Abstract
Two easy-to-use commercial diagnostic assays, a dipstick enzyme-linked immunosorbent assay (ELISA) (Integrated Diagnostics, Baltimore, Md.) and an immunochromatographic card assay (PanBio, Brisbane, Australia) were evaluated for detection of immunoglobulin M (IgM) antibody to dengue virus with an in-house IgM antibody capture microplate ELISA as a reference assay. The dipstick ELISA was based on the indirect-ELISA format using dengue 2 virus as the only antigen and enzyme-labeled goat anti-human IgM antibody as the detector. The total assay time was 75 min. The immunochromatographic card assay was based on the antibody capture format and separately measured both anti-dengue virus IgM and IgG in the same test. Colloidal-gold-labeled anti-dengue virus monoclonal antibody bound with dengue virus 1 to 4 antigen cocktail was the detector, and anti-human IgM and IgG were the capture antibodies. The total assay time was <10 min. Sera from 164 individuals classified as either anti-dengue virus IgM positive (94) or anti-dengue virus IgM negative (70) in the reference microplate ELISA with a dengue virus 1 to 4 antigen cocktail were tested in the two commercial assays. The dipstick ELISA missed 7 of 94 positive samples, for a sensitivity of 92.6%, while the immunochromatographic card assay missed two positive samples, for a sensitivity of 97.9%. Of the 70 negative samples, four were false positive by the dipstick ELISA and two were false positive in the immunochromatographic card assay, resulting in specificities of 94.3 and 97.1%, respectively. Both commercial assays provide sensitive and specific detection of anti-dengue virus IgM antibody and could prove useful in settings where the microplate ELISA is impractical.
Dengue viruses, transmitted by Aedes aegypti and Aedes albopictus mosquitoes, are widely distributed throughout the tropical and subtropical areas of the world (6). The four distinct dengue virus serotypes (dengue virus 1, 2, 3, and 4) are estimated to cause up to 100 million infections annually (7). In children, infection is often subclinical or causes a self-limited febrile disease. However, if the patient is infected a second time with a different serotype, a more severe disease, dengue hemorrhagic fever or dengue shock syndrome, is more likely to occur. Dengue is considered to be the most important arthropod-borne viral disease due to the human morbidity and mortality it causes (5).
Traditionally, the serological diagnosis of an acute dengue virus infection has relied on showing a fourfold or greater rise in anti-dengue virus antibody between paired acute- and convalescent-phase sera from a patient. The hemagglutination inhibition test (4), which detects both anti-dengue virus immunoglobulin M (IgM) and IgG antibodies in serum, has been the most commonly used serological assay for dengue diagnosis. In fact, the World Health Organization has developed guidelines to aid in the interpretation of anti-dengue virus antibody titers obtained with the hemagglutination inhibition test (18). More recently, the IgM antibody capture microplate enzyme-linked immunosorbent assay (ELISA) formatted to detect anti-dengue virus IgM antibody has become the test of choice for the serological diagnosis of acute dengue virus infections in many laboratories (2, 3, 9). Serum samples are usually tested at a single dilution, and a presumptive diagnosis of a recent dengue virus infection is made if anti-dengue virus IgM antibody is detected in any sample because IgM antibody usually does not persist for more than 3 months following an acute infection (9). The World Health Organization has not defined standards for interpreting the microplate ELISA, and reagents and interpretation of results can vary considerably among laboratories using different in-house or commercial reagents and protocols.
The objective of this study was to evaluate two commercially available easy-to-perform diagnostic assays, a dipstick ELISA and an immunochromatographic card assay, for identifying anti-dengue virus IgM antibody in serum samples. We had previously evaluated a prototype dengue virus IgM dipstick ELISA (19). However, the modified format of the dengue virus IgM dipstick ELISA with shorter assay time has not been evaluated. The immunochromatographic card assay has also been previously evaluated in several studies (1, 11, 13, 14, 17). In this study, the immunochromatographic card assay and the modified format of the IgM dipstick ELISA were compared in parallel by using panels of sera classified as anti-dengue virus IgM antibody positive or antibody negative in a reference microplate ELISA.
MATERIALS AND METHODS
Human sera.
The 164 sera used in this study to evaluate the two commercial diagnostic assays were selected from existing collections and were verified as either anti-dengue virus IgM antibody positive (94 sera) or anti-dengue virus IgM antibody negative (70 sera) in a reference microplate ELISA (Table 1). Of the 94 different patients that the IgM antibody-positive samples were obtained from, 38 originally had been diagnosed with acute dengue virus infections by virus isolation (12 dengue 1, 11 dengue 2, 7 dengue 3, and 8 dengue 4) as well as by the detection of anti-dengue virus IgM antibody in serum samples. The remaining 56 patients were diagnosed originally with acute dengue based only on the detection of anti-dengue virus IgM antibody in serum samples. All 94 anti-dengue virus IgM antibody-positive sera were convalescent samples collected a mode of 19 days (range, 3 to 140 days) post-onset of illness. They were selected to represent a wide range of IgM reactivities, as shown in Table 2. Among them, 39 sera had low ELISA optical density (OD) (<0.500). Nine of the 39 low-IgM sera were collected within 8 days after the onset of disease, and 22 of the 39 sera were collected within a month after the onset of disease. Most of the anti-dengue virus IgM antibody-negative samples were obtained from long-term residents of the United States (35 sera) or from healthy residents of Peru who remained negative for anti-dengue virus antibody over a 1-year period following collection of the serum sample used in this study (24 sera). Eleven additional sera from Peru were from patients with recent cases of malaria identified by the detection of anti-Plasmodium falciparum IgM antibody.
TABLE 1.
Anti-dengue virus IgM antibody classifications and countries of origin of human sera used to evaluate the dipstick ELISA and immunochromatographic assay
| Anti-dengue virus IgM antibody statusa | Country of origin | No. of samples | Provider or reference |
|---|---|---|---|
| Dengue IgM− | USAb | 35 | Integrated Diagnostics, Inc. |
| Peruc | 35 | 8 | |
| Total | 70 | ||
| Dengue IgM+ | Peru | 30 | 8 |
| Somalia | 28 | 15 | |
| Haiti | 27 | 16 | |
| Thailand | 9 | A. Nisalakd | |
| Total | 94 | ||
| Total sera | 164 |
Determined by the IgM antibody capture microplate ELISA. An OD value of >0.100 was considered positive for anti-dengue virus IgM antibody.
Included one serum each positive for IgM antibody against varicella-zoster virus, rubella virus, Epstein-Barr virus, cytomegalovirus, and hepatitis B virus.
Included 11 sera positive for IgM antibody against P. falciparum malaria, (provided by D. Watts, Naval Medical Research Center Detachment, Lima, Peru).
Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand.
TABLE 2.
Dengue virus IgM antibody sensitivity of the dipstick ELISA and the immunochromatographic assay compared to that of the microplate ELISA
| Microplate ELISA OD rangea | No. of sera | No. of sera IgM+ (IgM−)
|
|
|---|---|---|---|
| Dipstick ELISAb | Immunochromatographic assayc | ||
| >0.100–0.499 | 39 | 32 (7) | 37 (2) |
| 0.500–0.999 | 26 | 26 | 26 |
| ≥1.000 | 29 | 29 | 29 |
| Total | 94 | 87 (7) | 92 (2) |
An OD value of >0.100 was considered positive for dengue virus IgM antibody.
Sensitivity, 92.6% (87 of 94); 95% confidence interval, 87.3 to 97.9%.
Sensitivity, 97.9% (92 of 94); 95% confidence interval, 95.0 to 100.8%.
IgM antibody capture microplate ELISA (reference assay).
All serum samples were tested at 1:100 dilution for anti-dengue virus IgM antibody by an IgM antibody capture microplate ELISA with dengue virus 1 to 4 antigen cocktail as previously described, and some samples were also tested with dengue 2 virus antigen only (19). Adjusted OD values were calculated by subtracting the mean OD of the uninfected antigen-coated wells from that of the corresponding dengue virus antigen-coated wells. Because the mean of adjusted OD values ± 2 standard deviations for the negative control sera were consistently below 0.10, test samples with adjusted OD values greater than 0.10 were considered positive for dengue virus IgM antibody.
IgM dipstick ELISA.
Integrated Diagnostics, Inc. (Baltimore, Md.), provided the IgM dipstick ELISA kits. This was a modified format of the prototype dipstick ELISA evaluated previously (19). The modifications included using a twofold-more-concentrated purified dengue 2 virus antigen preparation, removing IgG antibody from the test serum with goat anti-human IgG absorbent (ProSorb G) instead of using a protein G device, and reducing the total assay time from 3 h to 75 min. Each sample assay was performed in a series of four reaction cuvettes in a 50°C water bath, similar to the prototype IgM dipstick ELISA. Before the assay, 40 μl of goat anti-human IgG absorbent (ProSorb G) was added to cuvette no. 1 containing 10 μl of test serum and 2 ml of diluent (1:200 diluted) and incubated for 10 min to remove human IgG from the test serum. The incubation times for the test serum and enzyme conjugate were shortened from 90 and 45 min to 38 and 18 min, respectively. The dipstick had the same six-well format as the prototype, including one well spotted with positive reagent control, one well with negative reagent control, and four wells with serial dilutions of dengue 2 virus antigen. The number of reaction dots, which appeared as clearly defined purple-blue dots, was observed and recorded after the dipsticks were completely dry. Any test sample that showed one to four antigen-positive dots was considered positive.
Immunochromatographic card assay.
PanBio Pty. Ltd. (Brisbane, Australia) manufactured the dengue fever IgM and IgG rapid (<10 min) immunochromatographic test kits. Anti-dengue virus IgM and IgG antibodies in the test sample (30 μl of undiluted serum per test) were determined simultaneously on the same card by using an antibody capture format with a cocktail of all four dengue virus serotype antigens as described in detail previously (17). The intensities of lines observed were scored as follows: 0, no reactivity; 0.5, faintly positive; 1, distinctly positive; or 2, strongly positive. The test is considered positive for primary dengue virus infection if two lines (IgM and control) are seen in the viewing window. The test is considered positive for secondary dengue virus infection if three lines (IgM, IgG, and control) are seen in the viewing window. A strong suspicion of secondary dengue virus infection exists if only the IgG and control lines are seen. In this study, we were primarily interested in the IgM antibody results for comparison to the IgM antibody capture microplate ELISA and the dipstick ELISA.
Comparison of assays.
The reactivities of sera classified as positive and negative by the reference microplate ELISA were compared in both commercial assays to determine sensitivity and specificity. Sera were tested and read in a randomized fashion, blinded to the reference ELISA results (10). All these assays were run and interpreted by personnel at the Naval Medical Research Center. The confidence intervals for sensitivity and specificity were calculated according to the method of Kirkwood (10). Sensitivity and specificity and the overall performance, determined as the sum of the sensitivity and specificity for each assay, were compared by Fisher's exact test. The association between the number of reactive dots in the dipstick ELISA or the intensity of the immunochromatographic card assay and the microplate ELISA OD values was determined with Pearson's correlation coefficient.
RESULTS
Comparison of the dipstick ELISA, the immunochromatographic card assay, and the reference microplate ELISA.
The 94 sera classified as anti-dengue virus IgM antibody positive in the reference microplate ELISA formatted with dengue virus 1 to 4 cocktail antigen were tested in the dipstick ELISA and the immunochromatographic card assay (Table 2). Seven of the 94 positive samples were false negative by the dipstick ELISA, giving a sensitivity of 92.6%. Because the dipstick ELISA contained dengue 2 virus as the only antigen, these seven false-negative samples were retested in the microplate ELISA with dengue 2 virus antigen alone in place of the dengue virus 1 to 4 cocktail antigen. The mean OD value (x̄OD = 0.129 ± 0.082) with the dengue 2 virus antigen alone was significantly lower (paired t test; P < 0.001) than with the dengue virus 1 to 4 cocktail antigen (x̄OD = 0.281 ± 0.075), and three retested samples were negative in the dengue 2 virus microplate ELISA. Two samples were false negative by the immunochromatographic card assay (microplate ELISA OD values, 0.365 and 0.158), giving a sensitivity of 97.9%. The sensitivities of the dipstick ELISA (92.1%) and immunochromatographic card assay (97.4%) remained essentially unchanged when evaluated with only the 38 convalescent sera from patients with virus isolation-confirmed acute dengue infection (Table 3). In the immunochromatographic card assay, 74 (78.7%) of the convalescent sera from the 94 patients with acute dengue infection also were positive for anti-dengue virus IgG antibody, giving an antibody response pattern that would be expected in a secondary dengue virus infection.
TABLE 3.
Sensitivities of the dipstick ELISA and the immunochromatographic assay compared to that of the microplate ELISA with convalescent sera from patients with virus isolation-confirmed dengue infection
| Serotype of isolate | No. of sera | No. of sera IgM+ (IgM−)a
|
|
|---|---|---|---|
| Dipstick ELISAb | Immunochromatographic assayc | ||
| Dengue 1 | 12 | 9 (3) | 12 |
| Dengue 2 | 11 | 11 | 11 |
| Dengue 3 | 7 | 7 | 7 |
| Dengue 4 | 8 | 8 | 7 (1) |
| Total | 38 | 35 (3) | 37 (1) |
All sera were positive for anti-dengue virus IgM in the reference microplate ELISA.
Sensitivity, 92.1% (35 of 38); 95% confidence interval, 83.5 to 100.7%.
Sensitivity, 97.4% (37 of 38); 95% confidence interval, 92.3 to 102.5%.
Of the 70 microplate ELISA IgM antibody-negative sera, four were false positive by the dipstick ELISA (microplate ELISA x̄OD = 0.033) (Table 4), for a specificity of 94.3%. When these four dipstick ELISA-false-positive sera were retested in the microplate ELISA with dengue 2 virus antigen alone, they remained negative for anti-dengue virus IgM antibody. Two of the 70 sera were false positive in the immunochromatographic card assay (microplate ELISA x̄OD = 0.024), giving a specificity of 97.1%. Of the total of six false-positive sera by either assay, three (50%) were from the group positive for P. falciparum IgM antibody. Two of the four false-positive sera by dipstick ELISA and one false-positive serum by the immunochromatographic card assay were P. falciparum IgM antibody positive. The sensitivities, specificities, and overall performances of the dipstick ELISA and the immunochromatographic card assay did not differ significantly compared to those of the reference microplate ELISA.
TABLE 4.
Dengue virus IgM antibody specificity of the dipstick ELISA and the immunochromatographic assay compared to that of the microplate ELISA
| Origin of seraa | Total no. tested | No. of sera IgM− (IgM+)
|
|
|---|---|---|---|
| Dipstick ELISAb | Immunochromatographic assayc | ||
| USA | 35 | 35 | 34 (1) |
| Peru | 35 | 31 (4)d | 34 (1)e |
| Total | 70 | 66 (4) | 68 (2) |
All of these sera were negative for dengue virus IgM antibody by the microplate ELISA.
Specificity, 94.3% (66 of 70); 95% confidence interval, 88.9 to 99.7%.
Specificity, 97.1% (68 of 70); 95% confidence interval, 93.2 to 101.0%.
Two of the four false-positive samples were P. falciparum IgM antibody positive.
One false-positive sample was P. falciparum IgM antibody positive.
Correlation of rapid diagnostic assays and the reference microplate ELISA.
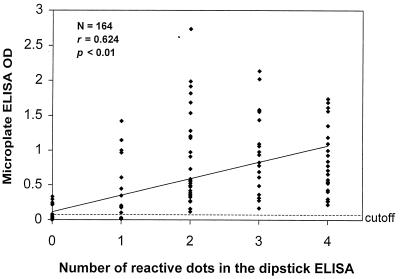
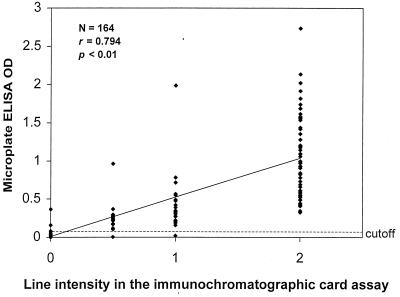
Both the number of positive antigen dots in the dipstick ELISA and the line intensity of the immunochromatographic card assay were significantly (P < 0.001) correlated with the OD values for the reference IgM microplate ELISA (r = 0.624 and 0.794, respectively) (Fig. 1 and 2).
FIG. 1.
Correlation of microplate ELISA OD values with number of reactive dots in the dipstick ELISA. A microplate ELISA OD value of >0.100 was considered positive for dengue virus IgM antibody.
FIG. 2.
Correlation of microplate ELISA OD values with line intensity in the immunochromatographic assay (0, negative; 0.5, faintly positive; 1, distinctly positive; 2, strongly positive). A microplate ELISA OD value of >0.100 was considered positive for dengue virus IgM antibody.
DISCUSSION
The commercially available IgM dipstick ELISA and the immunochromatographic assay are designed for rapid and simple serological diagnosis of dengue virus infections. From our parallel evaluation, both assays were shown to have excellent specificity and sensitivity (>90%) compared with our in-house reference antibody capture microplate ELISA for detection of anti-dengue virus IgM antibody (Tables 2 and 4). The two false-negative samples with the immunochromatographic assay and the seven false-negative samples with the dipstick assay all had OD values in the lowest-range group (>0.100 to 0.499) in the microplate ELISA (Table 2). The slightly lower sensitivity of the dipstick ELISA may be attributable to the use of dengue 2 virus as the only antigen to detect IgM antibody compared to the microplate ELISA and the immunochromatographic assay, which used a tetravalent cocktail antigen. The mean OD value of the seven dipstick ELISA false-negative samples was significantly higher in the microplate ELISA with the dengue virus 1 to 4 cocktail antigen compared to the mean OD values with dengue 2 virus as the only antigen. This is of particular relevance, since four of the false-negative samples were from Peru, where only dengue 1 virus was endemic at the time these sera were collected, and the other three false-negative samples were from patients from Haiti with isolation-confirmed dengue 1 virus infections. The high rate of detection (78.7%) of anti-dengue virus IgG in the immunochromatographic assay in the sera from the 94 patients with acute dengue infection is characteristic of secondary flavivirus infections (11, 14, 17). Some of the these secondary IgG responses were probably reflective of previous yellow fever (YF) and/or Japanese encephalitis (JE) exposure rather than past dengue virus infection. For example, 48 (87.3%) of the 55 sera from Somalia and Haiti were positive for anti-dengue virus IgG antibody. Most of these sera were from deployed U.S. military personnel who were likely to have been vaccinated against YF and possibly JE but unlikely to have been previously exposed to dengue (15, 16). Both YF and JE viruses share cross-reactive antigens with the dengue viruses, and individuals previously vaccinated with YF virus and subsequently infected with dengue virus have been shown to develop a typical secondary IgG antibody response in an antibody capture microplate ELISA (9). Secondary antibody response patterns were also seen in the sera from residents of Peru and Thailand. Although only dengue 1 virus was endemic in Peru at the time the study sera were collected, other dengue virus serotypes probably have circulated in the past and YF and other related flaviviruses are also present in Peru (8). In Thailand all four serotypes of dengue virus as well as JE virus are endemic.
All of the four false positives with the dipstick ELISA were sera from Peru, a country where dengue is endemic (Table 4). One of the two false positives with the immunochromatographic assay was also from Peru, and the other false-positive sample was from the United States, an area where dengue is nonendemic. None of these false-positive samples reacted strongly in either of the commercial assays. Although five of the six false-positive sera by either of the commercial assays were from a country where dengue is endemic, we believe these five samples are true anti-dengue virus IgM antibody-negative samples because of the very low OD values (x̄ = 0.015) obtained in the reference microplate ELISA. In addition, two of the samples from Peru represent baseline sera drawn from individuals who were rebled a year later and still remained negative for both anti-dengue virus IgM and IgG antibodies. The three other false-positive sera from Peru were all anti-P. falciparum IgM-positive samples. Other investigators also have reported a problem with false-positive IgM reactions (10%) for the immunochromatographic card assay with sera from patients with malaria (11). Another commercial IgM immunoblotting kit (Dengue Blot IgM; Diagnostic Biotechnology Ltd., Singapore) was reported to cross-react weakly with malaria-positive samples (7 of 30, or 23.3%) even though they were negative in a microplate IgM ELISA for anti-dengue virus antibody (12).
Other investigators who have evaluated the immunochromatographic card assay for dengue diagnosis have reported high levels of sensitivity and specificity, similar to what we found in this study (1, 11, 13, 14, 17). The sensitivity and specificity of the modified IgM dipstick ELISA were similar to what we had found previously for the prototype IgM dipstick (19).
These two commercially available assays are faster and simpler to perform and also provide acceptable sensitivity and specificity (>90%) compared to the standard microplate ELISA for detection of dengue virus IgM antibody. The immunochromatographic card assay is faster than the dipstick ELISA and requires no special equipment. The immunochromatographic card assay requires 30 μl of serum for each test, while the dipstick ELISA requires only 10 μl of serum or 20 μl of whole blood. Both assays could be useful as screening tests for dengue fever in health facilities or laboratories without expensive microplate readers and washers. This study suggests that both assays would be useful for testing acute or convalescent sera from small numbers of patients clinically suspected to have dengue infection or for instances where the diagnostic results are desired on the same day. As with any antibody-based diagnostic assay for dengue, these assays will report negative during the first 7 to 10 days of fever when IgM antibody may be nondetectable and cannot guide clinical care during the acute phase of illness. It is recommended that the patients be retested 7 days after the first specimen is taken. Another diagnostic assay based on dengue antigen detection would meet this need during the acute phase of illness and would complement these assays.
ACKNOWLEDGMENTS
We thank E. Henchal for administrative coordination and for providing the test sera from Haiti. We also thank A. Nisalak, D. Watts, and J. Burans for providing sera.
Cheryl Kung was supported by the Department of Defense Science and Engineering Apprentice Program as a summer apprentice. This research was funded by U.S. Naval Medical Research Center Work Unit 62787A.870.L.1441 and the U.S. Army Medical Research and Materiel Command.
REFERENCES
- 1.Berry N, Chakravarti A, Gur R, Mathur M D. Serological investigation of a febrile outbreak in Delhi, India, using a rapid immunochromatographic test. J Clin Microbiol. 1998;36:2795–2796. doi: 10.1128/jcm.36.9.2795-2796.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bundo K, Igarashi A. Antibody-capture ELISA for detection of immunoglobulin M antibodies in sera from Japanese encephalitis and dengue hemorrhagic fever patients. J Virol Methods. 1985;11:15–22. doi: 10.1016/0166-0934(85)90120-x. [DOI] [PubMed] [Google Scholar]
- 3.Chungue E, Boutin J P, Roux J. Antibody capture ELISA for IgM antibody titration in sera for dengue serodiagnosis and surveillance. Res Virol. 1989;140:229–240. doi: 10.1016/s0923-2516(89)80100-1. [DOI] [PubMed] [Google Scholar]
- 4.Clarke D H, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 5.Halstead S B. Immunological parameters of togavirus disease syndromes. In: Schlesinger R W, editor. The togaviruses. New York, N.Y: Academic Press, Inc.; 1980. pp. 107–173. [Google Scholar]
- 6.Halstead S B. Selective primary health care: strategies for control of disease in the developing world. XI. Dengue. Rev Infect Dis. 1984;6:251–264. doi: 10.1093/clinids/6.2.251. [DOI] [PubMed] [Google Scholar]
- 7.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 8.Hayes C G, Phillips I, Callahan J, Griebenow W, Hyams K C, Wu S-J, Watts D M. The epidemiology of dengue virus infection among urban, jungle, and rural populations in the Amazon region of Peru. Am J Trop Med Hyg. 1996;55:459–463. doi: 10.4269/ajtmh.1996.55.459. [DOI] [PubMed] [Google Scholar]
- 9.Innis B L, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke C H. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 10.Kirkwood B R. Essentials of medical statistics. Boston, Mass: Blackwell Scientific Publications; 1988. p. 84. [Google Scholar]
- 11.Lam S K, Devine P L. Evaluation of capture ELISA and rapid immunochromatographic test for the detection of IgM and IgG antibodies produced during dengue infection. Clin Diagn Virol. 1998;10:75–81. doi: 10.1016/s0928-0197(98)00002-6. [DOI] [PubMed] [Google Scholar]
- 12.Lam S K, Fong M Y, Chungue E, Doraisingham S, Igarashi A, Khin M A, Kyaw Z T, Nisalak A, Roche C, Vaughn D W, Vorndam V. Multicentre evaluation of dengue IgM dot enzyme immunoassay. Clin Diagn Virol. 1996;7:93–98. doi: 10.1016/s0928-0197(96)00257-7. [DOI] [PubMed] [Google Scholar]
- 13.Palmer C J, King S D, Cuadrado R R, Perez E, Baum M, Ager A L. Evaluation of the MRL diagnostics dengue fever virus IgM capture ELISA and the PanBio rapid immunochromatographic test for diagnosis of dengue fever in Jamaica. J Clin Microbiol. 1999;37:1600–1601. doi: 10.1128/jcm.37.5.1600-1601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sang C T, Hoon L S, Cuzzubbo A, Devine P L. Clinical evaluation of a rapid immunochromatographic test for the diagnosis of dengue virus infection. Clin Diagn Lab Immunol. 1998;5:407–409. doi: 10.1128/cdli.5.3.407-409.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp T W, Wallace M R, Hayes C G, Sanchez J L, DeFraites R F, Arthur R R, Thornton S A, Batchelor R A, Rozmajzl P J, Hanson R K, Wu S-J, Iriye C, Burans J P. Dengue fever in U.S. troops during Operation Restore Hope, Somalia, 1992–1993. Am J Trop Med Hyg. 1995;53:89–94. [PubMed] [Google Scholar]
- 16.Rossi C A, Drabick J J, Gambel J M, Sun W, Lewis T E, Henchal E A. Laboratory diagnosis of acute dengue fever during the United Nations Mission in Haiti, 1995–1996. Am J Trop Med Hyg. 1998;59:275–278. doi: 10.4269/ajtmh.1998.59.275. [DOI] [PubMed] [Google Scholar]
- 17.Vaughn D W, Nisalak A, Kalayanarooj S, Solomon T, Dung N M, Cuzzubbo A, Devine P L. Evaluation of a rapid immunochromatographic test for diagnosis of dengue virus infection. J Clin Microbiol. 1998;36:234–238. doi: 10.1128/jcm.36.1.234-238.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Dengue haemorrhagic fever: diagnosis, treatment, and control. Geneva, Switzerland: World Health Organization; 1986. [Google Scholar]
- 19.Wu S-J L, Hanson B, Paxton H, Nisalak A, Vaughn D W, Rossi C, Henchal E A, Porter K R, Watts D M, Hayes C G. Evaluation of a dipstick enzyme-linked immunosorbent assay for detection of antibodies to dengue virus. Clin Diagn Lab Immunol. 1997;4:452–457. doi: 10.1128/cdli.4.4.452-457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]