Abstract
Background
Leprosy is primarily a disease of peripheral nerves. Some isolated case reports and case series have communicated imaging changes in the central nervous system (CNS) and brachial plexus in patients with leprosy.
Objectives
To study the neuroimaging abnormalities in patients with lepra bacilli-positive neuropathy in the context of CNS, spinal root ganglion, and brachial plexus.
Design
Prospective observational study
Methods
We screened newly-diagnosed patients with multibacillary leprosy presenting with neuropathy. Patients with bacilli-positive sural nerve biopsies were included in the study and subjected to magnetic resonance imaging (MRI) of the brain and spinal cord.
Results
A total of 54 patients with bacteriologically confirmed multibacillary leprosy were screened; Mycobacterium leprae was demonstrated in the sural nerve biopsies of 29 patients. Five patients (5/29; 17.24%) had MRI abnormalities in CNS, spinal root ganglion, and/or brachial plexus. Three patients had MRI changes suggestive of either myelitis or ganglionitis. One patient had T2/FLAIR hyperintensity in the middle cerebellar peduncle while 1 had T2/FLAIR hyperintensity in the brachial plexus.
Conclusion
CNS, spinal root ganglion, and brachial plexus are involved in patients with leprous neuropathy. Immunological reaction against M leprae antigen might be a plausible pathogenetic mechanism for brachial plexus and CNS imaging abnormalities.
Keywords: Brain, brainstem, Hansen’s disease, Mycobacterium leprae, spinal cord, myelitis
Introduction
Leprosy is a chronic disabling condition caused by a bacillus, Mycobacterium leprae. Leprosy predominantly affects the peripheral nerves, skin, mucosa of the upper respiratory tract, eyes, and the reticuloendothelial system. According to World Health Organization1 Southeast Asian region report, 71% of all the global cases are from Southeast Asia.
Generally, it is believed that Mycobacterium leprae does not affect the central nervous system. GHA Hansen, himself, always believed that the brain was never affected by leprosy. However, later several autopsy reports indicated a possible affliction of the brain by lepra bacilli. In a series of autopsies performed on 10 lepers, adhesions between the dura mater and brain were observed in 4 patients; lepra bacilli were demonstrated in 3 of these 4 patients.2 In a Japanese autopsy study, Aung and co-workers explored CNS involvement in 67 clinically-cured patients with lepromatous leprosy. Paraffin sections of the medulla oblongata and spinal cord were subjected to hematoxylin and eosin staining, Fite acid-fast staining, and antiphenolic glycolipid-I immunostaining. Sixty-seven percent (44/67) had vacuolar changes in motor neurons of the medulla oblongata and/or spinal cord. The polymerase chain reaction (PCR) test demonstrated the presence of Mycobacterium leprae DNA in almost all cases with vacuolated changes. Even in the majority of patients without vacuolated changes, Mycobacterium leprae DNA was isolated. In controls, leprosy-related findings were not recorded.3 Lee and colleagues reported a patient who had a cystic lesion in the right frontal lobe and was erroneously diagnosed with glioma. Histopathology of the resected brain tissue demonstrated red granulomatous inclusion on Fite acid-fast staining indicative of lepra bacilli. Nested polymerase chain reaction amplification and DNA sequencing further proved the presence of M leprae genome.4 Neuroimaging abnormalities of the brain, brainstem and spinal cord have also been shown in some case reports and case-series.5-7
In the absence of any systematic assessment, we planned this prospective study to evaluate neuroimaging abnormalities of the brain, spinal cord, and brachial plexus in patients with multibacillary leprosy.
Material and methods
The study was conducted between September 2017 and January 2020 in King George’s Medical University, Lucknow, India; a tertiary care University hospital situated in a leprosy endemic area. Ethical approval for the study (Ref. Code: 88th ECM IIB-Thesis/P39) was obtained from the Institutional Ethics Committee of King George’s Medical University, U.P., Lucknow (Registration No.: ECR/262/Inst/UP/2013/RR-16). Written informed consent to participate in the study, and publication of anonymised data was obtained from every subject or their legal guardian.
Inclusion criteria
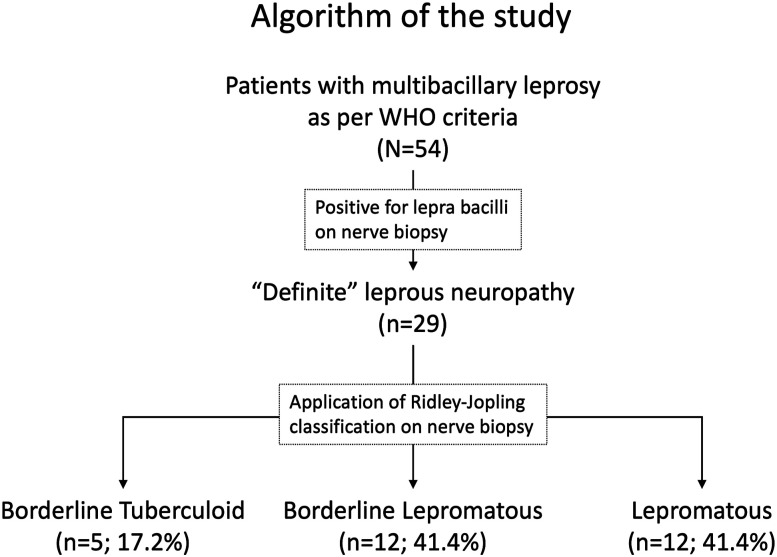
In this prospective study, consecutive newly-diagnosed patients of peripheral neuropathy with multibacillary leprosy were screened. As per World Health Organization8 guidelines, a multibacillary case was defined as those with >5 skin lesions; those with bacilli-positive slit skin smear irrespective of several skin lesions; or those having neuropathy (either pure neuritis or a combination of any number of skin lesions and neuritis). Sural nerve biopsy was performed in all cases; those patients where bacilli were demonstrated on nerve biopsy were finally included for further evaluation by neuroimaging in the study. Such patients were labeled as having “definite” leprous neuropathy. The algorithm of the study is provided in Figure 1.
Figure 1.
Algorithm of the study.
Exclusion criteria
(1) History of Diabetes mellitus/alcoholism/toxin exposure/drug-induced
(2) Hepatitis B or Hepatitis C
(3) Human Immunodeficiency virus infection
(4) Vasculitis/Connective tissue disorder
Work-Up
A detailed clinical evaluation of every patient with multibacillary leprosy was performed. Cardinal features of leprosy - hypopigmented or erythematous skin patch with loss of sensation, positive slit skin smear, and thickened or enlarged peripheral nerves were evaluated. Supraorbital, great auricular, ulnar, superficial radial, and superficial peroneal were examined to detect thickened peripheral nerves. Patients were also evaluated for disability. For disability assessment, the Eye, Hand, and Feet (EHF) disability scale was used.9
Haematological evaluation, including complete blood count, liver function test, renal function test, erythrocyte sedimentation rate (ESR), anti-nuclear antibody (ANA), vasculitis screen, hepatitis B and C testing, and human immunodeficiency virus-enzyme-linked immunosorbent assay was performed in every patient.
Sural nerve biopsies, measuring at least 3.0 cm in length, were taken and dispatched for histopathological examination in glass or plastic vials containing 2.5% glutaraldehyde solution. Hematoxylin and Eosin Staining, and Wade-Fite Staining were done as per standard protocol. The nerve tissue sections were examined for granuloma, lymphocytic infiltrations, foamy histiocytes, perivascular and perineural inflammation.
Ridley-Jopling criteria was applied to bacilli-positive nerve biopsies and based on histopathogical characteristics these were classified into mid borderline leprosy (BB), borderline lepromatous leprosy (BL), and lepromatous leprosy (LL) groups.10,11 Figure 1 BB was diagnosed if nerves biopsy showed the presence of scattered epithelioid cells, lymphoid cells, and foamy histiocytes. Well-formed granulomas or necrosis were absent and lepra bacilli were demonstrated in such cases. In the BL category nerve tissue demonstrated intense endoneurial infiltrates of foamy histiocytes. Epithelioid histiocytes, necrosis, or giant cells were absent and lepra bacilli were demonstrated in abundance. LL was diagnosed if nerves tissues showed the diffuse presence of foamy cells. No lymphocytes, epithelioid histiocytes, necrosis, or giant cells were seen. Numerous lepra bacilli could be demonstrated.11 No patient was classified into tuberculoid leprosy (TT) and borderline tuberculoid leprosy (BT), categories that are essentially negative for lepra bacilli.
Lepra reactions
A type-1 lepra reaction was characterized by erythema and edema of the skin lesion. A type-2 lepra reaction was characterized by the appearance of new tender erythematous lesions anywhere on the body, along with destructive neuritis, malaise, and fever. In Type-2 reaction systemic involvement in form of lymphadenopathy, arthralgia, iridocyclitis, and epididymo-orchitis. Neuritis was characterized by pain, tenderness, or loss of function.12
Magnetic resource imaging
Magnetic resonance imaging was performed on a Signa Explorer 1.5 T instrument (General Electric Medical Systems, USA). The MRI protocol consisted of T1 and T2 axial and sagittal images, and coronal short tau inversion recovery (STIR) sequences for the spine and plexus. For the assessment of the brain, T1 and T2 axial and sagittal images, T2 coronal images, fluid attenuated inversion recovery (FLAIR), diffusion-weighted and gradient recall echo axial images were obtained. Gadolinium-enhanced images were used to look for contrast uptake.
Treatment
All patients were treated with multi-drug therapy, consisting of dapsone, rifampicin and clofazimine, along with steroids.13
Statistical analysis
Data were collected and analyzed using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) software. Descriptive statistics for variables were described using frequencies and percentages.
Results
A total of 54 patients with multibacillary leprosy were assessed. Of these, Wade-Fite positivity in sural nerve biopsies was observed in 29 patients (“definite” leprous neuropathy) and included in the study for further assessment; these patients were subjected to neuroimaging (n = 29). Slit skin smear was positive in 82.76% (24/29) patients.
The median age at the time of presentation was 30 years. The majority of included patients were male (26/29). Mononeuropathy multiplex (82.7%) was the most frequent pattern of nerve involvement. Polyneuropathy and mononeuropathy were observed in 13.8% and 3.5%, respectively. (Table 1) Nerve conduction studies showed a mixed pattern (axonal plus demyelinating) in 62.1% (18/29) patients while pure axonal and demyelinating patterns were noted in 20.7% (6/29) and 17.24% (5/29) patients, respectively. All had deformities, with or without trophic ulcers, and their mean EHF score was 6.8.
Table 1.
Baseline clinical characteristics of the leprosy patients that were evaluated for central nervous system, spinal root ganglion and brachial plexus involvement (n = 29).
| Clinical Characteristics | Values |
|---|---|
| Age (in years) | |
| Mean ± SD | 34.59 ± 13.91 |
| Median | 30 |
| Range | 14-63 |
| Sex | |
| Male | 26 (89.7%) |
| Female | 3 (10.3%) |
| Type of neuropathy | |
| Mononeuropathy multiplex | 24 (82.7%) |
| Distal symmetrical polyneuropathy | 4 (13.8%) |
| Mononeuropathy | 1 (3.5%) |
| Duration of illness (months) | |
| Mean ± SD | 14.13 ± 14.7 |
| Range | 1-84 |
| Cranial nerve involvement | |
| Facial nerve palsy | 3 (10.35%) |
| Trophic ulcersa | 13 (44.8%) |
| Upper limb | 7 |
| Lower limb | 8 |
| Claw hand | 17 (58.6%) |
| Foot drop | 11 (37.9%) |
| Hypopigmented/Erythematous patches | |
| >10 | 7 (24.1%) |
| ≤10 | 22 (75.9%) |
| Lepra reaction | 3 (10.35%) |
| Type-1 | 0 |
| Type-2 | 3 |
| Eye, hand and feet disability (EHF) score | |
| Mean | 4.01 ± 2.14 |
| Median | 4 |
| Ridley jopling classification | |
| Borderline tuberculoid | 5 (17.2%) |
| Borderline lepromatous | 12 (41.4%) |
| Lepromatous | 12 (41.4%) |
a= 2 patients had lesions both in upper and lower limbs.
Five patients (17.24%) had definite CNS neuroimaging abnormalities. One patient had T2/FLAIR hyperintensity of the middle cerebellar peduncle. (Figure 2) 3 patients had MRI changes suggestive of myelitis and/or ganglionitis. (Figure 3, Figure 4 and Figure 5) Contrast enhancement was seen in 3 patients with brachial plexus, dorsal root ganglion and spinal cord lesion. (Figure 6) (Table 2)
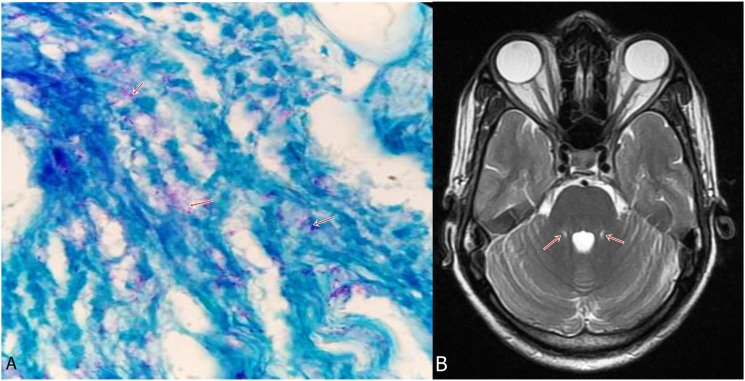
Figure 2.
A 40-year-old man presented with sensory motor polyneuropathy, lower motor neuron facial palsy and thickened bilateral peroneal nerves. He also had an erythematous hypo-anesthetic patch on his face. There was no suggestion of appendicular or gait ataxia. Sural nerve biopsy demonstrated lepra bacilli (A, arrows) (Wade Fite stain, x1000). MRI brain demonstrated bilateral symmetrical hyperintensities in middle cerebellar peduncles (B, arrows). (Case 1).
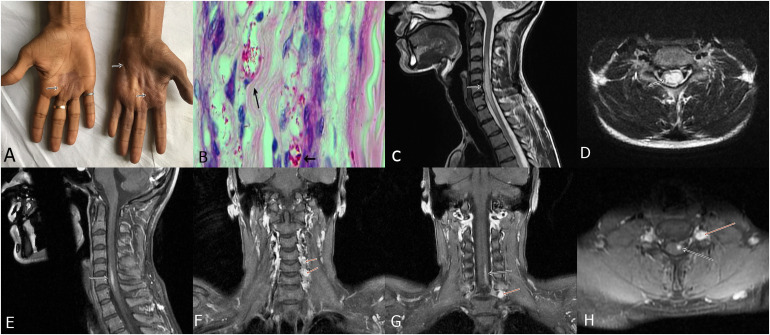
Figure 3.
A 28-year-old man presented with multiple anesthetic patches on the body, weakness of both the upper limbs and clawing. Ulnar nerves were thickened. Hyperpigmented and anesthetic skin lesions were present on the hands along with wasting of hand muscles (A, arrows). Sural nerve biopsy demonstrated lepra bacilli forming globi (Wade Fite stain, x1000) (B, arrows). MRI of the spine demonstrated a long segment hyper intensity along with cord swelling (myelitis) in the cervical region (C, arrow). Axial cuts showed involvement of the central spinal cord (D, arrow); post-contrast image showed enhancement of the lesion (E, arrow). Coronal post-contrast images depicted enhancement of the dorsal root ganglion (Ganglionitis) (F, arrows). Coronal and axial post contrast image demonstrated contrast enhancement of spinal cord lesion as well as ganglion (G and H, arrows). (Case-2).
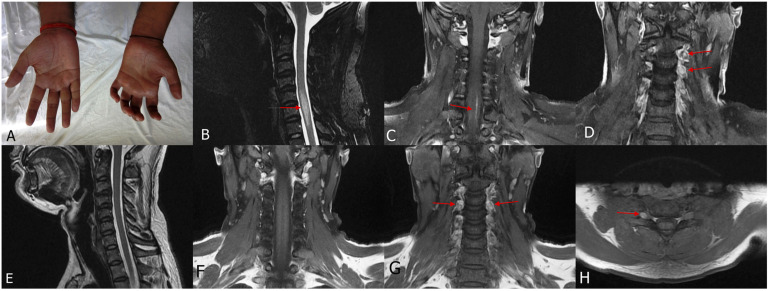
Figure 4.
A 28-year-old man presented with polyneuropathy, thickened nerves and trophic ulcers. Claw hand deformity was present (A). MRI cervical spine T2 sagittal (B) and post contrast coronal (C and D) showed hyperintensity in the cervical spinal cord with contrast enhancement (myelitis) along with contrast enhancement of dorsal root ganglion (ganglionitis). MRI was repeated after 6 months, which showed resolution of myelitis (E and F) but persistence of ganglionitis (G and H). (Case 3).
Figure 5.
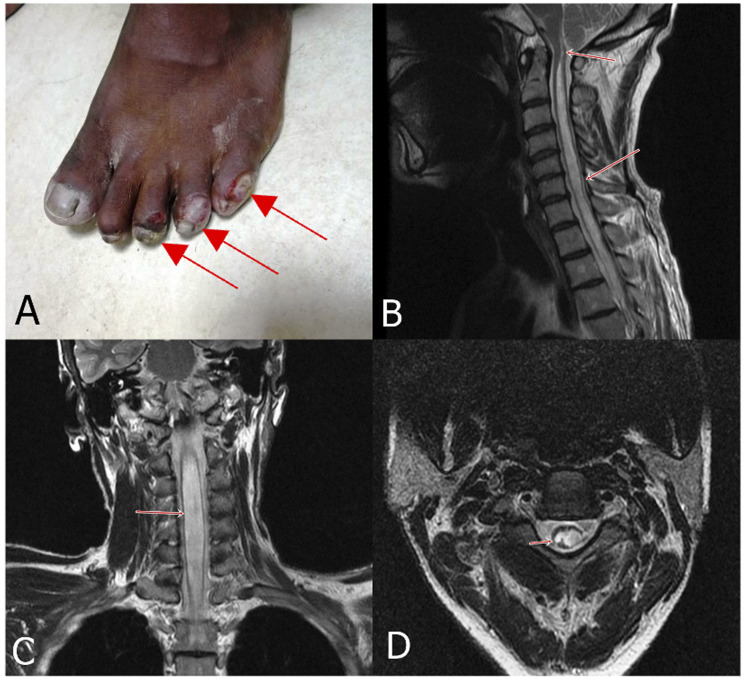
A 58-year-old man, well-controlled hypertensive, presented with mononeuritis multiplex with multiple thickened nerves and trophic ulcers over foot. Besides brisk deep tendon jerks of the lower limbs, no features suggestive of spinal cord involvement were noted. (A) MRI cervical spine showed long segment hyperintensity involving the cervical spinal cord, which extended up to the brain stem. Sagittal (B), Coronal (C) and axial (D) T2 images showed hyperintensity within the cervical spinal cord. (Case 4).
Figure 6.
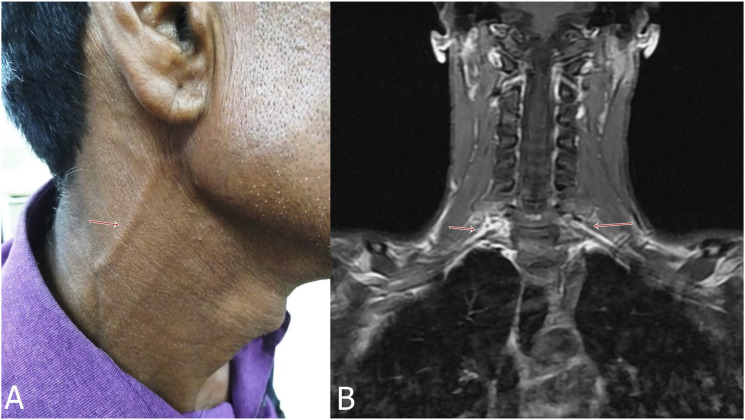
A 25-year-old man presented with mononeuritis multiplex. He also had proximal weakness of both the upper limbs. There was thickening of great auricular nerve (A, arrow), besides thickening of ulnar and superficial radial nerves. MRI demonstrated contrast enhancement of bilateral brachial plexus (B, arrows). (Case-5).
Table 2.
Characteristics of leprosy patients with central nervous system, spinal root ganglion and brachial plexus involvement.
| Clinical Characteristics | Case-1 (VP) | Case-2 (RK) | Case-3 (SM) | Case-4 (KS) | Case- 5 (AR) |
|---|---|---|---|---|---|
| Age (Years) | 40 | 28 | 28 | 58 | 25 |
| Sex | Male | Male | Male | Male | Male |
| Type of neuropathy | Polyneuropathy | Mononeuritis multiplex | Polyneuropathy | Mononeuritis multiplex | Mononeuritis multiplex |
| Cranial nerve involvement | Facial palsy | Facial palsy | None | None | None |
| Eye hand and feet disability score | 6 | 8 | 8 | 8 | 4 |
| Ridley jopling category | Lepromatous | Lepromatous | Borderline lepromatous | Lepromatous | Lepromatous |
| CNS manifestation | Middle cerebellar peduncle hyperintensity | Myelitis and ganglionitis | Myelitis and ganglionitis | Myelitis | Brachial plexitis |
CNS= Central nervous system; CSF= cerebrospinal fluid.
Discussion
We prospectively evaluated 29 patients, with bacilli-positive nerve biopsy, with a dedicated neuroimaging protocol. Three patients had spinal cord involvement similar to that of longitudinally extensive transverse myelitis. One patient each had T2 hyperintensity involving both middle cerebellar peduncles and evidence of brachial plexitis.
Our study is noteworthy in performing a dedicated prospective analysis in the absence of any CNS features. To increase the probability of detecting any neuroimaging abnormality, we screened the cases as per the WHO definition for multibacillary leprosy and included only those where nerve biopsies were positive for lepra bacilli.8,14 The reason to include only bacilli-positive nerve biopsy cases was to increase the level of conviction in associating peripheral neural tissue involvement with that of CNS. The biopsies, in turn, were categorized by applying Ridley-Jopling criteria11; in a subsequent study, such categorization was further validated.15 The results of this study also address the concerns associated with mislabelling multibacillary leprosy as paucibacillary.14
As a standard procedure, we did a sural nerve biopsy in all patients screened in our study. The sural nerve, besides being superficial and sensory in the constitution, provides a larger substrate as a biopsy specimen.11,16 Importantly, it is 1 of those nerves that is routinely sampled to rule out alternative diagnoses such as vasculitis, amyloidosis, gammopathies, neurolymphomatosis, etc., the significance of which continues to be reiterated.17,18
Some earlier reports had similar observations. Polavarapu and co-workers reported 8 cases of leprosy with CNS involvement. Two patients had brainstem lesions, 1 with enhancing facial nuclei and nerves, while another had a lesion in the nucleus ambiguus.6 Lesions in the local or regional area were described in a few cases; only 1 of our patients (Case 1), had an erythematous hypo-anesthetic patch over the face. Polavarapu et al.6 also described a case with multiple lower cranial nerve palsies. Generally, facial and trigeminal nerves are the 2 most frequently involved cranial nerves.19 In a report, abnormalities in brain stem auditory-evoked potentials were detected indicating that the brainstem can be affected in severe forms of leprosy.20 In an autopsy-based study, Mycobacterium leprae-specific DNA has been demonstrated in the brainstem. It has been postulated that the bacteria travels along the cranial nerves, to reach the brainstem.3
In addition to the imaging abnormalities of the spinal cord, imaging changes in dorsal root ganglion and brachial plexus have also been described.21 Dorsal root ganglion and brachial plexus are parts of the peripheral nervous system but these structures are not typically affected in leprosy.6 Khadilkar and colleagues described a case of multibacillary leprosy where an MRI of the spinal cord showed hyperintensity in the cervical cord at the C5-6 level. There was enlargement along with enhancement of the left dorsal root ganglion at the C5-6 level. In this case, cutaneous lesions were present in the upper limbs and the patient had dominant involvement in the upper extremities.7 In another report, Rice and co-workers described a patient with mononeuritis multiplex involving dominantly upper limbs. MRI demonstrated expansion of the cervical cord with intramedullary T2 hyperintensity, at the C5-C7 level. Spinal cord lesions were remarkably apparent on the STIR sequence.22 Enhancing gray-matter spinal cord lesions were reported by Polavarapu et al in 7 patients. Follow-up MRI performed in 3 cases showed complete resolution of the cord lesion in 2 cases, whereas faint enhancement was still observed in 1 case. Affected MRI spinal cord segmental levels largely corresponded to an affected cutaneous dermatome. The patient having cutaneous lesions involving lower limbs had linear hyperintensity in the conus medullaris region as well.6
The Polavarapu group postulated 3 possible pathogenetic mechanisms for MRI abnormalities in leprosy: (1) a retrograde spread of Mycobacterium leprae via peripheral nerve, plexus, nerve roots, and then to the spinal cord; (2) transection of axons of peripheral nerves or nerve roots may result in reactive changes in the spinal cord; and (3) immunological reaction against bacterial antigen as the most plausible cause of CNS involvement.6 We hypothesize that the Mycobacterium leprae antigen might have triggered auto-reactive T cells and subsequently CNS damage. Jacob and co-workers described a young patient with leprosy who developed a demyelinating disorder of the central and peripheral nervous system. CSF examination showed inflammatory changes. In addition to multidrug therapy, the patient was treated with intravenous methylprednisolone. He responded well and was able to walk independently. Possibly, CNS demyelinating disorder was produced by the phenomenon of molecular mimicry by cross-reacting epitopes. Autoreactive T cells attack the myelin sheath of nerve axons.23
An earlier publication also discusses the possibility of lacunar lesions in the brain secondary to endothelial involvement as a result of Mycobacterium leprae. This was concluded based on computed tomography of the brain done in 6 patients with leprosy. We feel that these findings need review in light of non-specific white matter changes, asymptomatic lacunar infarcts, and enlarged perivascular spaces of unknown significance.24
None of the patients with CNS involvement had any clinical feature referable to brain or spinal cord involvement, except 1 patient who had hyperreflexia of lower limbs (Case 4). It may be noted that with a high the eye-hand-foot impairment score (EHF score), symptoms of neuropathy might have overshadowed CNS-related manifestations.
Our study had some limitations. It was difficult to analyze the significant predictors of CNS manifestations in leprosy as we could identify only 4 patients with CNS involvement. Follow-up imaging was performed in only 1 patient (Case 3).
To conclude, CNS involvement may be seen in patients with (definite) leprous neuropathy. The spinal cord and brainstem can be involved besides the involvement of brachial plexus and ganglions. Immunological reaction against Mycobacterium leprae antigen appears more likely as a pathogenic mechanism responsible for MRI abnormalities of the brain, spinal cord, and brachial plexus than the retrograde spread of lepra bacilli.
Acknowledgments
We acknowledge the cooperation and support of our patients with leprosy without whom the conduct of this study was not possible
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Ravindra Kumar Garg https://orcid.org/0000-0003-0044-7083
Hardeep Singh Malhotra https://orcid.org/0000-0003-0438-4446
References
- 1.World Health Organization . Leprosy: New Data Show a Steady Decline in New Cases; 2019. https://www.who.int/neglected_diseases/news/Leprosy-new-data-show-steady-decline-in-new-cases/en/ [Google Scholar]
- 2.Ashmead AS. Lepra Bacilli Found in the Brain. JAMA. 1899;XXXII(1):40. doi: 10.1001/jama.1899.02450280048015 [DOI] [Google Scholar]
- 3.Aung T, Kitajima S, Nomoto M, et al. Mycobacterium leprae in neurons of the medulla oblongata and spinal cord in leprosy. J Neuropathol Exp Neurol. 2007;66(4):284-294. [DOI] [PubMed] [Google Scholar]
- 4.Lee KH, Moon KS, Yun SJ, et al. Brain involvement by presenting as a frontal cystic lesion. J Neurosurg. 2014;121:184-188. [DOI] [PubMed] [Google Scholar]
- 5.Baveja S, Sandhu S, Vashisht D. A Rare Case of Possible Vacuolar Degeneration of Leprosy in Brain with Segmental Necrotizing Granulomatous Neuritis and Horner's Syndrome. Indian Dermatol Online J. 2019;10:444-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polavarapu K, Preethish-Kumar V, Vengalil S, et al. Brain and Spinal Cord Lesions in Leprosy: A Magnetic Resonance Imaging-Based Study. Am J Trop Med Hyg. 2019;100(4):921-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khadilkar SV, Kasegaonkar PS, Ursekar M. Spinal cord involvement and ganglionitis in leprosy. Neurol India. 2007;55:427-428. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . Global Leprosy Strategy 2016-2020. Monitoring and Evaluation Guide. Downloaded from https://apps.who.int/iris/bitstream/handle/10665/208824/9789290225096_en.pdf, 2022. [Google Scholar]
- 9.Brandsma JW, Van Brakel WH. WHO disability grading: operational definitions. Lepr Rev. 2003;74(4):366-373. [PubMed] [Google Scholar]
- 10.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34(3):255-273. [PubMed] [Google Scholar]
- 11.Hui M, Uppin MS, Challa S, Meena AK, Kaul S. Pure neuritic leprosy: Resolving diagnostic issues in acid fast bacilli (AFB)-negative nerve biopsies: A single centre experience from South India. Ann Indian Acad Neurol. 2015;18(3):292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The International Federation of Anti-Leprosy Associations (ILEP) . How to Recognise and Manage Leprosy Reactions. : The International Federation of Anti-Leprosy Associations (ILEP); 2002. Downloaded from https://www.leprosy-information.org/resource/ilep-learning-guide-two-how-recognise-and-manage-leprosy-reactions [Google Scholar]
- 13.World Health Organization . Global Leprosy Strategy 2016-2020. Operational Manual. https://www.who.int/publications/i/item/9789290225096 [Google Scholar]
- 14.Lockwood DN, Sarno E, Smith WC. Classifying leprosy patients—searching for the perfect solution? Lepr Rev. 2007;78:317-320. [PubMed] [Google Scholar]
- 15.Kulshreshtha D, Malhotra KP, Malhotra HS, et al. Mandating nerve biopsy: A step towards personalizing therapy in pure neuritic leprosy. J Peripher Nerv Syst. 2018;23(3):190-196. [DOI] [PubMed] [Google Scholar]
- 16.Dyck PJ, Lofgren EP. Nerve biopsy. Choice of nerve, method, symptoms, and usefulness. Med Clin. 1968;52(4):885-893. [PubMed] [Google Scholar]
- 17.Oh SJ. Diagnostic usefulness and limitations of the sural nerve biopsy. Yonsei Med J. 1990;31(1):1-26. doi: 10.3349/ymj.1990.31.1.1 [DOI] [PubMed] [Google Scholar]
- 18.Nathani D, Spies J, Barnett MH, Pollard J, Wang MX, Sommer C, Kiernan MC. Nerve biopsy: Current indications and decision tools. Muscle Nerve. 2021;64:41-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Alexander M, Gnanamuthu C. Cranial nerve involvement in patients with leprous neuropathy. Neurol India. 2006;54(3):283-285. [DOI] [PubMed] [Google Scholar]
- 20.Celik O, Yalcin S, Gok U, Yavrucuoglu E, Ozturk A, Akyol A. Auditory brain stem evoked potentials in patients with leprosy. Int J Lepr Other Mycobact Dis. 1997;65(2):166-169. [PubMed] [Google Scholar]
- 21.Bafna P, Sahoo RR, Manoj M, Wakhlu A. Ganglionitis and myelitis: myriad neurological manifestations of Hansen's disease. BMJ Case Rep. 2020;13(8):e236813. doi: 10.1136/bcr-2020-236813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice CM, Oware A, Klepsch S, et al. Leprous ganglionitis and myelitis. Neurol Neuroimmunol Neuroinflamm. 2016;3(3):e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacob J, Alexander M, Aaron S, Pulimood S, Walter N, Gnanamuthu C. Acute disseminated encephalomyelitis in Hansen's disease. Ann Indian Acad Neurol. 2006;9:166-168. [Google Scholar]
- 24.Dumas JL, Hugon J, Brugieres P, et al. Impairment of central nervous system in leprosy: CT and MRI. In: du Boulay G, Molyneux A, Moseley I, eds Proceedings of the XIV Symposium Neuroradiologicum. Berlin, Heidelberg: Springer; 1991:222-224. [Google Scholar]