Highlights
-
•
IGFBP3 inhibits lung cancer cell spheroid growth (3D proliferation).
-
•
IGFBP3 inhibits invasion by reduction of MMP activity.
-
•
Higher IGFBP3 plasma levels correlates with a less aggressive tumor.
-
•
Increased plasma levels is associated with improved overall cancer patient survival.
Abstract
The insulin-like growth factor (IGF)-pathway is involved in tumor cell proliferation, metastasis, and survival. We aimed to find out what effects IGF binding protein 3 (IGFBP3) exerted on H1299 lung cancer (LC) cells in terms of tumor growth and invasion and whether IGFBP3 was associated with clinical and pathological parameters in a prospective cohort of LC patients. H1299 cells were transfected with an IGFBP3-expressing vector. Its influence on apoptosis induction via flow cytometry annexin V FITC assay, cell proliferation in 2D and 3D cell culture, and invasion were examined. Expression of several matrix metalloproteinases (MMPs) and inhibitors (TIMP-1) were also investigated in IGFBP3-transfected LC cells. Further, data on LC patients (n = 131), tumor characteristics, and survival were prospectively collected and correlated with IGFBP3 plasma levels. IGFBP3 did not influence apoptosis induction and 2D cell proliferation. However, both spheroid growth (3D proliferation) and invasion of IGFBP3-transfected cells planted in an extracellular matrix-based gel were significantly inhibited. IGFBP3 inhibited MMP-1 release, and the total MMP activity. In LC patients, higher IGFBP3 plasma levels correlated with both lower clinical tumor stage, grading, Ki-67 staining, and the absence of necrosis (P < 0.05, respectively). Increased IGFBP3 plasma levels were associated with improved overall survival (hazard ratio 0.37, P = 0.01). In conclusion, overexpressed IGFBP3 in a LC cell line inhibited tumor growth and invasion. Translating from bench to bedside, investigation of clinicopathological parameters confirmed these experimental results showing that higher IGFBP3 plasma levels were associated with less aggressive tumor growth, reduced tumor spread, and improved survival of LC patients.
Introduction
Lung cancer (LC) is the leading cause of cancer–related death in the world [1]. Despite the development of new targeted therapies, such as tyrosine kinase inhibitors and immune checkpoint inhibitors, and the progress in surgical and radiotherapeutical techniques, survival rates of LC patients have hardly changed. Consequently, a better understanding of biological events leading to tumor progression is needed and can contribute to the development of novel therapeutic strategies.
Insulin-like growth factor 1 (IGF1) and its main binding protein IGFBP3 have an important function in cell growth, and survival. IGF1 binds to its transmembrane receptor (IGFR1) and thereby the PI3K/Akt signaling pathway is activated stimulating cell growth and blocks apoptosis [2]. Results of various studies suggested that high circulating IGF-1 concentrations are associated with an increased risk of cancer including LC [3,4].
IGFBP-3 is the dominant circulating binding partner for IGF‑1 [3,5]. Contrary to IGF-1, IGFBP3 can induce apoptosis in LC cells in vitro and in vivo, and inhibits the growth of non-small cell LC [2]. Previous own investigation also have shown, that IGFBP3 overexpression is involved in LC growth regulation [6]. Further, IGFBP‑3 serum concentration is significantly diminished in LC patients and has an inverse correlation with the risk of LC [3,7]. A recent study has shown that IGFBP3 reversed EMT (epithelial mesenchymal transition) through vimentin destabilization and thereby suppresses tumor migration [8]. However, only few reports have been published with regard to the effects of IGFBP-3 on invasion and migration of LC cells - one of them showed that XBP1-IGFBP3 signaling promotes invasion, and metastasis [9].
The present study was designed to determine and verify the effects of IGFBP3 overexpression on cell growth, apoptosis, and cell invasion in a LC cell line, to investigate involved mechanisms, and to prove possible results in vivo in LC patients.
Material and methods
Cell lines and cell culture
H1299 cell lines was purchased from ATCC. The cells were maintained in RPMI 1640, supplemented with 10% FCS (fetal calf serum) and 2 mM glutamine at 37 °C, 5% CO2 in a humidified atmosphere. For experiments, cells were seeded, treated, and analyzed as indicated.
Clinical LC cohort
Patients with newly diagnosed LC were prospectively enrolled at the Department of Respiratory Medicine at the University Hospital Leipzig.
For this observational study, all procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration, and its later amendments or comparable ethical standards. It received ethical approval from the local ethics committee at the Medical Faculty of Leipzig (IRB00001750, AZ: 024/17-ek). All participants gave their written informed consent prior to enrollment.
Clinical and pathologic characterization of LC patients
Participants were characterized clinically including sex, age, and body mass index (BMI). The latter was subdivided into the four categories defined by World Health Organization (WHO): underweight (<18.5 kg/m2), normal weight (≥ 18.5 to < 25.0 kg/m2), overweight (≥ 25.0 to < 30.0 kg/m2), obesity (>30.0 kg/m2).
Participants were characterized pathologically including histology, tumor stage, tumor grading, Ki-67 staining (clone MiB-1; monoclonal mouse from Dako, dilution 1:100).
Tumor tissue was characterized histopathologically and tumor stage was established according to the 4th edition of World Health Organization classification [10], the 8th edition of TNM classification of malignant tumors [11], respectively.
Survival analysis of LC patients
Survival data were retrieved from all patients with LC and included the following parameters: Observation period was defined as the time from the date of patient enrollment to the date of an event (i.e. progress, death, lost-to-follow up). Survival analyses comprised two endpoints: progression-free survival (PFS) and overall survival (OS).
Analysis of blood samples of LC patients
At the time of study enrollment, participants were drawn 10 mL blood collected in ethylenediaminetetraacetic acid (EDTA) tubes. Cells and plasma were immediately separated by centrifugation (500 g for 15 min [min] at 4 °C) and plasma samples were instantly frozen at -80 °C until further analysis.
RT-PCR for IGFBP3
Total RNA was isolated from H1299 cells using the RNeasy Mini Kit (Quiagen). 1 µg RNA was digested with DNase (GIBCO) for 15 min at room temperature. Digested RNA was reverse transcribed at 42 °C for 30 min (Reverse Transcription System, Promega). Amplification for IGFBP3 was performed using the following primer: IGFBP3 (s): 5´-ACT GGT GTA TTT CCT TGA CC, IGFBP3 (as): 5´-AAA ACG GGG GCT TCT TCC TG. PCR was carried out in a final volume of 20 µL, with 1 µL cDNA, 200 µM dNTP, 1U Taq polymerase (Thermo fisher), and 10 pmol of each oligonucleotide primer. PCR for IGFBP3 was performed for 30 cycles with an initial denaturation at 94 °C for 2 min, and cycling times of 30 s at 94 °C, 30 s at 52 °C, and 30 s at 72 °C. Following amplification, PCR products were analyzed in 2% agarose gels containing ethidium bromide with the Chemi Doc System (Bio-Rad).
Plasmids construction, transfection, and prove of functionality
IGFBP3 cDNA was generated by RT-PCR as described above. The PCR product was subcloned using the TOPO TA Cloning kit (Invitrogen). To express IGFBP3 cDNA insert of pCR®2.1-TOPO® was digested with EcoRI and cloned to the expression vector pIRES2-EGFP (Clontech). The DNA sequence of the inserts were analyzed using DNA Sequencing (ABI PRISM® 377).
H1299 cells were transfected with pIRES2-EGFP (Invitrogen) with or without IGFBP3 cDNA using EffecteneTM transfection reagent. After 72 h, cells were harvested and sorted by fluorescence cell sorting system (FACSVantage SE, Becton Dickinson). Then, 72 h after sorting the rate of EGFP positive cells was redetermined with flow cytometry. Lysates and supernatants of these cells were tested for IGFP3 with western blot and enzyme-linked immunosorbent assay (ELISA).
To determine the functionality of IGFBP3, supernatants of IGFBP3 positive cells were incubated (37 °C) with IGF-1 (2.5 ng/mL). After 2 h, ELISA was used to analyze these supernatants for free IGF-1.
ELISA assays
Culture supernatants of all probes were prepared in order to quantify the levels of IGFBP3, IGF-1, MMP-1, MMP-3, MMP-9 and TIMP-1. Supernatants were spun at 14.000 g, 4 °C, 10 min, and frozen at -80 °C. Concentrations of all factors were measured using specific ELISA assays (Human IGFBP-3 DuoSet ELISA, Human IGF-I/IGF-1 Quantikine ELISA Kit, Human Total MMP-1 DuoSet ELISA, Human Total MMP-3 DuoSet ELISA, Human MMP-9 DuoSet ELISA, and Human TIMP-1 DuoSet ELISA from RD-Systems). Further, MMP activity in all supernatants was determined using MMP Activity Assay Kit (Abcam). Assay procedures were performed according to user manuals.
Western blot
Cells were lysed on ice. Protein concentration of cell extracts was determined by DC Protein Assay (PIERCE). For Western blotting, 10 µg of protein per lane were resolved by SDS-PAGE, and transferred onto nitrocellulose membranes (Hybond-C, Amersham). Primary monoclonal antibody against IGFBP3 was purchased from Santa Cruz. The biotinylated secondary antibody was purchased from DAKO. Detection was performed with streptavidin-coupled peroxidase (DAKO) and ECL (Amersham). The intensity of bands was determined using the ChemiDoc XRS System (Bio-Rad).
Growth of IGFBP3 positive cells as 2D monolayer and 3D spheroid
All experiments were performed with cells, which were 85% positive for EGFP/IGFBP3. To determine 2D monolayer growth, cells were seeded (75 × 103 cells) in six-well plates. After 72 h, cells were counted. The difference in cell number at beginning (T1) and the end (T2) of experiment was divided by T1-cell number (proliferation rate = [T2-T1]/T1).
Spheroids were formed from 50,000 cells in 50 µL drops in reversed 10 cm culture plates. After 3–5 days, spheroids were transferred into an extracellular matrix-based hydrogel (Corning®Matrigel®) and both growth and invasion were documented by microscopy (Nikon Eclipse E400, 40x).
For all experiments, H1299 cells and H1299 cells transfected with pIRES-EGFP (without insert) as controls were used.
Apoptosis analysis
Analysis for apoptosis was performed using an Annexin V-FITC Apoptosis Detection Kit (Immunotech). Cells were harvested, washed with PBS, resuspended (5 × 105 cells in 100 µL assay binding buffer) and incubated with 1 µL Annexin V-FITC and 5 µL propidium iodide (PI) for 15 min in the dark on ice. Then, 400 µL of binding buffer was added. Cells were analyzed by flow cytometry (Cytomics FC500, Beckman Coulter).
Statistical analysis
Data with normal distribution and equal variances from two experimental groups were analyzed by using Student's t-test. For comparing more than two experimental groups, one-way analysis of variance (ANOVA) with Bonferroni post hoc analysis was performed. Wilcoxon signed-rank test or Kruskal Wallis test were applied for data that were not normally distributed or with unequal variances. Results are expressed as means ± standard deviation (SD) or medians with interquartile range. Correlation analysis was performed computing the Spearman's rho (ρ).
Survival analyses were performed using Kaplan-Meier estimator and log-rank test to calculate whether survival cures were significantly different. Cox proportional hazards regression analysis was used to determine the hazard ratio (HR) and the 95% confidence interval (95%CI). To test metric variables for survival analysis, IGFBP3 plasma levels were grouped into four quartiles, while the second and the third quartile were merged in one group; thus, three groups of IGFBP3 plasma levels were established: termed low, intermediate, and high.
Receiver operating characteristics (ROC) analyses including the calculation of the area under the curve (AUC), sensitivity, and specificity were performed to test for discrimination purposes of higher and lower IGFBP3 plasma cutoff values.
Two-sided P values < 0.05 were considered statistically significant. Statistical analysis was performed using the software package of SigmaPlot 14.0 (Systat Software), GraphPad Prism (v9.3.0 for macOS, La Jolla, California, USA), and SPSS (v27.0, IBM Corporation, Chicago, IL, USA).
Results
Construction, identification, and functionality of pIRES2-EGFP-IGFBP3
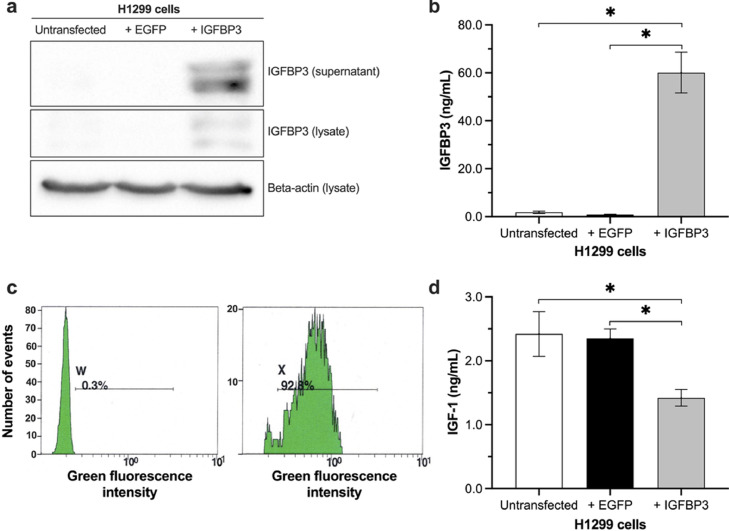
The full-length IGFBP3 was amplified with RT-PCR, and cloned in pIRES2-EGFP. The transfection rate of H1299 cell with pIRES-EGFP and pIRES-EGFP-IGFBP3 was examined by flow cytometry and was accepted at a transfection rate of > 85% (Fig. 1c). Expression of IGFBP3 was validated with Western blot, and ELISA. The results showed high concentration of IGFBP3 especially in the supernatants of transfected cells (Fig. 1a, b).
Fig. 1.
Analysis of IGFBP3 expression in H1299 cells.
pIRES2-EGFP-IGFBP3 was transfected into H1299 cells. At 24 h post-transfection, IGFBP3 was analyzed (a) by Western Blot in H1299 lysates and supernatants, and (b) by ELISA in supernatants. (c) The transfection rate was determined by flow cytometry, and (d) the function of secreted IGFBP3 was validated by its ability to bind free IGF-1. Two-sided P-value < 0.05 was considered statistically significant (n = 7; *P < 0.05).
In terms of IGFBP3 functionality, cells were incubated with recombinant IGF-1. We found significantly less free IGF in the supernatants of H1299 cells transfected with IGFBP3 (untransfected H1299 cells: 2.3 ± 0.3 ng/mL; cells transfected with EGFP: 2.3 ± 0.1 ng/mL; cells transfected with IGFBP3: 1.4 ± 0.1 ng/mL) compared to controls (Fig. 1d).
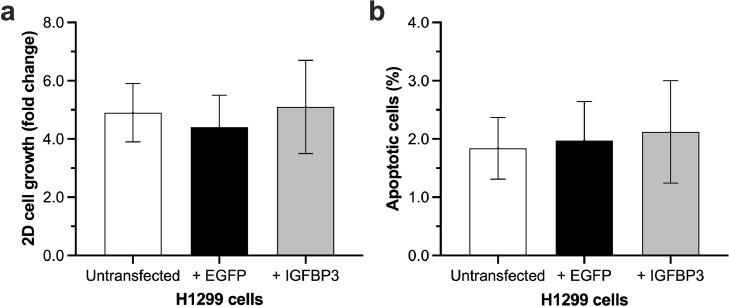
IGFBP3 has no influence on cell growth in 2D monolayer culture and apoptosis
All groups showed a good growth in the 2D monolayer cell culture (Fig. 2a). We found no significant differences in cell proliferation between H1299 cells transfected with IGFBP3 and the control groups (untransfected H1299 cells: 4.9 ± 1.0; transfected with EGFP: 4.4 ± 1.1; transfected with IGFBP3: 5.1 ± 1.6; shown as fold change to day 0).
Fig. 2.
Influence of IGFBP3 on H1299 2D cell growth and apoptosis.
IGFBP3 transfected H1299 cells were seeded out in 6-well culture plates (75 × 103/well). After 72 h (a) cells were counted, and (b) apoptosis was determined by annexin V-FITC staining (n = 6).
Because IGFBP3 is able to induce apoptosis in LC cells [12], we also determined apoptosis in IGFBP3 transfected cells. As shown in Fig. 2b, there were no significant differences between H1299 cells transfected with IGFBP3 and the control groups (non-transfected H1299 cells: 1,84 ± 0,53 %; cells transfected with EGFP: 1,97 ± 0,67 %; cells transfected with IGFBP3: 2.12 ± 0,88 %) in 2D monolayer culture.
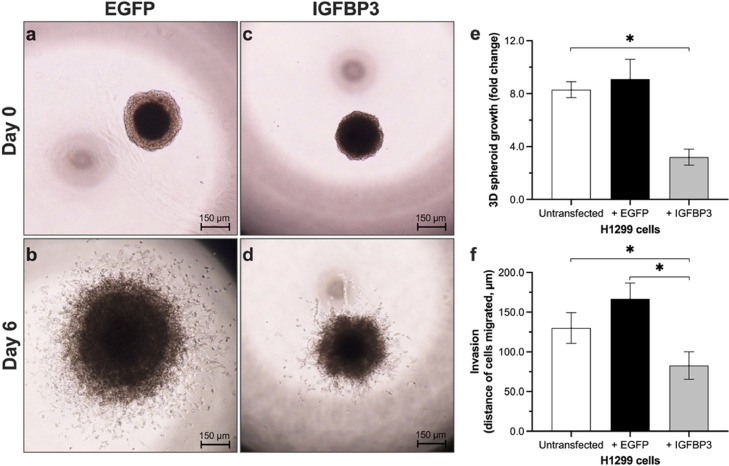
IGFBP3 reduces the cell growth in 3D spheroids and invasion
To determine the cell growth in 3D spheroids, the size of spheroids was measured after 6 days. Spheroid growth was determined as fold change referenced to the size on day 0. We observed an inhibition of cell growth in 3D spheroids transfected with IGFBP3 (Fig. 3a–d). As shown in Fig. 3e, the fold change of spheroid growth was significantly lower in H1299 cells transfected with IGFBP3 compared to controls (non-transfected H1299 cells: 8.3 ± 0.6; cells transfected with EGFP: 9.1 ± 1.5; cells transfected with IGFBP3: 3.2 ± 0.6).
Fig. 3.
Influence of IGFBP3 on H1299 3D spheroid growth and invasion.
Spheroids, formed from 50 × 103 cells in 50 µL drops in reversed 10 cm culture plates over 5 days, were transferred in a solubilized basement membrane preparation. Spheroids were observed over 6 days (a: pIRES2-EGFP day 0; b: pIRES2-EGFP day 6; c: pIRES-EGFP-IGFBP3 day 0; d: pIRES-EGFP-IGFBP3 day 6) by microscopy (40x). Each time (e) size of spheroids, and (f) migration of cells into the gel were measured. Two-sided P-value < 0.05 was considered statistically significant (n = 7; *P < 0.05).
Wang et al. have shown that IGFBP3-null mice exhibit a more aggressive tumor growth than wild-type mice [12]. We, therefore, investigated the influence of IGFBP3 on invasion in H1299 cells (Fig. 3f). Tumor cells of all groups migrated in the extracellular matrix gel. The distance of migrated cells was significantly shorter in the IGFBP3 group compared with controls (untransfected H1299 cells: 130.8 ± 9.3 μm; cells transfected with EGFP: 166.7 ± 20.1 μm; cells transfected with IGFBP3: 82.7 ± 17.4 μm).
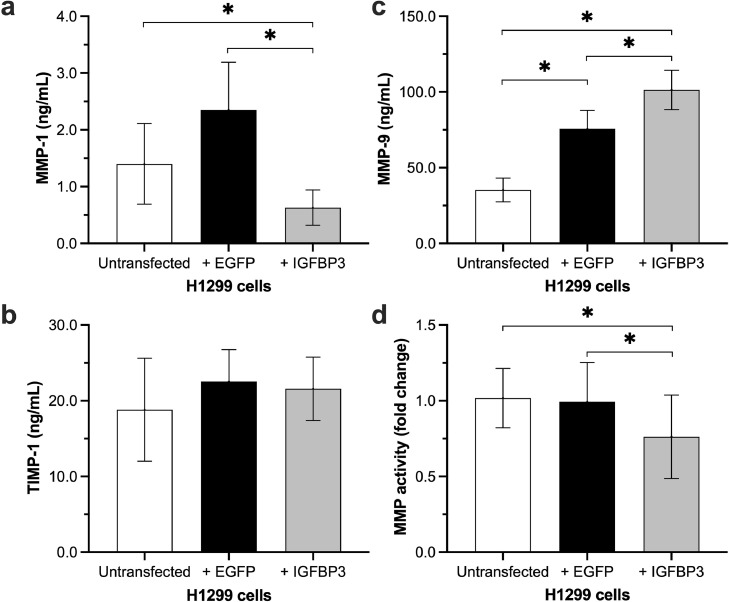
IGFBP3 reduces the expression of matrix metalloproteinases 1 and the total MMP activity
Metalloproteinases (MMPs) are involved in many central processes of tumorigenesis and cancer progression including tumor growth, survival, angiogenesis, and invasion [13]. Therefore, we investigated the influence of IGFBP3 on secretion of several MMPs (MMP-1, MMP-3, MMP-9), and tissue inhibitor of metalloproteinases (TIMP)-1 (Fig. 4). H1299 cells transfected with IGFBP3 inhibited the secretion of MMP-1 compared to control groups (untransfected H1299 cells: 1.4 ± 0.71 ng/mL; cells transfected with EGFP: 2.35 ± 0.84 ng/mL; cells transfected with IGFBP3: 0.63 ± 0.31 ng/mL). In contrast, the secretion of MMP-9 was increased in IGFBP3 transfected cells (non-transfected H1299 cells: 35.3 ± 7.84 ng/mL; cells transfected with EGFP: 75.71 ± 12.11 ng/mL; cells transfected with IGFBP3: 101.4 ± 13.02 ng/mL). However, levels of MMP-9 were also significantly higher in the supernatants of vector-transfected cells compared to untransfected H1299 cells. TIMP-1 and MMP-3 levels showed no significant differences between all groups.
Fig. 4.
Influence of IGFBP3 on MMP expression and activity.
IGFBP3 transfected H1299 cells were seeded in 6-well culture plates (75 × 103/well). After 72 h, concentration of (a) MMP-1; (b) MMP-9, and (c) TIMP-1 in supernatants was measured by ELISA. (d) The total MMP activity in supernatants was determined by MMP activity assay. Two-sided P-value < 0.05 was considered statistically significant (n = 7; *P < 0.05).
Because of the different results of MMP expression, we determined the total MMP activity in supernatants. We found approximately 25% lower MMP activity in H1299 cells transfected with IGFBP3 (p = 0.028) compared to controls (Fig. 4d).
IGFBP3 is associated with tumor grading, proliferation, and metastasis in LC patients
From a prospective clinical cohort of patients with LC, 131 participants were included in the analysis (86 male, 45 female). Clinical and pathological characteristics are shown in Table 1. Female LC patients showed higher levels of IGFBP3 as compared to the male counterparts. There were no differences in BMI categories or LC histology types.
Table 1.
Patients' characteristics.
| Characteristics | Participants | IGFBP3 | IGF1 | |||||
|---|---|---|---|---|---|---|---|---|
| ng/mL | P | ng/mL | P | |||||
| N | 131 | 498.6 | (382.7–604.7) | 39.0 | (24.8–62.9) | |||
| Male | 86 | (66) | 443.3 | (333.8–565.3) | <0.01 | 41.4 | (24.4–65.5) | 0.119 |
| Female | 45 | (34) | 562.9 | (468.4–637.2) | 34.0 | (22.1–58.5) | ||
| Age, years (range) | 66 | (46–86) | - | - | ||||
| BMI, kg/m2 (range) | 24.1 | (15.2–36.1) | - | - | ||||
| Underweight | 9 | (7) | 505.3 | (445.5–591.7) | 0.343 | 26.7 | (19.5–41.5) | 0.065 |
| Normal weight | 67 | (51) | 469.3 | (348.6–570.1) | 35.9 | (23.8–59.8) | ||
| Overweight | 42 | (32) | 528.8 | (401.1–631.5) | 40.3 | (33.3–73.1) | ||
| Obesity | 13 | (10) | 564.2 | (449.9–629.0) | 54.9 | (24.8–66.0) | ||
| Histology | ||||||||
| Adenocarcinoma | 74 | (56) | 519.2 | (394.0–626.4) | 0.305 | 45.5 | (25.0–70.8) | 0.196 |
| Squamous cell carcinoma | 39 | (30) | 461.9 | (367.2–568.5) | 34.7 | (24.0–49-6) | ||
| NSCLC, NOS | 8 | (6) | 490.9 | (322.4–620.7) | 38.9 | (23.2–95.0) | ||
| SCLC | 10 | (8) | 460.3 | (395.4–602.1) | 33.0 | (24.7–46.6) | ||
| Grading | ||||||||
| 1 | 8 | (7) | 642.1 | (461.1–731.4) | 0.032 | 57.2 | (46.0–86.8) | 0.165 |
| 2 | 49 | (41) | 516.5 | (398.4–605.9) | 38.0 | (28.7–61.0) | ||
| 3 | 53 | (44) | 461.9 | (349.7–588.2) | 40.3 | (20.9–66.5) | ||
| 4 | 10 | (58) | 460.3 | (395.4–602.1) | 33.0 | (24.7–46.6) | ||
| Tumor stage | ||||||||
| I | 17 | (13) | 536.4 | (386.4–701.6) | 0.039 | 47.8 | (16.5–54.1) | 0.605 |
| II | 6 | (5) | 638.7 | (543.0–694.0) | 46.8 | (36.7–112.1) | ||
| III | 25 | (19) | 483.2 | (431.4–635.3) | 37.6 | (24.6–54.7) | ||
| IV | 83 | (63) | 471.0 | (354.2–598.5) | 39.0 | (24.0–67.3) | ||
LC patients' characteristics. Data are number (N) and percentage (%) or median with interquartile range (IQR, Q1-Q3), unless otherwise defined. Student's t-test or Mann-Whitney-U test were used to compared two groups with each other, while one-way analysis of variance or Kruskal-Wallis test to compare more than two groups. BMI, Body mass index; IGF1, insulin-like growth factor; IGFBP3, insulin-like growth factor binding protein 3; NSCLC, non-small cell lung cancer; NOS, not otherwise specified; P, P-value; SCLC, small cell lung cancer.
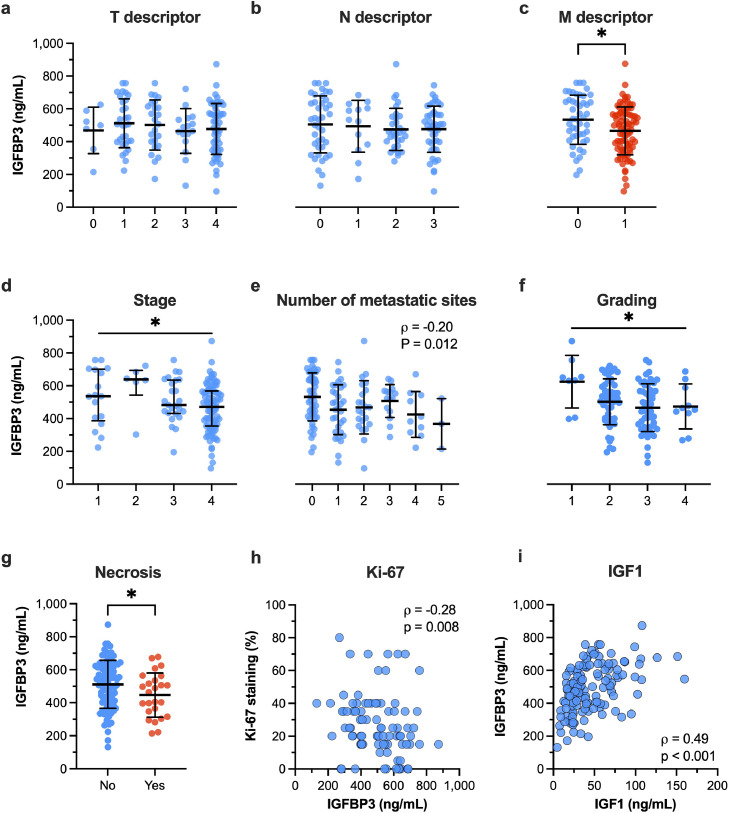
While IGFBP3 plasma levels did not change across the T or N descriptors, IGFB3 plasma levels were significantly higher in LC patients with M0 disease compared to those with M1 disease (531.9 ± 149.7 ng/mL vs. 464.2 ± 145.1 ng/mL, P = 0.0128, Fig. 5a–c). Higher IGFBP3 plasma levels correlated with lower tumor stages both in Spearman correlation and ANOVA on ranks (ρ = -0.22, P = 0.01; ANOVA P = 0.039), as shown in Fig. 5d. In addition, the number of metastatic sites correlated inversely with IGFBP3 plasma levels (ρ = -0.21, P = 0.012, Fig. 5e). The histopathological markers such as tumor grading, the percentage of Ki-67-stained LC cells, and the presence of tumor necrosis were associated with lower IGFBP3 plasma levels (Fig. 5f–h). Of note, we found a moderate association between IGFBP3 and IGF1 plasma levels (Fig. 5i).
Fig. 5.
IGFBP3 and histopathological characteristics in LC patients.
We investigated the associations of IGFBP3 plasma levels with (a) tumor size (T descriptor), (b) locoregional lymph nodes (N descriptor), (c) distant metastasis (M descriptor), (d) clinical tumor stage, (e) number of metastatic sites, (f) tumor grading, (g) necrosis, (h) proliferation index (Ki-67 stain), and IGF1 plasma levels. Data are shown as mean with SD or median wit interquartile range. Two-sided P-value < 0.05 was considered statistically significant (*P < 0.05). Spearman's ρ is depicted in the panels (h) and (i). The legend in panel (a) and (d) applies to its entire row.
There were no significant differences between IGF1 plasma levels and the patients' characteristics: e.g. distribution among males and females, BMI categories, LC histology, tumor grading, and stage (Table 1).
Improved survival in LC patients with increased IGFBP3 plasma levels
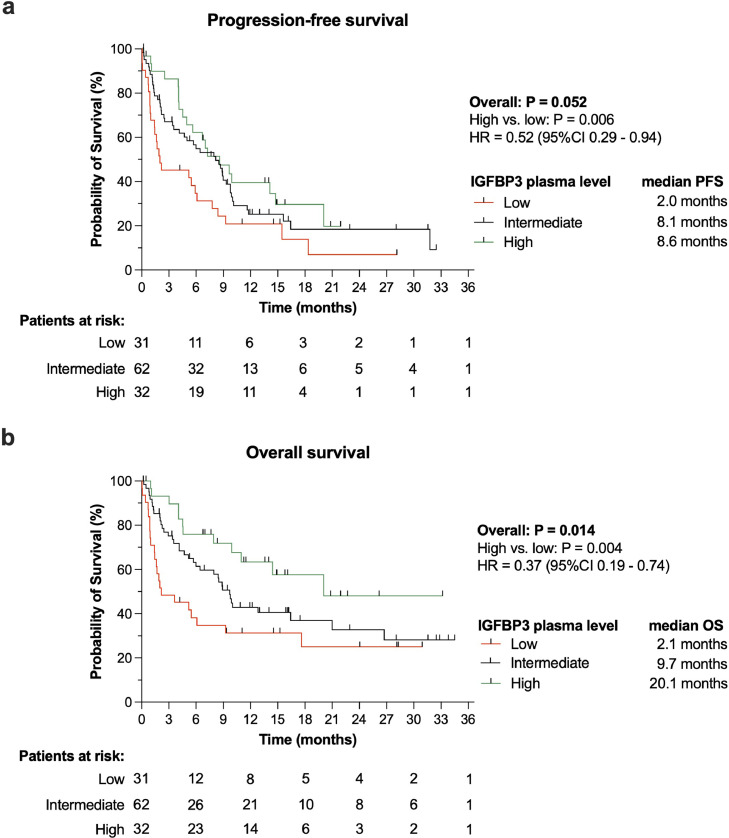
In the LC cohort of 133 participants, higher IGFBP3 plasma levels were observed with significantly longer PFS and OS compared to lower IGFBP3 plasma levels (Fig. 6). Cox regression analysis revealed that higher IGFBP3 plasma levels (>604.7 ng/mL [Q4]) were associated with a 48% risk reduction for disease progression or death, i.e. PFS, (HR 0.52, 95% CI 0.29–0.94) and even a 63% risk reduction for death alone (HR 0.37, 95% CI 0.19–0.74) when compared to lower IGFBP3 plasma levels (<382.7 ng/mL [Q1]).
Fig. 6.
IGFBP3 is associated with improved survival.
Both (a) progression-free (PFS) and (b) overall survival (OS) were longer in LC patients who were measured increased plasma concentrations of IGFBP3 (95%CI: 95% confidence interval; HR: hazard ratio).
Analysis of the IGFBP3 plasma cutoff values by employing ROC curve analysis revealed for OS an AUC of 0.63 (95% CI 0.53–0.723). The upper IGFBP3 plasma level threshold limit of >604.7 ng/mL yielded a sensitivity of 0.83 (95% CI 0.73–0.90) and a specificity of 0.37 (95% CI 0.25–0.50) while the lower threshold limit of < 382.7 ng/mL, a sensitivity of 0.31 (95% CI 0.21–0.42), and a specificity of 0.83 (95% CI 0.71–0.91).
Discussion
By binding to IGF, IGFBP3 blocks its actions, and thereby regulates cellular proliferation, differentiation, apoptosis, and carcinogenesis. Several studies have shown that overexpression of IGFBP3 inhibits LC cell growth and carcinogenesis in vivo and in vitro [5,14,15]. However, there have also been reports about close association of IGFBP3 with increased invasion and metastasis [9,16], as well as poor prognosis in tumor patients [17,18]. Therefore, we investigated the effect of IGFBP3 overexpression in a non-small cell LC (NSCLC) cell line in vitro and proved these results in a LC patient cohort.
Our experiments showed that overexpression of IGFBP3 significantly inhibited 3D cell growth, and invasion. It has previously been shown that IGFBP3 is able to inhibit cell growth in vitro and in vivo [14,15]. Hochscheid et al. described the inhibitory effect in the monolayer (2D) culture of the NSCLC H23 cells, which do not express IGFBP3 [15]. In line with the results from Oh et al. and Lee et al. our results confirmed theirs observations that IGFBP3 overexpression showed minimal to no inhibitory effects on cell growth in a H1299 monolayer culture [5,14]. The in vivo growth results reported by Lee et al. were similar to our data in the spheroid (3D) cell growth model. Both experiments showed a significant increase of 3D cell growth. In contrast to our results, Lee et al. found induction of apoptosis in H1299 cells by IGFBP3 overexpression, while we did not find any change in apoptotic cells between untransfected cells and H1299 cells transfected with IGFBP3. The vector systems used could explain the different results since adenoviral (Ad) vectors are able to induce cell cycle alterations [19]. Further, induction of apoptosis through IGFBP3 seems to depend on p53 status [20]. Price et al. have shown that IGFBP3 induced apoptosis in a p53-dependent manner in A549 cells, but not in H1299 (p53-null) cells. We hypothesized that the Ad vector modulates cellular actions required for apoptosis induction, as seen for p53. These effects were absent in our model.
The discussion about IGFBP3′s role on invasion needs to be led differently. In line with the results of Oh et al. [5], we found that IGFBP3 inhibited invasion of LC cells. Recent reports have shown that upregulation of IGFBP3 promotes invasion and metastasis [9,16]. Reasons for these differences are not completely clear. In contrast to our experiments, Luo et al. induced indirect upregulation of IGFBP3 by XBP1 (X-box binding protein 1) overexpression [9]. They found that IGFBP3/MMP-9 axis is responsible for promoting invasion, and metastasis. Likewise, we found not only an increase of MMP-9, but also a decrease of both MMP-1 and total MMP activity compared to controls. Yang et al. also overexpressed IGFBP3, but observed stimulation of invasion, and metastasis [16]. In contrast to Oh's and our results, Yang and colleagues used a lentivirus for transfection, which incorporates IGFBP3 and induces a permanent expression. Like Oh et al., we used a system for a transient expression of IGFBP3. We can only hypothesize that the circumstance of different transfection systems might be responsible for the different results.
To validate the IGFBP3 in vitro results of its clinical importance, we prospectively investigated the associations of IGFBP3 plasma levels to clinical and pathological tumor parameters and survival of LC patients.
We found higher IGFBP3 levels to be associated with lower tumor grades, and early tumor stages. Lower T, N, M, and overall tumor stage were previously reported by Wang et al. to be associated with higher IGFBP3 levels [21]. Interestingly, investigations of tissue expression of IGFBP3 showed also that higher IGFBP3 tissue expression was associated with lower T, N, M, and overall tumor stage, while tumor location (central or peripheral) or tumor grade were not related to IGFBP3 or IGF1 expression [21].
Necrosis is commonly observed in tumor cells that exhibit uncontrolled cell proliferation leading to deficient blood supply and as a result extensive cell death. The presence of necrosis was observed in LC patients who expressed lower IGFBP3 plasma levels. Consistent with the notion of necrosis as an indirect feature of rapid tumor growth, we found a larger amount of proliferating LC cells (Ki67 staining) in patients with lower IGFBP3 plasma levels, as well. Morphologically, the grade of tumor cell dedifferentiation was associated with lower IGFBP3 plasma levels. Reversely, higher IGFBP3 plasma levels were observed in LC cells with lower degree of anaplasia, lower proliferation, and thus necrosis. In the view of literature, we report those association for the first time.
We did not identify differences in histological entities (adenocarcinoma versus squamous cell carcinoma). In line with these observations, others also found neither an association of IGFBP3 plasma levels with histology, nor with tumor stage [21,22]. However, it was yet reported that patients with non-squamous cell carcinoma were found to have higher IGFBP3 levels than patients with pulmonary adenocarcinoma [4]. Evidently, those inter-study differences may largely be associated with differences in sample size and its low quantity.
The survival benefit observed in LC patients who were measured with higher IGFBP3 plasma levels compared to those with lower levels was quite remarkable considering the differences in median OS and PFS (8.6 versus 2.0 months or 20.1 versus 2.1 months, respectively), and the pronounced risk reduction for progression/death and death (48% or 63%, respectively). For OS, we found for the upper threshold of 604.7 ng/mL IGFBP3 plasma level a solid sensitivity (0.83) but a rather low specificity of 0.37. In contrast to the upper threshold, the lower threshold of <382.7 ng/mL showed opposing sensitivity and specificity values (0.31 or 0.83, respectively). Hence, by its very nature, the lower threshold may underestimates the putative protective effect of IGFBP3, while the upper threshold may overestimate it.
Previous work attributed higher IGFBP3 plasma levels (≥5312 ng/mL) in LC patients to both significantly improved PFS and OS (multivariate RR 0.45, each) [4]. Those results show comparable effect size in survival benefit compared to ours. Even in LC patients, who expressed higher level of IGFBP3 in LC tissue, a higher probability of disease specific 5-year survival was found compared to those, who showed loss of IGFBP3 expression (85.7% versus 64.6%, P = 0.019) [23]. Moreover, the post-treatment values of the IGF1-IGFBP3 ratio were statistically reduced in those LC patients who had responded to treatment [24]. However, there are other clinical reports as well showing no influence of IGFBP3 plasma levels on survival or treatment response [25,26].
In conclusion, we provide evidence that overexpressed IGFBP3 inhibits tumor growth and invasion of LC-cells. These results are confirmed by correlation of high IGFBP3 plasma levels with clinical and pathological tumor parameters of LC-patients. Furthermore, increased IGFBP3 plasma levels in LC patients are associated with improved patient survival. Thus, IGFBP3 may be regard as a laboratory parameter associated with improved LC survival. IGFBP3 should be further investigated as a potential surrogate of survival in LC patients.
Consent
Studies on patients or volunteers require ethics committee approval and fully informed written consent which should be documented in the paper.
Authors must obtain written and signed consent to publish the case report from the patient (or, where applicable, the patient's guardian or next of kin) prior to submission. We ask Authors to confirm as part of the submission process that such consent has been obtained, and the manuscript must include a statement to this effect in a consent section at the end of the manuscript, as follows: "Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request”.
This observational clinical study received ethical approval from the local ethics committee at the Medical Faculty of Leipzig (IRB00001750, AZ: 024/17-ek). All participants gave their written informed consent prior to enrollment to the study. No images of patients was used that could lead to their identification (their anonymity was protected).
CRediT authorship contribution statement
Hartmut Kuhn: Conceptualization, Methodology, Writing – original draft, Formal analysis, Project administration. Armin Frille: Conceptualization, Methodology, Writing – original draft, Formal analysis, Visualization, Funding acquisition. Marie Anna Petersen: Investigation, Visualization. Jonas Oberhuber-Kurth: Methodology, Investigation, Writing – review & editing. Lukas Hofmann: Investigation, Writing – review & editing. Albrecht Gläser: Investigation, Writing – review & editing. Sabine Taubenheim: . Sabine Klagges: . Sebastian Kraemer: Investigation, Writing – review & editing. Johannes Broschewitz: Investigation, Writing – review & editing. Maximilian von Laffert: Investigation, Resources, Writing – review & editing. Hubert Wirtz: Project administration, Resources, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
For this work, A. Frille was supported by the postdoctoral fellowship “MetaRot program” from the Federal Ministry of Education and Research (BMBF), Germany (FKZ 01EO1501, IFB Adiposity Diseases), a research grant from the Mitteldeutsche Gesellschaft für Pneumologie (MDGP) e.V. (2018‐MDGP‐PA‐002), and a junior research grant from the Medical Faculty, University of Leipzig (934100-012).
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Pollak M.N., Schernhammer E.S., Hankinson S.E. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 3.Yu H., Spitz M.R., Mistry J., Gu J., Hong W.K., Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J. Natl. Cancer Inst. 1999;91:151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- 4.Han J.Y., Choi B.G., Choi J.Y., Lee S.Y., Ju S.Y. The prognostic significance of pretreatment plasma levels of insulin-like growth factor (IGF)-1, IGF-2, and IGF binding protein-3 in patients with advanced non-small cell lung cancer. Lung Cancer. 2006;54:227–234. doi: 10.1016/j.lungcan.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Oh S.H., Lee O.H., Schroeder C.P., Oh Y.W., Ke S., Cha H.J., Park R.W., Onn A., Herbst R.S., Li C., Lee H.Y. Antimetastatic activity of insulin-like growth factor binding protein-3 in lung cancer is mediated by insulin-like growth factor-independent urokinase-type plasminogen activator inhibition. Mol. Cancer Ther. 2006;5:2685–2695. doi: 10.1158/1535-7163.MCT-06-0142. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn H., Bräunlich J., Hammerschmidt S., Wirtz H. Candidate genes upregulated in density dependent growth inhibition of lung cancer cells. Int. J. Oncol. 2004;25:1481–1487. [PubMed] [Google Scholar]
- 7.Chen B., Liu S., Xu W., Wang X., Zhao W., Wu J. IGF-I and IGFBP-3 and the risk of lung cancer: a meta-analysis based on nested case-control studies. J. Exp. Clin. Cancer Res. 2009;28:89. doi: 10.1186/1756-9966-28-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le H.T., Lee H.J., Cho J., Min H.Y., Lee J.S., Lee S.J., Lee H.Y. Insulin-like growth factor binding protein-3 exerts its anti-metastatic effect in aerodigestive tract cancers by disrupting the protein stability of vimentin. Cancers. 2021;13 doi: 10.3390/cancers13051041. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo Q., Shi W., Dou B., Wang J., Peng W., Liu X., Zhao D., Tang F., Wu Y., Li X., Li J., Wen S., Zhang C., Duan C. XBP1- IGFBP3 signaling pathway promotes NSCLC invasion and metastasis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.654995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travis W.D., Brambilla E., Nicholson A.G., Yatabe Y., Austin J.H.M., Beasley M.B., Chirieac L.R., Dacic S., Duhig E., Flieder D.B., Geisinger K., Hirsch F.R., Ishikawa Y., Kerr K.M., Noguchi M., Pelosi G., Powell C.A., Tsao M.S., Wistuba I. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 11.Goldstraw P., Chansky K., Crowley J., Rami-Porta R., Asamura H., Eberhardt W.E.E., Nicholson A.G., Groome P., Mitchell A., Bolejack V. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y.A., Sun Y., Palmer J., Solomides C., Huang L.C., Shyr Y., Dicker A.P., Lu B. IGFBP3 modulates lung tumorigenesis and cell growth through IGF1 signaling. Mol. Cancer Res. 2017;15:896–904. doi: 10.1158/1541-7786.MCR-16-0390. [DOI] [PubMed] [Google Scholar]
- 13.Hadler-Olsen E., Winberg J.O., Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol. 2013;34:2041–2051. doi: 10.1007/s13277-013-0842-8. [DOI] [PubMed] [Google Scholar]
- 14.Lee H.Y., Chun K.H., Liu B., Wiehle S.A., Cristiano R.J., Hong W.K., Cohen P., Kurie J.M. Insulin-like growth factor binding protein-3 inhibits the growth of non-small cell lung cancer. Cancer Res. 2002;62:3530–3537. [PubMed] [Google Scholar]
- 15.Hochscheid R., Jaques G., Wegmann B. Transfection of human insulin-like growth factor-binding protein 3 gene inhibits cell growth and tumorigenicity: a cell culture model for lung cancer. J. Endocrinol. 2000;166:553–563. doi: 10.1677/joe.0.1660553. [DOI] [PubMed] [Google Scholar]
- 16.Yang L., Li J., Fu S., Ren P., Tang J., Wang N., Shi X., Wu J., Lin S. Up-regulation of insulin-like growth factor binding protein-3 is associated with brain metastasis in lung adenocarcinoma. Mol. Cells. 2019;42:321–332. doi: 10.14348/molcells.2019.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Hu Z.G., Li D., Xu J.X., Zeng Z.G. Gene expression and prognosis of insulin‑like growth factor‑binding protein family members in non‑small cell lung cancer. Oncol. Rep. 2019;42:1981–1995. doi: 10.3892/or.2019.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao L., Liu H., You B., Gu M., Shi S., Shan Y., Li L., Chen J., You Y. Overexpression of IGFBP3 is associated with poor prognosis and tumor metastasis in nasopharyngeal carcinoma. Tumor Biol. 2016;37:15043–15052. doi: 10.1007/s13277-016-5400-8. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn H., Liebers U., Gessner C., Karawajew L., Ruppert V., Schumacher A., Witt C., Wolff G. Infection of cells with replication deficient adenovirus induces cell cycle alterations and leads to downregulation of E2F-1. Biochim. Biophys. Acta. 2002;1542:106–115. doi: 10.1016/s0167-4889(01)00171-9. [DOI] [PubMed] [Google Scholar]
- 20.Price D., Muterspaugh R., Clegg B., Williams A., Stephens A., Guthrie J., Heyl D., Evans H.G. IGFBP-3 blocks hyaluronan-CD44 signaling, leading to increased acetylcholinesterase levels in A549 cell media and apoptosis in a p53-dependent manner. Sci. Rep. 2020;10:5083. doi: 10.1038/s41598-020-61743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Wang Z., Liang Z., Liu J., Shi W., Bai P., Lin X., Magaye R., Zhao J. Expression and clinical significance of IGF-1, IGFBP-3, and IGFBP-7 in serum and lung cancer tissues from patients with non-small cell lung cancer. OncoTargets Ther. 2013;6:1437–1444. doi: 10.2147/OTT.S51997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izycki T., Chyczewska E., Naumnik W., Ossolinska M. Serum levels of IGF-I and IGFBP-3 in patients with lung cancer during chemotherapy. Oncol. Res. 2006;16:49–54. doi: 10.3727/000000006783981251. [DOI] [PubMed] [Google Scholar]
- 23.Chang Y.S., Kong G., Sun S., Liu D., El-Naggar A.K., Khuri F.R., Hong W.K., Lee H.Y. Clinical significance of insulin-like growth factor-binding protein-3 expression in stage I non-small cell lung cancer. Clin. Cancer Res. 2002;8:3796–3802. [PubMed] [Google Scholar]
- 24.Kotsantis I., Economopoulou P., Psyrri A., Maratou E., Pectasides D., Gogas H., Kentepozidis N., Mountzios G., Dimitriadis G., Giannouli S. Prognostic significance of IGF-1 signalling pathway in patients with advanced non-small cell lung cancer. Anticancer Res. 2019;39:4185–4190. doi: 10.21873/anticanres.13578. [DOI] [PubMed] [Google Scholar]
- 25.Han J.Y., Kim J.Y., Lee S.H., Yoo N.J., Choi B.G. Association between plasma hepatocyte growth factor and gefitinib resistance in patients with advanced non-small cell lung cancer. Lung Cancer. 2011;74:293–299. doi: 10.1016/j.lungcan.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Masago K., Fujita S., Togashi Y., Kim Y.H., Hatachi Y., Fukuhara A., Nagai H., Irisa K., Sakamori Y., Mio T., Mishima M. Clinical significance of epidermal growth factor receptor mutations and insulin-like growth factor 1 and its binding protein 3 in advanced non-squamous non-small cell lung cancer. Oncol. Rep. 2011;26:795–803. doi: 10.3892/or.2011.1354. [DOI] [PubMed] [Google Scholar]