Abstract
Objective
To identify carbapenem-resistant Enterobacteriaceae (CRE) in patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; COVID-19) and to determine whether they had different risk factors for the acquisition of CRE than patients without COVID-19.
Methods
This retrospective single-centre, case–control study enrolled patients with and without COVID-19. The demographic, clinical, infection, colonization and mortality data were compared between the two groups.
Results
A total of 38 patients with COVID-19 and 26 patients without COVID-19 were enrolled. The majority of isolates detected in COVID-19 patients were Klebsiella spp. Leukopenia at admission (odds ratio [OR] 4.70; 95% confidence interval [CI] 1.37, 16.10), invasive mechanical ventilation (OR 5.74; 95% CI 1.07, 30.63), carbapenem treatment (OR 5.09; 95% CI 1.21, 21.27) and corticosteroid treatment (OR 7.06; 95% CI 1.53, 32.39) were independent risk factors for CRE acquisition in COVID-19 patients. Intensive care unit (ICU) mortality was significantly higher in COVID-19 patients compared with patients without COVID-19 (OR 20.62; 95% CI 5.50, 77.23). Length of ICU stay increased the risk of death in patients with COVID-19 (subdistribution hazard ratio 3.81; 95% CI 1.33, 10.92).
Conclusion
CRE strains were more common in patients with COVID-19 and they had different risks for CRE compared with patients without COVID-19.
Keywords: Carbapenem-resistant Enterobacteriaceae, COVID-19, risk factors
Introduction
The virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first discovered in 2019 in Wuhan, China.1 In Romania, the virus was first detected on 26 February 2020 and in Constanta it was detected on 11 March 2020, the day of the declaration of the SARS-CoV-2 pandemic by the World Health Organization.2
A considerable amount of information is now known about infection with SARS-CoV-2 (COVID-19) and the subsequent acquisition of bacterial or fungal superinfections.3 Superinfections are associated with several risk factors such as lung damage with decreased mucociliary clearance, impaired immunity, decreased lymphocytes, decreased B cells, natural killer cells and cytotoxic helper and T cells counts, with low CD4 and CD8.3,4 Clinical data suggest that a selective decrease in CD8 lymphocytes may be associated with a negative prognosis in patients with SARS-CoV-2 infection.5 A previous study demonstrated that patients with COVID-19 that had low CD8 lymphocyte levels, associated with lymphopenia and obesity, had a worse prognosis.5
Morbidity and mortality have been increased in patients with SARS-CoV-2 infection compared with non-COVID-19 patients, with an increased number of patients being admitted to the intensive care unit (ICU).6 In 2017, more than 13 000 hospitalized patients in the US had carbapenem-resistant Enterobacteriaceae (CRE), which resulted in 1100 deaths and healthcare costs of approximately $130 million.7 The mortality rate is higher than 50% for patients infected with carbapenemase-producing Enterobacterales (CP-CRE).8 As a result of which CRE/CP-CRE have been designated an ‘Urgent Threat’, which is the highest level of risk according to Veterans Health Administration disseminated guideline.8
The aim of this current study was to identify the strains of CRE in the Clinical Infectious Diseases Hospital, Constanța, Romania and to identify the risk factors associated with acquiring these strains of CRE.
Patients and methods
Study design
This retrospective single-centre, case–control study enrolled consecutive patients with CRE strains that were hospitalized in the ICU, Clinical Infectious Diseases Hospital, Constanța, Romania between September 2017 and September 2021. The patients were enrolled in two phases to form two groups: before the COVID-19 pandemic and during the COVID-19 pandemic. The inclusion criterion was as follows: (i) patients hospitalized in the ICU where CRE bacterial strains were detected from September 2017 to September 2019. The exclusion criteria were as follows: (i) admission to the ICU < 24 h; (ii) all positive tests with the same strains of CRE identified in the same patient (iii) patients that were not hospitalized in ICU.
The Clinical Infectious Diseases Hospital has 10 ICU beds. During the COVID-19 pandemic, the number of beds in the ICU was not supplemented, although the occupancy rate was very high, with numerous transfers from other hospitals. Regarding bacterial colonization, there were five strains of CP-CRE colonization detected by using Rosco discs. These microbiological data were selectively performed starting with 2020, in bacteriologically screened patients. Until 2020, for bacterial colonization detected with CP-CRE, the Modified Hodge test was performed as a confirmation method. In patients with CRE infection, 14 strains of CRE were shown to produce carbapenemase by using Rosco discs.
Ethical review and approval were waived for this study by the Ethics Committee of the Clinical Infectious Diseases Hospital, Constanța, Romania because of the retrospective design of the study. The study was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided their written informed consent for the use of their personal data at admission into the hospital. The anonymity of the patients was guaranteed during the whole process of data analysis and results reporting. The reporting of this study conforms to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.9
CRE isolates: definition and microbiology
The CRE cultures that were positive for Klebsiella spp. and Escherichia coli were found in sputum, urine, blood samples and rectum swabs. In the ICU ward, bacteriological screening using rectal swabs was performed for the detection of extended-spectrum β-lactamases, vancomycin-resistant Enterococcus and CRE. Bacteriological screening of nasal exudate and pharyngeal exudate was performed for the detection of methicillin-resistant Staphylococcus aureus. These bacteriological screenings were performed upon admission and at 7 days after hospitalization according to the national protocol.
According to clinical and laboratory data, CRE infection was detected in the lungs, urinary tract and blood; and CRE colonization was detected in the rectum and urinary tract. CRE from rectal swab samples were detected using carbapenem-resistant Enterobacteriaceae chromogenic media (CHROMID® CARBA SMART Agar; bioMérieux SA, Marcy-l'Étoile, France). Isolates from sputum, urine culture and blood culture were identified using VITEK® 2 (bioMérieux SA) and MALDI-TOF mass spectrometry (MALDI TOF Autof MS 1000; Autobio Diagnostics, Zhengzhou, China). Rosco discs (Rosco kits; Rosco Diagnostica, Albertslund, Denmark) confirmed carbapenemase production.
Antibiogram was not performed for isolates detected by rectal swab screening, but only for isolates detected in the urinary tract, sputum and blood. The antibiogram was interpreted according to the European Committee for Antimicrobial Sensitivity Testing guidelines10 and the susceptibility to antibiotics was tested by standard microdilution. The diagnosis of SARS-CoV-2 infection was confirmed following a positive result from qualitative real-time SARS-CoV-2 reverse transcriptase polymerase chain reaction with CFX96 Dx Real-Time PCR Detection Systems for In Vitro Diagnostics (Bio-Rad, Hercules, CA, USA).
The decision to interpret a CRE result as an infection or colonization was made by the infectious diseases specialist after analysing the patient's clinical and paraclinical data. In isolates that were determined to be bacterial colonization, patients showed no clinical signs of infection and serum inflammatory parameters were within normal limits. Patients with CRE infection showed clinical symptoms suggestive of an infection, and paraclinical data such as erythrocyte sedimentation rate, C-reactive protein (CRP) and fibrinogen were increased. The definitions of infection were based on the US Centers for Disease Control and Prevention criteria.11
Variables used for risk factor analysis
The variables that were suspected to be risk factors for CRE in the ICU were numerous. Patient data were taken from the hospital's computer system. From the demographic, epidemiological and clinical data, the year of admission to the ICU, sex, age, days of hospitalization, previous hospitalization, previous antibiotic therapy, Charlson Comorbidity Index (CCI) and the use of invasive mechanical ventilation were considered potential risk factors.
Regarding paraclinical data, leukopenia at admission and CRP >5 mg/l at admission were suspected to be risk factors. Regarding treatment, the predictive risk factors that were investigated were as follows: (i) anti-inflammatory treatment with corticosteroids (dexamethasone or methylprednisolone); (ii) antibiotic treatment with ceftazidime-avibactam, carbapenems, cephalosporins, metronidazole, fluoroquinolones, piperacillin-tazobactam, vancomycin, amikacin or treatment with a reserve antibiotic.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). All patient data were collected from the hospital’s computer system and imported in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). The study was undertaken in two phased to generate two groups of patients: cases (COVID-19 positive patients with CRE strains) and controls (non-COVID-19 patients with CRE strains). Univariate analysis was performed using χ2-test, followed by multivariate analysis with binary logistic regression or time-varying covariate. All tests were two-tailed. A P-value ≤0.05 was considered statistically significant.
Results
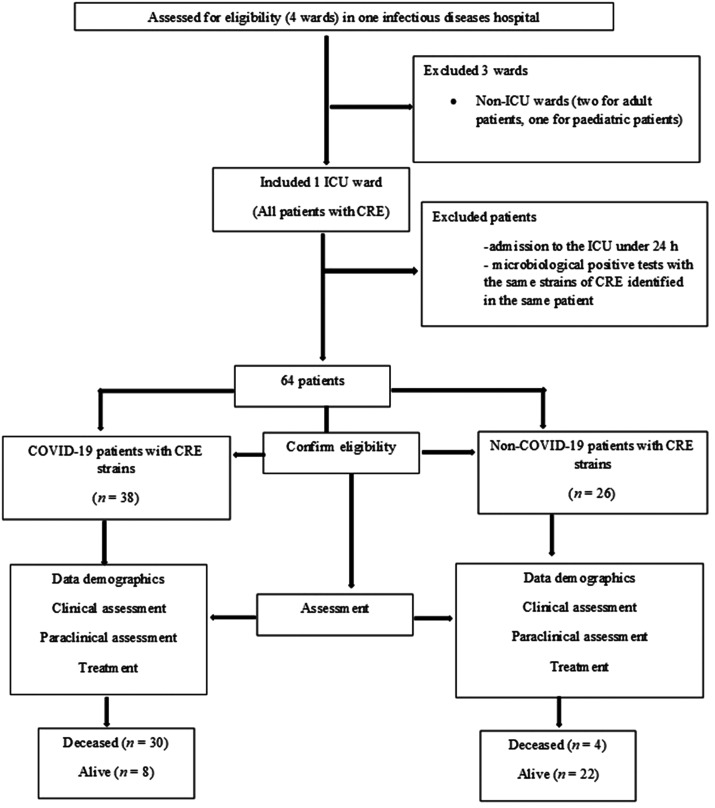
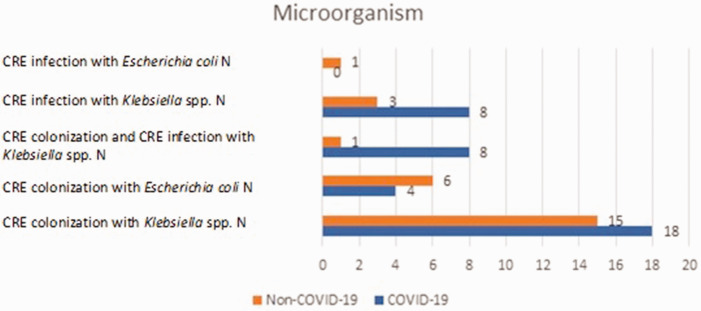
This retrospective single-centre, case–control study enrolled 64 patients with CRE strains: 38 (59.38%) patients with SARS-CoV-2 COVID-19 infection and 26 (40.63%) patients without SARS-CoV-2 COVID-19 infection (Figure 1). Figure 2 presents the data on the microorganisms detected in patients with CRE strains in the ICU. Of the 38 patients with COVID-19, 18 (47.37%) patients were colonized with Klebsiella spp. and four (10.53%) patients with E. coli. Of the 38 patients with COVID-19, eight (21.05%) patients were infected with Klebsiella spp. and eight (21.05%) patients had colonization and infection with Klebsiella spp. None of the patients with COVID-19 had an E. coli infection. Of the 26 patients without COVID-19, 15 (57.69%) were colonized with Klebsiella spp., six (23.08%) were colonized with E. coli, 1 (3.84%) with Klebsiella spp. colonization and infection, 3 (11.53%) with Klebsiella spp. Infection and one (3.85%) had an E. coli infection. Univariate analysis demonstrated that there was no significant association between the microorganisms identified in COVID-19 patients and non-COVID-19 patients (Table 1). CRE colonization or CRE infection did not have a significantly increased prevalence in patients with COVID-19. There were eight patients from the group of patients with COVID-19 that also had colonization and infection with CRE.
Figure 1.
Flow chart showing the number of patients, inclusion criteria, exclusion criteria and data analysis in a retrospective case–control study that aimed to identify the strains of carbapenem-resistant Enterobacteriaceae (CRE) and the risk factors associated with acquiring these infections in the Clinical Infectious Diseases Hospital, Constanța, Romania. ICU, intensive care unit; COVID-19, SARS-CoV-2.
Figure 2.
Microorganisms identified in patients with carbapenem-resistant Enterobacteriaceae (CRE) with or without concomitant SARS-CoV-2 (COVID-19) that were included in a retrospective case–control study that aimed to identify the strains of CRE and the risk factors associated with acquiring these infections. The colour version of this figure is available at: http://imr.sagepub.com.
Table 1.
Univariate analysis of the strains of carbapenem-resistant Enterobacteriaceae (CRE) in patients with or without concomitant SARS-CoV-2 (COVID-19) that were included in a retrospective case–control study that aimed to identify the strains of CRE and the risk factors associated with acquiring these infections.
| Isolates with CRE strains | Patients with COVID-19n = 38 | Patients without COVID-19n = 26 |
|---|---|---|
| CRE colonization | 30 (78.95) | 22 (84.62) |
| CRE infection | 16 (42.11) | 5 (19.23) |
Data presented as n of patients (%).
No significant between-group differences (P > 0.05); χ2-test.
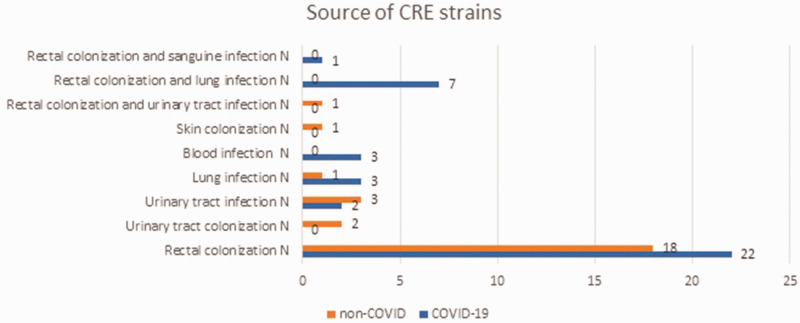
In the 38 COVID-19 patients, 22 (57.89%) had rectal colonizations, two (5.26%) had urinary tract infections, three (7.89%) had lung infections and three (7.89%) had blood infections (Figure 3). Associations between colonization and infection were found in patients with COVID-19. For example, rectal colonization and lung infection were detected in seven (18.42%) of 38 patients and rectal colonization and a blood infection were detected in one patient (2.63%). No urinary colonization was detected in COVID-19 patients. patients. In the 26 patients without COVID-19, there were 18 (69.23%) patients had rectal colonization, two (7.69%) had urinary tract colonization, three (11.54%) had a urinary tract infection, one (3.85%) had a lung infection, one (3.85%) had skin colonization and one (3.85%) had rectal colonization and urinary tract infection.
Figure 3.
Source of carbapenem-resistant Enterobacteriaceae (CRE) stains in patients with or without concomitant SARS-CoV-2 (COVID-19) that were included in a retrospective case–control study that aimed to identify the strains of CRE and the risk factors associated with acquiring these infections. The colour version of this figure is available at: http://imr.sagepub.com.
With regard to year of hospital admission, in 2020 there were four of 38 (10.53%) patients with COVID-19 and in 2021 there were 34 (89.47%) patients with COVID-19 (Table 2). Univariate analysis demonstrated that 2021 was the predominant year for COVID-19 patients (odds ratio [OR] 28.33; 95% confidence interval [CI] 7.12, 112.67; P = 0.0001).
Table 2.
Univariate analysis of the year of hospital admission in patients with or without concomitant SARS-CoV-2 (COVID-19) that were included in a retrospective case–control study that aimed to identify the strains of carbapenem-resistant Enterobacteriaceae and the risk factors associated with acquiring these infections.
| Year of admission | Patients with COVID-19n = 38 | Patients without COVID-19n = 26 | Statistical analysisa | Odds ratio | 95% confidence interval |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| 2017 | 0 (0.00) | 2 (7.69) | – | – | – | – |
| 2018 | 0 (0.00) | 7 (26.92) | – | – | – | – |
| 2019 | 0 (0.00) | 11 (42.31) | – | – | – | – |
| 2020 | 4 (10.53) | 0 (0.00) | NS | – | – | – |
| 2021 | 34 (89.47) | 6 (23.08) | P = 0.0001 | 28.33 | 7.12 | 112.67 |
Data presented as n of patients (%).
aχ2-test; NS, no significant between-group difference (P > 0.05).
The demographic and clinical characteristics of the two patient groups are shown in Table 3. The mean age of the COVID-19 patients was 64.86 years. The majority of patients (78.95%; 30 of 38 patients) with COVID-19 were males. The median CCI score of the patients with COVID-19 was 3.23; with 35 patients (92.11%) having a CCI score of 1–4 points and 18 patients (47.36%) having a CCI score >4. Leukopenia at admission and invasive mechanical ventilation were shown by univariate analysis to significant risk factors for CRE in COVID-19 patients (P ≤ 0.05 for both comparisons). Female sex, CCI score 1–4 points, CCI score > 4, previous antibiotic therapy, CRP > 5 mg/l at admission and length of stay (LOS) in ICU > 3 days were not associated with CRE in patients with COVID-19.
Table 3.
Univariate analysis of the demographic and clinical characteristics of patients with or without concomitant SARS-CoV-2 (COVID-19) that were included in a retrospective case–control study that aimed to identify the strains of carbapenem-resistant Enterobacteriaceae and the risk factors associated with acquiring these infections.
| Characteristic | Patients with COVID-19n = 38 | Patients without COVID-19n = 26 | Statistical analysisa |
|---|---|---|---|
| Sex, male | 30 (78.95) | 15 (57.69) | NS |
| Mean age, years | 64.86 | 64.15 | NS |
| Median Charlson Comorbidity Index score | 3.23 | 3.77 | – |
| Charlson Comorbidity Index score 1–4 | 35 (92.11) | 22 (84.62) | NS |
| Charlson Comorbidity Index score >4 | 18 (47.37) | 16 (61.54) | NS |
| Previous hospitalization | 6 (15.79) | 14 (53.85) | P < 0.001 |
| Previous antibiotic therapy | 24 (63.16) | 12 (46.15) | NS |
| Leukopenia at admission | 32 (84.21) | 14 (53.85) | P = 0.008 |
| C-reactive protein >5 mg/l at admission | 35 (92.11) | 24 (92.31) | NS |
| Invasive mechanical ventilation | 12 (31.58) | 2 (7.69) | P = 0.023 |
| Median length of stay in ICU, days | 10.40 | 9.46 | – |
| Length of stay in ICU > 3 days | 34 (89.47) | 19 (73.08) | NS |
Data presented as n of patients (%), mean or median.
aχ2-test; NS, no significant between-group difference (P > 0.05).
ICU, intensive care unit.
Univariate analysis identified treatment with reserve antibiotics, carbapenem, quinolones, linezolid and corticosteroids as significant risk factors for CRE in COVID-19 patients (P ≤ 0.05 for all comparisons) (Table 4). Other antibiotics such as ceftazidime-avibactam, colistin, cephalosporins, piperacillin-tazobactam, aminoglycosides, doxycycline and azithromycin were not significant risk factors for patients with COVID-19 and CRE. Previous hospitalization, metronidazole and vancomycin treatment were significantly more frequent in patients without COVID-19 compared with patients with COVID-19 (P ≤ 0.05 for all comparisons).
Table 4.
Univariate analysis of the treatments used in patients with or without concomitant SARS-CoV-2 (COVID-19) that were included in a retrospective case–control study that aimed to identify the strains of carbapenem-resistant Enterobacteriaceae and the risk factors associated with acquiring these infections.
| Patients’ treatment | Patients with COVID-19n = 38 | Patients without COVID-19n = 26 | Statistical analysisa |
|---|---|---|---|
| Treatment with reserve antibiotics | 29 (76.32) | 13 (50.00) | P = 0.029 |
| Ceftazidime-avibactam | 4 (10.53) | 2 (7.69) | NS |
| Colistin | 4 (10.53) | 3 (11.54) | NS |
| Carbapenem | 21 (55.26) | 4 (15.38) | P = 0.001 |
| Cephalosporins | 10 (26.32) | 4 (15.38) | NS |
| Piperacillin-tazobactam | 1 (2.63) | 1 (3.85) | NS |
| Quinolones | 16 (42.11) | 3 (11.54) | P = 0.009 |
| Linezolid | 9 (23.68) | 1 (3.85) | P = 0.032 |
| Vancomycin | 1 (2.63) | 6 (23.08) | P = 0.010 |
| Metronidazole | 1 (2.63) | 8 (30.77) | P = 0.001 |
| Aminoglycosides | 5 (13.16) | 5 (19.23) | NS |
| Doxycycline | 5 (13.16) | 1 (3.85) | NS |
| Azithromycin | 1 (2.63) | 0 (0.00) | NS |
| Corticosteroids | 32 (84.21) | 11 (42.31) | P < 0.001 |
Data presented as n of patients (%).
aχ2-test; NS, no significant between-group difference (P > 0.05).
Multivariate analysis demonstrated that leukopenia at admission (OR 4.70; 95% CI 1.37, 16.10; P = 0.014) and invasive mechanical ventilation (OR 5.74; 95% CI 1.07, 30.63; P = 0.041) were independent risk factors for CRE acquisition in COVID-19 patients (Table 5).
Table 5.
Multivariate analysis of risk factors for carbapenem-resistant Enterobacteriaceae in patients with or without concomitant SARS-CoV-2 (COVID-19) that were included in a retrospective case–control study that aimed to identify the strains of carbapenem-resistant Enterobacteriaceae and the risk factors associated with acquiring these infections.
| Risk factors | Statistical analysisa | Odds ratio | 95% confidence interval |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Leukopenia at admission | P = 0.014 | 4.70 | 1.37 | 16.10 |
| Invasive mechanical ventilation | P = 0.041 | 5.74 | 1.07 | 30.63 |
aBinary logistic regression analysis.
Multivariate analysis demonstrated that carbapenem treatment (OR 5.09; 95% CI 1.21, 21.27; P = 0.026) and corticosteroid treatment (OR 7.06; 95% CI 1.53, 32.39; P = 0.012) were independent risk factors for CRE acquisition in COVID-19 patients (Table 6). Treatment with reserve antibiotics, quinolones and linezolid were not significantly associated with CRE acquisition in COVID-19 patients.
Table 6.
Multivariate analysis of treatment-related risk factors for carbapenem-resistant Enterobacteriaceae in patients with or without concomitant SARS-CoV-2 (COVID-19) that were included in a retrospective case–control study that aimed to identify the strains of carbapenem-resistant Enterobacteriaceae and the risk factors associated with acquiring these infections.
| Risk factors | Statistical analysisa | Odds ratio | 95% confidence interval |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Reserve antibiotics | NS | 0.54 | 0.11 | 2.56 |
| Carbapenem treatment | P = 0.026 | 5.09 | 1.21 | 21.27 |
| Quinolone treatment | NS | 5.67 | 1.00 | 35.32 |
| Linezolid treatment | NS | 1.79 | 0.15 | 21.41 |
| Corticosteroid treatment | P = 0.012 | 7.06 | 1.53 | 32.39 |
aBinary logistic regression analysis; NS, no significant association (P > 0.05).
According to the Ambler classification, 16 of 64 (25.00%) patients had CP-CRE (Table 7). Of the 64 patients, 11 (17.19%) had CP-CRE infection, two (3.13%) had CP-CRE colonization and three (4.69%) had CP-CRE infection and colonization. The results of the tests were confirmed by using Rosco discs. Of the 38 COVID-19 patients, nine (23.68%) had CP-CRE infection, two (5.26%) had CP-CRE colonization and three (7.89%) had CP-CRE infection and colonization. In the COVID-19 patients, there were four (10.53%) bacterial strains producing Klebsiella pneumoniae carbapenemase (KPC), two (5.26%) producing oxacillinase-48 (OXA-48), one (2.63%) metallo-β-lactamase (MBL), five (13.16%) producing KPC/OXA-48 and two (5.26%) producing KPC/MBL. Univariate analysis demonstrated that CP-CRE isolates were significantly more frequent in patients with COVID-19 compared with patients without COVID-19 (P = 0.023).
Table 7.
Mechanism of carbapenem resistance in bacterial strains of carbapenemase-producing Enterobacterales (CP-CRE) in patients with or without concomitant SARS-CoV-2 (COVID-19) that were included in a retrospective case–control study that aimed to identify the strains of carbapenem-resistant Enterobacteriaceae and the risk factors associated with acquiring these infections.
| Mechanism of resistance of CP-CRE | Patients with COVID-19n = 38 | Patients without COVID-19n = 26 | Statistical analysisa |
|---|---|---|---|
| Total CP-CRE | 14 (36.84) | 2 (7.69) | P = 0.023 |
| CP-CRE (KPC) | 4 (10.53) | 0 (0.00) | – |
| CP-CRE (OXA-48) | 2 (5.26) | 0 (0.00) | – |
| CP-CRE (MBL) | 1 (2.63) | 2 (7.69) | – |
| CP-CRE (KPC/OXA-48) | 5 (13.16) | 0 (0.00) | – |
| CP-CRE (KPC/MBL) | 2 (5.26) | 0 (0.00) | – |
Data presented as n of patients (%).
aχ2-test.
KPC, Klebsiella pneumoniae carbapenemase; OXA-48, oxacillinase-48; MBL, metallo-β-lactamase.
Regarding antimicrobial resistance, all 16 CRE strains in patients with COVID-19 were resistant to carbepenem and fluoroquinolone (Table 8); and 14 (87.5%) of 16 strains were resistant to aminoglycosides and one (50.0%) of one strain was resistant to colistin. One (100.0%) of one strain was resistant to tigecycline and one (100.0%) of one strain was sensitive to imipenem-cilastatin-relebactam, ceftazidime-avibactam and ceftolozane-tazobactam.
Table 8.
Antimicrobial resistance of the strains of carbapenem-resistant Enterobacteriaceae (CRE) in patients with concomitant SARS-CoV-2 (COVID-19) that were included in a retrospective case–control study that aimed to identify the strains of CRE and the risk factors associated with acquiring these infections.
| Antibiotics | Antibiotic resistance tests in patients with COVID-19 and infection with CREn = 16 | Number of resistant bacteria |
|---|---|---|
| Carbapenem | 16 (100.00) | 16 (100.00) |
| Fluoroquinolone | 16 (100.00) | 16 (100.00) |
| Aminoglycoside | 16 (100.00) | 14 (87.50) |
| Colistin | 2 (12.50) | 1 (50.00) |
| Tigecycline | 1 (6.25) | 1 (100.00) |
| Imipenem-cilastatin-relebactam | 1 (6.25) | 0 (0.00) |
| Ceftazidim-avibactam | 1 (6.25) | 0 (0.00) |
| Ceftolozane-tazobactam | 1 (6.25) | 0 (0.00) |
Data presented as n of patients (%).
Regarding patient mortality, 30 (78.95%) of 38 patients with COVID-19 and four (15.38%) of 26 patients without COVID-19 died whilst admitted to the ICU. Multivariate analysis performed using binary logistic regression demonstrated that COVID-19 patients with CRE had an increased risk of death compared with patients without COVID-19 (OR 20.62; 95% CI 5.50, 77.23; P ≤ 0.05). Other risk factors that might increase mortality were investigated using multivariate Cox time-dependent analysis. LOS in ICU in the patients with CRE and concomitant COVID-19 was associated with a significant increase in the subdistribution hazard ratio for ICU mortality (subdistribution hazard ratio 3.81; 95% CI 1.33, 10.92; P = 0.012) (Table 9).
Table 9.
Cox time-dependent analysis of the association between mortality and length of stay (LOS) in the intensive care unit (ICU) in patients with carbapenem-resistant Enterobacteriaceae (CRE) and concomitant SARS-CoV-2 (COVID-19) that were included in a retrospective case–control study that aimed to identify the strains of CRE and the risk factors associated with acquiring these infections.
| Risk factor | Statistical analysisa | SHRb | 95% confidence interval |
|
|---|---|---|---|---|
| Lower | Upper | |||
| LOS in ICU | P = 0.012 | 3.81 | 1.33 | 10.92 |
aCox time-dependent analysis.
bSHR, subdistribution hazard ratio.
Discussion
Previous studies have reported CP-CRE strains in patients with SARS-CoV-2 infection, especially in ICUs.3,6,12 Possible factors associated with in-hospital transmission of CP-CRE infections in the ICU were the rapid increase in hospital admissions, insufficient amount of protective equipment, overwork and the lack of medical staff.5 Other studies suggest that among the factors that increase the risk of CP-CRE infection are hospitalization with a severe illness, prolonged hospitalization, the presence of invasive medical devices, antibiotic treatment, old age and severe comorbidities.13–15 The main target of primary prophylaxis is the prevention of CP-CRE infections, because resistance genes can spread to other bacteria of the same species or to other species with serious consequences.7 Experts in the US and Europe recommend the implementation of infection control programmes, with well-defined structures and procedures, and a continuous evaluation of the measures implemented to prevent the spread of carbapenemase-producing Enterobacterales (CPE) in acute care units.16,17
In this current case–control study, the predominant Enterobacteriaceae included Klebsiella spp. The growth of Enterobacteriaceae such as Klebsiella spp. is also reported by other studies.3,18–20 In this current study, the prevalence of CRE was higher in COVID-19 patients, a fact also found in a study undertaken in a New York City hospital.18 The independent risk factors demonstrated in this current study were also found in other studies. For example, a previous study found that risk factors such as mechanical ventilation and antimicrobial use were more common in COVID-19 patients.3 In a study undertaken in a Korean ICU, multivariate analysis found that pneumonia/chronic pulmonary disease, previous use of fluoroquinolone and previous use of nasogastric tube were risk factors for CPE infection or colonization.19 A study undertaken in Vietnamese hospitals reported that the risk factors for colonization with CRE were carbapenem treatment and long hospitalization.20 The same study also reported that patients colonized with CRE had an increased risk of healthcare-acquired infection and death.20 A meta-analysis reported that the risk factors for carbapenem-resistant Klebsiella pneumoniae infection were longer LOS, ICU admission, previous antibiotic use and exposure to carbapenems.21 Regarding infection control measures, a previous study in an ICU found that misuse of gloves and the absence of changing personal protective equipment in the context of COVID-19 contributed to cross-transmission.22 Another study demonstrated that patients with COVID-19 admitted to an ICU were more susceptible to multidrug-resistant microorganism colonization compared with other patients hospitalized in these units because of their special characteristics and the fact that most of them require long hospital stays.23
Consumption of antibiotics during COVID-19 meant that the consumption of penicillin with β-lactamase inhibitors and carbapenems in the ICU ward increased during the pandemic and the prevalence of CRE isolated from clinical trials in the ICU increased significantly.24 The COVID-19 pandemic is associated with increased antibiotic use and has influenced the prevalence of infections caused by multidrug-resistant isolates.24
A previous observational cohort study demonstrated that colonized patients had a 1.79-times higher risk of dying in the ICU.25 Regarding the mortality rate in the patients with CRE and COVID-19 in the current study, the risk of death was higher compared with patients with CRE strains without COVID-19 (OR 20.62; 95% CI 5.50, 77.23; P ≤ 0.05). In addition, the length of hospitalization in the ICU increased the risk of death in patients with COVID-19 (subdistribution hazard ratio 3.81; 95% CI 1.33, 10.92; P = 0.012). Active epidemiological surveillance is needed to limit CPE, the most important measures being active surveillance cultures, the distribution of dedicated medical staff and the isolation of CPE carriers.26
This current study had several limitations. First, the number of patients was small because there were only 10 beds in the ICU and few patients with CRE were detected. However, all patients in the ICU were screened according to the national screening protocol and associated symptoms, so all patients that had CRE were detected. Another limitation of this study is that the study does not describe the infection control measures and the risks associated with non-compliance with protection measures. The study was carried out on patients in the ICU from September 2017, the year of the opening of the ICU ward, until its closure on 1 October 2021, due to a fire disaster.
In conclusion, this current study demonstrated that leukopenia at admission, invasive mechanical ventilation and treatment with carbapenems or corticosteroids were independent risk factors of CRE in COVID-19 patients. The risk of ICU mortality was 3.81-times higher in COVID-19 patients than in patients without COVID-19. Knowing the risk factors and eliminating them might improve the prognosis of patients with COVID-19 and thus reduce their risk of death.
Footnotes
Author contributions: Conceptualization: N.D.V.; methodology: A.D.; formal analysis: S.V., I.M.D and N.D.V.; investigation: R.C.C., S.R. and C.N.; resources: S.C.; data curation: R.M.; writing original draft: N.D.V., R.C.C. and I.M.D.; writing review and editing: N.D.V.; supervision: R.C.C., S.V. and I.M.D. All authors have read and agreed to the published version of the manuscript.
The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Nicoleta-Dorina Vlad https://orcid.org/0000-0003-1230-6819
References
- 1.Sharma A, Tiwari S, Deb MK, et al. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents 2020; 56:106054. doi: 10.1016/j.ijantimicag.2020.106054. Epub 2020 Jun 10. PMID: 32534188; PMCID: PMC7286265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Europe. Emergencies. Coronavirus disease (COVID-19) pandemic, https://www.who.int/europe/emergencies/situations/covid-19 (accessed 20 June 2022).
- 3.Pintado V, Ruiz-Garbajosa P, Escudero-Sanchez R, et al. Carbapenemase-producing Enterobacterales infections in COVID-19 patients. Infect Dis (Lond) 2022; 54: 36–45. doi: 10.1080/23744235.2021.1963471. Epub 2021 Aug 12. PMID: 34382910; PMCID: PMC8425444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nestler M, Godbout E, Lee K, et al. Fungal superinfection in patients with COVID-19: Role of antifungal stewardship? Am J Infect Control 2021; 49: 279–280. doi: 10.1016/j.ajic.2020.11.015. Epub 2020 Nov 19. PMID: 33220338; PMCID: PMC7674015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urra JM, Cabrera CM, Porras L, et al. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin Immunol 2020; 217: 108486. doi: 10.1016/j.clim.2020.108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farfour E, Lecuru M, Dortet L, et al. Carbapenemase-producing Enterobacterales outbreak: Another dark side of COVID-19. Am J Infect Control 2020; 48: 1533–1536. doi: 10.1016/j.ajic.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2022–2020 data. Copenhagen: WHO Regional Office for Europe, chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.ecdc.europa.eu/sites/default/files/documents/ECDC-WHO-AMR-report.pdf (2022, accessed 15 March 2022). [Google Scholar]
- 8.Goedken CC, Guihan M, Brown CR, et al. Evaluation of carbapenem-resistant Enterobacteriaceae (CRE) guideline implementation in the Veterans Affairs Medical Centers using the consolidated framework for implementation research. Implement Sci Commun 2021; 2: 69. doi: 10.1186/s43058-021-00170-5. PMID: 34187592; PMCID: PMC8243642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 10.European Committee on Antimicrobial Susceptibility Testing (EUCAST). Expert rules and expected phenotypes, https://www.eucast.org/expert_rules_and_expected_phenotypes (accessed 15 June 2022).
- 11.Centers for Disease Control and Prevention. The CDC Prevention Guidelines Database, https://wonder.cdc.gov/wonder/prevguid/prevguid.html (accessed 20 June 2022).
- 12.Dumitru IM, Dumitrascu M, Vlad ND, et al. Carbapenem-Resistant Klebsiella pneumoniae Associated with COVID-19. Antibiotics (Basel) 2021; 10: 561. doi: 10.3390/antibiotics10050561. PMID: 34065029; PMCID: PMC8151469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thuy DB, Campbell J, Nhat LTH, et al. Hospital-acquired colonization and infections in a Vietnamese intensive care unit. PLoS One 2018; 13: e0203600. doi: 10.1371/journal.pone.0203600. PMID: 30192894; PMCID: PMC6128614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariappan S, Sekar U, Kamalanathan A. Carbapenemase-producing Enterobacteriaceae: Risk factors for infection and impact of resistance on outcomes. Int J Appl Basic Med Res 2017; 7: 32–39. doi: 10.4103/2229-516X.198520. PMID: 28251105; PMCID: PMC5327604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segagni Lusignani L, Presterl E, Zatorska B, et al. Infection control and risk factors for acquisition of carbapenemase-producing enterobacteriaceae. A 5 year (2011-2016) case-control study. Antimicrob Resist Infect Control 2020; 9: 18. Published 2020 Jan 17. doi:10.1186/s13756-019-0668-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Implementation manual to prevent and control the spread of carbapenem-resistant organisms at the national and health care facility level: interim practical manual supporting implementation of the Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities, https://apps.who.int/iris/bitstream/handle/10665/312226/WHO-UHC-SDS-2019.6-eng.pdf?sequence=1&isAllowed=y (accessed 22 June 2022).
- 17.Centers for Disease Control and Prevention. Carbapenem-resistant Enterobacterales (CRE), https://www.cdc.gov/infectioncontrol/index.html (accessed 22 June 2022).
- 18.Gomez-Simmonds A, Annavajhala MK, McConville TH, et al. Carbapenemase-producing Enterobacterales causing secondary infections during the COVID-19 crisis at a New York City hospital. J Antimicrob Chemother 2021; 76: 380–384. doi: 10.1093/jac/dkaa466. PMID: 33202023; PMCID: PMC7717307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YA, Lee SJ, Park YS, et al. Risk Factors for Carbapenemase-Producing Enterobacterales Infection or Colonization in a Korean Intensive Care Unit: A Case-Control Study. Antibiotics (Basel) 2020; 9: 680. Published 2020 Oct 8. doi:10.3390/antibiotics9100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran DM, Larsson M, Olson L, et al. High prevalence of colonisation with carbapenem-resistant Enterobacteriaceae among patients admitted to Vietnamese hospitals: Risk factors and burden of disease. J Infect 2019; 79: 115–122. doi: 10.1016/j.jinf.2019.05.013. Epub 2019 May 21. PMID: 31125639. [DOI] [PubMed] [Google Scholar]
- 21.Liu P, Li X, Luo M, et al. Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Infection: A Meta-Analysis. Microb Drug Resist 2018; 24: 190–198. doi:10.1089/mdr.2017.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miltgen G, Garrigos T, Cholley P, et al. Nosocomial cluster of carbapenemase-producing Enterobacter cloacae in an intensive care unit dedicated COVID-19. Antimicrob Resist Infect Control 2021; 10: 151. doi: 10.1186/s13756-021-01022-6. PMID: 34674756; PMCID: PMC8529563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández P, Moreno L, Yagüe G, et al. Colonization by multidrug-resistant microorganisms in ICU patients during the COVID-19 pandemic. Med Intensiva (Engl Ed) 2021; 45: 313–315 [Article in English, Spanish]. doi: 10.1016/j.medin.2021.02.015. Epub 2021 Mar 10. PMID: 33775434. [Google Scholar]
- 24.Jeon K, Jeong S, Lee N, et al. Impact of COVID-19 on Antimicrobial Consumption and Spread of Multidrug-Resistance in Bacterial Infections. Antibiotics (Basel) 2022; 11: 535. doi: 10.3390/antibiotics11040535. PMID: 35453286; PMCID: PMC9025690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dautzenberg MJ, Wekesa AN, Gniadkowski M, et al. The association between colonization with carbapenemase-producing enterobacteriaceae and overall ICU mortality: an observational cohort study. Crit Care Med 2015; 43: 1170–1177. doi: 10.1097/CCM.0000000000001028. PMID: 25882764; PMCID: PMC4431676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzouvelekis LS, Markogiannakis A, Psichogiou M, et al. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25: 682–707. doi:10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]