Abstract
Male accessory gland infection (MAGI) represents a frequent disease, commonly treated with antibiotics alone. However, in approximately 40% to 50% of patients, persistent infection is detected. Intestinal dysbiosis is involved in the pathogenesis of prostatitis. We aimed to evaluate the efficacy of antibiotic treatment in association with a specific probiotic supplementation. A total of 104 infertile patients, with microbiological analysis on semen and/or prostatic secretions positive for Gram-negative bacteria, have been enrolled. All patients received antibiotic treatment with fluoroquinolones. In total, 84 patients received a commercial association of Enterococcus faecium and Saccharomyces boulardii during antibiotic treatment, followed by treatment with Lactobacilli. After the treatment, a complete microbiological analysis was repeated. Polymicrobial infections have been observed in 11% of patients, while infections due to a single germ were reported in 89% of the patients. After the treatment was performed, a complete eradication with negative semen culture and microbiological analysis on prostatic secretion was observed in 64 of 84 patients (76.2%), while only 10 of 20 patients receiving antibiotics alone (50%; p < .05) reported negative microbiological analysis. Persistent infections have been observed only in patients with infections due to Enterococcus faecalis and Escherichia coli. This study represents the first approach demonstrating the efficacy of a specific probiotic treatment in reducing the rate of persistent infections in patients with MAGI.
Keywords: prostatitis, probiotic, MAGI, Saccharomyces boulardii, Lactobacilli
Introduction
Male accessory gland infection (MAGI)/inflammation represents a common andrological disease in clinical practice. It is defined as a syndrome characterized by symptoms of inflammation of the prostate, seminal vesicles, ductus deferens, and epididymis. Seminal microbiological results permit to classify this clinical entity into bacterial and abacterial forms (Krause, 2008).
The association between MAGI and infertility is frequently observed in clinical practice (La Vignera et al., 2011; Milardi et al., 2012). It has been estimated that up to 15% of male fertility disorders are linked to infectious and inflammatory conditions (La Vignera et al., 2011). Male tract infections may be often asymptomatic. The impact on fertility depends on the etiology (the type of pathogen), the course of the disease (acute or chronic), and the affected tract (epididymis, prostate, or seminal vesicles). The inflammatory process modifies the secretory activity of the accessory glands (Grande et al., 2018) and it may induce anatomical obstructions of the ejaculatory ducts (Rusz et al., 2012). Moreover, the presence of an inflammatory microenvironment, initiated by oxidative stress, may induce a dysregulation in spermatogenesis leading to infertility (Alshahrani et al., 2013; La Vignera et al., 2013; Tremellen, 2008).
According to World Health Organization (WHO) criteria, the diagnosis of MAGI is done in the presence of one or more abnormalities of seminal parameters (oligo- or astheno- or teratozoospermia), associated with two or more of the following criteria (La Vignera et al., 2012; Rowe et al., 1993):
A: history of urinary tract infections and/or epididymitis and/or sexually transmitted diseases and/or abnormal physical urogenital examination (in terms of thickened or tender epididymis and/or thickened vas deferens and/or abnormal rectal examination).
B: alterations in the number of leukocytes or bacteria in prostatic secretion and/or urinary sediment after prostate massage.
C: signs of infection in ejaculate (leukocyte concentration >1 × 106 mL); significant culture for pathogenic bacteria in the seminal plasma (diluted 1:2); altered secretive function of the accessory sex glands in terms of abnormal physicochemical and/or biochemical seminal plasma properties.
Commonly, patients are treated with antibiotics alone as recommended by the European Urology guidelines (Naber et al., 2001). The choice of the specific antibiotic treatment should be driven by the result of the drug-sensitivity test and the patient clinical feature (e.g., drug allergy, renal insufficiency). For chronic bacterial prostatitis, fluoroquinolones, thanks to their favorable pharmacokinetic properties, prostate penetration, bioavailability, and excellent activity against typical and atypical pathogens together with a good safety profile, are considered the gold standard for starting the treatment (Busetto et al., 2014; Mazzoli, 2007). Usually, high doses and a long-lasting therapy are preferred because only low-molecular-weight and lipid-soluble drugs, not tightly connected to plasma proteins, are able to penetrate the epithelial membrane, even considering reported side effects (gastrointestinal disorders and development of antibiotics resistance) (Busetto et al., 2014; Wagenlehner & Naber, 2003). This approach, although recommended, may not be enough, considering patients’ and urologists’ high rate of dissatisfaction (Busetto et al., 2014). Additional treatment options include anti-inflammatory (Milardi et al., 2017) and/or fibrinolytic drugs (La Vignera et al., 2019).
However, in approximately 40% to 50% of patients, antibiotic treatment is unable to eradicate the pathogen and persistent infection is often detected at semen culture after the treatment (Stamatiou et al., 2019). A growing number of studies have focused on the concomitant presence of prostatitis syndromes and irritable bowel syndrome (IBS). IBS patients had a significantly higher frequency (82%) of MAGI (Vicari et al., 2012). Although the pathophysiology of this correlation is complex and multifactorial, intestinal dysbiosis and mucosal inflammation due to modified commensal gut flora might be involved in both IBS and infective prostatitis (Vicari et al., 2014).
The purpose of the study was to evaluate the efficacy of a therapy with a fluoroquinolone drug, recommended as first choice in this disease, in association with a specific probiotic supplement.
Materials and Methods
The study was approved by the Institutional Review Board and conducted in accordance with the guidelines of the Declaration of Helsinki. Informed consent was obtained from each patient.
Patients
We retrospectively evaluated in the study a population of 20- to 55-year-old patients seeking medical care for primary infertility, with normal testicular volume and follicle-stimulating hormone (FSH) values (<8 mUI/L), associated with scrotal ultrasound (US) and transrectal ultrasound (TRUS) signs of prostate-vesicular-epididymitis (PVE) and with microbiological analysis on semen and/or prostatic secretions positive for Gram-negative bacteria (growth of more than 103 CFU/mL of pathogenic bacteria in cultures of diluted seminal plasma and/or secretions obtained after prostatic massage).
Exclusion criteria included history of cryptorchidism, orchitis, testicular torsion or trauma, hypogonadism, occupational chemical exposure, Y chromosome microdeletions, karyotype abnormalities and Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) mutations, ultrasound testicular volume <12 mL, FSH >8 mUI/L, fever, or drug use within 3 months prior to the enrollment in this study and azoospermia.
All patients underwent a complete physical and andrological examination, standard semen analysis, complete microbiological analysis (including culture for bacteria, mycoplams, and chlamydia in semen and in prostatic secretion after prostate massage), and ultrasound evaluation. The validated Italian version of the International Prostate Symptom Score (IPSS) (Badia et al., 1997) was administered to each patient and self-completed.
Design of the Study
A total of 104 patients, fulfilling the inclusion criteria, have been considered for this study. All patients received antibiotic treatment with fluoroquinolones (ciprofloxacin 1 g daily for 14 days or levofloxacin 500 mg daily for 14 days). Eighty-four patients received a probiotic treatment and namely a commercial association of Enterococcus faecium and Saccharomyces boulardii (Enterelle plusTM: Enterococcus faecium EF41, Saccharomyces boulardii LYO SB28, Saccharomyces boulardii SP92 and Lactobacillus acidophilus LA14; Bromatech, Milano, Italy; 2 tablets daily for 14 days) during antibiotic treatment, followed by treatment with Lactobacilli (FemelleTM: Lactobacillus jensenii KS121.1, Lactobacillus rhamnosus LR32, Lactobacillus plantarum LP115, Lactobacillus acidophilus LA14; Bro-matech, Milano, Italy; 1 tablet daily for 14 days after antibiotic treatment). This regimen has been chosen with the aim to reduce the treatment time and to cover both the period of treatment with antibiotics (since Saccharomyces boulardii is often prescribed in association with antibiotics) and the following period, to positively modify the microbiome.
Twenty patients received antibiotic treatment alone. Association treatment has been proposed to all patients and the treatment option (probiotics + antibiotic or antibiotic alone) was chosen according to patient preference. After the treatment, a complete microbiological analysis (including culture for bacteria, mycoplams, and chlamydia in semen and in prostatic secretion after prostate massage) was repeated. We evaluated the prevalence of polymicrobic/unimicrobic infections, the prevalence of identified germs, and for each group the percentage of eradication after treatment.
Statistics
All statistical analyses were performed using SPSS software (Version 27, IBM, Segrate [MI], Italy). All data have been analyzed for normality of distribution using the Kolmogorov–Smirnov test of Normality. Results were expressed as mean ± standard deviation (SD) when normally distributed, or as median [quartiles] when not normally distributed. The differences between continuous variables were analyzed by analysis of variance (ANOVA). The differences between discrete variables were analyzed by chi-square test or Fisher test (if the expected count was <5). Values of p <.05 were considered statistically significant.
Results
The baseline clinical and seminal data of patients are reported in Table 1. No significant variations have been noted among the studied populations.
Table 1.
Clinical and Seminal Parameters of Patients.
| Clinical/Seminal parameters | Antibiotic + probiotics (N = 84) | Antibiotic only (N = 20) |
|---|---|---|
| Age (years) | 34.28 ± 6.21 | 37.21 ± 4.24 |
| IPSS score | 12.52 ± 4.10 | 13.0 ± 3.70 |
| Seminal volume (mL) | 3.1 ± 0.97 | 3.70 ± 2.10 |
| Sperm concentration (×106/mL) | 28.85 ± 11.28 | 19.1±7.69 |
| Total sperm motility (%) | 35.5 ± 19.11 | 25.0 ± 16.83 |
| Normal morphology (%) | 10.75 ± 9.77 | 15.5 ± 12.73 |
Note. Association of probiotic treatment with antibiotics in male accessory gland infections. IPSS = International Prostate Symptom Score.
Mild symptoms have been reported in 11 of 104 patients (10.6%) and moderate symptoms in 93 patients (89.4%). No differences have been observed for IPSS score among the two studied groups. The treatment was performed by all patients without any side effect.
Polymicrobial infections have been observed in 11% of patients, while infections due to a single germ were reported in 89% of the patients. Table 2 reports the identified germs and their prevalence in our study population.
Table 2.
Identified Germs and Prevalence in Our Study Population (N = 104).
| Type of germ | No. of patients (N = 104) |
% |
|---|---|---|
| Enterococcus faecalis | 57 | 54.98 |
| Escherichia coli | 24 | 22.64 |
| Klebsiella pneumoniae | 10 | 9.7 |
| Citrobacter | 10 | 9.7 |
| Morganella morganii | 3 | 3.23 |
| Proteus mirabilis | 3 | 3.23 |
| Pseudomonas aeruginosa | 3 | 3.23 |
After the treatment was performed, a complete eradication with negative semen culture and microbiological analysis on prostatic secretion was observed in 64 of 84 patients (76.2%), while only 10 of 20 patients receiving antibiotics alone (50%; p < .05) reported negative microbiological analysis (Figure 1).
Figure 1.
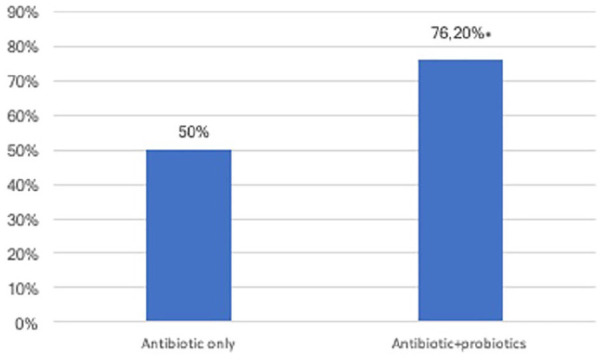
Eradication Rate in Patients Treated With Antibiotics and With Antibiotics Associated With Probiotics (*p < .005).
Persistent infections have been observed only in patients with infections due to Enterococcus faecalis and Escherichia coli. We therefore retrospectively analyzed these populations of patients in the two groups.
Despite the eradication rate was higher in patients with infections due to E. faecalis and E. coli treated with probiotics than in patients treated with antibiotics alone, the difference was not statistically different.
In fact, 47 patients with MAGI due to E. faecalis received the association therapy, while 10 patients received antibiotics alone. After the treatment, negative microbiological examinations have been demonstrated in 34 of 47 (72.3%) patients treated with the association therapy, while only in 5 of 10 (50%) patients who received antibiotic treatment alone.
A total of 20 patients with MAGI due to E. coli received the association therapy, while four patients received antibiotics alone. After the treatment, negative microbiological examinations have been demonstrated in 13 of 20 (65%) patients treated with the association therapy, while only 2 of 4 (50%) patients received antibiotic treatment alone.
Discussion
Increasing interest has been directed toward the study of the human microbiome, defined as the ecological community of commensal, symbiotic, and pathogenic microorganisms and their genetic content inhabiting the human body (Ochoa-Repáraz & Kasper, 2018). While our own human genome contains approximately 20,000 protein-encoding genes, it has been estimated that the sheer number of microbiota living on and inside of us is at least 10 times the number of somatic and germ cells in our bodies (Turnbaugh et al., 2007). As we are beginning to understand the role of the microbiome in healthy humans, it is becoming increasingly clear that there exists interplay and symbiotic relationships between our bodies and these microorganisms, the most abundant of which can be found in the gut. Deviations from the “normal” human gut microbiome have been discovered in a variety of diseases and conditions, including inflammatory bowel disease, colorectal cancer, obesity/metabolic syndrome, type 2 diabetes mellitus, breast cancer, autoimmune disease, autism spectrum disorder, post-traumatic stress disorder, and responsiveness to visceral pain (Li et al., 2016).
Dysbiosis of the microbiome within a particular anatomic site may not solely affect that site. This imbalance may cause expression of proinflammatory cytokines that spread via the circulatory system to distant sites in the body, leading to systemic inflammation (Forbes et al., 2016; Porter et al., 2018). The gastrointestinal microbiome plays an important role in the immune system, and dysbiosis of the gastrointestinal microbiota can lead to systemic inflammation (Tilg & Kaser, 2011). Namely, the gastrointestinal microbiome composition is significantly different in men with symptoms of prostatitis from those without (Arora et al., 2017). In a comprehensive approach to evaluating changes in the gut microbiome in men with prostatitis, Shoskes et al. demonstrated several individual bacterial taxa over-represented and under-represented in the gut of patients with prostatitis versus controls. They observed an increase in alphaprotobacteria in the gut microbiome in patients with prostatitis. The most significant species underrepresented was Prevotella (genus), known to colonize the gastrointestinal tract and suspected to play a role in mitigating inflammation, as compared to controls (Shoskes et al., 2016).
In 2017, Mändar et al. (2017) studied seminal microbiome in patients with prostatitis and demonstrated in seminal plasma an increase in Firmicutes and a reduction in Lactobacilli. Moreover, the increased level of Firmicutes has been previously associated with gut and systemic inflammation (Spychala et al., 2018).
According to these preliminary data, the goal for a complementary probiotic treatment in patients with prostatitis should be to improve the level of Lactobacilli and Prevotella and to reduce the level of Firmicutes. Previous data in mice demonstrated the role of S boulardii in modulating gut microbiota and namely in reducing the percentage of Firmicutes (Dong et al., 2019).
The barrier effect to infection of Lactobacillus species in the female urogenital tract has been demonstrated to contribute to the control of vaginal microbiota (Boris & Barbés, 2000). The regulatory roles attributed to Lactobacillus species in the vaginal microbiota have attracted interest because of potential therapeutic applications (Reid et al., 2003). Lactobacillus inhibits the anaerobes Peptostreptococcus anaerobius and Prevotella bivia in vivo (Skarin & Sylwan, 1986; Strus et al., 2002). In vitro studies have been reported about the ability of human vaginal isolates of Lactobacilli (Lactobacillus acidophilus, Lactobacillus jensenii, Lactobacillus gasseri, and Lactobacillus crispatus) of inhibiting the growth of P. bivia by a multifaceted mechanism, including the production of hydrogen peroxide, lactic acid, and antibacterial compounds, including bacteriocins or bacteriocin-like molecules, nonbacteriocin molecules, and non-lactic acid molecules (Servin, 2004).
Based on these premises, we evaluated whether the administration of a specific probiotics, namely S. boulardii and vaginal Lactobacilli, might be effective in reducing the rate of persistent infections in patients with infective prostatitis. We demonstrated an increase in the eradication rate in patients receiving probiotic treatment versus controls, confirming the effectiveness of this complementary treatment.
Some persistent infections have been observed in the group receiving probiotics only in patients with infections due to E. faecalis and E. coli. Several studies reported that E. faecalis and E. coli are the most frequent pathogens isolated from ejaculates of patients with MAGI. Although a high percentage of persistent infections after antibiotic treatment has been observed (50%), the association of antibiotic treatment with a sequential probiotic treatment significantly reduced the rate of persistent infections (40% for E. faecalis and 25% for E. coli) also in this populations of patients.
Conclusion
Despite the limitations of this study, retrospectively performed on a relatively small sample scale and without randomization of the patients, this preliminary study represents the first approach in using specific probiotic drugs as effective in reducing the rate of persistent infections in patients with MAGI. Moreover, perspective studies are needed to analyze the effect of the associated treatment, not only on the rate of persistent infections but also on clinical and seminal parameters (such as symptoms of prostatitis and seminal markers of inflammation). These results might help in future in orienting clinical choices for an integrated approach in the treatment of patients with male accessory gland infections.
Acknowledgments
The authors thank Esther Mahoney (Cardiff, UK) for her kind and careful English editing.
Footnotes
Author Contributions: D.M.: Conceptualization; D.M., G.G., A.L.A., G.P.: Methodology; G.G. and D.M.: Investigation; G.G.: Data Curation; G.G. and D.M.: Writing—Original Draft Preparation; A.L.A., G.P., and A.P.: Writing—Review & Editing; A.P.: Supervision.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Giuseppe Grande  https://orcid.org/0000-0003-3264-0937
https://orcid.org/0000-0003-3264-0937
References
- Alshahrani S., McGill J., Agarwal A. (2013). Prostatitis and male infertility. Journal of Reproductive Immunology, 100(1), 30–36. 10.1016/j.jri.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Arora H. C., Eng C., Shoskes D. A. (2017). Gut microbiome and chronic prostatitis/chronic pelvic pain syndrome. Annals of Translational Medicine, 5(2), Article 30. 10.21037/atm.2016.12.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia X., García-Losa M., Dal-Ré R. (1997). Ten-language translation and harmonization of the International Prostate Symptom Score: Developing a methodology for multinational clinical trials. European Urology, 31(2), 129–140. 10.1159/000474438 [DOI] [PubMed] [Google Scholar]
- Boris S., Barbés C. (2000). Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes and Infection, 2(5), 543–546. 10.1016/S1286-4579(00)00313-0 [DOI] [PubMed] [Google Scholar]
- Busetto G. M., Giovannone R., Ferro M., Tricarico S., Giudice F., Del Matei D. V., De Cobelli O., Gentile V., De Berardinis E. (2014). Chronic bacterial prostatitis: Efficacy of short-lasting antibiotic therapy with prulifloxacin (Unidrox®) in association with saw palmetto extract, lactobacillus sporogens and arbutin (Lactorepens®) (Vol. 14). 10.1186/1471-2490-14-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J. P., Zheng Y., Wu T., He Q., Teng G. G., Wang H. H. (2019). Protective effect of Saccharomyces boulardii on intestinal mucosal barrier of dextran sodium sulfate-induced colitis in mice. Chinese Medical Journal, 132(16), 1951–1958. 10.1097/CM9.0000000000000364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes J. D., Van Domselaar G., Bernstein C. N. (2016). The gut microbiota in immune-mediated inflammatory diseases. Frontiers in Microbiology, 7, Article 1081. 10.3389/fmicb.2016.01081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande G., Vincenzoni F., Mancini F., Baroni S., Luca G., Calafiore R., Marana R., Castagnola M., Pontecorvi A., Milaradi D. (2018). Semen proteomics reveals the impact of Enterococcus faecalis on male fertility. Protein and Peptide Letters, 25, 472–477. [DOI] [PubMed] [Google Scholar]
- Krause W. (2008). Male accessory gland infection. Andrologia, 40(2), 113–116. 10.1111/j.1439-0272.2007.00822.x [DOI] [PubMed] [Google Scholar]
- La Vignera S., Calogero A. E., Condorelli R. A., Vicari L. O., Catanuso M., D’Agata R., Vicari E. (2012). Ultrasonographic evaluation of patients with male accessory gland infection. Andrologia, 44, 26–31. 10.1111/j.1439-0272.2010.01132.x [DOI] [PubMed] [Google Scholar]
- La Vignera S., Condorelli R. A., Cannarella R., Giacone F., Mongioi L. M., Cimino L., Defeudis G., Mazzilli R., Calogero A. E. (2019). Urogenital infections in patients with diabetes mellitus: Beyond the conventional aspects. International Journal of Immunopathology and Pharmacology, 33, Article 866582. 10.1177/2058738419866582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Vignera S., Condorelli R. A., Vicari E., Tumino D., Morgia G., Favilla V., Cimino S., Calogero A. E. (2013). Markers of semen inflammation: Supplementary semen analysis? Journal of Reproductive Immunology, 100(1), 2–10. 10.1016/j.jri.2013.05.001 [DOI] [PubMed] [Google Scholar]
- La Vignera S., Vicari E., Condorelli R. A., D’Agata R., Calogero A. E. (2011). Male accessory gland infection and sperm parameters (review). International Journal of Andrology, 34(5 Pt. 2), e330–e347. 10.1111/j.1365-2605.2011.01200.x [DOI] [PubMed] [Google Scholar]
- Li D., Wang P., Wang P., Hu X., Chen F. (2016). The gut microbiota: A treasure for human health. Biotechnology Advances, 34(7), 1210–1224. 10.1016/j.biotechadv.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Mändar R., Punab M., Korrovits P., Türk S., Ausmees K., Lapp E., Preem J. K., Oopkaup K., Salumets A., Truu J. (2017). Seminal microbiome in men with and without prostatitis. International Journal of Urology, 24(3), 211–216. 10.1111/iju.13286 [DOI] [PubMed] [Google Scholar]
- Mazzoli S. (2007). Conventional bacteriology in prostatitis patients: Microbiological bias, problems and epidemiology on 1686 microbial isolates. Archivio Italiano di Urologia e Andrologia, 79(2), 71–75. [PubMed] [Google Scholar]
- Milardi D., Grande G., Sacchini D., Astorri A. L., Pompa G., Giampietro A., De Marinis L., Pontecorvi A., Spagnolo A. G., Marana R. (2012). Male fertility and reduction in semen parameters: A single tertiary-care center experience. International Journal of Endocrinology, 2012, Article 649149. 10.1155/2012/649149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milardi D., Luca G., Grande G., Ghezzi M., Caretta N., Brusco G., De Filpo G., Marana R., Pontecorvi A., Calafiore R., Foresta C., Garolla A. (2017). Prednisone treatment in infertile patients with oligozoospermia and accessory gland inflammatory alterations. Andrology, 5(2), 268–273. 10.1111/andr.12300 [DOI] [PubMed] [Google Scholar]
- Naber K. G., Bergman B., Bishop M. C., Bjerklund-Johansen T. E., Botto H., Lobel B., Cruz F. J., Selvaggi F. P. (2001). EAU guidelines for the management of urinary and male genital tract infections: Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). European Urology, 40(5), 576–588. 10.1159/000049840 [DOI] [PubMed] [Google Scholar]
- Ochoa-Repáraz J., Kasper L. H. (2018). The microbiome and neurologic disease: Past and future of a 2-way interaction. Neurotherapeutics, 15(1), 1–4. 10.1007/s13311-018-0604-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. M., Shrestha E., Peiffer L. B., Sfanos K. S. (2018). The microbiome in prostate inflammation and prostate cancer. Prostate Cancer and Prostatic Diseases, 21(3), 345–354. 10.1038/s41391-018-0041-1 [DOI] [PubMed] [Google Scholar]
- Reid G., Jass J., Sebulsky M. T., McCormick J. K. (2003). Potential uses of probiotics in clinical practice. Clinical Microbiology Reviews, 16(4), 658–672. 10.1128/CMR.16.4.658-672.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe P., Comhaire F., Hargreave T., Mellows H. (1993). WHO manual for the standardized investigation and diagnosis of the infertile couple. Press Syndicate of the University of Cambridge. [Google Scholar]
- Rusz A., Pilatz A., Wagenlehner F., Linn T., Diemer T., Schuppe H. C., Lohmeyer J., Hossain H., Weidner W. (2012). Influence of urogenital infections and inflammation on semen quality and male fertility. World Journal of Urology, 30(1), 23–30. 10.1007/s00345-011-0726-8 [DOI] [PubMed] [Google Scholar]
- Servin A. L. (2004). Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiology Reviews, 28(4), 405–440. 10.1016/j.femsre.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Shoskes D. A., Wang H., Polackwich A. S., Tucky B., Altemus J., Eng C. (2016). Analysis of gut microbiome reveals significant differences between men with chronic prostatitis/chronic pelvic pain syndrome and controls. The Journal of Urology, 196(2), 435–441. 10.1016/j.juro.2016.02.2959 [DOI] [PubMed] [Google Scholar]
- Skarin A., Sylwan J. (1986). Vaginal lactobacilli inhibiting growth of Gardnerella vaginalis, Mobiluncus and other bacterial species cultured from vaginal content of women with bacterial vaginosis. Acta Pathologica Microbiologica Scandinavica Series B: Microbiology, B, 94(1–6), 399–403. 10.1111/j.1699-0463.1986.tb03074.x [DOI] [PubMed] [Google Scholar]
- Spychala M. S., Venna V. R., Jandzinski M., Doran S. J., Durgan D. J., Ganesh B. P., Ajami N. J., Putluri N., Graf J., Bryan R. M., McCullough L. D. (2018). Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Annals of Neurology, 84(1), 23–36. 10.1002/ana.25250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatiou K., Magri V., Perletti G., Trinchieri A., Lacroix R., Rekleiti N., Moschouris H. (2019). Prostatic calcifications are associated with a more severe symptom burden in men with type II chronic bacterial prostatitis. Archivio Italiano di Urologia e Andrologia, 91(2), 79–83. 10.4081/aiua.2019.2.79 [DOI] [PubMed] [Google Scholar]
- Strus M., Malinowska M., Heczko P. B. (2002). In vitro antagonistic effect of Lactobacillus on organisms associated with bacterial vaginosis. The Journal of Reproductive Medicine, 47(1), 41–46. [PubMed] [Google Scholar]
- Tilg H., Kaser A. (2011). Gut microbiome, obesity, and metabolic dysfunction. The Journal of Clinical Investigation, 121(6), 2126–2132. 10.1172/JCI58109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremellen K. (2008). Oxidative stress and male infertility: A clinical perspective. Human Reproduction Update, 14(3), 243–258. 10.1093/humupd/dmn004 [DOI] [PubMed] [Google Scholar]
- Turnbaugh P. J., Ley R. E., Hamady M., Fraser-Liggett C. M., Knight R., Gordon J. I. (2007). The human microbiome project. Nature, 449(7164), 804–810. 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari E., Calogero A. E., Condorelli R. A., Vicari L. O., La Vignera S. (2012). Male accessory gland infection frequency in infertile patients with chronic microbial prostatitis and irritable bowel syndrome. International Journal of Andrology, 35(2), 183–189. 10.1111/j.1365-2605.2011.01216.x [DOI] [PubMed] [Google Scholar]
- Vicari E., La Vignera S., Castiglione R., Condorelli R. A., Vicari L. O., Calogero A. E. (2014). Chronic bacterial prostatitis and irritable bowel syndrome: Effectiveness of treatment with rifaximin followed by the probiotic VSL#3. Asian Journal of Andrology, 16(5), 735–739. 10.4103/1008-682X.131064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenlehner F. M. E., Naber K. G. (2003). Antimicrobial treatment of prostatitis. Expert Review of Anti-infective Therapy, 1(2), 275–282. 10.1586/14787210.1.2.275 [DOI] [PubMed] [Google Scholar]


