literature data suggest that curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization, and mitochondrial events.45,47
Antibacterial Activity
According to the literature, Curc has pronounced antibacterial activity against a series of pathogenic Gram-negative and Gram-positives bacteria, such as Escherichia coli and S. aureus, and fungi, including Candida albicans.48−50 Here, we performed a series of tests to determine the PEG600-Curc minimum inhibitory concentration (MIC). As presented in Figure 10, the PEG600-Curc MIC was found to be 74 μg/mL according to the amount of bound curcumin (210 μg/mL according to the conjugate). This value was lower than the MIC of Curc (15 μg/mL) that was found previously.51
Figure 10.
Antibacterial activity of PEG600-Curc against S. aureus.
As a concluding remark on the PEG600-Curc biological properties, it was found that the biological activity of Curc was preserved after the chemical modification. The lower values in terms of cytotoxic concentration and MIC might be explained with the covalent bonding involvement of one part of Curc functional groups in PEG600-Curc. In accordance with the literature, pegylation of small hydrophobic bioactive molecules may slightly decrease their therapeutic index. Such is the case of paclitaxel pegylation, where its improved solubility was an acceptable compromise accompanied with lower anticancer activity.52,53
Conclusions
In the present study, successful synthesis of a water-soluble PEG600-Curc conjugate was performed by direct esterification of PEG diacid and Curc in the presence of N,N′-dicyclohexylcarbodiimide. The obtained product was nearly amorphous with good chemical stability (water solubility and pH stability) and preserved Curc biological properties. Moreover, the conjugate PEG600-Curc exhibited a cytotoxic effect against the Graffi tumor cell lines and antibacterial activity against Gram-positive microorganisms such as S. aureus. The obtained prodrug product is suitable for the preparation of dosage forms with antitumor and antibacterial activity and chemical stability (water solubility and pH stability) as part of the cancer prevention and in situ imaging clinical practice. It is to be stressed that the synthesis applied here has brought a pegylated conjugate with the highest reported anticancer and antimicrobial activities, thus opening the gate for more clinically oriented studies.
Acknowledgments
The authors gratefully acknowledge the bilateral cooperation between the Bulgarian Academy of Sciences and WBI/FRS-FNRS-Belgium. The members of the SMPC laboratory A.T., R.M., J.-M.R., and P.D. also acknowledge the support from Wallonia and the European Community (FEDER) for general support in the frame of LCFM-BIOMAT. J.-M.R is a F.R.S.-FNRS Maître de recherches.
Author Present Address
# Materia Nova R&D Center, 1 Nikolai Copernic str., 7000 Mons, Belgium
Author Present Address
⊥ Konstantin Preslavsky University of Shumen, 115, Universitetska St, BG-9712 Shumen, Bulgaria
The authors declare no competing financial interest.
References
- GLOBOCAN. Global Cancer Observatory. Age-Standardized (World) Incidence and Mortality Rates, Top 10 Cancer, 2020. https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf.
- Vasan N.; Baselga J.; Hyman D. M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W. K.; Sporn M. B. Recent advances in chemoprevention of cancer. Science 1997, 278, 1073–1077. 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- Choudhari A. S.; Mandave P. C.; Deshpande M.; Ranjekar P.; Prakash O. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614 10.3389/fphar.2019.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz S.; Jabeen S.; Ilyas S.; Manzoor F.; Aslam F.; Ali A. Antibacterial activity of Curcuma longa varieties against different strains of bacteria. Pak. J. Bot. 2010, 42, 455–462. [Google Scholar]
- Kim D.-C.; Ku S.-K.; Bae J.-S. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012, 45, 221–226. 10.5483/BMBRep.2012.45.4.221. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Zhao Y.; Zhang Y.; Chen W. Anti-tumor effect of curcumin on human cervical carcinoma HeLa cells in vitro and in vivo. Chin. J. Cancer Res. 2007, 19, 32–36. 10.1007/s11670-007-0032-6. [DOI] [Google Scholar]
- Tang H.; Murphy C. J.; Zhang B.; Shen Y.; Van Kirk E. A.; Murdoch W. J.; Radosz M. Curcumin polymers as anticancer conjugates. Biomaterials 2010, 31, 7139–7149. 10.1016/j.biomaterials.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Menon V. P.; Sudheer A. R.. Antioxidant and Anti-Inflammatory Properties of Curcumin. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease, Advances in Experimental Medicine and Biology; Springer, 2007; Vol. 595, pp 105–125. [DOI] [PubMed] [Google Scholar]
- Kohli K.; Ali J.; Ansari M. J.; Raheman Z. Curcumin: A natural antiinflammatory agent. Indian J. Pharmacol. 2005, 37, 141–147. 10.4103/0253-7613.16209. [DOI] [Google Scholar]
- Prasad S.; Tyagi A.; Aggarwal B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.; Wang N.; He H.; Tang X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Controlled Release 2019, 316, 359–380. 10.1016/j.jconrel.2019.10.053. [DOI] [PubMed] [Google Scholar]
- Heger M.; van Golen R. F.; Broekgaarden M.; Michel M. C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2014, 66, 222–307. 10.1124/pr.110.004044. [DOI] [PubMed] [Google Scholar]
- Ratnatilaka Na Bhuket P.; El-Magboub A.; Haworth I. S.; Rojsitthisak P. Enhancement of curcumin bioavailability via the prodrug approach: challenges and prospects. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 341–353. 10.1007/s13318-016-0377-7. [DOI] [PubMed] [Google Scholar]
- Safavy A.; Raisch K. P.; Mantena S.; Sanford L. L.; Sham S. W.; Krishna R.; Bonner J. A. Design and development of water-soluble curcumin conjugates as potential anticancer agents. J. Med. Chem. 2007, 50, 6284–6288. 10.1021/jm700988f. [DOI] [PubMed] [Google Scholar]
- Wichitnithad W.; Nimmannite U.; Callery P.; Rojsitthisak P. Effects of different carboxylic ester spacers on chemical stability, release characteristics, and anticancer activity of mono-pegylated curcumin conjugates. J. Pharm. Sci. 2011, 100, 5206–5218. 10.1002/jps.22716. [DOI] [PubMed] [Google Scholar]
- Qiao H.; Fang D.; Chen J.; Sun Y.; Kang C.; Di L.; Li J.; Chen Z.; Chen J.; Gao Y. Orally delivered polycurcumin responsive to bacterial reduction for targeted therapy of inflammatory bowel disease. Drug Delivery 2017, 24, 233–242. 10.1080/10717544.2016.1245367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Wang Y.; Yang C.; Wang P.; Oelschlager D. K.; Zheng Y.; Tian D.-A.; Grizzle W. E.; Buchsbaum D. J.; Wan M. Polyethylene glycosylated curcumin conjugate inhibits pancreatic cancer cell growth through inactivation of Jab1. Mol. Pharmacol. 2009, 76, 81–90. 10.1124/mol.109.054551. [DOI] [PubMed] [Google Scholar]
- Gao M.; Chen C.; Fan A.; Zhang J.; Kong D.; Wang Z.; Zhao Y. Covalent and non-covalent curcumin loading in acid-responsive polymeric micellar nanocarriers. Nanotechnology 2015, 26, 275101 10.1088/0957-4484/26/27/275101. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Chen C.; Zhang Q.; Gao M.; Zhang J.; Kong D.; Zhao Y. Tuning the architecture of polymeric conjugate to mediate intracellular delivery of pleiotropic curcumin. Eur. J. Pharm. Biopharm. 2015, 90, 53–62. 10.1016/j.ejpb.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Zhang H.-Y.; Sun C.-Y.; Adu-Frimpong M.; Yu J.-N.; Xu X.-M. Glutathione-sensitive PEGylated curcumin prodrug nanomicelles: Preparation, characterization, cellular uptake and bioavailability evaluation. Int. J. Pharm. 2019, 555, 270–279. 10.1016/j.ijpharm.2018.11.049. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Chen B.; Zhu Y.; Sun C.; Adu-Frimpong M.; Deng W.; Yu J.; Xu X. Enhanced oral bioavailability of self-assembling curcumin-vitamin E prodrug-nanoparticles by conanoprecipitation with vitamin E TPGS. Drug. Dev. Ind. Pharm. 2020, 46, 1800–1808. 10.1080/03639045.2020.1821049. [DOI] [PubMed] [Google Scholar]
- Li M.; Gao M.; Fu Y.; Chen C.; Meng X.; Fan A.; Kong D.; Wang Z.; Zhao Y. Acetal-linked polymeric prodrug micelles for enhanced curcumindelivery. Colloids Surf., B 2016, 140, 11–18. 10.1016/j.colsurfb.2015.12.025. [DOI] [PubMed] [Google Scholar]
- Cao Y.; Gao M.; Chen C.; Fan A.; Zhang J.; Kong D.; Wang Z.; Peer D.; Zhao Y. Triggered-release polymeric conjugate micelles for on-demand intracellular drug delivery. Nanotechnology 2015, 26, 115101 10.1088/0957-4484/26/11/115101. [DOI] [PubMed] [Google Scholar]
- Meng X.; Gao M.; Deng J.; Lu D.; Fan A.; Ding D.; Kong D.; Wang Z.; Zhao Y. Self-immolative micellar drug delivery: the linker matters. Nano Res. 2018, 11, 6177–6189. 10.1007/s12274-018-2134-5. [DOI] [Google Scholar]
- Banerjee S. S.; Aher N.; Patil R.; Khandare J. Poly(ethylene glycol)-prodrug conjugates: concept, design, and applications. J. Drug Delivery 2012, 2012, 1–17. 10.1155/2012/103973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese F. M.; Mero A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. 10.2165/00063030-200822050-00004. [DOI] [PubMed] [Google Scholar]
- Vargason A. M.; Anselmo C.; Mitragotri S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. 10.1038/s41551-021-00698-w. [DOI] [PubMed] [Google Scholar]
- Kim C. Y.; Bordenave N.; Ferruzzi M. G.; Safavy A.; Kim K.-H. Modification of curcumin with polyethylene glycol enhances the delivery of curcumin in preadipocytes and its antiadipogenic property. J. Agric. Food Chem. 2011, 59, 1012–1019. 10.1021/jf103873k. [DOI] [PubMed] [Google Scholar]
- Shiraishi K.; Yokoyama M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: a review. Sci. Technol. Adv. Mater. 2019, 20, 324–336. 10.1080/14686996.2019.1590126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobylinska L.; Patereha I.; Finiuk N.; Mitina N.; Riabtseva A.; Kotsyumbas I.; Stoika R.; Zaichenko A.; Vari G. Comb-like PEGcontaining polymeric composition as low toxic drug nanocarrier. Cancer Nanotechnol. 2018, 9, 11 10.1186/s12645-018-0045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H. A.; Petroni M. L.; Abu-Hamdiyyah M.; Jazrawi R. P.; Northfield T. C. Hydrophobic/hydrophilic balance of proteins: a major determinant of cholesterol crystal formation in model bile. J. Lipid Res. 1994, 35, 211–219. 10.1016/S0022-2275(20)41209-X. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. lmmunol. Methods 1983, 65, 55–63. 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Wang Y.-J.; Pan M.; Cheng A.; Lin L.; Ho Y.; Hsieh C.; Lin J. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. 10.1016/S0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- Yakub G.; Toncheva A.; Manolova N.; Rashkov I.; Kussovski V.; Danchev D. Curcumin-loaded poly(l-lactide-co-D,l-lactide) electrospun fibers: Preparation and antioxidant, anticoagulant, and antibacterial properties. J. Bioact. Compat. Polym. 2014, 29, 607–627. 10.1177/0883911514553508. [DOI] [Google Scholar]
- Chhouk K.; Wahyudiono; Kanda H.; Kawasaki S.-I.; Goto M. Micronization of curcumin with biodegradable polymer by supercritical anti-solvent using micro swirl mixer. Front. Chem. Sci. Eng. 2018, 12, 184–193. 10.1007/s11705-017-1678-3. [DOI] [Google Scholar]
- Matos R. L.; Lu T.; Prosapio V.; McConville C.; Leeke G.; Ingram A. Coprecipitation of curcumin/PVP with enhanced dissolution properties by the supercritical antisolvent process. J. CO2 Util. 2019, 30, 48–62. 10.1016/j.jcou.2019.01.005. [DOI] [Google Scholar]
- Kharat M.; Du Z.; Zhang G.; McClements D. J. Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and 3 molecular environment. J. Agric. Food Chem. 2016, 65, 1525–1532. 10.1021/acs.jafc.6b04815. [DOI] [PubMed] [Google Scholar]
- Wulandari A.; Sunarti T. C.; Fahma F.; Enomae T. The potential of bioactives as biosensors for detection of pH. IOP Conf. Ser.: Earth Environ. Sci. 2020, 460, 012034 10.1088/1755-1315/460/1/012034. [DOI] [Google Scholar]
- Anand P.; Kunnumakkara A. B.; Newman R. A.; Aggarwal B. Bioavailability of curcumin problems and promises. Mol. Pharm. 2007, 4, 807–818. 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Tonnesen H.; Karlsen J. Studies on curcumin and curcuminoids V alkaline degradation of curcumin. Z. Lebensm.-Unters. -Forsch. 1985, 180, 132–134. 10.1007/BF01042637. [DOI] [PubMed] [Google Scholar]
- Stankovic I. In CURCUMIN. Chemical and Technical Assessment, 61st JECFA, 2004; pp 1–8.
- Pourreza N.; Golmohammadi H. Application of curcumin nanoparticles in a lab-on-paper device as a simple and green pH probe. Talanta 2015, 131, 136–141. 10.1016/j.talanta.2014.07.063. [DOI] [PubMed] [Google Scholar]
- Sunarti T. C.; Fahma F.; Enomae T. The potential of bioactives as biosensors for detection of pH. IOP Conf. Ser.: Earth Environ. Sci. 2020, 460, 012034 10.1088/1755-1315/460/1/012034. [DOI] [Google Scholar]
- Nagahama K.; Kumano T.; Oyama N.; Kawakami J. Curcumisome nanovesicles generated by self-assembly of curcumin amphiphiles toward cancer theranostics. Biomater. Sci. 2015, 3, 1566–1578. 10.1039/C5BM00212E. [DOI] [PubMed] [Google Scholar]
- Jankun J.; Wyganowska-Świątkowska M.; Dettlaff K.; Jelińska A.; Surdacka A.; Wątróbska-Świetlikowska D.; Skrzypczak-Jankun E. Determining whether curcumin degradation/condensation is actually bioactivation (Review). Int. J. Mol. Med. 2016, 37, 1151–1158. 10.3892/ijmm.2016.2524. [DOI] [PubMed] [Google Scholar]
- Goyal P.; Kumar P.; Gupta A. Amphipathic methoxypolyethylene glycol-curcumin conjugate as effective drug delivery system useful for colonic diseases. Colloid Polym. Sci. 2021, 299, 1757–1766. 10.1007/s00396-021-04892-9. [DOI] [Google Scholar]
- Moustapha A.; Pérétout P. A.; Rainey N. E.; Sureau F.; Geze M.; Petit J.-M.; Dewailly E.; Slomianny C.; Petit P. X. Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discovery 2015, 1, 15017 10.1038/cddiscovery.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukvik T.; Bruzell E.; Kristensen S.; Tønnesen H. H. Photokilling of bacteria by curcumin in selected polyethylene glycol 400 (PEG 400) preparations Studies on curcumin and curcuminoids. Pharmazie 2010, 65, 600–606. [PubMed] [Google Scholar]
- Dahl T. A.; McGowan W. M.; Shand M. A.; Srinivasan V. S. Photokilling of bacteria by the natural dye curcumin. Arch. Microbiol. 1989, 151, 183–185. 10.1007/BF00414437. [DOI] [PubMed] [Google Scholar]
- Dovigo L. N.; Pavarina A. C.; Ribeiro A. P. D.; Brunetti I. L.; Costa C. A. d. S.; Jacomassi D. P.; Bagnato V. S.; Kurachi C. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. 10.1111/j.1751-1097.2011.00937.x. [DOI] [PubMed] [Google Scholar]
- Yakub G.; Toncheva A.; Kussovski V.; Toshkova R.; Georgieva A.; Nikolova E.; Manolova N.; Rashkov I. Curcumin-PVP loaded electrospun membranes with conferred antibacterial and antitumoral activities. Fibers Polym. 2020, 21, 55–65. 10.1007/s12221-020-9473-z. [DOI] [Google Scholar]
- Greenwald R. B.; Pendri A.; Bolikal D. Highly water soluble taxol derivatives: 7 Polyethylene glycol carbamates and carbonates. J. Org. Chem. 1995, 60, 331–336. 10.1021/jo00107a010. [DOI] [Google Scholar]